Hygrothermal materials for heat and moisture control in buildings
Abstract:
This chapter seeks to explain what makes a ‘hygrothermal’ material, with reference to their specific ability to regulate indoor environmental conditions of air temperature and relative humidity. The volume averaged physical properties of these materials is addressed along with the experimental and numerical techniques employed to characterize and study their behaviour. The main material classes of candidate hygrothermal materials are explained in detail. Finally, the application of these materials in relation to buildings is explored along with predicted future research trends in this field. Details of the main active research groups and numerical modelling techniques available are provided for those wishing to engage in the subject.
14.1 Introduction
The balance (or buffering) of indoor air temperature and relative humidity (RH) in buildings is very important from the point of view of respiratory health and comfort of the occupants (Toftum et al., 1998a), healthy skin by avoiding dryness (Toftum et al., 1998b), perceived indoor air quality (Fang et al., 1998a), to avoid deterioration of the building materials (Lucas et al., 2002), and to avoid mould growth and the problems associated with condensation (Sedlbauer, 2002). The use of mechanical systems for heating, cooling, ventilation and air conditioning for air temperature and RH control is not always the best solution due to the levels of energy consumption required, the issue of noise for occupants, the need for regular maintenance, and the potential threats associated with security of energy supply needed to run the equipment.
14.1.1 What makes a material hygrothermal?
Hygrothermal behaviour, with respect to materials and buildings, considers the simultaneous (and inter-dependent) occurrence of heat absorption, storage and release, and moisture (liquid/vapour) absorption, storage and release. It is by definition a fully transient approach normally tackled through a combination of experimental testing, physical modelling and numerical modelling solutions, as detailed in Chapter 2. The approach is typically applied to materials such as timber, plasterboard, insulation materials, masonry, concrete, brick, lime plaster, etc., and more recently earth materials.
All materials and adjacent control volumes (e.g. a room or zone in a building) can be assessed hygrothermically, but not all exhibit desirable properties for the given application. The ones that do are referred to as ‘hygrothermal materials’ and have good capacity for enthalpy buffering, i.e. simultaneous buffering of heat energy (sensible and latent energy in air) and mass concentration (vapour and associated partial pressure). In order to predicatively model hygrothermal behaviour, knowledge of several material functional properties is required:
• bulk porosity and apparent porosity
• dry state and moisture-dependent heat capacity at constant pressure
• dry state and moisture-dependent thermal conductivity
• sorption isotherms including absorption/desorption moisture storage functions at relevant temperatures
Much experimental data has been recorded and published for these properties for many conventional and existing materials, though there is much work still to do. The data is included in the databases for various computer software packages as listed in Section 14.6.
The key advantages to using hygrothermal materials in buildings include:
14.1.2 Buffering capacities
Conventionally, we can study thermal (heat) energy buffering and moisture vapour buffering. Both involve absorption, desorption and volumetric storage capacity changing as a function of time in response to driving potentials in the adjacent control volume. For details of the fundamentals behind these processes, refer to Chapter 2. In the case of buildings, the material constitutes a portion of the indoor environment (e.g. walls) and the control volume is an occupied space filled with air containing moisture vapour and other gases. We know that for thermal energy, the driving potentials include the heat energy differential between air and material with heat therefore travelling from the hotter body to the cooler body, e.g. warm air heating a cooler surface. The increase in surface temperature of the material generates a temperature gradient within that material resulting in transfer of heat, principally by conduction. The rate of energy transfer is determined by the thermal conductivity of the material and also the steepness of the temperature gradient. If the material density is high, normally accompanied by a high specific heat capacity, the energy requirement for temperature gradient formation is higher. Hence, if the rate of heat flow is constant from the control volume (assumed steady state) the response time of the material to distribute heat throughout its mass can be given by the thermal diffusivity, α (m2/s), which is simply calculated from λ/ρcp. Thermal effusivity, β, is the ability of the material to exchange heat with its surroundings (i.e. heat entering or leaving the material) and is given by ![]()
In the same way that ‘thermal mass’ can be used to stabilize the temperature of occupied spaces, hygroscopic materials have a hygric capacity that can be used to buffer relative humidity fluctuations. Excessive humidity can result from activities such as bathing, washing and drying clothes and occupant perspiration, while low humidity is associated with heated spaces in the winter months. Humidity buffering is therefore a desirable property of a material to absorb water vapour from the air, when relative humidity is high, and release this water as vapour when relative humidity falls. This property can be quantified using the experimentally-determined moisture buffer value (MBV) as defined by Rode (2003) and the NORDTEST Project. For this test a sample of material is subjected to a high humidity (23 °C 75% RH) environment for a period of 8 h followed by a low humidity environment (23 °C 33% RH) for 16 h. This cycle is repeated for at least three days and until the change of mass between cycles is less than 5%. Specimens are sealed on all but one exposed surface over which the air velocity should be 0.10 m/s ± 0.05. An example of experimental test data for the MBV test is shown in Fig. 14.1. The MBV (g/m2 %RH) is calculated from the change in mass per square metre per % relative humidity (Rode, 2003):
Using this approach, materials may be classified under one of five categories based on their ability to contribute to the control of humidity in a room as shown in Table 14.1.
Table 14.1
Moisture buffer value classification scheme

(adapted from Nordic Innovation Centre, 2005)
A numerical method for estimating the MBV using material physical properties was also proposed by the NORDTEST Project (Nordic Innovation Centre, 2005). It was based on a mass transport solution and assumes no boundary layer resistance. For these reasons it is distinguished by being called the ideal moisture buffer value or MBVideal and represents a theoretical maximum value where:
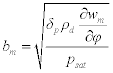
Note that the value, bm, is the moisture (or mass) effusivity, which is analogous to the thermal effusivity described above, and is calculated by:
Enthalpy buffering is the minimization of ∆H, where H = U + PV. In the case of psychrometrics, for the air in a building, internal energy, air pressure and air volume can change as a consequence of air temperature, and also partial pressure due to changes in mass concentration (moisture content of the air). This air of course interacts with the material fabric inside the building. The rates at which energy can be transported (in the form of heat) from the air to the material and back again, and the rate at which mass can be transported (in the form of water vapour) in and out of the material, can effectively create a balance effect in indoor air enthalpy. Hygrothermal materials derive their name from the fact that their hygrothermal functional properties are particularly useful for enthalpy buffering, as previously mentioned. The first most important set of functional properties relates to the control of absorption and desorption of water vapour from the air in order to maintain small changes in the humidity ratio. The second most important parameter relates to the control of absorption and desorption of energy (as heat) from the air in order to maintain small changes in dry bulb temperature. This can be visualized using a psychrometric chart (as discussed in Chapter 4) in which the influence of both humidity ratio and dry bulb temperature on air enthalpy at constant relative humidity can clearly be seen.
14.2 Characterization of hygrothermal functional properties
The following section explains how hygrothermal functional properties can be characterized along with various appropriate test methods and procedures for providing data.
14.2.1 Volume-averaged properties
The main functional properties of a hygrothermal material are listed in Section 14.1, and the fundamentals behind these properties are described in detail in Chapter 2. A good hygrothermal material has, amongst other things, a high moisture buffer value (MBV) and the equivalent of a high thermal buffer value (TBV) which manifests itself as a delayed thermal response and fabric energy storage. These things are controlled by material physical properties at the microstructural level which are effectively quantified as volume-averaged properties at the macrostructural level. The important concept behind hygrothermal behaviour, however, is that the absorption, storage and release of both heat energy and mass (moisture) are interdependent and occur simultaneously, i.e. thermal performance becomes more complicated and more important when moisture is present, and vice versa.
Empirical relationships between λ and constituent volume fractions, bulk density and moisture content have been obtained from experimental testing of a wide range of porous building materials by various researchers. Auto coherent homogenization has been used to try simple predictions of λ for two phase (air and solid) or three phase (air and mixture of solid A and solid B) porous materials by assuming that the heat transfer is analogous to that occurring across a multilayered sphere of concentric homogenized phases (Bederina et al., 2007). The cubic lattice and capillary bridge model (Ewing & Horton, 2007) proposes a mathematical description of λ for dry porous materials (when the void ratio is varied), and for moist porous materials when the degree of saturation is varied. The Random Mixed Cube Model (Zhang et al., 2006) uses the three phase continuum model as a base to represent relative quantities of each phase as small discrete cubes randomly arranged within a larger finite volume cube (i.e. analogous to a Rubik’s cube). The heat transfer between adjacent cubes is modelled to provide a cumulative effect. This allows good consideration of the boundary effects but cannot accurately measure the effects of void geometry which is crucial in determining the independent functions of the matrix structure. There is much research work still to do in order to fully understand how and why the volume-averaged hygrothermal properties that we measure for a given material originate by understanding their microstructure.
14.2.2 Measurement techniques
Many standard modelling techniques for thermal and hygric properties of porous materials are detailed, along with the fundamentals behind them, in Chapter 1. The important properties for a hygrothermal material are listed in Section 14.1 and the techniques for measuring them are described here. Thermal conductivity can generally be assessed using a heat flow meter (HFM), dual probe heat pulse meter (DPHP), or a guarded hot box. Thermal conductivity testing using computer-controlled heat flow meter apparatus, for example, must comply with ISO 8301 Thermal Insulation – Determination of Steady State Thermal Resistance and Related Properties (ISO 8301, 1991). For moisture-dependent thermal conductivity testing, the same HFM apparatus is used but the methodology is adapted to comply with ISO 10051 Determination of Thermal Transmissivity of a Moist Material (ISO 10051, 1996). An alternative to direct measurement is ISO 10456 Procedures for Determining Declared and Design Thermal Values (ISO 10456, 1999) which provides methodologies for the theoretical calculation of the change in thermal conductivity when influenced by, for example, variations in specimen moisture content, temperature and age.
The rate of water vapour flow through a porous specimen can be determined using the wet cup/dry cup method in a temperature and humidity controlled environmental chamber (BS EN ISO 12572, 2001). By using a saturated salt solution for the ‘test’ vessel, a vapour pressure gradient can be imposed across the sample thickness thus inducing diffusive mass transfer and allowing the water vapour permeability to be calculated. Sorption and desorption isotherms can be determined using small representative samples that have been oven dried to constant mass. Using an array of sealed desiccators containing different saturated salt solutions, to provide a wide range of stable relative humidity environments, the dry specimens can be progressively placed in each of the desiccators and permitted to absorb moisture vapour until equilibrium moisture content is achieved. The relationship between the moisture content of a porous material and the relative humidity of the surrounding environment is quoted using the moisture storage function (MSF). Sorptivity and rate of liquid water flow in unsaturated specimens can be measured using the partial immersion test described in Chapter 1. The hydraulic conductivity, as a function of moisture content, can be calculated based on the MSF value. Much more precise physical modelling of liquid water movement can be performed using gamma ray scanning devices and nuclear magnetic resonance (NMR) spectrometry (Hall & Hoff, 2002).
14.3 Material classes
Several engineering materials can be described as ‘hygrothermal’ exhibiting strong behaviour. In the case of building physics applications, these chiefly fall amongst the categories described in the following sub-sections although there is still much need for further research and many institutions are now actively pursuing this field.
14.3.1 Clay materials
The term ‘clay’ can mean many things. It can, for example, refer to a clay soil which can be a formal geological classification, or it can refer to the behaviour of the material (clay-like) in terms of cohesion and plasticity. In terms of chemical composition, clay is a mineral belonging to the group know as phyllosilicates on account of their layered sheet-like structure. In civil engineering terms, clay is a soil material originating from a parent rock mineral and has a mean particle diameter of 2 microns or less.
Clays are naturally-occurring phyllosilicates formed from various parent rock minerals, usually as the result of weathering. The many variations of naturally-occurring clay minerals are formed from layers of silicate and aluminate minerals that produce either 1:1 or 2:1 lamellar structures. They form discrete particles known as micelles, as shown by the examples given in Fig. 14.2. For further details on clay chemistry and clay mineral classification, refer to Velde (1992). Since water is a polar molecule, it is attracted to the net electrostatic charge on the surfaces of clay platelets. H+ in H2O is attracted to O− in the platelet shell. The higher the specific surface area of the clay, the more water molecules will be attracted both in terms of quantity and distance of the effect. The accumulation of water (often containing hydrated metal cations) forms what is known as a double diffuse layer around the clay platelets. The thickness of this layer (or distance from the platelet surface) is determined by the surface potential of the clay, i.e. the magnitude of the net electrical charge. Some clay minerals have very low surface potentials, such as kaolinite, and hence their double diffuse layer is relatively thin in relation to the platelet size. This means that the quantity of water absorbed, when equilibrium is achieved between charged surface and water molecules, is relatively small. Other clays, such as montmorillonite, have very large specific surface areas (up to 800 m2/g) and consequently their surface potential and double diffuse layer thickness is extremely high, see Fig. 14.3. These minerals can absorb very large quantities of water but obviously they expand considerably in volume whereas less reactive clays do not.
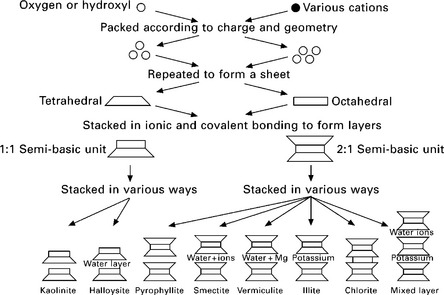
14.2 Composition of typical clay mineral structures (Mitchell, 1993).

14.3 Relative sizes of adsorbed water layers surrounding a montmorillonite clay particle (left) and a kaolinite particle (right) (adapted from Holtz & Kovacs, 1981).
Clays generally have a small range of achievable λ, and a slightly bigger range for dry density/porosity. This means that thermal resistance, R, cannot change much for a given thickness (in the dry state) between materials. However, when water is introduced, even in small amounts it, can vary considerably depending upon pore structures and their properties. This means that thermal storage and also thermal diffusivity can be altered, and hence thermal buffering can be manipulated. Crucially, clay minerals are very hygroscopic due to their chemical composition as described above.
Unfired clay bricks are often referred to as ‘green bricks’ as they use less energy during the production process and are simply air-dried rather than being fired in a kiln. They can be formed by hand or mechanically extruded and are dried until the equilibrium moisture content is reduced to around 2%. Many shapes and sizes can be created through the use of a variety of different moulds. They are designed to be used for non-structural, internal applications because they degrade when in long-term contact with water, i.e. exposure to weather. The bricks create little waste during production, require less energy to produce them, and can be either recycled or cleanly disposed of when no longer needed (Morton, 2006). Bricklaying can be done following conventional trade practices, although the bricks must either be left exposed or coated with a highly breathable paint, surface treatment or plaster.
Unfired clay bricks are a relatively new idea in Great Britain; however, in other parts of Europe, particularly Germany, it has developed a good reputation and been perceived as a viable building solution. A number of UK suppliers have started to produce earth bricks to meet the ever-increasing interest in these methods. Ibstock, for example, have released a line of earth bricks known as ‘Ecoterre bricks’ and even equip buyers with a list of retailers who supply materials such as mortars and paints to be used alongside their products. The Errol Brick Company in Scotland also produces a range of green clay bricks and, along with Arc Architects, Fife, have successfully been used in a small-scale trial building to regulate relative humidity over the period of a year. The test house was designed to passively regulate internal relative humidity between 40% and 60%, and tests confirmed that in the bathroom humidity regulation was the same after a shower whether or not mechanical ventilation was in use (Morton, 2005). Water vapour sorption of 1.5–2.3% wt at 80% RH and a vapour permeability of between 8 and 16 × 10− 12 kg/(Pa s m) is reported by Hansen & Hansen (2002). In addition, they found that an active depth (i.e. the depth from the surface involved in sorption/desorption) of between 4.1 and 5.1 mm was available on green clay bricks for humidity buffering.
Clays can be used in combination with fibre reinforcement (to prevent expansion/contraction cracking) as interior plaster for buildings and occupied spaces. A good example of this is the range of clay plasters produced by CLAYTEC, Germany, although other manufacturers now make similar products. One of the main advantages of clay plasters are that they have the ability to absorb and diffuse water vapour for buffering of interior relative humidity fluctuations. The plasters are also perceived to be more environmentally friendly because they are not calcined (heated at high temperatures to dissociate CO2) and do not contain any energy-intensive binders such as Portland cement or lime.
14.3.2 Stabilized earth materials
It is claimed that rammed earth (RE) or stabilized rammed earth (SRE) walls can be used as a building-integrated source of ‘passive air conditioning’, which is a combination of indoor moisture buffering and air temperature buffering (Allinson & Hall, 2007). They are ‘building integrated’ because the walls themselves act as a load-bearing external envelope. SRE structures are increasingly accepted in other developed countries such as the USA, Canada and Australia where in some regions they account for up to 20% of all new build (Easton, 1996); see the example in Fig. 14.4. In the UK, the Rammed Earth Design & Construction Guidelines, published by BRE Bookshop (Walker et al., 2005) were developed as part of a DTi Partners in Innovation Project under the title ‘Developing Rammed Earth for UK Housing’.

14.4 Interior of an SRE house with exposed surfaces to allow hygrothermal buffering (© 2000–2009 Trevor Mein).
These materials have a strong ability to passively cool a building by naturally absorbing heat gains, and re-radiating the stored heat energy when the ambient temperature falls, i.e. the thermal flywheel effect. The porous nature of the material coupled with the presence of hygroscopic clay minerals also enables the walls to passively control the humidity of a building’s internal environment, i.e. a humidity-based version of the thermal wheel. Current research by the author is investigating how to control SRE material properties such that the passive cooling and dehumidification could be specified, thus enabling these to be tailored to the design of a building. The potential future outcome could be buildings that regulate themselves so that only a relatively small intervention from mechanical building services is required. An electronically monitored SRE house in Sydney, for example, which was left unoccupied and with no curtains for over a year had an average annual indoor temperature range of 18–27 °C, when the outdoor temperature range was 7–42 °C (Mortensen, 2000). A two-storey SRE office in New South Wales also displayed good indoor environmental performance over a hot summer due to passive cooling and dehumidification (Taylor & Luther, 2004).
14.3.3 Timber
Timber is a naturally-occurring cellular fibre composite that chiefly comprises cellulose (fibre) and lignin (matrix). Timber is a light, strong material, used in building structures for millennia because it is renewable, simple to manufacture, easy to use, stable and safe. It has an inherently low thermal conductivity although both its structural behaviour and durability may be adversely affected by moisture. The physical properties of timber vary greatly between species. Hardwoods are normally from broad-leaved deciduous trees (e.g. oak, ash, beech, elm, mahogany) that are slow growing and therefore have a dense, close-grained cellular structure but that can be prone to fungal attack. Softwoods are from fast-growing coniferous trees (e.g. normally evergreens such as spruce, pine, douglas fir) that have lower density, a much more open grained structure, and are mostly softer than their hardwood counterparts. The coefficient of variability for timber materials can be greatly reduced when knots and other defects are removed and/or lamination techniques are used.
In hardwoods purpose-grown large vessels occur whose function is to transport fluids through the plant, whereas in softwoods this function is achieved within the main cellular structure. For further details on timber microstructure, refer to Dinwoodie (2000). Within the cellular structure of timber there are individual openings known as a ‘torus’ which can open and close to allow in response to moisture movement. The electron microscope image in Fig. 14.5 clearly shows the vertically aligned cellular tissue of a piece of European spruce where at least one torus is visible inside each broken vessel. Note that the specimen was prepared by tensile failure which clearly exposes the fibre composite nature of the cellular tissue. One can appreciate that the equilibrium moisture content, at a known relative humidity (which of course can be used to plot a sorption isotherm; see Chapter 1), is normally very high for timber in comparison with other building materials such as brick, concrete and plaster.
Gaur and Bansal (2002) used a simplistic model of a spruce-lined room to demonstrate that it was necessary to consider moisture transfer if room air temperature was to be modelled. They found that neglecting the hygroscopic fabric resulted in temperature errors of 2–3 °C for climatic conditions in Delhi, India. Osanyintola and Simonson (2006) suggested that spruce plywood can reduce both the heating and cooling demand in a building. Heating demand could be reduced while the building was occupied (as moisture accumulated in the fabric releases heat) but increased while the building was unoccupied as energy was needed to dry out the walls. Good system control produced an overall saving. The cooling load could be reduced by a greater amount due to lowering of the enthalpy of the air through the reduced humidity. Further savings were expected from improved indoor air quality allowing for increasing indoor temperatures during the summer, reducing them in winter and lowering ventilation rates.
14.3.4 Natural fibre materials and composites
Many natural fibres have the ability to absorb, store and release water vapour from the air and therefore make good candidates for hygrothermal materials in buildings. These include straw, hemp, sisal, flax, jute and many others. The issue of bio-degradable materials such as these is protecting the fibre from decay when moisture is present by use of stabilizing materials. ‘Hemcrete®’ is a lime–hemp composite material made using hemp shiv and a hydrated lime binder (see the cross section shown in Fig. 14.6). They have a high thermal insulation and high water vapour permeability that can reduce the development of condensation and trapped moisture within the building. As with all good humidity buffering materials, it can also improve the indoor air quality by controlling the relative humidity as well and reducing the potential for mould growth. A hygrothermal analysis of a lime–hemp composite was conducted by the Fraunhofer Institute for Building Physics in Holzkirchen, Germany using WUFI software and three theoretical case studies. Evrard (2006) reports an experimentally determined MBV of 1.39 g/m2∙%RH for lime–hemp materials and a dry thermal conductivity of 0.115 W/m K. Some manufacturers are now marketing a commercial lime–hemp product as Hemcrete®, an example application of which is shown in Fig. 14.7.
14.3.5 Smart materials
The work conducted during the NORDTEST Project involved determining the relative humidity buffering capacity of conventional building materials such as concrete, wood, gypsum plaster and brick. Following on from this, some researchers have attempted to develop ‘smart materials’ with very high buffering capacity that can be used to better regulate the indoor environmental conditions without the use of air conditioning systems. Candidate absorbent materials included silica desiccants, zeolites and molecular sieves. Others include sodium polyacrylate, with a reported MBV of 8.97 g/m2 %RH, and cellulose with an MBV of 3.07 g/m2 %RH (Cerolini et al., 2009). These materials were evaluated for an exposure to cyclic fluctuation between 75% RH and 33% RH over test periods of 8 h and 16 h. Some hysteresis was involved, as expected from looking at a sorption isotherm; sodium polyacrylate suffers from high hysteresis whereas the cellulose-based has a lower hysteresis.
14.4 Applications in buildings and occupied spaces
Many researchers, architects and engineers are making increasing use of hygrothermal materials for the passive regulation of indoor relative humidity, to reduce/eliminate problems associated with condensation and/or degradation of materials and furniture, and to increase the quality of indoor air. This applies not only to domestic buildings but also, for example, to office buildings with relatively high daytime air moisture loads, where the ‘hygrothermal’ material can be both cooled and dried at night time/off-peak times through the use of natural ventilation and night cooling strategies.
14.4.1 Thermal buffering, comfort and energy efficiency in buildings
Thermal buffering is the combined effect of materials absorbing, storing and releasing heat energy to their surroundings in response to changes in environmental conditions. The use of exposed thermal mass in buildings to reduce peak summertime temperatures and cooling loads in buildings is well established. Peak indoor temperatures are reduced when the heat gains (especially solar and internal heat gains) are absorbed by the building fabric which is in turn cooled overnight in readiness for the next day. During the heating season, the same thermal mass tends to absorb heat intended for the interior space and thereby increases energy demand. Good, passive solar design (BRESCU, 1998) and thermally efficient buildings with continuous low level heating (TCC, 2006) can be used to overcome this problem. Ultimately thermal mass can reduce overall energy demand, and improve thermal comfort, in properly designed buildings. In the UK, climate change is predicted to increase the summertime cooling load and reduce winter heating, reinforcing the case for thermally heavyweight buildings (Arup R + D, 2005). What appears to be less understood is that effective control of the air enthalpy, by controlling air moisture content, can significantly reduce the peak cooling loads and hence allow the thermal mass to become more effective. In addition, the material properties, such as thermal conductivity, density and heat capacity, are moisture content-dependent and can vary significantly and in a non-uniform manner. Hence, more accurate consideration and predictive modelling of thermal mass behaviour, and indeed the future design of thermal mass material technologies, will require a more sophisticated understanding using the hygrothermal approach.
14.4.2 Humidity buffering and perceived comfort in buildings
Similarly, the use of exposed hygroscopic building materials has the potential to control the relative humidity of the interior of a building and its structure. Peak indoor humidity is reduced as internal and external moisture gains are absorbed by the building fabric to be returned during periods of low humidity. This passive humidity control has the potential to reduce the energy needed for mechanical systems through improving the perceived indoor air quality and thermal comfort for building occupants as well as directly reducing the sensible and latent heating and cooling loads. Toftum et al. (1998a) found that the humidity of the air that we inhale has a significant effect on the perceived acceptability of that air. Discomfort was thought to be due to reduced evaporative and convective cooling of the mucous membranes in the respiratory tract when breathing high humidity air (Toftum et al., 1998b). By reducing the humidity, higher indoor temperatures will be acceptable, reducing the cooling load of a building. Fang et al. (1998a) also found that the temperature and humidity of inhaled air had a significant effect on the perception of indoor air quality though little influence on the odour intensity of pollutants from building materials. Acceptability was found to be linearly correlated with the enthalpy of the air for a constant pollution level. Similar, though less distinct, results were found for whole body exposure (Fang et al., 1998b). Therefore, by reducing air temperature and humidity, acceptable air quality may be achieved at reduced ventilation rates. Reducing ventilation would lead to a lowering of the heating demand in winter and air conditioning in summer.
14.4.3 Dampness and mould control in buildings
In an experimental study of Swedish apartment buildings, Hameury and Lundström (2004) found that exposed massive wood contributed to the thermal mass effect and buffered indoor temperature variations. Modelling of the heat and mass transfer processes suggested that the contribution of the wood to moisture buffering was significant, especially at low air ventilation rates (Hameury, 2005). Further potential benefits of hygroscopic building materials include a reduction in dust mite populations and mould growth (beneficial for asthma and allergies) (Cunningham, 1996) and a reduction in structural degradation caused by moisture ingress (Lucas et al., 2002). This form of passive relative humidity control may be particularly appropriate to museums, art galleries and libraries, and also to heritage structures (e.g. historic buildings that allow visitor access) where the displayed items are sensitive to moisture. The use of mechanical systems (e.g. air conditioning) in these environments can often be inappropriate, especially in important historic buildings, and the need for a reliable, non-powered humidity control system is vital for the preservation of museum artefacts or sensitive media such as cloth and paper.
14.5 Future trends
There is a clear need to carefully match hygrothermal materials and their behaviour patterns with mechanical systems engineering design. Firstly, this will maximize the ability of hygrothermal materials to passively regulate indoor environmental conditions and reduce the energy use from mechanical systems, and secondly it will avoid any conflict between the operation of the mechanical system and the material response which could (in the worst case) result in over-sizing the dehumidification and cooling loads. Barbosa and Mendes (2008) demonstrate, using a whole building hygrothermal model, that it is important to consider the hygrothermal behaviour of the building material when sizing the heating, ventilating and air conditioning (HVAC) system. Their work, based on a case study in Brazil, shows that disregarding moisture may oversize the system by 13% and underestimate the energy required for cooling by 4%. Künzel et al. (2003) use validated computer modelling to show that the reduction in cooling load may not be as simple as many first think because the moisture absorbed during air conditioning shut off periods is re-emitted when the system is turned back on – which increases the latent heat capacity of the air.
14.6 Sources of further information and advice
In addition to research being conducted in this field by the author, numerous very well-established research teams and organizations are involved directly or indirectly with hygrothermal materials. A selection of them is presented in Table 14.2, although this list is by no means exhaustive.
Table 14.2
A list of institutions and research teams involved with hygrothermal materials research
| Institute | Location |
| Fraunhofer Institute for Building Physics | Germany |
| Oak Ridge National Laboratory (DOE) | USA |
| National Research Council Canada – Institute for Research in Construction (NRCC-IRC) | Canada |
| University of Saskatchewan | Canada |
| VTT Technical Research Centre | Finland |
| Technical University of Denmark | Denmark |
| Royal Institute of Technology | Sweden |
| Helsinki University of Technology | Finland |
| Tampere University of Technology | Finland |
| Tallinn Technical University | Estonia |
| Lund University | Sweden |
| Dresden University | Germany |
| University of Helsinki | Finland |
| Pontifical Catholic University of Parana | Brazil |
| University of Nottingham | UK |
A number of computer models have been developed for use with hygrothermal material properties and in tandem with building/occupied space assessment and analysis. A selection of the most widely used and validated models is given in Table 14.3.
Table 14.3
A selection of the most widely used computer models for use with hygrothermal material properties.
| Model | Institution | Country |
| 1D-HAM | Chalmers University of Technology | Sweden |
| DELPHIN 4, DIM 3.1 | Technical University of Dresden | Germany |
| EMPTIED | Canada Mortgage and Housing Corporation | Canada |
| GLASTA | Physibel Software | Belgium |
| hygIRC | Institute for Research in Construction | Canada |
| Hygran24 | Catholic University of Leuven | Belgium |
| LATENTITE | National Research Centre | Canada |
| MATCH | Technical University of Denmark | Denmark |
| MOIST 3 | National Institute of Standards and Technology | USA |
| MOISTURE-EXPERT | Oak Ridge National Laboratory | USA |
| TCCC2D | VTT Technical Research Centre | Finland |
| TRATMO | VTT Technical Research Centre | Finland |
| UMIDUS | Pontifical Catholic University of Parana | Brazil |
| WALLDRY | Canada Mortgage and Housing Corporation | Canada |
| WUFI Pro/Plus | Fraunhofer Institute for Building Physics | Germany |
| WUFI ORNL/IBP | Oak Ridge National Laboratory | USA |
14.7 Acknowledgements
The author wishes to acknowledge the assistance and helpful discussion of Dr David Allinson during the preparation of this chapter.
14.8 References
Allinson, D., Hall, M., Investigating the optimisation of stabilised rammed earth materials for passive air conditioning in buildings. Proceedings for the International Symposium of Earthen Structures, Bangalore 22–24 August, 2007.
Arup R+D. UK housing and climate change: heavyweight vs. lightweight construction. London: A technical report for Ove Arup and Partners Ltd; 2005.
Barbosa, R.M., Mendes, N. Combined simulation of central HVAC systems with a whole-building hygrothermal model. Energy and Buildings. 2008; 40(3):276–288.
Bederina, M., Marmoret, L., Mezreb, K., Khenfer, M.M., Bali, A., Quéneudec, M. Effect of the addition of wood shavings on thermal conductivity of sand concretes: experimental study and modelling. Construction and Building Materials. 2007; 21(3):662–668.
BRESCUPlanning for Passive Solar Design. BRE, Watford: BRECSU, 1998.
BS EN ISO 12572Hygrothermal performance of building materials and products – Determination of water vapour transmission properties. London: British Standards Institute, 2001.
Cerolini, S., D’Orazio, M., Di Perna, C., Stazi, A. Moisture buffering capacity of highly absorbing materials. Energy and Buildings. 2009; 41(2):164–168.
Cunningham, M.J. Controlling dust mites psychrometrically – a review for building scientists and engineers. Indoor Air. 1996; 6(4):249–258.
Dinwoodie, J.M. Timber: its nature and behaviour, 2nd edn. London: Taylor and Francis, 2000.
Easton, D.The Rammed Earth House. Vermont: The Chelsea Green Publishing Company, 1996.
Evrard, A., Sorption behaviour of lime–hemp concrete and its relation to indoor comfort and energy demand. PLEA2006 – 23rd Conference on Passive and Low Energy Architecture, Geneva, Switzerland, 6–8 September, 2006.
Ewing, R.P., Horton, R. Thermal conductivity of a cubic lattice of spheres with capillary bridges. Journal of Physics D: Applied Physics. 2007; 40(16):4959–4965.
Fang, L.G., Clausen, G., Fanger, P.O. Impact of temperature and humidity on the perception of indoor air quality. Indoor Air. 1998; 8(2):80–90.
Fang, L.G., Clausen, G., Fanger, P.O. Impact of temperature and humidity on perception of indoor air quality during immediate and longer whole-body exposures. Indoor Air. 1998; 8(4):276–284.
Gaur, R.C., Bansal, N.K. Effect of moisture transfer across building components on room temperature. Building and Environment. 2002; 37:11–17.
Hall, C., Hoff, W.D.Water Transport in Brick, Stone and Concrete. London: Taylor & Francis, 2002.
Hameury, S. Moisture buffering capacity of heavy timber structures directly exposed to an indoor climate: a numerical study. Building and Environment. 2005; 40(10):1400–1412.
Hameury, S., Lundström, T. Contribution of indoor exposed massive wood to a good indoor climate: in situ measurement campaign. Energy and Buildings. 2004; 36(3):281–292.
Hansen, E.J.dP., Hansen, K.K., Unfired clay bricks – moisture properties and compressive strength. Building Physics 2002 – 6th Nordic Symposium, 17–19 June, Trondheim, Norway, 2002.
Holtz, R.D., Kovacs, W.D.An Introduction to Geotechnical Engineering. Englewood Cliffs, NJ: Prentice-Hall, 1981.
ISO 8301Thermal insulation – Determination of steady-state thermal resistance and related properties – Heat flow meter apparatus. Geneva, Switzerland: International Organization for Standardization, 1991.
ISO 10051Thermal Insulation – Moisture effects on heat transfer – Determination of thermal transmissivity of a moist material. Geneva, Switzerland: International Organization for Standardization, 1996.
ISO 10456Building materials and products: Procedures for determining declared and design thermal values. Geneva, Switzerland: International Organization for Standardization, 1999.
Künzel, H.M., Zirkelbach, D., Sedlbauer, K., Predicting indoor temperature and humidity conditions including hygrothermal interactions with the building envelope. Proceedings of 1st International Conference on Sustainable Energy and Green Architecture, BSRC, Bangkok, 2003.
Lucas, F., Adelard, L., Garde, F., Boyer, H. Study of moisture in buildings for hot humid climates. Energy and Buildings. 2002; 34:345–355.
Mitchell, J.K.Fundamentals of Soil Behaviour. London: John Wiley & Sons, 1993.
Mortensen, N. The naturally air conditioned house. Earth Building Research Forum, University of Technology Sydney, Australia, available via: http://www.dab.uts.edu.au/ebrf/, 2000.
Morton, T. Unfired earth brick building. Building for the Future. 2005; 24–27.
Morton, T. Feat of clay. Materials World. 2006; 14(1):23–24.
Nordic Innovation Centre (NIC), Moisture buffering of building materials Technical report BYG-DTU R-126. Technical University of Denmark, 2005.
Osanyintola, O.F., Simonson, C.J. Moisture buffering capacity of hygroscopic building materials: experimental facilities and energy impact. Energy and Buildings. 2006; 38(10):1270–1282.
Rode, C.Workshop on Moisture Buffer Capacity – Summary Report R-067. Technical University of Denmark, 2003. [Department of Civil Engineering].
Sedlbauer, K. Prediction of mould growth by hygrothermal calculation. Journal of Thermal Envelope and Building Science. 2002; 25:321–335.
Taylor, P., Luther, M.B. Evaluating rammed earth walls: a case study. Solar Energy. 2004; 76:79–84.
TCCThermal Mass for Housing. Surrey: The Concrete Centre, 2006.
Toftum, J., Jorgensen, A.S., Fanger, P.O. Upper limits of air humidity for preventing warm respiratory discomfort. Energy and Buildings. 1998; 28(1):15–23.
Toftum, J., Jorgensen, A.S., Fanger, P.O. Upper limits for indoor air humidity to avoid uncomfortably humid skin. Energy and Buildings. 1998; 28(1):1–13.
Velde, B.Introduction to Clay Minerals. Berlin: Springer, 1992.
Walker, P., Keable, R., Marton, J., Maniatidis, V.Rammed Earth Design and Construction Guidelines. Watford: BRE Bookshop, 2005.
Zhang, H., Ge, X., Ye, H. Randomly mixed model for predicting the effective thermal conductivity of moist porous media. Journal of Physics D: Applied Physics. 2006; 39:220–226.