Chapter 7. Scale Inhibitors
In certain operations in petroleum industries, such as production, stimulation, and transport, there is a risk of scale deposition. This can occur when a solution becomes supersaturated, which occurs mostly if the temperature changes in the course of injection operations.
Scale is also formed, if two chemicals that will form a precipitate are brought together, e.g., if a hydrogen fluoride solution meets calcium ions. From a thermodynamic perspective, there is a stable region, a metastable region, and an unstable region, separated by the binodal curve and the spinodale curve, respectively.
Scales may consist of calcium carbonate, barium sulfate, gypsum, strontium sulfate, iron carbonate, iron oxides, iron sulfides, and magnesium salts (Keatch 1998). There are relevant monographs, e.g., the Corrosion and Scale Handbook (Becker 1998), as well as reviews (Crabtree et al. 1999) available in the literature. Case studies have been presented for North Sea carbonate reservoirs (Jordan et al., 2003 and Jordan et al., 2005) and the Gulf of Mexico (Jordan et al., 2002). A more recent publication focuses on green systems (Frenier and Hill, 2004).
Classification and Mechanism
The problem is basically similar to preventing scale in washing machines and similar chemicals are used. Inhibition can be achieved, either by adding substances that react with potential scale-forming substances so that thermodynamically, a table region is reached, or by adding substances that suppress crystal growth.
Conventional scale inhibitors are hydrophilic, i.e., they dissolve in water. In the case of downhole squeezing, the scale inhibitor should be adsorbed on to the rock to prevent it washing out before it can act as desired, but this may change the surface tension and the wettability of the system. To overcome these disadvantages, oil-soluble and coated scale inhibitors have been developed.
Frequently, scale inhibitors are applied in combination with corrosion inhibitors (Martin et al. 2005). Scale inhibitors can be classified into two main groups:
• Thermodynamic inhibitors and
• Kinetic inhibitors.
Thermodynamic inhibitors are complexing and chelating agents, suitable for specific scales. The action of kinetic inhibitors may be understood in terms of stereospecific and nonspecific mechanisms.
Scale prevention is important to ensure continuous production from existing reserves that produce brine. Wells can be abandoned prematurely because of poor management of scale and corrosion (Kan and Tomson, 2010). The scale inhibitor operates in two ways (Viloria et al. 2010):
1. Adsorption effects and
2. Morphologic changes of the growing sites.
The adsorption effects are caused by the inhibitor molecules occupying nucleation sites that are preferred by the scale-forming molecules. Crystals cannot find active places at which to adhere to the surface, therefore crystal nucleation is prevented.
Another absorption-based inhibitor mechanism is based on morphological changes that can prevent the formation of crystals in the presence of the inhibitor. Depending on the inhibitor characteristics, and the nature of the substrate, it is possible for it to be adsorbed over the crystalline net, forming complex surfaces or nets, which have difficulty remaining and growing in active places.
Sea water often reacts with the formation water in offshore fields to produce barium, calcium, and strontium sulfate deposits, which hinder oil production. In some fields, CaCO3 is a major problem.
In some regions, the formation water chemistry varies considerably (Duccini et al., 1997). For example, in the Central North Sea Province, levels of barium ions vary from a few mgl−1 to gl−1, and the pH varies from 4.4 to 7.5; a pH as high as 11.7 has been measured. In the southern region of the North Sea, the waters have a high salinity and are rich in sulfate and acidic compounds. The ideal scale inhibitor should have the following properties (Duccini et al., 1997):
• Effective scale control at low inhibitor concentration,
• Compatibility with sea and formation water,
• Balanced adsorption-desorption properties, allowing the chemicals to be slowly and homogeneously released into the production water,
• High thermal stability,
• Low toxicity and high biodegradability, and
• Low cost.
Scale inhibitors are broadly classified as organic or inorganic (Viloria et al. 2010). Inorganic types include condensed phosphate, such as polymetaphosphates or phosphate salts. Suitable organic scale inhibitors are polyacrylic acid (PAA), phosphinocarboxylic acid, sulfonated polymers, and phosphonates (Duccini et al., 1997).
Phosphonates are maximally effective at high temperatures, whereas sulfonated polymers are better at low temperatures (Talbot et al. 2009). Copolymers that contain both phosphonate and sulfonate moieties can operate well over a range of temperatures. A phosphonate end-capped vinyl sulfonic acid/acrylic acid copolymer has been shown to be particularly useful in the scale inhibition of barium sulfate scale in water-based systems. (Talbot et al. 2009). The basic limitations of scale inhibitors are given in Table 7.1.
| Inhibitor Type | Limitations |
|---|---|
| Inorganic polyphosphates | Suffer hydrolysis and can precipitate as calcium phosphates because of temperature, pH, solution quality, concentration, phosphate type, and the presence of some enzymes. |
| Organic polyphosphates | Suffer hydrolysis with temperature. Not effective at high calcium concentrations. Must be applied in high doses. |
| Polymers based on carboxylic acids | Limited calcium tolerance (2000 ppm) although some can work at concentrations higher than 5000 ppm. Larger concentrations are needed. |
| Ethylene diamine tetraacetic acid | Expensive |
Thermodynamic Inhibitors
Thermodynamic inhibitors are complexing and chelating agents, suitable for specific scales. For example, common chemicals for the inhibition of barium sulfate are ethylene diamine tetraacetic acid (EDTA) and nitrilo triacetic acid. The solubility of calcium carbonate can be influenced by varying the pH or the partial pressure of carbon dioxide (CO2). The solubility increases with decreasing pH and increasing partial pressure of CO2, and it decreases with temperature.
Kinetic Inhibitors
Kinetic inhibitors for hydrate formation may also be effective for preventing scale deposition (Sikes and Wierzbicki 1996). This mode of operation may be understood in terms of stereospecific and nonspecific mechanisms of scale inhibition.
Adherence Inhibitors
Another mechanism of scale inhibition is based on adherence inhibitors, in which surface active chemicals simply suppress the adherence of crystals to the metal surfaces.
Mathematical Models
Mathematical models have been developed (Mackay and Sorbie, 1998 and Mackay and Sorbie, 1999; Mackay et al. 1998; Shuler and Jenkins 1989), including simulation scale formation of iron carbonate and iron monosulfide by thermodynamic and electrochemical means (Anderko 2000; Mackay and Sorbie 1998; Malandrino et al. 1998; Zhang et al. 2000). An accurate model to predict pH, scale indices, density, and inhibitor needs has been discussed, experimental data to validate the model have been examined, and an estimation of the error in analysis has been presented (Kan and Tomson, 2010).
The scaling tendency of sulfates, barite, celestite, and halites are not a strong function of the pH of the brine. In contrast, carbonates, such as calcite, dolomite, and siderite, and sulfide scales are acid-soluble, hence their scaling tendencies are strongly dependent on the pH of the brine. Scale prediction is more complicated in these cases (Kan and Tomson, 2010).
Optimal Dose
A method to estimate the optimal dose of a scale inhibitor has been described (Mikhailov et al. 1987). The method starts by measuring the chemical composition and temperature of the water and using these parameters to calculate a stability index predicting the optimal dose of a scale inhibitor.
Precipitation Squeeze Method
In the precipitation squeeze method, the scale inhibitor reacts to form an insoluble salt, which precipitates in the pores of the formation rock. For example, a phosphonate scale inhibitor and a calcium chelate are employed as a precipitation squeeze treatment, as has phosphinic polycarboxylate and polyepoxysuccinic acid (Brown and Brock 1995).
An anionic scale inhibitor and a multivalent cation salt are dissolved in an alkaline aqueous liquid to provide a solution, which contains both scale-inhibiting anions and multivalent cations that are mutually soluble under alkaline conditions. However, at lower pH the inhibitor is not soluble. One compound that reacts at a relatively slow rate to reduce the pH of the alkaline solution is dissolved in the solution. The rate at which the pH of the solution is reduced can be adjusted by the formulation (Collins 2000).
Near-well squeeze treatment models assume that the flow pattern around the well is radial. It has been investigated whether strictly non-radial flow patterns around the well have a major effect on the squeeze treatment. It has been found that fractured wells have longer squeeze lifetimes than non-fractured wells.
Further, the calculations reveal that for fractured wells, inhibitor adsorption on the face of the fracture itself has no impact on the treatment lifetime. In a fractured well, the inhibitor is more retarded by contact with rock over a greater distance in comparison to a matrix with radial treatment (Rakhimov et al., 2010).
Inhibitor Chemicals
Chemically, inhibitors can be broadly subdivided into acids and complexing agents. Scale inhibitors described in the recent literature are summarized in Table 7.2.
| a)In borate crosslinked fracturing fluids | |
| b)High temperature applications | |
| c)Oil-soluble | |
| Compound | References |
|---|---|
| 1-Hydroxyethylidene-1,1-diphosphonic acid | He et al. (1999) |
| Carbonic dihydrazide, H2N-NH-CO-NH-NH2 | Mouche and Smyk (1995) |
| Polyaminealkylphosphonic acid and carboxymethyl cellulose or polyacrylamide | Kochnev et al. (1993) |
| Polyacrylic acid and chromium | Yan (1993) |
| Polyacrylatesa | Watkins et al. (1993) |
| Amine methylene phosphonateb | Graham et al. (2000) |
| Phosphonomethylated polyamine | Singleton et al. (2000) |
| Sulfonated polyacrylate copolymer | Chilcott et al. (2000) |
| Bis[tetrakishydroxymethylphosphonium] sulfate | Larsen et al. (2000) |
| Phosphonates | Holzner et al. (2000), Jordan et al. (1997) |
| Carboxymethyl inulin | Kuzee and Raaijmakers (1999) |
| Polycarboxylic acid salts | Dobbs and Brown (1999) |
| Phosphoric acid esters of rice bran extract | Zeng and Fu (1998) |
| Polyphosphino maleic anhydride | Yang and Song (1998) |
| N,N -Diallyl-N -alkyl-N -sulfoalkyl ammoniumbetaine copolymer (with N -vinylpyrrolidone or acrylamide (AAm)), diallylmethyltaurine hydrochloride (CH2=CH-CH2Cl×CH3-NH-CH2-CH2-SO3−Na+) | Fong et al. (2001) |
| Aminotrimethylenephosphonic acid | Kowalski and Pike (2001), Tantayakom et al., 2005 and Tantayakom et al., 2004 |
| Polyaspartates | Fan et al. (1998) |
| Polyacrolein | Siegmeier et al. (1998) |
| Naphthylamine polycarboxylic acids | Carter et al. (1998) |
| Phosphonic acid and hydrofluoric acid | Dean et al. (1998) |
| Tertiary aminesc | Reizer et al. (2002) |
| Diethylentrilopentrakismethylenephosphonic acid | Tantayakom et al. (2005) |
| Tetrakis hydroxyorgano phosphonium salts | Jones and Grech (2004), Jones and Taylor (2004), Talbot and Grech (2005) |
| Phosphino-polycarboxylic acid | Andrei and Gagliardi (2004), Andrei and Malandrino (2003) |
| Diethylentriaminepentaacetic acid | Mendoza et al. (2002) |
| Ethylene diamine tetraacetic acid | Mendoza et al. (2002) |
| Vinylsulfonate copolymer | Jordan et al. (2005) |
| Phosphinated maleic copolymer | Gupta and Kirk (2009) |
Water-soluble Inhibitors
Acids
Both inorganic acids, such as hydrochloric acid and hydrofluoric acid, and organic acids, such as formic acid, can be used to increase the pH. They are used in combination with surfactants.
Acids, when used as scale inhibitors, are extremely corrosive as has been evaluated in the laboratory. Parameters included acid type, metallurgy, temperature, inhibitor type and concentration, duration of acid-metal contact, and the effect of other chemical additives (Burger and Chesnut 1992). Lead and zinc sulfide scale deposits can be removed by an acid treatment (Jordan et al. 2000).
Hydrofluoric Acid
It is known that permeability impairment may be improved by injecting acid formulations containing HF into the formation. Such methods are known to improve production from both subterranean calcareous and siliceous formations.
Most sandstone formations are composed of over 70% sand quartz, i.e. silica, bonded together by various amount of cementing material including carbonate, dolomite, and silicates. Suitable silicates include clays and feldspars. A common method of treating sandstone formations involves the introduction of hydrofluoric acid into the wellbore and allowing it to react with the surrounding formation.
Hydrofluoric acid exhibits high reactivity toward siliceous minerals, such as clays and quartz fines, and reacts very quickly with authigenic clays, such as smectite, kaolinite, illite, and chlorite, especially at temperatures above 65∘C. It is therefore capable of attacking and dissolving siliceous minerals, but undesirable precipitation reactions occur if hydrofluoric acid contacts metallic ions present in the formation, such as sodium, potassium, calcium, and magnesium.
Sandstone or siliceous formations and calcareous formations may be treated with an aqueous well treatment composition containing a hydrofluoric acid source in combination with a boron containing compound and a phosphonate acid, ester, or salt in order to increase the permeability of the formation being treated by inhibiting or preventing the formation of undesirable inorganic scales, such as calcium fluoride, magnesium fluoride, potassium fluorosilicate, sodium fluorosilicate, fluoroaluminate, etc. (Ke and Qu 2010)
Encapsulated Scale Inhibitors
This type of scale inhibitor allows chemical release over an extended period of time (Hsu et al. 2000; Powell et al. 1995). Microencapsulated formulations may have a gelatin coating with a multipurpose cocktail, such as (Kowalski and Pike, 1999 and Kowalski and Pike, 2001):
• Scale inhibitor,
• Corrosion inhibitor,
• Biocide,
• Hydrogen sulfide scavengers,
• Demulsifier, and
• Clay stabilizer.
Chelating Agents
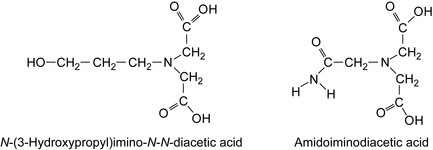
Trace amounts of chelating agents, such as EDTA, citric acid, or gluconic acid may lower the efficiency of scale inhibitors (Barthorpe 1993). The concentration of calcium ions and magnesium ions affects the inhibition of barium sulfate (Boak et al. 1999). Pentaphosphonate, hexaphosphonate, phosphinopolycarboxylic acid (PPCA) salts, and polyvinyl sulfonate (PVS) scale inhibitors were studied. String chelating agents, given in Table 7.3, also stabilize the coating of encapsulated formulations (Kowalski and Pike 1999). Some chelating agents based on imino acids are shown in Figure 7.1.
| Chelating agent | Acronym |
|---|---|
| N -3-Hydroxypropylimino-N,N -diacetic acid | 3-HPIDA |
| N -2-Hydroxypropylimino-N,N -diacetic acid | 2-HPIDA |
| N -Glycerylimino-N,N -diacetic acid | GLIDA |
| Dihydroxyisopropylimino-N,N -diacetic acid | DHPIDA |
| Methylimino-N,N -diacetic acid | MIDA |
| 2-Methoxyethylimino-N,N -diacetic acid | MEIDA |
| Amidoiminodiacetic acid = sodium amidonitrilo triacetic acid | SAND |
| Acetamidoiminodiacetic acid | AIDA |
| 3-Methoxypropylimino-N,N -diacetic acid | MEPIDA |
| Trishydroxymethylmethylimino-N,N -diacetic acid | TRIDA |
EDTA
A conventional scale dissolver for barite consists of a concentrated solution of potassium carbonate, potassium hydroxide, and the potassium salt of EDTA. Carbonate scales, on the other hand, may be dissolved using simple mineral acids, such as HCl (Jones et al. 2008). In addition, surfactants, e.g., N -erucyl-N,N -bis-2-hydroxyethyl-N -methyl ammonium chloride are advantageous for controlling the viscosity of the fluids (Jones et al. 2008).
These surfactants can form worm-like micelles when mixed with brines. These structures contribute significantly to the viscoelasticity of the fluid. This is lost rapidly when the fluid contacts hydrocarbons, since they cause the micelles to change structure or disband.
The difference in viscosity of the fluid when in contact with hydrocarbons and water allows a selective placement of the scale treatment. As a result, scale may be preferentially removed from hydrocarbon-bearing zones. This can lead to a stimulation of hydrocarbon production without a substantial increase in the water cut of produced fluids (Jones et al. 2008).
The EDTA can also be regenerated. Eq. 7.1 illustrates in simplified form the dissolution and subsequent isolation of a barium sulfate scale and the regeneration of EDTA (Keatch 2008).
(7.1)
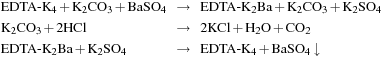
Phosphonates
Previous studies have strongly indicated that amine methylene phosphonic acid-based inhibitor species, such as pentaphosphonate and hexaphosphonate, are considerably less thermally stable than polymeric species, such as PVS and the S-Co species, so the phosphonate-based species were reported to be less applicable for deployment in high-temperature reservoir systems. However, species based on different amine methylene phosphonic acid revealed that certain species are thermally stable at temperatures exceeding 160∘C (Graham et al., 2002).
In studies, a series of phosphonate-based scale inhibitors were thermally aged at 160∘C, and it was reported that the scale inhibitors were still able to prevent carbonate scale in dynamic test after aging. However, thermal aging did reduce the performances of some of the phosphonate compounds against sulfate scale (Dyer et al., 2004).
Esterified phosphono or phosphino acids with a long chain alcohol are effective as oil-soluble scale inhibitors, and as wax or asphaltene inhibitors or dispersants in oil production. The esters can be prepared by azeotropic condensation of the phosphino acids with the alcohol or by telomerizing an ester of an unsaturated carboxylic acid with a phosphite or hypophosphite telogen (Woodward et al. 2004).
In contrast, laboratory studies demonstrate that changing from a phosphonate to a vinylsulfonate copolymer-based scale inhibitor could significantly extend the lifetime of a treatment (Jordan et al. 2005).
Alkaline Earth Sulfates
In dissolution studies of barite, using EDTA -based and diethylentriaminepentaacetic acid-based chelating agents, it has been verified that the presence of dicarboxylic acid additives, such as oxalate ion, improve the performance of the chelating agents. However, other related additives such as malonate and succinate reduce the effectiveness.
Oxalate ions catalyze the surface complexation reaction between the chelant and the barite surface by the formation of a two-ligand surface complex. The adverse effect observed for the other dicarboxylic acids is believed to arise because of steric effects, which prevent the formation of such a complex.
In extended studies with other barite related scales, such as celestite (SrSO4), gypsum (CaSO4× 2 H2O), and anhydrite (CaSO4), it was observed that scale dissolvers, which are optimized for their effectiveness against one type of scale, such as barite, may not be the most effective against other scales (Mendoza et al. 2002).
Biodegradable Scale Inhibitors
Many oil companies are requesting environmentally friendly fracturing fluids. Fracturing fluids are composed from a variety of compounds, each having a special function and usually, contain scale inhibitors. Fracturing fluids are explained in Chapter 17. Biodegradable chelants can be selected from a variety of compounds (Crews 2006).
Sodium Iminodisuccinate
This compound is a maleic acid derivative used as a chelant for divalent and trivalent ions. It complexes ions that can cause emulsions, form scale, can denature enzyme breakers, and cause crosslinked gel instability, and thus it can prevent these ions from having these undesirable effects.
Disodium Hydroxyethyleneiminodiacetic Acid
This is one of the few amino carboxylic acid chelants that is readily biodegradable. It is useful for the chelation of divalent and trivalent ions that cause scale, can denature enzymes, and create crosslinked gel instability.
Sodium Gluconate and Sodium Glucoheptonate
These polyols are commonly used for chelation of mineral vitamins such as calcium, magnesium, iron, manganese, and copper. They have been also found to be useful to complex titanate, zirconate, and borate ions for crosslink delay purposes. They are also excellent iron complexors for enzyme breaker stability and crosslinked gel stability.
Sodium Polyaspartate
This compound is also known as polymerized aspartic amino acid. It chelates with multiple types of divalent and trivalent ions, and is useful in breaking emulsions and scale prevention.
Polyaspartic acid-based chemicals are environmentally friendly and biodegradable oil field chemicals. They can be used both as corrosion inhibitors and scale inhibitors in brine-injection petroleum recovery. They exhibit a good calcium compatibility. At pH 5, polyaspartates are resistant to calcium ion concentrations of 8500–7500 ppm, in comparison with 5000 ppm for phosphonate and maleic acid polymer products.
Calcium compatibility is superior to that of phosphonate and maleic acid polymer products at concentrations of 5%. Polyaspartates also do not interfere with the oil-water separation process (Fan et al., 2001).
These chemicals are used as scale inhibitors and also as preconditioning solutions for other scale inhibitors. It has been claimed that a polyaspartate preconditioning solution at low pH enhances the adsorption of a phosphonate scale inhibitor to a rock material (Montgomerie et al. 2004).
Bioreactors near the site of the borehole have been suggested for the synthesis well treatment chemicals could even well treatment be achieved by introducing downhole thermophilic Archea or other thermophilic bacteria or organisms which are capable of generating well treatment chemicals (Kotlar and Haugan 2005).
Oil-soluble Scale Inhibitors
Phosphonic acids, such as diethylene triamine tetramethylene phosphonic acid, or bis-hexamethylene triamine pentakismethylene phosphonic acid or acrylic copolymers, PAA, PPCA, or phosphate esters are suitable oil-soluble scale inhibitors. These basic compounds are blended with amine compounds to form an oil-soluble mix (Reizer et al. 2002). tert -Alkyl primary amines with 12–16 carbon atoms are oil soluble and effect the oil solubility of the scale inhibitor.
Aloe-based Scale Inhibitor
An aloe gel dissolved in water has been used as a scale inhibitor. It comprises polysaccharides, solubilized in water between 60∘C–90∘C. Carboxyl and alcohol functional groups are present in the chains that interact with divalent ions such as Ca2+ and Mg2+.
Unlike chemically synthesized inhibitors, the active ingredients in the aloe plant gel are naturally occurring compounds. The scale inhibitor can be applied at low and high calcium concentrations and will not precipitate because of hydrolysis. Hydrolysis, in fact, favors the interaction with ions in the solution and thus its efficiency as a scale inhibitor may even increase (Viloria et al. 2010).
Reaction with calcium to form calcium-encapsulating gels is believed to occur via an egg-box model, as shown in Figure 7.2. In general, gels can be formed by the interaction of multivalent ions with polymers. This phenomenon is also known as physical crosslinking.
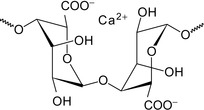 |
| Figure 7.2 Egg-box model Viloria et al. (2010). |
The chains of the gel interact via Ca2+, hence conferring stability towards systemic forces or other conditions.
The model assumes that calcium ions serve as an ionic bridge between carboxyl groups from two different chains in close contact. According to this polysaccharide model, the chains interact via Ca2+ allowing a structure coordinated packaging.
Inhibitors for Special Tasks
Iron Sulfide
Ferrous sulfide deposits are a major source of economic loss in the oil industry. The deposits are mainly the result of a reaction between hydrogen sulfide, formed by sulfate-reducing bacteria, and ferrous metal oil field equipment or an iron compound in the formation. They obstruct the flow of oil through wells and in the adjacent strata, and also in pipelines and in processing and refinery plants. Ferrous sulfide particles also tend to stabilize oil water emulsions that often form, especially during secondary oil recovery, and present major problems to oil producers.
The simplest way to dissolve such deposits is by contact with a strong acid. Unfortunately this method generates large volumes of highly toxic hydrogen sulfide gas, which in the past has been responsible for fatalities.
An alternative method of treating the deposits is with powerful oxidizing agents, which avoids the toxicity hazards but produces oxidation products, including elemental sulfur, which is so corrosive to pipework that it has not generally been practiced.
It has been found that trishydroxymethylphosphine (THP) is capable of solubilizing iron sulfide by forming a bright red, water-soluble complex. THP is believed to be formed in oil wells, which are treated with tetrakishydroxmethylphosphonium salts.
Such salts, especially the sulfate salts, are commonly added to oil wells as biocides. They are highly effective for killing the sulfate-reducing bacteria, whose activity was largely responsible for the original formation of the iron sulfide deposits. However, the effectiveness of THP as a solubilizing agent for iron sulfides varies considerably from well to well because complexation with iron sulfide requires the presence of ammonium ions. Although normally present in oil field water, their concentration is frequently less than the optimum for iron sulfide removal. The pH is critical to the formation of the complex.
Water-soluble condensates of THP with co-condensable organic nitrogen compounds such as urea and thiourea are also capable of solubilizing iron sulfide. These condensates provide more consistent performance than THP ammonia mixtures, but they may also cause deposition of polymers if used in high concentration.
THP and amino carboxylic acids or amino phosphonic acids act synergistically to dissolve iron sulfide deposits, even in the absence of ammonia. Moreover, THP is stable in the presence of amino phophonates even when the two are formulated together, and stored for extended periods prior to use (Fidoe et al. 2005).
Lead Sulfide
Scales from lead sulfide are much more difficult to inhibit than those of calcium carbonate or barium sulfate. Test methods have been developed in order to test the performance of the inhibition of lead sulfide (Chen et al., 2010).
Scale inhibitors, including phosphonate-based scale inhibitors, PPCA, polymaleic acid, PAA, polyaspartate, PVS, and acrylic copolymers have been tested. Their performance with respect to the inhibition of lead sulfide can be classified as follows (Chen et al., 2010):
1. Dispersion inhibitors,
2. Nucleation inhibitors, and
3. Poor performance inhibitors.
Dispersion inhibitors exhibit a dispersion effect on the formation of lead sulfide. Nucleation and growth scale inhibitors inhibit the nucleation and the growth of lead sulfide.
Zinc Sulfide
Generally, a zinc bromide (ZnBr2) brine will be used when the brine is required to have a density of about 1.7 kg l-1 (14.0ppg) or above. If a reservoir contains hydrogen sulfide, then zinc sulfide (ZnS) scales can form (Wang et al. 2008). Usually an acid treatment is performed to remove such scales, but there are significant risks associated with acid treatments in high-temperature, high-pressure gas wells. The acid treatment may restore production to its previous level, but new zinc sulfide deposits can be formed in the well in a short period of time, and a re-treatment is required.
Phosphonate and phosphonic acid type scale inhibitors control the ZnS scale at relatively low concentrations. Polymeric scale inhibitors, such as a copolymer of the sodium salt 2-acrylamido-2-methyl-1-propane sulfonic acid and acrylic acid (AA) are also effective in fresh water and low density brines, but not high-density brines. For these brines, copolymers from AAm and diallyidimethylammonium salts are more effective (Wang et al. 2008). The incorporation of a nitrogen heterocyclic compound in the copolymer improves its thermal stability. Such heterocyclic compounds include N -vinylpyrrolidone, N -vinylcaprolactam, N -vinylimidazole, and N -vinylpyridine.
The cationic nature of the copolymer greatly improves its compatibility for use as a scale inhibitor with high-density brines.
Naturally Occurring Radioactive Materials
In oil and gas fields, uranium, as 238U and 235U, and thorium, as 232Th, are present in immobile chemical forms, whereas radium and its isotopes, and their γ-emitting daughter nuclides, can easily be transported with chloride-rich formation waters. Once radium isotopes are leached from their lithological origin, they are no longer supported by their ancestors, and thus they decay.
Radium and its isotopes tend to co-precipitate alongside sparingly soluble alkaline cations mainly as the sulfate, or carbonate, or silicate. Formation and produced waters can therefore become radioactive due to the transportation of radium isotopes. External (near any processing equipment) and internal (during maintenance or workovers) radioactive hazards could exist due to naturally occurring radioactive materials that adhere to scale during processing (Bader 2006). For these reasons, it is highly desirable to establish an effective scale control.
High Reservoir Temperatures
Conventional polymer and phosphonate scale inhibitors may not be appropriate for the application in high-pressure and high-temperature reservoirs. Only a limited range of commercially available oil field scale inhibitor chemicals are sufficiently thermally stable at temperatures above 150∘C. They include homopolymers of vinylsulfonate and copolymers of AA and vinylsulfonate. Other polymers, such as polymaleic acid, polyitaconic acid, and maleic acid/AA copolymers, may offer similar thermal stability (Collins 1995). Thermal stability tests, influence on pH, ionic strength, and oxygen on conventional polymer and phosphonate scale inhibitors, for example, on phosphinopolycarboxylate, PVS, pentaphosphonate, and hexaphosphonate, have been presented (Dyer et al. 1999; Graham et al., 1998a, Graham et al., 1998b and Graham et al., 1997).
As pointed out above, it was originally believed that phosphonate scale inhibitors would not work in high-temperature inhibition applications, but it has been more recently shown that phosphonate inhibitors are somewhat effective at 200∘C under strictly anoxic conditions and in NaCl brines (Fan et al., 2010). In contrast, phosphonate inhibitors may precipitate with Ca2+ ions in a brine at high temperatures.
Characterization
Spectroscopic Methods
Field desorption mass spectrometry (Shen and Al-Saeed 1990), 13C nuclear magnetic resonance, and Fourier transform infrared spectroscopy (Newton 1988) have all been used to characterize oil field chemicals, including scale inhibitors. Ion chromatography is suitable for the simultaneous determination of hydroxyethylsulfonate, sodium vinylsulfonate, chloride, and sulfate reaction byproducts (Atwood 1992; Weber 1987).
Turbidimetry
Phase diagrams of a polyacrylate-phosphonate system with temperature and calcium ion concentration can be established by turbidimetric measurements (Weber 1987). Conductometric titrations also are suitable to characterize the phase behavior of scale inhibitors (Drela et al. 1998).
Static Bottle Test
Using alkaline surfactant polymer floods in sandstone reservoirs may cause silicate scaling. Silicate scaling has been a significant problem in alkaline surfactant polymer flooded fields in China (e.g., Daqing field) and Canada.
Methods for static and dynamic testing under specific field conditions using alkaline surfactant polymer floods have been reviewed. The tests serve to screen chemical inhibitors for the prevention of magnesium silicate scaling (Arensdorf et al., 2010).
In a static bottle test, water samples that serve for connate cationic and connate anionic waters are mixed together and afterwards mixed with an alkaline surfactant polymer solution. The compositions of these solutions are shown in Table 7.4.
| Compound | ASP | g l−1 | Cationic |
|---|---|---|---|
| NaCl | 2.5 | 2.4 | 3.5 |
| KCl | 0.08 | 016 | |
| NaHCO3 | 2,8 | 5.6 | |
| Na2SO4 | 0.3 | 0.6 | |
| Na2SiO3× 5H2O | 14.2 | ||
| MgCl2× 6H2O | 2 | ||
| CaCl2× 2H2O | 0.6 | ||
| BaCl2× 2H2O | 0.06 | ||
| pH | 10.7 | 7.0 |
The mixtures are initially clear, but slowly develop turbidity over the course of several hours, which is monitored with a photometer. At elevated temperatures, the scale formation is sometimes very fast. Typically, in a static test, silicate slowly forms from the beginning of the test and calcium carbonate forms after two hours.
It is interesting to note that none of the chemicals tested acted as a threshold inhibitor and prevented scaling at low doses. Rather, the inhibitors tended to delay the scaling process (Arensdorf et al., 2010).
It turned out that the static and dynamic tests correlate well for the individual inhibitors. The dynamic tests reveal that calcium carbonate is formed slowly, while a silicate scale is formed more quickly in other studies (Arensdorf et al., 2010).
In other studies dramatic differences have been observed for dynamic and static test conditions, indicating that the structures, which cause adherence and blocking of the pipework during flow may be different to those that dominate under bulk conditions (Senthilmurugan et al., 2010).
References
Anderko, A., Simulation of FeCO3/FeS scale formation using thermodynamic and electrochemical models, In: Proceedings Volume, NACE International Corrosion Conference, Corrosion 2000Orlando, FL, March 26–31, 2000. (2000).
Andrei, M.; Gagliardi, F., Redissolution studies in bulk and in coreflood for PPCA scales inhibitor, J. Pet. Sci. Eng. 43 (2004) 35–55.
Andrei, M.; Malandrino, A., Comparative coreflood studies for precipitation and adsorption squeeze with ppca as the scales inhibitor, Pet. Sci. Technol. 21 (2003) 1295–1315.
Arensdorf, J.J.; Hoster, D.; McDougall, D.B.; Yuan, M., Static and dynamic testing of silicate scale inhibitors, In: International Oil and Gas Conference and Exhibition in China (2010) Society of Petroleum Engineers, Beijing, China; Paper Number 132212-MS, pp. 1–7. http://www.onepetro.org/mslib/app/Preview.do?paperNumber=SPE-132212-MS&societyCode=SPE.
Atwood, S.E., 1992. Identification of sulfonation by-products by ion chromatography. US Patent 5 133 868, assigned to Marathon Oil Co., July 28, 1992.
Bader, M.S., 2006. Methods to solve alkaline-sulfate scales and related-gases problems. US Patent 7 093 663, August 22, 2006.
Barthorpe, R.T., The impairment of scale inhibitor function by commonly used organic anions, In: Proceedings Volume, SPE Oilfield Chem. International SymposiumNew Orleans, LA, March 2–5, 1993. (1993), pp. 69–76.
Becker, J.R., Corrosion and Scale Handbook. (1998) Pennwell Publishing Co, Tulsa.
Boak, L.S.; Graham, G.M.; Sorbie, K.S., The influence of divalent cations on the performance of BaSO4 scale inhibitor species, In: Proceedings Volume, SPE Oilfield Chem. International SymposiumHouston, TX, February 16–19, 1999. (1999), pp. 643–648.
Brown, J.M., Brock, G.F., 1995. Method of inhibiting reservoir scale. US Patent 5 409 062, assigned to Betz Laboratories, Inc., Trevose, PA, April 25, 1995.
Burger, E.D.; Chesnut, G.R., Screening corrosion inhibitors used in acids for downhole scale removal, Mater. Perf. 31 (7) (1992) 40–44.
Carter, C.G., Kreh, R.P., Fan, L.D.G., 1998. Composition and method for inhibiting scale and corrosion using naphthylamine polycarboxylic acids. EP Patent 538 969, assigned to Betzdearborn Inc., May 27, 1998.
Chen, T.; Chen, P.; Montgomerie, H.; Hagen, T.H.; Jeffrey, C., Development of test method and inhibitors for lead sulfide, In: SPE International Conference on Oilfield ScaleSociety of Petroleum Engineers, Aberdeen, UK. (2010); Paper Number 130926-MS, pp. 1–13. http://www.onepetro.org/mslib/app/Preview.do?paperNumber=SPE-130926-MS&societyCode=SPE.
Chilcott, N.P.; Phillips, D.A.; Sanders, M.G.; Collins, I.R.; Gyani, A., The development and application of an accurate assay technique for sulphonated polyacrylate co- polymer oilfield scale inhibitors, In: Proceedings Volume, 2nd Annual SPE Oilfield Scale International SymposiumAberdeen, Scotland, January 26–27, 2000. (2000).
Collins, I.R., Scale inhibition at high reservoir temperatures, In: Proceedings Volume, IBC Tech. Serv. Ltd Advances in Solving Oilfield Scaling International ConferenceAberdeen, Scotland, November 20–21, 1995. (1995).
Collins, I.R., 2000. Oil and gas field chemicals. US Patent 6 148 913, assigned to BP Chemicals Limited, London, GB, November 21, 2000.
Crabtree, M.; Eslinger, D.; Fletcher, P.; Miller, M.; Johnson, A.; King, G., Fighting scale – removal and prevention, Oilfield Rev. 11 (3) (1999) 30–45.
Crews, J.B., 2006. Biodegradable chelant compositions for fracturing fluid. US Patent 7 078 370, assigned to Baker Hughes Incorporated, Houston, TX, July 18, 2006.
Dean, G.D.; Nelson, C.A.; Metcalf, S.; Harris, R.; Barber, T., New acid system minimizes post acid stimulation decline rate in the wilmington field, los angeles county, california, In: Proceedings Volume, 68th Annual SPE Western Regional MeetingBakersfield, CA, May 10–13, 1998. (1998).
Dobbs, J.B.; Brown, J.M., An environmentally friendly scale inhibitor, In: Proceedings Volume, NACE International Corrosion Conference, Corrosion 99San Antonio, April 25–30, 1999. (1999).
Drela, I.; Falewicz, P.; Kuczkowska, S., New rapid test for evaluation of scale inhibitors, Water Res. 32 (10) (1998) 3188–3191.
Duccini, Y.; Dufour, A.; Harm, W.M.; Sanders, T.W.; Weinstein, B., High performance oilfield scale inhibitors, In: Corrosion97 (1997) NACE International, New Orleans, LA.
Dyer, S.J.; Anderson, C.E.; Graham, G.M., Thermal stability of amine methyl phosphonate scale inhibitors, J. Pet. Sci. Eng. 43 (2004) 259–270.
Dyer, S.J.; Graham, G.M.; Sorbie, K.S., Factors affecting the thermal stability of conventional scale inhibitors for application in high pressure/high temperature reservoirs, In: Proceedings Volume, SPE Oilfield Chem. International SymposiumHouston, TX, February 16–19, 1999. (1999), pp. 167–177.
Fan, J.C.; Fan, L.D.G.; Liu, Q.W.; Reyes, H., Thermal polyaspartates as dual function corrosion and mineral scale inhibitors, Polym. Mater. Sci. Eng. 84 (2001) 426–427.
Fan, C.; Kan, A.T.; Zhang, P.; Lu, H.; Work, S.; Yu, J.; Tomson, M.B., Scale prediction and inhibition for unconventional oil and gas production, In: SPE International Conference on Oilfield ScaleSociety of Petroleum Engineers, Aberdeen, UK. (2010); Paper Number 130690-MS, pp. 1–22. http://www.onepetro.org/mslib/app/Preview.do?paperNumber=SPE-130690-MS&societyCode=SPE.
Fan, G.; Koskan, L.P.; Ross, R.J., Polyaspartates: An emerging green technology in oil production, In: Book of Abstr (ACS), no. ENVR 029, 215th ACS Nat. Mtg.Dallas, March 29–April 2, 1998. (1998).
Fidoe, S.D., Talbot, R.E., Jones, C.R., Gabriel, R., 2005. Treatment of iron sulphide deposits. US Patent 6 926 836, assigned to Rhodia Consumer Specialties Limited, Watford, GB, August 9, 2005.
Fong, D.W., Marth, C.F., Davis, R.V., 2001. Sulfobetaine-containing polymers and their utility as calcium carbonate scale inhibitors. US Patent 6 225 430, assigned to Nalco Chemical Co., May 1, 2001.
Frenier, W.W.; Hill, D.G., Green inhibitors-development and applications for aqueous systems, In: Proceedings Volume, Vol. 3 of Reviews on Corrosion Inhibitor Science and Technology, Corrosion-2004 SymposiumNew Orleans, LA, United States, March 28–April 1, 2004. (2004), pp. 6/1–6/39.
Graham, G.M.; Dyer, S.J.; Shone, P., Potential application of amine methylene phosphonate based inhibitor species in HP/HT (high pressure/high temperature) environments for improved carbonate scale inhibitor performance, In: Proceedings Volume, 2nd Annual SPE Oilfield Scale International SymposiumAberdeen, Scotland, January 26–27, 2000. (2000).
Graham, G.M.; Dyer, S.J.; Shone, P., Potential application of amine methylene phosphonate based inhibitor species in hp/ht environments for improved carbonate scale inhibitor performance, SPE Prod. Facil. 17 (2002) 212–220.
Graham, G.M.; Dyer, S.J.; Sorbie, K.S.; Sablerolle, W.; Graham, G.C., Practical solutions to scaling in HP/HT (high pressure/high temperature) and high salinity reservoirs, In: Proceedings Volume, 4TH IBC UK Conf. Ltd Advances in Solving Oilfield Scaling International ConferenceAberdeen, Scotland, January 28–29, 1998. (1998).
Graham, G.M.; Dyer, S.J.; Sorbie, K.S.; Sablerolle, W.R.; Shone, P.; Frigo, D., Scale inhibitor selection for continuous and downhole squeeze application in HP/HT (high pressure/ high temperature) conditions, In: Proceedings Volume, Annual SPE Technical ConferenceNew Orleans, LA, September 27–30, 1998. (1998), pp. 645–659.
Graham, G.M.; Jordan, M.M.; Sorbie, K.S.; Bunney, J.; Graham, G.C.; Sablerolle, W.; Hill, P., The implication of HP/HT (high pressure/high temperature) reservoir conditions on the selection and application of conventional scale inhibitors: Thermal stability studies, In: Proceedings Volume, SPE Oilfield Chem. International SymposiumHouston, TX, February 18–21, 1997. (1997), pp. 627–640.
Gupta, D.V.S., Kirk, J.W., 2009. Method of inhibiting or controlling formation of inorganic scales. US Patent 7 491 682, assigned to BJ Services Company, Houston, TX, February 17, 2009.
He, S.; Kan, A.T.; Tomson, M.B., Inhibition of calcium carbonate precipitation in NaCl brines from 25 to 90°C, Appl. Geochem. 14 (1) (1999) 17–25.
Holzner, C., Kleinstueck, R., Spaniol, A., 2000. Phosphonate-containing mixtures (phosphonathaltige mischungen). WO Patent 0 032 610, assigned to Bayer AG, June 8, 2000.
Hsu, J.F.; Al-Zain, A.K.; Raju, K.U.; Henderson, A.P., Encapsulated scale inhibitor treatments experience in the ghawar field, saudi arabia, In: Proceedings Volume, 2nd Annual SPE Oilfield Scale International SymposiumAberdeen, Scotland, January 26–27, 2000. (2000).
Jones, C.R., Grech, J.M., 2004. Formulation for corrosion and scale inhibition. WO Patent 2 004 083 131, assigned to Rhodia Cons Spec. Ltd., Jones Christopher Raymond, and Grech Jason Mark, September 30, 2004.
Jones, C.R., Taylor, R.S., 2004. Sludge control in crude oil. WO Patent 2 004 104 367, assigned to Rhodia Cons Spec. Ltd., Jones Christopher Raymond, and Taylor Robert S, December 2, 2004.
Jones, T.G.J., Tustin, G.J., Fletcher, P., Lee, J.C.-W., 2008. Scale dissolver fluid. US Patent 7 343 978, assigned to Schlumberger Technology Corporation, Ridgefield, CT, March 18, 2008.
Jordan, M.M.; Kemp, S.; Sorhaug, E.; Sjursaether, K.; Freer, B., Effective management of scaling from and within carbonate oil reservoirs, North Sea basin. Chem. Eng. Res. Des. 81 (2003) 359–372.
Jordan, M.M.; Sjursaether, K.; Bruce, R.; Edgerton, M.C., Inhibition of lead and zinc sulphide scale deposits formed during production from high temperature oil and condensate reservoirs, In: Proceedings Volume, SPE Asia Pacific Oil & Gas ConferenceBrisbane, Australia, October 16–18, 2000. (2000).
Jordan, M.M.; Sjursaether, K.; Collins, I.R., Scale control within the north sea chalk/ limestone reservoirs–-the challenge of understanding and optimizing chemical-placement methods and retention mechanisms: Laboratory to field, SPE Prod. Facil. 20 (4) (2005) 262–273.
Jordan, M.M.; Sjuraether, K.; Collins, I.R.; Feasey, N.D.; Emmons, D., Life cycle management of scale control within subsea fields and its impact on flow assurance Gulf of Mexico and the North Sea basin, Spec. Publ. R. Soc. Lond. 280 (2002) 223–253.
Jordan, M.M.; Sorbie, K.S.; Chen, P.; Armitage, P.; Hammond, P.; Taylor, K., The design of polymer and phosphonate scale inhibitor precipitation treatments and the importance of precipitate solubility in extending squeeze lifetime, In: Proceedings Volume, SPE Oilfield Chem. International SymposiumHouston, TX, February 18–21, 1997. (1997), pp. 641–651.
Kan, A.T.; Tomson, M.B., Scale prediction for oil and gas production, In: International Oil and Gas Conference and Exhibition in ChinaSociety of Petroleum Engineers, Beijing, China. (2010), pp. 1–29; Paper Number 132237-MS, http://www.onepetro.org/mslib/app/Preview.do?paperNumber=SPE-132237-MS&societyCode=SPE.
Ke, M., Qu, Q., 2010. Method for controlling inorganic fluoride scales. US Patent 7 781 381, assigned to BJ Services Company LLC, Houston, TX, August 24, 2010.
Keatch, R.W., 1998. Removal of sulphate scale from surface. GB Patent 2 314 865, January 14, 1998.
Keatch, R., 2008. Method for dissolving oilfield scale. US Patent 7 470 330, assigned to M-1 Production Chemicals UK Limited, Aberdeen, GB, Oilfield Mineral Solutions Limited, Edinburgh, GB, December 30, 2008.
Kochnev, E.E., Merentsova, G.I., Andreeva, T.L., Ershov, V.A., 1993. Inhibitor solution to avoid inorganic salts deposition in oil drilling operations-contains water, carboxymethylcellulose or polyacrylamide and polyaminealkyl phosphonic acid and has improved distribution uniformity. SU Patent 1 787 996, assigned to Sibe Oil Ind. Res. Inst., January 15, 1993.
Kotlar, H.K., Haugan, J.A., 2005. Genetically engineered well treatment microorganisms. GB Patent 2 413 797, assigned to Statoil Asa, November 9, 2005.
Kowalski, T.C., Pike, R.W., 1999. Microencapsulated oil field chemicals. US Patent 5 922 652, July 13, 1999.
Kowalski, T.C., Pike, R.W., 2001. Microencapsulated oil field chemicals. US Patent 6 326 335, assigned to Corsicana Technologies Inc., December 4, 2001.
Kuzee, H.C., Raaijmakers, H.W.C., 1999. Method for preventing deposits in oil extraction. WO Patent 9 964 716, assigned to Cooperatie Cosun Ua, December 16, 1999.
Larsen, J.; Sanders, P.F.; Talbot, R.E., Experience with the use of tetrakishydroxy- methylphosphonium sulfate (thps) for the control of downhole hydrogen sulfide, In: Proceedings Volume, NACE International Corrosion Conference, Corrosion 2000Orlando, FL, March 26–31, 2000. (2000).
Mackay, E.J.; Sorbie, K.S., Modelling scale inhibitor squeeze treatments in high crossflow horizontal wells, J. Can. Pet. Technol. 39 (10) (1998) 47–51.
Mackay, E.J.; Sorbie, K.S., An evaluation of simulation techniques for modelling squeeze treatments, In: Proceedings Volume, Annual SPE Technical ConferenceHouston, TX, October 3–6, 1999. (1999), pp. 373–387.
Mackay, E.J.; Sorbie, K.S.; Jordan, M.M.; Matharu, A.P.; Tomlins, R., Modelling of scale inhibitor treatments in horizontal wells: Application to the alba field, In: Proceedings Volume, SPE Formation Damage Contr. Int. SymposiumLafayette, LA, February 18–19, 1998. (1998), pp. 337–348.
Malandrino, A.; Andrei, M.; Gagliardi, F.; Lockhart, T.P., A thermodynamic model for PPCA (phosphino-polycarboxylic acid) precipitation, In: Proceedings Volume, 4th IBC UK Conf. Ltd., Advances in Solving Oilfield Scaling International ConferenceAberdeen, Scotland, January 28–29, 1998. (1998).
Martin, R.L., Brock, G.F., Dobbs, J.B., 2005. Corrosion inhibitors and methods of use. US Patent 6 866 797, assigned to BJ Services Company, March 15, 2005.
Mendoza, A.; Graham, G.M.; Farquhar, M.L.; Sorbie, K.S., Controlling factors of EDTA and DTPA based scale dissolvers against sulphate scale, Prog. Min. Oilfield Chem. 4 (2002) 41–58.
Mikhailov, S.A.; Khmeleva, E.P.; Moiseeva, E.V.; Sleta, T.M., Determination of the optimal dose of salt deposition inhibitors, Neft Khoz (7) (1987) 43–45.
Montgomerie, H.T.R., Chen, P., Hagen, T., Wat, R.M.S., Selle, O.M., Kotlar, H.K., 2004. Method of controlling scale formation. WO Patent 2 004 011 772, assigned to Champion Technology Inc., Statoil Asa, Montgomerie Harry Trenouth Rus, Chen Ping, Hagen Thomas, Wat Rex Man Shing, Selle Olav Martin, and Kotlar Hans Kristian, February 5, 2004.
Mouche, R.J., Smyk, E.B., 1995. Noncorrosive scale inhibitor additive in geothermal wells. US Patent 5 403 493, assigned to Nalco Chemical Co., April 4, 1995.
Newton, J.A., Applications of spectroscopic quality control techniques for oilfield chemicals, In: Proceedings Volume, NACE Corrosion 88St Louis, March 21–25, 1988. (1988).
Powell, R.J.; Fischer, A.R.; Gdanski, R.D.; McCabe, M.A.; Pelley, S.D., Encapsulated scale inhibitor for use in fracturing treatments, In: Proceedings Volume, Annual SPE Technical ConferenceDallas, October 22–25, 1995. (1995), pp. 557–563.
Rakhimov, A.Z.; Vazquez, O.; Sorbie, K.S.; Mackay, E.J., Impact of fluid distribution on scale inhibitor squeeze treatments, In: SPE EUROPEC/EAGE Annual Conference and ExhibitionSociety of Petroleum Engineers, Barcelona, Spain. (2010).
Reizer, J.M., Rudel, M.G., Sitz, C.D., Wat, R.M.S., Montgomerie, H., 2002. Scale inhibitors. US Patent 6 379 612, assigned to Champion Technology Inc., April 30, 2002.
Senthilmurugan, B.; Ghosh, B.; Graham, G.M.; Kundu, S.S., The influence of maleic acid copolymers on the growth and microstructure of calcite scale, In: SPE International Conference on Oilfield ScaleSociety of Petroleum Engineers, Aberdeen, UK. (2010).
Shen, J.; Al-Saeed, A.S., Study of oil field chemicals by combined field desorption/ collisionactivated dissociation mass spectrometry via linked scan, Anal. Chem. 62 (2) (1990) 116–120.
Shuler, P.J.; Jenkins, W.H., Prevention of downhole scale deposition in the ninian field, In: Proceedings Volume, Vol. 2, SPE Offshore Europe ConferenceAberdeen, Scotland, September 5–8, 1989. (1989).
Siegmeier, R.; Kirschey, M.; Voges, M., Acrolein based polymers as scale inhibitors, In: Proceedings Volume, no. PAP 7, NACE International Corrosion Conference, Corrosion 98San Diego, March 22–27, 1998. (1998).
Sikes, C.S.; Wierzbicki, A., Stereospecific and nonspecific inhibition of mineral scale and ice formation, In: Proceedings Volume, 51st Annual NACE International Corrosion Conference, Corrosion 96Denver, March 24–29, 1996. (1996).
Singleton, M.A.; Collins, J.A.; Poynton, N.; Formston, H.J., Developments in phosphonomethylated polyamine (PMPA) scale inhibitor chemistry for severe BaSO4 scaling conditions, In: Proceedings Volume, 2nd Annual SPE Oilfield Scale International SymposiumAberdeen, Scotland, January 26–27, 2000. (2000).
Talbot, R.E., Grech, J.M., 2005. Formulation for corrosion and scale inhibition. WO Patent 2 005 040 050, assigned to Rhodia Cons Spec. Ltd., Talbot Robert Eric, and Grech Jason Mark, May 6, 2005.
Talbot, R.E., Jones, C.R., Hills, E., 2009. Scale inhibition in water systems. US Patent 7 572 381, assigned to Rhodia UK Limited, Hertfordshire, GB, August 11, 2009.
Tantayakom, V.; Fogler, H.S.; Chavadej, S., Scale inhibitor precipitation kinetics, In: Proceedings Volume, 7th World Congress of Chemical EngineeringGlasgow, United Kingdom, July 10–14, 2005. (2005), pp. 85704/1–85704/8.
Tantayakom, V.; Fogler, H.S.; de Moraes, F.F.; Bualuang, M.; Chavadej, S.; Malakul, P., Study of Ca-ATMP precipitation in the presence of magnesium ion, Langmuir 20 (6) (2004) 2220–2226.
Viloria, A., Castillo, L., Garcia, J.A., Biomorgi, J., 2010. Aloe derived scale inhibitor. US Patent 7 645 722, assigned to Intevep, S.A., Caracas, VE, January 12, 2010.
Wang, X., Qu, Q., Ke, M., 2008. Method for inhibiting or controlling inorganic scale formations with copolymers of acrylamide and quaternary ammonium salts. US Patent 7 398 824, assigned to BJ Services Company, Houston, TX, July 15, 2008.
Watkins, D.R., Clemens, J.J., Smith, J.C., Sharma, S.N., Edwards, H.G., 1993. Use of scale inhibitors in hydraulic fracture fluids to prevent scale build-up. US Patent 5 224 543, assigned to Union Oil Co. California, July 6, 1993.
In: (Editor: Weber, E.) Molecular Inclusion and Molecular Recognition – Clathrates 1, Vol. 140 of Topics in Current Chemistry (1987) Springer Verlag, Berlin.
Woodward, G., Jones, C.R., Davis, K.P., 2004. Novel phosphonocarboxylic acid esters. WO Patent 2 004 002 994, assigned to Rhodia Consumer Specialities L, Woodward Gary, Jones Christopher Raymond, and Davis Keith Philip, January 8, 2004.
Yan, T.Y., 1993. Process for inhibiting scale formation in subterranean formations. WO Patent 9 305 270, assigned to Mobil Oil Corp., March 18, 1993.
Yang, L.; Song, B., Phosphino maleic anhydride polymer as scale inhibitor for oil/gas field produced waters, Oilfield Chem. 15 (2) (1998) 137–140.
Zeng, Y.B.; Fu, S.B., The inhibiting property of phosphoric acid esters of rice bran extract for barium sulfate scaling, Oilfield Chem. 15 (4) (1998) 333–335; 365.
Zhang, H.; Mackay, E.J.; Sorbie, K.S.; Chen, P., Non-equilibrium adsorption and precipitation of scale inhibitors: Corefloods and mathematical modelling, In: Proceedings Volume, SPE Oil & Gas International Conference in ChinaBeijing, China, November 7–10, 2000. (2000), p. 18.
Tradenames
| Tradename | |
|---|---|
| Description | Supplier |
| BRIQUEST® 543 | Rhodia Consumer Specialties Ltd. |
| Sodium diethylene triamine pentakismethylene phosphonate (Fidoe et al. 2005) | |
| Dequest® 2060 | Monsanto |
| Diethylene triamine pentamethylene phosphonic acid (Collins 2000) | |
| Empol™ (Series) | Henkel |
| Oligomeric oleic acid (Jones et al. 2008) | |
| Gyptron® KT-178 | Champion Technologies |
| Diethylene triamine tetramethylene phosphonic acid (DETA), Scale inhibitor (Reizer et al. 2002) | |
| Primene® | Rohm & Haas |
| Primary aliphatic amines with highly branched alkyl chains (Reizer et al. 2002) | |
| Rhodafac® RS-410 | Rhodia |
| Polyoxy-1,2-ethandiyl tridecyl hydroxy phosphate (Martin et al. 2005) | |
| Scaletreat® XL14FD | TR Oil Services Ltd. |
| polymaleate (Collins 2000) |
..................Content has been hidden....................
You can't read the all page of ebook, please click here login for view all page.