Appendix G
A General Theorem for Mixtures with Associating or Solvation Molecules
Many fluid mixtures of practical interest contain molecules that exhibit strong interactions (e.g., hydrogen bonding). The properties of such mixtures can often be interpreted by assuming that the molecules in the mixture are not only monomers – as given by the “apparent” (stoichiometric) composition – but also dimers, trimers. etc. and complexes containing dissimilar components. It is farther assumed that all “true” species are in chemical equilibrium.
These attractive assumptions lead to an immediate problem: What is the relation between the chemical potential of the “apparent” monomer and that of the “true” monomer? Standard thermodynamic measurements give us only the composition and chemical potential of the “apparent” monomer. How are these related to the composition and chemical potential of the “true” monomer that we (usually) do not know experimentally? The simple proof below shows that without any additional assumptions, the chemical potential of the “apparent” monomer is always equal to that of the “true” monomer.
Consider a binary mixture containing nA moles of component A and nB moles of component B. The chemical potentials are μA and μB. These quantities (nA, nB, μA, and μB) are obtained in typical thermodynamic measurements. We assume that component A exists not only as monomer A1 but also, because of association, as dimer A2, trimer A3, etc. We make a similar assumption for component B.
In addition, we assume that molecules of components A and B may solvate to form complexes of the type AiBj, where i and j are positive integers. For each component there is a material balance
(G.1)
![]()
(G.2)
![]()
By assumption, all “true” species are in chemical equilibrium. That is, every Association reaction
![]()
and every solvation reaction
![]()
attains its equilibrium state:
(G.3)
![]()
(G.4)
![]()
(G.5)
![]()
To relate μA to μA1 and μB to μB1, we use the exact differential of the Gibbs energy at constant temperature and pressure. First, we write this differential for the “apparent” (stoichiometric) case, i.e., where we are concerned only with components A and B as such, without consideration of the molecular forms of these components:
(G.6)
![]()
Next, we write the same differential for the “true” case, i.e., where we postulate the existence of dimers, trimers, etc.:
(G.7)
![]()
Substituting the chemical equilibria [Eqs. (G-3), (G-4), and (G-5)] into Eq. (G-7), we obtain
(G.8)
![]()
Substituting the material balances [Eqs. (G-l) and (G-2)] into Eq. (G-8), we obtain
(G.9)
![]()
We now compare Eq. (G-6) with Eq. (G-9). Because the two equations must be identical for all values of dnA and dnB it follows that
(G.10)
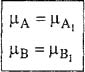
For convenience, the proof given here is for a binary mixture. However, the same proof can be extended to mixtures containing any number of components, yielding the same results.
Equation (G-10) is important because it relates a readily measurable quantity (left-hand side) to another, not readily measured quantity that is useful for construction of “chemical” models to explain nonideal behavior. Equation (G-10) provides the key for relating common thermodynamic quantities (such as fugacity coefficients φA and φB or activity coefficients γA and γB) to mixture models that postulate the existence of associated or solvated molecules.
Equation (G-10) assumes only that all postulated monomers, dimers, etc. are in chemical equilibrium. It is independent of any assumption concerning the mode of association (linear or cyclic) or of any assumption concerning physical interactions between the postulated “true” species. In particular, Eq. (G-10) is not limited to the so-called “ideal” associated (or solvated) mixture where the “true” species form an ideal mixture, i.e., one where, at constant temperature and pressure, the fugacity of a true species is proportional to its concentration.
Although Eq. (G-10) was derived early in the twentieth century, it was not until 1954 that its importance became well known. In that year, D. H. Everett published his edited translation of Chemical Thermodynamics by I. Prigogine and R. Defay (1954),1 originally published in French. Chapter 26 of that splendid book gives an excellent discussion of the properties of associated and solvated liquid mixtures.
1 (London: Longmans & Green).
