Chapter 15. Odorization
The hazards of odorless combustible gases were probably first realized by miners. The idea of the odorization of combustible gases results from Julius Quaglio in 1880, who was engaged in researching various aspects of water gas (Quaglio, 1880). The gas produced at that time already contained impurities that created the typical odor of gas, but later it was obtained in such a purity that dangers emerged in handling it. Some aspects concerning the history of this issue are given in the literature (Usher, 1999). The primary objective of gas odorization is safety. It allows natural gas in air to be detected before it reaches combustible levels and hence acts as a warning. Naturally, odorization is a part of risk management for pipelines of natural gas (Muhlbauer, 2004).
Certain federal pipeline safety regulations, including those of the national fire protection association (US), require that combustible gases in pipelines be detectable at one-fifth of the lower explosive limit by a person with a normal sense of smell, either by the natural odor of the gas or by means of artificial odorization (Fant, 1993). Therefore proper odorization and odorants are integral parts of safety (Henderson, 1993; Toth, 1989).
Odorization is a primary concern for any gas transmission company (Henderson, 1993; Oudman, 1993). Accurate injection of the odorant, proper monitoring techniques, and complete record maintenance are important factors in developing and sustaining a successful odorization program.
A review has been presented concerning most aspects of odorization. Important points to consider are which pipelines require odorization, the detectable limits of gas odor, odorants and odorizing considerations, and the monitoring of the pipeline system to ensure that the odorization program is meeting regulatory requirements (Fant, 1993).
General Aspects
Limits of Explosion
The approximate lower explosion limits of certain gases in air are shown in Table 15.1. There is also an explosion upper limit, which is dependent on the oxygen content – of course a constant for ordinary air. However, the limits of explosion are dependent on the total pressure (Holtappels et al., 2001).
There are mathematical models in order to calculate the limits of explosion (Askar et al., 2008), for example, the Software GasEq® and some additional software has been used to calculate the flammability limits of mixtures with, e.g., ethylene oxide, air, and inert gases at temperatures between 20°C and 100°C and pressures between 0.4 bar and 1.0 bar.
GasEq® is a Microsoft Windows-based program with a Microsoft Excel interface. The program can be used to calculate the equilibrium of combustion. It is intended primarily for gas phase calculations, although there is some limited facility for condensed phases, such as soot (Morley, 2005).
Desirable Properties of Odorants
An ideal odorant should have the following physical and functional properties (Kato, 2007):
• Low perceptual threshold,
• Preferably distinguishable from the smells of daily life and able to function as a warning smell,
• Low boiling point (essential for hydrogen gas),
• Low corrosivity,
• Little or no olfactory fatigue, and
• Low toxicity.
Odorants should allow leaks to be detected without any external equipment, so the end user need not worry about maintaining any measuring equipment. In this way equipment failures will not cause leaks to go undetected. Consequently, odorants allow the detection of leaks in places where it may be difficult to position detectors, and they can be used in small concentrations because of the sensitivity of the human olfactory system (Kopasz, 2007).
Furthermore, a fuel-gas odorant should be easily distinguishable from smells encountered in daily life, i.e., those smells that are experienced in daily life situations and are not perceived as a foreign or unusual odor.
By contrast, a warning smell is in general an unpleasant smell that is perceived as an odor that indicates an unusual situation, clearly distinguishable from the smells in daily life. In this way, the smell acts as a warning signal (Kato, 2007).
Measurement and Odor Monitoring
The methods of odor monitoring are reviewed by Klusmann (1993) and by Wetteman and Wilson (1993).
In the early days of the coal mining industry, open flames were take taken into the mine. When the candle started burning irregularly, a possible danger of a near explosion could be realized. However, often this technique indicated a danger too late. A significant step forward was the invention of the safety lamp in 1815 by Sir Humphry Davy. The presence of combustible gases could be still detected, but the explosive reaction only happened inside the wired cage of the safety lamp.
Another method of monitoring utilized the extraordinary sensitivity of canary birds to methane. These birds were taken by the miners into the mines, admittedly involuntary, in small cages. The canary stopped singing in the presence of even small amounts of coal gas, and died at higher levels that were still harmless to the human body (Kopasz, 2007).
The addition of odorants to liquid petroleum gas and natural gas gives an improved level of safety, but their use suffers from certain limitations and disadvantages. Firstly, for an odor to be detected, a human being must be present in the vicinity of the leak. Secondly, not every individual is able to detect the odors at the same mandatory level. Most dangerous is that some individuals are not able detect the odor at all.
The sensitivity of an individual may be affected by a seasonal illness, such as influenza or a cold. Also, the exposure to other odors lowers one's overall sensitivity and during sleep the olfactory response decreases.
Olfactoric Response
Odorants are chemicals that stimulate the olfactoric sense. The human olfactory system is much less sensitive than that of animals, such as dogs, which are notorious for their sense of smell. Nevertheless, a human being can still detect certain odorants in concentrations in the air in the parts per trillion (ppt) range.
There are several definitions that are relevant to quantifying the odor. The threshold odor concentration is the absolute perception threshold at which a substance can be barely identified, however faint the impression. The odor recognition threshold is the concentration at which a representative odor for a certain substance can be detected (Patnaik, 2007).
There is a difference between the minimum detectable concentration and the minimum identifiable concentration. The former is defined as the lowest concentration at which 50% of the human population is able to smell something. This concentration is sometimes known as the perceptual threshold or odor recognition threshold.
In contrast, the recognition threshold is the minimum concentration at which a certain, predefined percentage of people can identify the substance coarsely. At the detection and recognition thresholds there will still be a large number of individuals who do not detect or recognize the odor. Data indicate that, as a rule of the thumb, the recognition threshold is roughly a factor of 10 higher than the detection threshold (Kopasz, 2007).
Perceptual Threshold and Olfactoric Intensity
The perceptual threshold of an odorant is the minimum concentration of the odorant in the air, expressed in ppm, etc., at which a human being can easily notice the smell of the odorant. In general, the values refer to ratios by volume and not by weight, as is usual in gas analysis.
This threshold value can be determined by panelists, who assess the olfactory intensity of a test substance in an odorless chamber. The air in the chamber is stirred until the concentration of the test substance becomes constant and is then left to stand. The olfactory intensity is assessed, for instance, on a scale of 0 to 5 for smell pollution. The olfactoric response of human males and females between the ages of 16 and 82 years were tested with various odorants, including tert-butylmercaptan, thiophene, ethylmercaptan, dimethyl sulfide, isopropylmercaptan, and mixtures of these odorants. The perceptual and recognition thresholds of selected chemicals are summarized in Table 15.2.
| Perceptual thresholds (Kato, 2007) | Concentration |
|---|---|
| p-Cresol | 51.3 ppt |
| 5-Ethylidene-2-norbornene | 4 ppb |
| Methylmercaptan | 1.6 ppb |
| γ-Undecalactone | 22.8 ppt |
| 3-Hydroxy-4-methyl-5-ethyl-2(5H)-furanone | 7.4 ppt |
| trans-2-trans -4-Decadienal | 87 ppt |
| Recognition thresholds (Patnaik, 2007, pp. 14) | Concentration |
| Ethane | 1500 ppm |
| Propane | 11,000 ppm |
| Butane | 5000 ppm |
| Pentane | 900 ppm |
| Octane | 200 ppm |
| Methanol | 6-000 ppm |
| Ethanol | 6-000 ppm |
| Octanol | 2 ppb |
| Geosmin | 5 ppt |
| Diethylether | 300 ppb |
| Ethyl acrylate | 2 ppb |
| Hydrogen sulfide | 1 ppm |
| Methylmercaptan | 35 ppb |
| Ethylmercaptan | 2 ppb |
| Butylmercaptan | 0.8 ppb |
| Ethyl sulfide | 4 ppb |
The goal was to establish the warning levels below the explosion limit in the event of a gas leak (Ripley et al., 1990). The study suggests that ethylmercaptan is the most suitable odorant. Trained dogs can detect odorizing agents in concentrations as small as 10–18 ppb (Bissell et al., 1993; Quaife and Moynihan, 1990; Quaife et al., 1992a).
Odor Index
The odor index (OI) is the ratio of the vapor pressure to the odor recognition threshold (Patnaik, 2007, p. 13).
(15.1)
Thus, the odor index is a dimensionless number, and a value less than 1 means that the substance has a vapor pressure that is too small for the substance to be detected.
Olfactory Power
There is another definition of the intensity of a certain smell called, the olfactory power (Flynn and Sprague, 2009), which is defined as the negative decadic logarithm of the detection threshold. Olfactory power tables are given in Table 15.3.
| Functionality | Compound | Olfactory Power |
|---|---|---|
| Hydrocarbons | Ethane | 2.00 |
| Propane | 2.57 | |
| Butane | 3.69 | |
| Halides | Chloromethane | 4.99 |
| Ethylchloride | 5.39 | |
| Alcohols | Methanol | 3.85 |
| Ethanol | 4.54 | |
| 1-Propanol | 5.62 | |
| Esters | Methyl formate | 4.03 |
| Methyl acetate | 5.21 | |
| Ketones | Acetone | 4.84 |
| Aldehydes | Formaldehyde | 6.06 |
| Acetaldehyde | 6.73 | |
| Amines | Methylamine | 7.73 |
| Dimethylamine | 7.09 | |
| Ethylamine | 6.49 | |
| Diethylamine | 6.73 | |
| Propylamine | 7.96 | |
| Thiols | Methylmercaptan | 8.98 |
| Ethylmercaptan | 8.97 | |
| Isobutylmercaptan | 8.95 | |
| tert -Butylmercaptan | 9.48 | |
| Sulfides | Dimethyl sulfide | 8.65 |
| Methylethyl sulfide | 8.42 | |
| Diethyl sulfide | 8.41 | |
| Selenides | Diethylselenide | 9.13 |
| Selenols | Ethylselenol | 10.74 |
Physiological Methods
An example of a specific method for determining the perceptual threshold is as follows. A test odorant in a dish is left in an odorless chamber for a given period of time. The air in the chamber is agitated until the concentration of the test substance becomes constant, and then it is left standing for 1 min. Panelists then enter the chamber and assess the olfactory intensity on a scale of 0 to 5. This procedure is then repeated for different concentrations of the test odorant. A perceptual threshold is then obtained by determining the concentration of the odorant that corresponds to the olfactory intensity of 2, at which the smell can easily be identified as described below. The olfactory intensity is assessed on a scale of 0 to 5 (Kato, 2007):
0: Odorless,
1: Slight smell, but not identified,
2: Easily noticed and can be identified,
3: Obvious smell,
4: Strong smell, and
5: Intolerably strong smell.
Triangle Odor Bag Method
The measurement of the odor threshold by the triangle odor bag method has been described in detail (Nagata, 2003). The data were collected over a comparatively long period of time, i.e., from 1976 to 1988, and 223 substances were tested in total for this study.
It was found that isoamylmercaptan exhibited the lowest threshold, of 0.77 ppt, and propane exhibited the highest threshold of 1500 ppm. The distribution of the thresholds of the substances investigated follows a Gaussian normal distribution. As expected from common sense, sulfur compounds, apart from sulfur dioxide and carbon disulfide, exhibit a comparatively low threshold.
There is a relationship between the molecular weight of the substance and its odor threshold, and there is a great difference in threshold between isomers. The thresholds may differ by up to four powers of ten. Extensive listings of the odor thresholds measured in this study have been presented (Nagata, 2003).
For comparison, Table 15.4 presents selected odor thresholds measured by the triangle odor bag method. Observe that these values differ appreciably from those given in Table 15.2. This may be caused by the different methods used in the acquisition, but considerable variation was discovered when tests were repeated on the same substance.
| Substance | Concentration |
|---|---|
| Propane | 1500 ppm |
| Butane | 1200 ppm |
| Pentane | 1.4 ppm |
| Octane | 1.7 ppm |
| Methanol | 33 ppm |
| Ethanol | 0.52 ppm |
| Octanol | 2.7 ppb |
| Methylmercaptan | 70 ppt |
| Ethylmercaptan | 8.7 ppt |
| n-Propylmercaptan | 13 ppt |
| Isopropylmercaptan | 6 ppt |
| n-Butylmercaptan | 2.8 ppt |
| Isobutylmercaptan | 6.8 ppt |
| Butylmercaptan | 30 ppt |
| tert -Butylmercaptan | 29 ppt |
| n -Amylmercaptan | 0.78 ppt |
| Isoamylmercaptan | 0.77 ppt |
| n-Hexylmercaptan | 15 ppt |
Standardized Methods
Sensory thresholds have been established in order to determine the potential of substances at low concentrations to impart odor, taste, haptic, etc., to some form of matter (ASTM E 0679-04, 2009).
Procedures for referencing the odor intensities of materials have been standardized, and the so-called ASTM Odor Intensity Referencing Scale has been developed (ASTM E 544-99(2004), 2009). This scale is a geometric progression scale. Reference odorant vapors are evaluated by a panel of at least eight independent judges, who compare the odor intensity of the sample to the odor intensities of a series of concentrations of n-butanol, the reference odorant.
Two methods are used to create the smell. In the dynamic scale method, a dynamic-dilution apparatus is used, equipped with a series of sniffing ports from which constant concentrations of 1-butanol emerge at constant volumetric flow rates in air. In the static-scale method, a series of Erlenmeyer flasks containing known concentrations of n-butanol dissolved in water is used.
Chemical and Physical Methods
Chromatographic and Spectroscopic Methods
The concentration of odorants in gases can be measured by their absorbance in the ultraviolet region (Shimokawatoko et al., 1998a and Shimokawatoko et al., 1998b). The absorbance of odorized gas is much higher than that of untreated gas.
An integrated natural gas pipeline leak detector based on near-infrared diode laser absorption spectroscopy, wavelength modulation spectroscopy, and harmonic detection has been used (Gao et al., 2006). Direct absorption spectroscopy has the drawback of low sensitivity.
It is difficult to detect a small flux pipeline leak of natural gas, so the second harmonic signal is used in a modulated wave. The method directly accesses the absorbance by methane, so the presence of an odorant is immaterial.
Gas chromatography with an electrochemical detector is also suitable (Wallace et al., 1991) for the analysis of mercaptans. Portable equipment for the measurement and analysis of odorants in gas distribution networks is available.
The analysis and characterization of odorants plays an important role in the food industry and in the perfume and oil and gas industries. Remarkably the sensitivity of an analytical instrument, such as a mass spectrometer and the sensitivity of the human nose do not correlate at all. Substances with an intense smell may cause only a small mass spectrometric peak and vice versa.
Colorimetric Methods
The classical analysis of traces of gases is by colorimetric methods, originally invented by the Dräger company in Lübeck, Germany. An indicator is placed inside a thin glass tube that is sealed at both ends by melting the glass. In the test procedure, the sealed ends of the glass tube are broken and a known amount of gas is sucked through. The indicator will react with any impurity and change color. The distance up the tube that changes color is proportional to the concentration of impurity present. On the tube itself, a scale is printed so that the concentration of the respective impurity can be read out directly in ppm, provided the correct volume of gas has been sucked through.
Electronic Nose
The term electronic nose was created around the late 1980s (Gardner and Bartlett, 1994). It is based on a chemical sensor (Junichi et al., 2006), a device that converts chemical information into an analytically useful signal.
Chemical sensors are important for a variety of industrial and environmental applications, including the detection of hazardous chemicals, quality control in the food, perfume, and beverage industries, and medical applications (McGill et al., 2009). These types of sensors include a sorbent layer deposited on the active area of a signal transducer.
Some chemical sensors have been described that are potentially suitable for the detection of natural gas (Ameer and Adeloju, 2005; Munoz et al., 2009). For example, conducting polypyrrol films have been found useful for the detection of methane, since the sensitivity of the film is dependent on the CH4 concentration and the pressure.
Nanocomposites based on iron oxide and polypyrrol have been used for sensing various gases (Suri et al., 2002), and could potentially be used for the sensing of methane in oil fields, natural gas pipelines and joints, and waste water treatment plants (Ameer and Adeloju, 2005).
Commercial metal-oxide-based gas sensors that are specifically sensitive to methane are available. They are recommended as sensors for domestic gas alarms for the detection of methane (TGS 842, 2008). Suitable sensor systems have also been described for the detection of water traces in natural gas (May, 2009), which operate by the change in the electric capacitance in the presence of water vapor.
Additives for Odorization
Sulfur Compounds
Certain organic sulfur compounds are used for odorization because of their inherent penetrating smell. Skunk repellents contain sulfur compounds such as trans-2-butene-1-thiol and 3-methyl-1-butanethiol. Ethylmercaptan, because of its extremely low odor threshold, is the main compound used as an odorant in natural gas and liquid propane for leak detection, although tetrahydrothiophene is also often used. Common odorization reagents are summarized in Table 15.5 and Figure 15.1 and Figure 15.2.
| a)From wastes | |
| Additive | References |
|---|---|
| Ethylmercaptan or mixture of ethyl-, propyl-, and butylmercaptans dimethyl disulfide, diethyl disulfide, and methyl ethyl disulfide a | Arkema Inc. (2008), Fakhriev et al., 1993 and Fakhriev et al., 1994, Ismagilov et al. (1995) and Mazelli (1977) |
| Diethyl sulfide and ethyl propyl sulfide | McClure (1958) |
| Cyclohexylmercaptan | Oister (1970) |
| Dimethyl sulfide | Quaife et al. (1992b) |
| Tetrahydrothiophene, thiophenemer- captans with additional pyridine and picoline | Yashchenko et al. (1997) |
| Mixture of ethyl-, propyl-, butyl-, and amylmercaptan | Fakhriev et al. (1995) and Yoshida et al., (1984) |
| 2-Methoxy-3-isobutyl pyrazine and 4-methyl-4-mercapto-2-pentanone | Yoshida et al. (1984) |
tert-Butylmercaptan is very common in single component odorant, with good soil penetration and a high resistance to oxidation. Its high freezing point means that it needs to be used in mixtures with other components.
Isopropylmercaptan has a strong odor and a good resistance to oxidation. It is usually blended with tert-butylmercaptan to depress the freezing point of the latter compound (Usher, 1999).
Alkyl sulfides are resistant to oxidation, but they do not have as strong an odor as the mercaptans, so they are not used as stand-alone odorants. They are usually added to lower the freezing point of mercaptans. The commonly used odorant blends fall into one of the following main categories (Usher, 1999):
Mercaptan blends,
Mercaptan–alkyl sulfide blends, and
Tetrahydrothiophene–mercaptan blends.
Thermodynamic Properties of Odorants
The effectiveness of an odorant depends on its partition coefficients and solubility. Vapor-liquid equilibria data for sulfur compounds in liquefied natural gas are available (Guilbot et al., 1997; Kedzierski, 1996).
Structure Property Relationships
The power of the odor depends on certain properties of the respective molecule. A number of structure-odor relationships for odor intensity and quality have been established (Chastrette, 1997) using statistical methods.
Other Compounds
Sulfur-containing compounds are widely known as odorants used for fuel gases, but they usually generate sulfur dioxide when the fuel gases are burned. Also, if the fuel gases are used in fuel cells, a desulfurizer must be installed to remove odorant components that would cause catalyst poisoning (Kato, 2007).
Attempts have been undertaken to provide sulfur-free gas odorizing compositions, including the use of alkyl acrylates, vinyl or alkyl ethers, n-valeric acid, ethyl acrylate, cyclohexene, and norbornene derivatives (Mansfeld et al., 2006). These odorants have certain disadvantages, however. For example, acrylic ester odorants are chemically unstable. The content of cyclohexene or ethylidene norbornene must be larger than those of mercaptans (Kato, 2007). On the other hand, nitrogen-containing odorants may cause the enhanced formation of nitrogen oxides, which are toxic and react with sunlight to form ozone.
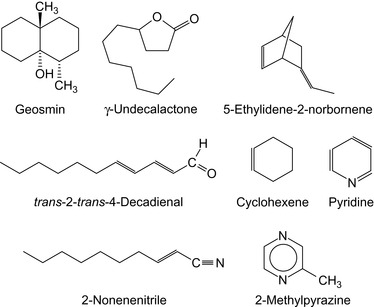
Among the alcohols that are suitable as odorants, geosmin is a preferred compound. Geosmin actually means earth smell. It is a naturally occuring organic compound produced by microorganisms. The human nose is extremely sensitive to geosmin. Its structured name is 2,6-dimethylbicyclo [4.4.0]decan-1-ol. The structures of geosmin and other sulfur-free odorants are shown in Figure 15.3.
trans-2-trans-4-Decadienal also has a low perceptual threshold (Kato, 2007). Mixtures of acrylates and pyrazines have been proposed as sulfur-free odorants (Mansfeld et al., 2006). The odorizing compositions may contain antioxidants, e.g., butylhydroxyanisole, ionol, i.e., tert-butyl hydroxytoluene, hydroquinone monomethyl ether, and α-tocopherol to protect against undesired oxidation. Examples are shown in Figure 15.4.
Sulfur-free odorants are shown in Table 15.6. Commercial sulfur-free odorants (or compositions with a reduced sulfur content) include a mixture of ethyl acrylate and methyl acrylate (Gasodor S Free™) and a mixture of ethyl acrylate and tetrahydrothiophen (Spotleak Z) (Heimlich et al., 2008).
| Additive | References |
|---|---|
| trans -2-trans -4-Decadienal | Kato (2007) |
| Geosmin | Kato (2007) |
| Cyclohexene | Mansfeld et al. (2006) |
| n-Valeric acid | Mansfeld et al. (2006) |
| Ethyl acrylate | Charles (2008) and Mansfeld et al. (2006) |
| 1-Methoxy-buten-3-yne | Müller and Short (2008) |
| Methyl ethyl pyrazine | Müller and Short (2008) |
Sulfur-free odorants can be smelled readily, but the odor rather resembles garlic. Human common sense does not associate this type of smell with combustible gas, because people are accustomed to the mercaptan smell.
Some cities have changed their odorant in the natural gas pipelines to sulfur-free odorants, with reports of success (Wagner, 2005), but others have returned to the conventional sulfur-based odorants.
The behavior of Gasodor S Free™ during reforming of methane that has been odorized with just this odorant has been tested with respect to the application of methane in fuel gas systems. It has been verified that odorization with Gasodor S Free would not have a negative impact on its subsequent use in fuel gas systems (Hennings and Reimert, 2007).
Industrial Synthesis of Odorants
The chemistry of thiols has been reviewed by Roberts (1997). Ethylmercaptan is readily formed by the reaction of ethyl bromide with hydrogen sulfide. Mercaptans can also be produced by the reaction of hydrogen sulfide and an olefin in the presence of a catalyst. Ethylmercaptan can be prepared by reaction of pure ethylene and hydrogen sulfide without the need for separation because it is the only mercaptan product. The basic reactions are
(15.2)
(15.3)
However, where the reactants are a mixture of more than one olefin, such as ethylene and propylene, the reaction is likely to produce both ethylmercaptan and propylmercaptan resulting in separation difficulty, because these mercaptans cannot be easily separated by distillation, extraction, filtration, or membrane diffusion. However, the selective production of ethylmercaptan from a fuel gas mixture can be achieved by special catalysts, i.e., oxides of cobalt and molybdenum (Sattich, 1994).
Mercaptans and sulfides can be selectively produced from alcohols by an electrophilic substitution reaction with hydrogen sulfide, in the presence of a catalyst blend. The alcohols can include primary and secondary alcohols (Hasenberg and Refvik, 2008).
(15.4)
In addition, mercaptans are formed by the reaction of sulfides with hydrogen sulfide:
(15.5)
Uses and Properties
Odorant Injection Techniques
Odorants are typically provided in liquid form, and are added to the gas where the distribution gas is taken from a main gas pipeline and transferred to a distribution pipeline. In such circumstances, the gas pressure may be stepped down through a regulator to a lower pressure. The odorants added to natural gas are extremely concentrated. Odorants such as tert-butylmercaptan and other blends are mildly corrosive and are also very noxious.
If a leak of odorant were to occur at an injection site, people in the surrounding area would assume that a gas leak had occurred with areas being evacuated and commerce being interrupted. However, if such mistakes become commonplace, people in the surrounding area will become desensitized to potential gas leaks and will fail to report them.
Three techniques are commonly used for odorizing incidents natural gas in a main distribution pipeline. A liquid odorant can be injected directly into the pipeline by a high-pressure injection pump, which pumps the odorant from a liquid storage tank into a small pipe that empties directly into the main gas pipeline. Because the odorant is extremely volatile, drops injected into the pipeline immediately disperse and spread throughout the gas in the pipeline. In this way, the drops of liquid odorant are dispersed in gaseous form within a few seconds (Marshall and Zeck, 2001).
The flow of gas in the pipeline is typically metered, so that liquid odorant can be injected periodically. For example, a few drops of odorant will suffice for a 30 m3 flow of natural gas. When the gas flow meter indicates that such an amount of natural gas has flowed through the pipe, another aliquot of liquid odorant is injected into the pipeline. This process is then repeated, even though the injection is performed periodically, the odorant diffusion within the gas provides adequate levels of odorant throughout the pipeline, assuming the time between injections is not too great.
Another odorization technique involves bypassing a small amount of natural gas at a slightly higher pressure than that of the main distribution pipeline through a tank containing a liquid odorant. This bypass gas absorbs relatively high concentrations of odorant while it is in the tank, and then returns to the main pipeline. The odorant, now volatilized, diffuses throughout the pipeline in much the same manner as described in the previous method (Arnold, 2000).
A third method for odorizing natural gas is to inject the odorant into the pipeline at a controlled rate. The system includes an odorant storage tank containing the odorant to be injected. A pressurized source of inert gas, such as nitrogen, maintains the tank at a desired positive pressure above that of the natural gas pipeline. An injection conduit communicates the odorant storage tank with the pipeline. A photooptic metering means, located within the injection conduit, meters the odorant to be injected into the pipeline (Zeck, 2006).
In an improved version of this method, the chemical is metered on a drop wise basis, with individual drops being counted as they pass through a measuring unit into the injection conduit and hence the pipeline. The measuring unit includes ultrasonic transmitters and receivers, which act as either proximity sensors or by measuring the transit time, to provide a measurement of the flow rate of the odorant on either a drop basis or in a steady state flow condition. Alternatively, liquid drops may land on the diaphragm of a piezoelectric sensor and thereby generate sound waves. These are then transmitted to an associated crystal, which generates a proportional electric charge resulting in a voltage difference between the two electrodes. The resulting voltage spikes can be counted and measured (Zeck, 2008).
Leak Detection
Leaks in pipelines can be detected by means of a test fluid. The test fluid, a mixture of dimethyl sulfide in solvent, is injected into a pipeline. It will escape through any leak, and the odorant is released from the closed compartments (Quaife et al., 1991 and Quaife et al., 1993).
Fuel Cells
With the advent of hydrogen-based fuels cells, the odorization of hydrogen has become an issue (Kopasz, 2007). Here another problem emerges, since common odorants may have a negative impact on the performance of the fuel cells, since commercial odorants act as poisons for the catalysts used in hydrogen-based fuel cells, most specifically for proton exchange membrane fuel cells. Chemical compounds based on mixtures of acrylic acid and nitrogen compounds have been adopted to achieve sulfur-free odorization of the gas (Puri, 2007).
In the use of natural gas and other petroleum gases to generate hydrogen for fuel cell applications, sulfur-free natural or petroleum gases are needed, or else a desulfurization step must be incorporated in the reforming process, which adds further cost to hydrogen generation.
Fuel cells are sulfur intolerant due to sulfur poisoning of the noble metal catalysts used. If sulfur-containing odorants are used, it would be necessary to remove sulfur-containing materials, like mercaptan odorants, from the feed gas using materials like zinc oxide. However, some sulfur-containing materials, like thiophenes, cannot be removed by zinc oxide and may require a specific hydrodesulfurization process, using hydrogen gas, to remove sulfur.
A further complexity for hydrogen fuel comes from the nature of the hydrogen flame propagation. When gases burn in air, their flames propagate upward with greater ease than they propagate downward. This is primarily due to the natural upward convection of hot burnt gases. For petroleum gases, propane and methane, the upward and downward propagating lean limits of combustion are approximately the same.
However, for hydrogen, since they differ by a factor of 2.5, the amount of odorant needed for leak detection in hydrogen could be >2.5 times that needed for methane or propane. The higher quantity of the odorant needed for hydrogen odor detection further complicates the sulfur poisoning problems for hydrogen gas used in fuel cells (Puri, 2007).
Odor-fading
One specific problem of odorization is odor-fading. The gas may be satisfactorily odorized at the source, but if it no longer has the necessary odor impact and intensity by the time it reaches the customer, escaping gas can remain undetected and result in a serious fire or explosion hazard. Basically three causes of fading may arise (Usher, 1999):
Oxidation, the formation of disulfides in the presence of iron oxide and traces of oxygen;
New pipe materials may cause adsorption or absorption of the odorant on to the surface of a plastic pipe; and
Gas quality problems may cause masking, or reaction of odorant components with impurities in the gas stream.
The presence of rust and air within a pipeline may act as a catalyst for the oxidation of mercaptans, resulting in compounds that do not smell at all. On the other hand, sulfide components are much more resistant to oxidation.
Dry gas is the easiest to odorize and does not cause odor-fade. Condensed liquids in the pipeline may absorb components of the odorant. Odor masking may also occur because of the odor imparted by any impurities present in the gas.
Odor-fading from odorized liquefied petroleum gas stored in carbon steel containers can occur by catalytic effects of the containers. To postpone this effect, the respective steel surfaces can be deactivated by treating them with a deactivating agent (Nevers, 1990) before exposure to the liquefied petroleum gas.
Examples of such deactivating agents are benzotriazole, tolyl triazole, mercaptobenzothiazole, benzothiazyl disulfide, or mixtures of these compounds (Nevers, 1987). It has been suggested that a mathematical model and adequate software should be developed to predict odorant fade (Altpeter, 1997).
Environmental Problems
If natural gas for storage in natural reservoirs is odorized with sulfur compounds, then a possible environmental impact can result. Some of the odorant is lost in the formation (Sasnanand, 1993). If the loss occurs in a reservoir adjacent to an aquifer, it could contaminate the water and cause environmental problems. When gas is drawn off, water is also often injected into the reservoir. A case was described in which the respective water had a strong characteristic odor (Girod et al., 1996). A stripping column has been recommended to overcome this problem.
Contaminated ground water can be decontaminated by reaction with iron (Huang and Lee, 1997). This technique was proposed to remedy ground water that was contaminated with ethylmercaptan in situ. Studies suggest chemical reactions with iron rather than an irreversible surface adsorption. Gas odorizers can be removed by extraction, similar to the usual glycol dehydration and desulfurization process (Jullian et al., 1997; Rojey et al., 1998).
Another cleaning process for the removal of tetrahydrothiophene uses an advanced oxidation technique, consisting of water treatment by UV radiation in combination with a dose of hydrogen peroxide (Panneman et al., 1997). It is possible to keep the concentrations of odorant and condensate in the effluent below 0.1 ppb.
Altpeter Jr., L.L., Research recommended to develop odorant-fade model, Pipe Line Gas Ind. 80 (2) (1997) 39–40.
Ameer, Q.; Adeloju, S.B., Polypyrrole-based electronic noses for environmental and industrial analysis, Sens. Actuators B 106 (2) (2005) 541–552.
Arkema Gas Odorants, 2008. Arkema Inc., Philadelphia, PA. http://arkema-inc.com/literature/pdf/802.pdf (accessed 29.05.08).
Arnold, J.F., 2000. System and method for odorizing natural gas. US Patent 6 142 162, assigned to Odoreyes Technology, Inc., Birmingham, AL, November 7, 2000.
ASTM E 0679-04, Practice for determination of odor and taste thresholds by a forced-choice ascending concentration series method of limits, In: ASTM Standard, Book of Standards, vol. 15.08 (2009) ASTM International, West Conshohocken, PA.
ASTM E 544-99(2004), Standard practices for referencing suprathreshold odor intensity, In: ASTM Standard, Book of Standards, vol. 15.08 (2009) ASTM International, West Conshohocken, PA.
Askar, E.; Schröder, V.; Acikalin, A.; Steinbach, J., Calculation of flammability limits of gas phases with ethylene oxide in sterilisers, Biomed. Eng. 53 (6) (2008) 265–269.
Bissell, J.; Acker, D.; Quaife, L.R., Pipeline leak-location technique utilizing a novel test fluid and trained dogs, In: Proceedings Volume, no. 9, 5th Pipe Line Ind. & Pipes Pipelines Int. Pipeline Pigging & Integrity Monit Int. Conf.Houston, February 1–4, 1993. (1993).
Charles, P., 2008. Gas odorant. US Patent Application 20080295404, assigned to Arkema France, Colombes, December 4, 2008.
Chastrette, M., Trends in structure-odor relationship, SAR QSAR Environ. Res. 6 (3) (1997) 215–254.
Fakhriev, A.M., Ismagilov, F.R., Latypova, M.M., 1995. Odorant for natural gas. RU Patent 2 041 243, assigned to Bashkirskoe Sp Kt B Kontserna, August 9, 1995.
Fakhriev, A.M., Latypova, M.M., Ismagilov, F.R., Navalikhin, P.G., 1993. Odorisation of liquefied hydrocarbon gases – using waste from oxidising demercaptanisation of light hydrocarbon material. RU Patent 2 000 313, September 7, 1993.
Fakhriev, A.M., Latypova, M.M., Nasteka, V.I., Berdnikov, A.I., Klimov, V.Y., 1994. Odorising agent for compressed hydrocarbon gas – contains ethyl-mercaptan or mixed mercaptans, and additionally waste from process of oxidising de-mercaptanisation of light hydrocarbons. RU Patent 2 009 178, assigned to Hydro Carbon Raw Mat. Res. and Orenburg Gas Process Wks, March 15, 1994.
Fant, E.E., Odorization – a regulatory perspective, In: (Editors: Wilson, G.G.; Attair, A.A.) Odorization, vol. 3 (1993) Institute of Gas Technology, Chicago, IL, pp. 109–118.
Flynn, P.J., Sprague, M., 2009. Hydrogen odorants and odorant selection method. US Patent Application 20090179177, assigned to Enersol, Inc. N.A., L.P. Fairfax VA, July 16, 2009.
Gao, X.; Fan, H.; Huang, T.; Wang, X.; Bao, J.; Li, X.; et al., Natural gas pipeline leak detector based on NIR diode laser absorption spectroscopy, Spectrochim. Acta Part A 65 (1) (2006) 133–138.
Gardner, J.W.; Bartlett, P.N., A brief history of electronic noses, Sens. Actuators B 18 (1–3) (1994) 210–211.
Girod, J.F.; Leclerc, J.P.; Muhr, H.; Paternotte, G.; Corriou, J.P., Removing a small quantity of THT (tetrahydrothiophene) from gas storage groundwater through air stripping and gas-phase carbon adsorption, Environ. Progr. 15 (4) (1996) 277–282.
Guilbot, P.; Valtz, A.; Richon, D., Partition coefficients at infinite dilution for different sulfur compounds in various solvents, In: Proceedings Volume, 76th Annu. Gpa ConvSan Antonio, March 10–12, 1997. (1997), pp. 33–39.
Hasenberg, D.M., Refvik, M.D., 2008. Process and catalyst for synthesis of mercaptans and sulfides from alcohols. US Patent 7 399 893, assigned to Chevron Phillips Chemical Company LP, The Woodlands, TX, July 15, 2008.
Heimlich, F.; Niebialek, S.; Schulz, C., Odorization with spotleak Z, Gas und Wasserfach Gas Erdgas 149 (3) (2008) 165.
Henderson, D.F., Large volume odorization, installation, operation, and maintenance, In: (Editors: Wilson, G.G.; Attair, A.A.) Odorization, vol. 3 (1993) Institute of Gas Technology, Chicago, IL, pp. 239–249.
Hennings, U.; Reimert, R., Behaviour of sulfur-free odorants in natural gas fed PEM fuel cell systems, Fuel Cells 7 (1) (2007) 63–69.
Holtappels, K.; Brinkmann, C.; Dietlen, S.; Schröder, V.; Stickling, J.; Schönbucher, A., Messung und Simulation des Inertgaseinflusses auf Explosionsgrenzen bei erhöhten Anfangsdrücken, Chem. Ing. Tech. 73 (3) (2001) 270–274.
Huang, F.; Lee, R., Degradation of ethyl mercaptan in the presence of zero-valence iron, In: Proceedings Volume, 4th US DOE, Tulsa Univ, et al Petrol. Environ. Conf.San Antonio, September 9–12, 1997. (1997).
Ismagilov, F.R., Kazantsev, A.V., Akhunov, R.R., Rygalov, V.A., Navalikhin, P.G., Andrianov, V.V., et al., 1995. Odorant for liquefied hydrocarbon gases and method for its production. RU Patent 2 051 168, assigned to Bashkirskoe Sp Kt B Kontserna, December 27, 1995.
Jullian, S., Thomas, M., Rojey, A., 1997. Process for the complete treatment of natural gas on a production site. EP Patent 0 781 832, assigned to Inst. Francais Du Petrole, May 2, 1997.
Junichi, K.; Masayuki, O.; Hisamitsu, A.; Motoo, K., Electronic nose, J. Jpn. Assoc. Odor Env. 37 (3) (2006) 172–178; (in Japanese).
Kato, Y., 2007. Odorant for fuel gas. US Patent 7 182 796, assigned to Soda Aromatic Co., Ltd. (JP), February 27, 2007.
Kedzierski, S., The solubility of odorants in natural gas (rozpuszczalnosc preparatow nawaniajacych w gazie ziemnym), Nafta Gaz (Pol) 52 (8) (1996) 357–360.
Klusmann, E.B., Odor monitoring at southern california gas company, In: (Editors: Wilson, G.G.; Attair, A.A.) Odorization, vol. 3 (1993) Institute of Gas Technology, Chicago, IL, Southern California Gas C., pp. 407–423.
Kopasz, J.P., Fuel cells and odorants for hydrogen, Int. J. Hydrogen Energy 32 (13) (2007) 2527–2531.
Mansfeld, G., Rohde, U., Henke, F., Kaesler, H., 2006. Gas odorization method. US Patent 7 108 803, assigned to Symrise GmbH & Co. KG (DE), September 19, 2006.
Marshall, S.E., Zeck, M.V., 2001. Chemical injection system. US Patent 6 208 913, assigned to YZ Systems, Inc., Conroe, TX, March 27, 2001.
May, R.D., 2009. System and method for detecting water vapor within natural gas. US Patent 7 504 631, assigned to SpectraSensors, Inc., Rancho Cucamonga, CA, March 17, 2009.
Mazelli, J.R., 1977. Method of odorizing liquid natural gas. US Patent 4 025 315, assigned to San Diego Gas & Electric Co. (San Diego, CA) Dual Fuel Systems, Inc. (Los Angeles, CA), May 24, 1977.
McClure, J.S., 1958. Warning agent and process for the odorization of a hydrocarbon fuel gas therewith. US Patent 2 823 104, assigned to California Research Corp., February 11, 1958.
McGill, R.A., Voiculescu, I., Fedder, G.K., 2009. Microelectro-mechanical chemical sensor. US Patent 7 556 775, assigned to The United States of America as represented by the Secretary of the Navy (Washington, DC), July 7, 2009.
Morley, C., 2005. GasEq: A chemical equilibrium program for windows. http://www.gaseq.co.uk (accessed 27.04.11).
Muhlbauer, W.K., Distribution systems, In: Pipeline Risk Management Manualthird ed. (2004) Gulf Professional Publishing, Burlington, pp. 223–242; (Ch. 11).
Müller, U., Short, J.N., 2008. Gas odorant. US Patent Application 20080256847, October 23, 2008.
Munoz, B.C., Pierce, K.J., Galloway, C.P., 2009. Sensors with improved properties. US Patent 7 501 091, assigned to Smiths Detection Inc., Pasadena, CA, March 10, 2009.
Nagata, Y., Measurement of odor threshold by triangle odor bag method, In: International Symposium on Odor Measurement, Asian Network on Odor Measurement and ControlJapan Association on Odor Environment, Tokyo, JP. (2003), pp. 118–127; http://www.env.go.jp/en/air/odor/measure/02_3_2.pdf..
Nevers, A.D., 1987. Odor-fading prevention from organosulfur-odorized liquefied petroleum gas. US Patent 4 701 303, October 20, 1987.
Nevers, A.D., 1990. Odor-fading prevention from organosulfur-odorized liquefied petroleum gas. CA Patent 1 274 692, assigned to Atochem North America Inc., October 2, 1990.
Oister, W.H., 1970. Odorized gas. US Patent 3 545 949, assigned to Pennwalt Corp., December 8, 1970.
Oudman, P., Odorization and odorant monitoring practices at canadian western natural gas company limited, In: (Editor: Wilson, G.G.) Odorization, vol. 3 (1993) Institute of Gas Technology, Chicago, IL, Southern California Gas C, pp. 389–405.
Panneman, H.J.; Pot-Gerritsen, R.C.; Kuiper-Van Loo, E.M.; Pastoor, H.; Janssen- Van Rosmalen, R., UV (ultraviolet)-oxidation process for water treatment at gas plant sites, In: Proceedings Volume, Pt. B, 20th Int. Gas Union World Gas Conf.Copenhagen, Denmark, June 10–13, 1997. (1997), p. 269; 271–285.
Patnaik, P., In: A Comprehensive Guide to the Hazardous Properties of Chemical Substancesthird ed. (2007) John Wiley & Sons, New York.
Puri, P.S., 2007. Leak site odorization for gas leak detection. US Patent 7 229 831, assigned to Air Products and Chemicals, Inc., Allentown, PA, June 12, 2007.
Quaife, L.R.; Moynihan, K.J., A new pipeline leak-locating technique utilizing a novel odourized test-fluid (patent pending) and trained domestic dogs, In: Proceedings Volume, 1st US Environ. Protect Agency et al Oil & Gas Explor & Prod. Waste Manage Pract Int. Symp.New Orleans, September 10–13, 1990. (1990), pp. 647–657.
Quaife, L.R.; Moynihan, K.J.; Larson, D.A., A new pipeline leak-location technique utilizing a novel (patented) test-fluid and trained domestic dogs, In: Proceedings Volume, 71st Annu. Gpa Conv.Anaheim, California, March 16–18, 1992. (1992), pp. 154–161.
Quaife, L.R., Szarka, J., Moynihan, K.J., Moir, M.E., 1991. Test-fluid composition and method for detecting leaks in pipelines and associated facilities. US Patent 5 049 312, assigned to Exxon Production Research Co., September 17, 1991.
Quaife, L.R., Szarka, J., Moynihan, K.J., Moir, M.E., 1992b. Test-fluid composition and method for detecting leaks in pipelines and associated facilities. US Patent 5 167 867, assigned to Exxon Production Research Company, December 1, 1992.
Quaife, L.R., Szarka, J., Moynihan, K.J., Moir, M.E., 1993. Test-fluid composition and method for detecting leaks in pipelines and associated facilities. CA Patent 2 052 242, February 14, 1993.
Quaglio, J., 1880. Wassergas als Brennstoff der Zukunft: Strong's Patent zur Bereitung von Heizgas in Verwendung mit Lowe's Verfahren für Leuchtgas, Bericht 252123468. Bergmann, Wiesbaden.
Ripley, D.L., Goetzinger, J.W., Whisman, M.L., 1990. Human response research evaluation of alternate odorants for LP-gas. GPA Res. Rep. RR-129.
Roberts, J.S., Thiols, In: (Editor: Kroschwitz, J.I.) fourth ed.Kirk-Othmer Encyclopedia of Chemical Technology, vol. 24 (1977) John Wiley & Sons, New York, p. 19.
Rojey, A., Thomas, M., Jullian, S., 1998. Process for treatment of natural gas at a storage site. US Patent 5 803 953, assigned to Inst. Francais Du Petrole, September 8, 1998.
Sasnanand, S., 1993. Adsorption of tetrahydrothiophene in porous media: an experimental approach. Ph.D. thesis, New Mex Inst Mining Techn, Socorro, New Mexico.
Sattich, W.E., 1994. Selective production of ethyl mercaptan. US Patent 5 352 838, assigned to Phillips Petroleum Company, Bartlesville, OK, October 4, 1994.
Shimokawatoko, T., Sumida, K., Ueda, H., 1998a. Method and apparatus for measuring odorant concentration and oderant adding system. US Patent 5 844 124, assigned to Osaka Gas Co. Ltd., December 1, 1998.
Shimokawatoko, T., Sumida, K., Ueda, H., 1998b. Method and apparatus for measuring odorant concentration and odorant adding system. EP Patent 836 091, assigned to Osaka Gas Co. Ltd., April 15, 1998.
Suri, K.; Annapoorni, S.; Sarkar, A.K.; Tandon, R.P., Gas and humidity sensors based on iron oxide-polypyrrole nanocomposites, Sens. Actuators B 81 (2–3) (2002) 277–282.
Toth, J.M., Natural gas odorization and its techniques, In: Proceedings Volume, 49th Annu. Appalachian Gas Meas Short CourseCoraopolis, Penn, August 15–18, 1989. (1989), pp. 170–174.
TGS 842 – for the detection of methane, 2008. Tech. rep., Figaro USA, Inc., Arlington Heights, IL 60005. http://www.figarosensor.com/products/842pdf.pdf (accessed 17.01.08).
Usher, M.J., Odor fade – possible causes and remedies, In: Proceedings Volume, no. 285CGA Gas Measurement School, Canadian Gas Association, Ontario, CA. (1999).
Verschueren, K., In: Handbook of Environmental Data on Organic Chemicalsfifth ed. (2009) Wiley & Sons, Hoboken, NJ.
Wagner, T., Erfahrungsbericht zur regionalen Zentralodorierung mit schwefelfreiem Odoriermittel, Gas und Wasserfach Gas Erdgas 146 (10) (2005) 560–563.
Wallace, J.R., Stetter, J.R., Nacson, S., Findlay Jr., M.W., 1991. Odorant analyzer system. EP Patent 0 445 927, assigned to Gas Res. Inst., September 11, 1991.
Wetteman, A.J.; Wilson, J.R., Operation of large volume odorizers, In: Odorization, vol. 3 (1993) Institute of Gas Technology, Chicago, IL, pp. 251–255.
Yashchenko, V.L., Vakulin, V.I., Berdnikov, A.I., Grunvald, V.R., Nikolaev, V.V., Klimov, V.Y., et al., 1997. Odorant for natural gas. RU Patent 2 076 137, assigned to Aktsionernoe Obshchestvo Zakry, March 27, 1997.
Yoshida, T., Katz, I., Warren, C.B., Wiener, C., 1984. Odorization of combustible hydrocarbon gases. US Patent 4 487 613, assigned to International Flavors & Fragrances Inc., New York, NY, December 11, 1984.
Zeck, M., 2006. Optical odorization system. US Patent 7 056 360, June 6, 2006.
Zeck, M., 2008. Ultrasonic and sonic odorization systems. US Patent 7 389 786, June 24, 2008.
Tradenames
| Tradename Description | Supplier |
|---|---|
| Black Pearls® Carbon black (Munoz et al., 2009) | Cabot Corp. |
..................Content has been hidden....................
You can't read the all page of ebook, please click here login for view all page.