Chapter 1. Drilling Muds
According to the American Petroleum Institute (API), a drilling fluid is defined as a circulating fluid, used in rotary drilling to perform any or all of the various functions required in drilling operations.
Drilling fluids are mixtures of natural and synthetic chemical compounds used to cool and lubricate the drill bit, clean the hole bottom, carry cuttings to the surface, control formation pressures, and improve the function of the drill string and tools in the hole. They are divided into two general types: waterbased drilling muds (WBMs) and oil-based drilling muds (OBMs). The type of fluid base that is used depends on drilling and formation needs, as well as the requirements for disposing of the fluid after it is no longer needed. Drilling muds are a special class of drilling fluids used to drill most deep wells. The term mud is used because of the thick consistency of the formulation.
Drilling fluids serve several fundamental functions (Brazzel, 2009; Melbouci and Sau, 2008):
• Control of downhole formation pressures,
• Overcoming the fluid pressure of the formation,
• Avoiding damage to the producing formation,
• Removal of cuttings generated by the drill bit from the borehole, and
• Cooling and lubricating the drill bit.
In order to perform their fundamental functions, drilling fluids should possess several desirable characteristics, which greatly enhance the efficiency of the drilling operation.
These include desired rheological properties (plastic viscosity, yield value, low-end rheology, and gel strengths), fluid loss prevention, stability under various temperature and pressure operating conditions, stability against contaminating fluids, such as salt water, calcium sulfate, cement, and potassium contaminated fluids (Melbouci and Sau, 2008).
The drilling fluid should also have penetration enhancement characteristics that wet the drill string and keep the cutting surfaces of the drill bit clean (whether it is a roller cone or other configuration).
Wetting ability is at least in part a function of the surface tension of the fluid. The fluid should also have a high degree of lubricity and to minimize friction between the drill string and the wall of the borehole to minimize of differential sticking. In this situation, the hydrostatic pressure of the drilling fluid column must be sufficiently higher than the formation pressure so that the drill string is forced against the wall of the borehole and stuck.
It should also prevent the solids of the formation, primarily shales and clays, from swelling, so reducing the incidence of drill sticking, undergauge holes etc.
Classification of Muds
The classification of drilling muds is based on their fluid phase alkalinity, dispersion, and the type of chemicals used in their formulation. The classification according to (Lyons, 1996) is reproduced in Table 1.1.
| dDispersed systems | |
| nNondispersed systems | |
| Class | Description |
|---|---|
| Fresh water mudsd | pH from 7–9.5, include spud muds, bentonite-containing muds, phosphate-containing muds, organic thinned muds (red muds, lignite muds, lignosulfonate muds), organic colloid muds |
| Inhibited mudsd | Water-based drilling muds that repress hydration of clays (lime muds, gypsum muds, sea water muds, saturated salt water muds) |
| Low-solids mudsn | Contain less than 3–6% of solids. Most contain an organic polymer |
| Emulsions | Oil in water and water in oil (reversed phase, with more than 5% water) |
| OBMs | Contain less than 5% water; mixture of diesel fuel and asphalt |
Drilling muds are usually classified as either WBMs or OBMs, depending upon the continuous phase of the mud. However, WBMs may contain oil and OBMs may contain water (Guichard et al., 2008).
OBMs generally use hydrocarbon oil as the main liquid component, with other materials such as clays or colloidal asphalts being added to provide the desired viscosity together with emulsifiers, polymers, and other additives including weighting agents. Water may also be present, but in an amount not usually greater than 50% by volume of the entire composition. If more than about 5% of water is present, the mud is often referred to as an invert emulsion, i.e., a water-in-oil emulsion.
WBMs conventionally contain viscosifiers, fluid loss control agents, weighting agents, lubricants, emulsifiers, corrosion inhibitors, salts, and pH control agents. Water makes up the continuous phase of the mud, and is usually present as at least 50 volume percent of the entire composition. Oil is also usually present in small amounts, but will typically not exceed the amount of the water, so that the mud will retain its character as a water-continuous-phase material.
Potassium muds are the most widely accepted water mud system for drilling water sensitive shales. K+ ions attach to clay surfaces and lend stability to the shale that is exposed to drilling fluids by the bit. The ions also help to hold the cuttings together, minimizing its dispersion into finer particles. Potassium chloride, KCl is the most widely used source of potassium, with others being potassium acetate, potassium carbonate, potassium lignite, potassium hydroxide, and potassium salt of partially hydrolyzed polyacrylamide (PHPA).
For rheological control, different types of polymers are used, such as xanthan gum and PHPA. For fluid loss control, mixtures of starch and polyanionic cellulose (PAC) are often used. Carboxymethyl starch, hydroxypropyl starch, carboxymethyl cellulose (CMC), and sodium polyacrylate are also used. PHPA is widely used for shale encapsulation.
Salt water muds contain varying amounts of dissolved sodium chloride (NaCl) as a major component. Undissolved salt may also be present in saturated salt muds to increase density or to act as a bridging agent over permeable zones. Starch and its derivatives for fluid loss control, and xanthan gums for hole cleaning are among the few additives that are effective for salt water muds.
Sea water mud is a WBM designed for offshore drilling whose make-up water is taken from the ocean. Sea water has relatively low salinity, containing about 3–4% of NaCl, but has a high hardness because of the presence of Mg+2 and Ca+2 ions. This hardness is removed from sea water by adding NaOH (sodium hydroxide), which precipitates Mg+2 as Mg(OH)2 (magnesium hydroxide) and by adding Na2CO3 (sodium carbonate), which removes Ca+2 as CaCO3 (calcium carbonate). The additives are the same as those used in fresh water muds (Guichard et al., 2008), namely
• Bentonite clay,
• Lignosulfonate,
• Lignite,
• CMC, or
• PAC, and
• Caustic soda.
Xanthan gum may be used in place of bentonite. Silicate-mud is a type of shale-inhibitive water mud that contains sodium or potassium silicate as the inhibitive component. If this material is used, then a high pH is a necessary characteristic of silicate muds in order to control the amount and type of polysilicates that are formed. This is achieved by the addition of NaOH (or KOH) and the appropriate silicate solution. Silicate anions and colloidal silica gel combine to stabilize the wellbore by sealing microfractures, forming a silica layer on shales and possibly acting as an osmotic membrane, which can produce in-gauge holes through troublesome shale sections that otherwise might require an oil mud.
Lime mud is a type of WBM that is saturated with lime (Ca(OH)2), and has excess, undissolved lime solids maintained in reserve. Fluid loss additives include starch, hydroxypropyl starch, CMC, or PAC (Guichard et al., 2008).
Dispersed Noninhibited Systems
Drilling fluids used in the upper hole sections are referred to as dispersed noninhibited systems. They are formulated from fresh water and may contain bentonite. The classification of bentonite-based muds is shown in Table 1.2. The flow properties are controlled by a flocculant or thinner, and the fluid loss is controlled with bentonite and CMC.
Phosphate-treated Muds
Phosphates are only effective in small concentrations, and the mud temperature must be less than 55°C. The salt contamination must be less than 500 ppm sodium chloride. The concentration of calcium ions should be kept as low as possible. The pH should be between 8 and 9.5. Some phosphates may decrease the pH, so more NaOH must be added.
Lignite Muds
Lignite muds are temperature resistant up to 230°C. Lignite can control viscosity, gel strength, and fluid loss. The total hardness must be lower than 20 ppm.
Quebracho Muds
Quebracho is a natural product extracted from the heartwood of the Schinopsis trees that grow in Argentina and Paraguay. It is a well-characterized polyphenolic, readily extracted from the wood by treatment with hot water, and is widely used as a tanning agent. It is also used as a mineral dressing, as a dispersant in drilling muds, and in wood glues. Quebracho is commercially available as a crude hot water extract, either in lump, ground, or spray-dried form, or as a bisulfite-treated, spray-dried product that is completely soluble in cold water. It is also available in a bleached form, which can be used in applications where the dark color of unbleached quebracho is undesirable (Shuey and Custer, 1995).
Quebracho-treated fresh water muds were originally used at shallow depths. It is also referred to as red mud because of the deep red color. Quebracho acts as a thinner. Polyphosphates are also added when Quebracho is used. Quebracho is active at low concentrations and consists of tannates.
Lignosulfonate Muds
Lignosulfonate fresh water muds contain ferrochrome lignosulfonate for viscosity and gel strength control. These muds are resistant to most types of drilling contamination because of the thinning efficiency of the lignosulfonate in the presence of large amounts of salt and at extreme hardnesses.
Lime Muds
Lime muds contain caustic soda, an organic thinner, hydrated lime, and a colloid for filtrate loss. From this a pH of 11.8 can result, with calcium ions at a concentration of 3–20 ppm in the filtrate. Lime muds exhibit low viscosity, low gel strength, and good suspension of weighting agents. They can carry a larger concentration of clay solids at lower viscosities than other types of mud. At high temperatures, lime muds present a danger of gelation.
Sea Water Muds
The average composition of sea water is shown in Table 1.3. Most of the hardness in sea water is caused by magnesium. Sea water muds have sodium chloride concentrations above 10,000 ppm. They also contain bentonite, thinner (lignosulfonate or lignosulfonate with lignite), and an organic filtration control agent.
Nondispersed Noninhibited Systems
In nondispersed systems no special agents are added to deflocculate the solids in the fluid. The main advantages of these systems are the higher viscosities and the higher yield point-to-plastics viscosity ratio. These alterated flow properties provide a better cleaning of the borehole, allow a lower annular circulating rate, and minimize the washout of the borehole.
Low-solids Fresh Water Muds
Clear fresh water is the best drilling fluid in terms of penetration rate. Therefore, it is desirable to achieve a maximal drilling rate using a minimal amount of solid additives. Originally, low-solids mud formulations were used in hard formations, but they now also tend to be used in other formations. Several types of flocculants are used to promote the settling of drilled solids by flocculation.
Variable Density Fluids
Variable density fluids are those that have a density which varies as a function of the pressure in the subterranean formation. Such a fluid comprises a base fluid and a proportion of elastic particles.
These elastic particles allow the density of the variable density fluid to vary as a function of pressure. For instance, as the elastic particles encounter higher downhole pressures, they become compressed, thereby decreasing the volume and in turn increasing the density of the fluid that contains them. When the elastic particles are fully compressed, the density increases considerably.
The increase in volume of the elastic particles in turn reduces the overall density of the variable density drilling fluid. The resulting change in density may be sufficient to permit the return of the variable density fluid through the riser to the surface without the need for any additional pumps or subsurface additives (Ravi et al., 2009).
The elastic particles are usually either a copolymer of styrene and divinyl-benzene, a copolymer of styrene and acrylonitrile, or a terpolymer of styrene, vinylidene chloride, and acrylonitrile (Ravi et al., 2009).
Gas-based Muds
Although natural gas (methane) exhaust or other combustion gases can be used, air is the most common gas to be used in such drilling fluids. It is used to produce so-called foam muds, in which air bubbles are surrounded by a film of water containing a foam-stabilizing substance or film-strengthening material, such as an organic polymer or bentonite.
This type of mud is not recirculated and is often used for reduced-pressure drilling to improve the hole stability in caving formations. However, this type of mud has some limitations, since the drilling water produces wet formations, and it has a limited salt tolerance.
Drill-in Fluids
After drilling a well to the total depth, it is a normal practice to replace the drilling mud with a completion fluid. This fluid is a clean, solids-free, or acid soluble, non-damaging formulation, intended to minimize reductions in permeability of the producing zone. Prior to producing from the formation, it is usually necessary to clean up what is left by the original mud and the completion fluid, by breaking and degrading the filter cake with an oxidizer, enzyme, or an acid solution.
Nowadays, many wells exploit the pay-zone formations for long distances horizontally. It is no longer practical in these wells to drill the pay-zone with conventional, solids-laden muds, as the extended clean-up process afterwards is much more difficult. Consequently, the current generation of drill-in fluids was developed.
Drill-in fluids are completion fluids, but they also act as drilling muds. As the pay-zone is penetrated horizontally, these fluids must provide the multifunctional requirements of drilling fluids in addition to the non-damaging attributes of completion fluids. In practice, the normal drilling mud is replaced with a drill-in fluid just before the pay-zone is penetrated, and used until the end of the operation.
Mud Compositions
Commercial products are listed in the literature. The additional components include bactericides, corrosion inhibitors, defoamers, emulsifiers, fluid loss and viscosity control agents, and shale control additives (Anonymous, 1991a, Anonymous, 1991b, Anonymous, 1991c, Anonymous, 1992 and Anonymous, 1996).
Inhibitive Water-based Muds
Minimizing the environmental impact of the drilling process is a highly important part of drilling operations, in order to comply with environmental regulations which have become stricter throughout the world. In fact, this is a mandatory requirement for the North Sea sector. The drilling fluids industry has made significant progress in developing new fluids and ancillary additives to fulfill the increasing technical demands for drilling oil wells. Additives now have very little or no adverse effects on the environment or on drilling economics.
New drilling fluid technologies have been developed to allow the continuation of oil-based performance with regard to formation damage, lubricity, and wellbore stability aspects and thus penetration rates. These aspects were greatly improved by incorporating polyols or silicates as shale inhibitors in the fluid systems.
Polyol-based fluids contain a glycol or glycerol as a shale inhibitor, commonly used in conjunction with conventional anionic and cationic fluids to provide additional inhibition of swelling and dispersing of shales. They also provide some lubrication properties.
Sodium or potassium silicates are known to provide levels of shale inhibition comparable to that of OBMs. This type of fluid is characterized by a high pH (> 12), for optimum stability of the mud system. The inhibition properties of such fluids are due to the precipitation or gelation of silicates that occurs on contact with divalent ions and lower pH in the formulation, providing an effective water barrier that prevents hydration and dispersion of the shales.
Water-based Muds
These muds have water as the continuous phase, which may contain several dissolved substances such as alkalies, salts and surfactants, organic polymers in colloidal state, droplets of emulsified oil, and various insoluble substances, such as barite, clay, and cuttings in suspension.
The mud composition that is selected for use often depends on the dissolved substances present in the most economically available make-up water, or on the soluble or dispersive materials in the formations to be drilled. Several mud types or systems are recognized and described in the literature such as:
• Spud muds,
• Dispersed/deflocculated muds,
• Lime muds,
• Gypsum muds,
• Salt water muds,
• Nondispersed polymer muds,
• Inhibitive potassium muds,
• Cationic muds, and
• Mixed metal hydroxide muds.
Despite their environmental acceptability, conventional WBMs exhibit major deficiencies relative to OBMs/pseudo oil-based drilling muds (POBMs) because of their relatively poor shale inhibition, lubricity, and thermal stability characteristics. To overcome these deficiencies, specific additives may be added to the WBM compositions to bring their properties close to that of OBMs/POBMs while minimizing their environmental impact.
Components of WBMs are shown in Table 1.4. Various methods for the modification of lignosulfonates have been described in the literature, for example, condensation with formaldehyde (Martyanova et al., 1997) or modification with iron salts (Ibragimov et al., 1998). It has been found that chromium-modified lignosulfonates, as well as mixed metal lignosulfonates of chromium and iron, are highly effective as dispersants. They are therefore useful for controlling the viscosity of drilling fluids and reducing their yield point and gel strength. Because chromium is potentially toxic, its release into the natural environment is continuously being reviewed by various government agencies around the world.
| Compound | References |
|---|---|
| Glycol-based | Lee et al. (1997) |
| Alkali silicates | Mullen and Gabrysch (2001), Urquhart (1997) |
| Polyacrylamide, carboxymethyl cellulose | Kotelnikov et al. (1996) |
| Carboxymethyl cellulose, zinc oxide | Gajdarov and Tankibaev (1996) |
| Acrylamide copolymer, polypropylene glycol (PPG) (water-based mud) | Patel and Muller (1996) |
Therefore, less toxic substitutes are desirable. These can be prepared by combining tin or cerium sulfate with an aqueous solution of calcium lignosulfonate, thereby producing a solution of tin or cerium sulfonate and a calcium sulfate precipitate (Patel, 1994b).
Compositions with Improved Thermal Stability
To avoid the problems associated with viscosity reduction in polymer-based aqueous fluids, formates, such as potassium formate and sodium formate, are commonly added to enhance their thermal stability, but this is very expensive, and thermal stabilities of polymer-based aqueous fluids can be improved by other means (Maresh, 2009).
The stability of a wellbore treatment fluid may be maintained up to temperatures of 135–160°C (275–325°F) by introducing various polysaccharides into the fluid. The apparent viscosities of some drilling fluids containing xanthan gum and polyacrylamide (PAM) before and after rolling at 120°C are shown in Table 1.5.
| Composition | Before | After |
|---|---|---|
| Brine/XC | 13 | 3 |
| Brin/PA | 8.5 | 6 |
| Brine/Filtercheck | 4 | 4 |
| Brine/FLC/XC | 16 | 10.5 |
| Brine/FLC/PA | 14.6 | 9 |
| Brine/XC/CLAYSEAL | 12.5 | 3 |
| XC/PA | 30 | 28.5 |
| XC/PA/FLC | 38.5 | 16.5 |
| XC/PA/FLC/CLAYSEAL | 34 | 28 |
| XC/PA/FLC/CLAYSEAL/Barite | 38.5 | 38.5 |
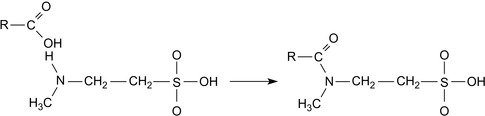
Shale Encapsulator
A shale encapsulator is added to a WBM in order to reduce the swelling of the subterranean formation in the presence of water. It must be at least partially soluble in the aqueous continuous phase in order to be effective.
A conventional encapsulator is a quaternary PAM, preferably a quaternized polyvinyl alcohol. Useful anions include halogen, sulfate, nitrate, and formate (Patel et al., 2009).
By varying the molecular weight and the degree of amination, a wide variety of products can be produced. It is possible to create shale encapsulators for use in low salinity conditions, including fresh water (Patel et al., 2009). The repeating units of quaternized, etherified polyvinyl alcohol and quaternized PAM are shown in Figure 1.1.
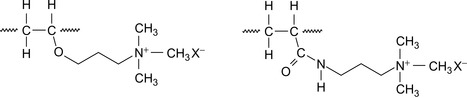 |
| Figure 1.1 Quaternized etherified polyvinyl alcohol and quaternized polyacrylamide (Patel et al., 2009). |
Membrane Formation
In order to increase wellbore stability, formulations for water-based drilling fluids can be provided that form a semi-permeable osmotic membrane over a specific shale formation (Schlemmer, 2007). This membrane allows the comparatively free movement of water through the shale, but significantly restricts the movement of ions across the membrane and thus into the shale.
Membrane formation involves the application of two reactants to form a relatively insoluble Schiff base in situ, which deposits the shale as a polymer film. This Schiff base coats the clay surfaces as a polymer membrane.
The first reactant is a soluble monomer, oligomer, or polymer with ketone, aldehyde, aldol functionalities, or precursors to those. Examples are carbohydrates, such as dextrin and linear or branched starch. The second reactant is a primary amine. These compounds react via a condensation reaction to form an insoluble crosslinked polymer. The formation of a Schiff base is shown in Figure 1.2.
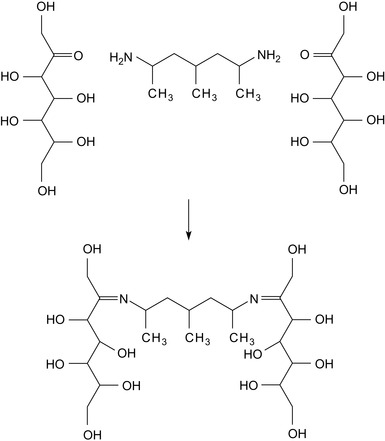 |
| Figure 1.2 Formation of a Schiff base (Schlemmer, 2007). |
Figure 1.2 shows the reaction of a dextrine with a diamine, but other primary amines and polyamines will of course react in the same way. Long chain amines, diamines, or polyamines with a relatively low amine ratio may require pH adjustment, using materials such as sodium hydroxide, potassium hydroxide, sodium carbonate, potassium carbonate, or calcium hydroxide (Schlemmer, 2007). The Schiff base formed in this way must be essentially insoluble in the carrier brine in order to deposit a sealing membrane on the shale during the drilling of a well.
By carefully selecting the primary polymer and the crosslinking amine, their relative concentrations, and the pH, the required degree of crosslinking, polymerization, and precipitation of components occurs, effectively forming an osmotically effective membrane on or within the face of the exposed rock.
Oil-based Drilling Muds
These materials have oil as their continuous phase, usually diesel oil, mineral oil or low toxicity mineral oil. Because some water will always be present, the OBM must contain water-emulsifying agents. Various thickening and suspending agents as well as barite are added. The emulsified water may contain alkalies and salts. If water is purposely added (for economical reasons), the OBM is called an invert emulsion mud.
Due to the character of their continuous phase, OBMs provide unequaled performance attributes with respect to the rate of penetration, shale inhibition, wellbore stability, high lubricity, high thermal stability, and high salt tolerance. However, they are subjected to strict environmental regulation regarding their discharge and recycling.
OBMs are now being replaced by synthetic muds. Diesel oil is harmful to the environment, particularly the marine environment in offshore applications. The use of palm oil derivatives could be considered as a harmless alternative (Yassin and Kamis, 1990), or hydrated castor oil can be used as a viscosity promoter instead of organophilic quaternized clays (Mueller et al., 1991).
An OBM can be made more viscous with maleated ethylene-propylene elastomers (Jones and Acker, 1999). The elastomers are ethylene-propylene copolymers or ethylene-propylene-diene terpolymers. These compounds are far more effective oil mud viscosifiers than the organophilic clays orginally used. However, specific organophilic clays can provide a drilling fluid composition that is less sensitive to high temperatures (Dino and Thompson, 2001).
Poly-α-olefins (PAOs) are biodegradable and non-toxic to marine organisms. They also meet viscosity and pour point specifications for OBM formulations (Ashjian et al., 1995). The hydrogenated dimer of 1-decene (Mercer and Nesbit, 1992) can be used instead of conventional organic fluids, as can n-1-octene (Lin, 1996).
Polyethercyclicpolyols
Polyethercyclicpolyols possess molecular properties and characteristics that permit the preparation of enhanced drilling fluids, which inhibit the formation of gas hydrates, prevent shale dispersion, and reduce the swelling of the formation to enhance wellbore stability, reduce fluid loss, and reduce filter cake thickness.
Drilling muds that incorporate these compounds are substitutes for OBMs in many applications (Blytas and Frank, 1995; Blytas et al., 1992; Blytas et al., 1992; Zuzich and Blytas, 1994; Zuzich et al., 1995). Polyethercyclicpolyols are prepared by thermally condensing a polyol, for example glycerol, to oligomers and cyclic ethers.
Emulsifier for Deep Drilling
Two major problems are encountered when using OBMs for drilling very deep wells (Dalmazzone, 2007). The first is a problem with the stability of the emulsions at elevated temperatures. The emulsion must be stable up to temperatures of 200°C. If the emulsion coalesces, the fluid loses its rheological properties.
The second problem is their environmental impact. The emulsification agents must not only be effective, but also as non-toxic as possible.
Fatty acid amides consisting of N-alkylated polyether chains are used as emulsifiers. For those the term ‘polyalkoxylated superamides’ has been coined (Le Helloco et al., 2004). As a cosurfactant, tall oil fatty acids or their salts can be used.
Biodegradable Composition
Some oil-based drilling fluids are biodegradable. The main oil phase component of these materials is a mixture of methyl esters from biodegradable fatty acids. A typical formulation of a biodegradable drilling fluid is shown in Table 1.6.
| Compound | Amount/[%] | Function | ||
|---|---|---|---|---|
| Soybean methylate | 55 | to | 70 | Oil component |
| D -Limonene | 1 | to | 5 | Pour point depressant |
| 2,6-Di-tert-butyl-p-cresol | 0.1 | to | 0.5 | Antioxidant |
| Hydrogenated castor oil | 0.3 | to | 1 | Oil component |
| Fatty acid salts | 3 | to | 6 | Puffer |
| Magnesium oxide | 1 | to | 3 | In situ soap former |
| NaCl Brine | 26 | to | 30 | Aqueous component |
| Organophilic clay | 0.5 | to | 1 | Viscosifier |
| Succinimide copolymer | 0.1 | to | 0.5 | Fluid loss agent |
| Sodium polyacrylate | 0.1 | to | 0.5 | Fluid loss agent |
| Citric acid | 0.1 | to | 1.5 | Puffer |
| Barium sulfate | 0.1 | to | 25 | Weighting agent |
Electric Conductive Nonaqueous Mud
A wellbore fluid has been developed that has a nonaqueous continuous liquid phase and exhibits an electrical conductivity that is a factor of 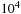 to
to 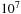 greater than a conventional invert emulsion. 0.2–10% by volume of carbon black particles and emulsifying surfactants are used as additives. Information from electrical logging tools, including measurements while drilling, can be obtained (Sawdon et al., 2000).
greater than a conventional invert emulsion. 0.2–10% by volume of carbon black particles and emulsifying surfactants are used as additives. Information from electrical logging tools, including measurements while drilling, can be obtained (Sawdon et al., 2000).
Water Removal
Water can be removed from OBMs by the action of magnesium sulfate (Smith and Jeanson, 2001).
Synthetic Muds
Synthetic muds are expensive. Two factors influence the direct cost, namely the costs per barrel and mud losses. Synthetic muds are the technical equivalent of OBMs when drilling intermediate hole sections. They are technically superior to all water-based systems when drilling reactive shales in directional wells. With efficient solids-control equipment, optimized drilling, and good housekeeping practices, the cost of the synthetic mud can be brought to a level that is comparable to OBM (Munro et al., 1993).
POBMs or synthetic oil-based drilling muds are made on the same principle as OBMs. They have been developed to maintain the performance characteristics of OBMs while reducing their environmental impact. The objective behind the design of these drilling fluids is to exchange the diesel oil or mineral oil base with an organic fluid that has a lower environmental impact. The organic fluids used are esters, polyolefins, acetal, ether, and linear alkyl benzenes. As with OBMs, POBMs may contain various ingredients, such as thickening and suspending agents and emulsifying agents as well as weighting agents.
POBMs were developed to maintain the technical performance characteristics of OBMs and reduce their environmental impact. They are, however, not as stable as OBMs depending upon the continuous phase present. From an environmental perspective, legislation is becoming as strict for POBMs as for OBMs. The mud selection process is based on the mud's technical performance and environmental and financial impact.
Skeletally isomerized linear olefins exhibited a better high-temperature stability in comparison to a drilling fluid prepared from a conventional PAO. Fluid loss properties are good, even in the absence of fluid loss additives (Gee et al., 1992, Gee et al., 1998 and Gee et al., 2000; Williamson et al., 1995). Although normal α-olefins are not generally useful, mixtures of mostly linear olefins are minimally toxic and are highly effective as the continuous phase of drilling fluids (Gee et al., 1995 and Gee et al., 1992).
Acetals as mineral oil substitutes exhibit good biodegradability and are less toxic than mineral oils (Hille et al., 1992 and Hille et al., 1998). Acrylic acid (AA) salts are formed by the neutralization reaction of AA in aqueous solution (Shimomura et al., 1990).
Alginates are hydrocolloids, which are extracted from brown marine microalgae. Water-soluble alginates are prepared as highly concentrated, pumpable suspensions in mixtures of propylene glycol and water by using hydroxypropylated guar gum in combination with carboxymethylated cellulose, which is used as a suspending agent (Kehoe and Joyce, 1993).
Inverted Emulsion Drilling Muds
Inverted emulsion muds are used in 10–20% of all drilling jobs. Historically, first of all crude oils, then diesel oils and mineral oils were used to formulate invert drilling fluids. Considerable environmental damage may occur when the mud gets into the sea. Drilling sludge and the heavy mud sink to the seabed and partly flow with the tides and sea currents to the coasts. All of these hydrocarbons contain no oxygen and are not readily biodegraded (Hille et al., 1998).
Because of problems of toxicity and persistence, alternative drilling oils have been developed. Examples of such oils are fatty acid esters and branched chain synthetic hydrocarbons such as PAOs. Fatty acid ester-based oils have excellent environmental properties, but drilling fluids made with these esters tend to have lower densities and are prone to hydrolytic instability.
PAO-based drilling fluids can be formulated to high densities with good hydrolytic stability and low toxicity. They are, however, somewhat less biodegradable than esters and they are expensive. The fully weighted, high-density fluids tend to be too viscous (Lin, 1996).
Esters
Esters of C6 to C11 monocarboxylic acids (Müller et al., 1990; Mueller et al., 1990a, Mueller et al., 1990b and Mueller et al., 1994), acid-methyl esters (Mueller et al., 1990a), and polycarboxylic acid esters (Mueller et al., 1991), as well as oleophilic monomeric and oligomeric diesters (Mueller et al., 1991), have all been proposed as basic materials for inverted emulsion muds. Natural oils are triglyceride ester oils (Wilkinson et al., 1995) and are similar to synthetic esters. Diesters also have been proposed (Mueller et al., 1991, Mueller et al., 1992, Mueller et al., 1993 and Mueller et al., 1995; Muller et al., 1993).
Acetals
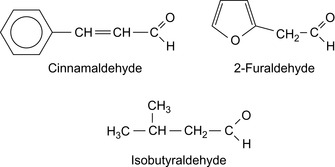
Acetals and oleophilic alcohols or oleophilic esters are suitable for the preparation of inverted emulsion drilling muds and emulsion drilling muds. They may replace the base oils, diesel oil, purified diesel oil, white oil, olefins, and alkyl benzenes (Hille et al., 1996 and Hille et al., 1998). Examples are isobutyraldehyde, di-2-ethylhexyl acetal, dihexyl formal. Also mixtures with coconut alcohol, soya oil, and α-methyldecanol are suitable. Some aldehydes are shown in Figure 1.3.
Inverted emulsion muds are more useful in stable, water sensitive formations and in inclined boreholes. They are stable up to very high temperatures and provide excellent corrosion protection. Their disadvantages are their higher price, the greater risk if gas reservoirs are bored through, the more difficult handling for the team at the tower, and their greater environmental problems.
The high setting point of linear alcohols and the poor biodegradability of branched alcohols limit their use as an environment-friendly mineral oil substitute. Higher alcohols, which are slightly water-soluble, are eliminated for use in offshore muds because of their high toxicity to fish.
Esters and acetals can be degraded anaerobically on the seabed. This possibility minimizes the environmentally damaging effect on the seabed. When such products are used, rapid recovery of the ecology of the seabed takes place after the end of drilling. Acetals, which have a relatively low viscosity and in particular a relatively low setting point, can be prepared by combining various aldehydes and alcohols (Hille et al., 1998; Young and Young, 1994).
Anti-settling Properties
Ethylene-AA copolymer, neutralized with amines such as triethanol amine or N-methyl diethanol amine, enhances anti-settling properties (McNally et al., 1999; Santhanam and MacNally, 2001).
Glycosides
If glycosides are used in the internal phase, then much of the concern over the ionic character of the internal phase is not necessary. If water is limited in the system, then the hydration of the shales is greatly reduced.
The reduced water activity of the internal phase of the mud and the improved efficiency of the shale is an osmotic barrier if the glycoside interacts directly with the shale. This helps to lower the water content of the shale, thus increasing rock strength, lowering effective mean stress, and stabilizing the wellbore (Hale and Loftin, 1996).
Methyl glucosides also could find applications in water-based drilling fluids and have the potential to replace OBMs (Headley et al., 1995). The use of such a drilling fluid could reduce the need for the disposal of oil-contaminated drilling cuttings, minimize health and safety concerns, and minimize adverse environmental effects.
Miscellaneous
Other proposed base materials are listed in Table 1.7. Quaternary oleophilic esters of alkylolamines and carboxylic acids improve the wettability of clay (Ponsati et al., 1992 and Ponsati et al., 1994). Nitrates and nitrites can replace calcium chloride in inverted emulsion drilling muds (Fleming and Fleming, 1995).
| Base material | References |
|---|---|
| Ethers of monofunctional alcohols | Mueller et al. (1990) |
| Branched didecyl ethers | Godwin and Mathys (1993), Godwin and Sollie (1993) |
| Mueller et al. (1996) | |
| Oleophilic alcohols | Mueller et al. (1990b), Muller et al., 1990 and Muller et al., 1990 |
| Oleophilic amides | Mueller et al. (1990c) |
| Hydrophobic side chain polyamide from N,N-didodecylamine and sodium polyacrylate or polyacrylic acid | Monfreux et al. (2000) |
| Polyether amine | Wall et al. (1995) |
| Phosphate ester of a hydroxy polymer | Brankling (1994) |
Reversible Phase Inversion
Invert emulsion fluids, in which the emulsion can be readily and reversibly converted from a water-in-oil type emulsion to an oil-in-water type emulsion, have been developed. The essential ingredient is an amine-based surfactant, which may be diethoxylated tallow amine, diethoxylated soya amine, or N-tallow-1,3-diaminopropane (Patel, 2008).
The invert emulsion is admixed with an acid that can protonate the amine surfactant. When sufficient quantities of the acid are present, the invert emulsion is converted so that the oleaginous fluid becomes the discontinuous phase and the non-oleaginous fluid becomes the continuous phase.
The phase inversion is reversible, so that on addition of a base capable of deprotonating the protonated amine surfactant, a stable invert emulsion is formed, where the oleaginous liquid becomes the continuous phase and the non-oleaginous fluid become the discontinuous phase (Patel, 2008).
In other words, when the drilling fluid is converted into an oil-in-water type emulsion, solids, now substantially water-wet, may now be separated from the fluid, by gravity or mechanical means, for further processing or disposal. The fluid may then be mixed with a base, which can deprotonate the protonated amine surfactant, and so converts the oil-in-water type emulsion back to a water-in-oil emulsion. The resulting water-in-oil emulsion may then be used as it is, or may be reformulated into a drilling fluid that is suitable for use in another well (Patel, 2008).
Foam Drilling
Drilling low-pressure reservoirs with nonconventional methods can use low-density dispersed systems, such as foams, to achieve underbalanced conditions. Selection of an adequate foam formulation, requires not only the reservoir characteristics but also the foam properties to be taken into account.
Parameters such as stability of foam, and the interactions between rock-fluid and drilling fluid-formation fluid are among the properties to consider when designing the drilling fluid (Aguilar et al., 2000).
A composition with a specific pH, an ionic surfactant, and a polyampholytic polymer whose charge depends on the pH, is circulated in a well. By varying the pH, it is possible to destabilize the foam in such a way as to more easily break it back at the surface, and potentially to recycle the foaming solution (Argillier and Roche, 2000).
Chemically Enhanced Drilling
Chemically enhanced drilling offers substantial advantages over conventional methods in carbonate reservoirs. Coiled tubing provides the perfect conduit for chemical fluids that can accelerate the drilling process and provide stimulation while drilling (Rae and Di Lullo, 2001). The chemical fluids are mainly acidic in order to dissolve or disintegrate the carbonate rock.
Supercritical Carbon Dioxide Drilling
The efficiency of drilling operations can be increased using a drilling fluid material that exists as supercritical fluid, or a dense gas at temperature and pressure conditions occurring in the drill site, such as carbon dioxide.
A supercritical fluid exhibits physiochemical properties intermediate between those of liquids and gases. Mass transfer is rapid with supercritical fluids, and their dynamic viscosities are nearer to those of normal gaseous states.
In the vicinity of the critical point, the diffusion coefficient is more than 10 times that of a liquid. Carbon dioxide can be compressed readily to form a liquid, and under typical borehole conditions, it is a supercritical fluid.
The viscosity of carbon dioxide at its critical point is only 0.02 cP. This value increases with pressure to about 0.1 cP at 70 MPa (about 10,000 psi). Because the diffusivity of carbon dioxide is so high, and the rock associated with petroleum-containing formations is generally porous, the carbon dioxide is effective in penetrating the formation.
Carbon dioxide therefore is often used to stimulate the production of oil wells, because it tends to dissolve in the oil, reducing the oil viscosity while providing a pressure gradient that drives the oil from the formation.
Carbon dioxide can be used to reduce mechanical drilling forces, to remove cuttings, or to jet erode a substrate. Supercritical carbon dioxide is used with coiled-tube drilling equipment. The very low viscosity of supercritical carbon dioxide provides efficient cooling of the drill head and efficient cuttings removal.
Furthermore, the diffusivity of supercritical carbon dioxide within the pores of petroleum formations is significantly higher than that of water, making jet erosion much more effective than water. Supercritical carbon dioxide jets can be used to assist mechanical drilling, for erosion drilling, or for scale removal. Spent carbon dioxide can be vented to the atmosphere, collected for reuse, or directed into the formation to aid in the recovery of petroleum (Kolle, 2002).
Additives
Thickeners
A variety of compounds that are useful as thickeners is shown in Table 1.8 and the individual compounds are explained in detail in the following sections.
| aStable up to temperatures of about 180°C | |
| bSolubilized in acidic solution | |
| Compound | References |
|---|---|
| A water-soluble copolymer of hydrophilic and hydrophobic monomers, acrylamide (AAm)-acrylate of silane or siloxane | Meyer et al. (1999) |
| Carboxymethyl cellulose, polyethylene glycol | Lundan et al. (1993), Lundan and Lahteenmaki (1996) |
| Combination of a cellulose ether with clay | Rangus et al. (1993) |
| Amide-modified carboxyl-containing polysaccharide | Batelaan and van der Horts (1994) |
| Sodium aluminate and magnesium oxide | Patel (1994a) |
| Thermally stable hydroxyethyl cellulose (HEC) 30% ammonium or sodium thiosulfate and 20% HEC | Lukach and Zapico (1994) |
| AA copolymer and oxyalkylene with hydrophobic group | Egraz et al. (1994) |
| Copolymers acrylamide-acrylate and vinylsulfonate–vinylamide | Waehner (1990) |
| Cationic polygalactomannans and anionic xanthan gum | Yeh (1995) |
| Copolymer from vinyl urethanes and AA or alkyl acrylates | Wilkerson et al. (1995) |
| 2-Nitroalkyl ether–modified starch | Gotlieb (1996) |
| Polymer of glucuronic acid | Courtois-Sambourg et al. (1993) |
| Ferrochrome lignosulfonate and carboxymethyl cellulose | Kotelnikov et al. (1992) |
| Cellulose nanofibrilsa | Langlois, 1998 and Langlois, 1999 |
| Quaternary alkyl amido ammonium salts | Subramanian et al. (2001) |
| Chitosanb | House and Cowan (2001) |
Polymers
Thickener polymers include polyurethanes (PUs), polyesters, PAMs, natural polymers, and modified natural polymers (Doolan and Cody, 1995).
pH Responsive Thickeners
The viscosity of ionic polymers is dependent on their pH. In particular, pH responsive thickeners can be prepared by copolymerization of acrylic or methacrylic acid ethyl acrylate or other vinyl monomers and tristyrylpoly(ethyleneoxy)x methyl acrylate. Such a copolymer provides a stable, aqueous, colloidal dispersion at a pH lower than 5.0, but becomes an effective thickener for aqueous systems on adjustment to a pH of 5.5 to 10.5 or higher (Robinson, 1996 and Robinson, 1999).
Mixed Metal Hydroxides
By addition of mixed metal hydroxides, typical bentonite muds are transformed to an extremely shear-thinning fluid (Lange and Plank, 1999). At rest, these fluids exhibit a very high viscosity but are thinned to an almost water-like consistency when shear stress is applied.
The shear thinning rheology of mixed metal hydroxides and bentonite fluids is due to the formation of a three-dimensional, fragile network of mixed metal hydroxides and bentonite.
The positively charged, mixed metal hydroxide particles attach themselves to the surface of negatively charged bentonite platelets. Typically, magnesium aluminum hydroxide salts are used as mixed metal hydroxides.
Mixed metal hydroxides demonstrate the following advantages in drilling (Felixberger, 1996):
• High cuttings removal,
• Suspension of solids during shutdown,
• Lower pump resistance,
• Stabilization of the borehole,
• High drilling rates, and
• Protection of the producing formation.
Mixed metal hydroxide drilling muds have been used successfully in horizontal wells; in tunneling under rivers, roads, and bays; for drilling in fluids; for drilling large-diameter holes; with coiled tubing; and to ream out cemented pipe.
Mixed metal hydroxides can be prepared from the corresponding chlorides by treatment with ammonia (Burba and Strother, 1991). Experiments with various drilling fluids showed that the mixed metal hydroxides system, coupled with propylene glycol (Deem et al., 1991), caused the least skin damage of the drilling fluids tested.
Thermally activated mixed metal hydroxides, made from naturally occurring minerals, especially hydrotalcites, may contain small or trace amounts of metal impurities besides the magnesium and aluminum components, which are particularly useful for activation (Keilhofer and Plank, 2000).
Mixed hydroxides of bivalent and trivalent metals with a three-dimensional spaced-lattice structure of the garnet type (Ca3Al2[OH]12) have been described (Burba et al., 1992; Mueller et al., 1997).
Lubricants
Bit lubricants are dealt with in detail in Chapter 4. During drilling, the drill string may develop an unacceptable rotational torque or, in the worst case, become stuck. When this happens, the drill string cannot be raised, lowered, or rotated. Common factors leading to this situation include:
• Cuttings or slough build-up in the borehole,
• An undergauge borehole,
• Irregular borehole development embedding a section of the drill pipe into the drilling mud wall cake, or
• Unexpected differential formation pressure.
Differential pressure sticking occurs when the drill pipe becomes embedded in the mud wall cake opposite a permeable zone.
The difference between the hydrostatic pressure in the drill pipe and the formation pressure holds the pipe in place, resulting in a sticking pipe. Differential sticking may be prevented, and a stuck drill bit may be freed by using an OBM, or an oil-based, or water-based surfactant composition.
Such a composition reduces friction, permeates drilling mud wall cake, destroys binding wall cake, and reduces the differential pressure. Unfortunately, many such compositions are toxic to marine life.
Bacteria
Bacterial contamination of drilling fluids contributes to a number of problems. Many of the muds contain sugar-based polymers in their formulation that provide an effective food source for bacterial populations. This can lead to direct degradation of the mud.
In addition, the bacterial metabolism can generate deleterious products. Most notable among these is hydrogen sulfide, which can lead to the decomposition of mud polymers, the formation of problematic solids such as iron sulfide, and corrosive action on drilling tubes and drilling hardware (Elphingstone and Woodworth, 1999). Moreover, hydrogen sulfide is a toxic gas.
Many polymers are used in drilling fluids as fluid loss control agents or vis-cosifiers. Because of the degradation of these polymers by bacteria in drilling fluids, an increase in fluid loss can occur. All naturally occurring polymers are capable of being degraded by bacterial action, but some are more susceptible than others. One solution, besides using bactericides, is to replace the starch with low viscosity PAC, polyanionic lignin, or other enzyme-resistant polymer (Hodder et al., 1992).
Certain additives are protected from biodegradation while drilling deep wells by quaternary ammonium salts (Rastegaev et al., 1999), which considerably reduces consumption of the additives needed.
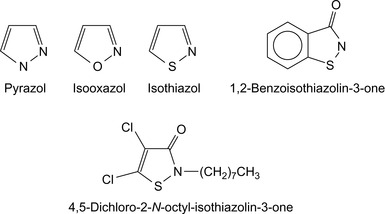
Bacterial control is important not only in drilling fluids, but also for other oil and gas operations. The topic is treated more extensively in Chapter 5. Some bactericides especially recommended for drilling fluids are summarized in Table 1.9 and sketched out in Figure 1.4.
| aAbsorbed on solid | |
| bSynergistically effective with organic acids | |
| cSynergistically effective with organic acids | |
| dAlgicide | |
| Bactericide | References |
|---|---|
| Bis[tetrakis(hydroxymethyl) phosphonium] sulfatea | Elphingstone and Woodworth (1999) |
| Dimethyl-tetrahydro-thiadiazine-thione | Karaseva et al. (1995) |
| 2-Bromo-4-hydroxyacetophenoneb | Oppong and King (1995) |
| Thiocyanomethylthio-benzothiazolec | Oppong and Hollis (1995) |
| Dithiocarbamic acid, | Austin and Morpeth (1992) |
| Hydroxamic acidc | Austin and Morpeth (1992) |
| 1,2-Benzoisothiazolin-3-one | Morpeth and Greenhalgh (1990) |
| 3-(3,4-Dichlorophenyl)-1,1-dimethylurea | Morpeth and Greenhalgh (1990) |
| Di-iodomethyl-4-methylphenyl sulfoned | Morpeth and Greenhalgh (1990) |
| Isothiazolinones | Downey et al. (1995), Hsu, 1990 and Hsu, 1995, Morpeth (1993) |
Corrosion Inhibitors
Corrosion inhibitors are the subject of several topics in petroleum industries, such as transport and completion. They are detailed in Chapter 6.
Viscosity Control
Bentonites are highly colloidal and swell in water to form thixotropic gels. This property results from their micaceous sheet structure. Because of these viscosity-building characteristics, bentonites are used as viscosity enhancers or builders in such areas as drilling muds and fluids, concrete and mortar additives, foundry and molding sands, and compacting agents for gravel and sand, as well as cosmetics. Most bentonites that are found in nature are in their sodium or calcium form.
The performance of a calcium bentonite as a viscosity builder can often be enhanced by conversion to the sodium form. Crude bentonite can be upgraded to a range of solutions with unusually high aqueous viscosities (Bauer et al., 1993). The crude material is sheared and dried. Sodium carbonate is then dry-blended with the material and pulverized. The resulting bentonite clays are self-suspending, self-swelling, and self-gelatinizing when mixed with water.
The modification of bentonite with alkylsilanes also improves their dispersing properties (Kondo and Sawada, 1996). Incorporation of phosphonate-type compounds in bentonites for drilling mud permits the removal of free calcium ions in the form of soluble and stable complexes, and the preservation or restoration of the initial fluidity of the mud (Michelson and Vattement, 1999). The phosphonates also have dispersing and fluidizing effects on the mud.
Clay Stabilization
Selected clay stabilizers are shown in Table 1.10. Thermally treated carbohydrates are suitable as shale stabilizers (Sheu and Bland, 1992). They may be formed by heating an alkaline solution of the carbohydrate, and the reaction product may be reacted with a cationic base. The inversion of non-reducing sugars may be first effected on selected carbohydrates, with the inversion catalyzing the browning reaction.
| aWater sensitive smectite or illite shale formations | |
| Additive | References |
|---|---|
| Modified poly-amino acida | Bruton and McLaurine (1993) |
| Polyacrylamide | Ballard et al. (1994) |
| Amphoteric acetates and glycinates | Jarrett (1997a) |
| Capryloamphoglycinate | Alonso-Debolt and Jarrett (1995) |
| Cocoamphodiacetate | Alonso-Debolt and Jarrett (1995) |
| Disodium cocoamphodiacetate | Alonso-Debolt and Jarrett (1995) |
| Lauroamphoacetate | Alonso-Debolt and Jarrett (1995) |
| Sodium capryloamphohydroxypropyl sulfonate | Alonso-Debolt and Jarrett (1995) |
| Sodium mixed C8 amphocarboxylate | Alonso-Debolt and Jarrett (1995) |
| Alkylamphohydroxypropyl sulfonate | Alonso-Debolt and Jarrett (1995) |
| Polyvinylpyrrolidone | |
| Polyvinyl alcohol | |
| Starches | |
| Cellulosic material | Patel et al. (1995) |
| Partially hydrolyzed polyacrylamide and PPG, or a betaine | Patel et al. (1995) |
| Quaternized trihydroxyalkyl amine | Patel et al. (1995) |
| Polyfunctional polyamine | McGlothlin and Woodworth (1996) |
Formation Damage
Polyacrylates are often added to drilling fluids to increase their viscosity and limit formation damage. The filter cake is critical to preventing reservoir invasion by mud filtrate. Polymer invasion of the reservoir has been shown to have a great impact on permeability reduction (Audibert et al., 1999). The invasion of filtrate and solids in drilling in fluid can cause serious reservoir damage.
Shale Stabilizer
Swelling due to shale hydration is one of the most important causes of borehole instability. Three processes are known to contribute to shale instability (Bailey et al., 1994):
Adding a shale stabilizer to drilling fluids is an effective way to control clay swelling (Fu and Hu, 1997). A copolymer of AAm and acrylonitrile has been found to be effective in this regard. Experimental results show that the inhibitors are effective in inhibiting shale hydration swelling, especially their quaternized product. 2-Hydroxybutyl ether and polyalkyl ether modified polygalactomannans have been described as useful shale hydration inhibitors (Dino, 1997).
A copolymer of styrene and maleic anhydride (MA) with alkylene oxide based side chains is effective as a shale stabilizer (Smith and Balson, 2000), as are a variety of polyoxyalkylene amines. It was found that polyoxypropylenediamine H2N–CH(CH3)CH2[–OCH2CH(CH3)]x–NH2 (Patel et al., 2001) is the best, with x < 15. Surfactants are used to change the interfacial properties. Suitable surfactants are given in Table 1.11.
| aControlling foam formation, drilling muds | |
| Compound | References |
|---|---|
| Alkylpolyglycosides | Lecocumichel and Amalric (1995) |
| Amphoteric surfactants | Dahanayake et al. (1996) |
| Acetal or ketal adduct hydroxy polyoxyalkylene ethera | Felix (1996) |
| Amphoteric anion ethoxy and propoxy units | Hatchman (1999) |
| Alkanolamine | Hatchman (1999) |
Fluid Loss Additives
Filtration control is an important property of a drilling fluid, particularly when drilling through permeable formations, where the hydrostatic pressure exceeds the formation pressure. It is important for a drilling fluid to quickly form a filter cake to effectively minimize fluid loss, but which also is thin and erodable enough to allow product to flow into the wellbore during production (Jarrett and Clapper, 2010). Fluid loss additives are detailed in Chapter 2. Here a few fluid loss additives are summarized for quick reference.
There are a number of methods that have been proposed to help prevent the loss of circulation fluid (Messenger, 1981). Some of these methods use fibrous, flaky, or granular materials to plug the pores as the particulate material settles out of the slurry.
Other methods use materials that interact in the fissures of the formation to form a plug of increased strength. Lost circulation additives are summarized in Table 1.12.
| Material | References |
|---|---|
| Encapsulated lime | Walker (1986) |
| Encapsulated oil-absorbent polymers | Delhommer and Walker (1987a) |
| Hydrolyzed polyacrylonitrile | Yakovlev and Konovalov (1987) |
| Divinylsulfone, crosslinked | |
| Poly(galactomannan) gum | Kohn (1988) |
| PU foam | Glowka et al. (1989) |
| Partially hydrolyzed polyacrylamide 30% hydrolyzed, crosslinked with Cr3+ | Sydansk (1990) |
| Compound | References |
| Oat hulls | House et al. (1991) |
| Rice products | Burts Jr, 1992 and Burts Jr, 1997 |
| Waste olive pulp | Duhon (1998) |
| Nut cork | Fuh et al. (1993), Rose (1996) |
| Pulp residue waste | Gullett and Head (1993) |
| Petroleum coke | Whitfill et al. (1990) |
| Shredded cellophane | Burts Jr (2001) |
Water Swellable Polymers
Certain organic polymers absorb comparatively large quantities of water, for example, alkali metal polyacrylate or crosslinked polyacrylates (Green, 2001). Such water-absorbent polymers, insoluble in water and in hydrocarbons, can be injected into the well so that they encounter naturally occurring or added water at the entrance to and within an opening in the formation. The resultant swelling of the polymer forms a barrier to the continued passage of the circulation fluid through that opening into the formation.
The hydrocarbon carrier fluid initially prevents water from contacting the water-absorbent polymer until such water contact is desired. Once the hydrocarbon slug containing the polymer is properly placed at the lost circulation zone, water is mixed with it so that the polymer will absorb the water and substantially increase in size to close off the lost circulation zone (Bloys and Wilton, 1991; Delhommer and Walker, 1987b; Walker, 1989). The situation is similar to an oil-based cement. The opposite mechanism is used by a hydrocarbon-swellable elastomer (Wood, 2001).
Anionic Association Polymer
Another type of lost circulation agent is a combination of an organic phosphate ester and an aluminum compound, for example, aluminum isopropoxide. The alkyl phosphate ester becomes crosslinked by the aluminum compound to form an anionic association polymer, which serves as a gelling agent (Reid and Grichuk, 1991), hence preventing fluid loss.
Fragile Gels
A fragile gel is one that can be easily disrupted or thinned under shear stress, etc, but can quickly return to a gel when the stress is alleviated or removed, such as when the circulation of the fluid is stopped. Fragile gels may be disrupted simply by a pressure or a compression wave during drilling. They break instantaneously when disturbed, turning from a gel back into a liquid with minimum pressure, force, and time.
Metal crosslinked phosphate esters impart a fragile progressive gel structure to a variety of oil and invert emulsion-based drilling fluids, both at neutral or acidic pH.
The amount of phosphate ester and metal crosslinker that is used in a drilling fluid depends on the oil type and the desired viscosity of the product. Generally, however, more phosphate ester and metal crosslinker is used for gelling or enhancing the viscosity of the fluid for transport than is used for imparting fragile progressive gel structure to the drilling fluid (Bell and Shumway, 2009).
Aphrons
Other lost-circulation additives can be present in an encapsulated form. The encapsulation is then dissolved and the material swells to close fissures. Microbubbles in a drilling fluid can be generated by certain surfactants, and polymers known as aphrons are a different approach to reduce the fluid loss (Ivan et al., 2001).
An aphron drilling fluid is similar to a conventional drilling fluid, but the drilling fluid system is converted to an energized air-bubble mud system before drilling (Kinchen et al., 2001).
Permanent Grouting
Lost circulation also can be suppressed by grouting permanently, either with cement or with organic polymers that cure in situ (Allan and Kukacka, 1995; Cowan and Hale, 1994).
Scavengers
Oxygen Scavengers
Oxygen corrosion is often underestimated, but studies have shown that the corrosion can be limited when proper oxygen scavengers are used. Hydrazine leads the group of chemicals that are used for oxygen removal. Because of its special properties, it is used for corrosion control in heating systems and in drilling operations, well workover, and cementing (Sikora, 1994).
Hydrogen Sulfide Removal
It is sometimes necessary to remove hydrogen sulfide from a drilling mud. Techniques using iron compounds that form sparingly soluble sulfides have been developed, for example, the use of iron (II) oxalate (Sunde and Olsen, 2000) and iron sulfate (Prokhorov et al., 1993), where the sulfur is precipitated out as FeS. Alternatively, ferrous gluconate is an organic iron-chelating agent, stable at pH levels as high as 11.5 (Davidson, 2001).
Zinc compounds have a high reactivity with regard to H2S and therefore are suitable for the quantitative removal of even small amounts of hydrogen sulfide (Wegner and Reichert, 1990). However, at high temperatures they may negatively affect the rheology of drilling fluids.
Surfactants
Surfactant in Hydrocarbon Solvent
Methyl-diethyl-alkoxymethyl ammonium methyl sulfate has high foam extinguishing properties (Fabrichnaya et al., 1997).
Biodegradable Surfactants
Alkylpolyglucosides (APGs) are highly biodegradable surfactants (Nicora and McGregor, 1998). The addition of APGs, even at very low concentrations, to a polymer mud can drastically reduce fluid loss even at high temperatures. Moreover, both fluid rheology and temperature resistance are improved.
Deflocculants and Dispersants
Deflocculants have a relatively low molecular weight. Complexes of tetravalent zirconium with organic acids, such as citric, tartaric, malic, and lactic acids, and a complex of aluminum and citric acid have been claimed to be active as dispersants.
Polymers composed of sodium styrene sulfonate, MA, and a zwitterionic functionalized MA (Grey, 1993; Peiffer et al., 1991, Peiffer et al., 1992 and Peiffer et al., 1993) are also suitable. The dispersant is especially useful in dispersing bentonite suspensions (Burrafato and Carminati, 1994).
Polymers with amine sulfide terminal moieties are synthesized by using aminethiols as chain transfer agents in aqueous addition polymerization reactions. The polymers are useful as mineral dispersants (McCallum and Weinstein, 1994).
Shale Stabilizing Surfactants
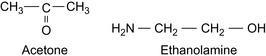
There are special shale stabilizing surfactants consisting of non-ionic alkanolamides (Jarrett, 1997b), for example, acetamide monoethanolamines and diethanol amines. Acetone and ethanolamine are shown in Figure 1.5.
Toxicity
Alkyl phenol ethoxylates are a class of surfactants that have been used widely in the drilling fluid industry. The popularity of these surfactants is based on their cost effectiveness, availability, and range of obtainable hydrophilic-lipophilic balance values (Getliff and James, 1996).
However, studies have shown that alkyl phenol ethoxylates exhibit oestrogenic effects and can cause sterility in some male aquatic species. This may have subsequent human consequences, and such possibilities have led to their use being banned in some countries, and agreements to phase out their use have been drawn up. Alternatives are available, and in some cases they show an even better technical performance.
Defoamers
Defoamers are covered in Chapter 22.
Hydrate Inhibitors
Hydrate inhibitors for drilling fluids are summarized in Chapter 13.
Weighting Materials
There are many weighting materials, including barite and iron oxides, which are used to increase the specific weight of a slurry. Conversely, the specific weight can be reduced by foaming or by the addition of hollow glass particles.
Barite
Barite has been used as a weighting agent in drilling fluids since the 1920s. It is preferred over other materials because of its high density, low production costs, low abrasiveness, and ease of handling. Other weighting materials have been used, but they are problematic or costly. Finished barite producers sometimes blend ores from different sources to obtain the desired average density to meet API specifications.
Some barite ores contain alkaline-soluble carbonate minerals that can be detrimental to a drilling fluid, such as iron carbonate (siderite), lead carbonate (cerussite), and zinc carbonate (smithsonite) (Kulpa et al., 1992). Details of how to characterize barite have been worked out (Recommended practice for chemical analysis of barite, 1996). Barite can be modified to become oleophilic (Shen et al., 1998 and Shen et al., 1999).
To recover barite from drilling muds, a direct flotation without prior dewatering and washing of the drilling muds has been described (Heinrich, 1992). An alkyl phosphate is used as a collecting and frothing reagent.
Ilmenite
Environmental considerations suggest replacing barite with ilmenite. However, the use of this as weighting material can cause severe erosion problems. Using ilmenite with a narrow particle size distribution around 10 μ can reduce the erosion to a level experienced with barite (Saasen et al., 2001).
Carbonate
It is possible to replace barite and iron-based weighting material with carbonate if a high degree of weighting is not required. Besides being cheaper than barite, such materials are less abrasive, which is especially important when drilling is performed in producing formations. It is also readily soluble in hydrochloric acid. The main shortcomings of carbonate powders are due to the presence of a coarsely divided fraction, and also of noncarbonate impurities (Lipkes et al., 1996).
Zinc Oxide, Zirconium Oxide, and Manganese Tetroxide
Zinc oxide (ZnO), is a particularly suitable material for weighting because it has a high density; 5.6 g ml−1 versus 4.5 g ml−1 for barite. It is soluble in acids (e.g., HCl), and its particle size can be set so that it does not invade the formation. Acid solubility is particularly useful because dissolved ZnO can be pass through a production screen without plugging it. A high density means less weighting material is needed per unit mud volume to achieve a desired density.
The particle size, around 10 μ, is such that the ZnO particles do not invade the formation core with the filtrate. On the other hand, the particle size is not large enough to settle out of suspension.
Zirconium oxide possesses similar properties to ZnO. It has a density of 5.7 g ml−1 and is soluble in nitric acid and hot concentrated hydrochloric, hydrofluoric, and sulfuric acids. Therefore, a filter cake formed from zinc or zirconium oxide can be dissolved. The high solubility of ZnO in acids makes it particularly suitable as weighting material (Lau et al., 1997). On the other hand, manganese tetroxide (Mn3O4) is so fine that it invades the formation with the filtrate.
Hollow Glass Microspheres
Initially, glass microspheres were used in the 1970s to overcome severe lost circulation problems in the Ural Mountains. The technology has subsequently been used in other sites (McDonald et al., 1999). Hollow glass beads reduce the density of a drilling fluid and can be used for underbalanced drilling (Medley Jr. et al., 1997 and Medley Jr. et al., 1995). Field applications have been reported (Arco et al., 2000).
Organoclay Compositions
It has long been known that organophilic clays can be used to thicken a variety of organic compositions. Such clays are prepared by the reaction of an organic cation with a clay. If this cation contains at least one alkyl group of at least 8–10 carbon atoms, then the clays produced have the property of increasing the viscosity of organic liquids and thus imparting desired rheological properties to a wide variety of such liquids, including paints, coatings, adhesives, and similar products.
It is also well known that such organoclays may function to thicken polar or nonpolar solvents, depending on the organic salt. Their efficiency in nonaqueous systems can be further improved by adding a polar organic material of low molecular weight to the composition. Such materials have been called dispersants, dispersion aids, and solvating agents. Low molecular weight alcohols and ketones, particularly methanol and acetone, have been found to be the most efficient.
Organophilic clays are generally prepared by reacting a hydrophilic clay with an organic cation, usually a quaternary ammonium salt compound produced from a fatty nitrile. Examples of hydrophilic clays include bentonite, attapulgite, and hectorite.
Native clay surfaces have negatively charged sites and cationic counter ions such as sodium and calcium cations. Thus, they may be treated with a cationic surfactant to displace the cations that are naturally present at the clay surfaces. The cationic surfactant becomes tightly held to the surfaces through electrostatic charges. In this manner, the hydrophilic nature of the clay is reversed, making it more soluble in oil. Bentonite, when treated with sodium cations, is known as sodium bentonite. Those monovalent sodium cations may be easily displaced from the clay, making a large number of anionic sites available (Miller, 2009).
Quaternary ammonium compounds contain nitrogen moieties in which one or more of the hydrogen atoms attached to the nitrogen are substituted by organic radicals. One of the most popular quaternary ammonium compounds for organophilic clays is dimethyl dihydrogenated tallow ammonium chloride. Tallow contains unsaturated and saturated fatty acids, including oleic acid, palmitic acid, stearic acid, and other minor fatty acids.
The hydrocarbon structure of this compound and the two long chain alkyl groups makes it very oil-soluble. Further, the presence of two methyl groups prevent steric interference, thus allowing close packing of the ammonium cation at the clay surface.
The dimethyl dihydrogenated tallow ammonium chloride surfactant, however, cannot be activated efficiently at relatively low temperatures. Improved cationic surfactants have been developed in which the ammonium compounds have greater numbers of alkyl groups. Inclusion of a benzyl group greatly enhances the performance of organophilic clays at low temperatures (Miller, 2009).
Two or more types of organic salts in the presence of an organic anion act synergistically. The combination of hydrophobic and hydrophilic organic salts and an organic anion produces an organophilic clay gellant, which exhibits improved gelling properties in nonaqueous systems (Nae et al., 1995).
Examples are dimethyl dihydrogenated tallow quaternary ammonium chloride and methyl bis-polyoxyethylene (15 units) cocoalkyl quaternary ammonium chloride, and the salts stearic, succinic, and tartaric acids (Mardis et al., 1997; Nae et al., 1993 and Nae et al., 1999).
Biodegradable Organophilic Clay
Organophilic clays are treated with a quaternary ammonium surfactant having an amide linkage. Examples of such surfactants are shown in Figure 1.6.
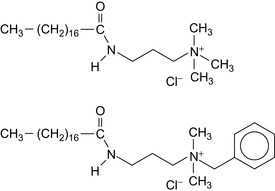 |
| Figure 1.6 Quaternary ammonium surfactants (Miller, 2009). |
The surfactants are based on stearamides. The benzyl group greatly enhances the performance of organophilic clays at temperatures near 7°C.
This type of cationic surfactant is substantially biodegradable, meaning that it is capable of being decomposed by natural biological processes. In particular, it undergoes aerobic biodegradation, which is the breakdown of organic chemicals by microorganisms when oxygen is present.
In this process, aerobic bacteria use oxygen as an electron acceptor and degrade organic chemicals into smaller compounds, producing carbon dioxide and water as the final product (Miller, 2009).
Clays treated in this way may therefore be used in drilling fluids without concern that the surfactant could accumulate in the environment. The surfactant will usually not reach toxic levels that could harm the surrounding environment and the life supported by it (Miller, 2009). The organophilic clay is suitable for both oil-based fluids and invert emulsions.
Polyvinyl neodecanoate
Organophilic clays have been considered as necessary for the suspension of drill cuttings. However, formulations have been developed recently that have improved suspension properties, without organophilic clays (Miller and Kirsner, 2009).
Additives for clayless formulations are emulsified copolymers of 2-ethylhexyl acrylate (EHA) and AA. However, at elevated temperatures it is likely that some acrylate will hydrolyze to AA, thus raising the level of AA moieties in the copolymer. For certain applications, vinyl neodecanoate may be substituted for EHA (Miller and Kirsner, 2009).
These drilling fluids do not need viscosifiers or additional suspension agents and generally do not need fluid loss control agents or filtration control additives. Their rheological properties remain stable over a broad temperature range, even after exposure to high temperatures (Miller and Kirsner, 2009).
Since space is limited at some well sites, such as offshore platforms, it may be advantageous to use efficient drilling fluid additives, which can be formulated using as few additives as possible.
Miscellaneous
Reticulated Bacterial Cellulose
Reticulated bacterial cellulose may be used in place of a conventional gellant, or in combination with conventional gellants to produce enhanced drilling muds (Westland et al., 1992). Only relatively small quantities of this material is needed to enhance their rheologic properties.
Scleroglucan
Scleroglucan is a polysaccharide secreted by the mycelia of certain microorganisms, produced by aerobic fermentation of d-glucose. It has been proposed as a better alternative to xanthan gum for drilling fluid compositions (Gallino et al., 1996).
For drilling fluid applications, scleroglucan can be used in unrefined form. It is an effective thickener for water (Vaussard et al., 1997) and enhances the lubricating and cleaning power of WBMs. In the drilling of deviated wells, scleroglucan permits better cleaning of the well (Donche et al., 1994; Vaussard et al., 1991). It can also be used in drilling jobs with large-diameter wells (Lacret and Donche, 1991; Ladret and Donche, 1991 and Ladret and Donche, 1996).
Uintaite
Uintaite is a naturally occurring, hydrocarbon mineral that is classified as an asphaltite. It is a natural product whose chemical and physical properties vary and depend strongly on the uintaite source. It is also called Gilsonite, which is a registered trademark of American Gilsonite Co., Salt Lake City, Utah.
General purpose Gilsonite brand resin has a softening point of about 175°C, Gilsonite HM has a softening point of about 190°C, and Gilsonite Select 300 and Select 325 have softening points of about 150°C and 160°C, respectively. The softening points of these natural uintaites depend primarily on the source vein that is mined when the mineral is produced.
Uintaite is described by Kirk-Othmer (Neel, 1980). The typical material used in drilling fluids is mined from an area around Bonanza, Utah, and has a specific gravity of 1.05 with a softening point ranging from 190–205°C), although a lower softening point (165°C) material is sometimes used. It has a low acid value, a zero iodine number, and is soluble or partially soluble in aromatic and aliphatic hydrocarbons, respectively.
For many years uintaite and other asphaltic-type products have been used in water-based drilling fluids as additives to assist in borehole stabilization. These additives can minimize hole collapse in formations that contain water sensitive, sloughing shales. Uintaite and asphalt-type materials have been used for many years to stabilize sloughing shales and to reduce borehole erosion. Other benefits derived from these products include borehole lubrication and reduction in the need for filtration.
Uintaite is not easily water-wet with most surfactants. Thus, stable dispersions of uintaite are often difficult to achieve, particularly in the presence of salts, calcium, solids, and other drilling fluid contaminants and in the presence of diesel oil. The uintaite must be readily dispersible and must remain water-wet; otherwise it will coalesce and be separated from the drilling fluid, along with cuttings at the shale shaker or in the circulating pits. Surfactants and emulsifiers are often used with uintaite drilling mud additives.
Loose or poor bonding of the surfactant to the uintaite will lead to it being washed off during use, possible agglomeration, and the removal of uintaite from the mud system with the drilling wastes. Thus, the importance of the wettability, rewettability, and storage stability criteria is evident.
A preferred product comprises about 2 parts Gilsonite HM, about 1 part Gilsonite Select, about 1 part causticized lignite, and about 0.1–0.15 part of a non-ionic surfactant (Christensen et al., 1991 and Christensen et al., 1993).
Sodium Asphalt Sulfonate
Neutralized sulfonated asphalt (i.e., salts of sulfonated asphalt and their blends with materials such as Gilsonite, blown asphalt, lignite, and mixtures of the latter compounds) are commonly used as additives in drilling fluids. These additives, however, cause some foaming in water or water-based fluids, and they are only partially soluble in the fluids.
Liquid additives have therefore been developed to overcome some of the problems associated with the use of dry additives. However, liquid compositions containing polyglycols can give rise to stability problems. Stable compositions can be obtained by special methods of preparation (Patel, 1996). In particular first the viscosifier is mixed with water, then the polyglycol, and finally the sulfonated asphalt is added.
Formation Damage by Gilsonite and Sulfonated Asphalt
Laboratory experiments have been conducted with a chromium lignite/chromium lignosulfonate mud system both without and with solid lubricants. These studies looked at filtration loss, cake quality, and their impact on the formation.
A comparative evaluation has led to the conclusion that Gilsonite is a better additive compared with sulfonated asphalt, as it results in less filtration loss and compact cake formation, thereby reducing formation damage. Flow studies have indicated that the addition of these solid lubricants can be used in drilling fluids without adversely impacting the producing zones (Garg et al., 1995).
Multicomponent Additives
Multi-component additives for drilling fluids have been proposed, containing three primary components, namely a (Brazzel, 2009)
1. Rate of penetration enhancer,
2. Lubricant, and
3. Clay inhibitor or stabilizer.
Penetration enhancers are ester-based oils, which are potential carriers for other additives, such as surfactants. The lubricant is a chlorinated wax. The clay stabilizer is a polyglycol. The three components are premixed in a single container, for ease of use in adding to a drilling fluid system.
The pre-blended additive has significant advantages. Once a desired specific ratio is blended, then adding it at that ratio to a drilling fluid system is very much simplified and maintaining the desired volumetric concentrations in the system is much easier. Adding it to the overall system, is much quicker than adding each component singly (Brazzel, 2009).
Cleaning Operations
Cuttings Removal
When drilling deviated and horizontal wells, gravity causes deposits of drill cuttings and especially fines, or smaller sized cuttings, to build up along the lower side or bottom of the wellbore. Such deposits are commonly called cuttings beds. Buildup of cuttings beds can lead to undesirable friction, and possibly to sticking of the drill string.
Removing the drill cuttings from a deviated well, in particular when drilled at a high angle, can be difficult. Limited pump rate, eccentricity of the drill pipe, sharp build rates, high bottom hole temperatures, and oval-shaped wellbores can all contribute to inadequate hole cleaning.
Well treatments by circulating fluids that have been specially formulated to remove such cuttings beds are periodically necessary to prevent buildup to the point that the cuttings or fines interfere with the drilling apparatus.
Usually, the drilling operation must be stopped while such treatment fluids are swept through the wellbore to remove the fines. Alternatively, special viscosifier drilling fluid additives have been proposed to enhance the ability of the drilling fluid to transport cuttings, but such additives at best merely delay the buildup of cuttings beds and they can be problematic in themselves if they change the density of the drilling fluid. Removal of cutting beds has also performed mechanically wherein the drill string is pulled back along the well, pulling the bit through the horizontal or deviated section of the well.
Barium sulfate can be used as a sweep material. It should be ground and sieved to a size range that is sufficiently small to enable it to be suspended in the drilling fluid. After adding it to the drilling fluid, the fluid is circulated in the wellbore, where it removes the small cuttings or cuttings beds from the borehole and delivers them to the well surface.
The composition and the cuttings are then removed from the drilling fluid in a manner that prevents a significant change in its density. This is done by sieving or screening, preferably by the principal shale shaker of the drilling operation (West et al., 2001).
Junk Removal
Drilling equipment that is broken or stuck in the hole can be dissolved by means of nitric and hydrochloric acids mixed in a proportion of 1:3. To accelerate the dissolving of the metal, a mixture containing 1.1 parts of sodium nitrate and 1.0 part of monoethanolamine is added initially to the acids in the amount of 0.05–13.0 parts per 100 parts of acid mixture. The acidic residue in the hole is neutralized by addition of alkali and converted into drilling fluid by addition of polymer solution (Dolganskaya and Sharipov, 1992).
Filter Cake Removal
As the drilling fluid is circulated, a layer of solids, referred to as a filter cake, is usually formed on the walls of the wellbore. A certain degree of cake buildup usually is desirable to isolate formations from drilling fluids. Once the wellbore has been drilled to the desired depth, the drill string and bit are removed, and a pipe string, e.g., casing, liners, etc., are introduced into the wellbore.
Eventually, the wellbore may be conditioned by circulating the drilling fluid. The purpose of this conditioning is to remove as much of the filter cake and the gelled drilling fluid from the walls of the wellbore as possible. However, sometimes this is not enough to remove the undesired material completely. Problems with subsequent processing, e.g., in primary cementing operations may also arise, because in general, cement compositions are not compatible with the drilling fluid and the filter cake.
To mitigate these problems, a special chemical wash composition containing surfactants can be introduced, sometimes known as preflushes.
An aqueous chemical wash solution contains sulfonated bisulfite lignin, and a taurate, present in amounts of 0.1–5% (Dealy and Chatterji, 2010). The sulfonated lignin is produced by the bisulfite process, or by sulfomethylation of a lignosulfonate with formaldehyde.
Taurates, or taurides, are generally based on taurine, or 2-aminoethane-sulfonic acid. Taurine occurs naturally in food, being first isolated from ox bile in 1827. Taurine derivates have biological and medial roles (Azuma et al., 2009), and are used in cosmetics and as surfactants.
Examples of taurates useful for preflushes are N-methyl-N-cocoyl taurate, N-methyl-N-palmitoyl taurate, and N-methyl-N-oleyl taurate and their metal salts. They are obtained by the acylation of N-methyl taurine with the corresponding long chain acids (Walele and Syed, 1995), c.f., Figure 1.7.
Additional additives may be included in the chemical wash compositions, such as (Dealy and Chatterji, 2010):
• Viscosifying agents,
• Defoamers,
• Curing agents,
• Corrosion inhibitors,
• Scale inhibitors, and
• Formation conditioning agents.
Viscosifying agents may be clays, diatomaceous earth, starches, or polymers.
Drilling Fluid Disposal
Toxicity
Drilling fluids are known to be potentially toxic and are therefore environmentally damaging. They are composed, unlike other most toxic agents, of a wide variety of chemicals, thus making it difficult to predict the actual risk of a specific drilling mud, but methods for assessing this toxicity have been developed (Kanz and Cravey, 1985).
Studies under laboratory conditions revealed that in 9% of cases, drilling muds were acutely toxic at concentrations of 1,000–10,000 ppm. However, in the natural environment, concentrations drop rapidly to background levels of around 200–1,000ppm, hence low levels may be tolerated in the environment.
In a more recent study, soil samples from oil and gas drilling and production sites were analyzed for contaminating substances associated with drilling fluids and petroleum products. The results revealed that contamination of the soil was widespread and persistent. However, it is generally localized in the immediate vicinity of drilling and production activity. Most prominently, heavy metals, such as barium, chromium, lead, and zinc were detected. Further problems may be caused by salinity, pH, and petroleum hydrocarbons.
No discernible pattern of contamination between well sites was observed due to the variability of methods and materials used in the drilling of individual wells. It was considered that the levels of contaminating agents that were found do not represent an immediate environmental threat. However, the long-term cumulative effects are largely unknown (Carls et al., 1995).
The most consistently toxic bioassay phase is the suspended solid phase. This phase consists of bentonite, cuttings, and soluble components. The toxicity has been explained by specific chemical toxicity of a given mud component, or by physical toxicity generated by abrading or clogging epithelial tissue, i.e., respiratory or digestive body surfaces. In addition, the danger to marine animals from exposure to waste drilling muds may also originate from chemical toxicity. Further details are beyond the scope of this text and the reader is referred to the literature for more information (Kanz and Cravey, 1985, p. 329).
In a long-term study, the influence of increased levels of petroleum hydrocarbons upon soil and plants has been studied. Different doses of drilling fluids and crude oil were applied to clean soil, and the changes in some chemical parameters of the soil, plant density, and crop yields were measured. Drilling fluids showed a stronger impact on the chemical properties of the studied soil, while the plant density and yield were more strongly affected by the levels of crude oil. The soil levels of petroleum hydrocarbons, mineral oils, and polycyclic aromatic hydrocarbons were significantly reduced after the first trial year (Kisic et al., 2009).
For the reasons illustrated above, there is a need to take care in the waste management of drilling muds. Selected solutions for the waste management of these materials will now be discussed.
Conversion Into Cements
Water-based drilling fluids may be converted into cements using hydraulic blast furnace slag (Bell, 1993; Cowan and Hale, 1995; Cowan et al., 1994; Cowan and Smith, 1993; Zhao et al., 1996), a unique material that has low impact on the rheological and fluid loss properties of drilling fluids. It can be activated to set in drilling fluids that are difficult to convert to cements by other solidification technologies.
Hydraulic blast furnace slag has a more uniform and consistent quality than Portland well cements, and it is available in large quantities from multiple sources. Fluid and hardened solid properties of blast furnace slag and drilling fluids mixtures used for cementing operations are comparable with the properties of conventional Portland cement compositions.
Environmental Regulations
In response to effluent limitation guidelines promulgated by the Environmental Protection Agency for the discharge of drilling wastes offshore, alternatives to WBMs and OBMs have been developed. Thus, synthetic-based muds are more efficient than WBMs for drilling difficult and complex formation intervals, and they have lower toxicity and lower environmental impacts than diesel or conventional mineral OBMs.
Synthetic drilling fluids may present a significant pollution prevention opportunity, because they are recycled, and smaller volumes of metals are discharged with the cuttings. A framework for a comparative risk assessment for the discharge of synthetic drilling fluids has been developed that will help to identify potential impacts and benefits associated with the use of specific drilling muds (Meinhold, 1998).
Characterization of Drilling Muds
Important parameters for characterizing the properties of a drilling mud are viscosity, specific weight, gel strength, and filtration performance.
Viscosity
Viscosity is measured by means of a Marsh funnel. The funnel is dimensioned so that the outflow time of 1 qt (926 ml) fresh water at 70°F (21°C) is 26 s.
Viscosity is also measured with a rotational viscometer. The mud is placed between two concentric cylinders. One cylinder rotates with constant velocity, while the other is connected by a spring. The torque on this cylinder results in a deviation of its position from rest, which may serve as a measure of viscosity.
Gel strength is measured by a rotational viscometer, if the maximal deflection of the pointer is monitored when the motor is turned on with low speed, the liquid being at rest for a prolonged time before, for example, for 10 min. This maximal deflection measurement is referred to as a 10-minute gel.
API Filtration
A filter press is used to determine the wall-building characteristics of a mud. This press consists of a cylindrical chamber, which is resistant to alkaline media. A filter paper is placed on the bottom of the chamber. The mud is placed into the chamber and a pressure of 0.7 MPa is applied. After 30 min the volume of filtrate is reported. The filter cake is inspected visually and the consistency is noted as hard, soft, tough, rubbery, or firm.
There is another procedure suitable for OBMs under high-pressure and hightemperature conditions. Here, filtration is performed at 100 psi (7 MPa) and at temperatures of 200°F (93°C). It should be noted that research has shown that there may be significant differences between static and dynamic filtering.
Alkalinity and pH
Alkalinity is measured by acid-base titration, with methylorange or phenolphthalein as an indicator. Phenolphthalein changes color at pH 8.3, whereas methylorange changes color at pH 4.3. At pH 8 the neutralization of the strongly alkaline components such as NaOH is essentially complete.
Further reduction of the pH to 4 will also measure the levels of carbonates and bicarbonates that are present. Colorimetric tests and glass electrode systems are used to determine pH. Some indicators are shown in Figure 1.8.
Total Hardness
The sum of calcium and magnesium ions in the mud determines its total hardness. These ions are analyzed by complexometric titrations using ethylene diamine tetraacetic acid.
Roller Oven
The effects of temperature and various chemical additives on the rheological, filtration, and chemical properties of fluids and muds under simulated circulating conditions can be elucidated in a roller oven (Schroeder, 1987 and Schroeder, 1992). Its basic construction is shown in Figure 1.9. A more detailed view can be found in the literature (Schroeder, 1987).
In this oven, motorized rollers rotate a cylindrical cell, which contains the sample under investigation. A heating element is situated beneath the rollers, which heats the chamber to a predetermined temperature. A timer may be preset to start and end the test automatically without having an operator in constant attendance (Schroeder, 1987). The roller oven is a versatile tool to monitor ageing, and the change in properties of fluids used in the petroleum industry, as a function of temperature (Mueller et al., 2003).
Aguilar, K.; Colina, R.; B., M.; A., A.; Rojas, Y., Evaluation criteria to formulate foam as underbalanced drilling fluid, In: Proceedings Volume on CD ROM, Iadc et al Underbalanced Drilling Conf.Houston, TX, 8/28–29/2000. (2000).
Allan, M.L.; Kukacka, L.E., Calcium phosphate cements for lost circulation control in geothermal, Geothermics 24 (2) (1995) 269–282.
Alonso-Debolt, M., Jarrett, M.A., 1995. Drilling fluid additive for water-sensitive shales and clays, and method of drilling using the same. EP Patent 668 339, assigned to Baker Hughes Inc., August 23, 1995.
Anonymous, Drilling fluids product directory, Offshore Incorporating Oilman (Int. Ed.) 51 (9) (1991) 43–44; 46.
Anonymous, Drilling fluids product directory, Offshore Incorporating Oilman (Int. Ed.) 51 (10) (1991) 62; 64–65, 67–68, 70, 72–73.
Anonymous, World oil's 1991 guide to drilling, completion and workover fluids, World Oil 212 (6) (1991) 75–112.
Anonymous, 1992-93 environmental drilling and completion fluids directory, Offshore Incorporating Oilman (Int. Ed.) 52 (9) (1992) 41–42; 45–46, 48, 50–56.
Anonymous, World oil's 1996 drilling, completion and workover fluids, World Oil 217 (6) (1996) 85–126.
Arco, M.J.; Blanco, J.G.; Marquez, R.L.; Garavito, S.M.; Tovar, J.G.; Farias, A.F.; Capo, J.A., Field application of glass bubbles as a density-reducing agent, In: Proceedings Volume, Annu. SPE Tech. Conf.Dallas, TX, 10/1–4/2000. (2000), pp. 115–126.
Argillier, J.F., Roche, P., 2000. Drilling method using a reversible foaming composition (procede de forage utilisant une composition moussante reversible). EP Patent 1 013 739, assigned to Inst. Francais Du Petrole, June 28, 2000.
Ashjian, H., Peel, L.C., Sheerin, T.J., Williamson, R.S., 1995. Non toxic, biodegradable well fluids. WO Patent 9 509 215, assigned to Mobil Oil Corp., April 06, 1995.
Audibert, A.; Argillier, J.F.; Ladva, H.K.J.; Way, P.W.; Hove, A.O., Role of polymers on formation damage, In: Proceedings Volume, SPE Europe Formation Damage Conf.The Hague, Neth, 5/31/1999-6/1/1999. (1999), pp. 505–516.
Austin, P.W., Morpeth, F.F., 1992. Composition and use. EP Patent 500 352, assigned to Imperial Chemical Inds Pl, August 26, 1992.
In: (Editors: Azuma, J.; Schaffer, S.W.; Ito, T.) Taurine 7, Vol. 643 of Advances in Experimental Medicine and Biology (2009) Springer Verlag, Heidelberg.
Bailey, L.; Reid, P.I.; Sherwood, J.D., Mechanisms and solutions for chemical inhibition of shale swelling and failure, In: Proceedings Volume, Recent Advances in Oilfield Chemistry, 5th Royal Soc. Chem. Int. Symp.Ambleside, Engl, 4/13–15/94. (1994), pp. 13–27.
Ballard, T.J.; Beare, S.P.; Lawless, T.A., Shale inhibition with water-based muds: The influence of polymers on water transport through shales, In: Proceedings Volume, Recent Advances in Oilfield Chemistry, 5th Royal Soc. Chem. Int. Symp.Ambleside, Engl, 4/13–15/94. (1994), pp. 38–55.
Batelaan, J.G., van der Horts, P.M., 1994. Method of making amide modified carboxyl-containing polysaccharide and fatty amide-modified polysaccharide so obtainable. WO Patent 9 424 169, assigned to Akzo Nobel NV, October 27, 1994.
Bauer, P.M., Hanlon, D.J., Menking, W.R., 1993. Process for producing bentonite clays exhibiting enhanced solution viscosity properties. US Patent 5 248 641, assigned to Southern Clay Products In, September 28, 1993.
Bell, S., Mud-to-cement technology converts industry practices, Pet. Eng. Int. 65 (9) (1993) 51–52; 54–55.
Bell, S.A., Shumway, W.W., 2009. Additives for imparting fragile progressive gel structure and controlled temporary viscosity to oil based drilling fluids. US Patent 7 560 418, assigned to Halliburton Energy Services, Inc. (Duncan, OK), July 14, 2009.
Bloys, J.B., Wilton, B.S., 1991. Control of lost circulation in wells. US Patent 5 065 820, assigned to Atlantic Richfield Co., November 19, 1991.
Blytas, G.C., Frank, H., 1995. Copolymerization of polyethercyclicpolyols with epoxy resins. US Patent 5 401 860, assigned to Shell Oil Co., March 28, 1995.
Blytas, G.C., Frank, H., Zuzich, A.H., Holloway, E.L., 1992. Method of preparing polyethercyclicpolyols. EP Patent 505 000, assigned to Shell Internat. Res. Mij BV, September 23, 1992.
Blytas, G.C., Zuzich, A.H., Holloway, E.L., Frank, H., 1992. Method of preparing polyethercyclicpolyols. EP Patent 505 002, assigned to Shell Internat. Res. Mij BV, September 23, 1992.
Brankling, D., 1994. Drilling fluid. WO Patent 9 402 565, assigned to Oilfield Chem. Technol. Ltd., February 03, 1994.
Brazzel, R.L., 2009. Multi-component drilling fluid additive, and drilling fluid system incorporating the additive. US Patent 7 635 667, assigned to Ambar Lonestar Fluid Services, LLC (Layfayette, LA), December 22, 2009.
Bruton, J.R.; McLaurine, H.C., Modified poly-amino acid hydration suppressant proves successful in controlling reactive shales, In: Proceedings Volume, 68th Annu. SPE Tech. Conf.Houston, 10/3–6/93. (1993), pp. 127–135.
Burba, J.L.I., Hoy, E.F., Read Jr., A.E., 1992. Adducts of clay and activated mixed metal oxides. WO Patent 9 218 238, assigned to Dow Chemical Co., October 29, 1992.
Burba, J.L.I., Strother, G.W., 1991. Mixed metal hydroxides for thickening water or hydrophilic fluids. US Patent 4 990 268, assigned to Dow Chemical Co., February 05, 1991.
Burrafato, G., Carminati, S., 1994. Aqueous drilling muds fluidified by means of zirconium and aluminium complexes. EP Patent 623 663, assigned to Eniricerche SPA and Agip SPA, November 09, 1994.
Burts Jr., B.D., 1992. Lost circulation material with rice fraction. US Patent 5 118 664, assigned to Bottom Line Industries In, June 02, 1992.
Burts Jr., B.D., 1997. Lost circulation material with rice fraction. US Patent 5 599 776, assigned to M & D Inds Louisiana Inc., February 04, 1997.
Burts Jr., B.D., 2001. Well fluid additive, well fluid made therefrom, method of treating a well fluid, method of circulating a well fluid. US Patent 6 323 158, assigned to Bottom Line Industries In, November 27, 2001.
Carls, E.G.; Fenn, D.B.; Chaffey, S.A., Soil contamination by oil and gas drilling and production operations in Padre Island National Seashore, Texas, U.S.A, Environ. J. Manage. 45 (3) (1995) 273–286.
Christensen, K.C., Davis, N.I., Nuzzolo, M., 1991. Water-wettable drilling mud additives containing uintaite. US Patent 5 030 365, assigned to Chevron Research Co., July 09, 1991.
Christensen, K.C., Davis, N.I., Nuzzolo, M., 1993. Water-wettable drilling mud additives containing uintaite. AU Patent 636 334, assigned to American Gilsonite Co., April 29, 1993.
Courtois-Sambourg, J., Courtois, B., Heyraud, A., Colin-Morel, P., Rinaudo-Duhem, M., 1993. Polymer compounds of the glycuronic acid, method of preparation and utilization particularly as gelifying, thickening, hydrating, stabilizing, chelating or flocculating means. WO Patent 9 318 174, assigned to Picardie Univ., September 16, 1993.
Cowan, K.M., Hale, A.H., 1995. High temperature well cementing with low grade blast furnace slag. US Patent 5 379 840, assigned to Shell Oil Co., January 10, 1995.
Cowan, M.K., Hale, A.H., 1994. Restoring lost circulation. US Patent 5 325 922, assigned to Shell Oil Co., July 05, 1994.
Cowan, K.M., Hale, A.H., Nahm, J.J.W., 1994. Dilution of drilling fluid in forming cement slurries. US Patent 5 314 022, assigned to Shell Oil Co., May 24, 1994.
Cowan, K.M.; Smith, T.R., Application of drilling fluids to cement conversion with blast furnace slag in Canada, In: Proceedings Volume, no. 93-601, Cade/caodc Spring Drilling Conf.Calgary, Can, 4/14–16/93. (1993); Proc..
Dahanayake, M., Li, J., Reierson, R.L., Tracy, D.J., 1996. Amphoteric surfactants having multiple hydrophobic and hydrophilic groups. EP Patent 697 244, assigned to Rhone Poulenc Inc., February 21, 1996.
Dalmazzone, C., Audibert-Hayet, A., Langlois, B., Touzet, S., 2007. Oil-based drilling fluid comprising a temperature-stable and non-polluting emulsifying system. US Patent 7 247 604, assigned to Institut Francais du Petrole (Rueil Malmaison Cedex, FR) and Rhodia Chimie (Aubervilliers Cedex, FR), July 24, 2007.
Davidson, E., 2001. Method and composition for scavenging sulphide in drilling fluids. WO Patent 0 109 039, assigned to Halliburton Energy Serv., February 08, 2001.
Dealy, S.T., Chatterji, J., 2010. Chemical wash compositions for removing drilling fluids. US Patent 7 662 752, assigned to Halliburton Energy Services, Inc. (Duncan, OK), February 16, 2010.
Deem, C.K.; Schmidt, D.D.; Molner, R.A., Use of mmh (mixed metal hydroxide)/propylene glycol mud for minimization of formation damage in a horizontal well, In: Proceedings Volume, no. 91-29, 4th Cade/caodc Spring Drilling Conf.Calgary, Can, 4/10–12/91. (1991); Proc..
Delhommer, H.J., Walker, C.O., 1987a. Encapsulated oil absorbent polymers as lost circulation additives for oil based drilling fluids. US Patent 4 704 213, November 03, 1987.
Delhommer, H.J., Walker, C.O., 1987b. Method for controlling lost circulation of drilling fluids with hydrocarbon absorbent polymers. US Patent 4 633 950, January 06, 1987.
Dino, D.J., 1997. Modified polygalactomannans as oil field shale inhibitors. US Patent 5 646 093, assigned to Rhone Poulenc Inc., July 08, 1997.
Dino, D., Thompson, J., 2001. Organophilic clay additives and oil well drilling fluids with less temperature dependent rheological properties containing said additives. EP Patent 1 138 740, assigned to Elementis Specialties Inc., October 04, 2001.
Dolganskaya, S.I., Sharipov, A.U., 1992. Removal from hole of junk and stuck drilling equipment – by dissolving latter with mixture of nitric and hydrochloric acids with added sodium nitrate and ethanolamine. SU Patent 1 782 271, assigned to W Sibe Deep Pros Dril Des., December 15, 1992.
Donche, A., Vaussard, A., Isambourg, P., 1994. Application of scleroglucan muds to drilling deviated wells. US Patent 5 330 015, assigned to Soc. Natl. Elf Aquitaine, July 19, 1994.
Doolan, J.G., Cody, C.A., 1995. Pourable water dispersible thickening composition for aqueous systems and a method of thickening said aqueous systems. US Patent 5 425 806, assigned to Rheox Inc., June 20, 1995.
Downey, A.B., Willingham, G.L., Frazier, V.S., 1995. Compositions comprising 4,5-dichloro-2-noctyl-3-isothiazolone and certain commercial biocides. EP Patent 680 695, assigned to Rohm & Haas Co., November 08, 1995.
Duhon, J.J.S., 1998. Olive pulp additive in drilling operations. US Patent 5 801 127, September 01, 1998.
Egraz, J.B., Grondin, H., Suau, J.M., 1994. Acrylic copolymer partially or fully soluble in water, cured or not and its use (copolymere acrylique partiellement ou totalement hydrosoluble, reticule ou non et son utilisation). EP Patent 577 526, assigned to Coatex SA, January 05, 1994.
Elphingstone, E.A., Woodworth, F.B., 1999. Dry biocide. US Patent 6 001 158, assigned to Baker Hughes Inc., December 14, 1999.
Fabrichnaya, A.L., Shamraj, Y.V., Shakirzyanov, R.G., Sadriev, Z.K., Koshelev, V.N., Vakhrushev, L.P., Tavrin, A.E., 1997. Additive for drilling solutions with high foam extinguishing properties – containing specified surfactant in hydrocarbon solvent with methyl-diethyl-alkoxymethyl ammonium methyl sulphate. RU Patent 2 091 420, assigned to Etn Co. Ltd., September 27, 1997.
Felix, M.S., 1996. A surface active composition containing an acetal or ketal adduct. WO Patent 9 600 253, assigned to Dow Chemical Co., January 04, 1996.
Felixberger, J., Mixed metal hydroxides (MMH) – an inorganic thickener for water-based drilling muds (Mixed Metal Hydroxide (MMH) – Ein anorganisches Verdickungsmittel für wasserbasierte Bohrspülungen), In: Proceedings Volume, DMGK Spring Conf.Celle, Ger, 4/25–26/96. (1996), pp. 339–351.
Fleming, J.K., Fleming, H.C., 1995. Invert emulsion drilling mud. WO Patent 9 504 788, assigned to J K F Investments Ltd. and Hour Holdings Ltd., February 16, 1995.
Fu, M.; Hu, X., An investigation into shale stability by utilizing copolymer of acrylamide and acrylonitrile and its derivatives. Jianghan, J., Pet. Inst. 19 (1) (1997) 70–73.
Fuh, G.F., Morita, N., Whitfill, D.L., Strah, D.A., 1993. Method for inhibiting the initiation and propagation of formation fractures while drilling. US Patent 5 180 020, assigned to Conoco Inc., January 19, 1993.
Gajdarov, M.M., Tankibaev, M.A., 1996. Non-clayey drilling solution – contains organic stabiliser, caustic soda, water and mineral additive in form of zinc oxide, to improve its thermal stability. RU Patent 2 051 946, assigned to Aktyubinsk Oil Gas Inst., January 10, 1996.
Gallino, G.; Guarneri, A.; Poli, G.; Xiao, L., Scleroglucan biopolymer enhances wbm (waterbase mud) performances, In: Proceedings Volume, Annu. SPE Tech. Conf.Denver, 10/6–9/96. (1996), pp. 105–119.
Garg, V.; Ralhan, M.; Tewari, H.C.; Srivastava, A.; Nanda, S.K.; Rawat, H.S., Impact assessment of solid lubricants used in drilling fluids on the producing zone, In: Proceedings Volume, Vol. 3, 1st India Oil & Natur Gas Corp Ltd et al Int. Petrol. Conf. (Petrotech 95)New Delhi, India, 1/9–12/95. (1995), pp. 55–60.
Gee, J.C., Lawrie, C.J., Williamson, R.C., 1995. Drilling fluids comprising mostly linear olefins. WO Patent 9 521 226, assigned to Chevron Chemical Co., August 10, 1995.
Gee, J.C., Williamson, R.C., Lawrie, C.J., 1992. Drilling fluids comprising mostly linear olefins. US Patent 6 057 272, assigned to Chevron Chem. Co., November 19, 1992.
Gee, J.C., Williamson, R.C., Lawrie, C.J., Miller, S.J., 1998. Skeletally isomerized linear olefins. US Patent 5 741 759, assigned to Chevron Chemical Co., April 21, 1998.
Gee, J.C., Williamson, R.C., Lawrie, C.J., Miller, S.J., 2000. Skeletally isomerized linear olefins. US Patent 6 054 415, assigned to Chevron Chem. Co., April 25, 2000.
Getliff, J.M.; James, S.G., The replacement of alkyl-phenol ethoxylates to improve the environmental acceptability of drilling fluid additives, In: Proceedings Volume, Vol. 2, 3rd SPE et al Health, Safety & Environ. Int. Conf.New Orleans, 6/9–12/96. (1996), pp. 713–719.
Glowka, D.A., Loeppke, G.E., Rand, P.B., Wright, E.K., 1989. Laboratory and Field Evaluation of Polyurethane Foam for Lost Circulation Control, Vol. 13 of The Geysers – Three Decades of Achievement: A Window on the Future, Geothermal Resources Council, Davis, Calif, pp. 517–524.
Godwin, A.D., Mathys, G.M.K., 1993. Ester-free ethers. WO Patent 9 304 028, assigned to Exxon Chemical Patents In, March 04, 1993.
Godwin, A.D., Sollie, T., 1993. Load bearing fluid. EP Patent 532 128, assigned to Exxon Chemical Patents In, March 17, 1993.
Goncalves, J., De Oliveira, M.F., Aragäo, t.F.L., 2007. Compositions of oil-based biodegradable drilling fluids and process for drilling oil and gas wells. US Patent 7 285 515, assigned to Petroleo Brasileiro S.A. – Petrobras (BR), October 23, 2007.
Gotlieb, K.F., Bleeker, I.P., van Doren, H.A., Heeres, A., 1996. 2-nitroalkyl ethers of native or modified starch, method for the preparation thereof, and ethers derived therefrom. EP Patent 710 671, assigned to Coop Verkoop Prod. Aard De, May 08, 1996.
Green, B.D., 2001. Method for creating dense drilling fluid additive and composition therefor. WO Patent 0 168 787, assigned to Grinding & Sizing Co. Inc., September 20, 2001.
Grey, R.A., 1993. Process for preparing alternating copolymers of olefinically unsaturated sulfonate salts and unsaturated dicarboxylic acid anhydrides. US Patent 5 210 163, assigned to Arco Chemical Technol. Inc., May 11, 1993.
Guichard, B., Wood, B., Vongphouthone, P., 2008. Fluid loss reducer for high temperature high pressure water based-mud application. US Patent 7 449 430, assigned to Eliokem S.A.S. (Villejust, FR), November 11, 2008.
Gullett, P.D., Head, P.F., 1993. Materials incorporating cellulose fibres, methods for their production and products incorporating such materials. WO Patent 9 318 111, assigned to Stirling Design Intl Ltd., September 16, 1993.
Hale, A.H., Loftin, R.E., 1996. Glycoside-in-oil drilling fluid system. US Patent 5 494 120, assigned to Shell Oil Co., February 27, 1996.
Hatchman, K., 1999. Drilling fluid concentrates. EP Patent 903 390, assigned to Albright & Wilson Ltd., March 24, 1999.
Headley, J.A.; Walker, T.O.; Jenkins, R.W., Environmentally safe water-based drilling fluid to replace oil-based muds for shale stabilization, In: Proceedings Volume, SPE/IADC Drilling Conf.Amsterdam, Neth, 2/28/95–3/2/95. (1995), pp. 605–612.
Heinrich, G., 1992. Process for recovering barite from drilling muds. CA Patent 1 310 144, November 10, 1992.
Hille, M., Wittkus, H., Weinelt, F., 1996. Application of acetal-containing mixtures (verwendung von acetal enthaltenden mischungen). EP Patent 702 074, assigned to Hoechst AG, March 20, 1996.
Hille, M., Wittkus, H., Weinelt, F., 1998. Use of acetal-containing mixtures. US Patent 5 830 830, assigned to Clariant GmbH, November 03, 1998.
Hille, M., Wittkus, H., Windhausen, B., Scholz, H.J., Weinelt, F., 1992. Application of acetals (verwendung von acetalen). EP Patent 512 501, assigned to Hoechst AG, November 11, 1992.
Hille, M., Wittkus, H., Windhausen, B., Scholz, H.J., Weinelt, F., 1998. Use of acetals. US Patent 5 759 963, assigned to Hoechst AG, June 02, 1998.
Hodder, M.H.; Ballard, D.A.; Gammack, G., Controlling drilling fluid enzyme activity, Pet. Eng. Int. 64 (11) (1992) 31; 33, 35.
House, R.F., Cowan, J.C., 2001. Chitosan-containing well drilling and servicing fluids. US Patent 6 258 755, assigned to Venture Innovations Inc., July 10, 2001.
House, R.F., Wilkinson, A.H., Cowan, C., 1991. Well working compositions, method of decreasing the seepage loss from such compositions, and additive therefor. US Patent 5 004 553, assigned to Venture Innovations Inc., April 02, 1991.
Hsu, J.C., 1990. Synergistic microbicidal combinations containing 3- isothiazolone and commercial biocides. US Patent 4 906 651, assigned to Rohm & Haas Co., March 06, 1990.
Hsu, J.C., 1995. Biocidal compositions. EP Patent 685 158, assigned to Rohm & Haas Co., December 06, 1995.
Ibragimov, F.B., Kolesov, A.I., Konovalov, E.A., Rud, N.T., Gavrilov, B.M., Mojsa, J.N., Rjabokon, A.A., Shcherbaeva, O.M., 1998. Preparation of lignosulphonate reagent – for drilling solutions, involves additional introduction of water-soluble salt of iron, and anti-foaming agent. RU Patent 2 106 383, March 10, 1998.
Ivan, C.D.; Blake, L.D.; Quintana, J.L., Aphron-base drilling fluid: Evolving technologies for lost circulation control, In: Proceedings Volume, Annu. SPE Tech. Conf.New Orleans, LA, 9/30/2001-10/3/2001. (2001).
Jarrett, M., 1997a. Amphoteric acetates and glycinates as shale stabilizing surfactants for aqueous well fluids. US Patent 5 593 952, assigned to Baker Hughes Inc., January 14, 1997.
Jarrett, M., 1997b. Nonionic alkanolamides as shale stabilizing surfactants for aqueous well fluids. US Patent 5 607 904, assigned to Baker Hughes Inc., March 04, 1997.
Jarrett, M., Clapper, D., 2010. High temperature filtration control using water based drilling fluid systems comprising water soluble polymers. US Patent 7 651 980, assigned to Baker Hughes Incorporated (Houston, TX), January 26, 2010.
Jones, C.K., Acker, D.B., 1999. Oil-based drilling muds with increased viscosity. EP Patent 922 743, assigned to Nalco Exxon Energy Chem. L, June 16, 1999.
Kanz, J.E.; Cravey, M.J., Oil well drilling fluids: Their physical and chemical properties and biological impact, In: (Editors: Saxena, J.; Fisher, F.) Hazard Assessment of Chemicals, vol. 5 (1985) Elsevier, New York, pp. 291–421.
Karaseva, E.V., Dedyukhina, S.N., Dedyukhin, A.A., 1995. Treatment of water-based drilling solution to prevent microbial attack – by addition of dimethyl- tetrahydro-thiadiazine-thione bactericide. RU Patent 2 036 216, May 27, 1995.
Kehoe, J.D., Joyce, M.K., 1993. Water soluble liquid alginate dispersions. US Patent 5 246 490, assigned to Syn Chem. Inc., September 21, 1993.
Keilhofer, G., Plank, J., 2000. Solids composition based on clay minerals and use thereof. US Patent 6 025 303, assigned to Skw Trostberg AG, February 15, 2000.
Kinchen, D.; Peavy, M.A.; Brookey, T.; Rhodes, D., Case history: Drilling techniques used in successful redevelopment of low pressure H2S gas carbonate formation, In: Proceedings Volume, Vol. 1, SPE/IADC Drilling Conf.Amsterdam, Netherlands, 2/27/2001-3/1/2001. (2001), pp. 392–403.
Kisic, I.; Mesic, S.; Basic, F.; Brkic, V.; Mesic, M.; Durn, G.; Zgorelec, Z.; Bertovic, L., The effect of drilling fluids and crude oil on some chemical characteristics of soil and crops, Geoderma 149 (3–4) (2009) 209–216.
Kohn, R.S., 1988. Thixotropic aqueous solutions containing a divinylsulfone-crosslinked polygalactomannan gum. US Patent 4 752 339, June 21, 1988.
Kolle, J.J., 2002. Coiled tubing drilling with supercritical carbon dioxide. US Patent 6 347 675, assigned to Tempress Technologies Inc., February 19, 2002.
Kondo, M., Sawada, T., 1996. Readily dispersible bentonite. US Patent 5 491 248, assigned to Hojun Kogyo Co. Ltd., February 13, 1996.
Kotelnikov, V.S., Demochko, S.N., Fil, V.G., Marchuk, I.S., 1996. Drilling mud composition - contains carboxymethyl cellulose, acrylic polymer, ferrochrome lignosulphonate, cement and water. SU Patent 1 829 381, assigned to Ukr. Natural Gas Res. Inst., April 20, 1996.
Kotelnikov, V.S., Demochko, S.N., Melnik, M.P., Mikitchak, V.P., 1992. Improving properties of drilling solution - by addition of ferrochrome- lignosulphonate and aqueous solution of cement and carboxymethyl cellulose. SU Patent 1 730 118, assigned to Ukr. Natural Gas Res. Inst., April 30, 1992.
Kulpa, K.; Adkins, R.; Walker, N.S., New testing vindicates use of barite, Am. Oil Gas Report. 35 (4) (1992) 52–54.
Lacret, A., Donche, A., 1991. Use of scleroglucan muds for the drilling of large diameter holes (application des boues au scleroglucane au forage des puits a gros diametre). FR Patent 2 662 447, assigned to Soc. Natl. Elf Aquitaine, November 29, 1991.
Ladret, A., Donche, A., 1991. Use of scleroglucan muds for the drilling of large diameter holes (application des boues au scleroglucane au forage des puits a gros diametre). EP Patent 459 881, assigned to Soc. Natl. Elf Aquitaine, December 04, 1991.
Ladret, A., Donche, A., 1996. Application of muds containing scleroglucan to drilling large diameter wells. US Patent 5 525 587, assigned to Soc. Natl. Elf Aquitaine, June 11, 1996.
Lange, P.; Plank, J., Mixed metal hydroxide (MMH) viscosifier for drilling fluids: Properties and mode of action (Mixed Metal Hydroxide (MMH) – Eigenschaften undWirkmechanismus als Verdickungsmittel in Bohrspülungen), Erdöl Erdgas Kohle 115 (7–8) (1999) 349–353.
Langlois, B., 1998. Fluid comprising cellulose nanofibrils and its use for oil mining. WO Patent 9 802 499, assigned to Rhone Poulenc Chimie, January 22, 1998.
Langlois, B., Guerin, G., Senechal, A., Cantiani, R., Vincent, I., Benchimol, J., 1999. Fluid comprising cellulose nanofibrils and its use for oil mining (fluide comprenant des nanofibrilles de cellulose et son application pour l'exploitation de gisements petroliers). EP Patent 912 653, assigned to Rhodia Chimie, May 06, 1999.
Lau, H.C., Hale, A.H., Bernardi Jr., L.A., 1997. Drilling fluid. US Patent H168, assigned to Shell Oil Comp., October 7, 1997.
Le Helloco, J.-G., Joye, J.-L., Taverna, C.C., 2004. Polyalkoxylated superamides optionally functionalized, use as emulsifiers. US Patent 6 689 908, assigned to Rhodia Chimie (Courbevoie Cedex, FR), February 10, 2004.
Lecocumichel, N., Amalric, C., 1995. Concentrated aqueous compositions of alkylpolyglycosides, and applications thereof. WO Patent 9 504 592, assigned to Seppic SA, February 16, 1995.
Lee, L.J., Patel, A., Stamatakis, E., 1997. Glycol based drilling fluid. WO Patent 9 710 313, assigned to M I Drilling Fluids Llc., March 20, 1997.
Lin, K.-F., 1996. Synthetic paraffinic hydrocarbon drilling fluid. US Patent 5 569 642, assigned to Albemarle Corp., October 29, 1996.
Lipkes, M.I.; Mezhlumov, A.O.; Shits, L.A.; Avdeev, G.E.; Fomenko, V.I.; Shvetsov, A.M., Carbonate weighting material for drilling-in producing formations and well overhaul, Stroit Neft Gaz Skvazhin Sushe More (5–6) (1996) 34–41.
Lukach, C.A., Zapico, J., 1994. Thermally stable hydroxyethylcellulose suspension. EP Patent 619 340, assigned to Aqualon Co., October 12, 1994.
Lundan, A.O., Anas, P.H., Lahteenmaki, M.J., 1993. Stable cmc (carboxymethyl cellulose) slurry. WO Patent 9 320 139, assigned to Metsa Serla Chemicals Oy, October 14, 1993.
Lundan, A.O., Lahteenmaki, M.J., 1996. Stable cmc (carboxymethyl cellulose) slurry. US Patent 5 487 777, assigned to Metsa Serla Chemicals Oy, January 30, 1996.
Lyons, W.C., In: Standard Handbook of Petroleum and Natural Gas Engineering, vol. 1–2 (1996) Gulf Publishing Co., Burlington.
Müller, H., Herold, C.P., von Tapavicza, S., Neuss, M., Zöllner, W., Burbach, F., 1990. Invert drilling muds. WO Patent 9 010 681, assigned to Henkel KG Auf Aktien, September 20, 1990.
Mardis, W., Sanchaz, J., Basson, H., 1997. Organoclay compositions manufactured with organic acid ester-derived quaternary ammonium compounds, their preparation and non-aqueous fluid systems containing such compositions. EP Patent 798 267, assigned to Rheox International Inc., October 01, 1997.
Maresh, J.L., 2009. Wellbore treatment fluids having improved thermal stability. US Patent 7 541 316, assigned to Halliburton Energy Services, Inc. (Duncan, OK), June 2, 2009.
Martyanova, S.V., Chezlov, A.A., Nigmatullina, A.G., Piskareva, L.A., Shamsutdinov, R.D., 1997. Production of lignosulphonate reagent for drilling muds – by initial heating with sulphuric acid, condensation with formaldehyde, and neutralisation of mixture with sodium hydroxide. RU Patent 2 098 447, assigned to Azimut Res. Prod. Assoc., December 10, 1997.
McCallum, T.F.I., Weinstein, B., 1994. Amine-thiol chain transfer agents. US Patent 5 298 585, assigned to Rohm & Haas Co., March 29, 1994.
McDonald, W.J., Cohen, J.H., Hightower, C.M., 1999. New lightweight fluids for underbalanced drilling, DOE/FETC Rep 99-1103, Maurer Engineering Inc.
McGlothlin, R.E., Woodworth, F.B., 1996. Well drilling process and clay stabilizing agent. US Patent 5 558 171, assigned to M I Drilling Fluids Llc., September 24, 1996.
McNally, K., Nae, H., Gambino, J., 1999. Oil well drilling fluids with improved anti-settling properties and methods of preparing them. EP Patent 906 946, assigned to Rheox Inc., April 07, 1999.
Medley Jr., G.H.; Haston, J.E.; Montgomery, R.L.; Martindale, I.D.; Duda, J.R., Field application of lightweight, hollow-glass-sphere drilling fluid, Pet. J. Technol. 49 (11) (1997) 1209–1211.
Medley Jr., G.H.; Maurer, W.C.; Garkasi, A.Y., Use of hollow glass spheres for underbalanced drilling fluids, In: Proceedings Volume, Annu. SPE Tech. Conf.Dallas, 10/22–25/95. (1995), pp. 511–520.
Meinhold, A.F., 1998. Framework for a comparative environmental assessment of drilling fluids, Brookhaven Nat Lab Rep BNL-66108, Brookhaven Nat Lab (November 1998).
Melbouci, M., Sau, A.C., 2008. Water-based drilling fluids. US Patent 7 384 892, assigned to Hercules Incorporated (Wilmington, DE), June 10, 2008.
Mercer, J.D., Nesbit, L.L., 1992. Oil-base drilling fluid comprising branched chain paraffins such as the dimer of 1-decene. US Patent 5 096 883, assigned to Union Oil Co. California, March 17, 1992.
Messenger, J.U., Lost Circulation. (1981) PennWell Publishing Co., Tulsa, OK; pp. 44–56.
Meyer, V., Audibert-Hayet, A., Gateau, J.P., Durand, J.P., Argillier, J.F., 1999. Water-soluble copolymers containing silicon. GB Patent 2 327 946, assigned to Inst. Francais Du Petrole, February 10, 1999.
Michelson, A., Vattement, H., 1999. Bentonite-based drilling mud and drilling method making use thereof (boue de forage a base de bentonite et procede de forage la mettant en oeuvre). EP Patent 936 263, assigned to Cie Du Sol, August 18, 1999.
Miller, J.J., 2009. Drilling fluids containing biodegradable organophilic clay. US Patent 7 521 399, assigned to Halliburton Energy Services, Inc. (Duncan, OK), April 21, 2009.
Miller, J., Kirsner, J., 2009. Drilling fluid comprising a vinyl neodecanoate polymer and method for enhanced suspension. US Patent 7 572 755, assigned to Halliburton Energy Services, Inc. (Ducan, OK), August 11, 2009.
Monfreux, N., Perrin, P., Lafuma, F., Sawdon, C., 2000. Invertible emulsions stabilised by amphiphilic polymers and application to bore fluids (emulsions inversables stabilisees par des polymers amphiphiles. application a des fluides de forage). WO Patent 0 031 154, assigned to Sofitech NV, Schlumberger Canada Ltd., and Dowell Schlumberger SA, June 02, 2000.
Morpeth, F.F., 1993. Biocide composition and its use. EP Patent 542 721, assigned to Imperial Chemical Inds Pl, May 19, 1993.
Morpeth, F.F., Greenhalgh, M., 1990. Composition and use. EP Patent 390 394, assigned to Imperial Chemical Inds Pl, October 03, 1990.
Mueller, H., Breuer, W., Herold, C.P., Kuhm, P., von Tapavicza, S. 1997. Mineral additives for setting and/or controlling the rheological properties and gel structure of aqueous liquid phases and the use of such additives. US Patent 5 663 122, assigned to Henkel KG Auf Aktien, September 02, 1997.
Mueller, H., Herold, C.-P., Fues, J.F., 1996. Use of surface-active alpha-sulfo-fatty acid di-salts in water and oil based drilling fluids and other drill-hole treatment agents. US Patent 5 508 258, assigned to Henkel KG, April 16, 1996.
Mueller, H., Herold, C.P., von Tapavicza, S., 1990a. Monocarboxylic acid-methyl esters in invertemulsion muds (monocarbonsaeure-methylester in invert-bohrspuelschlaemmen). EP Patent 382 071, assigned to Henkel KG Auf Aktien, August 16, 1990.
Mueller, H., Herold, C.P., von Tapavicza, S., 1990b. Oleophilic alcohols as components of invert emulsion drilling fluids (oleophile alkohole als bestandteil von invert-bohrspuelungen). EP Patent 391 252, assigned to Henkel KG Auf Aktien, October 10, 1990.
Mueller, H., Herold, C.P., von Tapavicza, S., 1990c. Oleophilic basic amine derivatives as additives in invert emulsion muds (oleophile basische aminverbindungen als additiv in invertbohrspuelschlaemmen). EP Patent 382 070, assigned to Henkel KG Auf Aktien, August 16, 1990.
Mueller, H., Herold, C.P., von Tapavicza, S., 1991. Use of hydrated castor oil as a viscosity promoter in oil-based drilling muds. WO Patent 9 116 391, assigned to Henkel KG Auf Aktien, October 31, 1991.
Mueller, H., Herold, C.P., von Tapavicza, S., Fues, J.F., 1991. Fluid borehole-conditioning agent based on polycarboxylic acid esters. WO Patent 9 119 771, assigned to Henkel KG Auf Aktien, December 26, 1991.
Mueller, H., Herold, C.P., von Tapavicza, S., Grimes, D.J., Braun, J.M., Smith, S.P.T., 1990a. Use of selected ester oils in drilling muds, especially for offshore oil or gas recovery (verwendung ausgewaehlter esteroele in bohrspuelungen insbesondere zur off-shore- erschliessung von erdoel- bzw. erdgasvorkommen (i)). EP Patent 374 671, assigned to Henkel KG Auf Aktien and Baroid Ltd., June 27, 1990.
Mueller, H., Herold, C.P., von Tapavicza, S., Grimes, D.J., Braun, J.M., Smith, S.P.T., 1990b. Use of selected ester oils in drilling muds, especially for offshore oil or gas recovery (verwendung ausgewaehlter esteroele in bohrspuelungen insbesondere zur off-shore- erschliessung von erdoel- bzw. erdgasvorkommen (ii)). EP Patent 374 672, assigned to Henkel KG Auf Aktien and Baroid Ltd., June 27, 1990.
Mueller, H., Herold, C.P., von Tapavicza, S., Neuss, M., Burbach, F., 1994. Use of selected ester oils of low carboxylic acids in drilling fluids. US Patent 5 318 954, assigned to Henkel KG Auf Aktien, June 07, 1994.
Mueller, H., Herold, C.-P., von Tapavicza, S., Stoll, G., Jeschke, R., Fues, J.F., 2003. Use of selected oleophilic ethers in water-based drilling fluids of the o/w emulsion type and corresponding drilling fluids with improved ecological acceptability. US Patent 6 596 670, assigned to Cognis Deutschland GmbH & Co. KG, Duesseldorf and Baroid Limited, London, July 22, 2003.
Mueller, H., Herold, C.P., Westfechtel, A., von Tapavicza, S., 1991. Free-flowing drill hole treatment agents based on carbonic acid diesters. WO Patent 9 118 958, assigned to Henkel KG Auf Aktien, December 12, 1991.
Mueller, H., Herold, C.P., Westfechtel, A., von Tapavicza, S., 1992. Fluid drill-hole treatment agents based on carbonic acid diesters. ZA Patent 9 104 341, assigned to Henkel KG Auf Aktien, January 20, 1992.
Mueller, H., Herold, C.P., Westfechtel, A., von Tapavicza, S., 1993. Free-flowing drill hole treatment agents based on carbonic acid diesters (fliessfaehige bohrlochbehandlungsmittel auf basis von kohlensaeurediestern). EP Patent 532 570, assigned to Henkel KG Auf Aktien, March 24, 1993.
Mueller, H., Herold, C.P., Westfechtel, A., von Tapavicza, S., 1995. Fluid-drill-hole treatment agents based on carbonic acid diesters. US Patent 5 461 028, assigned to Henkel KG Auf Aktien, October 24, 1995.
Mueller, H., Stoll, G., Herold, C.P., von Tapavicza, S., 1990. Application of selected ethers of monofunctional alcohols in drilling fluids (verwendung ausgewaehlter ether monofunktioneller alkohole in bohrspuelungen). EP Patent 391 251, assigned to Henkel KG Auf Aktien, October 10, 1990.
Mullen, G.A., Gabrysch, A., 2001. Synergistic mineral blends for control of filtration and rheology in silicate drilling fluids. US Patent 6 248 698, assigned to Baker Hughes Inc., June 19, 2001.
Muller, H., Herold, C.P., von Tapavicza, S., 1990. Drilling fluids. ZA Patent 9 002 669, assigned to Henkel KG Auf Aktien, October 08, 1990.
Muller, H., Herold, C.P., Westfechtel, A., von Tapavicza, S., 1993. Free-flowing drill hole treatment agents based on carbonic acid diesters. AU Patent 643 299, assigned to Henkel KG Auf Aktien, November 11, 1993.
Muller, H., Stoll, G., Herold, C.P., von Tapavicza, S., 1990. Drilling fluids. ZA Patent 9 002 665, assigned to Henkel KG Auf Aktien, October 08, 1990.
Munro, R.; Hanni, G.; Young, A., The economics of a synthetic drilling fluid for exploration drilling in the UK sector of the North Sea, In: Proceedings Volume, IBC Tech. Serv. Ltd Prev. Oil Discharge from Drilling Oper. – The Options Conf.Aberdeen, Scot, 6/23–24/93. (1993).
Nae, H., Reichert, W.W., Eng, A.C., 1993. Organoclay compositions prepared with a mixture of two organic cations and their use in non-aqueous systems. EP Patent 542 266, assigned to Rheox International Inc., May 19, 1993.
Nae, H.N., Reichert, W.W., Eng, A.C., 1995. Organoclay compositions containing two or more cations and one or more organic anions, their preparation and use in non-aqueous systems. US Patent 5 429 999, assigned to Rheox Inc., July 04, 1995.
Nae, H., Reichert, W., Eng, A.C., 1999. Organoclay compositions containing two or more cations and one or more organic anions, their preparation and use in non-aqueous systems. EP Patent 681 990, assigned to Rheox International Inc., November 24, 1999.
Neel, K.R., Gilsonite, In: third ed.Kirk-Othmer Encyclopedia of Chemical Technology, vol. 11. (1980) J. Wiley & Sons, New York, pp. 802–806.
Nicora, L.F.; McGregor, W.M., Biodegradable surfactants for cosmetics find application in drilling fluids, In: Proceedings Volume, Iadc/SPE Drilling Conf.Dallas, 3/3–6/98. (1998), pp. 723–730.
Oppong, D., Hollis, C.G., 1995. Synergistic antimicrobial compositions containing (thiocyanomethylthio) benzothiazole and an organic acid. WO Patent 9 508 267, assigned to Buckman Labs Internat. Inc., March 30, 1995.
Oppong, D., King, V.M., 1995. Synergistic antimicrobial compositions containing a halogenated acetophenone and an organic acid. WO Patent 9 520 319, assigned to Buckman Labs Internat. Inc., August 03, 1995.
Patel, B.B., 1994a. Fluid composition comprising a metal aluminate or a viscosity promoter and a magnesium compound and process using the composition. EP Patent 617 106, assigned to Phillips Petroleum Co., September 28, 1994.
Patel, B.B., 1994b. Tin/cerium compounds for lignosulfonate processing. EP Patent 600 343, assigned to Phillips Petroleum Co., June 08, 1994.
Patel, B.B., 1996. Liquid additive comprising a sulfonated asphalt and processes therefor and therewith. US Patent 5 502 030, assigned to Phillips Petroleum Co., March 26, 1996.
Patel, A.D., 2008. Methods for using reversible phase oil-based drilling fluid. US Patent 7 377 721, assigned to M-I L.L.C. (Houston, TX), May 27, 2008.
Patel, A.D., McLaurine, H.C., Stamatakis, E., Thaemlitz, C.J., 1995. Drilling fluid additive and method for inhibiting hydration. EP Patent 634 468, assigned to M I Drilling Fluids Co., January 18, 1995.
Patel, B.B., Muller, G.T., 1996. Compositions comprising an acrylamide-containing polymer and process therewith. EP Patent 728 826, assigned to Phillips Petroleum Co., August 28, 1996.
Patel, A.D., Stamatakis, E., Davis, E., 2001. Shale hydration inhibition agent and method of use. US Patent 6 247 543, assigned to M I Llc., June 19, 2001.
Patel, A.D., Stamatakis, E., Young, S., 2009. High performance water-based drilling mud and method of use. US Patent 7 514 389, assigned to M-I L.L.C. (Houston, TX), April 7, 2009.
Peiffer, D.G., Bock, J., Elward-Berry, J., 1991. Zwitterionic functionalized polymers as deflocculants in water based drilling fluids. US Patent 5 026 490, assigned to Exxon Research & Eng. Co., June 25, 1991.
Peiffer, D.G., Bock, J., Elward-Berry, J., 1992. Thermally stable hydrophobically associating rheological control additives for water-based drilling fluids. US Patent 5 096 603, assigned to Exxon Research & Eng. Co., March 17, 1992.
Peiffer, D.G., Bock, J., Elward-Berry, J., 1993. Thermally stable hydrophobically associating rheological control additives for water-based drilling fluids. CA Patent 2 055 011, assigned to Exxon Research & Eng. Co., May 07, 1993.
Ponsati, O., Trius, A., Herold, C.P., Mueller, H., Nitsch, C., von Tapavicza, S., 1992. Use of selected oleophilic compounds with quaternary nitrogen to improve the oil wettability of finely divided clay and their use as viscosity promoters. WO Patent 9 219 693, assigned to Henkel KG Auf Aktien, November 12, 1992.
Ponsati, O., Trius, A., Herold, C.P., Mueller, H., Nitsch, C., von Tapavicza, S., 1994. Use of selected oleophilic compounds with quaternary nitrogen to improve the oil wettability of finely divided clay and their use as viscosity promoters (verwendung ausgewaehlter oleophiler verbindungen mit quartaerem stickstoff zur verbesserung der oelbenetzbarkeit feinteiliger tone und deren anwendung als viskositaetsbildner). EP Patent 583 285, assigned to Henkel KG Auf Aktien, February 23, 1994.
Prokhorov, N.M., Smirnova, L.N., Luban, V.Z., 1993. Neutralisation of hydrogen sulphide in drilling solution - by introduction of additive consisting of iron sulphate and additionally sodium aluminate, to increase hydrogen sulphide absorption. SU Patent 1 798 358, assigned to Polt. Br. Ukr. Geoprosp. Inst., February 28, 1993.
Rae, R.; Di Lullo, G., Chemically-enhanced drilling with coiled tubing in carbonate reservoirs, In: Proceedings Volume, SPE/Int. Coiled Tubing Ass. Coiled Tubing RoundtableHouston, TX, 3/7–8/2001. (2001).
Rangus, S., Shaw, D.B., Jenness, P., 1993. Cellulose ether thickening compositions. WO Patent 9 308 230, assigned to Laporte Industries Ltd., April 29, 1993.
Rastegaev, B.A.; Andreson, B.A.; Raizberg, Y.L., Bactericidal protection of chemical agents from biodegradation while drilling deep wells, Stroit Neft Gaz Skvazhin Sushe More (7–8) (1999) 32–34.
Ravi, K.M., Whitfill, D.L., Reddy, B.R., 2009. Methods of drilling wellbores using variable density fluids comprising coated elastic particles. US Patent 7 482 309, assigned to Halliburton Energy Services, Inc. (Duncan, OK), January 27, 2009.
Recommended practice for chemical analysis of barite. 1996. API Standard API RP 13K. American Petroleum Institute, Washington, DC.
Reid, A.L., Grichuk, H.A., 1991. Polymer composition comprising phosphorous- containing gelling agent and process thereof. US Patent 5 034 139, assigned to Nalco Chemical Co., July 23, 1991.
Robinson, F., 1996. Polymers useful as ph responsive thickeners and monomers therefor. WOPatent 9 610 602, assigned to Rhone Poulenc Inc., April 11, 1996.
Robinson, F., 1999. Polymers useful as ph responsive thickeners and monomers therefor. US Patent 5 874 495, assigned to Rhodia, February 23, 1999.
Rose, R.A., 1996. Method of drilling with fluid including nut cork and drilling fluid additive. US Patent 5 484 028, assigned to Grinding & Sizing Co. Inc., January 16, 1996.
Saasen, A.; Hoset, H.; Rostad, E.J.; Fjogstad, A.; Aunan, O.; Westgard, E.; Norkyn, P.I., Application of ilmenite as weight material in water based and oil based drilling fluids, In: Proceedings Volume, Annu. SPE Tech. Conf.New Orleans, LA, 9/30/2001-10/3/2001. (2001).
Santhanam, M., MacNally, K., 2001. Oil and oil invert emulsion drilling fluids with improved antisettling properties. EP Patent 1 111 024, assigned to Rheox Inc., June 27, 2001.
Sawdon, C., Tehrani, M., Craddock, P., 2000. Electrically conductive non-aqueous wellbore fluids. GB Patent 2 345 706, assigned to Sofitech NV, July 19, 2000.
Schlemmer, R.F., 2007. Membrane forming in-situ polymerization for water based drilling fluids. US Patent 7 279 445, assigned to M-I L.L.C. (Houston, TX), October 9, 2007.
Schroeder, R.E., 1987. Roller oven for testing fluids. US Patent 4 677 843, assigned to OFI Testing Equipment Inc. (Houston, TX), July 7, 1987.
Schroeder, R.E., 1992. Thermal test liner apparatus and method. US Patent 5 152 184, assigned to OFI Testing Equipment, Inc. (Houston, TX), October 6, 1992.
Shen, W.; Pan, H.; Du, T.; Jia, D., Preparation and applications of oleophilic modified barite, Univ. J. Pet. China 22 (1) (1998) 66–69; 114.
Shen, W.; Pan, H.F.; Qin, Y.Q., Advances in chemical surface modification of barite, Oilfield Chem. 16 (1) (1999) 86–90.
Sheu, J.J., Bland, R.G., 1992. Drilling fluid with stabilized browning reaction anionic carbohydrate. US Patent 5 110 484, assigned to Baker Hughes Inc., May 05, 1992.
Shimomura, T., Irie, Y., Takahashi, H., Kajikawa, K., Saga, J., Fujiwara, T., Hatsuda, T., 1990. Process for production of acrylate and acrylate- containing polymer. EP Patent 372 706, assigned to Nippon Shokubai Kag Kog C, June 13, 1990.
Shuey, M.W., Custer, R.S., 1995. Quebracho-modified bitumen compositions, method of manufacture and use. US Patent 5 401 308, assigned to Saramco Inc., March 28, 1995.
Sikora, D., Hydrazine – a universal oxygen scavenger (hydrazyna – uniwersalny inhibitor korozji tlenowej w pluczkach wiertniczych), Nafta Gaz (Pol) 50 (4) (1994) 161–168.
Smith, C.K., Balson, T.G., 2000. Shale-stabilizing additives. GB Patent 2 340 521, assigned to Sofitech NV and Dow Chemical Co., February 23, 2000.
Smith, R.J., Jeanson, D.R., 2001. Dehydration of drilling mud. US Patent 6 216 361, assigned to Newpark Canada Inc., April 17, 2001.
Subramanian, S., Islam, M., Burgazli, C.R., 2001. Quaternary ammonium salts as thickening agents for aqueous systems. WO Patent 0 118 147, assigned to Crompton Corp., March 15, 2001.
Sunde, E., Olsen, H., 2000. Removal of H2S in drilling mud. WO Patent 0 023 538, assigned to Den Norske Stats Oljese A, April 27, 2000.
Sydansk, R.D., 1990. Lost circulation treatment for oil field drilling operations. US Patent 4 957 166, assigned to Marathon Oil Co., September 18, 1990.
Urquhart, J.C., 1997. Potassium silicate drilling fluid. WO Patent 9 705 212, February 13, 1997.
Vaussard, A., Ladret, A., Donche, A., 1991. Scleroglucan drilling mud (boue de forage au scleroglucane). FR Patent 2 661 186, assigned to Soc. Natl. Elf Aquitaine, October 25, 1991.
Vaussard, A., Ladret, A., Donche, A., 1997. Scleroglucan based drilling mud. US Patent 5 612 294, assigned to Elf Aquitaine, March 18, 1997.
Waehner, K., Experience with high temperature resistant water based drilling fluids (erfahrungen beim einsatz hochtemperatur-stabiler wasserbasischer bohrspülung), Erdöl Erdgas Kohle 106 (5) (1990) 200–201.
Walele, I.I., Syed, S.A., 1995. Process for making N-acyl taurides. US Patent 5 434 276, assigned to Finetex, Inc. (Elmwood Park, NJ), July 18, 1995.
Walker, C.O., 1986. Encapsulated lime as a lost circulation additive for aqueous drilling fluids. US Patent 4 614 599, September 30, 1986.
Walker, C.O., 1989. Method for controlling lost circulation of drilling fluids with water absorbent polymers. CA Patent 1 259 788, assigned to Texaco Development Corp., September 26, 1989.
Wall, K., Zard, P.W., Barclay-Miller, D.J., Martin, D.W., 1995. Surfactant composition. WO Patent 9 530 722, assigned to Burwood Corp. Ltd., November 16, 1995.
Wegner, C.; Reichert, G., Hydrogen sulfide scavenger in drilling fluids (schwefelwasserstoffscavenger in bohrspülungen), In: Proceedings Volume, BASF et al Chem. Prod. in Petrol. Prod. Mtg. H2S – A Hazardous Gas in Crude Oil Recovery DiscussClausthal-Zellerfeld, Ger, 9/12–13/90. (1990).
West, G.C., Valenziano, R., Lutgring, K.A., 2001. Method and composition for sweep of cuttings beds in a deviated borehole. US Patent 6 290 001, assigned to Halliburton Energy Serv., September 18, 2001.
Westland, J.A., Penny, G.S., Lenk, D.A., 1992. Drilling mud compositions. WO Patent 9 222 621, assigned to Weyerhaeuser Co., December 23, 1992.
Whitfill, D.L., Kukena Jr., E., Cantu, T.S., Sooter, M.C., 1990. Method of controlling lost circulation in well drilling. US Patent 4 957 174, assigned to Conoco Inc., September 18, 1990.
Wilkerson, J.M.I., Verstrat, D.W., Barron, M.C., 1995. Associative monomers. US Patent 5 412 142, assigned to Natl. Starch Chem. Inv. Corp., May 02, 1995.
Wilkinson, A.O., Grigson, S.J., Turnbull, R.W., 1995. Drilling mud. WO Patent 9 526 386, assigned to Heriot Watt Univ., October 05, 1995.
Williamson, R.C., Lawrie, C.J., Miller, S.J., 1995. Skeletally isomerized linear olefins. WO Patent 9 521 225, assigned to Chevron Chemical Co., August 10, 1995.
Wood, R.R., 2001. Improved drilling fluids. WO Patent 0 153 429, July 26, 2001.
Yakovlev, S.S.; Konovalov, E.A., Plugging mixtures on a base of hydrolyzed polyacrylonitrile, Neft Khoz (4) (1987) 25–27.
Yassin, A.A.M.; Kamis, A., Palm oil derivative as a based fluid in formulating oil based drilling mud, In: Proceedings Volume, vol. 2, 4th SPE et al Latin Amer. Petrol. Eng. Conf.Rio De Janeiro, Brazil, 10/14–19/90. (1990).
Yeh, M.H., 1995. Compositions based on cationic polymers and anionic xanthan gum (compositions a base de polymeres cationiques et de gomme xanthane anionique). EP Patent 654 482, assigned to Rhone Poulenc Spec. Chem. C, May 24, 1995.
Young, S.; Young, A., Recent field experience using an acetal based invert emulsion fluid, In: Proceedings Volume, IBC Tech. Serv. Ltd Prev. of Oil Discharge from Drilling Oper. – The Options Conf.Aberdeen, Scot, 6/15–16/94. (1994).
Zhao, L.; Xie, Q.; Luo, Y.; Sun, Z.; Xu, S.; Su, H.; Wang, Y., Utilization of slag mix mud conversion cement in the karamay oilfield, xinjiang. Jianghan, J., Pet. Inst. 18 (3) (1996) 63–66.
Zuzich, A.H., Blytas, G.C., 1994. Polyethercyclicpolyols from epihalohydrins, polyhydric alcohols and metal hydroxides or epoxy alcohol and optionally polyhydric alcohols with addition of epoxy resins. US Patent 5 286 882, assigned to Shell Oil Co., February 15, 1994.
Zuzich, A.H., Blytas, G.C., Frank, H., 1995. Polyethercyclicpolyols from epihalohydrins, polyhydric alcohols, and metal hydroxides or epoxy alcohols and optionally polyhydric alcohols with thermal condensation. US Patent 5 428 178, assigned to Shell Oil Co., June 27, 1995.
Tradenames
| Tradename Description | Supplier |
|---|---|
| Accolade® Drilling fluid (Bell and Shumway, 2009) | Halliburton Energy Services, Inc. |
| Adapta® Filtration control agent (Bell and Shumway, 2009; Miller and Kirsner, 2009) | Halliburton Energy Services, Inc. |
| Aquagel® Sodium montmorillonite clay (Ravi et al., 2009) | Halliburton Energy Services, Inc. |
| AquaPAC® Polyanionic cellulose (Melbouci and Sau, 2008) | Aqualon Corp. |
| BARABUF® Buffer (Ravi et al., 2009) | Halliburton Energy Services, Inc. |
| BARACARB® Ground marble (Miller and Kirsner, 2009) | Halliburton Energy Services, Inc. |
| BARASIL® S Sodium silicate shale stabilizer (Ravi et al., 2009) | Halliburton Energy Services, Inc. |
| BARAZAN® Polysaccharide (Ravi et al., 2009) | Halliburton Energy Services, Inc. |
| BAROID® 41 Ground barium sulfate (Bell and Shumway, 2009; Miller and Kirsner, 2009) | Halliburton Energy Services, Inc. |
| CELLEX Carboxymethyl cellulose (Ravi et al., 2009) | Halliburton Energy Services, Inc. |
| Celpol® (Series) Polyanionic cellulose (Melbouci and Sau, 2008) | Noviant, Nijmegen |
| Clay Sync™ Shale stabilizer (Maresh, 2009) | Baroid |
| ClaySeal® Shale stabilizer (Maresh, 2009) | Baroid Fluid Services |
| COLDTROL™ Fatty alcohol thinner (Miller and Kirsner, 2009) | Halliburton Energy Services, Inc. |
| Disponil® Ether sulfonates (Emulsifyer) (Guichard et al., 2008) | Henkel |
| Driltreat™ Wetting agent (Miller and Kirsner, 2009) | Halliburton Energy Services, Inc. |
| EDC95® n-Alkane cuts (Dalmazzone, 2007) | BHI |
| EZMUD® Partially hydrolyzed polyacrylamide (Ravi et al., 2009) | Halliburton Energy Services, Inc. |
| FACTANT™ Concentrated emulsifier (Miller and Kirsner, 2009) | Halliburton Energy Services, Inc. |
| FILTER-CHEK® Modified cellulose (Maresh, 2009; Ravi et al., 2009) | Halliburton Energy Services, Inc. |
| Geltone® (Series) Organophilic clay (Bell and Shumway, 2009; Miller, 2009; Miller and Kirsner, 2009) | Halliburton Energy Services, Inc. |
| Grabber® Flocculant (Maresh, 2009) | Baroid |
| Hydro-Guard® Inhibitive water-based-fluid (Maresh, 2009) | Halliburton Energy Services, Inc. |
| IMPERMEX Pregelatinized cornstarch (Ravi et al., 2009) | Halliburton Energy Services, Inc. |
| Interdrill Emul HT® Emulgator (Dalmazzone, 2007) | Dowell Schlumberger |
| Interdrill® LORM Emulsification system (Dalmazzone, 2007) | Dowell Schlumberger |
| Invermul® Blends of oxidized tall oil and polyaminated fatty acids (Bell and Shumway, 2009; Ravi et al., 2009) | Halliburton Energy Services, Inc. |
| Kleemul® Emulsifier (Guichard et al., 2008) | BW Group |
| Kraton® Styrenic block copolymer (Guichard et al., 2008) | Shell |
| LE BASE™ Base drilling fluid (Miller and Kirsner, 2009) | Halliburton Energy Services, Inc. |
| LE SUPERMUL™ Emulsifier (Bell and Shumway, 2009; Miller and Kirsner, 2009) | Halliburton Energy Services, Inc. |
| LIQUI-VIS Hydroxyethyl cellulose (Ravi et al., 2009) | Baroid |
| Lorm® Emulsifier (Dalmazzone, 2007) | Dowell Schlumberger |
| MICRO MATRIX® Cement (Dealy and Chatterji, 2010) | Halliburton Energy Services, Inc. |
| N-Dril™ HT Plus Filtration control agent (Maresh, 2009) | Baroid |
| PAC™ -L Filtration control agent (Maresh, 2009) | Baroid |
| PAC Polyanionic cellulose (Ravi et al., 2009) | Halliburton Energy Services, Inc. |
| PETROFREE® LV Ester-based invert emulsion (Miller and Kirsner, 2009) | Halliburton Energy Services, Inc. |
| PETROFREE® SF Olefin-based invert emulsion (Miller and Kirsner, 2009) | Halliburton Energy Services, Inc. |
| Plex® Acrylate resin (Guichard et al., 2008) | Rohm & Haas |
| Plioflex® Styrene butadiene rubber (Guichard et al., 2008) | Goodyear Chemicals |
| Pliolite® DF01 Styrene-butadiene copolymer (Guichard et al., 2008) | Goodyear Tire & Rubber Co. |
| POLYAC® Polyacrylate (Ravi et al., 2009) | Halliburton Energy Services, Inc. |
| Resinoline® BD2 Tall oil fatty acid (Dalmazzone, 2007) | DRT-GRANEL |
| RHEMOD™ L Modified fatty acid (Bell and Shumway, 2009) | Halliburton Energy Services, Inc. |
| Scotchlite™ Reflective glass (Ravi et al., 2009) | 3M Comp. |
| SF BASE® Base drilling fluid (Miller and Kirsner, 2009) | Halliburton Energy Services, Inc. |
| Silwet® Ethyleneoxy surfactants (Patel, 2008) | O Si Specialities, Inc. |
| Staflo® PAC (Melbouci and Sau, 2008) | Akzo Nobel |
| Suspentone™ Attapulgite clay (Miller, 2009; Miller and Kirsner, 2009) | Diversity Technologies Corp. |
| Ultidrill® Hydrocarbon cuts (Dalmazzone, 2007) | Dowell Schlumberger |
| Versawet® NS Wetting agent (Patel, 2008) | M-I Drilling Fluids L.L.C. |
| XP07® n-Alkane cuts (Dalmazzone, 2007; Miller and Kirsner, 2009) | Baroid |
| X-VIS™ Suspension agent (Bell and Shumway, 2009; Miller and Kirsner, 2009) | Halliburton Energy Services, Inc. |
| Zeogel® Attapulgite clay (Ravi et al., 2009) | Halliburton Energy Services, Inc. |
..................Content has been hidden....................
You can't read the all page of ebook, please click here login for view all page.