Chapter 4. Lubricants
One of the greatest challenges in the formulation of specialty lubricants for drilling applications is the prevention of drill bit bearing wear in subterranean formations. In such applications, lubrication takes place in an abrasive environment of mud and rock particles deep below the earth's surface.
The journal bearings are subject to extremely high loads, because the bit generally turns at slow speeds and has the weight of the drill string on top of it. Furthermore, there is shock loading due to the bouncing and vibrating of the drill string (Willey et al., 2007).
Synthetic Greases
There are monographs on the issues of synthetic greases, including their application in petroleum industries (Rudnick, 2006). Synthetic greases have considerable advantages over conventional hydrocarbon-based greases including (Willey et al., 2007):
• High viscosity with good pumpability,
• Lower torque,
• Ability to function at lower operating temperatures, and
• Excellent thermal and oxidative stability.
Many of these advantages arise from the use of controlled synthesis, which yields products with exact properties. These benefits have led to the development of many commercial synthetic types of grease for a variety of uses.
Rock bit bearings are generally lubricated with greases to assist the seals in keeping out the drilling muds. These greases are prepared by adding a thickener to a lubricating oil.
Thickeners consist mostly of metal soaps, formed by the saponification of fatty oils into the corresponding fatty acids then subsequent neutralization with a metal hydroxide. A grease formulation typically includes various additives (Willey et al., 2007):
• Extreme pressure,
• Anti-wear,
• Corrosion,
• Solubility,
• Anti-seize protection, and
• Oxidation protection.
Base Fluids
Synthetic base fluids are low molecular weight poly-α-olefins (PAOs) with a high viscosity index (Willey et al., 2007). Preferred blends typically combine a high viscosity and a low viscosity component. Preferably, the high viscosity index PAO is the high viscosity component and the low viscosity component is an alkylated naphthalene.
So-called unconventional base stocks can be used as additional base fluids. These fluids are hydroprocessed, highly refined paraffinic base stocks, containing extremely low amounts of aromatic compounds, and sulfur and nitrogen levels relative to conventional hydroprocessed and solvent refined base stocks. They exhibit a high resistance to oxidation and thermal degradation, very high viscosity indices, superior viscosity and film strength at high temperatures, substantially reduced volatility, and improved lubricity (Willey et al., 2007).
Dibasic acid esters also exhibit good thermal stability, but are usually used in combination with additives to enhance the resistance to hydrolysis and oxidation. Polyol esters contain two or more alcohol moieties, such as trimethylolpropane, neopentylglycol, and pentaerythritol esters. They are the reaction product of a fatty acid derived from either animal or plant sources and a synthetic polyol and have excellent thermal stability, and generally resist hydrolysis and oxidation better than other base stocks.
Naturally occurring triglycerides or vegetable oils are in the same chemical family as polyol esters. However, polyol esters tend to be more resistant to oxidation than such oils, and thus tend to function better under severe conditions and high temperatures. The instability normally associated with vegetable oils is generally due to their high content of linoleic and linolenic fatty acids, both of which are unsaturated compounds. As the degree of unsaturation in the fatty acids in vegetable oils increases, the resulting esters tend to be less thermally stable (Willey et al., 2007). Ester-based lubricants have some advantages over oil-based lubricants (Rudnick, 2006, p. 71) in that they:
Extreme Pressure Agents
Extreme pressure agents (EPs) are additives for lubricants that decrease the wear on parts gears that are exposed to very high pressures. They modify the surface of the exposed parts under high pressure conditions by a chemical reaction. EPs include:
• Sulfurized fatty compounds and hydrocarbons,
• Chlorinated hydrocarbons,
• Chlorendic acid esters,
• Polymeric esters,
• Polysulfides, and
• Molybdenum compounds.
There are two types of EP (Willey et al., 2007); firstly compounds that become active at high temperatures, such as lead dithiocarbamate, organosulfur compounds, and organophosphorus sulfur compounds, and secondly inorganic EPs such as molybdenum disulfide, graphite, metal oxides, and powered metals such as copper and lead. The particles of solid EPs act by forming layers between the two bearing surfaces, and so protect them under load by sliding against each other in a way that is similar to cards in a stack sliding against each other. Solid EPs improve the load carrying capacity, but they contribute to excessive seal and hub wear and drill bit seal failure.
Drill bit lubricant compounds containing a copper EP caused seal failures due to copper that was deposited close to the seal area. This accumulated near the seal area until it became abraded by the contact with the copper deposit. Eventually, the grease composition is ejected from the journal area, and a metal-to-metal contact occurs between the roller cone and the journal, which causes a drill bit failure.
On the other hand, lubricants that reduce seal and hub wear do not have a sufficient film strength, i.e., load carrying capacity, to be used as drill bit lubricants. In general, any additives made up from heavy metal complexes exhibit an adverse environmental impact.
Alteratively, zirconium 2-ethylhexanoate or bismuth 2-ethylhexanoate can be added in amounts up to 20% as an additive. These compounds are non-toxic, which gives easier handling in the storage, use, and final deposit of the lubricant.
In addition, zirconium compounds impart some corrosion resistance to metal surfaces, which contributes to reducing the hub wear, and improves the sealing properties. Seals may also wear out more slowly when a zirconium-based lubricant is used.
Zirconium compounds also modify the viscosities of the base stock, which will extend the range of operating temperatures.
Anti-seize Agents
Anti-seize compounds are used to mitigate damage from high bearing stresses by providing a dissimilar metal or other material between like substrates. Such a compound inhibits the welding that may otherwise occur from the temperatures, pressures, and stresses normally incurred during proper make-up (Oldiges and Joseph, 2003).
Conventional anti-seize thread compounds include greases, which contain substantial amounts of heavy metals or their oxides, carbonates, or phosphates. The metals include (Oldiges and Joseph, 2003):
• Copper,
• Zinc,
• Lead,
• Nickel,
• Molybdenum, and
• Aluminum.
However, environmental regulations have begun to discourage or prohibit the use of anti-seize compounds that contain such materials. Organic fluid additives containing antimony, zinc, molybdenum, barium, and phosphorus have also become the subject of environmental scrutiny, partly because of the way that they are used.
Oil field threaded connections are usually coated with an excess amount of the thread compound to ensure complete connection coverage. The excess compound is sloughed off and ends up downhole, where it is then included with the other materials pumped out of the wellhole and into a containment area. From there, materials contaminated with heavy metals must be removed and deposited to a hazardous waste disposal site.
Alternatively, threaded connections can be protected by coating the threads, prior to their make-up, with a solvent-thinned, resin-based coating and bonding composition, composed of a suspending agent, a bonding agent, a thinning agent, and a metallic flake. The coated threads are dried to bond the coating and the bonding composition to the threads. Prior to their make-up, the threads are coated with an excess of an environmentally friendly lubricating composition. A typical formulation is shown in Table 4.1.
| Ingredient | Amount/[%] |
|---|---|
| Suspending agent, ethyl cellulose | 2 |
| Bonding agent, silicone thermoset | 6 |
| Thinning agent (xylene/trichloroethane) | 84 |
| Seizing-agent, copper flakes | 8 |
Using such a method, the anti-seize metallic film adheres to the thread surface to provide an anti-seize protection while minimizing the amount of metal emitted, since only thread wear discharges metal into the environment. Thus the metal contamination is substantially reduced in comparison to conventional methods.
In summary, the use of anti-seize metallic films, in conjunction with environmentally friendly lubricating compositions reduces the potential for environmental damage, while still providing an optimum protection in critical operations.
Fluorides of alkaline or earth alkaline metals such as CaF2 have been proposed as anti-seize agents for nickel and chrome ferrous alloys that are prone to galling under high contact stress (Oldiges et al., 2006). Typical grease compositions are formulated as shown in Table 4.2.
| Ingredient | Amount/[%] | Examples |
|---|---|---|
| Thixotropic base material | 40–90 | Metal salt complex greases |
| Metal complex grease | 50–90 | Lithium complex grease, aluminum calcium complex grease |
| Anti-seize agents | 5–50 | Metal fluorides |
| Boundary lubricant | 5–50 | Metal borates, molybdates, carbonates, acetates, stearates, etc. |
| Friction adjusters | 0–12 | polytetrafluoroethylene (PTFE), graphitic materials, natural or powders and synthetic fibers, molybdenum disulfide, fibers etc. |
| Anti-wear additives | 0–5 | Sulfurized isobutylene, phosphate esters, dithiocarbamates, dithiophosphates, naphthanates, or the like |
| Anti-degradant additives | 0–2 | Antioxidants and antiozonants |
Anti-wear Additives
Anti-wear additives are divided into two categories (Willey et al., 2007): those activated at a lower temperature than EPs, such as zinc dialkyl dithiophosphate, sorbitan monoleate, chlorinated hydrocarbons, and phosphate esters, and those that become active at lower loads than EPs, PTFE, and antimony trioxide.
Metal Deactivators
Metal deactivators protect against nonferrous corrosion and in some cases also against ferrous corrosion. Common metal deactivators are based on benzotriazole. Ferrous corrosion inhibitors include organic acids and esters, phenolates, and sulfonates (Willey et al., 2007).
Solubility Aids
Solubility aids make the additives dissolve in the oil or the soap. Common solubility aids include esters, such as trimellitic acid esters (Willey et al., 2007).
Antioxidants
Common antioxidants used in grease formulations include zinc dialkyl dithiophosphates, amine phosphates, aromatic amines, phenothiazine, or hindered phenols, such as tert-butylhydroxytoluene.
It is generally preferred not to employ a zinc dialkyl dithiophosphate antioxidant in the lubricating grease if the rock bit comprises an incompatible metal. They are, however, used in other lubricating applications (Willey et al., 2007).
Base stocks
A wide variety of grease compositions are known and often made from refined petroleum or hydrocarbon base stock, which gives them a low viscosity, and provides the base lubricity of the composition. The base stock may constitute about three-quarters of the total grease composition.
This base stock is thickened with a conventional metal soap or a metal complex soap. Light base stocks with low viscosity are used for low temperature greases, and heavier, higher viscosity base stocks are used for high temperature greases (Denton et al., 2007). In order to enhance the film lubricating capacity of base stocks, solid additives such as molybdenum disulfide, copper, lead, or graphite can be added (Newcomb, 1982).
Synthetic polymer EPs and high viscosity synthetic polymers may also be used. Such materials enhance the ability of the lubricant base stock to form a friction-reducing film between the moving metal surfaces under conditions of extreme pressure, and to increase the load carrying capacity of the lubricants.
Lubricant Compositions
Molybdenum disulfide
Molybdenum disulfide is used traditionally in greases for bit lubrication. In addition, polymers of 2-methylpropene (i.e., isobutene) and metal soaps are used to formulate synthetic greases (Denton and Fang, 1996).
A viscosity of 600–750 cP at 120°C is desirable. However, in the severe environment of a rock bit bearing, the viscosity of the composition should be at least 200 cP at 100°C (Delton and Hooper, 1994). Other heavy-duty greases based on molybdenum sulfide also contain calcium fluoride (Landry and Koltermann, 1991a and Landry and Koltermann, 1991b) and metal soaps as thickeners.
Specialized lubricating greases have been developed for the bearing assemblies of roller bits. They are prepared from petroleum oils thickened with alkali and alkaline earth metal soaps (Lyubinin et al., 1995). The greases contain additives and fillers, such as synthetic dichalcogenides of refractory metals, which exhibit the necessary service characteristics. Tests have shown that such greases out perform the initial grease by 7 to 12 times (Table 4.3).
| aSealing lubricant down to −40°C | |
| bSealing lubricant | |
| cWith metal soaps and Aerosil® as thickener | |
| dReaction product from pentaerythrite and paraformalehyde | |
| eEnvironmentally safe lubricating additives | |
| fAs mud additive for lubrication | |
| gAntioxidizing and anticorrosion additive to lubricating oils | |
| Compound | References |
|---|---|
| Carbon black, fatty acid esters | Runov et al. (1992) |
| Sodium ethyl siliconate based compositionsa | Goncharov et al. (1993) |
| Polyacrylamide, carboxymethyl cellulose, gypsumb | Kalashnikov (1994) |
| Olefinsc | Halliday and Schwertner (1997); Koltermann and Willey, 1999 and Koltermann and Willey, 2000 |
| 2,4,8,10-tetra-oxaspiro-5, 5-undecaned | Frolov et al. (1993) |
| Phosphatides or phospholipidse | Garyan et al. (1998) |
| Polypropylene glycolf | Enright et al. (1991); Fang et al. (1998); Sano (1997) |
| Fluoropolymers and zinc dioctyl phenyl dithiophosphateg | Chanshev et al. (1992) |
Polarized Graphite
Because of environmental concerns, molybdenum disulfide, regarding alternative compositions for solid lubricants have been developed. These compositions consist of graphite, sodium molybdate, and sodium phosphate (Holinski, 1995; Zaleski et al., 1998), or, more recently, polarized graphite.
Polarized graphite can be used as a lubricant additive for rock bits, since it exhibits extremely good load carrying ability and anti-wear performance.
Graphite consists of carbon in a layered structure, and its lack of polarity prevents graphite powder from forming a lubricant film and adhering to metal surfaces. The polarization of graphite results allows it to adhere to metal and thus form a lubricant film that can carry extremely high loads without failure.
Ordinary graphite has a laminar hexagonal crystal structure and the closed rings of carbon atoms do not normally have any electrical polarization. Hence, graphite has good lubricity because the layers may slip or shear readily, but the lack of polarity leads to a poor adhesion to metal surfaces.
Graphite can be treated with alkali molybdates or tungstenates to impart a polarized layer at its surface. Alternating positive and negative charges are formed. The treated graphite shows an extremely good load carrying capacity and anti-wear performance, somewhat similar to molybdenum disulfide, as well as a good adhesion of particles on metal surfaces and good film-forming properties (Denton and Lockstedt, 2006).
Ellipsoidal Glass Granules
The use of ellipsoidal glass granules instead of spherical glass beads increases the contact surface of antifrictional particles, reduces their ability to penetrate deeply into the mud cake, and increases their breaking strength (Kurochkin et al., 1990; Kurochkin et al., 1992a and Kurochkin et al., 1992b; Kurochkin and Tselovalnikov, 1994).
Calcium-Sulfonate-based Greases
Drilling muds have been changed significantly over the last few years due to environmental pressures and the increase in drilling operations in more extreme environments, where conventional grease formulations cannot withstand the enhanced demands. For these reasons, new materials have appeared that (Oldiges et al., 2007):
• Adhere more effectively to the threaded connections,
• To not degel at elevated temperatures and higher pH levels, and
• Impart galling resistance and corrosion resistance.
Typical grease thickeners are calcium acetate, lithium stearate, lithium 12-hydroxystearate, anhydrous and hydrous calcium soaps, sodium soaps, organophilic clays, and silica. It is important for a grease to be stable over a range of pHs, since the pH of drilling mud increases as oil well depths increase.
During drilling operations, the threaded connections are exposed to drilling fluids, which include drilling muds and shavings from the drilling operations. These fluids and shavings tend to dissolve, erode, or ablate the grease compounds thus removing their protection and increasing the likelihood of damage to the threaded connections.
Calcium-sulfonate-based grease formulations are a suitable alterative to conventional greases. These high performance sulfonate greases are superior carriers for controlled friction properties in oil field drilling and production thread compounds.
By reducing the thickener content, a cost-competitive calcium sulfonate grease can be formulated. Preferred base stocks include PAOs, polybutenes, polyolesters, vegetable oils, and animal oils (Oldiges et al., 2007). Thixotropic greases or grease-like overbased calcium sulfonate compositions have corrosion inhibiting properties (Olson et al., 1994).
When a drill bit is used in hard, tough formations, high pressures and temperatures are encountered. The total useful life of a drill bit in such severe environments is in the order of 20–200 h for bits of 6–28 in diameter, at depths of about 1,500–6000 m. Useful lifetimes of about 65–150 h are typical. When a drill bit wears out or fails as a borehole is being drilled, it is necessary to withdraw the drill string to replace the bit, which is a very expensive process. Prolonging the lives of drill bits minimizes the lost time in round tripping the drill string for replacing them (Denton et al., 2007).
The replacement of a drill bit can be required for a number of reasons, including wearing out or breakage of the structure contacting the rock formation. The journal bearings on which the roller cones are mounted may fail or wear severely. These bearings are lubricated with special formulations so that they may survive the severe conditions. A lubrication failure can sometimes be attributed to misfit of bearings or seal failure, as well as a problem with the grease itself (Denton et al., 2007).
Paraffins
Purified paraffins are non-toxic and biodegradable (Halliday and Clapper, 1998) and may be used as lubricants, rate of penetration enhancers, or spotting fluids for water-based drilling mud (WBM).
Olefins
Olefin isomers containing 8–30 carbon atoms are suitable, but isomers having fewer than 14 carbon atoms are more toxic, and isomers having more than 18 carbon atoms are more viscous. Therefore olefin isomers having 14–18 carbon atoms are preferred (Halliday and Schwertner, 1997).
Phospholipids
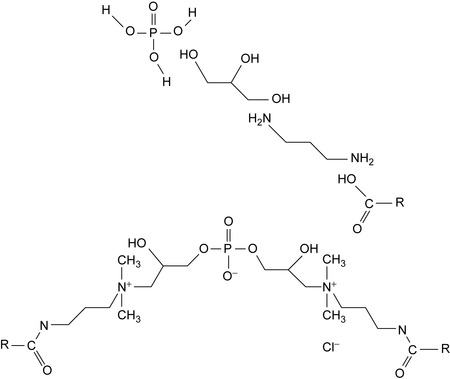
In aqueous drilling fluids, phospholipids are effective lubricating agents (Patel et al., 2006). They are naturally occurring compounds, for example, lecithin belongs to the class of phospholipids. An introduction to phospholipid chemistry was given by Hanahan (1997). They also find use as polymers (Nakaya and Li, 1999). The structural units of a phospholipid are shown in Figure 4.1.
Because of their ionic nature, some phospholipids are soluble in water. A preferred compound as lubrication additive for aqueous drilling fluids is cocoamidopropyl propylene glycol diammonium chloride phosphate (Patel et al., 2006). Phosphatides or phospholipids are environmentally safe lubricating additives (Garyan et al., 1998).
Alcohols
Alcohol Glucoside Mixture
Many oil-based fluids, or additives for such fluids, have caused environmental concerns and tend to be more costly than aqueous-based fluids, so aqueous-based fluids are often preferred (Fisk et al., 2006). However, these fluids tend to have more lubricity problems and adverse effects on the subterranean formation, such as causing swelling of clays, than encountered with oil-based fluids.
Silicate-based aqueous drilling fluids have long been known to inhibit the formation damage caused by water, but are also known to have poor lubricity properties. Lubricants commonly known and used in WBMs do not provide good lubricity in silicate muds (Fisk et al., 2006).
Recently, silicic-acid-based drilling fluids have been found to provide a membrane-efficient WBM, but they have high torque and drag values. Moreover, traditional mud lubricants show little to no effect in a high pH, silicic-acid-based mud.
The reaction mechanism for the polymerization of silicic acids derivatives is shown in Figure 4.2. A lubricant composition has been developed for silicicacid- based drilling fluids (Fisk et al., 2006), comprising 2-octyldodecanol, and 2-ethylhexylglucoside (Fisk et al., 2006). Alternative alcohols include oleyl alcohol, stearyl alcohol, and polyetherglycols.
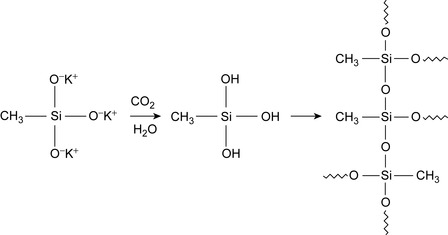 |
| Figure 4.2 Polymerization of silic acid Fleury et al. (1999). |
It is believed that the alcohol serves as the more active lubricant, and the alkylglucoside serves primarily as a wetting agent. These compounds exhibit their wetting properties even at high pH. Summarizing, the lubricant compositions is effective in a high pH environment, has low toxicity, and is environmentally acceptable.
Partial Glycerides
Historically, pure water-based systems are the oldest in the development of drilling fluids. However, their use is attended by such serious disadvantages that only limited application has been possible in technically demanding drilling operations. Most importantly, the interaction of the water-based drilling fluids with the water sensitive layers of rock, especially the layers of clay, leads to unacceptable interference with the drilling process (Müller et al., 2004a).
It has, however, been observed that, even in highly sensitive shale formations, adequate stability can be obtained in the case of purely water-based drilling fluids if soluble alkali metal silicates, i.e., water-glasses are used.
However, using water-based drilling fluids requires the addition of lubricants including mineral oils, animal and vegetable oils, and esters. Regulations with regard to the biodegradability of drilling fluids and their constituents are gradually restricting the use of otherwise particularly suitable mineral oils.
Fatty acid partial glycerides have been found to be lubricants suitable for both water-based and oil-based drilling muds (OBMs) for use at low temperatures. Basic WBMs and OBMs that have been used for testing the lubricants are given in Table 4.4.
| Water-based | Oil-based | ||
|---|---|---|---|
| Water | 4 l | Mineral oil | 675 ml |
| Xanthan gum | 20 g | Water | 225 ml |
| Bentonite | 56 g | CaCl2 | 95 g |
| Carboxymethyl cellulose | 40 g | Emulsifier | 35 g |
| Barite | 1.8 g | Fluid loss additive | 10 g |
| Viscosifier | 25 g | ||
| Lime | 17 g | ||
| Barite | 360 g | ||
The effectiveness of the lubricants can be measured by the Almen-Wieland test (Buyanovskii, 1994), the Falex pin and vee block method (ASTM, 2009), the Timken wear and lubricant test (ASTM, 2008), and the four ball test (Totten et al., 2003). The effect of various lubricants has been measured as shown in Figure 4.3.
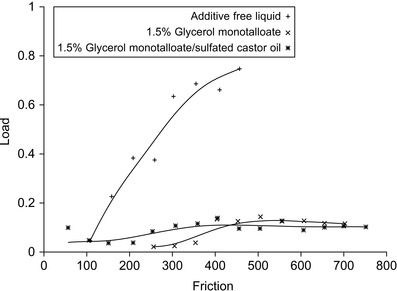 |
| Figure 4.3 Effect of various lubricants (arbitrary units) (Müller et al., 2004a). |
Aminoethanols
High pH values affect the stability of lubricating products, in particular those based on conventional esters, which hydrolyze at high pHs and temperatures, so instead of alcohols, amino alcohols can be used. For example, a lubricating composition has been synthesized by the reaction of polymerized linseed oil with diethanol amine at 160°C. A product with a viscosity of around 2,700 mPas at 40°C is obtained (Argillier et al., 2004), or this can be reduced by adding some methyl oleate to the reaction product.
When added to a silicate mud, good lubricating properties are obtained, even up to a pH of 12. Tests revealed that the addition of 3% of a lubricant to a base mud reduced the torque readings by 50%.
Polymeric Alcohols
Synthetic PAOs are non-toxic and effective in marine environments when used as lubricants, return-of-permeability enhancers, or spotting fluid additives for WBMs.
Both polyalkylene glycol (Alonso-Debolt et al., 1999) and side chain polymeric alcohols such as polyvinyl alcohol (PVA) have been suggested. These substances are comparatively environmentally safe (Penkov et al., 1999; Sano, 1997).
PVAs may be applied as they are, or in crosslinked form (Audebert et al., 1996). Crosslinkers can be aldehydes, e.g., formaldehyde, acetaldehyde, glyoxal, and glutaraldehyde, to form acetals, maleic acid or oxalic acid to form crosslinked ester bridges, or dimethylurea, polyacrolein, diisocyanate, and divinylsulfonate (Audebert et al., 1994 and Audebert et al., 1998).
An amine-terminated polyoxyalkylene with an average molecular weight of 600–10,000 Dalton can be acylated with a succinic acylating agent, e.g., hexadecenyl succinic anhydride or a Diels-Alder diacid, obtained from an unsaturated fatty acid (Forsberg and Jahnke, 1993a and Forsberg and Jahnke, 1993b). Similarly, alkyl-aryl sulfonate salts can be used in lubrication (Naraghi and Rozell, 1996).
The pendant hydroxy groups of ethylene oxide-propylene oxide copolymers of dihydroxy and trihydroxy alcohols may be sulfurized to obtain a sulfurized alcohol additive. This is effective as a lubricant when used in combination with oils and fats (Clark and Dye, 1997; Dye et al., 1995). The sulfurized alcohols may be obtained by the reaction of sulfur with an unsaturated alcohol.
Fatty alcohols and their mixtures with carboxylic acid esters have also been proposed as lubricant components (Müller et al., 1999a).
Ethers
2-Ethylhexanol can be epoxidized with 1-hexadecene epoxide. This additive also helps reduce or prevent foaming. By eliminating the need for traditional, oil-based components, the composition is non-toxic to marine life, biodegradable, environmentally acceptable, and capable of being disposed of at the drill site without the need for costly disposal procedures (Alonso-Debolt et al., 1995).
Esters
Esters are compounds of interest, as alternatives with better biodegradability. Some are listed in Table 4.5.
| Ester Compound | References |
|---|---|
| 2-Ethylhexyl oleate | Chapman and Ward (1997) |
| Triglyceride oil | Chapman and Ward (1997) |
| Soya oil sulfonate | Müller et al. (2004) |
| Glycerol monotalloate | Müller et al. (2004) |
| Sulfonated castor oil | Müller et al. (2004) |
The use of esters in water-based systems, particularly under highly alkaline conditions, can lead to considerable difficulties. Ester cleavage can result in the formation of components with a marked tendency to foam, which then introduces unwanted problems into the fluid systems.
Sulfonates of vegetable oils, in particular soya oil sulfonate, are also used as lubricants. Soya oil sulfonate can be used in water- and oil-based systems, but shows significant foaming, especially in water-based fluids, which restricts its usefulness (Müller et al., 2004a).
A lubricating composition that comprises components that can be obtained from by-products of manufacturing processes, so providing a use for them would also be advantageous (Breeden and Meyer, 2005).
A lubricating composition has been proposed that is obtained by reacting glycerol component comprising glycerol, glycerol oligomers, and a fatty acid component. The reaction product is neutralized with potassium hydroxide or ammonium hydroxide. The composition of the glycerol component is shown in Table 4.6.
| Compound | % |
|---|---|
| Glycerine | 10–13 |
| Diglycerine | 16–23 |
| Triglycerine | 5–7 |
| Tetraglycerine | 4–6 |
| Pentaglycerine | 3–4 |
| Heavier polyglycerines | 15 |
| NaCl | 2–4 |
| Na2CO3 | 0.3–1 |
| Water | 22–28 |
| Carboxylic acid salt | 11–14 |
The fatty acid component can be obtained from vegetable oils, wood pulp processing, animal fats processing, etc.
Catalysts for esterification include sulfuric acid, hydrochloric acid, nitric acid, and p-toluene sulfonic acid, although concentrated sulfuric acid is preferred (Breeden and Meyer, 2005). The composition can also be subjected to chain extension using diacids, such as maleic acid, succinic acid, or glutaric acid (Breeden and Meyer, 2005).
Ester-based Oils
Several ester-based oils are suitable as lubricants (Durr et al., 1994; Genuyt et al., 2001), as are branched chain carboxylic esters (Senaratne and Lilje, 1994). Tall oils can be transesterified with glycols (Runov et al., 1991) or condensed with monoethanolamine (Andreson et al., 1992). The ester class also comprises natural oils, such as vegetable oil (Argillier et al., 1999), spent sunflower oil (Kashkarov et al., 1998 and Kashkarov et al., 1997; Konovalov et al., 1993a and Konovalov et al., 1993b), and natural fats, for example, sulfonated fish fat (Bel et al., 1998). In WBM systems no harmful foams are formed from partially hydrolyzed glycerides of predominantly unsaturated C16 to C24 fatty acids.
The partial glycerides can be used at low temperatures and are biodegradable and non-toxic (Müller et al., 2000). A mixture of long chain polyesters and polyamides (PAs) is suitable for high temperature applications (Wall et al., 1995a).
In the case of esters from, for example, neopentylglycol, pentaerythrite, and trimethylolpropane with fatty acids, tertiary amines, such as triethanol amine, together with a mixture of fatty acids, improve the efficiency (Argillier et al., 1997).
Ester Alcohol Mixtures
In addition to esters, mixtures of fatty alcohols with carboxylic acid esters have been proposed as lubricating additives in WBMs. The alcohols include guerbet and oleyl alcohols with oleyl oleate or isotridecyl stearate as the ester component (Müller et al., 2004b). Fatty alcohols exhibit a foam-suppressing effect.
Phosphate Esters
It has been found that including polyether phosphate esters and polyethylene glycol (PEG) can give aqueous drilling fluids, which provide good lubricating properties in a wide range of drilling fluids (Dixon, 2009).
Typical synthetic routes to such esters involve the reaction of the polyether with a phosphating agent such as phosphorus pentoxide or polyphosphoric acid. The use of polyphosphoric acid in the synthesis gives higher proportions of the monoester, which is preferred. The optimal molecular weight of the PEG is 400 Dalton (Dixon, 2009). The compatibility of the lubricant may be adversely affected by other components of the drilling fluid, particularly by divalent cations such as calcium (Dixon, 2008 and Dixon, 2009).
Biodegradable Compositions
A biodegradable lubricating composition has been proposed, based on an aliphatic hydrocarbon oil and a fatty acid ester (Genuyt et al., 2006). It is important that the hydrocarbon is not aromatic because petroleum cuts with a high aromatic compound content present a risk to marine life due to their toxicity.
The composition is used as a continuous oil phase in an invert emulsion in a petroleum drilling fluid or mud. It is particularly useful in offshore drilling in deep water, or in inclined or long-range drilling. In the case of deep water drilling, the temperature of the water is around 4°C, hence the viscosity of drilling fluids needs to be controlled.
Triglyceride esters of animal or plant fatty acids are biodegradable, but the use of these compounds in invert emulsion drilling fluids shows them to be extremely susceptible to hydrolysis, which results in unwanted changes in the viscosity of emulsions.
Esters of saturated or unsaturated monocarboxylic acids, e.g., isononanic acid and of longer chain alcohols, e.g., isoheptanol, 2-ethyl hexanol, or n-octanol (Müller et al., 1990) are less sensitive to hydrolysis. However, they retain some susceptibility, particularly at temperatures above 160°C, as are encountered in rock drilling and deep offshore drilling.
Esters that have been condensed from rapeseed fatty acids and 2-ethyl hexanolFinagreen® BMDF (Totalfina) claim to have even better properties (Genuyt et al., 2006). Rapeseed oil consists mainly of oleic and linoleic acids. Mineral hydrocarbon cuts with different properties have been used to which the esters have been added. Some properties of these cuts are given in Table 4.7.
| Property | Cut A | Cut E |
|---|---|---|
| Typical chain length | C13–C15 | C14–C18 |
| Flash point/[° C] | 101 | 116 |
| Flow point/[° C] | − 51 | − 15 |
| Aromatics/[%] | < 0.01 | 0.9 |
| n -Paraffins/[%] | 3 | 27 |
| i -Paraffins/[%] | 44 | 19 |
| Naphthenes/[%] | 53 | 53 |
| Aromatics/[%] | 0 | 0.9 |
The kinematic viscosity as a function of temperature of cuts A and E, and of a mixture consisting of 70% of these cuts and 30% of Finagreen® BMDF are given in Table 4.8.
| a30% Finagreen® BMDF | |||||
| Temperature/[°C] | |||||
|---|---|---|---|---|---|
| 0 | 10 | 20 | 30 | 40 | |
| Composition | Viscosity/[mm2s−1] | ||||
| Cut A | 5.92 | 4.6 | 3.5 | 2.8 | 2.28 |
| Cut E | 10.3 | 7.2 | 5.3 | 3.9 | 3.2 |
| Cut A + estera | 8.4 | 6.1 | 4.6 | 3.7 | 3.0 |
| Cut E + estera | 13.5 | 9.25 | 6.8 | 5.1 | 4.07 |
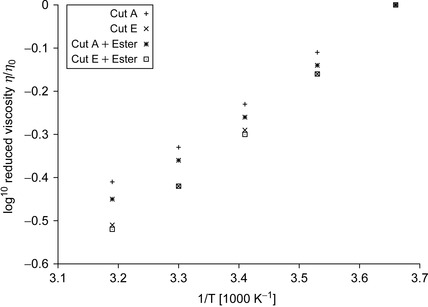
Inspection of the table reveals that cut A has a viscosity of 8.4mm2s−1 at 0°C, which is suitable for use in the low temperature conditions encountered in deep water drilling. Here, the maximum kinematic viscosity threshold at a temperature of 0°C must be less than 10mm2s−1 for a drilling fluid.
An Arrhenius plot of the data in Table 4.8 is presented in Figure 4.4. Here the viscosities are normalized to those measured at 0°C.
Polymers
Synthetic and natural polymers suitable for drilling muds are listed in Table 4.9 and Table 4.10, respectively. The structures of morpholine and methylenebisacrylamide are drawn in Figure 4.5.
| aDeep-drilling additives | |
| bThe polymer additive is characterized by increased viscosity at low shear rates and enhanced fluid loss control | |
| cSalt tolerance (above 10%) | |
| dThe additive also reduces drill string drag | |
| Polymer | References |
|---|---|
| 2-Acrylamido-2-methyl-1-propane sulfonic acid AMPS, diallyldimethylammonium chloride, N-vinyl-N-methylacetamide, AAms and acrylatesa | Hille et al. (1996); Li et al. (1996); Oswald et al. (2000) |
| AMPS/AAm/vinyl acetate copolymer | Matz et al. (2001) and Wang (1999) |
| AAm styrene sulfonate copolymerb | Patel and McLaurine (1991) |
| AAm, vinylpyrrolidone, N-vinyl lactam | Patel (2000) |
| Copolymer from AAm and AMPS, with methylenebisacry-lamide as the crosslinker | Patel (1998) |
| Copolymer from acryloylmorpholine and ammonium AMPS | Udarbe et al. (2000) |
| Styrene-butadiene copolymer latex and styrene-acrylate-methacrylate terpolymer latex | Bailey, 2001a and Bailey, 2001b |
| Polymers of amido sulfonic acid | Sopko and Lorentz (1991) |
| Acrylic polymer | Selikhanovich et al. (1997) |
| N-Vinyl-2-pyrrolidone, acrylamidopropane sulfonic acid, AAm, and acrylic acid copolymer | Stephens and Swanson (1992) |
| AMPS and N-vinyl amides of acrylics and methacrylics, or N-vinylcaprolactam | Heier et al. (2002) |
| Sulfonated chromium humate | Tan (1990) |
| Sulfonated phenolic resin and hydrolytic ammonium polyacrylate | Tan (1990) |
| PA and polyimide | Wall et al. (1995b) |
| Hydrolyzed polyacrylonitrile and cyan-ethylate carboxymethyl cellulosec | Liu et al. (1996) |
| PAOsd | Mensa-Wilmot et al. (1997) |
| Polymers of hydroxy carboxylic acids as a rheologic additive | Müeller et al. (1999b) |
| Dimethyl silicone fluids | Patel (1997) |
| aThe amylopectin starch may be crosslinked with epichlorohydrin to stabilize the starch molecule. The molecule may also be stabilized by hydroxypropylation, carboxymethylation, or both. | |
| bCombination for high temperature/high pressure. | |
| cFor example, with dimethyl aminopropyl methacrylamide, methacrylamido propyltrimethyl ammonium chloride, N-vinylformamide, N-vinylacetamide, diallyl dimethyl ammonium chloride, and diallylamine. | |
| Polymer | References |
|---|---|
| Amylopectina | Kok et al., 1999a and Kok et al., 1999b |
| Polyanionic cellulose sulfonate-containing polymerb | Hen (1991) |
| Hydroxyethyl and hydroxypropyl cellulose | Plank (1993) |
| Hydroxyethyl cellulose, hydrophobically modified | Audibert et al., 1995 and Audibert et al., 2000 |
| Carboxymethyl cellulose | Ryzhov et al. (1996) |
| Gellan | Dreveton et al. (1995) |
| Diutan | Navarrete et al. (2001); Navarrete and Shah (2001) |
| Cornstarch, carboxylated methyl, crosslinked hydroxypropyl cornstarch | Anderson et al. (1991) and Bernu (1998) |
| Graft copolymer of starch, AAm, and PVA | Gao et al. (1993) |
| Waxy maize starch, epichlorohydrin, crosslinked | Estes and Bernu (1999) |
| Crosslinked starches | Cobianco et al. (2001); Sifferman et al. (1999) |
| Amine-derivatized potato starch | Anderson et al. (1991) |
| Sulfonated chromium humate, sulfonated phenolic resin, and hydrolytic ammonium polyacrylate | Tan (1990) |
| Gellan, scleroglucan, xanthan gum | Dreveton et al. (1998) |
| Hydrophobically modified guars | Audibert and Argillier (1998) |
| Hydroxypropyl guar gum, hydrophobically modified | Audibert and Argillier (1996) |
| Deacetylated xanthan gum | Langlois (1999) |
| Vinyl grafted lignitec | Huddleston and Williamson, 1990 and Huddleston and Williamson, 1991 |
Polyacrylamides (PAMs) are eventually hydrolyzed over time and temperature, leading to a lack of tolerance toward electrolyte contamination and to rapid degradation. Modifications of PAM structures have been proposed to retain thermal stability at higher temperatures. Monomers such as 2-acrylamido-2-methyl-1-propane sulfonic acid (AMPS) or sulfonated styrene/maleic anhydride can be used to prevent acrylamide (AAm) comonomer from hydrolyzing (Audibert and Argillier, 1995).
Starch
Starch is a high molecular weight, natural polymer composed of repeating 1,4-α-D-glucopyranosyl units. It is typically a mixture of linear and branched polymers. Amylose is the linear component with a molecular weight of around 200 k Dalton, and amylopectin is the branched component with a molecular weight of around 1 M Dalton (Fanta et al., 2002).
Normal dent cornstarch contains about 25% amylose, and commercial cornstarch varieties are available that range in amylose content from 0% (waxy cornstarch) to about 70% (high amylose cornstarch).
Starch, as isolated in its native state, is insoluble in water at room temperature because of hydrogen bonding between polysaccharide macromolecules and areas of crystallinity within the starch granule. When a solution of starch is heated, granules initially take up water with limited swelling, then, at a definite temperature, typically about 70°C, the granules swell rapidly and irreversibly, and areas of crystallinity within the granule are lost. The temperature at which this occurs is referred to as the gelatinization temperature.
Near this temperature, the amylose component becomes soluble and diffuses out of the granule matrix. As the temperature is increased beyond about 70°C, a greater percentage of the starch becomes soluble. The granules become highly swollen, until, at a temperature of about 90–100°C, a viscous dispersion of starch in water is obtained. However, despite the overall apparent solubility, the starch is only partially soluble in water and usually occurs as highly swollen granules, thus granule fragments that may be easily separated from such a starch solution by centrifuging.
True solutions of starch in water are difficult to prepare using conventional cooking techniques, and require the application of specialized methods, such as autoclaving at elevated temperatures and pressures.
Steam jet cooking is another technique for preparing starch solutions. This is simpler and more economical than autoclaving, and is suitable for continuous processing. Because of these processing advantages, jet cooking has been used for decades to prepare starch solutions for commercial applications. The method involves pumping a water slurry of starch through an orifice located in a heating chamber, i.e., a hydroheater, where the starch slurry contacts a jet of high temperature, high pressure steam (Fanta et al., 2002). The amount of steam is carefully controlled in the process to achieve complete steam condensation. This means that only little or no excess steam passes through the cooker.
In the excess steam jet cooking technique, the steam entering the hydroheater exceeds the amount required to achieve the required cooking temperature and pressure, thus allowing considerable amounts of excess steam to pass through the cooker along with the cooked starch solution. The intense turbulence caused by the passage of this excess steam promotes mechanical shearing and degradation of starch molecules, especially those having the highest molecular weight, and produces starch solutions with a reduced viscosity (Fanta et al., 2002 and Fanta et al., 1999b).
The high degree of turbulence and mechanical shear of the excess steam jet cooking process also converts the water-immiscible lubricant phase to a homogeneous aqueous dispersion of micrometer-sized oleaginous droplets. These unique, aqueous, starch-oil dispersions form the basis for lubricant compositions that are suitable for oil field applications.
An inherent property of starch pastes and solutions is their tendency to form gels on cooling, and this property is commonly referred to as retrogradation. The phenomenon is caused by aggregation of starch molecules through hydrogen bonding and crystallization. The tendency of starch solutions to retrograde and form gels increases with its amylose content, because amylose is a straight chain polymer with little or no branching.
Although retrogradation has also been observed in amylopectin solutions, it is much slower here, and is generally observed only after such solutions have been allowed to stand for prolonged periods of time (Fanta et al., 2002). The starch can be crosslinked with epichlorohydrin or phosphorus oxychloride (Sifferman et al., 2001 and Sifferman et al., 2002).
Starch-oil compositions are prepared by mixing starch, water, and lubricating oil at room temperature, and then passing this mixture through an excess steam jet cooker. Alternatively, mixtures of starch and water are precooked in the steamcooker, and, after admixing the lubricating oil, the composition is again guided in a steamcooker.
For lubricant oils, a base olefin, a high molecular weight base olefin, a high molecular weight base olefin with ester, olefin blends with ester, or a viscous, liquid polybutene are used (Fanta et al., 2002). The resulting jet cooked compositions are stable with respect to separation and coagulation of oil droplets and are comprised of microscopic droplets of oil, about 1–10 μ in diameter, which are uniformly distributed in the starch-water phase (Fanta et al., 2002).
No emulsifying agents, dispersing agents, or surface active agents are used in the process. If the oil content is held within the preferred range of 20–40 phr, jet cooked compositions can be easily dried by drum drying. Outwardly dry, flake like products are obtained that can be easily reduced in size by milling. No separation of the oil from the dried starch matrix is observed.
These compositions can be easily dispersed in water to form smooth, stable, lump-free dispersions. Water dispersions do not phase separate into their oil and aqueous components on prolonged standing because of a thin layer or shell of starch that spontaneously forms around each oil droplet during the jet cooking process (Fanta et al., 1999a).
Preparation 4–1
The crosslinking of starch is achieved as follows (Sifferman et al., 2002): At room temperature, 1000 g of waxy maize starch is slurried in 1500 g of water. To the slurry, sodium hydroxide, as a 3% solution, is slowly added to reach a pH of 12. Then, 0.13% epichlorohydrin is added to the slurry. The reaction mixture is allowed to react at 40°C for completion, then cooled to room temperature, and neutralized to a pH of 6.0 with aqueous hydrochloric acid. Eventually the starch is filtered, washed, and dried to provide an ungelatinized dry powder.
Laboratory tests indicated that starch lubricant compositions lower both API and high temperature/high pressure fluid loss values. Results are represented in Table 4.11. The coefficients of friction are up to 45% lower than those of the untreated base muds; similar to those of OBMs. Only 0.5% of starch lubricant need be added to get satisfactory results (Sifferman et al., 2003).
| Friction | Fluid Loss | |||
|---|---|---|---|---|
| Composition | coeff. k | Reduction % | API ml | HTHP ml |
| Base mud | 0.3126 | – | 8.0 | 26 |
| Field mud + 3% lubricant | 0.2981 | 4.6 | 4.4 | 14 |
| Base mud + starch composite with 0.5% high MW olefin | 0.2732 | 12.6 | 3.1 | 12 |
| Base mud + starch composite with 0.5% base olefin | 0.2653 | 15.1 | 3.4 | 11 |
| Base mud + starch composite with 0.5% high MW olefin + ester | 0.2551 | 18.4 | 3.0 | 13 |
| Base mud + starch composite with 0.5% base olefin + ester olefin copolymer | 0.2473 | 20.9 | 3.2 | 12 |
| Base mud + starch composite with 0.5% polybutene | 0.1672 | 46.5 | 2.7 | 10 |
Amides
This group of nitrogen-containing additives comprises phenolic Mannich bases, phosphoric acid (Umutbaev et al., 1993), and oxalkylated alkyl phenols with nitrogen containing additives (Koshelev et al., 1993). Vinyl amide monomers for synthetic muds are shown in Figure 4.6.
Special Issues
Side Reactions
Extensive laboratory work has been carried out to determine the performance of a number of lubricants including tests to determine the potential for formation damage of several types of drilling fluids, as well as the reduction in the friction coefficient.
Certain polymer additives are also effective as lubrications as a side effect, but in many cases, additional lubricants must be added for the fluid to be successful in drilling to the total intended depth (Knox and Jiang, 2005).
Lubricants for water-based drilling are primarily chosen for their technical performance and environmental acceptability. Hydrocarbons and fatty acids were used mostly in the past, but nowadays a trend to more environmentally acceptable alternatives can be seen, in particular to esters and naturally occurring vegetable oils.
These chemicals are highly lubricating materials as they significantly reduce the coefficients of friction of both metal/metal and metal/rock contacts in water-based fluid environments, by up to 70%. Clearly, effective additives exhibit a high degree of surface activity. This property improves their adhesion to the metal casing or the drilling mud solids. On the other hand, this surface activity makes the lubricants more prone to reacting with other components of the mud.
Lubricants may act as an emulsifier in the presence of even small quantities of oil. Such a composition may turn into an invert emulsion, with the consistency of cottage cheese (Knox and Jiang, 2005). Of course, such events are highly undesirable as the formation of a highly viscous material is definitely a drilling hazard, and the production zone may be damaged.
Apart from this, the lubricant may react with divalent or multivalent ions, forming the ionic bonds as found in ionomers. This reaction results in the formation of a grease-like precipitate, which may formed at concentrations of calcium or magnesium ions as low as 1000 ppm, depending on the chemical nature of the lubricant. Such ionic concentrations are frequently observed even in fresh water. All these issues must be taken into account in the selection of suitable lubricants for water-based drilling fluids (Knox and Jiang, 2005).
Silicate-Based Muds
Silicate-based muds are notorious for their high coefficient of friction against rock or metal in comparison to oil or synthetic-based muds. However, the latter cause environmental concerns, and in certain locations it is not allowed to drill using lower friction muds. Thus, silicate-based muds are preferred for environmental reasons (Albrecht et al., 2008).
Synthetic muds are also more expensive than silicate-based muds. So it is desirable to lower the coefficients of friction of these muds in order to increase the drilling rates.
Suitable lubricants can be selected from glycosides, which may be functionalized. The amount of lubricant is typically in the range of 1–15%. Specific examples of glycosides are listed in Table 4.12 and the structure of glucopyranosides is shown in Figure 4.7.
| Glycoside |
|---|
| 4-Hydroxybutyl-D-glucopyranoside |
| 4-Aminobutyl-glycosides-D-glucopyranoside |
| Hexadecylphosphato-D-glucopyranoside |
| Trimethylammoniumcarboxymethyl-D-glucopyranoside |
| Triethylene-oxynonyl-D-glucopyranoside |
| 4-Hydroxy propylcarboxy-D-glucopyranoside |
| Stearyl-D-glucopyranoside |
Preparation 4–2
The preparation of alkylated an glucoside is basically an etherification. It can be performed by dissolving dry HCl in the respective alcohol. To this mixture, the glucose is added and allowed to react for 12 h (Brown et al., 1970). Then the glucoside is extracted with ethyl acetate. After drying, the major portion of the solvent is removed. The glucoside is somewhat reluctant to crystallize.
Alkylated polyglucosides act as surfactants in microemulsions (Ryan and Kaler, 2001). Microemulsions are thermodynamically stable, isotropic mixtures containing water, oil, and surfactant. They are utilized in a variety of industrial applications besides oil field applications, e.g., in solvent delivery, and polymerization techniques (Hill et al., 1997; Kjellin and Johansson, 2010; Ryan and Kaler, 2001).
Studies on Pipe Sticking
A study of the effect of various additives on pipe sticking is available (Pandey and Joshi, 1995), which investigates the effect of various available oil field additives in reducing downhole friction and their optimal concentration. As previously mentioned, the frictional forces present at the string-borehole interface are of prime importance. The friction at the string-borehole interface can be reduced through various chemicals incorporated in the drilling fluid system.
To obtain a mud cake in which sensitivity of various chemicals could be studied, a highly sticky cake was prepared from mud containing gypsum, kaolinite, sand, and shale powder. The ability of various mud additives to minimize friction at the string borehole interface, and thereby reduce the sticking tendency, was evaluated systematically with time. The study was extended to several mud systems.
Differential Sticking Reducer
Various additives have been proposed to assist in freeing a stuck drill pipe, the most common of which is diesel oil, added directly to the drilling mud as a spotting fluid. However, this is not always successful.
An additive comprising an oil-in-water microemulsion has been proposed. Sodium dodecyl benzene sulfonate may be used as a surfactant. Ethylene glycol or diethylene glycol act as cosurfactants (Davies et al., 1997).
Albrecht, M.S., Cowan, K.M., McNeil III, R.I., Van Oort, E., Rock Sr. R.L., 2008. Silica-based drilling mud comprising glycoside lubricants with amino-linked alkyl chains. US Patent 7 320 951, assigned to Shell Oil Company, Houston, TX, January 22, 2008.
Alonso-Debolt, M.A., Bland, R.G., Chai, B.J., Eichelberger, P.B., Elphingstone, E.A., 1995. Glycol and glycol ether lubricants and spotting fluids. WO Patent 9 528 455, assigned to Baker Hughes Inc., October 26, 1995.
Alonso-Debolt, M.A., Bland, R.G., Chai, B.J., Elchelberger, P.B., Elphingstone, E.A., 1999. Glycol and glycol ether lubricants and spotting fluids. US Patent 5 945 386, assigned to Baker Hughes Inc., August 31, 1999.
Andreson, B.A., Abdrakhmanov, R.G., Bochkarev, G.P., Umutbaev, V.N., Fryazinov, V.V., Kudinov, V.N., Valiakhmetov, F.M., 1992. Lubricating additive for water-based drilling solutions – contains products of condensation of monoethanolamine and tall oils, kerosene, monoethanolamine and flotation reagent. SU Patent 1 749 226, assigned to Bashkir Oil Ind. Res. Inst. and Bashkir Oil Proc. Inst., July 23, 1992.
Anderson, C.P.; Blenkinsopp, S.A.; Cusack, F.M.; Costerton, J.W., Drilling mud fluid loss – an alternative to expensive bulk polymers, In: Proceedings Volume, 4th Institute of Gas Technology, Gas, Oil, & Environmental Biotechnology International SymposiumColorado Springs, CO, December 9–11, 1991. (1991), pp. 481–489.
Argillier, J.F., Audibert, A., Marchand, P., Demoulin, A., Janssen, M., 1997. Lubricating composition including an ester-use of the composition and well fluid including the composition. US Patent 5 618 780, assigned to Inst. Francais Du Petrole, April 8, 1997.
Argillier, J.F., Demoulin, A., Audibert-Hayet, A., Janssen, M., 1999. Borehole fluid containing a lubricating composition – method for verifying the lubrification of a borehole fluid–application with respect to fluids with a high ph (fluide de puits comportant une composition lubrifiante – procede pour controler la lubrification d'un fluide de puits - application aux fluides a haut ph). WO Patent 9 966 006, assigned to Inst. Francais Du Petrole and Fina Research SA, December 23, 1999.
Argillier, J.-F., Demoulin, A., Audibert-Hayet, A., Janssen, M., 2004. Borehole fluid containing a lubricating composition–method for verifying the lubrification of a borehole fluid–application with respect to fluids with a high ph. US Patent 6 750 180, assigned to Institut Francais du Petrole, Rueil-Malmaison Cedex, FR, Oleon NV, Ertvelde, BE, June 15, 2004.
Audebert, R., Janca, J., Maroy, P., Hendriks, H., 1994. Chemically crosslinked polyvinyl alcohol (pva), process for synthesizing same and its applications as a fluid loss control agent in oil fluids. GB Patent 2 278 359, assigned to Sofitech NV, November 30, 1994.
Audebert, R., Janca, J., Maroy, P., Hendriks, H., 1996. Chemically crosslinked polyvinyl alcohol (pva), process for synthesizing same and its applications as a fluid loss control agent in oil fluids. CA Patent 2 118 070, assigned to Schlumberger Canada Ltd., April 14, 1996.
Audebert, R., Maroy, P., Janca, J., Hendriks, H., 1998. Chemically crosslinked polyvinyl alcohol (pva), and its applications as a fluid loss control agent in oil fluids. EP Patent 705 850, assigned to Sofitech NV, September 2, 1998.
Audibert, A.; Argillier, J.F., Thermal stability of sulfonated polymers, In: Proceedings Volume, SPE Oilfield Chem. International SymposiumSan Antonio, February 14–17, 1995. (1995), pp. 81–91.
Audibert, A., Argillier, J.F., 1996. Process and water-based fluid utilizing hydrophobically modified guar gums as filtrate (loss) reducer (procede et fluide a base d'eau utilisant des guars modifies hydrophobiquement comme reducteur de filtrat). EP Patent 722 036, assigned to Inst. Francais Du Petrole, July 17, 1996.
Audibert, A., Argillier, J.F., 1998. Process and water-base fluid utilizing hydrophobically modified guars as filtrate reducers. US Patent 5 720 347, assigned to Inst. Francais Du Petrole, February 24, 1998.
Audibert, A., Argillier, J.F., Bailey, L., Reid, P.I., 1995. Procedure and water-based fluid utilizing hydrophobically modified cellulose derivatives as filtrate reducer (fluide de traitement de puits de forage utilisant des derives cellulosiques modifies hydrophobiquement comme reducteur de filtrat, et procede d'utilisation). EP Patent 670 359, assigned to Inst. Francais Du Petrole and Sofitech NV, September 6, 1995.
Audibert, A., Argillier, J.F., Bailey, L., Reid, P.I., 2000. Process and water-base fluid utilizing hydrophobically modified cellulose derivatives as filtrate reducers. US Patent 6 040 276, assigned to Inst. Francais Du Petrole, March 21, 2000.
Bailey, L., 2001a. Latex additive for water-based drilling fluids. GB Patent 2 351 986, assigned to Sofitech NV, January 17, 2001.
Bailey, L., 2001b. Latex additive for water-based drilling fluids. WO Patent 0 104 232, assigned to Sofitech NV, January 18, 2001.
Bel, S.L.A., Demin, V.V., Kashkarov, N.G., Konovalov, E.A., Sidorov, V.M., Bezsolitsen, V.P., Gorjacheva, M.V., Gorlov, S.G., Ivchenko, A.M., Mal, T.L.S., Mojsa, Y.N., 1998. Lubricating composition – for treatment of clayey drilling solutions, contains additive in form of sulphonated fish fat. RU Patent 2 106 381, assigned to Shchelkovsk Agro Ent St C and Fakel Res. Prod. Assoc., March 10, 1998.
Bernu, C.J., 1998. High temperature stable modified starch polymers and well drilling fluids employing same. EP Patent 852 235, assigned to Chemstar Products Co., July 8, 1998.
Breeden, D.L., Meyer, R.L., 2005. Ester-containing downhole drilling lubricating composition and processes therefor and therewith. US Patent 6 884 762, assigned to Newpark Drilling Fluids, L.L.C., Houston, TX, April 26, 2005.
Brown, G.M.; Dubreuil, P.; Ichhaporiaa, F.M.; Desnoyers, J.E., Synthesis and properties of some α-D-alkyl glucosides and mannosides: Apparent molal volumes and solubilization of nitrobenzene in water at 25°C, Can. J. Chem. 48 (1970) 2525–2531.
Buyanovskii, I.A., Tribological test methods and apparatus, Chem. Technol. Fuels Oils 30 (3) (1994) 133–147.
Chanshev, R.F., Kovtunenko, S.V., Tsikunkov, F.D., Ismakov, R.A., Konesev, G.V., Mulyukov, R.A., 1992. Lubricant for cutter bit bearings – contains ethylene-propylene synthetic rubber, zinc dioctyl- phenyl dithio-phosphate, polytetrafluoroethylene and mineral oil. SU Patent 1 778 162, November 30, 1992.
Chapman, J., Ward, I., 1997. Lubricant for drilling mud. EP Patent 0 770 661, assigned to Mud B W Ltd., May 2, 1997.
Clark, D.E., Dye, W.M., 1997. Environmentally safe lubricated well fluid method of making a well fluid and method of drilling. US Patent 5 658 860, assigned to Baker Hughes Inc., August 19, 1997.
Cobianco, S., Bartosek, M., Guarneri, A., 2001. Non-damaging drilling fluids. EP Patent 1 104 798, assigned to Eni SPA and Enitecnologie SPA, June 6, 2001.
Davies, S.N., Meeten, G.H., Way, P.W., 1997. Water based drilling fluid additive and methods of using fluids containing additives. US Patent 5 652 200, assigned to Schlumberger Technol. Corp., July 29, 1997.
Delton, R.M., Hooper, M., 1994. Rock bit grease composition. GB Patent 2 276 884, assigned to Smith International Inc., October 12, 1994.
Denton, R.M., Fang, Z., 1996. Rock bit grease composition. US Patent 5 589 443, assigned to Smith International Inc., December 31, 1996.
Denton, R.M., Lockstedt, A.W., 2006. Rock bit with grease composition utilizing polarized graphite. US Patent 7 121 365, assigned to Smith International, Inc., Houston, TX, October 17, 2006.
Denton, R., Lockstedt, A.W., White, A.C. 2007. Drill bit lubricant with enhanced load carrying/anti wear properties. US Patent 7 267 183, assigned to Smith International, Inc., Houston, TX, September 11, 2007.
Dixon, J., 2008. Drilling fluids. US Patent 7 343 986, assigned to Croda International PLC, Goole, East Yorkshire, GB, March 18, 2008.
Dixon, J., 2009. Drilling fluids. US Patent 7 614 462, assigned to Croda International PLC, Goole, East Yorkshire, GB, November 10, 2009.
Dreveton, E., Lecourtier, J., Ballerini, D., Choplin, L., 1995. Process utilizing gellan as filtrate reducer for water-based drilling fluids (procede utilisant le gellane comme reducteur de filtrat pour les fluides de forage a base d'eau). EP Patent 662 563, assigned to Inst. Francais Du Petrole, July 12, 1995.
Dreveton, E., Lecourtier, J., Ballerini, D., Choplin, L., 1998. Process using gellan as a filtrate reducer for water-based drilling fluids. US Patent 5 744 428, assigned to Inst. Francais Du Petrole, April 28, 1998.
Durr Jr., A.M., Huycke, J., Jackson, H.L., Hardy, B.J., Smith, K.W., 1994. An ester base oil for lubricant compounds and process of making an ester base oil from an organic reaction by-product. EP Patent 606 553, assigned to Conoco Inc., July 20, 1994.
Dye, W., Clark, D.E., Bland, R.G., 1995. Well fluid additive. EP Patent 652 271, assigned to Baker Hughes Inc., May 10, 1995.
Enright, D.P., Dye, W.M., Smith, F.M., Perricone, A.C., 1991. Drilling fluid methods and composition. US Patent 5 007 489, assigned to Baker Hughes Inc., April 16, 1991.
Estes, B.L., Bernu, C.J., 1999. New and improved drilling fluids and additives therefor. WO Patent 9 951 701, assigned to Dresser Industries Inc., October 14, 1999.
Fang, Z., Peterson, S., Denton, R., 1998. O-ring seal with lubricant additives for rock bit bearings. GB Patent 2 318 139, assigned to Smith International Inc., April 15, 1998.
Fanta, G.F.; Felker, F.C.; Eskins, K.; Baker, F.L., Aqueous starch-oil dispersions prepared by steam jet cooking. Starch films at the oil-water interface, Carbohydr. Polym. 39 (1) (1999) 25–35.
Fanta, G.F., Muijs, H.M., Eskins, K., Felker, F.C., Erhan, S.M., 2002. Starch-containing lubricant systems for oil field applications. US Patent 6 461 999, assigned to The United States of America as represented by the Secretary of Agriculture (Washington, DC) Shrieve Chemical Products, The Woodlands, TX, October 8, 2002.
Fanta, G.F.; Shogren, R.L.; Salch, J.H., Steam jet cooking of high-amylose starch-fatty acid mixtures. An investigation of complex formation, Carbohydr. Polym. 38 (1) (1999) 1–6.
Fisk Jr., J.V., Kerchevile, J.D., Pober, K.W., 2006. Silicic acid mud lubricants. US Patent 6 989 352, assigned to Halliburton Energy Services, Inc. Duncan, OK, January 24, 2006.
Fleury, M.; Branlard, P.; Lenormand, R.; Zarcone, C., Intermediate wettability by chemical treatment, J. Pet. Sci. Eng. 24 (2–4) (1999) 123–130.
Forsberg, J.W., Jahnke, R.W., 1993a. Methods of drilling well boreholes and compositions used therein. US Patent 5 260 268, assigned to Lubrizol Corp., November 9, 1993.
Forsberg, J.W., Jahnke, R.W., 1993b. Methods of drilling well boreholes and compositions used therein. WO Patent 9 302 151, assigned to Lubrizol Corp., February 4, 1993.
Frolov, M.A., Molyavko, I.V., Spivak, A.I., Rakhmankulov, D.L., Rakhmatullin, V.R., Romanov, N.A., 1993. Lubricant for friction pairs working under heavy loads – contains mineral oil and additive in form of 2,4,8,10-tetra-oxaspiro-(5,5)-undecane, to improve anti-wear and antiscratch properties. SU Patent 1 817 788, assigned to Borehole Drill Constr. Tech., May 23, 1993.
Gao, J.; Guo, D.; Li, J.; Qiu, Z., The synthesis and properties of high temperature filtrate reducer, aps, Drill. Fluid Completion Fluid 10 (1) (1993) 21–23; 74.
Garyan, S.A.; Kuznetsova, L.P.; Moisa, Y.N., Experience in using environmentally safe lubricating additive fk-1 in drilling muds during oil and gas well drilling, Stroit Neft Gaz Skvazhin Sushe More (10) (1998) 11–14.
Genuyt, B., Janssen, M., Reguerre, R., Cassiers, J., Breye, F., 2001. Biodegradable lubricating composition and uses thereof, in particular in a bore fluid [composition lubrifiante biodegradable et ses utilisations, notamment dans un fluide de forage]. WO Patent 0 183 640, assigned to Total Raffinage Dist SA, November 8, 2001.
Genuyt, B., Janssen, M., Reguerre, R., Cassiers, J., Breye, F., 2006. Biodegradable lubricating composition and uses thereof, in particular in a bore fluid. US Patent 7 071 150, assigned to Total Raffinage Distribution S.A., Puteaux, FR, July 4, 2006.
Goncharov, S.V., Neradovskij, V.V., Zevakov, M.E., Babets, M.A., Bektimirov, E.I., Bezdenezhnykh, V.I., Shmavonyants, V.S., 1993. Sealing lubricant for profiled joints of e.g. casing strings – contains silico-organic liquid, diethylene glycol, graphite powder, mixture of derivatives of synthetic fatty acids and solution of polyacrylamide. SU Patent 1 796 648, February 23, 1993.
Halliday, W.S., Clapper, D.K., 1998. Purified paraffins as lubricants, rate of penetration enhancers, and spotting fluid additives for water-based drilling fluids. US Patent 5 837 655, November 17, 1998.
Halliday, W.S., Schwertner, D., 1997. Olefin isomers as lubricants, rate of penetration enhancers, and spotting fluid additives for water-based drilling fluids. US Patent 5 605 879, assigned to Baker Hughes Inc., February 25, 1997.
Hanahan, D.J., A Guide to Phospholipid Chemistry. (1997) Oxford University Press, New York.
Heier, K.H., Morschhaeuser, R., Tardi, A., Weber, S., Botthof, G., 2002. Copolymers and their use as drilling aids. US Patent 6 380 137, assigned to Clariant GmbH, April 30, 2002.
Hen, J., 1991. Sulfonate-containing polymer/polyanionic cellulose combination for high temperature/high pressure filtration control in water base drilling fluids. US Patent 5 008 025, assigned to Mobil Oil Corp., April 16, 1991.
Hill, K.; Rybinski, W.; Stoll, G., Alkyl Polyglycosides: Technology, Properties, and Applications. (1997) Wiely VCH, New York.
Hille, M., Wittkus, H., Tonhauser, J., Engelhardt, F., Riegel, U., 1996. Water-soluble copolymers useful in drilling fluids. US Patent 5 510 436, assigned to Hoechst AG, April 23, 1996.
Holinski, R., 1995. Solid lubricant composition. US Patent 5 445 748, assigned to Dow Corning GmbH, Wiesbaden, DE, August 29, 1995.
Huddleston, D.A., Williamson, C.D., 1990. Vinyl grafted lignite fluid loss additives. US Patent 4 938 803, assigned to Nalco Chemical Co., July 3, 1990.
Huddleston, D.A., Williamson, C.D., 1991. Vinyl grafted lignite fluid loss additives. US Patent 5 028 271, assigned to Nalco Chemical Co., July 2, 1991.
Kalashnikov, Y.T., 1994. Lubricant-sealer for profiled joints of casing pipes - contains soap plastic lubricant, polyacrylamide or carboxymethyl cellulose and additionally gypsum or cement powder, to increase sealing rate. RU Patent 2 007 438, February 15, 1994.
Kashkarov, N.G., Konovalov, E.A., Vjakhirev, V.I., Gnoevykh, A.N., Rjabokon, A.A., Verkhovskaja, N.N., 1998. Lubricant reagent for drilling muds - contains spent sunflower oil, and light tall oil and spent coolant-lubricant as modifiers. RU Patent 2 105 783, assigned to Tyumen Nat Gases Res. Inst., February 27, 1998.
Kashkarov, N.G., Verkhovskaya, N.N., Ryabokon, A.A., Gnoevykh, A.N., Konovalov, E.A., Vyakhirev, V.I., 1997. Lubricating reagent for drilling fluids – consists of spent sunflower oil modified with additive in form of aqueous solutions of sodium alkylsiliconate(s). RU Patent 2 076 132, assigned to Tyumen Nat Gases Res. Inst., March 27, 1997.
In: (Editors: Kjellin, M.; Johansson, I.) Surfactants from Renewable Resources (2010) Wiley, Chichester, West Sussex.
Knox, D.; Jiang, P., Drilling further with water-based fluids – selecting the right lubricant, In: Proceedings Volume, no. 92002-MS, International Symposium on Oilfield ChemistrySociety of Petroleum Engineers, Inc., The Woodlands, TX, 2005. (2005).
Kok, S.J., Guns, J., Kraan, L.C., Schuringa, G.E., Kesselmans, R.P.W., 1999a. Drilling fluids. WO Patent 9 952 990, assigned to Coop Verkoop Prod. Aard De, October 21, 1999.
Kok, S.J., Kraan, L.C., Schuringa, G.E., Guns, J., Kesselmans, R.P.W., 1999b. Drilling fluids. EP Patent 949 311, assigned to Coop Verkoop Prod. Aard De, October 13, 1999.
Koltermann, T.J., Willey, T.F., 1999. Lubricating grease. US Patent 5 891 830, assigned to Baker Hughes Inc., April 6, 1999.
Koltermann, T.J., Willey, T.F., 2000. Lubricating grease. US Patent 6 056 072, assigned to Baker Hughes Inc., May 2, 2000.
Konovalov, E.A., Ivanov, Y.A., Shumilina, T.N., Pichugin, V.F., Komarova, N.N., 1993a. Lubricating reagent for drilling solutions – contains agent based on spent sunflower oil, water, vat residue from production of oleic acid, and additionally water glass. SU Patent 1 808 861, assigned to Moscow Gubkin Oil Gas Inst., April 15, 1993.
Konovalov, E.A., Rozov, A.L., Zakharov, A.P., Ivanov, Y.A., Pichugin, V.F., Komarova, N.N., 1993b. Lubricating reagent for drilling solutions – contains spent sunflower oil as active component, water, boric acid as emulsifier, and additionally water glass. SU Patent 1 808 862, assigned to Moscow Gubkin Oil Gas Inst., April 15, 1993.
Koshelev, V.N., Krezub, A.P., Ponomarev, D.M., Mojsa, Y.N., Frolova, N.V., Penkov, A.I., Vasilchenko, S.V., 1993. Drilling solution with improved lubricating and rheology – contains additionally oxyalkylated alkylphenol with nitrogen-containing additive, in aromatic solvent. SU Patent 1 797 617, assigned to Borehole Consolidation Mu., February 23, 1993.
Kurochkin, B.M.; Kolesov, L.V.; Biryukov, M.B., Use of ellipsoidal glass granules as an antifriction mud additive, Neft Khoz (12) (1990) 61–64.
Kurochkin, B.M., Kolesov, L.V., Masich, V.I., Stepanov, N.V., Tselovalnikov, V.F., Alekperov, V.T., Kerimov, I.N., Ibragimov, O.N., Bulanov, B.M., 1992a. Solution for drilling gas and oil wells – contains ellipsoidal glass beads as additive reducing friction between walls of well and casing string. SU Patent 1 740 396, assigned to Drilling Tech. Res. Inst., June 15, 1992.
Kurochkin, B.M.; Simonyan, E.A.; Simonyan, A.A.; Khirazov, E.F.; Ozarchuk, P.A.; Voloshinivskii, V.O.; Glushakov, A.Y., New technology of drilling with the use of glass granules, Neft Khoz 7 (1992) 9–11.
Kurochkin, B.M.; Tselovalnikov, V.F., Use of ellipsoidal glass granules for drilling under complicated conditions, Neft Khoz (10) (1994) 7–13.
Landry, D.K., Koltermann, T.J., 1991a. Bearing grease for rock bit bearings. CA Patent 2 018 779, assigned to Hughes Tool Co., March 18, 1991.
Landry, D.K., Koltermann, T.J., 1991b. Bearings grease for rock bit bearings. US Patent 5 015 401, assigned to Hughes Tool Co., May 14, 1991.
Langlois, B., 1999. Fluids useful for oil mining comprising de- acetylated xanthane gum and at least one compound increasing the medium ionic strength (fluides utilisables dans l'exploitation du petrole comprenant de la gomme xanthane desacetylee et au moins un compose augmentant la force ionique du milieu). WO Patent 9 903 948, assigned to Rhodia Chimie, January 28, 1999.
Li, Y.G.; Li, S.L.; Wang, Z.L., New high temperature filtration reducer fla for drilling fluid, Drill. Fluid Completion Fluid 13 (3) (1996) 33–35.
Liu, Y.Y.; Hou, W.G.; Sun, D.J.; Wu, T.; Zhang, C.G., Study on function mechanism of filtration reducer: Research and development of new filtration reducer, Drill. Fluid Completion Fluid 13 (4) (1996) 12–14.
Lyubinin, I.A.; Gubarev, A.S.; Butovets, V.V.; Torgashov, A.V., Modern lubricants for roller bit bearings, Stroit Neft Gaz Skvazhin Sushe More (3) (1995) 14–25.
Matz, G.F., Melby, A.L., Loeffler, R.J., Vozza, N.F., Chen, S.R.T., 2001. Water soluble polymer composition and method of use. WO Patent 0 105 365, assigned to Calgon Corp., January 25, 2001.
Mensa-Wilmot, G.; Garrett, R.L.; Stokes, R.S., Pao (polyalphaolefin) lubricant inhibits bit balling, speeds drilling, Oil Gas J. 95 (16) (1997) 68–70.
Müller, H., Herold, C.P., Bongardt, F., Herzog, N., von Tapavicza, S., 2000. Lubricants for drilling fluids (schmiermittel fuer bohrspuelungen). WO Patent 0 029 502, assigned to Cognis Deutschland GmbH, May 25, 2000.
Müller, H., Herold, C.P., von Tapavicza, S., 1999a. Use of selected fatty alcohols and their mixtures with carboxylic acid esters as lubricant components in water-based drilling fluid systems for soil exploration (verwendung ausgewaehlter fettalkohole und ihrer abmischungen mit carbonsaeureestern als schmiermittelkomponente in wasserbasierten bohrspuelsystemen zum erdreichaufschluss). EP Patent 948 576, assigned to Henkel KG Auf Aktien, October 13, 1999.
Müller, H., Podubrin, S., Herold, C.P., Heidbreder, A., 1999b. Dispersions containing homopolymers or copolymers of hydroxy carboxylic acids as a rheological additive (dispersionen enthaltend homo- oder copolymere von hydroxycarbonsaeuren als rheologisches additiv). WO Patent 9 952 623, assigned to Cognis Deutschland GmbH, October 21, 1999.
Müller, H., Herold, C.-P., Bongardt, F., Herzog, N., von Tapavicza, S., 2004a. Lubricants for drilling fluids. US Patent 6 806 235, assigned to Cognis Deutschland GmbH & Co. KG, Duesseldorf, DE, October 19, 2004.
Müller, H., Herold, C.-P., von Tapavicza, S., 2004b. Use of selected fatty alcohols and their mixtures with carboxylic acid esters as lubricant components in water-based drilling fluid systems for soil exploration. US Patent 6 716 799, assigned to Cognis Deutschland GmbH & Co. KG, Duesseldorf, DE, April 6, 2004.
Müller, H., Herold, C.-P., von Tapavicza, S., Neuss, M., Zoellner, W., Burbach, F., 1990. Esters of medium chain size carboxylie acids as components of the oil phase of invert emulsion drilling fluids. EP Patent 0 386 636, assigned to Henkel KGAA, September 12, 1990.
Nakaya, T.; Li, Y.-J., Phospholipid polymers, Prog. Polym. Sci. 24 (1) (1999) 143–181.
Naraghi, A.R., Rozell, R.S., 1996. Method for reducing torque in downhole drilling. US Patent 5 535 834, assigned to Champion Technologies, July 16, 1996.
Navarrete, R.C.; Seheult, J.M.; Coffey, M.D., New biopolymers for drilling, drill-in, completions, spacer, and coil-tubing fluids: Pt. 2, In: Proceedings Volume, SPE Oilfield Chem. International SymposiumHouston, TX, February 13–16, 2001. (2001).
Navarrete, R.C.; Shah, S.N., New biopolymer for coiled tubing applications, In: Proceedings Volume, SPE/International Coiled Tubing Association, Coiled Tubing RoundtableHouston, TX, March 7–8, 2001. (2001).
Newcomb, A.L., 1982. Composite grease for rock bit bearings. US Patent 4 358 384, assigned to Smith International Inc., Newport Beach, CA, November 9, 1982.
Oldiges, D.A., Joseph, A.W., 2003. Methods for using environmentally friendly antiseize/lubricating systems. US Patent 6 620 460, assigned to Jet-Lube, Inc., Houston, TX, September 16, 2003.
Oldiges, D., McDonald, H., Blake, T., 2007. Use of calcium sulfonate based threaded compounds in drilling operations and other severe industrial applications. US Patent 7 294 608, assigned to Jet-Lube, Inc., DE, November 13, 2007.
Oldiges Jr., D.A., McDonald, H., Blake, T., Stroup, K., Oldiges III, D.A., 2006. Non-metallic thread sealant and anti-seize compound having improved anti-galling properties for metal alloys. US Patent 7 091 161, assigned to Jet-Lube, Inc., Houston, TX, August 15, 2006.
Olson, W.D., Muir, R.J., Eliades, T.I., Steib, T., 1994. Sulfonate greases. US Patent 5 308 514, assigned to Witco Corporation, New York, May 3, 1994.
Oswald, R.J., Morschhaeuser, R., Heier, K.H., Tardi, A., Tonhauser, J., Kayser, C., Patterson, D., 2000. Water-soluble copolymers and their use for the exploration and recuperation of oil and gas. EP Patent 1 059 316, assigned to Clariant GmbH, December 13, 2000.
Pandey, A.K.; Joshi, N.P., Effectivity of additives in reducing down hole friction and preventing sticking, In: Proceedings Volume, Vol. 3, 1st India Oil & Natural Gas Corporation Ltd et International Petroleum Conference, Petrotech 95New Delhi, India, January 9–12, 1995. (1995), pp. 45–54.
Patel, A.D., 1997. Silicone based fluids for drilling applications. EP Patent 764 709, assigned to M I Drilling Fluids LLC., March 26, 1997.
Patel, A.D., 1998. Water-based drilling fluids with high temperature fluid loss control additive. US Patent 5 789 349, assigned to M I Drilling Fluids LLC., August 4, 1998.
Patel, B.B., 2000. Drilling fluid additive and process therewith. WO Patent 0 020 527, assigned to Phillips Petroleum Co., April 13, 2000.
Patel, A.D., Davis, E., Young, S., Stamatakis, E., 2006. Phospholipid lubricating agents in aqueous based drilling fluids. US Patent 7 094 738, assigned to M-I L.L.C., Houston, TX, August 22, 2006.
Patel, A.D., McLaurine, H.C., 1991. Drilling fluid additive. EP Patent 427 107, assigned to M I Drilling Fluids Co., May 15, 1991.
Penkov, A.I.; Vakhrushev, L.P.; Belenko, E.V., Characteristics of the behavior and use of polyalkylene glycols for chemical treatment of drilling muds, Stroit Neft Gaz Skvazhin Sushe More (1–2) (1999) 21–24.
Plank, J., 1993. Drilling mud composition and process for reducing the filtrate of metal hydroxide mixtures containing drilling mud compositions. WO Patent 9 312 194, assigned to Skw Trostberg AG, June 24, 1993.
In: (Editor: Rudnick, L.R.) Synthetics, Mineral Oils, and Bio-Based Lubricants: Chemistry and Technology, Vol. 111 of Chemical Industries (2006) Taylor & Francis, Boca Raton.
Runov, V.A., Mojsa, Y.N., Subbotina, T.V., Pak, K.S., Krezub, A.P., Pavlychev, V.N., Istomin, N.N., Evdokimova, Z.A., Borzenko, V.I., 1991. Lubricating additive for clayey drilling solution - is obtained by esterification of tall oil or tall pitch with hydroxyl group containing agent, e.g. low mol. wt. glycol or ethyl cellulose. SU Patent 1 700 044, assigned to Volgo Don Br. Sintez Pav and Burenie Sci Prod. Assoc., December 23, 1991.
Runov, V.A., Subbotina, T.V., Mojsa, Y.N., Krezub, A.P., Samotoj, A.K., Morgunov, A.N., 1992. Lubricant additive for clayey drilling muds – contains chalk, carbon black or graphite as mineral component, and glycol ester(s) of synthetic higher fatty acids as organic component. SU Patent 1 726 491, assigned to Volgo Don Br. Sintez Pav and Burenie Sci Prod. Assoc., April 15, 1992.
Ryan, L.D.; Kaler, E.W., Alkyl polyglucoside microemulsion phase behavior, Colloids Surf. A 176 (1) (2001) 69–83.
Ryzhov, V.M., Mironyuk, V.S., Mazepa, T.Y., Muravev, V.V., 1996. Controlling water absorption of drilling solution – based on carboxymethyl cellulose, involves introduction of sodium and-or potassium chloride into aqueous solution of carboxymethyl cellulose. RU Patent 2 066 684, September 20, 1996.
Sano, M., Polypropylene glycol (PPG) used as drilling fluids additive, Sekiyu Gakkaishi 40 (6) (1997) 534–538.
Selikhanovich, A.M., Chuprina, G.A., Olejnikov, A.N., 1997. Reagent for treating drilling muds – contains acrylic polymer, sodium hydroxide, additional diethylene glycol, and water. RU Patent 2 087 515, assigned to Volgo Urals Hydrogen Gas, August 20, 1997.
Senaratne, K.P.A., Lilje, K.C., 1994. Preparation of branched chain carboxylic esters. US Patent 5 322 633, assigned to Albemarle Corp., June 21, 1994.
Sifferman, T.R.; Muijs, H.M.; Fanta, G.F.; Felker, F.C.; Erhan, S.M., Starch-lubricant compositions for improved lubricity and fluid loss in water-based drilling muds, In: Proceedings Volume, no. 80213-MS, International Symposium on Oilfield ChemistrySociety of Petroleum Engineers, Inc., Houston, TX, 2003. (2003).
Sifferman, T.R., Swazey, J.M., Skaggs, C.B., Nguyen, N., Solarek, D.B., 1999. Fluid loss control additives and subterranean treatment fluids containing the same. WO Patent 9 905 235, assigned to Monsanto Co. and Natl. Starch Chem. Inv. Corp., February 4, 1999.
Sifferman, T.R., Swazey, J.M., Skaggs, C.B., Nguyen, N., Solarek, D.B., 2001. Fluid loss control additives and subterranean treatment fluids containing the same. US Patent 6 180 571, assigned to Monsanto Company, St. Louis, MO, National Starch and Chemical Investment Holding Corporation, Wilmington, DE, January 30, 2001.
Sifferman, T.R., Swazey, J.M., Skaggs, C.B., Nguyen, N., Solarek, D.B., 2002. Method of controlling loss of a subterranean treatment fluid. US Patent 6 492 305, assigned to CP Kelco U.S., Inc., Wilmington, DE, National Starch and Chemical Investment Holding Corporation, Wilmington, DE, December 10, 2002.
Sopko, T.M., Lorentz, R.E., 1991. Method of using polymers of amido-sulfonic acid containing monomers and salts as drilling additive. US Patent 5 039 433, assigned to Lubrizol Corp., August 13, 1991.
ASTM, Standard test methods for measurement of extreme pressure properties of fluid lubricants (falex pin and vee block methods). ASTM Standard, Book of Standards, Vol. 5.01 ASTM D 3233(93). (2009) ASTM International, West Conshohocken, PA.
ASTM, Standard test method for measurement of extreme-pressure properties of lubricating fluids (timken method). ASTM Standard, Book of Standards, Vol. 5.01 ASTM D 2782-02. (2008) ASTM International, West Conshohocken, PA.
Stephens, M., Swanson, B.L., 1992. Drilling mud comprising tetrapolymer consisting of N-vinyl-2-pyrrolidone, acrylamidopropanesulfonic acid, acrylamide, and acrylic acid. US Patent 5 135 909, assigned to Phillips Petroleum Co., August 4, 1992.
Tan, D., Test and application of drilling fluid filtrate reducer polysulfonated humic acid resin, Oil Drill. Prod. Technol. 12 (1) (1990) 27–32; 97–98.
In: (Editors: Totten, G.E.; Westbrook, S.R.; Shah, R.J.) Fuels and Lubricants Handbook: Technology, Properties, Performance, and Testing, Vol. 37 of ASTM Manual Series (2003) American Society for Testing & Materials (ASTM), West Conshohocken, PA.
Udarbe, R.G., Hancock-Grossi, K., George, C.R., 2000. Method of and additive for controlling fluid loss from a drilling fluid. US Patent 6 107 256, assigned to Fritz Industries Inc., August 22, 2000.
Umutbaev, V.N., Kamaletdinov, M.G., Andreson, B.A., Abdrakhmanov, R.G., Sharipov, A.U., Utyaganov, I.V., 1993. Lubricant additive for water-based drilling muds – contains a mixture of phenolic mannich bases, additional phosphoric acid and water. SU Patent 1 799 895, assigned to Bashkir Oil Ind. Res. Inst., March 7, 1993.
Wall, K., Martin, D.W., Zard, P.W., Barclay-Miller, D.J., 1995a. Temperature stable synthetic oil. WO Patent 9 532 265, assigned to Burwood Corp. Ltd., November 30, 1995.
Wall, K., Zard, P.W., Barclay-Miller, D.J., Martin, D.W., 1995b. Amide and imide compounds and their use as lubricant oils. WO Patent 9 530 643, assigned to Burwood Corp. Ltd., November 16, 1995.
Wang, Z., Synthesis of AMPS/AM (acrylamide)/VAC (vinyl acetate) copolymer – a filtration reducer of drilling fluid, Drill. Prod. Technol. 22 (4) (1999) 55–56; 4A.
Willey, T.F., Willey, R.J., Willey, S.T., 2007. Rock bit grease composition. US Patent 7 312 185, assigned to Tomlin Scientific Inc. Santa Ana, CA, December 25, 2007.
Zaleski, P.L., Derwin, D.J., Weintritt, D.J., Russell, G.W., 1998. Drilling fluid loss prevention and lubrication additive. US Patent 5 826 669, assigned to Superior Graphite Co., October 27, 1998.
Tradenames
| Tradename Description | Supplier |
|---|---|
| Aldacide® G Biocide, glutaraldehyde (Fisk et al., 2006) | Halliburton Energy Services, Inc. |
| AquaPAC® Polyanionic cellulose (Sifferman et al., 2001 and Sifferman et al., 2002) | Aqualon Corp. |
| BARASIL® S Sodium silicate shale stabilizer (Fisk et al., 2006) | Halliburton Energy Services, Inc. |
| BIOZAN® Heteropolysaccaride (Fisk et al., 2006) | Merck |
| COLALIPID™ (Series) Quaternized amines (Patel et al., 2006) | Colonial Chemical, Inc. |
| COLALIPID™ RC Ricinoleamidopropyl PG-dimonium chloride phosphate (Patel et al., 2006) | Colonial Chemical, Inc. |
| Finagreen® BMDF 2-ethyl hexanol fatty acid esters (Genuyt et al., 2006) | Petrofina S.A. |
| Hatcol™ 2372 Polyol ester of dipentaerythritol (Willey et al., 2007) | Hatco Corp. of Fords, N.J. |
| Hatcol™ 2926 Polyol ester of dipentaerythritol (Willey et al., 2007) | Hatco Corp. of Fords, N.J. |
| Kopr-Kote®Aluminum complex lubricant with copper flakes (Oldiges et al., 2007) | Jet-Lube, Inc. |
| Lucant® HC-2000 Hydrocarbon-based nonpolar synthetic oil (Denton et al., 2007) | Mitsui Chemicals America, Inc. |
| Lucant® HC-600 Hydrocarbon-based nonpolar synthetic oil (Denton et al., 2007) | Mitsui Chemicals America, Inc. |
| Retsch® ZM-1 Grinding mill (Fanta et al., 2002) | Retsch GmbH |
| Rev Dust Artificial drill solids (Fisk et al., 2006) | Milwhite, Inc. |
| Seppic SIMULSOL AS-48™ Alkylglucoside (Fisk et al., 2006) | Seppic |
| Spectrasyn ULTRA™ Grease formulation (Willey et al., 2007) | Exxon Mobil |
| Supersyn™ (Series) Lubricating oil (Willey et al., 2007) | Mobil Oil Corp., Union Carbide Corp. |
| Synalox® PB-200 Chain alcohols (Fisk et al., 2006) | Dow Chemical Comp. |
| Unirex S2®Zirconium 2-ethylhexanoate grease (Denton et al., 2007) | Exxon Mobil |
| XAN-PLEX™ D Polysaccharide viscosifying polymer (Fanta et al., 2002) | Baker Hughes INTEQ |
..................Content has been hidden....................
You can't read the all page of ebook, please click here login for view all page.