Aerogel materials for insulation in buildings
Abstract:
Aerogel materials have recently received much attention since they give many exciting applications in a wide range of areas. This chapter highlights the processing of these materials, the resulting physicochemical properties and their applications. Thus, fundamental understandings in the techniques for processing of aerogel materials including conventional drying, supercritical drying, freeze-drying, ambient-pressure drying with regards to material density and void size distribution, thermal conductivity, optical and acoustic properties are provided. In addition, a number of chemical post-treatments for surface engineering of aerogel materials are included. Finally, potentially new applications of using these materials as thermal insulation for building, optical sensor, space dust collector and catalysis are discussed.
13.1 Introduction
13.1.1 General properties
Aerogels have received significant attention over the last few decades because they possess many unique and characteristic physicochemical properties. For example, solid aerogel can be fabricated at a lower density than any other solid materials, with a density ranging from 1.5 to 50 mg/cm3. It also gives exceptionally low thermal insulation, in the order of 0.005 W/mK, high dielectric constant, at 3–40 GHz with k = 1.008–2.27, low acoustic properties (sound velocities as low as 100 m/s) and good optical transmittance, over 90% even at 632.8 nm (Fricke and Emmerling, 1992; Kim and Hyun, 2001; Schultz et al., 2005, Dorcheh and Abbasi, 2008). Its extraordinarily high specific surface area, corresponding to a high surface-to-volume ratio, is particularly significant.
Recent commercial applications of aerogels include thermal window insulation (Duer and Svendsen, 1998; Wagh and Ingale, 2002), supercapacitors (Gouerec et al., 1999; Li et al., 2006), acoustic barriers (Vicente et al., 2005; Gronauer and Fricke, 1986), and catalysis (Pajonk, 1991; Cao et al., 2007; Schneider and Baiker, 1995). A number of possible new applications, such as modified materials for wall decoration, nuclear waste storage (Reynes et al., 2001), adsorbents (Carraher, 2005; Han et al., 2000) and dust capture (Tsou, 1995; Burchell et al., 2006) have been described. Modifying functional groups on the gel surface to carry foreign materials (Yu et al., 2009; Tsang et al., 2006) and total encapsulation of a range of different chemical species in the gel (Maury et al., 2004; Buisson et al., 2001; Orcaire et al., 2006; Yu et al., 2009) could offer new uses in many areas. Thus the range of technologies using aerogels is expanding rapidly to meet new and changing industrial demand.
13.1.2 Background
We start by defining terms related to the sol-gel process that will be used throughout this chapter. In a sol, (seed) colloidal particles with diameters in the range of 1–1000 nm are dispersed in a liquid. A gel consists of a sponge-like, three-dimensional solid network whose pores are filled with liquid. When gels are prepared by hydrolysis and condensation of metal or semi-metal alkoxides or other hydrolyzable metal compounds (through the sol stage), the liquid in the pores consists mainly of water and/or alcohols. The resulting wet gels are therefore called aquagels, hydrogels, or alcogels. When the pore liquid is replaced by air without decisively altering the network structure or the volume of the gel body, aerogels are obtained (or cryogels, when the pore liquid is removed by freeze-drying). A xerogel is formed upon conventional drying of wet gels, that is, by increase in temperature or decrease in pressure, and is accompanied by large shrinkage (and mostly destruction) of the initially uniform gel body. Thus the general term ‘aerogel’ describes a high surface area solid material with an internal structure of pores and networks that were originally filled with liquid during synthesis but this has since been replaced with air. Aerogel was first discovered by the scientist Samuel S. Kistler in the late 1920s. Its characteristic properties have been revealed gradually since the initial synthesis (Kistler, 1931, 1932, 1935). Kistler’s work led to many other new and fascinating materials of different composition being developed along the same lines. The first recorded commercial silica aerogel was produced by the Monsanto Chemical Co. at Boston, MA, in the 1940s (Ayers, 2000).
Synthesis of aerogel involves three steps: sol and gel formation, aging of the gel and solvent drying. This creates the internal porous structure, which gives the material its characteristic properties. The pores consist of cavities, voids, channels, holes or interstices which can be arranged regularly, similar to zeolite, or mesoporous and molecular sieves. However, aerogels more commonly have a rather irregular porous structure, as the different parameters of the chemical processes in seeding, aging and drying damage the internal ordering structure of the pores. Since the physical and chemical properties are greatly dependent on the conformation of pores, including pore size, shape, porosity, and density, etc., controlling the size and shape of the pores and their network is a major challenge. The drying process is undoubtedly the most important step affecting the internal porous structure of the final aerogel material. The gel typically shrinks during drying. Many researchers have devoted their efforts to investigating mild drying techniques in ambient pressure in order to maintain the porous network (Schwertfeger et al., 1998; Prakash et al., 1995; Rao et al., 2007; Shi et al., 2006). In recent studies, modifications of surface functional groups, solvent exchange and use of different precursors (Schwertfeger et al., 1998) have been explored as methods to maintain or improve the porosity of aerogels rather than using expensive and cumbersome supercritical techniques.
In this chapter, we first review the general synthesis of sol-gel materials and then discuss aerogel synthesis in order to elucidate the key parameters in controlling the internal porous structure of aerogels. For interested readers, more detailed information is available from the literature (e.g. Dorcheh and Abbasi, 2008; Husing and Schubert, 1998; Pierre and Pajonk, 2002, Pajonk and Venkateswara Rao, 2001).
13.2 Processing material and properties
13.2.1 Sol-gel processing
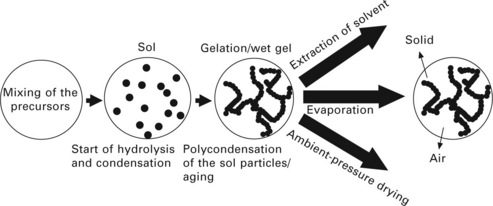
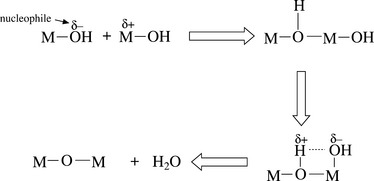
The sol-gel process is a wet-chemical technique which is widely adopted for making high surface area oxide materials. The sol-gel process is composed of three stages: hydrolysis, condensation and drying (see Fig. 13.1). A liquid sol particle (the smallest seed particles in the solvent) is first created by hydrolysis of a chemical precursor, followed by condensation to form a gel. The first precursors used in sol-gel processing were metallic salts MXn, in which a metal M is linked to some number n of anions X. In aqueous solution, the chemical precursors are presented as ionic species in which the metal atoms exist as solvated cations M[H2O]nz +. The reactions to form sol particles and gels comprise hydrolysis reactions, in which H2O groups are replaced with OH− groups (alkaline catalysed reactions, see Eq. 13.1) with the loss of protons, and condensation reactions, which lead to the construction of M![]() O
O![]() M ‘oxo’ bridges with the elimination of water molecules (nucleophilic attack of the metal cation, see Eq. 13.2). Thus, the sol particles gradually condense to form a three-dimensional continuous network (base-catalysed sols). In this stage, the porous gel structure is filled with solvent molecules. A more interconnected, wet gel (or hydrogel) forms during the aging period. During the final step, drying, solvent or liquid is removed from the pores of the wet-gel material. Conventional drying is typically accompanied by large shrinkage in volume.
M ‘oxo’ bridges with the elimination of water molecules (nucleophilic attack of the metal cation, see Eq. 13.2). Thus, the sol particles gradually condense to form a three-dimensional continuous network (base-catalysed sols). In this stage, the porous gel structure is filled with solvent molecules. A more interconnected, wet gel (or hydrogel) forms during the aging period. During the final step, drying, solvent or liquid is removed from the pores of the wet-gel material. Conventional drying is typically accompanied by large shrinkage in volume.
To understand particle agglomeration, it is important to appreciate the surface characteristics of the particle and the type of aqueous species surrounding it. In general, zero-charged particles give higher aggregation rates to form larger particles, which up to a critical size may settle as precipitate under gravity. Figure 13.2 shows zero-charge nanoparticles in the liquid phase, which collide with each other and form larger clusters (this is thermodynamically favourable because it reduces the overall surface energy of the system). If each particle carries the same electrical charge, mutual electrostatic repulsion will keep them apart in solution, as shown in Fig. 13.3. Typically, the small silica sol (nanoparticles) synthesized by the sol-gel method at pH 7.4 are in the form of a stable and homogeneous colloid which does not undergo aggregation. This is because of the lower isoelectronic point (pH ~ 2.0) of silica compared to the solvent medium, which renders the particles negatively charged. Particles possessing high charges can induce double layers in an aqueous environment and will remain discrete and well dispersed from each other.
13.2.2 Synthesis and structure of metal alkoxides
In core-shell nanoparticle morphology, a core metal particle is protected from oxidation and corrosion by a silica coating (Santra et al., 2001). This structure is becoming more popular, and is created using microemulsion techniques. Hydrolysis and condensation of sol-gel precursors such as silicon alkoxide displace the surfactant molecules in micelle assemblies and produce an ultra-thin porous coating on the core particle at the water/organic interface. This process is based on hydrolysis of precursors such as tetraethoxysilane (TEOS) in the presence of water and catalysts, followed by condensation with surface metal hydroxyls. An M![]() O
O![]() Si chemical linkage is established between surface metal atoms and TEOS, followed by the formation of a three-dimensional network of siloxane bonds (Si
Si chemical linkage is established between surface metal atoms and TEOS, followed by the formation of a three-dimensional network of siloxane bonds (Si![]() O
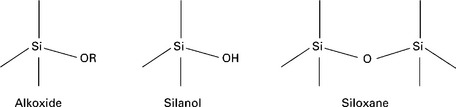
O![]() Si) with increasing TEOS concentration. Once the precursor has been condensed into a gel, the solvent is removed by drying. It is worth noting that the surfaces of silica gels can have different chemical functionalities, i.e. alkoxides, silanol and siloxane, depending on the preparation procedure, reagents used and post-treatments (see Fig. 13.4).
Si) with increasing TEOS concentration. Once the precursor has been condensed into a gel, the solvent is removed by drying. It is worth noting that the surfaces of silica gels can have different chemical functionalities, i.e. alkoxides, silanol and siloxane, depending on the preparation procedure, reagents used and post-treatments (see Fig. 13.4).
13.2.3 Possibility for advancement using other alkoxides for aerogels
Although silica alkoxide is most commonly used, other metal alkoxides (M-OR) can be used for the sol-gel process. For example, titanium, zirconium, tin, carbon and aluminium can be prepared in a similar way to give porous microstructures (Sanchez and Ribot, 1994). The rates of hydrolysis and condensation for these metal alkoxides are much faster than those for silicon as they are much stronger electrophiles. Carbon aerogels can be prepared from pyrolysis of an organic matrix. The surface area of synthesized carbon aerogels ranges from 400 to 800 m2g− 1. The pore size distribution is found to depend on the density of the carbon aerogel (Zhang et al., 1999). Carbon aerogel made using the pyrolysis process has been shown to have the largest specific area at 600 °C. After further pyrolysis at 2100 °C, the internal structure revealed by small-angle X-ray scattering (SAXS) contained a large amount of micropores (Reichenauer et al., 1998). Carbon aerogels also show density-dependent electrical conductivity, rendering them suitable for use as supercapacitors. They also find use as solar energy collectors because of their lower reflectance of radiation.
Alumina aerogel is synthesized using either Al(OsBu)3(ASB) or Al(OtBu)3(ATB) as a precursor (Husing and Schubert, 1998). These aerogels can be doped with metallic promoter(s) to introduce new physiochemical properties. For example, Kwak et al. (2005) used a platinum–cobalt–alumina aerogel for the selective oxidation of carbon monoxide to carbon dioxide in hydrogen gas. Controlling the homogeneity of zirconia silica aerogel was demonstrated by Miller et al. (1994). Aerogels based on titania–silica and MTiO2 (M = Mg, Mn, Fe, Co, Zn) were tested as catalysts for photocatalysis (Cao et al., 2007; Ahmed and Attia, 1995; Kapoor et al., 2005). Zirconia aerogels were prepared using combined alcohothermal and supercritical fluid drying techniques, resulting in materials that were characterized by mesopores with a narrow pore size distribution and high specific area (Cao et al., 2002).
13.3 Aerogel formation
13.3.1 Drying techniques
Making aerogels requires an efficient drying stage to replace trapped solvent molecules in the wet sol-gel materials with air without damaging the internal porous structure. The drying process is very complex, involving interplays between surface tension, capillarity and diffusion of the solvent with different pore size, shape and wall materials. When liquid is evaporated from occupied pores, it diffuses from the interior of the pore to the outer surface. A cohesion force holds the liquid molecules together (reflected by the phenomenon of surface tension), and adhesion forces stick the liquid molecules to the wall material. If different sizes of pores are present, the larger pores retain solvent molecules less easily than the smaller ones due to the smaller capillary potential under the same pressure conditions. The change in capillary force and the stress created on the wall of the pore are also different when solvent molecules leave the pore structure. A smaller pore network can become brittle, leading to cracking (see Fig. 13.5), since the capillary potential is inversely related to pore radius. Dynamic drying processes also affect the degree of shrinkage. A more rapid evaporation of solvent molecules from the interior pore surface will cause a greater pressure gradient change, which can easily lead to cracks in the pore structure. Thus, the large shrinkage of a gel body upon evaporation of the pore liquid is caused mainly by capillary forces acting on the pore walls as the liquid retreats into the gel body. This results in the collapse of the filigrane, the highly porous inorganic networks of aquagels and alcogels.
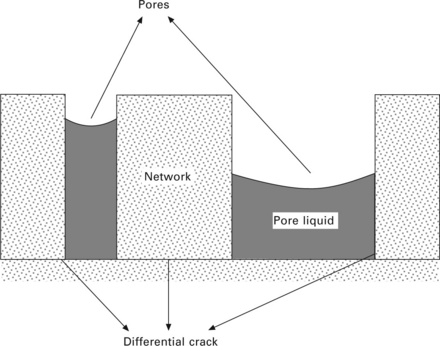
13.5 Evaporation of solvent molecules from smaller pores induces more structural damage (crack) than larger ones.
During the conventional drying process, there are also chemical changes that affect the degree of shrinkage of the aerogel materials. For example, the inner wall surface of a pore is likely to be covered with ![]() OH groups after wet chemical synthesis. In the case of silica gel, the surface Si–OH groups in close proximity can interact with each other to form siloxane bridges during drying. Thus, the porous network will become stiffer, with a smaller pore radius. Elevated temperatures will speed up these chemical changes. When the stress due to solvent evaporation is no longer capable of further deforming the network, the structure may collapse, creating substantial shrinkage in the gel material.
OH groups after wet chemical synthesis. In the case of silica gel, the surface Si–OH groups in close proximity can interact with each other to form siloxane bridges during drying. Thus, the porous network will become stiffer, with a smaller pore radius. Elevated temperatures will speed up these chemical changes. When the stress due to solvent evaporation is no longer capable of further deforming the network, the structure may collapse, creating substantial shrinkage in the gel material.
13.3.2 Supercritical drying
Supercritical drying is a very efficient method for producing aerogel. Two supercritical drying techniques have been demonstrated. One is described as high temperature supercritical drying (HTSCD) and the other low temperature supercritical drying (LTSCD).
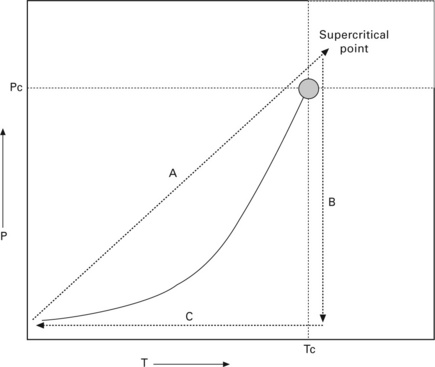
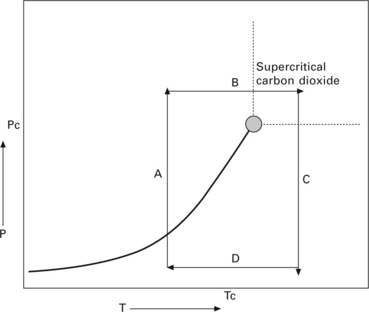
HTSCD was the first technique employed in the synthesis of silica aerogel by Kistler in the 1930s and is still widely used to prepare aerogel materials today (see Fig. 13.6). The drying principle is illustrated in Fig. 13.6, which shows a critical point in the phase diagram of a fluid at which the density of its gas and liquid forms is identical. A wet gel containing a large amount of solvent (e.g. methanol, ethanol, acetone, 2-propanol, or water) is first placed in an autoclave, and the temperature and pressure of the container are slowly increased to above the critical point of the solvent (Step A). The drying conditions are kept constant and this allows homogeneous extraction of the solvent because, at the supercritical drying stage, there is no interface between gas and liquid. Thus, the pressure/stress change imposed on the pore structure due to removal of solvent is not significant. The next step, B, is to slowly vent the contents of the autoclave at constant temperature until it reaches ambient pressure. The final step, C, is to decrease the temperature to room temperature, yielding a dried aerogel.
However, the supercritical states of most alcohols and acetones are characterized by critical temperatures about 250 °C and critical pressures between 5 and 8 MPa. It is important to note that the combination of high temperature and high pressure could cause undesirable chemical reactions or rearrangements in the surface of the gel network. Kocon et al. (1998) therefore studied the effect of pH on silica aerogel in an attempt to minimize material shrinkage during supercritical drying of ethanol from the structure. Under these conditions, a low density aerogel (3 kg/m3) can be obtained. Gross et al. (1998) used a rapid supercritical drying process to produce silica aerogel from tetramethoxysilane (TMOS) with ammonia mixed in alcohol. This process involved loading the sol in a stainless steel autoclave and immediately heating the mixture to supercritical conditions. The aging process took place and the reaction rate was very fast due to the high temperature. Once the supercritical point of the alcohol was reached, the supercritical fluid was vented rapidly. The whole process lasted less than one hour (Gross et al., 1998). An advantage of this technique is that the process is carried out in one single step, and a short period of time means an increase in production volume. The disadvantage is that this technique may still induce chemical change in the material due to the high temperature and pressure, but the fast supercritical drying stage may reduce undesirable chemical change to some extent (Poco et al., 1996; Scherer et al., 2002; Gauthier et al., 2004).
Another supercritical drying technique is the employment of carbon dioxide (LTSCD) for solvent extraction (Tang and Wang, 2005; Sui et al., 2004). The advantage of this method is that it takes place near to room temperature (< 32 °C) and under moderate pressure (< 80 bar). Figure 13.7 shows the phase diagram of carbon dioxide. A wet gel is placed in an autoclave and carbon dioxide is pumped in. The temperature is then raised to 40 °C and the pressure is kept at about 100 bars to reach the supercritical state of carbon dioxide. The time required for exchanging the trapped solvent with carbon dioxide depends on the dimension of the pores in the gel material. After extraction, the pressure of the applied carbon dioxide is slowly reduced and the temperature is dropped to room temperature. The fluid is then vented from the autoclave. Repeated extractions in the same manner are carried out. Silica aerogel can be obtained using this technique (Dorcheh and Abbasi, 2008). Since water and carbon dioxide are rather immiscible, modifier(s) such as alcohols are required to produce highly porous silica aerogels (Husing and Schubert, 1998).
13.3.3 Freeze-drying process
Freeze-drying is another mild drying method used to produce aerogels. The liquid solvent in the pores is rapidly frozen and then sublimed under reduced pressure. Using this drying method for the preparation of carbon or TiO2 aerogel materials is well documented (Mukai et al., 2004; Babic et al., 2004; Xu and Yang, 2003; Yamamoto et al., 2002). There are some prerequisite conditions that have to be achieved before the drying process can begin. First, the porous network of the wet material has to be extensively strengthened during the aging period. Secondly, the trapped solvent must have a low sublimation temperature, otherwise it will have to be exchanged with another solvent with lower sublimation temperature to facilitate diffusion and sublimation during drying. Salt or modifier may be used to lower the solvent freezing temperature. Maintaining the integrity of the porous network of a gel is challenging during crystallization of solvent molecules in the pores. The aerogel produced using this method is also known as ‘cryogel’.
13.3.4 Ambient-pressure drying process (APD)
Another common drying method in aerogel production is known as ‘ambient-pressure drying’ (APD). The drying is carried out at ambient pressure preferably using reduced or elevated pressures. As a potentially cheap and safe drying process for large-scale production of aerogels, this method has received considerable attention from industry. However, reducing shrinkage of the material during the drying process is a significant challenge, and the porous network has to be strengthened considerably, probably through surface modification, prior to drying. In addition, the contact angle between the trapped solvent and the pore wall must be measured to decide whether chemical modification of the inner surface is needed in order to minimize the change in capillary forces following solvent removal (Dorcheh and Abbasi, 2008). The general method for chemical modification of the surface is to use a mixture of precursors before gelation, although modifying the surface after gelation by using silylating agents is also effective. As the silylation reaction commonly takes place in an organic solvent, multi-step processes for solvent exchange are sometimes necessary. For example, mixed water and alcohol is used as a bulk solvent to exchange the trapped water in the gel pores before the silanol group on the surface is silylated by adding a modifier (e.g. CTMS, HMDS, HMDZ or others) (see Fig. 13.8). After the surface reaction, the material becomes hydrophobic due to the presence of surface capping groups (Si![]() O
O![]() R). Multi-stepped exchange processes also require long pre-treatment time and large amounts of solvents, which causes economic and environmental concerns.
R). Multi-stepped exchange processes also require long pre-treatment time and large amounts of solvents, which causes economic and environmental concerns.

13.8 A typical surface silylating process on aerogel by chlorotrimethylsilane (CTMS) or hexamethyldisilazane (HMDZ).
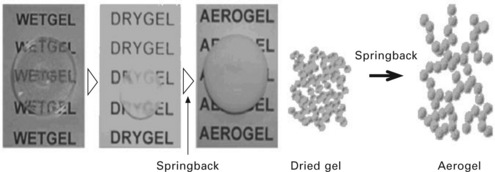
Ambient-pressure drying still results in a degree of material shrinkage, because severe stress is created in the gel material due to the unavoidable pore narrowing with the formation of siloxane. The pore structure can be returned to its original form upon re-wetting under defined conditions, the so-called spring-back effect (Lee et al., 2002) (see Fig. 13.9). Schwertfeger et al. (1998) presented a competitive method using inexpensive waterglass, a simple exchange solvent technique, and drying in ambient pressure with modification of the internal surface. They employed hexamethyldisiloxane (HMDSO)/trimethylchlorosilane (TMCS) solution to modify the inner surface of the wet gel. IPA/TMCS/n-hexane solution was used to prepare a silica aerogel by Kim and Hyun (2003) and Lee et al. (2002). Rao et al. (2005b) developed a two-step drying process at ambient pressure applicable to various solvents, including hexane, cyclohexane, heptane, benzene, toluene and xylene. They prepared aerogel from TEOS and modified the surface with hexamethyldisilazane (HMDZ) in different solvents. They reported that heptane gave highly transparent, porous but low density, low thermal conductive and hydrophobic aerogels. FTIR spectra of their pre-treated aerogels clearly indicated the change in surface functionalities (the IR bands at 1600 and 3500 cm− 1 corresponding to Si![]() OH and H
OH and H![]() OH were substantially attenuated but new bands at 840 and 1250 cm− 1, corresponding to Si
OH were substantially attenuated but new bands at 840 and 1250 cm− 1, corresponding to Si![]() C and C
C and C![]() H were formed (Rao et al., 2005a)).
H were formed (Rao et al., 2005a)).
13.3.5 Controlling density and void size distribution
When a wet gel is dried by removing trapped solvent molecules, an inherent porous structure can be reclaimed. In the case of inorganic gel, the skeletal structure becomes more rigid during solvent evaporation. Liquid in the pores is removed to form an extensive porous network. However, heat treatment will concomitantly cause the porous structure to collapse, making the network more dense. The chemical reactions that take place during heat treatment occasionally isolate the pore structure, resulting in closed pores. It is important to avoid cracking and pore blockage in order to maintain an ordered porous structure in an aerogel.
A sharp void size distribution can be obtained by adding reagents such as formamide, oxalic acid or glycerol to the gel precursors. The size of macroporous silica aerogel can be tailored by tuning the concentration of polymer added. For example, Nakanishi et al. (1998) successfully prepared a narrow void distributed aerogel by adding polyethylene glycol (PEG) to their sol precursor. A high concentration of PEG improved the strength of the solid matrix, whereas low concentrations of PEG reduced its strength. Harreld et al. (2002) modified silica gels by exchanging a mixture of solvents in order to reduce structural shrinkage during drying at ambient pressure. The porosity, pore size distribution and surface area of their gels were much improved by using appropriate ratios of acetone/alkane. Tabata et al. (2005) dried an alcogel (less shrinkage than aerogel) at ambient temperature to produce an extremely low density gel material of 9 kg/m3, which corresponded to a reflect index of 1.002. The world’s lowest density silica aerogel was prepared by Larry Hrubesh at Lawrence Livermore National Laboratory (LLNL) in the late 1980s. The claimed density of the aerogel was 3 kg/m3, which is approximately only three times that of air (Arlon and Michael, 2004). Widenmeyer and Anwander (2002) used surfactants ([CH3(CH2)nNMe2(CH2)mNMe2(CH2)nCH3]2 +2Br−) of different chain lengths under hydrothermal methods at pH > 9 to prepare tuneable pore sizes and highly ordered cubic mesoporous silicas (MCM-48). Kim and Ryoo (1999) prepared tuneable pore sizes of cubic mesoporous silica SBA-1 under acidic conditions using tetraethyl orthosilicate and hexadecyltriethylammonium bromide. The pore diameter of the SBA-1 materials was controlled over 1.4–2.7 nm by tuning the surfactant concentration. Rao et al. (2003) used methyltrimethoxysilane (MTMS) precursor, methanol (MeOH) solvent and ammonium hydroxide (NH4OH) catalyst to prepare aerogels. The optimum reagent ratio was obtained to yield a low density but highest contact angle (~ 173°) aerogel. BET is the most common method for characterizing porosity, pore size distribution and surface area of a synthesized aerogel.
13.4 Physical properties of aerogels
13.4.1 Thermal conductivity
Kistler was the first researcher to discuss the low thermal conductivity of silica aerogel (Kistler, 1932, 1935). This led to a fundamental analysis of the factors affecting the overall thermal transportation of aerogels consisting of gas phase, solid phase and radiation components. Figure 13.10 shows a model of the thermal transportation properties of an aerogel as a function of density (Husing and Schubert, 1998). There is a sharp increase in conductivity when the density of the gel material is raised. However, gas and radiation components show the opposite trend for thermal conductivity with increasing density. Thus, the lowest overall thermal conductivity peaks should be minimized at a density of about 150 kg/m3.

13.10 Thermal conductivity of aerogels: total, solid, gas and radiation transport λ, are dependent on the density ρ of aerogel materials (Husing and Schubert, 1998; Fricke, 1988).
It was anticipated that smaller pore size played an important role in lowering gaseous thermal conductivity due to the Knudsen effect (Pierre and Pajonk, 2002), and that gas thermal conductivity could be further minimized under reduced pressure. This led to the production of a new silica aerogel which showed thermal conductivity as low as 0.020 W/mK at ambient pressure, with a further decrease to 0.010 W/mK under vacuum at 300 K (Husing and Schubert, 1998; Fricke, 1988). Thermal conductivity also relates to the nature of the porous structure: whether the pores are closed or open will give different thermal transport properties. Heat transport through infrared radiation is an important factor affecting the thermal conductivity of silica aerogels. This is affected by density, temperature and optical thickness. Reducing thermal conductivity by doping aerogels with infrared opacifiers such as carbon or TiO2 is well documented (Husing and Schubert, 1998).
Silica aerogels show the lowest thermal conductivity of all non-flammable solid materials (see Fig. 13.11), thus they can be used as a coating to modify construction materials. Aerogels can be used to coat the walls of buildings. The poor infrared radiation prevents heat from a heated room penetrating outside, but visible and UV radiations from external daylight can pass through the material. Aerogels are therefore used for window insulation, daylight windows (for example car windshields, or bathroom, staircase or ceiling windows) and cooling and heating storage applications (Husing and Schubert, 1998; Pierre and Pajonk, 2002). Solar energy can be captured by panelling house walls (see Fig. 13.12), or by solar collectors that use aerogel materials (Fricke et al., 1987; Svendsen, 1992).

13.11 Comparing thermal conductivity of aerogel with other commercially available materials. PUR: polyurethane foam, CFC: chlorofluorohydrocarbons; EPS, XPS: expanded and extruded polystyrene (Husing & Schubert, 1998).
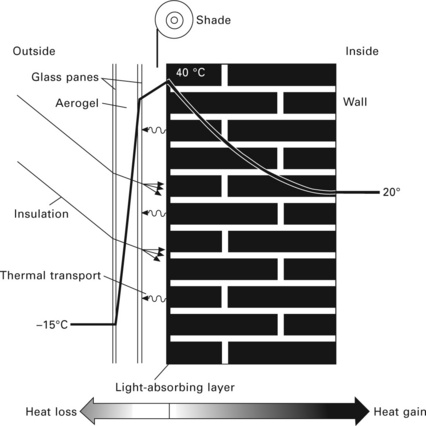
13.12 Principle of energy saving by using aerogel to capture solar energy (Husing and Schubert, 1998).
It is well known that transmitted light suffers from the scatter phenomenon, causing poor optical quality in conventional materials, but significant improvements in the optical quality of aerogels have been reported recently (Duer and Svendsen, 1998). Schultz and co-workers reported a super insulating glazing using a monolithic SiO2 aerogel sheet of approximately 55 × 55 cm2. Anti-reflective treatment of aerogel-coated glass can also improve their visual quality. Solar energy transmittance can reach 76% (Schultz et al., 2005; Jensen et al., 2004).
13.4.2 Optical properties
Silica aerogels are between transparent and translucent, depending on their internal structure and moisture content. The porous networks of ordered silica aerogels have smaller structural units than the wavelength of visible light, hence they should appear to be transparent. But if some amorphous structures are introduced in the ordered units, these lead to scattering in the visible light region, known as Rayleigh scattering (De Leo et al., 2001; Tajiri and Igarashi, 1998). Such non-homogeneity in silica aerogel is responsible for reduced transparency, and most silica aerogels appear yellowish under light and bluish under a dark background (Husing and Schubert, 1998). Their optical properties can be tuned by controlling the content of amorphous structures in the aerogel during synthesis.
Optical transparency is important for some applications. For example, a new type of solid-state Cerenkov particle detector requires a high quality, transparent aerogel (Adachi et al., 1995). Controlling the processing parameters during synthesis gives arerogels with different degrees of optical transparency. The drying process, degree of water adsorption on Si–OH groups, adsorbed organic components on Si![]() OCH3 or Si
OCH3 or Si![]() CH3, heat treatments (Buzykaev et al., 1999), type of precursors, sol-gel process, silation agent and solvent used were all found to affect the optical properties of aerogels (Rao et al., 2001). It was also claimed that two-step synthesis methods give more transparent aerogels than one-step methods, which could be related to the lower degree of amorphous silica present in the aerogel (Rao et al., 2005b; Danilyuk et al., 1999). Supercritical drying of the gel materials with CO2 commonly resulted in materials with better transparency than those dried using other organic fluids. Lower reaction temperature and lower amounts of water appeared to be effective in making aerogels that were more transparent (Husing and Schubert, 1998). Waterglass, a cheap aerogel precursor, gave a low transparency gel, probably because of the presence of large silica particles and sodium ions in the sample.
CH3, heat treatments (Buzykaev et al., 1999), type of precursors, sol-gel process, silation agent and solvent used were all found to affect the optical properties of aerogels (Rao et al., 2001). It was also claimed that two-step synthesis methods give more transparent aerogels than one-step methods, which could be related to the lower degree of amorphous silica present in the aerogel (Rao et al., 2005b; Danilyuk et al., 1999). Supercritical drying of the gel materials with CO2 commonly resulted in materials with better transparency than those dried using other organic fluids. Lower reaction temperature and lower amounts of water appeared to be effective in making aerogels that were more transparent (Husing and Schubert, 1998). Waterglass, a cheap aerogel precursor, gave a low transparency gel, probably because of the presence of large silica particles and sodium ions in the sample.
13.4.3 Acoustic properties
Overall, aerogels are excellent acoustic insulators. The acoustic propagation in aerogels depends on the interstitial gas type and pressure, density and, more generally, texture. The sound energy transferred from the gas to the solid phase is partly lost, thus reducing the amplitude and velocity of the acoustic wave. The longitudinal acoustic velocity is typically in the order of 100 m/s. This property makes silica aerogels suitable for applications in acoustic impedance ì/4 matching layers in ultrasonic transducers, range finders, speakers, etc. Their application in house floor-covering and for football pitch sound insulation has been mentioned (Husing and Schubert, 1998). Other applications concern acoustic insulation in transportation and machinery (Gross and Fricke, 1992; Gross et al., 1992). Silica aerogels have also been investigated for applications in anechoic chambers (Pierre and Pajonk, 2002; Forest et al., 1998; Zimmermann et al., 1995).
13.5 Applications of aerogels
13.5.1 Solid state insulation
As aerogels have an extraordinarily low thermal conductivity and high acoustic insulation, a striking number of applications have been developed in window glazing (Tewari et al., 1986; Duer and Svendsen, 1998). Reim et al. (2002, 2005) undertook a large programme to develop a highly insulating but translucent glazing material. A sandwich structure construction of gel filled with granules was set up. Krypton gas was used as the filler to optimize thermal insulation. The resulting heat transfer coefficient was less than 0.4 W/mK, with total solar energy transmittance of 35%.
13.5.2 Space applications
As low density and lightweight materials, aerogels can successfully capture cosmic dust particles in space. NASA’s stardust (spacecraft) mission programme employed silica aerogels for such capture. The speed of a cosmic dust particle is at least six times that of a bullet. Thus, when a dust particle hits the mechanically rigid silica aerogel, a bullet shaped track can be located in the gel. The extraordinarily lightweight silica aerogel also protects space mirrors (Hrubesh, 1998). The European Recoverable Carrier (EURECA) spacecraft is also using silica aerogel for catching falling cosmic dust (Tsou, 1995). This technology could be expanded to capture gases and dust in buildings and vehicles.
13.5.3 Optical applications
Transparent aerogels may find applications in solar windows (Tewari et al., 1986; Rubin and Lampert, 1983; Fricke, 1986), whilst translucent aerogels are proposed for use as solar covers and collectors (Svendsen, 1992; Jensen, 1992). Ultralow density aerogels are currently being considered as lightweight mirror backings (Hotaling, 1993; Hrubesh, 1998).
Silica aerogels doped with lanthanides are used as laser glasses (Tillotson et al., 1994). If they are doped with radioactive tritium and a phosphor, radioluminescent light/power sources can be obtained (Ashley et al., 1992). If treated with microwave-energized reducing gases, silica aerogels could become permanent visible photoluminescent sources with emission wavelengths ranging from 460 to 500 nm, due to oxygen-defective centres (Ayers and Hunt, 1997).
13.6 Future trends
Silica aerogels have high surface areas, are highly porous and lightweight but mechanically rigid and will no doubt find a wide range of applications from pharmaceutical to semiconductor industries. Silica aerogels can also be chemically modified to suit specific applications. A popular area of research is seeking applications for silica aerogel in biotechnology. Functionalizing the surface of silica aerogels to carry biomolecules can be divided broadly into two methods. The first method is based on non-covalent binding of bio-species by simple electrostatic interaction. At a pH above the isoelectric point (IEP), the surface of a silica aerogel becomes negatively charged, whereas at a pH below the IEP, the surface is positively charged. If the biomolecule of interest carries the opposite charge to the aerogel in solution, it attaches to the silica surface by electrostatic attraction. The advantage of this method is that it does not require complex processes for chemical binding of the biomolecule, which therefore maintains its original conformation and activity (see Fig. 13.13). The alternative technique is covalent linkage of the biomolecule onto the surface, which provides a much stronger binding affinity and site specificity for the biomolecule attachment. General methods for covalent conjugation of biomolecules with surface functional end groups such as ![]() NH2,
NH2, ![]() SH, and
SH, and ![]() COOH are available (see Fig. 13.14).
COOH are available (see Fig. 13.14).

13.13 Schematic to illustrate the formation of non-covalent biospecies conjugates through electrostatic attraction.

13.14 Schematic representation of covalent bio-conjugation protocols for the attachment of biomolecules on silica nanoparticles (Wang et al., 2006). Reproduced with the permission of American Chemical Society (ACS).
Silica aerogel can therefore carry a wide variety of bio-species following surface modification, including small drug, antigen and protein molecules. After the silica surface is tagged to the bio-species, interesting applications such as in vitro bio-separation and in vivo targeting of cancer cells can be envisaged, and chemical modification of aerogels has been studied intensively over the last decade. El Rassy et al. (2003) have recently tailored the surfaces of aerogels with different degrees of hydrophobicity and hydrophilicity through different coverage of Si![]() OH, Si
OH, Si![]() OCH3 or Si
OCH3 or Si![]() CH3 end groups. Bass and Katz (2006) have prepared silica surfaces with bi-functional groups consisting of thiol and primary amine groups, which were characterized using solid-state UV/Visble, Si-19 CP/MAS NMR and C-13 CP/MAS NMR spectroscopy. Zhang et al. (2008) prepared helical meso-structured silica as a drug carrier. Their report revealed that drug release rate could be controlled by the helicity and morphology of the meso-structured silica. Chandra and Bhaumik (2009) prepared a new functionalized mesoporous polymer with silica for the removal of pollutant anions. For example, mesoporous poly-triallylamine (MPTA-1) showed high anion exchange efficiency for the removal of pollutant anions such as MnO4−, CrO42 −, AsO33 −, NO3− and PO43 −. Attia et al. (1994) prepared alkaline earth oxide modified aerogels for the capture of greenhouse gases such as CO2, SO2 and NOX. The high surface area of silica areogel combined with the basicity of CaO or MgO has proved to be efficient in capture and/ or adsorption of these acidic pollutants. Other smart materials containing silica aerogel, such as silica-coated magnetic nanoparticles (Yu et al., 2007, 2009; Tsang et al., 2006) and luminescent silica aerogels (Fricke, 1992; Ashley et al., 1992; Leventis et al., 1999) have been described. Heavy metals have been removed from industrial effluents using novel magnetic nanocomposites synthesized by embedding metal nanoparticles in a silica aerogel (Xu and Dong, 2008). Applications of these materials in capturing hazardous substances from water have been demonstrated (Bryant et al., 2003; Wingenfelder et al., 2005; Perez-Quintanilla et al., 2006).
CH3 end groups. Bass and Katz (2006) have prepared silica surfaces with bi-functional groups consisting of thiol and primary amine groups, which were characterized using solid-state UV/Visble, Si-19 CP/MAS NMR and C-13 CP/MAS NMR spectroscopy. Zhang et al. (2008) prepared helical meso-structured silica as a drug carrier. Their report revealed that drug release rate could be controlled by the helicity and morphology of the meso-structured silica. Chandra and Bhaumik (2009) prepared a new functionalized mesoporous polymer with silica for the removal of pollutant anions. For example, mesoporous poly-triallylamine (MPTA-1) showed high anion exchange efficiency for the removal of pollutant anions such as MnO4−, CrO42 −, AsO33 −, NO3− and PO43 −. Attia et al. (1994) prepared alkaline earth oxide modified aerogels for the capture of greenhouse gases such as CO2, SO2 and NOX. The high surface area of silica areogel combined with the basicity of CaO or MgO has proved to be efficient in capture and/ or adsorption of these acidic pollutants. Other smart materials containing silica aerogel, such as silica-coated magnetic nanoparticles (Yu et al., 2007, 2009; Tsang et al., 2006) and luminescent silica aerogels (Fricke, 1992; Ashley et al., 1992; Leventis et al., 1999) have been described. Heavy metals have been removed from industrial effluents using novel magnetic nanocomposites synthesized by embedding metal nanoparticles in a silica aerogel (Xu and Dong, 2008). Applications of these materials in capturing hazardous substances from water have been demonstrated (Bryant et al., 2003; Wingenfelder et al., 2005; Perez-Quintanilla et al., 2006).
The possible applications of these smart and related materials to solving current environmental problems have been widely discussed (Zhu et al., 2007; Mureseanu et al., 2008; Puanngam and Unob, 2008; Shevchenko et al., 2008; Yantasee et al., 2008). As a result, study on grafting reactive species, including bio-molecules, inorganic catalyst species or composites, onto material surfaces has intensified. New applications, such as developing enzymatic or photo-catalytic paints for wall decorations to remove pollutants (e.g. incorporating photoactive TiO2 and ZnO into aerogels) may become possible in the near future.
13.7 Conclusions
This chapter only gives a limited amount of information on the chemistry and techniques involved in synthesizing aerogels. Many inorganic polymeric oxides are remarkable by themselves, with a very rich chemistry that can be tailored to a wide range of applications in fundamental science and modern technology. Recent developments in the preparation of ‘aerogel-like’ materials at ambient pressure, which eliminates the need to use an autoclave, will open new fields of applications at the industrial level and require further chemical characterization. Organic, inorganic and composite aerogels clearly deserve to be studied as a new class of chemically designed architectures, and much remains to be done for materials other than silica.
13.8 References
Adachi, I., Sumiyoshi, T., Hayashi, K., Iida, N., Enomoto, R., Tsukada, K., Suda, R., Matsumoto, S., Natori, K., Yokoyama, M., Yokogawa, H. Study of a threshold Cherenkov counter based on silica aerogels with low refractive-indexes. Nuclear Instruments & Methods in Physics Research Section A – Accelerators Spectrometers Detectors and Associated Equipment. 1995; 355:390–398.
Ahmed, M.S., Attia, Y.A. Aerogel materials for photocatalytic detoxification of cyanide wastes in water. J. Non-Cryst. Solids. 1995; 186:402–407.
Arlon, H., Michael, A., SilicaAeroGelsHistory of Silica Aerogels. Berkeley Lab, 2004.
Ashley, C.S., Reed, S.T., Brinker, C.J., Walko, R.J., Ellefson, R.E., Gill, J.T., Aerogel composites for radioluminescent light/power sources. Chem. Process. Adv. Mater. 1992:989–996.
Attia, Y.A., Ahmed, M.S., Zhu, M., Sol-gel prepared aerogels for the capture of pollution gases. Sol-Gel Process. Appl., [Proc. Int. Symp. Adv. Sol-Gel Process. Appl.]. 1994:311–321.
Ayers, M.The Pioneer: Samuel Kistler. Berkeley Lab, 2000.
Ayers, M.R., Hunt, A.J. Visibly photoluminescent silica aerogels. J. Non-Cryst. Solids. 1997; 217:229–235.
Babic, B., Kaluderovic, B., Vracar, L., Krstajic, N. Characterization of carbon cryogel synthesized by sol-gel polycondensation and freeze-drying. Carbon. 2004; 42:2617–2624.
Bass, J.D., Katz, A. Bifunctional surface imprinting of silica: thermolytic synthesis and characterization of discrete thiol-amine functional group pairs. Chem. Mater. 2006; 18:1611–1620.
Bryant, D.E., Stewart, D.I., Kee, T.P., Barton, C.S. Development of a functionalized polymer-coated silica for the removal of uranium from groundwater. Environmental Science & Technology. 2003; 37:4011–4016.
Buisson, P., Hernandez, C., Pierre, M., Pierre, A.C. Encapsulation of lipases in aerogels. J. Non-Cryst. Solids. 2001; 285:295–302.
Burchell, M.J., Graham, G., Kearsley, A. Cosmic dust collection in aerogel. Annual Review of Earth and Planetary Sciences. 2006; 34:385–418.
Buzykaev, A.R., Danilyuk, A.F., Ganzhur, S.F., Kravchenko, E.A., Onuchin, A.P. Measurement of optical parameters of aerogel. Nucl. Instrum. Methods Phys. Res., Sect. A. 1999; 433:396–400.
Cao, S., Yeung, K.L., Yue, P.L. An investigation of trichloroethylene photocatalytic oxidation on mesoporous titania-silica aerogel catalysts. Applied Catalysis B–Environmental. 2007; 76:64–72.
Cao, Y., Hu, J.C., Hong, Z.S., Deng, J.F., Fan, K.N. Characterization of high-surface-area zirconia aerogel synthesized from combined alcohothermal and supercritical fluid drying techniques. Catalysis Letters. 2002; 81:107–112.
Carraher, C.E., Jr. General topics: silica aerogels – properties and uses. Polym. News. 2005; 30:386–388.
Chandra, D., Bhaumik, A. A new functionalized mesoporous polymer with high efficiency for the removal of pollutant anions. J. Mater. Chem. 2009; 19:1901–1907.
Danilyuk, A.F., Kravchenko, E.A., Okunev, A.G., Onuchin, A.P., Shaurman, S.A. Synthesis of aerogel tiles with high light scattering length. Nucl. Instrum. Methods Phys. Res., Sect. A. 1999; 433:406–407.
De Leo, R., Capozzi, V., Casalino, C., Cisbani, E., Coluzza, C., Frullani, S., Garibaldi, F., Iodice, M., Lagamba, L., Nappi, E., Perna, G., Urciuoli, G.M. Chromatic aberration and forward scattering of light in silica aerogel. Nuclear Instruments & Methods in Physics Research Section A – Accelerators Spectrometers Detectors and Associated Equipment. 2001; 457:52–63.
Dorcheh, A.S., Abbasi, M.H. Silica aerogel; synthesis, properties and characterization. Journal of Materials Processing Technology. 2008; 199:10–26.
Duer, K., Svendsen, S. Monolithic silica aerogel in superinsulating glazings. Sol. Energy. 1998; 63:259–267.
El Rassy, H., Buisson, P., Bouali, B., Perrard, A., Pierre, A.C. Surface characterization of silica aerogels with different proportions of hydrophobic groups, dried by the CO2 supercritical method. Langmuir. 2003; 19:358–363.
Forest, L., Gibiat, V., Woignier, T. Biot’s theory of acoustic propagation in porous media applied to aerogels and alcogels. J. Non-Cryst. Solids. 1998; 225:287–292.
Fricke, J. Thermal transport in porous superinsulations. Springer Proc. Phys. 1986; 6:94–103.
Fricke, J. Aerogels. Scientific American. 1988; 258:92.
Fricke, J. Aerogels and their Applications. J. Non-Cryst. Solids. 1992; 147:356–362.
Fricke, J., Emmerling, A. Aerogels. J. Am. Ceram. Soc. 1992; 75:2027–2036.
Fricke, J., Caps, R., Buttner, D., Heinemann, U., Hummer, E., Kadur, A. Thermal loss coefficients of monolithic and antigranulocytes aerogel systems. Sol. Energy Mater. 1987; 16:267–274.
Gauthier, B.M., Bakrania, S.D., Anderson, A.M., Carroll, M.K. A fast supercritical extraction technique for aerogel fabrication. J. Non-Cryst. Solids. 2004; 350:238–243.
Gouerec, P., Miousse, D., Tran-Van, F., Dao, L.H. Characterization of pyrolized polyacrylonitrile aerogel thin films used in double-layer supercapacitors. J. New Mater. Electrochem. Syst. 1999; 2:221–226.
Gronauer, M., Fricke, J. Acoustic properties of microporous silica-aerogel. Acustica. 1986; 59:177–181.
Gross, J., Fricke, J. Ultrasonic velocity-measurements in silica, carbon and organic aerogels. J. Non-Cryst. Solids. 1992; 145:217–222.
Gross, J., Fricke, J., Hrubesh, L.W. Sound-propagation in SiO2 aerogels. Journal of the Acoustical Society of America. 1992; 91:2004–2006.
Gross, J., Coronado, P.R., Hrubesh, L.W. Elastic properties of silica aerogels from a new rapid supercritical extraction process. J. Non-Cryst. Solids. 1998; 225:282–286.
Han, S., Sohn, K., Hyeon, T. Fabrication of new nanoporous carbons through silica templates and their application to the adsorption of bulky dyes. Chem. Mater. 2000; 12:3337–3341.
Harreld, J.H., Ebina, T., Tsubo, N., Stucky, G. Manipulation of pore size distributions in silica and ormosil gels dried under ambient pressure conditions. J. Non-Cryst. Solids. 2002; 298:241–251.
Hotaling, S.P. Ultra-low density aerogel optical applications. J. Mater. Res. 1993; 8:352–355.
Hrubesh, L.W. Aerogel applications. J. Non-Cryst. Solids. 1998; 225:335–342.
Husing, N., Schubert, U. Aerogels – airy materials: chemistry, structure, and properties. Angew. Chem., Int. Ed. 1998; 37:22–45.
Jensen, K.I. Passive solar component based on evacuated monolithic silica aerogel. J. Non-Cryst. Solids. 1992; 145:237–239.
Jensen, K.I., Schultz, J.M., Kristiansen, F.H. Development of windows based on highly insulating aerogel glazings. J. Non-Cryst. Solids. 2004; 350:351–357.
Kapoor, P.N., Uma, S., Rodriguez, S., Klabunde, K.J. Aerogel processing of MTi2O5 (M = Mg, Mn, Fe, Co, Zn, Sn) compositions using single source precursors: synthesis, characterization and photocatalytic behavior. Journal of Molecular Catalysis A – Chemical. 2005; 229:145–150.
Kim, G.S., Hyun, S.H. Synthesis and application of nanoporous aerogel. Seramisutu. 2001; 4:5–16.
Kim, G.S., Hyun, S.H. Effect of mixing on thermal and mechanical properties of aerogel–PVB composites. J. Mater. Sci. 2003; 38:1961–1966.
Kim, M.J., Ryoo, R. Synthesis and pore size control of cubic mesoporous silica SBA-1. Chem. Mater. 1999; 11:487–491.
Kistler, S.S. Coherent expanded aerogels and jellies. Nature (London, U. K.). 1931; 127:741.
Kistler, S.S. Coherent expanded aerogels. J. Phys. Chem. 1932; 36:52–64.
Kistler, S.S. The relation between heat conductivity and structure in silica aerogel. J. Phys. Chem. 1935; 39:79–85.
Kocon, L., Despetis, F., Phalippou, J. Ultralow density silica aerogels by alcohol supercritical drying. J. Non-Cryst. Solids. 1998; 225:96–100.
Kwak, C., Park, T.J., Suh, D.J. Preferential oxidation of carbon monoxide in hydrogen-rich gas over platinum–cobalt–alumina aerogel catalysts. Chemical Engineering Science. 2005; 60:1211–1217.
Lee, C.J., Kim, G.S., Hyun, S.H. Synthesis of silica aerogels from waterglass via new modified ambient drying. J. Mater. Sci. 2002; 37:2237–2241.
Leventis, N., Elder, I.A., Rolison, D.R., Anderson, M.L., Merzbacher, C.I. Durable modification of silica aerogel monoliths with fluorescent 2,7-diazapyrenium moieties. Sensing oxygen near the speed of open-air diffusion. Chem. Mater. 1999; 11:2837–2845.
Li, J., Wang, X.Y., Huang, Q.H., Gamboa, S., Sebastian, P.J. Studies on preparation and performances of carbon aerogel electrodes for the application of supercapacitor. Journal of Power Sources. 2006; 158:784–788.
Maury, S., Buisson, P., Perrard, A., Pierre, A.C. Influence of the sol-gel chemistry on the activity of a lipase encapsulated in a silica aerogel. Journal of Molecular Catalysis B – Enzymatic. 2004; 29:133–148.
Miller, J.B., Rankin, S.E., Ko, E.I. Strategies in controlling the homogeneity of zirconia silica aerogels – effect of preparation on textural and catalytic properties. J. Catal. 1994; 148:673–682.
Mukai, S.R., Nishihara, H., Shichi, S., Tamon, H. Preparation of porous TiO2 cryogel fibers through unidirectional freezing of hydrogel followed by freeze-drying. Chem. Mater. 2004; 16:4987–4991.
Mureseanu, M., Reiss, A., Stefanescu, I., David, E., Parvulescu, V., Renard, G., Hulea, V. Modified SBA-15 mesoporous silica for heavy metal ions remediation. Chemosphere. 2008; 73:1499–1504.
Nakanishi, K., Minakuchi, H., Soga, N., Tanaka, N. Structure design of double-pore silica and its application to HPLC. J. Sol-Gel Sci. Technol. 1998; 13:163–169.
Orcaire, O., Buisson, P., Pierre, A.C. Application of silica aerogel encapsulated lipases in the synthesis of biodiesel by transesterification reactions. Journal of Molecular Catalysis B – Enzymatic. 2006; 42:106–113.
Pajonk, G.M. Aerogel catalysts. Applied Catalysis. 1991; 72:217–266.
Pajonk, G.M., Venkateswara Rao, A. From sol-gel chemistry to the applications of some inorganic and/or organic aerogels. Recent Res. Dev. Non-Cryst. Solids. 2001; 1:1–20.
Perez-Quintanilla, D., Del Hierro, I., Fajardo, M., Sierra, I. 2-Mercaptothiazoline modified mesoporous silica for mercury removal from aqueous media. Journal of Hazardous Materials. 2006; 134:245–256.
Pierre, A.C., Pajonk, G.M. Chemistry of aerogels and their applications. Chem. Rev. (Washington, DC, U.S.). 2002; 102:4243–4265.
Poco, J.F., Coronado, P.R., Pekala, R.W., Hrubesh, L.W. A rapid supercritical extraction process for the production of silica aerogels. Mater. Res. Soc. Symp. Proc. 1996; 431:297–302.
Prakash, S.S., Brinker, C.J., Hurd, A.J. Silica aerogel films at ambient-pressure. J. Non-Cryst. Solids. 1995; 190:264–275.
Puanngam, M., Unob, F. Preparation and use of chemically modified MCM-41 and silica gel as selective adsorbents for Hg(II) ions. Journal of Hazardous Materials. 2008; 154:578–587.
Rao, A.P., Pajonk, G.M., Rao, A.V. Effect of preparation conditions on the physical and hydrophobic properties of two step processed ambient pressure dried silica aerogels. J. Mater. Sci. 2005; 40:3481–3489.
Rao, A.P., Rao, A.V., Pajonk, G.M. Hydrophobic and physical properties of the 2 step processed ambient pressure dried silica aerogels with various exchanging solvents. J. Sol-Gel Sci. Technol. 2005; 36:285–292.
Rao, A.P., Rao, A.V., Pajonk, G.M. Hydrophobic and physical properties of the ambient pressure dried silica aerogels with sodium silicate precursor using various surface modification agents. Appl. Surf. Sci. 2007; 253:6032–6040.
Rao, A.V., Nilsen, E., Einarsrud, M.A. Effect of precursors, methylation agents and solvents on the physicochemical properties of silica aerogels prepared by atmospheric pressure drying method. J. Non-Cryst. Solids. 2001; 296:165–171.
Rao, A.V., Kulkarni, M.M., Amalnerkar, D.P., Seth, T. Superhydrophobic silica aerogels based on methyltrimethoxysilane precursor. J. Non-Cryst. Solids. 2003; 330:187–195.
Reichenauer, G., Emmerling, A., Fricke, J., Pekala, R.W. Microporosity in carbon aerogels. J. Non-Cryst. Solids. 1998; 225:210–214.
Reim, M., Beck, A., Korner, W., Petricevic, R., Glora, M., Weth, M., Schliermann, T., Fricke, J., Schmidt, C., Potter, F.J. Highly insulating aerogel glazing for solar energy usage. Sol. Energy. 2002; 72:21–29.
Reim, M., Korner, W., Manara, J., Korder, S., Arduini-Schuster, M., Ebert, H.P., Fricke, J. Silica aerogel granulate material for thermal insulation and daylighting. Sol. Energy. 2005; 79:131–139.
Reynes, J., Woignier, T., Phalippou, J. Permeability measurement in composite aerogels: application to nuclear waste storage. J. Non-Cryst. Solids. 2001; 285:323–327.
Rubin, M., Lampert, C.M. Transparent silica aerogels for window insulation. Sol. Energy Mater. 1983; 7:393–400.
Sanchez, C., Ribot, F. Design of hybrid organic-inorganic materials synthesized via sol-gel chemistry. New Journal of Chemistry. 1994; 18:1007–1047.
Santra, S., Tapec, R., Theodoropoulou, N., Dobson, J., Hebard, A., Tan, W.H. Synthesis and characterization of silica-coated iron oxide nanoparticles in microemulsion: the effect of nonionic surfactants. Langmuir. 2001; 17:2900–2906.
Scherer, G.W., Gross, J., Hrubesh, L.W., Coronado, P.R. Optimization of the rapid supercritical extraction process for aerogels. J. Non-Cryst. Solids. 2002; 311:259–272.
Schneider, M., Baiker, A. Aerogels in catalysis. Catal. Rev. – Sci. Eng. 1995; 37:515–556.
Schultz, J.M., Jensen, K.I., Kristiansen, F.H. Super insulating aerogel glazing. Sol. Energy Mater. Sol. Cells. 2005; 89:275–285.
Schwertfeger, F., Frank, D., Schmidt, M. Hydrophobic waterglass based aerogels without solvent exchange or supercritical drying. J. Non-Cryst. Solids. 1998; 225:24–29.
Shevchenko, N., Zaitsev, V., Walcarius, A. Bifunctionalized mesoporous silicas for Cr(VI) reduction and concomitant Cr(III) immobilization. Environmental Science & Technology. 2008; 42:6922–6928.
Shi, F., Wang, L., Liu, J. Synthesis and characterization of silica aerogels by a novel fast ambient pressure drying process. Mater. Lett. 2006; 60:3718–3722.
Sui, R.H., Rizkalla, A.S., Charpentier, P.A. Synthesis and formation of silica aerogel particles by a novel sol-gel route in supercritical carbon dioxide. Journal of Physical Chemistry B. 2004; 108:11886–11892.
Svendsen, S. Solar collector with monolithic silica aerogel. J. Non-Cryst. Solids. 1992; 145:240–243.
Tabata, M., Adachi, I., Fukushima, T., Kawai, H., Kishimoto, H., Kuratani, A., Nakayama, H., Nishida, S., Noguchi, T., Okudaira, K., Tajima, Y., Yano, H., Yokogawa, H., Yoshida, H., Development of silica aerogel with any density. 2005 IEEE Nuclear Science Symposium Conference Record, Vols 1–5. 2005:816–818.
Tajiri, K., Igarashi, K. The effect of the preparation conditions on the optical properties of transparent silica aerogels. Sol. Energy Mater. Sol. Cells. 1998; 54:189–195.
Tang, Q., Wang, T. Preparation of silica aerogel from rice hull ash by supercritical carbon dioxide drying. J. Supercrit. Fluids. 2005; 35:91–94.
Tewari, P.H., Hunt, A.J., Lofftus, K.D. Advances in production of transparent silica aerogels for window glazings. Springer Proc. Phys. 1986; 6:31–37.
Tillotson, T.M., Sunderland, W.E., Thomas, I.M., Hrubesh, L.W. Synthesis of lanthanide and lanthanide-silicate aerogels. J. Sol-Gel Sci. Technol. 1994; 1:241–249.
Tsang, S.C., Yu, C.H., Gao, X., Tam, K. Silica-encapsulated nanomagnetic particle as a new recoverable biocatalyst carrier. Journal of Physical Chemistry B. 2006; 110:16914–16922.
Tsou, P. Silica aerogel captures cosmic dust intact. J. Non-Cryst. Solids. 1995; 186:415–427.
Vicente, C.L., Choi, H.C., Xia, J.S., Halperin, W.P., Mulders, N., Lee, Y. A-B transition of superfluid He-3 in aerogel and the effect of anisotropic scattering. Physical Review B. 2005; 72:094519.
Wagh, P.B., Ingale, S.V. Comparison of some physico-chemical properties of hydrophilic and hydrophobic silica aerogels. Ceram. Int. 2002; 28:43–50.
Wang, L., Wang, K.M., Santra, S., Zhao, X.J., Hilliard, L.R., Smith, J.E., Wu, J.R., Tan, W.H. Watching silica nanoparticles glow in the biological world. Anal Chem. 2006; 78:646–654.
Widenmeyer, M., Anwander, R. Pore size control of highly ordered mesoporous silica MCM-48. Chem. Mater. 2002; 14:1827–1831.
Wingenfelder, U., Nowack, B., Furrer, G., Schulin, R. Adsorption of Pb and Cd by amine-modified zeolite. Water Research. 2005; 39:3287–3297.
Xu, J.J., Yang, J.S. Nanostructured amorphous manganese oxide cryogel as a high-rate lithium intercalation host. Electrochemistry Communications. 2003; 5:230–235.
Xu, Z., Dong, M., Synthesis, characterization, and application of magnetic nanocomposites for the removal of heavy metals from industrial effluents. Emerging Environmental Technologies. 2008:105–148.
Yamamoto, T., Sugimoto, T., Suzuki, T., Mukai, S.R., Tamon, H. Preparation and characterization of carbon cryogel microspheres. Carbon. 2002; 40:1345–1351.
Yantasee, W., Charnhattakorn, B., Fryxell, G.E., Lin, Y.H., Timchalk, C., Addleman, R.S. Detection of Cd, Pb, and Cu in non-pretreated natural waters and urine with thiol functionalized mesoporous silica and Nafion composite electrodes. Anal. Chim. Acta. 2008; 620:55–63.
Yu, C.H., Lo, C.C.H., Tam, K., Tsang, S.C. Monodisperse binary nanocomposite in silica with enhanced magnetization for magnetic separation. Journal of Physical Chemistry C. 2007; 111:7879–7882.
Yu, C.H., Al-Saadi, A., Shih, S.J., Qiu, L., Tam, K.Y., Tsang, S.C. Immobilization of BSA on silica-coated magnetic iron oxide nanoparticle. Journal of Physical Chemistry C. 2009; 113:537–543.
Zhang, L., Qiao, S.Z., Jin, Y.G., Cheng, L.N., Yan, Z.F., Lu, G.Q. Hydrophobic functional group initiated helical mesostructured silica for controlled drug release. Advanced Functional Materials. 2008; 18:3834–3842.
Zhang, S.Q., Wang, J., Shen, J., Deng, Z.S., Lai, Z.Q., Zhou, B., Attia, S.M., Chen, L.Y. The investigation of the adsorption character of carbon aerogels. Nanostruct. Mater. 1999; 11:375–381.
Zhu, X.B., Chang, X.J., Cui, Y.M., Zou, X.J., Yang, D., Hu, Z. Solid-phase extraction of trace Cu(II) Fe(III) and Zn(II) with silica gel modified with curcumin from biological and natural water samples by ICP-OES. Microchemical Journal. 2007; 86:189–194.
Zimmermann, A., Gross, J., Fricke, J. Constant-Q acoustic attenuation in silica aerogels. J. Non-Cryst. Solids. 1995; 186:238–243.