Chapter 12. Drag Reducers
This chapter deals almost exclusively with drag reduction in pipelines for liquid transportation. Pipeline flow improvers, or drag-reducing agents (DRAs), have been utilized in the petroleum industry for many years (Almond, 1989). They are important in oil drilling applications and the maintenance of pumping equipment in pipelines.
The first application of drag reducers was the use of guar in oil well fracturing, which is now a routine practice. One of the first large-scale pipeline applications was to increase the throughput of crude oil on the Trans-Alaskan pipeline in 1979 through the successful use of oil-soluble polymers.
Since then, DRA use has increased in refined products pipelines to offset power costs. The DRA cost incurred to move an additional barrel of product through a pipeline system can be less than $0.05/bbl. This has been made possible by the improved performance of commercially available DRAs and a nearly unchanging price structure.
In the subsea production of oil and gas, production piping presents a significant bottleneck because of the difficulty and expense associated with the installation of subsea piping. The resulting production decrease can have severe economic ramifications because the hydrocarbon production system cannot be run at full capacity (Milligan et al., 2008).
This bottlenecking could be alleviated by either increasing the diameter of the flow lines, increasing the number of flow lines, or reducing the amount of friction loss, thereby allowing more flow through the same diameter lines. The first two options are obviously very expensive, hence reducing friction losses is highly desirable (Milligan et al., 2008).
Operating Costs
Pipeline operators can decrease operating costs by using a drag-reducing flow improver to eliminate the need for underutilized intermediate or booster pump stations (Goudy, 1991; Muth and Kolby, 1985). Product lines operating below their capacity, or those that only use boosters intermittently, can also realize cost savings.
The overall benefits are likely to be most significant in 6–8 in. lines operating between 67% and 92% of their rated throughput capacity. Using computer modeling techniques, engineers have demonstrated potential power savings of up to 22% (from lower demand charges and reduced energy use) for systems using booster stations for 85% of the operating time. When stations operate only 70% of the time, total energy cost savings can approach 35%, depending on the diameter of the line and electricity costs.
Mechanism of Drag Reduction
A review of drag-reducing polymers is given in the literature (Al-Sarkhi, 2010; Oh-Kil and Ling-Siu, 1996). It has been suggested that drag reduction occurs by the interaction between elastic macromolecules and macrostructures in turbulent flow. In turbulent pipe flow, the region near the wall, which is composed of a viscous sublayer and a buffer layer, plays a major role in drag reduction.
The most serious problem in the effectiveness of drag reducers is the chain degradation of polymers by shear strains under conditions of turbulent flow. Ultra-high molecular weight polymers are more sensitive to shear-induced degradation (Gampert and Wagner, 1985), polymers with linear-chain structures are more vulnerable than branched polymers (Chang and Meng, 1987), and natural gums with semirigid structures (Deshmukh et al., 1985).
The mechanism of shear degradation is thought to be associated with chain elongation. Chain degradation is often observed when the shear rate is increased to a critical point, after which drag reduction decreases sharply.
The friction drag and heat-transfer-reduction phenomena associated with turbulent flows of so-called drag-reducing fluids are not well understood (Kostic, 1994). It is believed that elastic fluid properties are strongly related to these phenomena. However, not all drag-reducing fluids are viscoelastic, nor are all viscoelastic fluids drag-reducing, suggesting that drag reduction and viscoelasticity are probably phenomena that co-occur only incidentally.
It is argued that turbulence suppression, i.e., flow laminarization, is a determining factor in drag reduction, but the fluid elasticity is not, because of the flow-induced anisotropic fluid structure and associated properties. It may, however, be a major cause of laminar heat transfer augmentation.
Damping of Transmission of Eddies
Drag reduction can occur if the transmission of eddies is damped by the viscoelastic properties of fluids. The transfer process of an isolated eddy in viscoelastic Maxwell fluids was studied, and expressions describing such phenomena were obtained (Li, 1991). The results of the study showed that eddy transmission was damped significantly with an increase of the viscoelastic properties of the fluids.
Viscoelastic Fluid Thread
In the extensive literature on polymer drag reduction, it has occasionally been reported that a continuous thread of a high-concentration polymer solution, injected into the axis of a pipe, produces a drag reduction effect on the water flow in the pipe (Hoyt and Sellin, 1988). The thread seems to persist through the length of the pipe and little, if any, diffusion of polymer to the walls of the pipe is apparent.
A polyacrylamide (PAM) polymer was injected as a 0.5% solution from an axially placed nozzle at the bellmouth entrance. The experiments showed that the central thread provided drag reduction that was almost equivalent to premixed solutions of the same total polymer concentration flowing in the pipe. Overall concentrations of 1–20 ppm were used.
The effects were additive: 2 ppm overall thread concentration plus 2 ppm of premixed polymer gave drag reductions equivalent to 4 ppm of either type. Reynolds numbers of up to 300,000 were investigated. In other experiments, a number of different polymer fluids were injected on the centerline of a water pipe flow facility (Hoyt and Sellin, 1991). Two distinct flow regions were identified:
• Reynolds numbers above 25,000, at which centerline injection acted as a rather efficient mixing device for water-soluble polymer and no drag reduction, resulted from materials insoluble in water.
• Reynolds numbers from 10,000 to 25,000, at which strong evidence exists that under certain conditions, a viscoelastic fluid thread can interact with turbulence eddies and reduce the overall flow friction in the pipe.
Polymer Degradation in Turbulent Flow
Drag reduction in turbulent flow is of great potential benefit to many industrial processes, including the long distance transportation of liquids, oil well operations, and transportation of suspensions and slurries, but it is complicated by the problem of polymer degradation.
A capillary rheometer was used to investigate the effect of various parameters on polymer degradation in turbulent flow (Moussa and Tiu, 1994). These parameters included polymer concentration, contraction ratio, pipe length, pipe diameter, number of passes, solvent weight, and molecular weight of polymer. A commercial organic drag reducer, two grades of PAM, and a high molecular weight polyisobutylene were used.
In turbulent flow, the polymer degraded more in a poor solvent at low Reynolds numbers, whereas an opposite effect was observed at high Reynolds numbers. The critical Reynolds number, Rec or critical apparent shear extensional rate, V/d, was found to increase with polymer concentration and molecular weight, as represented by the dimensionless concentration c(η).
Polysaccharide guar gum is used as a turbulent drag reducer in aqueous systems. It reduces the friction drag tremendously in turbulent flow even in small amounts. A study on the mechanical degradation of guar gum has been presented, in which the effectiveness of drag reduction was measured as a function of time using a rotating disk apparatus.
Two different degradation models of a single-relaxation process and a stretched-exponential model were examined. The stretched-exponential model seemed to fit the experimental data better (Hong et al., 2010).
Drag Reduction in Two-phase Flow
The drag-reducing properties of a PAM were tested in two-phase air/water flow, using a horizontal pipe of 31 mm diameter (Saether et al., 1989). The properties of the polymer were tested in single-phase water flow, and the results were found to comply with the reduction in pressure drop found by other workers. Positive effects in two-phase flow were found to depend on the Reynolds number of the liquid flow.
The drag reduction in stratified flow was found to be small or negative. In slug flow, the drag reduction seems to occur in the liquid slug, not in the layer below the bubble. The flow regime seems to be unaffected by the polymer. It has been established that in multiphase flow, drag reducers also act as corrosion inhibitors because they smooth the flow profile near the walls (Kang et al., 1998).
Drag Reduction in Gas Flow
For storage or pipeline transportation of natural gas at pressures over 5.5 M Pa (800 psi), it is advantageous to add ammonia to the natural gas, but the ammonia should not create a liquid phase at the temperature and pressure used. A gaseous mixture of ammonia and natural gas can be compressed or pumped using less energy than would be needed for an equivalent volume of natural gas alone. When more than 4% by volume of ammonia is present, pumping through pipelines is also aided by the refrigerant effect of the ammonia, which reduces the temperature of the gas being transported (Morris and Perry, 2001).
Microfibrils
Friction loss in liquids can be reduced by adding a predetermined amount of selected organo-polymeric microfibrils to a liquid (Shinomura, 1988). These microfibrils are insoluble but highly dispersible in the liquid.
An organo-polymeric microfibril is a solid, organic polymer in the form of microfibrils, which have an average diameter in the range of 100–1000 Å, of an average length of 1–500 μ, and an aspect ratio (length/diameter) of 10–1,000,000. Polymeric materials to be processed into microfibrils should be insoluble but highly dispersible in a given liquid.
Drag-reducing Surfactant Solutions
The behavior of two types of drag-reducing surfactant solutions was studied under turbulent flow in pipes of different diameters (Bewersdorff and Ohlendorf, 1988). The surfactant systems contained rod-like micelles consisting of equimolar mixtures of n-tetradecyltrimethylammonium bromide, n-hexadecyltrimethylammonium bromide, and sodium salicylate.
The structure of the turbulence was studied using a laser-Doppler anemometer in a 50 mm pipe. In the regions of turbulent flow, both surfactant solutions exhibited characteristic flow regimes. In the regions of turbulent flow at low Reynolds numbers, velocity profiles similar to those observed for dilute polymer solutions were found, whereas at maximal drag reduction conditions, more S-shaped profiles that show deviations from a logarithmic profile occur.
Soapy Industrial Cleaner
Experiments have been conducted to investigate the effect of a soapy industrial cleaner on reducing the skin friction of a Jordanian crude oil flowing turbulently in pilot-scale pipes of different sizes. Experiments showed that a concentration of only 2 ppm of the additive injected into the crude oil line caused an appreciable amount of drag reduction (Mansour and Aldoss, 1988). The effects of additive concentration and pipe diameter on drag reduction were investigated.
Lyophobic Performance of the Lining Material
An experimental study was conducted on the characteristics of frictional drag for a lyophobic surface made of polytetrafluoroethylene (PTFE), with a working media of water and machine oil (Saether et al., 1989). The test results indicate that, depending on the lyophobic performance of the lining material, the pipes lined with PTFE have a better drag-reducing effect than conventional steel pipes.
A drag reduction of approximately 12% is achieved if the working medium is water or 6% with machine oil, respectively. In other words, PTFE has a higher lyophobic performance against water than against machine oil.
The theoretical analysis of the flow mechanism on the lyophobic surface shows that treatment can lower the surface energy level to such a degree that the attraction of the solid wall to liquid molecules becomes weaker than the liquid molecular absorption. This effect causes a gliding flow adjacent to the pipe wall, thus reducing the drag.
Interpolymer Complexes
It has been shown that hydrogen bonding-mediated interpolymer complexes can be powerful drag reducers. The drag reduction levels in such polymer systems are a factor of 2–6 greater than their nonassociating polymeric precursors. Their shear stability is also shown to be significantly enhanced (Malik and Mashelkar, 1995).
Hydrocarbon-soluble polymers containing small percentages of polar associating groups are used to determine the effects of polymer associations on solution drag reduction. Experimental data suggest that intrapolymer associations generally decrease the dilute solution drag reduction activities of single associating polymers with like polar groups (Kowalik et al., 1987).
Interpolymer complexes formed by a polymer with anionic groups and one with cationic groups can overcome this limitation and provide enhanced, dilute solution drag reduction activity as a result of favorable interpolymer associations, which build larger structures of higher apparent molecular weight. The latter associations may also increase the resistance of the polymers to degradation in turbulent flows.
Drag-reducing Chemicals
Ultra-high Molecular Weight Polyethylene
The flow of liquid hydrocarbons can be enhanced by introducing a nonagglomerating suspension of ultra-high molecular weight polyethylene (UHMWPE) (Dindi et al., 1996; Smith et al., 1995) in water with small amounts of surfactant. The finely divided UHMWPE is prepared by polymerization and then cryogrinded below its glass transition temperature.
Copolymers of α-Olefins
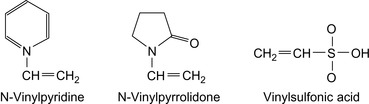
Several copolymers of α-olefins are used as drag reducers. Suggested recipes are summarized in Table 12.1 and monomers are shown in Figure 12.1.
| aSynthesis by a Ziegler-Natta process | |
| bUp to C30, Ziegler-Natta | |
| cMolecular weight of up to 15,000 and an isotacticity of 75% or greater | |
| dOil-soluble polymer | |
| eReduce friction in the flow of a hydrocarbon fluid by a factor of 5 at concentrations as low as 1–25 ppm | |
| fPolyampholytes | |
| Comonomer | References |
|---|---|
| Divinylbenzene/1-hexene, 1-octene, 1-decene, and 1-dodecenea | Aubanel and Bailly (1987), Brod et al. (1990), Eaton and Monahan (1999) and Gessell and Washecheck (1990) |
| Styrene/N-vinylpyridine (NVP) | Kowalik et al. (1986) |
| Ethene/α-olefinsb | Hostetler et al. (1989) |
| Homo- or copolymers that α-olefinsc | Rossi et al. (1993) |
| Polyisobutened | Martischius et al. (1990) |
| Meth acrylic acid esters | Ritter et al. (1991a), Ritter et al., 1991a, Ritter et al., 1989a, Ritter et al., 1991b, Ritter et al., 1989b, Ritter et al., 1991c and Ritter et al., 1995 |
| C12 to C18 acrylate or methacrylate/ionic monomere | Malik et al. (1992) and Malik et al. (1996) |
| tert-Butylstyrene/alkyl acrylate, methacrylic acid | Naiman and Chang (1991) |
| Acrylamide-acrylate | Schulz et al., 1986 and Schulz et al., 1987 |
| Ultra-high molecular weight polyolefin | Mack (1986) |
| Styrene/methyl styrene sulfonate/NVPf | Peiffer et al. (1987) |
Linear, low density polyethylene is a copolymer of ethylene and α-olefins, obtained by copolymerization utilizing Ziegler-Natta or metallocene catalysts. Concentrates may be prepared by precipitating the polymer from a kerosene solution with isopropanol (Fairchild et al., 1997). The resulting slurry concentrate dissolves rapidly in flowing hydrocarbon streams.
By coating poly-α-olefins with a fatty acid wax as a partitioning agent, and dispersing it in a long chain alcohol, a nonagglomerating, nonaqueous suspension can be obtained (Johnston and Lee, 1998).
Latex Drag Reducers
Latex drag reducers comprise a polymer that is formed via an emulsion-polymerization reaction dispersed in a continuous phase. Subsequent modifications can be applied in order to increase the solubility of the polymer in hydrocarbons.
2-ethylhexyl methacrylate is polymerized by conventional emulsion polymerization techniques, details of which can be found elsewhere (Milligan et al., 2008). The emulsion polymerization reaction yields an initial latex composition, as a stable colloidal dispersion.
The dispersed phase is made up of up to 50% of colloidal particles of the high molecular weight polymer. The continuous phase is water and surfactant. The latex can be modified or formulated with additional surfactants and organic solvents to increase the viscosity. The drag reducer may be injected into the pipeline using conventional or umbilical delivery systems.
Polyether Compounds for Oil-based Well Drilling Fluids
A liquid oil, an emulsifier, and a friction modifier, which includes certain polyether compounds, can be added to a drilling fluid consisting of a water-in-oil emulsion formed from a brine (Malchow, 1997). The friction modifier serves to decrease the coefficient of friction of the well drilling fluid.
Decreasing the coefficient of friction lowers the force required to turn the drill bit in the hole. Gravitational forces increase the coefficient of friction in deviated, horizontal, and extended-reach wells.
Tylose
Tylose is not as effective in drag reduction as other substances described in the literature. Detailed mean velocity, normal Reynolds stress, and pressure drop measurements were performed with 0.4–0.6% aqueous solutions of tylose, a methylhydroxyl cellulose (molecular weight 6 k Dalton), after a selection process from a set of low molecular weight fluids (Bewersdorff and Ohlendorf, 1988).
The measurements of the viscosity of these solutions showed shear thinning behavior, and the oscillatory and creep tests measured elastic components of the stress in the order of the minimal detectable values by the rheometer. These low molecular weight polymer solutions delay the transition from laminar to turbulent regimes and show drag reductions of approximately half those which occur with other low elasticity, shear thinning, high molecular, aqueous polymer solutions.
Microencapsulated Polymers
Highly concentrated DRAs may be prepared by microencapsulating a polymer or a monomer, which may be performed before, during, or after polymerization. If the encapsulation is done before or during polymerization, a catalyst must be present, but little or no solvent is required. The result is bulk polymerization within the microcapsule.
The inert capsule or shell may be removed before, during, or after the introduction of the microencapsulated drag reducer into a flowing liquid. No injection probes or other special equipment should be required to introduce the drag-reducing slurry into the liquid stream, nor is grinding (cryogenic or otherwise) of the polymer necessary to form a suitable DRA (Kommareddi and Rzeznik, 1999 and Kommareddi and Rzeznik, 2000).
Aluminum Carboxylate
Aluminum-carboxylate-based DRAs are non-polymeric drag-reducing agents. These additives are not subject to shear degradation and do not cause undesirable changes in the emulsion or fluid quality of the fluid being treated, or undesirable foaming.
The compositions consists of an aluminum carboxylate and fatty acids. The aluminum carboxylates are selected from aluminum salts of fatty acids, including octoates, stearates, oleates, or naphthenates (Jovancicevic et al., 2007). The fatty acids are selected from long chain carboxylic acids. Aluminum salts of a combination of short and long chain carboxylic acids may provide an optimum balance between drag reduction and change in viscosity.
Almond, N.E., Pipeline flow improvers, In: Proceedings Volume, API Pipeline ConferenceDallas, April 17–18, 1989. (1989), pp. 307–311.
Al-Sarkhi, A., Drag reduction with polymers in gas-liquid/liquid-liquid flows in pipes: a literature review, J. Nat. Gas Sci. Eng. 2 (1) (2010) 41–48.
Aubanel, M.L., Bailly, J.C., 1987. Amorphous high molecular weight copolymers of ethylene and alpha- olefins. EP Patent 243 127, October 28, 1987.
Bewersdorff, H.W.; Ohlendorf, D., The behaviour of drag-reducing cationic surfactant solutions, Colloid Polym. Sci. 266 (10) (1988) 941–953.
Brod, M., Venables, P., Lota, G.S., 1990. Crude and heavy fuel flow improvers. AU Patent 603 180, assigned to Exxon Chemical Patents In, November 08, 1990.
Chang, H.F.D.; Meng, J.S., Prediction of drag reduction from rheological properties of dilute polymer solutions, Physicochem. Hydrodyn. 9 (1987) 33.
Deshmukh, S.R.; Chaturved, P.N.; Singh, R.P., Novel biodegradable flocculants based on polysaccharides, J. Appl. Polym. Sci. 30 (1985) 4013–4420.
Dindi, A., Johnston, R.L., Lee, Y.N., Massouda, D.F., 1996. Slurry drag reducer. US Patent 5 539 044, assigned to Conoco Inc., July 23, 1996.
Eaton, G.B., Monahan, M.J., 1999. Composition of and process for forming polyalphaolefin drag reducing agents. US Patent 5 869 570, assigned to Energy & Environ. Int. Lc, February 09, 1999.
Fairchild, K., Tipton, R., Motier, J.F., Kommareddi, N.S., 1997. Low viscosity, high concentration drag reducing agent and method therefor. WO Patent 9 701 582, assigned to Baker Hughes Inc., January 16, 1997.
Gampert, B.; Wagner, P., The Influence of Polymer Additives on Velocity and Temperature Fluid. (1985) Springer-Verlag, Berlin, Germany; p. 71.
Gessell, D.E., Washecheck, P.H., 1990. Composition and method for friction loss reduction. US Patent 4 952 738, assigned to Conoco Inc., August 28, 1990.
Goudy, C.F.L., How flow improvers can reduce liquid line operating costs, Pipe Line Gas Ind. 74 (6) (1991) 49–51.
Hong, C.; Zhang, K.; Choi, H.; Yoon, S., Mechanical degradation of polysaccharide guar gum under turbulent flow, Ind. Eng. Chem. 16 (2) (2010) 178–180.
Hostetler, D.E., Kostelnik, R.J., Shanti In, Z.J., 1989. Polymerization process. US Patent 4 845 178, July 04, 1989.
Hoyt, J.W.; Sellin, R.H.J., Drag reduction by centrally-injected polymer “threads”, Rheol. Acta 27 (5) (1988) 518–522.
Hoyt, J.W.; Sellin, R.H.J., Polymer “threads” and drag reduction, Rheol. Acta 30 (4) (1991) 307–315.
Johnston, R.L., Lee, Y.N., 1998. Nonaqueous drag reducing suspensions. WO Patent 9 816 586, assigned to Conoco Inc., April 23, 1998.
Jovancicevic, V., Campbell, S., Ramachandran, S., Hammonds, P., Weghorn, S.J., 2007. Aluminum carboxylate drag reducers for hydrocarbon emulsions. US Patent 7 288 506, assigned to Baker Hughes Incorporated (Houston, TX), October 30, 2007.
Kang, C.; Jepson, W.P.; Gopal, M., The effect of drag reducing agents on corrosion in multiphase flow, In: Proceedings Volume, NACE International Corrosion Conference (Corrosion 98)San Diego, March 22–27, 1998. (1998); Paper Number 98054, pp. 1–11.
Kommareddi, N.S., Rzeznik, L.J., 1999. Microencapsulated drag reducing agents. WO Patent 9 937 396, assigned to Baker Hughes Inc., July 29, 1999.
Kommareddi, N.S., Rzeznik, L.J., 2000. Microencapsulated drag reducing agents. US Patent 6 126 872, assigned to Baker Hughes Inc., October 03, 2000.
Kostic, M., On turbulent drag and heat transfer reduction phenomena and laminar heat transfer enhancement in non-circular duct flow of certain non-newtonian fluids, Int. J. Heat Mass Transfer 37 (1994) 133–147.
Kowalik, R.M., Duvdevani, I., Kitano, K., Schulz, D.N., 1986. Drag reduction agents for hydrocarbon solutions. US Patent 4 625 745, assigned to Exxon Research & Eng. Co., December 02, 1986.
Kowalik, R.M.; Duvdevani, I.; Lundberg, R.D.; Schulz, D.N.; Peiffer, D.G.; Kitano, K., Enhanced drag reduction via interpolymer associations, J. Nonnewton. Fluid Mech. 24 (1) (1987) 1–10.
Li, Z., Effect of fluid viscoelasticity on isolated eddy transmission, J. Univ. Pet. China 15 (5) (1991) 33–38.
Mack, M.P., 1986. Improved use of flow improvers. EP Patent 196 350, October 08, 1986.
Malchow Jr., G.A., 1997. Friction modifier for oil-based (invert) well drilling fluids and methods of using the same. US Patent 5 593 953, assigned to Lubrizol Corp., January 14, 1997.
Malik, S.; Mashelkar, R.A., Hydrogen bonding mediated shear stable clusters as drag reducers, Chem. Eng. Sci. 50 (1) (1995) 105–116.
Malik, S., Shintre, S.N., Mashelkar, R.A., 1992. Process for the preparation of a new polymer useful for drag reduction in hydrocarbon fluids in exceptionally dilute polymer solutions. US Patent 5 080 121, assigned to Council Sci & Ind. Researc, January 14, 1992.
Malik, S., Shintre, S.N., Mashelkar, R.A., 1996. A polymer useful for drag reduction in hydrocarbon fluids and its preparations. EP Patent 471 116, assigned to Council Sci & Ind. Researc, March 06, 1996.
Mansour, A.R., Aldoss, T., 1988. Drag reduction in pipes carrying crude oil using an industrial cleaner. SPE Unsolicited Pap. (17918-MS), pp. 1–12.
Martischius, F.D., Raab, B., Karau, D., 1990. Process for improving the drag reducing properties of high-molecular weight polymer solutions in crude oil or refined products (verfahren zur verbesserung der fliesswiderstandsvermindernden eigenschaften hochmolekularer polymerloesungen in rohoel oder raffinerieprodukten). EP Patent 397 002, assigned to BASF AG, November 14, 1990.
Milligan, S.N., Harris, W.F., Smith, K.W., Burden, T.L., Johnston, R.L., Anderson, V.S., 2008. Remote delivery of latex drag-reducing agent without introduction of immiscible low-viscosity flow facilitator. US Patent 7 361 628, assigned to ConocoPhillips Company (Houston, TX), April 22, 2008.
Morris, I., Perry, G., 2001. High pressure storage and transport of natural gas containing added C2 or C3, or ammonia, hydrogen fluoride or carbon monoxide. US Patent 6 217 626, assigned to Jl Energy Transport Inc., April 17, 2001.
Moussa, T.; Tiu, C., Factors affecting polymer degradation in turbulent pipe flow, Chem. Eng. Sci. 49 (10) (1994) 1681–1692.
Muth, C.L.; Kolby, S.M., Cost saving by use of flow improver, In: Proceedings Volume, 13th International Pipeline Technology ConferenceHouston, February 5–7, 1985. (1985), pp. 353–357.
Naiman, M.I., Chang, J.C., 1991. Methods and compositions for reduction of drag in hydrocarbon fluids. US Patent 4 983 186, assigned to Petrolite Corp., January 08, 1991.
Oh-Kil, K.; Ling-Siu, C., Drag reducing polymers, In: (Editor: Joseph, C.S.) Concise Polymeric Materials Encyclopedia (1996) CRC Press, Inc., Boca Raton, FL, pp. 395–397.
Peiffer, D.G., Kowalik, R.M., Lundberg, R.D., 1987. Drag reduction with novel hydrocarbon soluble polyampholytes. US Patent 4 640 945, February 03, 1987.
Ritter, W., Meyer, C., Zoellner, W., Herold, C.P., Tapavicza, S.V., 1991a. Copolymers of (meth)- acrylic acid esters as flow improvers in oils. US Patent 5 039 432, assigned to Henkel KG Auf Aktien, August 13, 1991.
Ritter, W., Meyer, C., Zoellner, W., Herold, C.P., von Tapavicza, S., 1989a. Use of selected acrylic and/or methacrylic acid ester copolymers as flow enhancers in paraffin-rich crude oil and crude oil fraction (ii) (verwendung ausgewaehlter copolymeren der acryl- und/oder methacrylsaeureester als fliessverbesserer in paraffinreichen erdoelen und erdoelfraktionen (ii)). EP Patent 332 002, September 13, 1989.
Ritter, W., Meyer, C., Zollner, W., Herold, C.P., von Tapavicza, S., 1991b. Copolymers of acrylic acid and/or methacrylic acid esters as flow improvers. AU Patent 611 265, assigned to Henkel KG Auf Aktien, June 06, 1991.
Ritter, W., Pietsch, O., Zoellner, W., Herold, C.P., von Tapavicza, S., 1989b. Use of selected acrylic and/or methacrylic acid ester copolymer versions as flow enhancers in paraffin-rich crude oil and crude oil fractions (i) (verwendung ausgewaehlter copolymertypen der acryl- und/oder methacrylsaeureester als fliessverbesserer in paraffinreichen erdoelen und erdoelfraktionen (i)). EP Patent 332 000, September 13, 1989.
Ritter, W., Pietsch, O., Zoellner, W., Herold, C.P., von Tapavicza, S., 1991c. Copolymers of acrylic and/or methacrylic acid esters as flow improvers. AU Patent 610 700, assigned to Henkel KG Auf Aktien, May 23, 1991.
Ritter, W., Pietsch, O., Zoellner, W., Herold, C.P., von Tapavicza, S., 1995. Copolymers of (meth) acrylic acid esters as flow improvers in petroleum oils. CA Patent 1 334 013, assigned to Henkel KG Auf Aktien, January 17, 1995.
Rossi, A., Chandler, J.E., Barbour, R., 1993. Polymers and additive compositions. WO Patent 9 319 106, assigned to Exxon Chemical Patents In, September 30, 1993.
Saether, G.; Kubberud, K.; Nuland, S.; Lingelem, M., Drag reduction in two-phase flow, In: Proceedings Volume, 4th Bhra Multi-Phase Flow International ConferenceNice, Fr, June 19–21, 1989. (1989), pp. 171–184.
Schulz, D.N., Maurer, J.J., Bock, J., Kowalik, R.M., 1986. Process for the formation of novel acrylamide acrylate copolymers. CA Patent 1 213 606, November 04, 1986.
Schulz, D.N., Maurer, J.J., Bock, J., Kowalik, R.M., 1987. A process for the formation of novel acrylamide acrylate copolymers. EP Patent 116 779, January 14, 1987.
Shinomura, T., 1988. Method of reducing friction losses in flowing liquids. US Patent 4 751 937, June 21, 1988.
Smith, K.W., Haynes, L.V., Massouda, D.F., 1995. Solvent free oil soluble drag reducing polymer suspension. US Patent 5 449 732, assigned to Conoco Inc., September 12, 1995.
..................Content has been hidden....................
You can't read the all page of ebook, please click here login for view all page.