Desiccant materials for moisture control in buildings
Abstract:
Desiccant is a material for modifying humidity; the chapter explains the absorption and adsorption desiccant processes. The chapter reviews the commercial, solid and liquid desiccant processes, together with selected practical applications. Health, comfort and air quality, as well as energy coefficient of performance (COP), energy efficiency ratio (EER) and seasonal energy efficiency ratio (SEER) are discussed. Finally the chapter looks to the future and reviews the more natural approach to modifying building humidity with normal building materials and reviews practical applications.
15.1 Introduction
Sorption is the binding of one substance to another. Sorbents are materials that have the ability to attract and hold other gases or liquids. Desiccants are a subset of sorbents.
All desiccants behave in a similar way; they attract moisture until they reach equilibrium with their surroundings. Moisture may be removed from a desiccant by heating. Once the desiccant is dry it must be cooled before it can attract moisture once again.
Sorption generates sensible heat equal to the latent heat of the water taken up by a desiccant, isosteric enthalpy. In addition, the heat of sorption varies between 5 and 25% of the latent heat of water vapour which is dependent on the permeability/hygroscopicity of the desiccant. These are two distinct functions.
The process of attracting and holding moisture is described as either absorption or adsorption, depending on whether the desiccant undergoes change as it takes in moisture. Absorption changes the desiccant. An example of an absorbent is table salt which changes from a solid to a liquid as it absorbs moisture. Adsorption does not change the desiccant, except by the addition of the weight of water adsorbed. An example of adsorption is a sponge.
Virtually all materials are desiccants; that is they attract and hold water vapour. Wood, natural fibres, clays, and many synthetic materials attract and release moisture as commercial desiccants do, but they lack holding capacity. Commercial desiccants continue to attract moisture even when the surrounding air is quite dry, a characteristic that the other materials do not share.
Desiccants, whether liquid or solid, hold moisture through absorption or adsorption. Most absorbents are liquids and most adsorbents are solids.
15.2 Desiccant cycle
All desiccants function by moisture transfer caused by a difference between water vapour pressures at their surface and that of the surrounding air (Fig.15.1).
Liquid absorption is a dehumidification process. The vapour pressure of liquid absorption is directly proportional to temperature and inversely proportional to its concentration. The vapour pressure of a given concentration of absorbent solution approximates to the vapour pressure value of a fixed relative humidity line on a psychrometric chart. Higher concentrations provide a lower equilibrium and relative humidity, which allow the absorbent to dry air to lower levels (Fig. 15.2).
Adsorbents are solid materials with a tremendous internal surface area per unit of mass that attract moisture because of the electrical field at the material’s surface. This field is not uniform in either force or charge. When the complete surface is covered, the adsorbent can still hold more moisture because vapour continues to condense into the first layer and fill the capillaries throughout the material. As with absorbents, the ability of an adsorbent to attract moisture depends on the vapour pressure between its surface and the air.
Adsorption behaviour depends on total surface area, the total volume of the capillaries and the range of capillary diameters. A large surface area will give the adsorbent a larger capacity at low relative humidity. Large capillaries provide a high capacity at high relative humidity. A narrow range of capillary diameters makes an adsorbent more selective in the vapour molecules it can hold.
Water molecules migrate slowly through solid materials by diffusion, propelled by the difference between water vapour pressures on each side of the material. The molecules move slightly faster when the material is more permeable/hygroscopic and the greater the water vapour pressure difference. The unit that defines water vapour movement through a material is called ‘perm’. One perm is the number of nanograms per second that pass through one square metre with a pressure difference of one Pascal.
15.3 Desiccant applications
Desiccants can dry liquids or gases, including moisture from air and are used in many air conditioning applications. It is the air conditioning application that is of interest. Desiccant systems have already been implemented in many buildings. The technology promises to advance building technology in the 21st century and help the individual achieve ‘the right to healthy indoor air’ with minimal environmental impact.
15.4 Health and comfort
If humidity in the air has nowhere to go and levels rise, this can create an ideal environment for mites and mould, which start to thrive above 50% relative humidity (rainy day is 100% RH and middle of the desert is 0% RH). This is represented by a maximum absolute moisture content of 8 g/ kg at 21 °C in the space; below 40% RH the mites die. Humans are most comfortable between 40 and 60% RH, i.e. 6–9 g/kg at 21 °C.
The influence of moisture on people is one of the least understood of all the comfort factors.
• Comfort is affected by skin wetness; skin also requires water content to remain supple.
• Dry throats and noses lower the resistance to infection.
• Dry itchy eyes in very dry conditions lead to a breakdown in tear film.
• Clothing is influenced by moisture, both insulation and permeability. The electrical properties are also affected and can create electrostatic charges as well as in surrounding materials.
• Health and allergies are affected; very dry conditions lower the resistance to infection and damp conditions enable moulds and mites to flourish.
• Odour, irritant and dust effects are perceived worse at low humidities; the release of formaldehyde gas is also affected by humidity.
Figure 15.3 shows the modes of heat loss at various temperatures of a clothed sedentary person.
It is interesting to note that most comfort surveys treat moisture as an independent factor unrelated to comfort. Studies show that a 30% increase in relative humidity is equal to a 1 °C rise in temperature. Thus, if humidity levels are reduced then ‘comfort’ temperatures could be allowed to rise (Fig.15.4).
Moisture is an important factor in our comfort and health. Tests and studies confirm sensitivity to both dry and humid conditions. The time taken for these sensitivities to impact vary from 15 minutes to an hour for eye dryness to a few hours for body dryness and weeks for an overall feeling of unpleasant dryness. In humid conditions the sensitivities impact more quickly, skin becomes damp, the atmosphere becomes sultry and we overheat.
Research evidence indicates that at low temperatures of 20 °C and high relative humidities of 65% people are the most comfortable; however, the modern trend is to provide higher temperatures, especially in passive buildings, which makes it difficult to achieve healthy and comfortable moisture conditions (Fig. 15.5).
15.5 Air quality
Indoor air pollution poses many challenges to health. Studies have shown that people spend more than 90% of their time indoors. Volatile organic compounds (VOCs) are a class of pollutants that pose a risk to human health since they can be toxic at very low concentrations (ppb). The adverse health effects caused by these compounds can range from minor complaints, such as irritation of the nasal and ocular mucosa, to chronic complications such as the exacerbation of asthma.
Removal of VOCs by conventional means such as photo catalytic oxidation, ionization, condensation and ozone oxidation have proved insufficient due to the nature of VOCs and their very low concentrations in the air. The recent focus has been on sorption filtration using desiccant materials for the simultaneous removal of both water vapour and other pollutants from air (co-sorption) as it is an energy efficient process eliminating the need for cooling air to dew point temperatures to remove water vapour.
Experimental and predictive methods have been used to determine the suitability of adsorbents for pollutant removal. The results indicate an improved indoor environmental quality (IEQ) where desiccants were used to clean the air. Adsorption involves competition for binding sites on the surface of the adsorbent by the adsorbing molecules. Research has established that although competition exists between water and the pollutant molecules on desiccants, their capacity for adsorption remains relatively unaffected.
15.6 Natural and commercial desiccants: typical materials
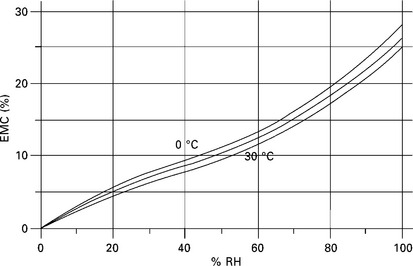
It is the air surrounding the material that controls its water content. The exact water content of a material depends on the temperature and moisture content of the surrounding air. The material permeability/hygroscopicity will determine its moisture content relative to that of the surrounding air. Each material has its own sorption isotherm which is a graph of equilibrium moisture content (EMC) versus relative humidity at a constant temperature (Fig. 15.6). Many materials produce similar results, e.g. cotton, lime, linen, silica gel, silk, wood, wool and concrete.
The amount of water in the air is a negligible portion of the water content in the material. Thus when the temperature is increased, the relative humidity of the air is only marginally increased. But the absolute moisture content of the air increases as a direct result of the change of temperature, whilst the moisture content of the material stays relatively constant. Thus the equilibrium curve moves horizontally across the graph. Removing the material from the air whilst reducing the temperature of the air will create an increase in relative humidity. If the material had remained whilst the air temperature was reduced, then the relative humidity would only have fallen slightly. In summary, water absorbent materials tend to keep relative humidity constant. A change in material temperature will cause a change in the surrounding air. Moisture mass is the equivalent of thermal mass in ‘passive design’. Natural moisture open, hygroscopic, materials such as clay, hemp, lime plaster, wood and mineral paints can provide ceilings, floors and walls in buildings that are reactive to moisture.
Mechanical ventilation is a crude tool for removing moisture and is less efficient than hygroscopic materials. The use of hygroscopic materials in buildings must be an essential part of ‘passive design’.
Commercial desiccants, liquids or solids hold moisture through absorption or adsorption. Most absorbents are liquids and most adsorbents are solids.
Liquid absorption dehumidification is best illustrated by comparison with an air washer. When air passes through an air washer, its dew point approaches that of the water temperature. In a similar manner, in a liquid absorption dehumidifier, as the vapour pressure of the solution is lower than the vapour pressure of the moisture in the air (at the same temperature), it is dehumidified. The concentration of an absorbent solution approximates to a fixed humidity line on a psychrometric chart.
In practice, the behaviour of a liquid desiccant is controlled by its temperature, concentration or both. Commercially available liquid desiccants have an especially high water holding capacity. For example each molecule of lithium chloride (LiCl) can hold two water molecules, in a dry state. Above two it becomes a liquid and continues to absorb water; in equilibrium with air at 90% RH 26 molecules of water is held. This is equivalent to 1000% of water absorption by weight.
Absorbents are solid materials with a tremendous internal surface area per unit of mass. They resemble a sponge; a single gram can have more than 5000 m2 of surface area. Adsorbents attract moisture due to the electrical field at the surface of the desiccant. The field is not uniform so specific sites attract water molecules that have a net opposite charge. As with liquid absorbents the ability of an adsorbent to attract moisture depends on the vapour pressure difference between its surface and the air.
The capacity of solid adsorbents is generally less than liquid absorbents. A typical molecular sieve can hold 17% of its dry weight in water with air at 20 °C and 20% RH compared with LiCl which can hold 130% of its mass at the same temperature and relative humidity.
Solid absorbents have many other favourable traits, e.g. molecular sieves continue to absorb moisture even when hot. Several solid absorbents can be manufactured to precise tolerances enabling pore diameters to be closely controlled to absorb molecules of specific diameter. This selective characteristic is useful as several desiccants with differing pore sizes can be combined to remove first water then other specific contaminants, such as VOCs. Silica gels and most other absorbents can be manufactured for a specific application, balancing capacity against strength, weight and other characteristics.
Typical solid absorbents comprise activated alumina, carbons, silica gels, synthetic polymers, synthetic zeolites (molecular sieves) and zeolites. Activated aluminas are oxides and hydrides of aluminium manufactured from a thermal process. Carbons are most frequently used for the absorption of gases as they have a greater affinity for the molecules typical of organic solvents. They also have a high capacity to absorb water vapour at high humidities. Silica gels are formed by condensing soluble silicates. They are low cost and easy to customize. Synthetic polymers have a capacity that exceeds many other solid desiccants due to long molecules, such as polystyrenesulfonic acid sodium salt (PSSASS) that are twisted together like strands of string. Each of the many sodium ions in the long PSSASS molecules has the potential to bind several water molecules. Synthetic zeolites or molecular sieves are crystalline aluminosilicates manufactured in a thermal process. Control of the manufacturing process permits close control of the structure and surface characteristics. This is a more costly product than naturally occurring zeolites. Zeolites and aluminosilicates occur in nature and are mined rather than processed. Zeolites have a very open crystalline lattice that allows water vapour molecules to be held in the lattice openings.
15.7 Practical applications of commercial desiccants
Examining ventilation rates of occupants in non-domestic buildings leads to the conclusion that the rates recommended were not determined by the respiratory needs of people (i.e. 0.015–0.10 l/s/m2), as various bodies recommend a minimum ventilation rate around 1.20 l/s/m2.
The moisture content of ventilation air can be significant. Anyone who has experienced a hot, humid day will know the discomfort of perspiring without any cooling effect. This is due to a saturated atmosphere being unable to absorb any more moisture, and thus any excess we produce simply stays on us, unable to evaporate. Dalton’s Law of Partial Pressures clearly confirms this and for those of us who have forgotten, it is as follows:
• Each constituent in a mixture of perfect gases exerts the same pressure as if it alone were present in the space occupied by the mixture, at the temperature of the mixture.
• Total pressure of the gases is the sum of their partial pressures, and the volume of the mixture of gases is the same as the volume occupied by each gas at its partial pressure.
• Totally enthalpy of the mixture is the sum of the enthalpy of each constituent at its partial pressure.
• Barometric pressure represents the total pressure of atmospheric air. This total pressure is composed of all the partial pressures of all the constituents, chiefly nitrogen, oxygen and water vapour.
A typical person, who works in an office, produces latent heat of around 60 W at 24 °C, which equates to 1.68 g/kg of moisture entering the atmosphere. Table 15.1 shows the effect of occupant density on dew point and humidity level.
The Comfort Zone Chart, published in 1924 by American Society of Heating and Ventilating Engineers (ASHVE) after studying physiological reactions of temperature humidity and air movement, clearly set out the relationship between humidity and temperature in relation to comfort. Basically, as temperature increases, humidity had to reduce to maintain the same relative comfort. Comfort lines similar to those in Fig. 15.7 were established in 1922.
Humidity and the influence it has with regard to comfort and energy saving when cooling is required is not always considered seriously. Air distribution rules may be relaxed where lower moisture content ventilation air is introduced at/or near to room temperature. Asymmetric temperature gradients may be ignored with vapour pressure differential doing the work, taking us back to Dalton’s Law of Partial Pressures.
Research and notable authors have concluded that a moisture content in air of not more than 7 g/kg is required to provide a healthy environment. This equates to a relative humidity of 40%, with a dry-bulb temperature of 23 °C. Too dry an environment, generally below 30% RH, is not recommended. It is therefore of critical importance that the moisture within the air is properly considered and suitably controlled. The desiccant system accommodates just this problem.
The psychrometric principals of a commercial desiccant system are illustrated in Fig. 15.8. Figure 15.9, shows an adsorbent (solid) desiccant air-handling system in summer, and Fig. 15.10 shows the desiccant air-handling system in winter. Both can be used in conjunction with the psychrometric description to demonstrate how a typical commercial desiccant system works.
Fresh air enters through the filter and then passes over the desiccant wheel, which absorbs some of the moisture in the air, which also adds heat (approx. SHF 0.6). The drier but warmer air then passes to the thermal wheel which cools it down; it may be further cooled by an evaporative cooler, and depending upon the required final conditions, may be further cooled by means of a sensible cooling coil. The cooler, now dehumidified air is introduced to the space via an air supply fan, where it provides all the latent cooling and some of the sensible cooling requirements depending upon which type of system is in use, either an all air, or air/water system.
The warm, moist return air leaving the space by way of the filter is passed through the evaporative cooler. This cool now humid air enters the thermal wheel enabling the sensible cooling to be transferred to the incoming air, which in turn is being heated by the incoming air. A regenerative heater further increases the air temperature before entering the desiccant wheel where it regenerates the desiccant. Some of the air could bypass the regeneration heater and desiccant wheel to save energy.
The system is environmentally friendly, using inert materials, i.e. salt, silica gel or titanium silicate, and can use direct or indirect gas, waste or solar heat to regenerate the desiccant. Desiccant wheels also have laminar flow characteristics, thus higher air volumes are permissible without an energy penalty.
During the winter months when the desiccant wheel may be inactive there needs to be an additional consideration. As supply air flows through the desiccant wheel without reactivation, moisture will build up in the desiccant wheel. Excess moisture supports fungal growth within the core of the material. Thus when dehumidification is required, the warm, humid wheel can give off odours reminiscent of wet wool until the regeneration heat kills off the microbial growth accumulated when the wheel was inactive. A regular exercise and purging regime is recommended for the wheel to prevent the build-up of microbial growth.
Commercial absorbent desiccant systems present a liquid to the air stream instead of a solid. This type of equipment is less common in a commercial application; however, it does provide some unique advantages. In a liquid system a solution of LiCl coats an extended surface similar to that of cooling tower media. The air flows through the media and the air gives up its moisture to the liquid desiccant. The diluted liquid desiccant is then sent to a regenerator to have its excess moisture, equal to the moisture taken out of the air, removed. The dilute liquid is first heated then put in contact with a small amount of outdoor air; the hot liquid desiccant releases its moisture to this regeneration air. The re-concentrated liquid desiccant flows back to the media where the process is repeated.
Control of the temperature and concentration of the solution enable the air to leave the liquid desiccant system at the precise temperature and moisture content needed without the after-cooling required by the dry adsorbent system of a desiccant wheel.
Around the world, over the past decade, desiccant cooling systems have advanced through development in polymer technology and novel developments in heat exchanger technology. These novel heat exchanger applications provide products that can heat and cool, both air and water, as well as recycle sensible and latent energy in a very efficient manner (Fig.15.11).
These novel desiccant cycles have more than doubled the coefficient of performance (energy efficiency ratio) of desiccant cooling systems. They are up to three times more energy efficient than conventional (Pennington) systems when operating on low temperature heat such as that from solar heat collectors, fuel cells or combined heat and power (CHP) systems.
15.8 Practical applications of natural desiccants for modifying building humidity
An alternative approach is to use the water absorption properties of building materials. The advantage of using water absorbing materials as a buffer during brief periods of increased water vapour is that during these periods the absorbent material may be used as a substitute for mechanical ventilation. The heat of evaporation being recovered by the heat of absorption released by the material provides for increased comfort.
The use of porous materials in walls, floors and ceilings may be used as a buffer to maintain relative humidity. Hygroscopic walls, floors and ceilings can aid stability of relative humidity and thus comfort, especially in rooms which are ventilated to less than 1 ac/h. A few centimetres of absorbent material are sufficient to buffer a daily RH cycle and about 40 cm of absorbent wall might buffer an annual cycle in a room with about 0.1 ac/h. The defining factors are the water capacity and permeability of the absorbent materials used. The common buffer building materials are wood, cut across the grain, and unfired clay brick. A specially designed lightweight clay made from bentonite mixed with perlite also gives an excellent performance (Padfield, 1998).
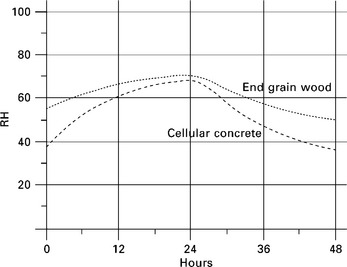
The best performer of the commonly used building materials is cellular concrete. Most other building materials have a negligible effect at moderate relative humidity. Cellular concrete is made by mixing silica, calcium oxide, aluminium powder and water. The end result is a mass of interlocking needle crystals of calcium aluminium silicate with relatively large air voids and a typical density of 750 kg/m3. These air voids provide plenty of sites for water absorption, making cellular concrete a moderately good buffer material. Figure 15.12 shows a typical absorption and desorption cycle.
The combination of porous materials laid over porous absorbent materials is interesting. A combination of gypsum plaster over cellular concrete provides a sort of a diode as it allows moisture to penetrate but hinders its return. The gypsum plaster conducts liquid water, because it is wettable and has a system of large pores. The cellular concrete has a similar structure on a finer scale and retains the water. This combination can only be advantageous if the cellular concrete can evaporate the moisture to the outside of the building.
The need to ventilate away moisture generated by human activities could be reduced by utilizing porous and absorbent materials to buffer the moisture and thus use a lower ventilation rate and less energy. In some instances this buffering may be so effective that mechanical systems are not necessary. However, in other circumstances the average RH provided by the passive buffers will be too variable and a relatively small and simple air conditioning system may assist the material buffers during the time of peak moisture gain.
There are no readily available design tools to explore the use of porous or absorbent materials to moderate indoor climate. However, when the room has absorbent buffers, the RH can be defined by the interaction of the materials with the various sources of water vapour within the room, as the RH is a consequence of the behaviour of the materials, not a controlling parameter.
The sorption curve of a material defines how much water will be released or absorbed when the material moves from equilibrium with one RH to another. The inverse argument is that this same curve also defines RH change in the air that surrounds the material resulting in the material losing a certain weight of water into the space. Where there is a reasonably even and moderate RH, only that portion of the absorption curve is relevant, i.e. between 40 and 65% RH. This part is fairly straight for most materials, so the absorption curve can be replaced by a constant: the water capacity.
The water capacity is defined as the weight of water that would be released from one cubic metre of material if the RH changed from 100 to 0%, assuming a sorption curve gradient constant at the value for 50% RH. Water vapour permeability is also a factor. The higher permeability makes for a better performance as a buffer; however, there are other considerations when the RH is changing: as the water vapour diffuses into the material it is attracted to the pore walls by van der Waals forces, according to its absorption coefficient. A material with both high permeability and high absorption will actually show a low permeability to water vapour under dynamic circumstances, because the water is absorbed before it is diffused. Calculation of this complex process is aided by defining a unit called diffusivity, which is the diffusion coefficient divided by the absorption coefficient. This is used to calculate an active depth for moisture exchange.
where ‘d’ is the active depth of water exchange, ‘t’ is the period of the water flux cycle and ‘D’ is the diffusivity. The active depth marks the distance into the wall at which the variation in RH in the pores has fallen to about one third of the variation in the room (Claesson and Hagentoft, 1994).
Unfortunately, the analogy between heat and moisture flow is not exact. Therefore the effective depth is just an indication of the order of the useful thickness of the material for buffering relative humidity. The amount of water available is now obtained by multiplying the water capacity by this active depth.
The best materials are not those generally used in building. Wood cut across the grain is by far the best buffer but may not be ideal because it outgases a variety of corrosive vapours. Clay is the second best, but some give off hydrogen sulphide. Clay walls smell much less than wood, as the hydrogen sulphide normally oxidizes to hydrogen sulphate. The active depth of clay for a daily cycle is 5 mm. Figure 15.13 provides a synopsis of the above.
Absorbent walls, floors and ceilings can significantly improve climatic stability of indoor spaces that have low ventilation rates. Thus, moisture mass must have equal consideration to thermal mass in passive buildings.
15.9 Bibliography
ASHRAE. Desiccant Cooling and Dehumidification, 1992.
ASHRAE Guide. Fundamentals, 2007. [Chapter 22].
Beggs, Warwicker. ‘Towards zero carbon dioxide emission: the environmental impact of fabric thermal storage in UK office buildings’. www.warwicker.com.
Beggs, Warwicker, Howarth, Elimination of air conditioning in existing buildings through fabric thermal storage: A Theoretical study. Building Serv. Eng. Res. Technol. 1995;16(4):215–220 www.warwicker.com
Brundrett, G.W.Criteria for Moisture Control. Butterworth’s, 1990.
CIBSE Desiccants the Future – Robert van Zyl Professor of Engineering, Professor Brian Warwicker. www.warwicker.com.
CIBSE Guide Volume A. 2006. [Section A7].
CIBSE Guide Volume B. 2005. [Section B2].
Claesson, Johan, Hagentoft, Carl-EricBasic building physics – mathematiucal modelling. University of Lund, Department of Building Science, 1994.
Evans, B. New cooling for old buildings. Architect Journal May, 1995 www.warwicker.com
Harriman, Brundrett, KittlerHumidity Control Design Guide. ASHRAE Publication, 2001.
Padfield. The role of absorbent building materials in moderating changes of relative humidity, October 1998.
Redman, B.Dehumidifying using Desiccants. CIBSE Journal, 1997. [June].
Warwicker, B.P. Low Temperature Air and Ice Storage. CIBSE Journal, 1989. www.warwicker.com [February].
Warwicker, B.P. Low Humidity Air & Air Conditioning. CIBSE Journal, 1995. www.warwicker.com [November].
World Health Organisation Report, Bilthoven, Nederlands. ‘The Right to Healthy Indoor Air’ 15–27 May 2000.
. Wood Science and technology Journal; 29, 1995. [Number 5/August].
15.10 Appendix: Energy efficiency ratio (EER) and coefficient of performance (COP)
Energy efficiency ratio (EER) is used in the USA, and is defined as the system output in Btu/h per watt of electrical energy. Coefficient of performance (COP) is the equivalent measure using SI units, which is widely used in the UK. A COP of 1.0 equates to an EER of 3.4.
When comparing technologies that use electrical energy with those that use heat energy, it is useful to consider the ‘equivalent COP’ that achieves the same carbon emission levels. In the UK the generation of electricity emits 2.6 times as much carbon per watt when compared with burning natural gas. In terms of carbon emissions, therefore, a heat driven device with a COP of 2.5 would be equivalent to an electrically driven device with a COP of 2.5 × 2.6 = 6.5.
The seasonal energy efficiency ratio (SEER) used in the USA and equivalent ‘seasonal COP’ is a measure of the annual energy efficiency taking into account the varying weather conditions. It is calculated using weather ‘bin’ values, with a given frequency of occurrence in each temperature ‘bin’. Very high SEER (and seasonal COP) values can be expected with new desiccant cycles, as for a large part of the year no dehumidification is required and no regeneration heat is used.
The SEER system is flawed, however, in that it does not consider the external humidity and therefore the requirement for dehumidification is not quantified. Until a standard is set, an objective view cannot be taken on what SEER to expect for desiccant cycles, but the SEER for novel desiccant systems could well be equivalent to an electrically driven device with a SEER in excess of 30, which is up to three times better than that of conventional air-conditioning systems.