Chapter 15
Global Warming Models

Make models to understand why the earth is warming.
As I sit writing this in 2018, I’ve just lived through the earth’s 18 hottest years on instrumental record with one exception. That’s not to say there were not hotter years back in prehistory. When the earth was just forming some 4.5 billion years ago, nonstop volcanoes and meteor showers made for some toasty summers. But the steady trend for the last century has been warming, and the rate of warming shot up beginning in the 1970s. What we’re seeing now is the fastest temperature change ever measured directly.
A number for the temperature of the earth is a pretty marvelously complex quantity, if you stop to think about it. Scientists arrive at this number with thermometers in the air, land, and sea, as well as satellite readings. Then the entire global scientific community watches the process and helps to make it more accurate. The vast majority of scientists agree that human creation of greenhouse gases like CO2 has skewed the natural balance of the earth’s heating and cooling. (Petroleum companies and those that grow wealthy from burning fossil fuels are scrambling to downplay the human impact. It’s always good to follow the money behind an argument.)
Timorese are not worried about inundation of their coastal cities and villages as the polar ice melts and the seas rise. The island of Timor itself happens to be rising around 10 cm per year! More on that in Chapter 17. Timorese are, however, extremely worried about the climate change already happening as a result of this warming. According to the climatologists working here, the island is experiencing a frighteningly rapid shift in growing seasons, less dependable rainfall, and more severe weather overall. Farmers are being advised to maximize flexibility and explore new crops that are more resilient to erratic weather patterns.
This can be hard advice to follow, especially for older farmers. After all, if it worked for Grandma and it’s worked for decades, why won’t it still work? But change is also apparent. I’ve spoken to several old-timers here who testify to things not being as predictable as they once were, and some are quite concerned about it. To explain what science understands about what’s happening is no easy trick. Here I’ll show models we’ve put in the curriculum to help understand the science behind global warming.
Gather stuff
- Three identical cups of water
- A bigger transparent plastic container that will cover the first one overturned
- Another one made from glass
- A thermometer that will go from the outside air temperature to around 100°F , if you can find one
Tinker
The setup here is pretty simple: put the three cups of water out in the sun, and then cover one with the plastic cup or container and one with the glass, and leave one alone.

Actually, wait a minute: first measure the temperature of the water. Use your fingers, and also a thermometer if you have one. All the cups should be the same temperature; if not, you should figure out why not. Measure the temperature of the air as well. Write these data down so you can compare them if you do this experiment on another day.

Then cover the two up again and wait a half hour or more.

Feel the temperature of the outside of the containers. Uncover the two covered cups. Measure the temperatures again with your fingers and the thermometer if you have one, and write down these observations.

What’s Going On?
Here are the results of our experiment (we’ll use degrees Celsius because that’s what people use here):
| Air temp: 31°C | Temp at start | Temp after one hour | Change in temp |
| Uncovered cup | 30°C | 40°C | +10°C |
| Cup covered with glass container | 30°C | 44°C | +14°C |
| Cup covered with plastic container | 30°C | 46°C | +16°C |
Big news: The sun warms things up when it shines on them. Thank your lucky stars this is true, for it would be quite unpleasant living, say, on Pluto, where the sun does shine but with an intensity 1,500 times less than it does here. Shoot, even on Mercury, the closest planet to the sun, the temperature goes down to –183 degrees Celsius on the side away from the sun. Kinda gives new meaning to the phrase “You can stick that where the sun don’t shine!” Brrr.
But it’s more complicated than the sun shining on things and warming them up. The earth is covered with a layer of air that is mostly, but not entirely, transparent. The larger, upturned containers in this activity represent that atmosphere, and if your experiment turned out like ours, the covered cups warmed up a lot more than the uncovered one. Here in the tropics, we didn’t need the thermometer to get to that conclusion; the original digital method of finger testing did the trick.
This makes sense when you think about it. The uncovered cup was getting the same heat radiation as the covered ones, but as it got hot, any little breeze that came by carried the heat away from it. There was also some evaporation that took away heat.1 The containers covering the other cups prevented air from circulating around their cups, so the air couldn’t carry the heat away. That’s one reason the covered cups got hotter. That concept is similar to the reason the earth doesn’t get super cold at night: the atmosphere holds the heat of the earth down instead of letting it all radiate away like it does on planet Mercury, which has no atmosphere.
But another thing was happening with the cups that is not so easy to visualize. Radiation2 from the sun was hitting those three cups, heating them up, and then some of it was reflected back out away from the cup. Well, on the uncovered cup, the radiation just took off back up into the atmosphere. But on the covered cups, some of that radiation was caught and kept by the walls of the containers. Some got back out through the walls—they are transparent, after all—but some got trapped. The containers themselves became hot, as you may have felt.
This is has a close parallel to what happens on Earth. The atmosphere is like a giant container covering the earth. Though it's not made of plastic or glass, some of its molecules act the same way; heat radiated from the earth and the warm air in the lower atmosphere hits these molecules and gets absorbed. The result is that those molecules are heated up.
In addition to preventing the sun’s radiation from escaping back out, the glass container stopped more of the radiation coming in, more so than the plastic container did. I think that’s why we got a lower temperature on the cup under the glass container.
Before I go on, let’s do the next activity.
Gather more stuff
- 20 rocks about the size of tennis balls
- 10 marbles
- Chalk or a rock that makes marks
- A flat, blank wall near a blank section of ground, concrete, tile, or something more or less flat
Tinker
First, make a table for the data you’ll collect during this activity. Here’s the template:
| Rocks in the rectangle | Marbles that stayed inside the line |
| 0 | |
| 5 | |
| 10 | |
| 15 | |
| 20 |
Mark off an area about 1.5 meters long and three-fourths of a meter wide, with one side as the wall.

Stand back maybe 2 or 3 meters and shoot marbles at the wall behind the rocks with the goal of getting them to bounce back out across the line. (If you’re not used to shooting marbles, spend some time practicing your thumb and finger action.) Shoot them low on the wall, not more than 10 cm from the ground. Shoot all 10, and then count how many stayed inside of the rectangle. If a marble goes out one of the sides of the rectangle, it counts as staying in. Only the ones that come back out the top of the rectangle (the side parallel with the wall, closest to the shooter) should not be counted.

Record this number in your data table.
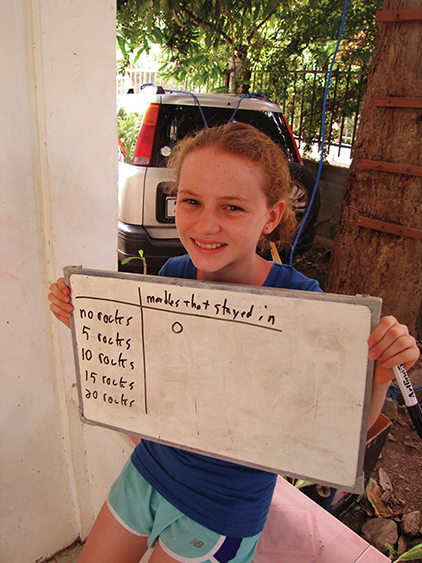
Now set five rocks at arbitrary spaces throughout the rectangle. Have the same person shoot 10 marbles again from the same position. (If a different person shoots them, his or her shooting skill may be better or worse than that of the first person, which would skew the results of the experiment.)

Record in your table the number of marbles that escaped.
Now add five more rocks spread out throughout the rectangle and repeat the activity. Then add five more two more times and repeat the shooting and recording so that the last time you’ve got all 20 rocks in there.




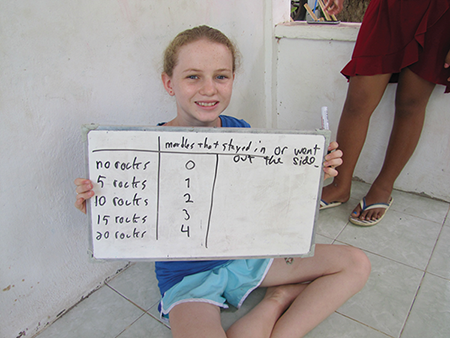
Now check out your data; here are ours:
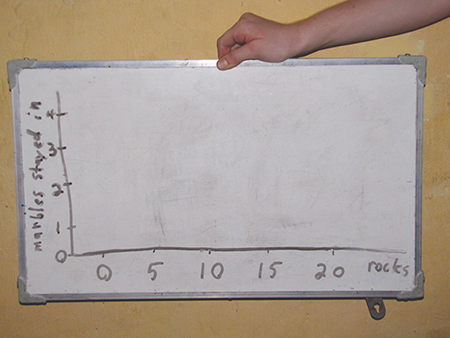
You can even make a bar graph of the data; here’s the template:
What’s Going On?
Here’s our graph:

I swear we didn’t make this up. Our marble shooter, Binoy, is pretty deadeye and found little cracks to shoot through to get most of the marbles out, but each time we added rocks, one more marble got stuck inside or diverted out an end.
The trend is pretty clear, eh? The more rocks in the rectangle, the less marbles get out. Now what the heck does this have to do with global warming?
Plenty. This little game nicely models the sun’s radiation hitting the earth. Here are representations in the model:
- Sun: You shooting marbles
- Sun’s radiation: The marbles
- Earth’s surface: The wall
- Atmosphere: The rectangle
- Greenhouse gas molecules: The rocks
Get it? Those marbles that hit the wall are like the radiation from the sun that hits the earth. Most of it makes it to the ground and heats up the earth and water and forests. Then those hot things heat up the air and the air moves up and then radiates some of the heat back out to space. This complex process is represented by the bouncing up of the marbles.
Well, the ones that make it back out into space, heading off to who knows where, certainly don’t do any more heating of our earth.3 But others of these photons on the way up happen to hit various molecules in the upper atmosphere and stop dead their tracks, giving their energy to warm those molecules and contributing to the overall temperature of the earth. (Sometimes this happens with photons on the way in, too.) Those molecules, called greenhouse gases, act like the rocks in our game. The more there are, the more marbles (photons) get stuck, and the hotter the earth grows.
Around 250 years ago the most disruptive species on Earth, us, began to chop trees and mine coal to burn in order to power various machines that mass-produced stuff or transported people or stuff. It was called the Industrial Revolution, and the lives of many of us humans changed forever. The atmosphere also began to change. All that burning put more carbon dioxide into the atmosphere, and CO2 happens to be a powerful greenhouse gas.
Well, we humans have never stopped, and in fact we’ve reproduced quite rapidly, multiplying our population by 10 times since the start of the industrial age. We also found all sorts of other things to burn, such as natural gas and oil. So now there are many more machines creating CO2. The earth has various ways to take that CO2 back out of the atmosphere, but they are limited, so it is piling up, together with other greenhouse gases, and the earth continues to warm.
This is not some vague theory. Scientists can dig out ice cores from Greenland and other massive ice shelves and check how much CO2 was in the air at various times in the past, and make pretty good estimates of what the temperature was back then. From these sorts of data, it’s abundantly clear that the CO2 has been ramping up and the temperature has been rising in lockstep.
So what do we do about it? Fortunately, there is a practical answer: work as fast as possible for a conversion to carbon-free energy—that is, getting energy from sources that do not produce carbon dioxide as a product. Technology has made this 100 percent possible, right now in 2018, in the areas of electricity and factory production. Solar, wind, water, tidal, and nuclear sources could power all our stationary energy needs, right now, if we made that a national policy priority.
If you agree, call up your representatives, local and national, and tell them this now. It doesn’t cost a cent to make your well-informed voice heard, and exercising this right feels good to every patriotic American. This is the single most important step you can take to stop global warming and climate change that is even now causing problems for millions of already poverty-stricken people.
Harder will be the conversion of mobile petroleum use: cars, trucks, trains, boats, and airplanes. That liquid petroleum is just so frigging convenient! Imagine squirting a few liters of a certain liquid into your car’s tank and using it to take you effortlessly 300 miles down the road. Unbelievable, eh? Like some kind of fabulous fantasy.
Unfortunately, aside from being unbelievable, it’s also darn near unstoppable. This is one of the most critical problems of our times, and it will take more technology and creative energy policy to make this conversion of mobile energy use. Batteries will be part of the solution, but not all. Still, it should be a top priority. I encourage you to devote your life to solving this problem, with both innovative technology and creative public policy. Then, when you’ve got the job done, if you happen to think of it, give a small word of credit for the one who inspired you…
It’s a mistake to say that global warming is ruining the earth. The earth’s biospheres will adapt and carry on in the event of a huge temperature rise. Don’t forget that a lot of plants love global warming! Turn it up! Bring on the radiation! Unfortunately, the changes will spell disaster for many habitats we humans have come to depend on, and the poorest will suffer the worst, and the first. So my family and I are doing our best to use less carbon and green the earth in order to curb global warming.
