Chapter 6. Corrosion Inhibitors
The history of corrosion inhibitors and neutralizers and their invention, development, and application in the petroleum industry has been reviewed by Fisher (1993). Early corrosion inhibitor applications in each of the various segments of the industry, including oil wells, natural gas plants, refineries, and product pipelines, are included.
Corrosion and scale deposition are the two most costly problems in oil industries. Corrodible surfaces are found throughout production, transport, and refining equipment. The Corrosion and Scale Handbook gives an overview of the problems and methods of prevention Becker (1998).
In many oil field operations, the contact of fluids with air is inevitable. A striking example is recovery stimulation by in situ combustion. Reducing agents can be used to remove oxygen. The conditions must be controlled so that oxygen removal is complete, yet little unreacted excess scavenger remains in the system. In addition, mechanical scavenging can be accomplished by vacuum deaeration or counter-current scrubbing with an oxygen free gas. For economical reasons there are many systems where a one step corrosion inhibitor would be preferred.
Even when oxygen is not present in the corrosion system, oil field corrosion is associated with deposition conditions. Iron sulfide or other solid particles can deposit on the steel surface and prevent access by corrosion inhibitors. In some cases, these deposits can act as harbors for anaerobic bacteria, which can also become involved in the corrosion process. Sulfate-reducing bacteria can even produce their own environment beneath a biofilm that is safe from turbulence and flow velocities. As the biofilm grows, it forms an exoskeleton, which provides a site for the growth of sessile bacteria Martin et al. (2005). Hydrogen sulfide is produced by these bacteria and is released to the protected environment where it reacts with the dissolved iron from the corrosion process to form iron sulfide.
The biofilm is formed from polysaccharides and other related molecules forming a semi-permeable matrix. Within the pores of this biofilm, the sulfate-reducing bacteria grow and produce locally high concentrations of H2S, which accelerates the corrosion process and causes severe pitting. Electrochemical polarization curves had been used to show the particular conditions responsible for ferrous metal corrosion when oxygen contacts both sour H2S and sweet CO2 production fluids (Martin et al., 2005).
Carbon dioxide sweet corrosion is a well-known problem in gas production. Carbon dioxide dissolves in brine to form carbonic acid, which ionizes to yield a low pH value. This acidic solution strongly enhances corrosion in carbon steel pipes and facilities. The presence of carbon dioxide would lead to corrosion rates of several mm/year if no proper corrosion protection methods were undertaken (Oberndorfer et al., 2007).
Classification of Corrosion Inhibitors
Corrosion inhibitors have been divided into many groups, such as (Dietsche et al., 2007):
• Cathodic and anodic inhibitors,
• Inorganic and organic corrosion inhibitors, or
• Filming and non-filming inhibitors.
Low molecular weight corrosion inhibitors often change the surface tension of water. Actually these groups act as surfactants, since they form a protective layer on the metal surfaces (Dietsche et al., 2007). Polymeric corrosion inhibitors act in the same way as ordinary low molecular weight inhibitors.
Polymeric film-forming corrosion inhibitors differ from polymer coatings as they exhibit a specific interaction with the surface before the dry film is formed. Polymeric corrosion inhibitors may not form a barrier layer against oxygen and water, but instead they change the corrosion potential of the metal (Dietsche et al., 2007).
From the chemists's point of view, corrosion inhibitors can be classified into the following broad groupings:
• Amides and imidazolines,
• Salts of nitrogenous molecules with carboxylic acids, i.e., fatty acids and naphthenic acids),
• Nitrogen quaternaries,
• Polyoxylated amines, amides, imidazolines, and
• Nitrogen heterocyclics.
Fields of Application
Corrosion problems may occur in numerous systems within the petroleum industry. These include:
Many anticorrosion compositions involve environmentally dangerous products, such as chromates, fatty amines of high molecular weights, imidazolines, etc. The use of some of the alternatives, for instance, polyphosphate or polyphosphonate, is limited because they precipitate in the presence of the salts of alkaline earth metals, or because of their high costs.
Acidization
Acidization is an oil reservoir stimulation technique for increasing well productivity. Stainless steels have been used successfully to combat hydrogen sulfide (H2S) and carbon dioxide (CO2) corrosion, but these materials are susceptible to hydrochloric acid (HCl). HCl is used in oil and gas production to stimulate the formation.
The downhole temperature may be in excess of 200°C in deep wells, and acid treatment occurs through steel tubes, hence this process requires a high degree of corrosion inhibition. Electrochemical measurements are nonpredictive in inhibited concentrated HCl at high temperatures (Hausler, 1986).
Oil Storage Tanks
Storage tank bottoms are protected from corrosion through the use of cathodic protection. In general, this method is successful, but problems arise when there is not complete contact with the soil. This occurs when the bottom buckles slightly, leaving air spaces, after the filling or emptying of the tank. Or, over time, a portion of the base may erode away. In either case, the electrical continuity is lost. Other methods of protection, such as protective coatings, are not suitable.
When the bottom plates are welded together, the coating is partially destroyed. Research and field work showed that protection can be achieved using volatile corrosion inhibitors under the tank (Gelner, 1996). This works alone or in combination with cathodic protection.
Double tank bottoms for leakage monitoring are often specified for new tanks, but the same problem of coating destruction occurs. Volatile corrosion inhibitors are an excellent solution from both a technical and an economic standpoint. This type of corrosion inhibitor has a long history of corrosion protection under the conditions of wet, corrosive environments in void spaces.
Pipelines
The normal industrial practice for controlling the internal corrosion of petroleum pipelines is to use coatings, nonmetallic pipeline materials, or corrosion inhibitors. Corrosion inhibitors, which are used for the protection of oil pipelines, are often complex mixtures.
The consequences of pipeline failure can include inventory loss, production shutdown, environmental damage, safety risks, and excessive repair and replacement costs (Kennard and McNulty, 1993). Chemical treatment can delay or inhibit the internal corrosion of a pipeline so that the line can fulfill its operating requirements over its design life.
Using pigs for corrosion inhibitor applications is particularly useful in gas and gas-condensate transmission pipelines, especially in multiphase flow service. Selecting a pig for inhibitor batching is based on its ability to create a good seal between the pig cups and the pipe wall. The thickness of the film deposited during inhibition must be known to correctly size the slug inhibitor. Epoxide resins with aromatic amines are used as coatings for pipelines (Camberlin et al., 1999a, Camberlin et al., 1999b and Camberlin et al., 1999c).
Production Wells
Unalloyed or low-alloyed steels of various strength are generally used in the production of oil and gas. Inhibitors must be injected into the borehole to increase the life of well casing, flow lines, and equipment of unalloyed and low-alloyed steels in corrosive media. If the inhibitor is improperly chosen, considerable corrosion damage may result, such as damage without hydrogen influence and hydrogen-induced damage in the presence of H2S.
Agitator autoclave tests can be used as screening tests despite the more intensive localized corrosion attack and the generally greater erosion rates in field tests. This test method elucidates the influences of certain test parameters including temperature, H2S/CO2 ratio, and flow rate (Faessler, 1990).
Scale Removal Treatments Using Acids
Acids injected downhole for scale removal treatments are extremely corrosive to the production tubing and casing liners. Inhibitors are added to the stimulation fluids to minimize this corrosion. The effectiveness of inhibitors can be estimated with laboratory screening methods (Burger and Chesnut, 1992).
Application Techniques
Application techniques include batch and continuous application.
Batch Versus Continuous Application
Batch treatment of pipelines with liquid or gel slugs of inhibitor, with continuous injection as a backup (or vice versa), are accepted methods of corrosion prevention (Kennard and McNulty, 1992). Batching liquid or gel inhibitors using pigs is more likely to attain complete coverage of the internal surface of the pipe wall than is continuous injection.
The film laid down is quite resilient and long casting. Important factors to optimize the application include determining film thickness and selecting an appropriate pigging system and program. Cleaning of the pipeline before inhibitor pigging is recommended.
Emulsions
Corrosion inhibitors are often emulsions that are able to form an organic film on the parts to be protected.
Application in Solid Form
The preparation of a corrosion inhibitor in solid form allows the development of a new technique of continuous intensive anticorrosive protection for gas and oil pipelines, as well as for acidizing operations of oil wells (Guimaraes et al., 1994). The controlled dissolution of the solid inhibitor creates a thin protective layer on the metallic surface, which prevents or at least minimizes undesirable corrosion reactions.
Characterization
The common method of treating rod-pumped wells is to periodically batch the inhibitor into them. The treatment period for a given well is selected using empirical rules based on well production volumes. To be successful and economic, the corrosion inhibition program must carefully control the inhibitor concentration in the well fluids.
Environmental aspects and efficacious inhibitor usage necessitate the measurement of very low corrosion inhibitor concentrations. Inhibitor concentrations as low as one part per million are significant, thus requiring an analytical technique that has a detection limit of a fraction of a part per million.
Accurate monitoring of the residual concentrations of the inhibitors is most important in systems in which the volume of water is unknown, or is highly variable. Frequent monitoring of the inhibitor concentration in the water exiting the pipeline is the simplest, and sometimes the only method that can be used to ensure that the line in fact is being protected.
Dye Transfer Method
The classic method for the determination of corrosion inhibitors in oil field brines is the dye transfer method. This method is basically sensitive to amines, but has many variations that the analyst may use to determine the amount of corrosion inhibitor, in either water or crude oil. Unfortunately these methods detect all amines present as corrosion inhibitors (Matherly et al., 1995).
Liquid Chromatography
Improved high-pressure liquid chromatography and high-performance liquid chromatography (HPLC) methods have been developed for the analysis of quaternary salt type corrosion inhibitors in brine waters (Cossar and Carlile, 1993), but they are not suitable for imidazolines and amido amines. A method based on fluorescence detection has been described for the quantitative analysis of the imidazoline-type and amido amine type corrosion inhibitors in both oil field water and crude oil samples by HPLC (Matherly et al., 1995).
Another analytic procedure based on HPLC has been developed for the quantitative determination of nitrogen-containing corrosion inhibitors (McKerrell and Lynes, 1988). The method was primarily developed for the analysis of certain oil pipeline condensate samples.
A fully automated instrumental procedure has been developed for analyzing residual corrosion inhibitors in production waters in the field, using ultraviolet and fluorescence spectrophotometric techniques.
Laboratory evaluations have shown that fluorescence is more suitable for field application because it minimizes errors from high salinity, contamination, and matrix effects. Comparison of the automated fluorescence technique with the classic extraction-dye transfer technique showed the former to be easier, faster, and to have greater to accuracy, and precision (Son and Chakravarty, 1996).
Thin Layer Chromatography
Attempts have been made using thin layer chromatography, to analyze amounts of residual inhibitors down to less than one part per million (Buck et al., 1993).
Ultraviolet Spectroscopy
Ultraviolet spectroscopy can be used to detect low levels of organic corrosion inhibitors in produced water. An analytic method has been developed using a diode array ultraviolet spectrophotometer (Fortenberry et al., 1993).
Corrosion Tests
Immersion tests, weathering, electrochemical measurements, and microscopy are all used to monitor the effect of different classes of organic inhibitors and their synergy with other additives (Dietsche et al., 2007).
Standard procedures have been developed to remove corrosion products without significant removal of the base metal layer. This allows an accurate determination of the mass loss of the metal or alloy that has occurred during exposure to a corrosive environment (ASTM Standard, 2010b).
Electrochemical measurements of the corrosion rate often provide results in terms of an electrical current or electrical resistance. Although the conversion of these current values into mass loss rates or penetration rates is based on the law of Faraday, calculations can be complex for alloys and metals with elements having multiple valence values. Guidance in calculating mass loss and penetration rates for such alloys has been provided, and some typical values of equivalent weights for a variety of metals and alloys have been compiled (ASTM Standard, 2010a).
There is a standard that specifies the mechanisms of corrosion and parameters for the selection of materials for pipes, tubes, and equipment for the transport and processing of hydrocarbons. Guidelines are given for International Organization for Standardization (2010):
1. Corrosion assessments,
2. Choice of materials for specific applications and systems,
3. Performance of specific materials, and
4. Corrosion tests.
Standards concerning materials for use in H2S -containing environments in oil and gas production have also been provided (General principles for selection of cracking-resistant materials, 2009, International Organization for Standardization, 2009b and International Organization for Standardization, 2009c).
Side Effects
Stabilizer for Emulsions
Some corrosion inhibitors have a side effect of stabilizing emulsions. This is sometimes undesirable.
Antisynergism with Alcohols
In stimulation fluid that contains concentrated HCl, the partial substitution of water by alcohols such as methanol, ethanol, and glycerol increases the corrosivity of the acid fluids; and so reduces the efficiency of the corrosion inhibitors (Mainier et al., 1990). This effect is especially important for fatty amine based inhibitors. For products containing acetylenic-type inhibitors the detrimental effect is less important and weight losses may be maintained within acceptable limits by using slightly higher, but still reasonable, levels of inhibitor.
Synergism with Surfactants
Certain surfactants greatly improve the performance of trans-cinnamaldehyde as a corrosion inhibitor for steel in HCl (Growcock, 1987; Shah et al., 1994 and Shah et al., 1992, by enhancing the adsorption at the surface of the steel. Increased solubility or dispersibility of the inhibitor is an incidental effect. N-dodecylpyridinium bromide is effective in this aspect far below its critical micelle concentration, probably as a result of electrostatic adsorption that leads to the formation of a hydrophobic monolayer, which attracts the inhibitor. On the other hand, an ethoxylated, nonyl phenol acts by incorporating the inhibitor into micelles, which themselves adsorb on the steel surface and facilitate the adsorption of trans-cinnamaldehyde.
Interactions with Kinetic Gas Hydrate Inhibitors
Gas hydrate inhibitors are often added together with corrosion inhibitors, but the two may be incompatible in the formulation.
It has been discovered that quaternary alkylaminoalkyl alkoxy esters and amides, respectively, both exhibit an excellent performance as corrosion inhibitors and gas hydrate inhibitors, as well as an improved film persistence and good biodegradability (Dahlmann and Feustel, 2008).
The general method for the preparation of alkylaminoalkyl alkoxy monoesters from dicarboxylic anhydrides has been described in detail (Dahlmann and Feustel, 2008; Leinweber and Feustel, 2009). The anhydride is heated in nitrogen atmosphere with an alkoxylated alkylenediamine. The products are then quaternized with dimethyl sulfate. For example, N,N-dibutylamino-N-tri(ethoxy)ethyl dodecenyl-tetradecenylsuccinate is obtained from dodecenyl-tetradecenylsuccinic anhydride and ethoxylated dibutylamine. The method is also suitable for polymer analog synthesis.
As a consequence of their ester and amide structure, these compounds have better biodegradability and can be used at a lower dosage (Dahlmann and Feustel, 2008). A series of compounds has been synthesized and tested both as gas hydrate inhibitors and corrosion inhibitors.
Interactions take place between kinetic gas hydrate inhibitors and corrosion inhibitors. Two theories concerning these interactions have been developed and tested. The first theory involves competition between them, which at the surface interface can be elucidated by measurements of the surface tension. The second theory postulates absorption of the corrosion inhibitor onto a polymeric kinetic gas hydrate inhibitor.
One commercially available kinetic gas hydrate inhibitor, poly(vinylcaprolactam) and three commercial available corrosion inhibitors, cocodimethyl benzyl ammonium chloride, aminoethyl fatty imidazoline, and an ethyoxylated phosphate ester were used to test these ideas (Moore et al., 2009).
The corrosion inhibitors had a varying negative impact on the kinetic gas hydrate inhibitor. However, the results from the corrosion testing indicate only a minimal interference of the performances. The efficiency of all corrosion inhibitors tested have been found to be dependent on the structure of the polymer (Moore et al., 2009), indicating that the second theory (absorption) is more sound.
Effect of Flow on Inhibitor Film Life
Experiments using low- and high-velocity conditions were performed in standard laboratory tests (Eaton and Sutton, 1994). It was found that corrosion is governed by the flow of reactants and products to and from the corroding surface. Corrosion in oxygenated fluids increases with the velocity of the fluid because a greater amount of oxygen is made available to the surface.
Corrosion of steel in fluids containing CO2 produces a protective iron carbonate film that initially results in decreased corrosion. However, at high velocities the protective layers are broken off, thus exposing the bare metal to the aggressive medium and increasing the corrosion rate. Inhibitor films are protective because they reduce the transfer rate of the corrosants, but they can become ineffective because of aging, removal, and dilution. In all of the previous examples the velocity is an important variable, governing the ability of the inhibitor to control the corrosion rate.
Inhibitor Chemicals
Amides and Imidazolines
Amides
An amide-type corrosion inhibitor is prepared as follows: methyl methacrylate is converted with tallow triamine or tallow tetramine at 80–90°C into the corresponding amides. After completion of this reaction, the temperature is raised to initiate polymerization (Niu et al., 1988), which is performed at temperatures up to 200°C. The polymer controls the corrosion of metal surfaces in contact with a corrosive hydrocarbon-containing medium.
Ammonium salts of alkenyl succinic half-amides have been described as corrosion inhibitors to combat corrosion in media containing CO2, H2S, and elemental sulfur (Oppenlaender et al., 1993). The inhibitor composition may contain a dispersing agent, such as a low molecular weight or polymeric anionic surfactant, such as an alkylsulfonic acid or an alkyl-aryl sulfonic acid.
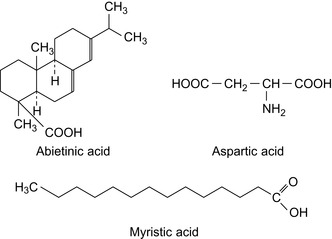
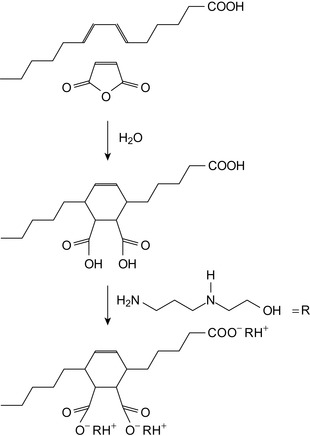
Ethoxylated and propoxylated alkyl phenol amines, converted into the amides with a fatty acid or similar long chain diacids, are effective in controlling sour and sweet corrosion (Valone, 1987a, Valone, 1987b, Valone, 1989a, Valone, 1989b, Valone, 1989c and Veldman and Trahan, 1999). Properties of fatty acids are shown in Table 6.1 and the structure of some acids are shown in Figure 6.1.
Tall oil is a waste product from the paper making industry. Tall oil derivates have been proposed as alterative biofuel materials (AltIparmak et al., 2007). Tall oil fatty acids consist of resinic acids and of a mixture of linolic acid, conjugated C18 fatty acids, oleic acid, 5,9,12-octadecatrienic acid, and saturated fatty acids. Resin acids are abietinic acid, dehydroabietic acid, and others. The overall composition of tall oil fatty acids is shown in Table 6.2.
| Component | Amount/[%] |
|---|---|
| Resinic acids | 40–50 |
| Fatty acids | 30–40 |
| Unsaponifiable material | 10 |
Polyimido amines
Corrosion inhibiting compositions for metals that are subjected to highly acidic environments may be produced by reacting a styrene/maleic anhydride (MA) copolymer with a poly(amine) in a condensation reaction to produce a polyimido amine inhibitor (Schilling, 1995). Such inhibitors exhibit film forming characteristics. Some relevant poly(amine)s are listed in Table 6.3. Diamines are shown in Figure 6.2.
Polypeptides
Polypeptides have been under consideration as corrosion inhibitors because of their environmental acceptability (Obeyesekere et al., 2001). Polyaspartate is the most efficient corrosion inhibitor known among the polypeptides (McMahon and Harrop, 1995). Its molecular weight (1–22 kDalton) does not affect its efficiency, but both high calcium ion concentration and high pH enhance the effectiveness. The performance was particularly good in batch treatment tests.
In another study, polyaspartic acid was examined as a corrosion inhibitor for steel over a range of pHs and temperatures (Silverman et al., 1995). At low to neutral pH values, it increases the corrosion rate of steel, but at pH values above 10, polyaspartic acid is a reasonably robust corrosion inhibitor.
Ampholytes
Corrosion inhibitors used in offshore oil production are highly cationic, but they are becoming less acceptable for environmental reasons. Cationic inhibitors are attracted to metal surfaces, thereby controlling the acid type corrosion. When these cationic corrosion inhibitors enter sea water, they are attracted to diatoms, which are type of algae. These algae are part of the food chain for mussels.
Betaines, shown in Figure 6.3 and ampholytes (Larsen, 1991) can be used instead of cationic inhibitors or can be neutralized with acids such as acetic acid, adipic acid, sebacic acid, naphthenic acids, paraffinic acids, tall oil acids, and free sulfur dioxide. They are claimed to prevent CO2 corrosion.
Slow-release Formulation
An amido amine obtained from the reaction of tetraethylenepentamine with stearic acid is modified with propylene oxide. The product is dispersed in a polymer matrix such as an acrylic or methacrylic polymer. This inhibitor is slowly released into the surrounding environment, such as in an oil or gas well, to prevent corrosion of metal equipment in the well.
Salts of Nitrogenous Bases
A corrosion inhibitor with excellent film forming and persistency characteristics is produced by first reacting C18 unsaturated fatty acids with MA or fumaric acid to produce the fatty acid Diels-Alder adduct or the fatty acid-ene reaction product (Alford et al., 1994). This reaction product is further reacted in a condensation or hydrolyzation reaction with a polyalcohol to form an acid anhydride ester corrosion inhibitor. The ester may be further reacted with amines, metal hydroxides, metal oxides, ammonia, and combinations thereof to neutralize the ester.
Surfactants may be added to tailor the inhibitor formulation in order to meet the specific needs of the user, for instance the corrosion inhibitor may be formulated to produce an oil-soluble, highly water-dispersible corrosion inhibitor or an oil-dispersible, water-soluble corrosion inhibitor. Suitable carrier solvents may be used as needed to disperse the corrosion inhibitor formulation.
Similarly, a salt of an ethoxylated amine and a reaction product of an alcohol and a fatty acid MA adduct produced by a reaction between MA and an unsaturated fatty acid has been described (Dougherty et al., 1996).
Nitrogen Quaternaries
Quaternary ammonium iodides were tested, alone and in combination with propargyl alcohol, with several steels in 15% HCl. The quaternary ammonium iodides showed a better inhibitor performance to that of propargyl alcohol (propargyl: –CH2–CCH) at identical dosage levels. Mixtures of propargyl alcohol and quaternary ammonium iodide showed a synergistic effect (Neemla et al., 1992), as did formic acid (Brezinski and Desai, 1998) and thiols (Vorderbruggen and Williams, 2000).
It has been shown that the corrosion rates of various steels can be reduced to less than 1 mg cm−2hr−1 by using ternary inhibitor mixtures containing quaternary ammonium salts, trans-cinnamaldehyde, and potassium iodide in amounts of 0.2% of each component (Trabanelli et al., 1988).
Thio-Substituted Salts
A thio-substituted, quaternary ammonium salt can be synthesized by the Michael addition of an alkyl thiol to acrylamide (AAm) in the presence of benzyl trimethyl ammonium hydroxide as a catalyst (Haslegrave and Sullivan, 1987). The reaction leads to the crystallization of the adducts in essentially quantitative yield.
Reduction of the amides by lithium aluminum hydride in tetrahydrofuran solution produces the desired amines, which are converted to desired halide by reaction of the methyl iodide with the amines. The inhibitor is useful in controlling corrosion such as that caused by CO2 and H2S.
Synergism of Thiosulfate
Laboratory observations have revealed that a combination of thiosulfate with cationic nitrogenous inhibitors has a significant effect on improving their performance (Phillips et al., 1996).
Polyoxylated Amines, Amides, and Imidazolines
A mixture of alkyl-ethylene diamine and di-alkyl-diethylene triamine, with an alkyl side chain of 8–26 carbon atoms is suitable (Young, 1993) as a corrosion inhibitor. This product can be further reacted with an alkylating agent or an alkylene oxide (Ho, 1993 and Ho, 1994).
The inorganic nitrite used as a corrosion inhibitor in aqueous alkylene glycol or polyalkylene glycol solutions can be replaced with polyoxyalkylene amines (Morris-Sherwood and Brink, 1987 and Morris-Sherwood and Brink, 1990). Such polyoxyalkylene amines impart corrosion inhibition to the liquid in contact with the metal and the metal in contact with the vapors of the aqueous composition. Aqueous compositions containing the glycol and the polyoxyalkylene amine also exhibit a low foaming tendency.
Mercaptan Modified Products
In highly acidic environments, a reaction product of an isobutyraldehyde and an alkylene amine compound with an alkylsulfopropionic amide group is recommended (Zetlmeisl and French, 1992a and Zetlmeisl and French, 1992b). The alkylene amine compound can be the product of a reaction of equimolar amounts of N-dodecylmercaptan, methyl methacrylate, and diethylene triamine.
Polyamine Derivatives
Fatty Amine Adducts
Dimerized fatty acid thioesters (with a dithiol), in combination with fatty amines, are sulfur-containing corrosion inhibitors Incorvia (1988b), which are best used in a hydrocarbon solvent.
Adducts of a fatty amine adduct to unsaturated acid in which the product contains only secondary or tertiary amine groups have a lower toxicity to the environment (Clewlow et al., 1992).
Adducts to Polymers
Polymeric polyolefins, such as polybutadiene, secondary amines, and synthesis gas, are reacted in the presence of a catalyst system comprising a ruthenium-containing compound, a rhodium-containing compound, a sterically hindered phosphine, and a solvent (McEntire and Knifton, 1987).
Preferred polybutadiene feedstocks are those with a predominance of straight chain, rather than pendant olefin groups and in particular, those polymers containing both the 1,2-polybutadiene and 1,4-polybutadiene units. These polymers of high amine content are useful as downhole corrosion inhibitors.
A low molecular weight, polyfunctional polymer can be formed by polymerizing a vinyl monomer in the presence of a mercaptan chain transfer agent (Wu and Gray, 1992). The vinyl monomer may be an unsaturated acid, acrylonitrile, vinylester, a variety of AAms, or N-vinyl-2-pyrrolidone. The molar ratio of the vinyl monomer to the mercaptan is preferably in the range of 2–40 mol of the vinyl monomer to 1 mole of the mercaptan. The composition and methods are useful for inhibiting corrosion of downhole metal surfaces present in oil and gas wells. Relevant vinyl monomers are shown in Figure 6.4.
Formaldehyde Condensates with Amines
Corrosion inhibitor compositions useful for oil and gas well applications are prepared by reacting 2,5-dimethylpyridine or 2,4,6-collidine with formaldehyde or acetone and an amine such as 1-dodecanamine (Treybig and Martinez, 1988 and Treybig and Martinez, 1989). A hydrocarbon-soluble corrosion inhibitor is obtained by the acid-catalyzed oligomerization of an alkylaniline and formaldehyde (Bacskai and Schroeder, 1988b).
These oligomers exhibit good initial inhibition of metal corrosion in aqueous environments, and this effect is more persistent than that observed for the corresponding monoamine starting material. Moreover, in an acidic environment, the products show superior persistence in inhibiting corrosion when compared with known monoamine corrosion inhibitors, such as tallow amine.
The oligomers can be formulated to be both hydrocarbon-soluble and water-dispersible. The water dispersibility can be controlled by varying the type and amount of the additional aromatic compound, such as ethoxylated alkyl phenol, that is included in the oligomerization reaction mixture (Bacskai and Schroeder, 1988a).
A corrosion inhibitor that is the adduct of a carbonyl compound, an amine, and a thiocyanate has been described (Petersen et al., 1990). The product provides protection against ferrous corrosion in severe environments at concentrations of 500ppm. The inhibitor is employed in wells that produce both oil as well as water in high-temperature environments around 120°C.
Lignin Amines
Lignin amines with high nitrogen content are water-soluble at both alkaline and acidic pH values, and have various useful properties. For instance, they are active as flocculants, filtration aids, scale inhibitors, fluid loss additives, oil well cement additives, and corrosion inhibitors among other potential uses. The nitrogen is introduced into the lignins by the Mannich reaction (Schilling and Brown, 1988).
Amido Amine Salts
Amido amine salts have been found to be effective corrosion inhibitors, and are essentially environmentally friendly. They are used in formate solutions, as long as the high pH environment does not hydrolyze the amido group.
The compounds can be prepared by the reaction of amines with a fatty acid. For example, soya fatty acid can be reacted with N-ethyl ethylene diamine by heating to 150°C for a period of 4 h. The reaction product is then solubilized by forming the salts with acetic acid (Miksic et al., 2004).
Tenax 2010 (Westvaco) is a commercially available inhibitor, which is obtained from the Diels Alder reaction of MA and a fatty acid and subsequently with amines. The amido diacid is solubilized with 2-amino-2-methyl-1-propanol or ethanolamine. The reaction is shown schematically in Figure 6.5.
 |
| Figure 6.5 Diels Alder reaction of a conjugated unsaturated fatty acid (Fischer and Boyd, 1998). |
Fatty Acid Amides
Propargyl alcohol has been found to be active in corrosion control, and a variety of formulations containing it have been proposed. Propargyl alcohol is shown in Figure 6.6.
A condensate of a polyamine, such as diethylene triamine, triethylenetetramine, or aminoethylethanol amine, with C21 or C22 carbon fatty acids or tall oil fatty acids, can be used as corrosion inhibitor base (Schilling and Braddon, 1986). Propargyl alcohol has been found to enhance the anticorrosive effects of this composition.
Most simply, a mixture of mainly 80–90% propargyl alcohol and cellosolve, with minor amounts of polyglycol, amine derivatives, a phenol-formaldehyde resin, and tar bases, has been described (Briggs, 1987 and Briggs, 1990).
Instead of propargyl alcohol, propargyl ether has been proposed as a corrosion inhibitor. Propargyl alcohol is added to olefins to form the corresponding ether (Karaev et al., 1996).
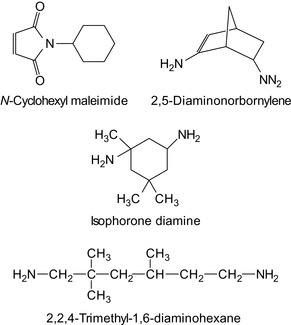
Fatty acid amides of isophorone diamine, 2,5-diaminonorbornylene, and 2,2,4-trimethyl-1,6-diaminohexane are particularly suitable for high-temperature and high-pressure applications (Kissel, 1999). The respective compounds are shown in Figure 6.7.
Nitrogen Heterocyclics
Hexamethylenetramine
Acetylenic alcohols such as propargyl alcohol, 1-hexyn-3-ol, and 5-decyne-4,7-diol have been used as corrosion inhibitors in HCl for the protection of ferrous metals. However, acetylenic alcohols are expensive and their use at temperatures in the range of 80–180°C (180–350°F) has been limited by the high concentrations that are needed to achieve the desired corrosion protection (Funkhouser et al., 2001).
The presence of a small amount of hexamethylenetetramine dramatically improves the performance of the acetylenic alcohols in reducing corrosion and enables their use at lower concentrations or higher temperatures than when used alone. Hexamethylenetetramine also acts as a sulfide scavenger, whereby the formation of free sulfur or the formation of ferrous sulfide precipitate is prevented.
The metal corrosion inhibiting compositions can also include solvents. The formation of ferric hydroxide precipitate, free sulfur, and other precipitates can be prevented.
Imidazolines
Quaternized imidazolines with an amido moiety are suitable corrosion inhibiting formulations for general oil and gas field applications. The synthesis of such compounds is detailed in the literature (Meyer, 2001). For aqueous systems that contain sulfide compounds, a mixture has been described that consists of an aqueous solution of an alcohol, such as diethylene glycol monobutyl ether, butyl cellosolve, additional orthophosphoric acid, a tall oil fatty acid, a substituted imidazoline, an ethoxylated fatty diamine, and a molybdate compound (Brown et al., 1996).
A modification of the previous formulation uses amine products preferably containing only tertiary amino groups Williams et al. (1994). These amines have favorable ecotoxicity levels in marine or fresh water environments. The ecotoxicity decreases with increasing substitutions on the nitrogen atoms present. It appears that tertiary groups are less toxic than secondary groups, which are in turn less toxic than primary groups. Combinations of imidazoles with wetting agents also have been described (Braga et al., 2000).
Water-soluble corrosion inhibitors are necessary to prevent corrosion of pipe walls, joints, pumps, and collection stations. An ampholytic, substituted imidazoline has been described for inhibiting corrosion in such systems (Byrne and Johnson, 1994). This type of corrosion inhibitor is intended for continuous treatment.
Pourable emulsions comprising up to 50% of a kerosene-containing corrosion inhibiting compound have been claimed to allow longer treatment intervals (French et al., 1991a and French et al., 1991b). A formulation that is resistant to sludge formation and does not tend to stabilize oil–water emulsions has been described in the literature. An imidazoline derivate, prepared from a long chain fatty acid and a polyamine, is dissolved in an aromatic solvent and dispersed with glycolic acid and hexylene glycol (McCullough, 1991).
In water systems, sulfate-reducing bacteria and sulfides are present. To prevent their growth, chlorine dioxide (ClO2) is added, but this is highly corrosive to the metallic components used in oil field equipment. Chromates are successful ClO2 corrosion inhibitors, but they are also undesirable because of their high toxicity. A mixture of an alcohol, an acid, a fatty imidazoline, an ethoxylated fatty diamine, and water can be used (Ohlsen et al., 1995) as an alternative. Such a composition has proved to be more effective than chromates inhibiting the corrosion caused by ClO2, without the serious toxicologic effects.
It has been reported that the corrosion rate of steel in the presence of H2S is greatly decreased by adding imidazoline compounds or quaternary ammonium salts into the drilling fluids (Jiashen and Jingmao, 1993). A concentration of 2 gl−1 gives an inhibition efficiency of 70–90%. If it is used together with the H2S scavenger–alkaline zinc carbonate, the inhibition becomes even more effective.
A synergistic effect is found between imidazoline inhibitors and calcium oxide. They also inhibit dioxide corrosion to some extent in H2S-free drilling muds. Moreover, imidazoline can improve the rheologic properties of a drilling mud.
Polyesters may be used (Alford et al., 1992, Alford et al., 1993a, Alford et al., 1993b and Alford et al., 1993c; Boyd et al., 1993) instead of a fatty acid modifier for imidazoline. In this way, a corrosion inhibitor that forms persistent films can be produced by first reacting a polybasic acid with a polyalcohol to form a partial ester. The partial ester is reacted with imidazoline or fatty diamines to result in a salt of the ester.
Pyridinium Compounds
Aliphatic pyridinium salts or aliphatic quinolinium salts in the presence of a sulfur-containing compound have been claimed to be active as corrosion inhibitors (Kennedy, 1987). N-(p-Dodecylphenyl)-2,4,6-trimethylpyridinium sulfoacetate is suitable as an inhibitor in aqueous media (Fisk and Tucker, 1991). Such pyridinium compounds exhibit greater thermal stability than N-aralkyl pyridinium compounds or N-alkyl pyridinium compounds, and the desired properties are retained both during and after exposure to elevated temperatures.
An α,β-ethylenically unsaturated aldehyde, together with organic amines, will form intermediate products, which are further reacted with a carboxylic acid, an organic halide, or an epoxide-containing compound (Treybig and Glass, 1988). The final products are suitable corrosion inhibitors for preventing corrosion of steel in contact with corrosive brine and oil and gas well fluids.
Still bottom residues, as produced in the distillation of quinoline from coal tar, can be oxidized and are then suitable as a metal corrosion inhibitor for use in aqueous acid solutions (Brezinski, 1998).
Azoles
The effectiveness of various chemicals, such as 1H-benzotriazole, 2-methylbenzotriazole, and 2-phenylbenzimidazole as a corrosion inhibitor for mild steel in 15% HCl was investigated, using weight loss and electrochemical techniques (Samant et al., 1989). 2-Phenylbenzimidazole showed the best performance of the azoles. A synergism of iodide and 2-phenylbenzimidazole was observed. Azoles are shown in Figure 6.8.
Aminopyrazine with Epoxide Compound
A condeasate of condensate aminopyrazine and an epoxide compound, such as the glycidyl ether of a mixture of C12 to C14 alkanols (Fischer, 1990) acts as an inhibitor during drilling and servicing of oil and gas wells.
Carbonyl Compounds
Aldehydes with Surfactants
Mixtures of aldehydes such as trans-cinnamaldehyde with surfactants are active in preventing corrosion, in particular in the presence of mineral or organic acids (Frenier and Growcock, 1988b). The surfactant used was N-dodecylpyridinium bromide or the reaction product of trimethyl-1-heptanol with ethylene oxide (Frenier and Growcock, 1988a). Such aldehyde and surfactant mixtures provide greater and more reliable corrosion inhibition than the respective compositions containing aldehydes alone.
Aldose Group Antioxidants
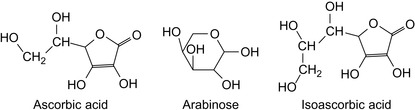
Sodium, ammonium, or calcium thiocyanate alone, or in combination with specific aldose group antioxidants can be used as corrosion inhibitors with calcium-free drilling, completion, and workover fluids in carbonate-containing or sulfate-containing wells (Dadgar, 1988a, Dadgar, 1988b and Dadgar, 1988c). Aldose group antioxidants include arabinose, ascorbic acid, isoascorbic acid, gluconic acid, and their corresponding salts. In addition, ammonium thioglycolate may be incorporated as another corrosion inhibitor. Thio groups and aldose group antioxidants exhibit synergistic properties (Shin, 1988). Aldose derivates are shown in Figure 6.9.
Similarly, a high-density brine, useful as a drilling fluid for deep wells, is made corrosion resistant by adding an aliphatic or aromatic aldehyde and thiocyanates (Henson and Doty, 1990). The aldehyde can be reacted with a primary amine before use.
Phosphate Esters
Phosphate ester-type inhibitors are produced by the reaction of ethoxylated, propoxylated, or butoxylated alcohols or phenols with phosphating agents (Naraghi, 1997; Naraghi and Grahmann, 1997; Walker et al., 2001). Inhibitors for both general corrosion and cracking-type corrosion are obtained by the reaction of a nitrogen base and a phosphate ester (Martin, 1988 and Martin, 1993).
Although the nitrogen bases and phosphate esters have good general corrosion inhibition properties, neither provide suitable inhibition for cracking-type corrosion, but the neutralization product of the two provides inhibition of both general and cracking-type corrosion. This inhibitor type is safe for aquatic organisms and is biodegradable.
Silicate-based Inhibitors
Silicates (Mainier et al., 1992) offer advantages with respect to low costs, low toxicity, and low environmental impact.
Thioacetals
Many corrosion inhibitors are useful only at selected temperature levels or pH ranges for the various heavy brines. Temperature changes, or any change that affects the pH of the brine often results in loss of the corrosion inhibition. Particular problems arise in the selection of corrosion inhibitors for use in zinc halide-containing heavy brine solutions.
Many common corrosion inhibitors, such as organic thiophosphates, quaternized amines, polyphosphate esters, and filming amines, form precipitates or are ineffective when admixed with zinc halide-containing heavy brine solutions (Welton and Cassidy, 2007). Thioacetals can be synthesized by the reaction of an aldehyde with a thiol.
Preparation 6–1
Thioacetal corrosion inhibitors are formed by the reaction of cinnamaldehyde with thioethanol in glacial acetic acid. p-Toluene sulfonic acid is added as catalyst (Welton and Cassidy, 2007).
The reaction is shown in Figure 6.10. In the same way, other thioacetal compounds have been synthesized and tested for corrosion. The various thioacetals are summarized in Table 6.4.
 |
| Figure 6.10 Formation of thioacetals (Welton and Cassidy, 2007). |
| Number | Name |
|---|---|
| 1 | Cinnamaldehyde 1,2-thioethanol dithioacetal |
| 2 | Cinnamaldehyde 1,2-dimercapto ethane dithioacetal |
| 3 | Cinnamaldehyde thioacetic acid dithioacetal |
| 4 | Crotonaldehyde 1,2-thioethanol dithioacetal |
| 5 | Crotonaldehyde 1,2-dithiolane dithioacetal |
To test their activity, a weighed coupon was suspended from a Teflon® holder inside a cell constructed of glass. The cell was then placed in an autoclave, 100 ml of 15% HCl was poured into the cell, and then enough kerosene was added such that the coupon was sufficiently submerged. Finally, the contents of the test cells were infused either with traditional corrosion inhibitors, no inhibitor, or an above-described thioacetal inhibitor at a concentration of 0.00378 mol. The autoclave was then pressurized to 68 atm (1000 psig) under a nitrogen atmosphere, and heated to 107°C for a total contact time of 3 h. The results are summarized in Table 6.5.
| Inhibitor | Corrosion Loss/[kg m−2] |
|---|---|
| None | 2.13 |
| Cinnamaldehyde | 0.81 |
| Thioglycolic acid | 4.11 |
| 1 in Table 6.4 | 0.07 |
| 2 in Table 6.4 | 0.10 |
| 3 in Table 6.4 | 0.18 |
| 4 in Table 6.4 | 0.13 |
| 5 in Table 6.4 | 0.38 |
The dithioacetal based on cinnamaldehyde and 1,2-thioethanol was very effective in preventing corrosion (Welton and Cassidy, 2007), but thioglycolic acid was found to enhance the rate of corrosion.
Miscellaneous Inhibitors
Antimony Halides
Antimony tribromide and other group VA halides minimize the corrosion rate effectively (Verma and Sandor, 2001). Unfortunately, antimony tribromide is toxic.
Aldol-amine Adducts
The corrosion of metal surfaces and the precipitation of a metal sulfide by an aqueous acid solution can be prevented by an aldol-amine adduct. Aldol (from acetaldehyde) CH3–CH(OH)CH2CHO has been utilized as a H2S scavenger that prevents the precipitation of metal sulfides from aqueous acid solutions. However, when the aldol or an aqueous solution of the aldol is stored, the solution separates quickly into two layers, with all of the aldol concentrated in the bottom layer. The bottom layer is not redispersible in the top layer or in water or acid. This bottom layer has very little activity as a sulfide scavenger, so the use of aldol as a H2S scavenger in aqueous acid solutions can give unsatisfactory results (Brezinski, 2001a and Brezinski, 2001b).
The aldol can be reacted with an amine, such as monoethanoleamine (= aminoethanol), to form an aldol-amine adduct to overcome these difficulties. The amine must be a primary amine, however. The aldol-amine adduct preferentially reacts with sulfide ions when they are dissolved in the acid compositions, thereby preventing the dissolved sulfide ions from reacting with dissolved metal ions and precipitating.
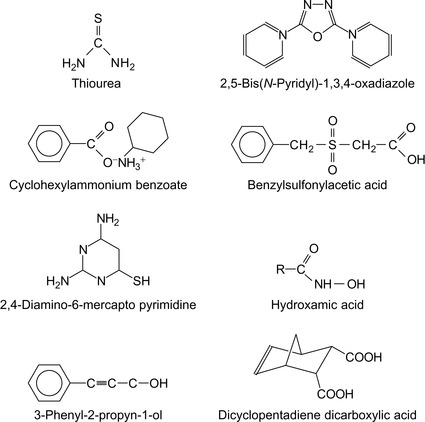
Some formulations that cannot be readily classified into any of the previous sections are summarized in Table 6.6. Some of these compounds are shown in Figure 6.11.
| aIn combination with ClO2treatment for bacteria control. | |
| bAqueous HCl | |
| c0.1–6% with antifreezers such as glycols | |
| dGas stream containing H2S or CO2 | |
| eForms a film of iron disulfide | |
| fRelatively non-toxic, substitution of chromate-based corrosion inhibitors, conventional phosphate, and organophosphonate inhibitors and the zinc-based inhibitors | |
| gCO2environment | |
| hCalcium chloride brine | |
| i10–500 ppm | |
| j5–200 ppm to inhibit naphthenic acid corrosion | |
| kIn drilling equipment | |
| Compound | References |
|---|---|
| Acetylinic alcohola | Teeters (1992) |
| Tall oil fatty acid anhydrides | Fischer and Parker (1997) |
| 3-Phenyl-2-propyn-1-olb | Growcock and Lopp (1988) |
| Dicyclopentadiene dicarboxylic acid saltsc | Darden and McEntire, 1986 and Darden and McEntire, 1990 |
| Hydroxamic acid | Fong and Shambatta (1993) |
| Cyclohexylammonium benzoate | Johnson and Ippolito, 1994 and Johnson and Ippolito, 1995 |
| Acyl derivatives of tris-hydroxy-ethyl-perhydro-1,3,5-triazine | Au (1986)& Au and Hussey (1989) |
| 2,4-Diamino-6-mercapto pyrimidine sulfate combined with oxysalts of vanadium, niobium, tantalum or titanium, zirconium, hafnium | Ramanarayanan and Vedage (1994) |
| Aqueous alkanol amine solutiond | Schutt (1990)& Veldman and Trahan (1999) |
| Quaternized fatty esters of alkoxylated alkyl-alkylene diamines | Wirtz et al. (1989) |
| Mercaptoalcohols | Ahn and Jovancicevic (2001) |
| Polysulfidee | Gay et al. (1993) |
| Polyphosphonohydroxybenzene sulfonic acid compoundsf | Kreh (1991) |
| 1-Hydroxyethylidene-1,1-diphosphonic acidg | Sekine et al. (1991) |
| 2-Hydroxyphosphono-acetic acidh | Zefferi and May, 1994a and Zefferi and May, 1994b |
| Water-soluble 1,2-dithiol-3-thionesi | Alink (1991)& Oude Alink (1993) |
| Sulfonated alkyl phenolj | Babaian-Kibala (1993) |
| Polythioether | Incorvia (1988a) |
| Thiazolidines | Alink and Outlaw (2001) |
| Substituted thiacrown ethers pendent on vinyl polymers | Minevski and Gaboury (1999) |
| Benzylsulfinylacetic acid or benzylsulfonylacetic acid | Lindstrom and Mark (1987) |
| Halohydroxyalkylthio-substituted and dihydroxyalkylthio-substituted polycarboxylic acidsk | Lindstrom and Louthan (1987) |
| Alkyl-substituted thiourea | Tang et al. (1995) |
| 2,5-Bis(N-pyridyl)-1,3,4-oxadiazoles | Bentiss et al. (2000) |
Encapsulated Types
Conventionally, corrosion inhibitors are applied by two basic modes: batch treatment and continuous chemical injection. Encapsulated time-release corrosion inhibitor has been shown to be highly effective against CO2 corrosion in a field trial, and combines the advantages of both methods.
Here, the corrosion inhibitor is released in a time-controlled manner from the water-hydrocarbon interfacial region in the annulus of the well. The product is delivered like a continuous corrosion inhibitor treatment while using a conventional batch application, and exhibits a significant increase in efficiency (Weghorn et al., 2007).
Anti-biofoulant Corrosion inhibitors
Formulations containing didecyl dimethyl ammonium chloride, poly(oxy-1,2-ethandiyl) tridecyl hydroxy phosphate, and potassium dimethyl dithiocarbamate were found to be very effective inhibitors. The thiocarbonyl compound provides additional inhibition (Martin et al., 2005).
These corrosion inhibitor compositions provide a tenacious, smooth, protective film that resists the adhesion of iron sulfide, sessile bacteria, and other solids, hence they are also referred to as antibiofoulant corrosion inhibitors.
The reduced corrosion rates observed were 4–5 milsy−1 ( ms−1) in contrast to 15–35 milsy−1 (
ms−1) in contrast to 15–35 milsy−1 ( ms−1) for uninhibited systems (Martin et al., 2005).
ms−1) for uninhibited systems (Martin et al., 2005).
Formic Acid Free Formulation
Acid corrosion inhibitors used for oil field applications normally contain formic acid components, or compounds that produce formic acid when exposed to well conditions. Although used successfully in well stimulation operations, they have been associated with the corrosion of pipelines and other equipment (Ali et al., 2010).
Unsaturated aldehydes and ketones have been found to be suitable replacements. Some examples are given in Table 6.7. The treatment fluid is substantially free of any formic acid or its precursor.
| Compound | Amount/[%] |
|---|---|
| Inhibitor 1 | |
| Isopropanol | 25 |
| Cinnamaldehyde | 35 |
| Benzyl quinolinium chloride | 15 |
| Ethoxylated C11 alcohols | 15 |
| Inhibitor 2 | |
| Mixture of methanol and isopropanol | 10 |
| Water | 8 |
| Naphthyl methyl quinolinium chloride | 25 |
| Ethoxylated tridecyl alcohol | 10 |
| 3-Methoxy-2-benzoyl-1-propene | 8 |
| Others | 1–3 |
| Inhibitor 3 | |
| Methanol | 35 |
| Propargyl alcohol | 5 |
| Others | 60 |
These types of inhibitors are used for viscoelastic diverting acids, formulated by mixing a viscoelastic surfactant (VES) with the acid prior to injection into the formation. The VES is a surfactant that under certain conditions can impart viscoelasticity to a fluid (Ali et al., 2010).
Intensifiers
Corrosion inhibitor intensifiers have been used to extend the performance range of a selected acid corrosion inhibitor. The term corrosion inhibitor intensifier refers to compounds that are capable of enhancing the performance of a selected acid corrosion inhibitor (Malwitz, 2008).
Most intensifiers do not perform universally with all corrosion inhibitors and many have temperature, time, and environmental drawbacks. For instance, formic acid, which is sometimes used as a corrosion inhibitor intensifier, is limited to a temperature range of 120–160°C in 15% HCl (Cassidy et al., 2009). Formic acid reduces the surface tension of a 15% HCl solution (Nasr-El-Din et al., 2004).
Antimony-based intensifiers can be used with 15% HCl, but not with stronger acids such as 28% HCl (Cassidy et al., 2009). In order to extend the effectiveness of acid corrosion inhibitors, metal salts of iodide and chloride have been suggested, sometimes even salts of mercury (Cizek, 1991). Cuprous iodide is effective up to about 160°C, but has limited solubility in acid solutions. Copper is a banned substance in some areas due to environmental considerations (Cassidy et al., 2009).
Besides environmental, these salts problems are not compatible with organic corrosion inhibitor formulations (Malwitz, 2008) and so they must be formulated separately and used in combination with organic-based corrosion inhibitors. Thus, the use of external intensifiers results in increased costs incurred in on-site formulation, handling, transport, and application (Malwitz, 2008). Compositions in which the intensifier is formulated directly into the composition have been developed, thereby eliminating or reducing the need for external intensifiers.
Various organic ammonium iodides, including phenyltrimethylammonium iodide, ethyl triphenylphosphonium iodide, and others have been tested as internal intensifiers. A variety of these iodide salts may be suitably formulated into corrosion inhibitor compositions (Malwitz, 2008).
The results suggest that tetramethylammonium iodide works well with formic acid as an external intensifier at a variety of temperatures and with several different metals (Malwitz, 2008).
Alternative corrosion inhibitor intensifiers have been used, based on 2-chloro-2,2-diphenylacetic acid and 2-bromo-isobutyric acid. The inhibitor in the formulation is a propargyl alcohol-based corrosion inhibitor and cinnamaldehyde (Cassidy et al., 2009). Formulations using 2-chloro-2,2-diphenylacetic acid reduced the rate of corrosion by a factor of 10 in certain systems.
In commercial corrosion inhibitors, e.g., a blend of quaternary salts, alcohols, formamide, and ethoxylated nonyl phenol, it has been shown that terpene compounds act as corrosion inhibitor intensifiers (Penna et al., 2006). Such compounds include carotene, limonene, pinene, farnesene, camphor, and menthol. They have the advantage of being naturally occurring and biodegradable.
Ahn, Y.S., Jovancicevic, V., 2001. Mercaptoalcohol corrosion inhibitors. WO Patent 0 112 878, assigned to Baker Hughes Inc., February 22, 2001.
Alford, J.A., Boyd, P.G., Fischer, E.R., 1992. Polybasic acid esters as oil field corrosion inhibitors. US Patent 5 174 913, December 29, 1992.
Alford, J.A., Boyd, P.G., Fischer, E.R., 1993a. Acid-anhydride esters as oil field corrosion inhibitors (ester acide anhidride comme inhibiteur de corrosion dans le domaine des huiles). FR Patent 2 692 283, December 17, 1993.
Alford, J.A., Boyd, P.G., Fischer, E.R., 1993b. Polybasic acid esters as oil field corrosion inhibitors. CA Patent 2 075 660, March 21, 1993.
Alford, J.A., Boyd, P.G., Fischer, E.R., 1993c. Polybasic acid esters as oil field corrosion inhibitors (esters d'acides polybasiques utilises comme inhibiteurs de corrosion dans les champs petroliferes). FR Patent 2 681 597, March 26, 1993.
Alford, J.A., Boyd, P.G., Fischer, E.R., 1994. Acid-anhydride esters as oil field corrosion inhibitors. GB Patent 2 268 487, January 12, 1994.
Ali, S., Reyes, J.S., Samuel, M.M., Auzerais, F.M., 2010. Self-diverting acid treatment with formicacid-free corrosion inhibitor. US Patent Application 20100056405, March 4, 2010.
Alink, B.A.M.O., Outlaw, B.T., 2001. Thiazolidines and use thereof for corrosion inhibition. WO Patent 0 140 205, assigned to Baker Hughes Inc., June 07, 2001.
Alink, B.A.O., 1991. Water soluble 1,2-dithio-3-thiones. EP Patent 415 556, assigned to Petrolite Corp., March 06, 1991.
AltIparmak, D.; Keskin, A.; Koca, A.; Gürü, M., Alternative fuel properties of tall oil fatty acid methyl ester-diesel fuel blends, Bioresour. Technol. 98 (2) (2007) 241–246.
ASTM Standard, Standard Practice for Calculation of Corrosion Rates and Related Information from Electrochemical Measurements. ASTM Standard, Book of Standards, vol. 03.02 ASTM G102. (2010) ASTM International, West Conshohocken, PA.
ASTM Standard, Standard Practice for Preparing, Cleaning, and Evaluating Corrosion Test Specimens. ASTM Standard, Book of Standards, vol. 03.02 ASTM G1-03. (2010) ASTM International, West Conshohocken, PA.
Au, A.T., 1986. Acyl derivatives of tris-hydroxy-ethyl-perhydro-1,3,5-triazine. US Patent 4 605 737, August 12, 1986.
Au, A.T., Hussey, H.F., 1989. Method of inhibiting corrosion using perhydro-s-triazine derivatives. US Patent 4 830 827, May 16, 1989.
Babaian-Kibala, E., 1993. Naphthenic acid corrosion inhibitor. US Patent 5 252 254, October 12, 1993.
Bacskai, R., Schroeder, A.H., 1988a. Alkylaniline/formaldehyde co-oligomers as corrosion inhibitors. US Patent 4 778 654, October 18, 1988.
Bacskai, R., Schroeder, A.H., 1988b. Alkylaniline/formaldehyde oligomers as corrosion inhibitors. US Patent 4 780 278, October 25, 1988.
Becker, J.R., Corrosion and Scale Handbook. (1998) Pennwell Publishing Co, Tulsa.
Bentiss, F.; Lagrenee, M.; Traisnel, M., 2,5-bis(N-pyridyl)-1,3,4-oxadiazoles as corrosion inhibitors for mild steel in acidic media, Corrosion 56 (7) (2000) 733–742.
Boyd, P.G., Fischer, E.R., Alford, J.A., 1993. Polybasic acid esters as oil field corrosion inhibitors. GB Patent 2 259 702, March 24, 1993.
Braga, T.G., Martin, R.L., McMahon, J.A., Oude Alink, B.A., Outlaw, B.T., 2000. Combinations of imidazolines and wetting agents as environmentally acceptable corrosion inhibitors. WO Patent 0 049 204, assigned to Baker Hughes Inc., August 24, 2000.
Brezinski, M.M., 1998. Metal corrosion inhibitor for use in aqueous acid solutions. US Patent 5 792 420, assigned to Halliburton Energy Serv., August 11, 1998.
Brezinski, M.M., 2001a. Methods and acidizing compositions for reducing metal surface corrosion and sulfide precipitation. US Patent 6 315 045, assigned to Halliburton Energy Serv. Inc., November 13, 2001.
Brezinski, M.M., 2001b. Well acidizing compositions. EP Patent 1 132 570, assigned to Halliburton Energy Serv., September 12, 2001.
Brezinski, M.M., Desai, B., 1998. Method and composition for acidizing subterranean formations utilizing corrosion inhibitor intensifiers. EP Patent 869 258, assigned to Halliburton Energy Serv., October 07, 1998.
Briggs, G.L., 1987. Corrosion inhibitor for well acidizing treatments. US Patent 4 698 168, October 06, 1987.
Briggs, G.L., 1990. Corrosion inhibitor for well acidizing treatments. CA Patent 1 274 379, September 25, 1990.
Brown, J.M., Ohlsen, J.R., McBride, R.D., 1996. Corrosion inhibitor composition and method of use. US Patent 5 512 212, April 30, 1996.
Buck, E.; Allen, M.C.; Sudbury, B.; Skjellerudsveen, B., Corrosion inhibitor detection by thin layer chromatography: Development of the technique, In: (Editor: Gundry, R.D.) Proceedings Volume, Annual NACE Corrosion Conference (Corrosion 93)New Orleans, March 7–12, 1993. (1993) NACE International, Houston, TX.
Burger, E.D.; Chesnut, G.R., Screening corrosion inhibitors used in acids for downhole scale removal, Mater. Perf. 31 (7) (1992) 40–44.
Byrne, N.E., Johnson, J.D., 1994. Water soluble corrosion inhibitors. US Patent 5 322 640, assigned to Nalco Chemical Co., June 21, 1994.
Camberlin, Y., Grenier, J., Poncet, S., Bonnet, A., Pascault, J.P., Sautereau, H., 1999a. Utilization of polymer compositions for the coating of surfaces, and surface coating containing such compositions (utilisation de compositions de polymeres pour le revetement de surfaces et revetement de surfaces comprenant ces compositions). EP Patent 931 819, assigned to Inst. Francais Du Petrole, July 28 1999.
Camberlin, Y., Grenier, J., Vallet, J., Bonnet, A., Pascault, J.P., Taha, M., 1999b. Polymer compositions, their preparation and their use (compositions de polymeres, leurs preparations et leurs utilisations). FR Patent 2 773 809, assigned to Inst. Francais Du Petrole, July 23 1999.
Camberlin, Y., Grenier, J., Vallet, J., Bonnet, A., Pascault, J.P., Taha, M., 1999c. Polymer compositions, their preparation and uses (compositions de polymeres, leurs preparations et leurs utilisations). EP Patent 931 803, assigned to Inst. Francais Du Petrole, July 28 1999.
Cassidy, J.M., Kiser, C.E., Wilson, J.M., 2009. Corrosion inhibitor intensifier compositions and associated methods. US Patent Application 20090156432, assigned to Halliburton Energy Services, Inc., June 18, 2009.
Cizek, A., 1991. Corrosion inhibition using mercury intensifiers. US Patent 4 997 040, assigned to Baker Hughes Incorporated, Houston, TX, March 5, 1991.
Clewlow, P.J., Haslegrave, A.J., Carruthers, N., Hedges, W.M., Bourland, B.I., Sullivan, D.S., Montgomerie, H.T.R., 1992. Amine adducts as corrosion inhibitors. EP Patent 520 761, December 30, 1992.
Cossar, J.; Carlile, J., A new method for oilfield corrosion inhibitor measurement, In: (Editor: Gundry, R.D.) Proceedings Volume, Annual NACE Corrosion Conference (Corrosion 93)New Orleans, March 7–12, 1993. (1993) NACE International, Houston, TX.
Dadgar, A., 1988a. Corrosion inhibitors for clear, calcium-free high density fluids. US Patent 4 784 779, November 15, 1988.
Dadgar, A., 1988b. Corrosion inhibitors for clear, calcium-free high density fluids. EP Patent 290 486, November 17, 1988.
Dadgar, A., 1988c. Corrosion inhibitors for clear, calcium-free high density fluids. WO Patent 8 802 433, April 07, 1988.
Dahlmann, U., Feustel, M., 2008. Corrosion and gas hydrate inhibitors having improved water solubility and increased biodegradability. US Patent 7 341 617, assigned to Clariant Produkte (Deutschland) GmbH Sulzbach, DE, March 11, 2008.
Darden, J.W., McEntire, E.E., 1986. Dicyclopentadiene dicarboxylic acid salts as corrosion inhibitors. EP Patent 200 850, assigned to Texaco Development Corp., November 12, 1986.
Darden, J.W., McEntire, E.E., 1990. Dicyclopentadiene dicarboxylic acid salts as corrosion inhibitors. CA Patent 1 264 541, January 23, 1990.
Dietsche, F.; Essig, M.; Friedrich, R.; Kutschera, M.; Schrepp, W.; Witteler, H.; Anchor, M.J.; Friedrich, K., Organic corrosion inhibitors for interim corrosion protection, In: CORROSION 2007 (2007) NACE International, Nashville, Tennessee; p. 12. NACE Paper 07358.
Dougherty, J.A., Outlaw, B.T., Alink, B.A.O., 1996. Corrosion inhibition by ethoxylated fatty amine salts of maleated unsaturated acids. US Patent 5 582 792, December 10, 1996.
Eaton, P.; Sutton, G., The effect of flow on inhibitor film life, In: (Editors: Morris, C.P.; Naraghi, A.; Nick, G.) Proceedings Volume, 49th Annual NACE International Corrosion Conference (Corrosion 94)Baltimore, February 27, 94–3, 4, 1994. (1994) NACE International, Houston, TX.
Faessler, K., Testing of corrosion inhibitors under pressure conditions in the presence of H2S and CO2 (Prüfung von Korrosionsinhibitoren Unter Druckbedingungen in Gegenwart von H2S und CO2), In: Proceedings Volume, BASF et al., Chem. Prod. in Petrol. Prod. Mtg. H2S – A Hazardous Gas in Crude Oil Recovery DiscussClausthal-Zellerfeld, Ger, September 12–13, 1990. (1990).
Fischer, E.R., Boyd, P.G., 1998. Water soluble corrosion inhibitors. US Patent 5 759 485, assigned to Westvaco Corporation New York, June 2, 1998.
Fischer, E.R.; Parker III, J.E., Tall oil fatty acid anhydrides as corrosion inhibitor intermediates, Corrosion 53 (1) (1997) 62–64.
Fischer, G.C., 1990. Corrosion inhibitor compositions containing inhibitor prepared from amino substituted pyrazines and epoxy compounds. US Patent 4 895 702, January 23, 1990.
Fisher, L.E., Corrosion inhibitors and neutralizers: Past, present and future, In: (Editor: Gundry, R.D.) Proceedings Volume, Annual NACE Corrosion Conference (Corrosion 93)New Orleans, March 7–12, 1993. (1993) NACE International, Houston, TX.
Fisk, T.E., Tucker, C.J., 1991. N-(hydrophobe aromatic) pyridinium compounds. US Patent 5 000 873, March 19, 1991.
Fong, D.W., Shambatta, B.S., 1993. Hydroxamic acid containing polymers used as corrosion inhibitors. CA Patent 2 074 535, assigned to Nalco Chemical Co., January 25, 1993.
Fortenberry Jr., C.L.; Grahmann, N.J.; Miller, C.D.; Son, A.J., Analysis of residual corrosion inhibitors in oilfield brines, In: Proceedings Volume, 68th Annual SPE Tech. ConferenceHouston, October 3–6, 1993. (1993) Society of Petroleum Engineers, Richardson, TX, pp. 965–979.
French, E.C., Fahey, W.F., Harte, J.G. 1991a. Method of oil well corrosion inhibition via emulsions and emulsions therefor. US Patent 5 027 901, July 02, 1991.
French, E.C., Fahey, W.F., Harte, J.G., 1991b. Method of oil well corrosion inhibition via emulsions and emulsions therefor. CA Patent 2 019 516, March 06, 1991.
Frenier, W.W., Growcock, F.B., 1988a. Mixtures of a,b-unsaturated aldehydes and surface active agents used as corrosion inhibitors in aqueous fluids. US Patent 4 734 259, March 29, 1988.
Frenier, W.W., Growcock, F.B., 1988b. Process and composition for inhibiting iron and steel corrosion. EP Patent 289 665, November 09, 1988.
Funkhouser, G.P., Cassidy, J.M., Lane, J.L., Frost, K., Gardner, T.R., King, K.L., 2001. Metal corrosion inhibitors, inhibited acid compositions and methods. US Patent 6 192 987, assigned to Halliburton Energy Serv., February 27, 2001.
Gay, R.J., Gay, C.C., Matthews, V.M., Gay, F.E.M., Chase, V., 1993. Dynamic polysulfide corrosion inhibitor method and system for oil field piping. US Patent 5 188 179, February 23, 1993.
Gelner, L., Protection of storage tank bottoms using volatile corrosion inhibitors (VCI), In: Proceedings Volume, vol. 1, 7th NACE International et al Middle East Corrosion ConferenceManama, Bahrain, February 26–28, 1996. (1996) NACE International, Houston, TX, pp. 102–109.
Growcock, F.B., Surfactants can affect corrosion inhibition of oil-field steel, In: Proceedings Volume, SPE Oilfield Chem. International SymposiumSan Antonio, February 4–6, 1987. (1987) Society of Petroleum Engineers, Richardson, TX, pp. 1–7; Paper No. 16 625.
Growcock, F.B.; Lopp, V.R., The inhibition of steel corrosion in hydrochloric acid with 3-phenyl-2-propyn-1-ol, Corros. Sci. 28 (4) (1988) 397–410.
Guimaraes, P.I.C.; Monteiro, A.P.; Mainier, F.B., New corrosion inhibitors in solid form to protect carbon steel pipes in acidizing operations, In: Proceedings Volume, vol. 2, no. TT-104, 5th Brazil Petrol. Congr. (Conexpo Arpel 94)Rio De Janeiro, Brazil, October 16–20, 1994. (1994) Instituto Brasileiro de Petroleo, Rio de Janeiro.
Haslegrave, J.A., Sullivan, D.S., 1987. N, S containing corrosion inhibitors. US Patent 4 673 436, June 16, 1987.
Hausler, R.H., On the use of linear polarization measurements for the evaluation of corrosion inhibitors in concentrated HCl at 200°F (93°C), Corrosion 42 (12) (1986) 729–739.
Henson, E.R., Doty, P.A., 1990. Corrosion inhibitors for aqueous brines. US Patent 4 980 074, assigned to Dow Chemical Co., December 25, 1990.
Ho, A.W., 1993. Derivatives of polyalkylenepolyamines as corrosion inhibitors. WO Patent 9 307 307, April 15, 1993.
Ho, A.W., 1994. Derivatives of polyalkylenepolyamines as corrosion inhibitors. US Patent 5 275 744, January 04, 1994.
Incorvia, M.J., 1988a. Polythioether corrosion inhibition system. US Patent 4 759 908, July 26, 1988.
Incorvia, M.J., 1988b. Thiol ester corrosion inhibition system. US Patent 4 744 948, May 17, 1988.
International Organization for Standardization, Petroleum and natural gas industries – materials for use in H2S-containing environments in oil and gas production – Part 1: General principles for selection of cracking-resistant materials. ISO Standard ISO 15156-2. (2009) International Organization for Standardization, Geneva, Switzerland.
International Organization for Standardization, Petroleum and natural gas industries – Materials for use in H2S-containing environments in oil and gas production – Part 2: Cracking-resistant carbon and low-alloy steels, and the use of cast irons. ISO Standard ISO 15156-2. (2009) International Organization for Standardization, Geneva, Switzerland.
International Organization for Standardization, Petroleum and natural gas industries – materials for use in H2S-containing environments in oil and gas production – Part 3: Cracking-resistant CRAs (corrosion-resistant alloys) and other alloys. ISO Standard ISO 15156-3. (2009) International Organization for Standardization, Geneva, Switzerland.
International Organization for Standardization, Petroleum, Petrochemical and Natural Gas Industries – Materials Selection and Corrosion Control for Oil and Gas Production Systems. ISO Standard ISO 21457. (2010) International Organization for Standardization, Geneva, Switzerland.
Jiashen, Z.; Jingmao, Z., Control of corrosion by inhibitors in drilling muds containing high concentration of H2S, Corrosion 49 (2) (1993) 170–174.
Johnson, D.M., Ippolito, J.S., 1994. Corrosion inhibitor and sealable thread protector end cap for tubular goods. US Patent 5 352 383, October 04, 1994.
Johnson, D.M., Ippolito, J.S., 1995. Corrosion inhibitor and sealable thread protector end cap for tubular goods. US Patent 5 452 749, September 26, 1995.
Karaev, S.F.O., Gusejnov, S.O.O., Garaeva, S.V.K., Talybov, G.M.O., 1996. Producing propargyl ether for use as metal corrosion inhibitors-by condensing propargyl alcohol with olefin in presence of phospho-tungstic acid. RU Patent 2 056 401, March 20, 1996.
Kennard, M.A.; McNulty, G., Depositing corrosion inhibitors effectively, Pipeline Gas J. 220 (4) (1993) 66–71.
Kennard, M.A.; McNulty, J.G., Conventional pipeline-pigging technology: Pt.2: Corrosioninhibitor deposition using pigs, Pipes Pipelines Int. 37 (4) (1992) 14–20.
Kennedy, Jr., W.C., 1987. Corrosion inhibitors for cleaning solutions. US Patent 4 637 899, January 20, 1987.
Kissel, C.L., 1999. Process and composition for inhibiting corrosion (verfahren und zusammensetzung zur inhibierung von korrosion). EP Patent 906 969, assigned to Degussa AG, April 07, 1999.
Kreh, R.P., 1991. Method of inhibiting corrosion and scale formation in aqueous systems. US Patent 5 073 339, December 17, 1991.
Larsen, A.L., 1991. Process for inhibiting corrosion in oil production fluids. EP Patent 446 616, assigned to Norol Hoechst Oil Chem. Assoc., September 18, 1991.
Leinweber, D., Feustel, M., 2009. Corrosion and gas hydrate inhibitors with an increased biological degradability and a reduced toxicity. US Patent 7 615 102, assigned to Clariant Produkte (Deutschland) GmbH Frankfurt, DE, November 10, 2009.
Lindstrom, M.R., Louthan, R.P., 1987. Inhibiting corrosion. US Patent 4 670 163, assigned to Phillips Petroleum Co., June 02, 1987.
Lindstrom, M.R., Mark, H.W., 1987. Inhibiting corrosion: Benzylsulfinylacetic acid or benzylsulfonylacetic acid. US Patent 4 637 833, January 20, 1987.
Mainier, F.; Saliba, C.A.; Gonzalez, G., Effectiveness of acid corrosion inhibitors in the presence of alcohols, SPE Unsolicited Pap SPE-20404. (1990) Petrobras Research Center, Rio de Janeiro, Brazil.
Mainier, F.B.; Lazaro, W.; Do, R.F.F., Silicate-based corrosion-inhibitor in drilling fluids: An environmentally-friendly option (inibidor de corrosao a base de silicato em fluidos de perfuracao: Uma opcao nao agressiva ao meio ambiente), In: Proceedings Volume, vol. 1, 8th Petrobras et al Latin Amer. Drilling Congr.Rio De Janeiro, Brazil, October 14–16, 1992. (1992), pp. 467–475.
Malwitz, M.A., 2008. Corrosion inhibitor composition comprising a built-in intensifier. US Patent Application 20080146464, June 19, 2008.
Martin, R.L., 1988. Multifunctional corrosion inhibitors. US Patent 4 722 805, February 02, 1988.
Martin, R.L., 1993. The reaction product of nitrogen bases and phosphate esters as corrosion inhibitors. EP Patent 567 212, October 27, 1993.
Martin, R.L., Brock, G.F., Dobbs, J.B., 2005. Corrosion inhibitors and methods of use. US Patent 6 866 797, assigned to BJ Services Company, March 15 2005.
Matherly, R.M.; Jiao, J.; Ryman, J.S.; Blumer, D.J., Determination of imidazoline and amidoamine type corrosion inhibitors in both crude oil and produced brine from oilfield production, In: Proceedings Volume, 50th Annual NACE International Corrosion Conference (Corrosion 95)Orlando, FL, March 26–31, 1995. (1995) NACE International, Houston, TX.
McCullough, T.M., 1991. Emulsion minimizing corrosion inhibitor for naphtha/water systems. US Patent 5 062 992, assigned to Betz Laboratories Inc., November 05, 1991.
McEntire, E.E., Knifton, J.F., 1987. Process for formation of dialkylaminomethylated internal olefin polymers. US Patent 4 657 984, April 14, 1987.
McKerrell, E.H.; Lynes, A., Development of an HPLC (high performance liquid chromatography) method for the determination of nitrogen containing corrosion inhibitors in a mixed hydrocarbon/glycol matrix, In: Proceedings Volume, no. 67, 3rd Royal Soc. Chem. Ind. Chem. in the Oil Ind. International SymposiumManchester, England, April 19–20, 1988. (1988), pp. 212–222.
McMahon, A.J.; Harrop, D., Green corrosion inhibitors: An oil company perspective, In: Proceedings Volume, 50th Annual NACE International Corrosion Conference (Corrosion 95)Orlando, FL, March 26–31, 1995. (1995) NACE International, Houston, TX.
Meyer, G.R., 2001. Corrosion inhibiting compositions. GB Patent 2 353 793, assigned to Nalco Exxon Energy Chem. L, March 07, 2001.
Miksic, B.A., Furman, A., Kharshan, M., Braaten, J., Leth-Olsen, H., 2004. Corrosion resistant system for performance drilling fluids utilizing formate brine. US Patent 6 695 897, assigned to Cortec Corporation St. Paul, MN, February 24, 2004.
Minevski, L.V., Gaboury, J.A., 1999. Thiacrown ether compound corrosion inhibitors for alkanolamine units. EP Patent 962 551, assigned to Betzdearborn Europe Inc., December 08, 1999.
Moore, J.; Vers, L.V.; Conrad, P., SS: Flow assurance: Understanding kinetic hydrate inhibitor and corrosion inhibitor interactions, In: Offshore Technology Conference, no. OTC 19869Houston, TX. (2009); Paper Number 19869-MS, pp. 1-21. http://www.onepetro.org/mslib/app/Preview.do?paperNumber=OTC-19869-MS&societyCode=OTC.
Morris-Sherwood, B.J., Brink, Jr., E.C., 1987. Corrosion inhibiting composition. EP Patent 221 212, May 13, 1987.
Morris-Sherwood, B.J., Brink, Jr., E.C., 1990. Corrosion inhibiting composition and method. CA Patent 1 264 539, January 23, 1990.
Naraghi, A., 1997. Corrosion inhibitor containing phosphate groups. US Patent 5 611 991, March 18, 1997.
Naraghi, A., Grahmann, N., 1997. Corrosion inhibitor blends with phosphate esters. US Patent 5 611 992, March 18, 1997.
Nasr-El-Din, H.A.; Al-Othman, A.M.; Taylor, K.C.; Al-Ghamdi, A.H., Surface tension of HCl-based stimulation fluids at high temperatures, J. Pet. Sci. Eng. 43 (1–2) (2004) 57–73.
Neemla, K.D.; Saxena, R.C.; Jayaraman, A., Corrosion inhibition of oil-well equipment during acidization, Corrosion Prev. Contr. 39 (3) (1992) 69–73.
Niu, J.H.Y., Edmondson, J.G., Lehrer, S.E., 1988. Method of inhibiting corrosion of metal surfaces in contact with a corrosive hydrocarbon containing medium. EP Patent 256 802, February 24, 1988.
Nogueira, J.M.F., Refining and separation of crude tall-oil components, Sep. Sci. Technol. 31 (17) (1996) 2307–2316.
Oberndorfer, M.; Thayer, K.; Havlik, W., Corrosion control in the oil and gas production – 5 successful case histories, In: CORROSION 2007 (2007) NACE International, Nashville, Tennessee, p. 17; NACE paper No. 07317.
Obeyesekere, N.; Naraghi, A.; McMurray, J.S., Synthesis and evaluation of biopolymers as low toxicity corrosion inhibitors for north sea oil fields, In: Proceedings Volume, NACE International Corrosion Conference [Corrosion 2001]Houston, TX, March 11–16, 2001. (2001) NACE International, Houston, TX, pp. 1–14; Paper Number 01049.
Ohlsen, J.R., Brown, J.M., Brock, G.F., Mandlay, V.K., 1995. Corrosion inhibitor composition and method of use. US Patent 5 459 125, October 17, 1995.
Oppenlaender, K., Wegner, B., Slotman, W., 1993. Ammonium salt of an alkenylsuccinic half-amide and the use thereof as corrosion inhibitor in oil and/or gas production technology. US Patent 5 250 225, assigned to BASF AG, October 05, 1993.
Oude Alink, B.A., 1993. Water soluble 1,2-dithio-3-thiones. US Patent 5 252 289, assigned to Petrolite Corp., October 12, 1993.
Penna, A., Arias, G.F.D.L., Rae, P.J., 2006. Corrosion inhibitor intensifier and method of using the same. US Patent Application 20060264335, assigned to BJ Services Company Houston, TX, November 23, 2006.
Petersen, P.R., Coker, L.G., Sullivan, D.S., 1990. Method of inhibiting corrosion using n-s containing compounds. GB Patent 2 221 458, February 07, 1990.
Phillips, N.J.; Renwick, J.P.; Palmer, J.W.; Swift, A.J., The synergistic effect of sodium thiosulphate on corrosion inhibition, In: Proceedings Volume, vol. 1, 7th NACE International et al Middle East Corrosion ConferenceManama, Bahrain, February 26–28, 1996. (1996) NACE International, Houston, TX, pp. 110–137.
Ramanarayanan, T.A., Vedage, H.L., 1994. Inorganic/organic inhibitor for corrosion of iron containing materials in sulfur environment. US Patent 5 279 651, January 18, 1994.
Samant, A.K.; Koshel, K.C.; Virmani, S.S., Azoles as corrosion inhibitors for mild steel in a hydrochloric acid medium, SPE Unsolicited Pap SPE-19022. (1989) KDM Inst. of Petroleum Exploration; February 1989.
Schilling, P., 1995. Polyamine condensates of styrene-maleic anhydride copolymers as corrosion inhibitors. US Patent 5 391 636, February 21, 1995.
Schilling, P., Braddon, D.V., 1986. Corrosion inhibitors. US Patent 4 614 600, assigned to Westvaco Corp., September 30, 1986.
Schilling, P., Brown, P.E., 1988. Cationic and anionic lignin amines corrosion inhibitors. US Patent 4 789 523, assigned to Westvaco Corp., December 06, 1988.
Schutt, H.U., 1990. Reducing stress corrosion cracking in treating gases with alkanol amines. US Patent 4 959 177, September 25, 1990.
Sekine, I., Yuasa, M., Shimode, T., Takaoka, K., 1991. Inhibition of corrosion. GB Patent 2 234 501, February 06, 1991.
Shah, S.S., Fahey, W.F., Alink, B.A.O., 1994. Corrosion inhibition in highly acidic environments by use of pyridine salts in combination with certain cationic surfactants. US Patent 5 336 441, August 09, 1994.
Shah, S.S., Fahey, W.F., Oude Alink, B.A., 1992. Corrosion inhibition in highly acidic environments by use of pyridine salts in combination with certain cationic surfactants. CA Patent 2 066 797, November 30, 1992.
Shin, C.C., 1988. Corrosion inhibiting composition for zinc halide-based clear, high density fluids. WO Patent 8 802 432, April 07, 1988.
Silverman, D.C.; Kalota, D.J.; Stover, F.S., Effect of pH on corrosion inhibition of steel by polyaspartic acid, In: Proceedings Volume, 50th Annual NACE International Corrosion Conference (Corrosion 95)Orlando, FL, March 26–31, 1995. (1995) NACE International, Houston, TX, pp. 1–8; Paper Number 95110818.
Son, A.J.; Chakravarty, J., Analysis of residual corrosion inhibitors by fluorescence and ultraviolet spectrophotometry, In: Proceedings Volume, 51st Annual NACE International Corrosion Conference (Corrosion 96)Denver, March 24–29, 1996. (1996) NACE International, Houston, TX, pp. 344/1–344/23.
Tang, Y.; Han, Z.; Wang, H.; Chen, H., Sp-2 acid corrosion inhibitor, J. Univ. Pet., China 19 (1) (1995) 98–101.
Teeters, S.M., 1992. Corrosion inhibitor. US Patent 5 084 210, assigned to Chemlink Inc., January 28, 1992.
Trabanelli, G.; Zucchi, F.; Brunoro, G., Inhibition of corrosion resistant alloys in hot hydrochloric acid solutions, Werkst. Korros. 39 (12) (1988) 589–594.
Treybig, D.S., Glass, T.W., 1988. Corrosion inhibitors. US Patent 4 784 796, November 15, 1988.
Treybig, D.S., Martinez, R.G., 1988. Process for preventing corrosion of a metal in contact with a well fluid. US Patent 4 740 320, April 26, 1988.
Treybig, D.S., Martinez, R.G., 1989. Compositions prepared from hydrocarbyl substituted nitrogen-containing aromatic heterocyclic compounds, an aldehyde and/or ketone and an amine. US Patent 4 871 848, October 03, 1989.
Valone, F.W., 1987a. Corrosion inhibiting system containing alkoxylated amines. EP Patent 207 713, January 07, 1987.
Valone, F.W., 1987b. Corrosion inhibiting system containing alkoxylated amines. US Patent 4 636 256, January 13 1987.
Valone, F.W., 1989a. Corrosion inhibiting system containing alkoxylated alkylphenol amines. US Patent 4 867 888, September 19, 1989.
Valone, F.W., 1989b. Corrosion inhibiting system containing alkoxylated amines. CA Patent 1 259 185, September 12, 1989.
Valone, F.W., 1989c. Corrosion inhibiting system containing alkoxylated dialkylphenol amines. US Patent 4 846 980, July 11, 1989.
Veldman, R.R., Trahan, D.O., 1999. Gas treating solution corrosion inhibitor. WO Patent 9 919 539, assigned to Coastal Fluid Technol. Llc., April 22, 1999.
Verma, S.K., Sandor, G.R., 2001. Corrosion inhibitors for use in oil and gas wells and similar applications. WO Patent 0 146 552, assigned to Fmc Corp., June 28, 2001.
Vorderbruggen, M.A., Williams, D.A. 2000. Acid corrosion inhibitor. US Patent 6 117 364, assigned to Nalco Exxon Energy Chem. Lp., September 12, 2000.
Walker, M.L., Martin, R.L., Poelker, D.J., 2001. High performance phosphorus-containing corrosion inhibitors for inhibiting corrosion by drilling system fluids. EP Patent 1 076 113, assigned to Baker Hughes Inc., February 14, 2001.
Weghorn, S.J.; Reese, C.W.; Oliver, B., Field evaluation of an encapsulated time-release corrosion inhibitor, In: CORROSION 2007 (2007) NACE International, Nashville, Tennessee; Paper Number 07321, pp. 1–10. http://www.onepetro.org/mslib/app/Preview.do?paperNumber=NACE-07321&societyCode=NACE..
Welton, T.D., Cassidy, J.M., 2007. Thiol/aldehyde corrosion inhibitors. US Patent 7 216 710, assigned to Halliburton Energy Services, Inc. Duncan, OK, May 15, 2007.
Williams, D.A., Looney, J.R., Sullivan, D.S., Bourland, B.I., Haslegrave, J.A., Clewlow, P.J., Carruthers, N., O'Brien, T.M., 1994. Amine derivatives as corrosion inhibitors. US Patent 5 322 630, assigned to Exxon Chemical Patents In, June 21, 1994.
Wirtz, H., Hoffmann, H., Ritschel, W., Hofinger, M., Mitzlaff, M., Wolter, D., 1989. Optionally quaternized fatty esters of alkoxylated alkyl-alkylene diamines (gegebenenfalls quaternierte fettsaeureester von oxyalkylierten alkyl-alkylendiaminen). EP Patent 320 769, June 21, 1989.
Wu, Y., Gray, R.A., 1992. Compositions and methods for inhibiting corrosion. US Patent 5 118 536, assigned to Phillips Petroleum Co., June 02, 1992.
Young, L.A., 1993. Low melting polyalkylenepolyamine corrosion inhibitors. WO Patent 9 319 226, September 30, 1993.
Zefferi, S.M., May, R.C., 1994a. Corrosion inhibition of calcium chloride brine. US Patent 5 292 455, assigned to Betz Laboratories Inc., March 08, 1994.
Zefferi, S.M., May, R.C., 1994b. Corrosion inhibition of calcium chloride brine. CA Patent 2 092 207, August 26, 1994.
Zetlmeisl, M.J., French, E.C., 1992a. Corrosion inhibition in highly acidic environments. US Patent 5 169 598, December 08, 1992.
Zetlmeisl, M.J., French, E.C., 1992b. Corrosion inhibition in highly acidic environments. CA Patent 2 067 313, November 30, 1992.
Tradenames
| Tradename Description | Supplier |
|---|---|
| Rhodafac® RS-410 Poly(oxy-1,2-ethandiyl) tridecyl hydroxy phosphate (Martin et al., 2005) | Rhodia |
..................Content has been hidden....................
You can't read the all page of ebook, please click here login for view all page.