Chapter 5. Bacterial Control
Major problems in oil and gas operations result from the biogenic formation of hydrogen sulfide (H2S) in the reservoir, which results in increased corrosion and iron sulfide formation leading to higher operating costs and reduced revenue. The gas also constitutes a serious environmental and health hazard.
In secondary oil recovery, which involves waterflooding of the oil-containing formation, biofilms can plug the oil-bearing formation, and severe corrosion can result from the production of acids associated with the growth of certain bacterial biofilms. These biofilms are often composed of sulfate-reducing bacteria, which grow anaerobically in water, often in the presence of oil and natural gases. Once biofilms are established, it is extremely difficult to regain biological control of the system.
When biofilms are formed on metallic surfaces, they can seriously corrode oil production facilities. Microbiologically influenced corrosion represents the most serious form of that degradation, and it is estimated that this type of corrosion may be responsible for 15–30% of failures caused by corrosion across all industries.
Effective control of bacteria is therefore mandatory. Several biocides and together with nonbiocidal techniques are available, and procedures and techniques to detect bacteria have been developed.
Mechanisms of Growth
Growth of Bacteria Supported by Oil Field Chemicals
Growth experiments have been conducted using bacteria from oil installations with several chemicals normally used in injection water treatments. These studies have revealed that some chemicals utilized as nitrogen, phosphorus, or carbon sources by those bacteria (Sunde et al. 1990). Therefore, it was concluded that the growth potential of water treatment additives may be substantial and this aspect should be investigated during their selection.
In other experiments it was established that the cultures of sulfate-reducing bacteria isolated from the waters around several oil fields have a greater capacity to form H2S than the standard collection culture. The stimulating effect of a given chemical product can vary considerably, depending on the species, activity, and adaptation of bacteria to the chemical in question.
Cultures of sulfate-reducing bacteria acquire relative resistance to toxic compounds, as a result of adaptation, which require higher doses of bactericide than those calculated for laboratory collection cultures to suppress the vital activity of sulfate-reducing bacteria in the bottom hole zone and reservoir (Kriel et al. 1993).
It has been shown that sulfidogenic bacteria injected into a reservoir with floodwater may survive high temperatures in the formation and can be recovered from producing well fluids (Salanitro et al. 1993). These organisms may colonize cooler zones and sustain growth by degrading fatty acids in the formation waters.
Mathematical Models
A mathematical model for reservoir souring, as caused by the growth of sulfate-reducing bacteria, is available. The model is a oneworddash dimensional numerical transport model based on conservation equations, and includes bacterial growth rates and the effect of nutrients, water mixing, transport, and adsorption of H2S in the reservoir formation. The adsorption of H2S by the rock has been considered. Two basic concepts for microbial H2S production were tested with field data (Sunde et al. 1993):
• H2S production in the mixing zone between formation water and injection water (mixing zone model), and
• H2S production caused by the growth of sulfate-reducing bacteria in a biofilm in the reservoir rock close to the injection well (biofilm model).
Field data obtained from three oil producing wells on the Gullfaks field correlated with H2S production profiles obtained using the biofilm model but could not be explained by the mixing zone model (Sunde et al. 1993).
Model of Colony Growth
The growth of bacteria with time in the presence of various amounts of copper sulfate is shown in Figure 5.1. The diameter of the colonies was used as an indicator of growth.
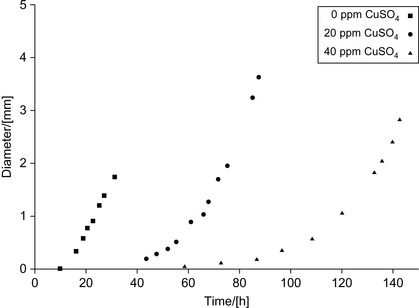 |
| Figure 5.1 Effect of copper sulfate on the growth of S. marcescens colonies (Rodin et al., 2005). |
A simplified model of colony growth has been presented (Rodin et al., 2005). According to this model, during growth, a colony passes successively through exponential and linear phases of growth, with the exponential phase persisting unless the concentration of nutritious substrates becomes limited. The increase of colony diameter d during the exponential phase can be described as:
(5.1)
In contrast, the linear phase of growth occurs under conditions of limited nutrients; beginning at a time tl, after which the colony diameter increases at a constant rate kd according to:
(5.3)
Detection of Bacteria
In oil field systems, the detection of living bacteria is necessary to evaluate the potential for microbially influenced corrosion, biogenic souring, and to evaluate the effectiveness of biocide treatment programs (Cowan 2005). If methods of measurement of the bacteria present is insufficient then the dosages of biocide will be too low or too high.
Uncontrolled growth and activity of sulfate-reducing bacteria can create safety, environmental, and operational problems, such as microbially influenced corrosion, solids production, and biogenic hydrogen sulfide generation. Rapid enumeration of living bacteria would allow quick biocide treatments, so optimizing bacterial control and minimizing, the environmental impact of the chemical treatment.
Microbiologically influenced souring (MIS) is the production of H2S through the metabolic activities of microorganisms. This problem is easier to control by using biocides if the problem is detected early in the souring process (Morris et al. 1994). However, if allowed to spread into the subsurface regions that are less accessible to biocides, i.e., profuse-stage MIS, the problem becomes more difficult to mitigate by conventional means.
API Serial Dilution Method
This is the most widely used method for the detection of microorganisms. Field test methods for estimating bacterial populations have been standardized, and a standard method dealing with the dose-response (time-kill) testing for evaluating biocides has been established. Effective sampling is essential to any successful analysis.
Enzymatic Assay
The enzymatic (luciferase) assay for adenosine triphosphate (ATP) is one method applied to biocidal control in oil production (Prasad 1988). Measuring the bioluminescence produced by the luciferin luciferase system is known to be a reliable method for the determination of adenosine triphosphate (ATP).
Electrochemical Determination
An electrochemical method has been developed to allow on-line monitoring of biofilm activity in aqueous environments.
Colorimetry
Laboratory data concerning the persistence of biocides formulated in glutaraldehyde and acrolein are available (Morris and Pope 1994). A colorimetric, general aldehyde detection method, based on m -phenylenediamine, was used. Such studies follow the demand for a better understanding of ecological systems for environmental protection.
In another study, a mathematical model was constructed, incorporating experimentally determined glutaraldehyde persistence, rates of water production, and other factors. The model was used to calculate levels of glutaraldehyde in a specified environment (lagoons) as a function of time, based on the amount of glutaraldehyde applied downhole (Derr et al. 1994).
Most Probable Number Technique
The traditional method for bacterial enumeration is the most probable number technique (Barton and Hamilton 2007; Oblinger and Koburger 1975; Postgate 1979). Serial dilution into bacterial culture media is the most common method that is used to enumerate viable oil field bacteria, but this method takes up to 4 weeks to obtain results for slow growing sulfate-reducing bacteria.
Direct microscopy is an alternative, faster method, but it does not differentiate between living and dead bacteria. A method for the rapid enumeration of living sulfate-reducing bacteria has been developed, based on the rehydration of dried nutrients with system water. This method gives results in 1–7 days (Cowan 2005).
DNA Sequencing
Bacterial enumeration and identification in diesel and naphtha pipelines located in northwest and southwest India has been reported. Traditional cultivation techniques and 16S rDNA gene sequencing was used, the latter using a Genetic Analyzer from PE Applied Biosystems.
The study included the phylogenetic analysis of 16S rRNA sequences of the isolated species. The sequences obtained were analyzed with BLAST search and 11 bacterial species were identified, as summarized in Table 5.1.
| Species |
|---|
| Serratia marcescens ACE2 |
| Bacillus subtilis AR12 |
| Bacillus cereus ACE4 |
| Pseudomonas aeruginosa AI1 |
| Klebsiella oxytoca ACP |
| Pseudomonas stutzeri AP2 |
| Bacillus litoralis AN1 |
| Bacillus sp. |
| Bacillus pumilus AR2 |
| Bacillus carboniphilus AR3 |
| Bacillus megaterium AR4 |
Sulfate-reducing bacteria were not detected in the samples. The dominant species were Bacillus cereus and Serratia marcescens.
It has been concluded that several types of bacteria may be involved in biocorrosion from natural biofilms in pipelines. Further localized pitting was observed by analysis with scanning electron microscopy (Rajasekar et al., 2010).
Sulfate-reducing Bacteria
Sulfate-reducing bacteria are chemolithotrophic bacteria (Barton and Fauque, 2009), of which 220 species in 60 genera are known. All use sulfate as a terminal electron acceptor. This makes them a unique physiological group of microorganisms, which couple anaerobic electron transport to ATP synthesis.
These bacteria can use a wide variety of compounds as electron donors, including proteins with metal groups that can be oxidized or reduced. In particular, they act on soluble electron transfer proteins and via transmembrane redox complexes. Their ability to utilize hydrocarbons offers the possibility to use them for the bioremediation of soils, which are contaminated with aromatic hydrocarbons.
Some strains of sulfate-reducing bacteria can even reduce chlorinated compounds, e.g., 3-chlorobenzoate, chloroethenes, and nitroaromatic compounds. Sulfate-reducing bacteria can also reduce some heavy metals, hence, several procedures have been proposed for using these strains in the bioremediation of materials contaminated with toxic metals.
High levels of hydrogen sulfide are produced by the metabolism, which contributes to the souring of the oil fields of these organisms, and the corrosion of casings and concrete (Barton and Fauque, 2009).
New strains of sulfate-reducing bacteria are being discovered all the time (Agrawal et al. 2010; Miranda-Tello et al. 2003; Youssef et al. 2009). For example in 2003, a new spirilloid sulfate-reducing bacterium, designated strain MET2T, was isolated from a Mexican oil field separator (Miranda-Tello et al., 2003).
Issues in the Oil field
Mesophilic and thermophilic sulfate-reducing bacteria are common inhabitants of oil field facilities. They may penetrate into oil reservoirs with the injection water, and so contaminate the well. Their sulfide production and hydrogen oxidation are responsible for serious and costly biocorrosion problems in the oil industry (Sarioglu et al., 1997). They are most often controlled in situ by biocides (Barton and Hamilton 2007; Hamilton 1983; Miranda-Tello et al. 2003; Postgate 1979).
The effect of temperature and pressure on a strain of sulfate-reducing bacteria isolated from an oil reservoir in Alaska has been investigated (Cheung et al., 1994). The highest bacterial growth rate was found at 37∘C at 100 atm. The temperature has a greater influence on the bacterial proliferation than the pressure.
The effect of various concentrations of biocides, i.e., isothiazolone and formaldehyde, has been tested. Both biocides are similarly effective, but formaldehyde is more effective at high pressures (Cheung et al., 1994).
Bacterial Corrosion
Bacterial corrosion is often referred to as microbiologically influenced corrosion. The metabolic products of microorganisms appear to affect most engineering materials, but the more commonly used corrosion-resistant alloys, such as stainless steel, seem to be particularly susceptible.
Its importance has been underestimated because most occurs as a localized, pitting-type attack. In general, it results in relatively low rates of weight loss, changes in electrical resistance, and changes in total area affected. This makes it difficult to detect and to quantify using traditional methods of corrosion monitoring (Pope et al. 1992).
To adequately address microbiologically influenced corrosion problems, interdisciplinary cooperation of specialists in microbiology, metallurgy, corrosion, and water chemistry is required; a single technique cannot provide all the answers in terms of corrosion mechanisms.
The problem of and importance of microbiologically influenced corrosion was not fully realized until recently. Even in the mid-1980s the statement was made that
The major problem encountered by the petroleum microbiologist working in the North Sea oil fields is that of convincing the oil field engineer that bacterial corrosion is a subject worthy of serious attention. (Maxwell 1986)
A reference guide on recognizing, evaluating, and alleviating corrosion problems caused by microorganisms has been compiled (Anonymous 1990). This manual provides a guide, training manual, and reference source for field and engineering personnel that deal with corrosion problems caused by microorganisms. Trends seen in the 1990s for dealing with microbiologically influenced corrosion have been reviewed in the literature (Farquhar 1990). The basic goal of a practicing corrosion engineer should be not to identify, count, or even kill the microorganisms, but to effectively control corrosion in an oil field.
Mechanisms of Microbial Corrosion
The role of microorganisms can be visualized directly in microbially induced corrosion in an electrochemical cell. Alternatively, the role can be indirect, in that it maintains a preexisting electrochemical cell by stimulating either the cathodic or the anodic reaction (Hamilton 1986).
Various microorganisms and mechanisms are thought to be involved, but most commonly, a differential aeration cell is built where concentrations of oxygen are low shielded beneath slime or colony growth, as compared with the high concentration externally in the bulk environment.
Under these conditions, the surface of the metal in the low concentration area becomes an anode due to the dissolution of metal, while the electrons react at the cathodic region with the high concentration of oxygen, giving rise to hydroxidious. Ultimately, metal oxides and hydroxides are characteristic for aerobic corrosion. Microbes influence the corrosion rate by the following mechanisms (Pope et al. 1990):
1. Cathodic depolarization,
2. Formation of occluded area on metal surface,
3. Fixing the anodic sites, and
4. Underdeposit acid attack.
Simultaneous Mechanisms of Corrosion
Microbiologically influenced corrosion almost always acts in concert with other corrosion mechanisms and may, at times, appear to be crevice corrosion, underdeposit acid attack, oxygen concentration cell corrosion, ion concentration cell corrosion, and CO2 corrosion (Pope 1997).
If microbiologically influenced corrosion is found on external surfaces, it is usually associated with disbonded coatings or other areas that are shielded from the potentially protective action of cathodic protection. Furthermore, pipelines are often in contact with wet clays, which have little scaling potential.
pH Regulation
Bacterial metabolism produces weak acids. Sulfate-reducing bacteria regulate the pH of their environment at levels that depend on potential secondary reactions, which are:
• Precipitation of iron sulfide,
• Oxidation of sulfide ions to thiosulfate by traces of oxygen, and
• Metabolism of this thiosulfate or of other sulfur compounds.
Biocide Enhancers
In order to effectively treat water against bacterial contamination, a fast-acting biocide is needed. This may be even more important for on-the-fly treatments, where biocides have a very short contact time with the water before other treating chemicals are added and the fluids are pumped downhole. In some instances it is believed to be helpful to include a biocide enhancer, to aid the biocide treatment or work synergistically with the biocide in order to kill the bacteria rapidly (Bryant et al. 2009).
Quaternary surfactants may act as biocide enhancers, for example, 19N™ is a cationic surfactant that also is a biocide enhancer. When used in combination with biocides such as sodium hypochlorite or glutaraldehyde, bacterial problems may sometimes be treated in times as short as 5 min.
Some quaternary surfactants may, however be fundamentally incompatible with anionic friction reducers, which are also used in subterranean operations. It is believed that this incompatibility may arise from charges present on both molecules that may cause the two to react and eventually form a precipitate. Some biocides, such as oxidizers, may also degrade certain friction reducers (Bryant et al. 2009).
Corrosion Monitoring
A critical review of the literature of monitoring techniques for microbiologically influenced corrosion has been presented (Borenstein and Licina 1994). The monitoring techniques in this review include measurements of electrochemical properties, measurements of physical metal loss, and enumeration of sessile organisms. The procedures for the study of microbiologically influenced corrosion, as well as the advantages and the disadvantages of each technique, are discussed.
Microbiologically influenced corrosion can be misdiagnosed as attack caused by conventional chloride crevice, or as pitting corrosion unless specialized techniques are used during the failure analysis (Borenstein and Lindsay 1994). These techniques include in situ sampling of residual water, bacterial analysis of corrosion products using analytical chemistry, culture growth, and scanning electron microscopy, as well as nondestructive examination using ultrasonic and radiographic techniques. Metallographic examination can reveal microbiologically influenced corrosion characteristics, such as dendritic corrosion attack in weld metal.
Bacterial Hydrogenase
Theoretical and experimental studies have shown that the removal of molecular hydrogen from cathodic surfaces is a primary driving force in microbiologically influenced corrosion. A rapid (1–4 h ) test has been developed for the presence of bacterial hydrogenase that detects the presence of a wide range of corrosion-causing bacteria in water, sludge, and adherent bacterial biofilms (Boivin et al. 1989).
This test can be used to monitor oil and gas systems for the development of potentially corrosive bacterial populations, and to assess the efficacy of control measures, including biocide treatment, because the hydrogenase test yields negative results when this pivotal, corrosion-causing enzyme has been denatured.
Lipid Biomarkers
Microbes of differing physiological types, acting in consortia, appear to be more destructive than monocultures. Methods for examining consortia are based on the detection of lipid biomarkers that are characteristic for different classes of microbes. These can be analyzed by gas chromatography coupled with mass spectrometry (Dowling et al. 1986).
Electron Microscopy
Side stream sampling devices can be used to collect biofilm and corrosion samples. The biofilm, inorganic passive layers, and metal attacked samples can be characterized with scanning electron microscopy and energy dispersive X-ray analysis. Results of one such study showed a correlation between biofouling and corrosion attack of carbon steel samples (Videla et al. 1991).
Electrochemical Impedance Spectroscopy
Electrochemical impedance, weight loss, and potentiodyne techniques can be used to determine the corrosion rates of carbon steel, and the activities of both sulfate-reducing and acid-producing bacteria in water injection field tests. One such study revealed that the corrosion rates as determined by the potentiodyne technique did not correlate with the bacterial activity, but those obtained by electrochemical impedance spectroscopy were comparable with the rates obtained by weight loss measurements (Elboujdaini and Sastri 1995).
Other electrochemical techniques that have been used include the measurements of the corrosion potential, redox potential, polarization resistance, electrochemical impedance, electrochemical noise, and polarization curves, including pitting scans. A critical review of the literature concerned with the application of electrochemical techniques in the study of microbiologically influenced corrosion is available (Mansfeld and Little 1990).
Assessment of the Activity of Biocides
Quantitative methods that use indirect parameters of the growth of cells have been developed. Initially, only a few rapid techniques were available. Although some disk diffusion techniques have been described that generated results within 4–6 h, most techniques required an incubation time of 18–24 h before a result was available (Wheat 2001).
One of the more rapid methods is based on impedance microbiology (Zhou and King, 1995). It uses a double-layer API agar medium, together with sodium thioglycolate as reducing agent. In comparison to the conventional API procedure (API 1975), which requires 28 days, this technique takes only 1 day to obtain test results.
Another rapid method for estimating the biocide potential of various chemicals toward certain microbes has been developed, based on the redox potential of live microbial cells. A water-soluble organic redox indicator, blue in the oxidized form and pink in the reduced form, was used as an indicator of the reducing potential of microbial cells (Novikov et al., 2001).
Turbidimetry can be also used to assess the growth of bacteria (Piddock 1990). The microcalorimetric measurement of microbial activity of biofilm samples allows easy testing of the efficacy of biocides (von Rège and Sand, 1998). Experiments with biofilm samples consisting of sulfate-reducing bacteria and chemo-organotrophic bacteria have been performed. Further, biofilms were produced in continuous culture on the surface of a flow-through gold tubing in the measuring cylinder of a calorimeter.
In separate experiments, the biofilm samples were treated with biocides, including formaldehyde, tetramethylammonium hydroxide, 1,8-dihydroxyanthraquinone, and glutaraldehyde at varying concentrations and incubation times. The heat produced in a typical experiment is illustrated in Figure 5.2.
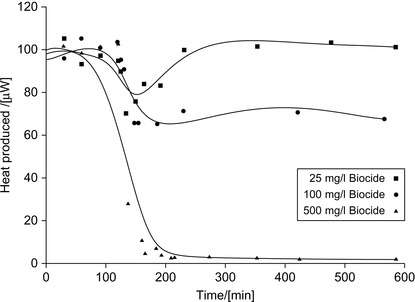 |
| Figure 5.2 Heat production by bacteria, biocide added at 2 h (von Rège and Sand, 1998). |
Synergistic Action of Biocides
The synergistic effects of biocides can be evaluated. A bacillus together with dehydrated nutrients and a growth indicating dye is put on a plastic strip, and strip then dipped into a fluid of interest. The medium is thereby rehydrated and the spores are activated. Incubation for 24 h should yield a visible growth in the absence of biocides, but in the presence of biocides, growth is inhibited.
The concentration of the biocide in the fluid can be adjusted by making a range of dilutions, which enables one to evaluate which concentration of biocide just inhibits bacterial growth. If more than one biocide is present, it is possible to distinguish between additive effects, antagonism, and enhancement. In many cases, it is possible to determine exact dosing of large systems from the first trial (Hill et al., 1989).
The method has been used to determine the synergistic effects of copper sulfate and kathon, (2-N -octyl-4-isothiazolin-3-one ) on S. marcescens (Rodin et al., 2005). Amino alcohols are not biocides themselves, but they enhance the performance of a wide range of biocides, which are used in water-based fluids (Coburn et al. 2010).
Treatments with Biocides
Previously Fractured Formations
A particular problem is the refracturing of a previously fractured formation that is contaminated with bacteria. In such a case, the fracturing fluid must be mixed with an amount of biocide that is sufficient to reach and to kill the bacteria contained in the formation. The refracturing of the formation causes the bactericide to be distributed throughout the formation and to contact and kill bacteria contained therein (McCabe et al. 1991).
Intermittent Addition of Biocide
The intermittent addition technique consists of Hegarty and Levy (1996):
• The addition of a slug dose of a biologically effective amount of a quick-kill biocide.
• Further, intermittent addition of biologically effective amounts of a control biocide.
• This means that the control biocide is dosed for a certain period of time, followed by a period of much lower or zero dosing. This cycle is repeated throughout the treatment.
This process reduces the amount of control biocide employed in the control of contamination of oil production system waters by sessile bacteria. The biocide may be applied at intervals of 2–15 d. The duration of biocide application is preferably 4–8 h(Moody and Montgomerie 1996).
Nonbiocidal Control
Chemical treatments for bacteria control represent a significant cost and environmental liability. Because the regulatory pressure on the use of toxic biocides is increasing, more environmentally acceptable control measures are being developed.
Biocompetitive Exclusion Technology
Besides adding biocides to wells, modifying the reservoir ecology also appears to be a promising approach to bacterial control. The production of sulfide can be decreased, and its concentration is reduced by the establishment and growth of an indigenous microbial population that replaces the population of sulfate-reducing bacteria.
Low concentrations of a water-soluble nutrient solution are added, which selectively stimulate the growth of an indigenous microbial population, thereby inhibiting the detrimental, sulfate-reducing bacteria population that generate H2S. This deliberate and controlled modification of the microflora and reservoir ecology has been termed biocompetitive exclusion(Hitzman and Dennis 1997; Sandbeck and Hitzman 1995).
Inhibitors for Bacterial Films
Laboratory tests with quaternary amine additives showed a very low surface colonization and lower corrosion rates (Enzien et al. 1996). On the other hand, the biocidal effect of quaternary amines in the test fluids appeared to be minimal. These results suggest that quaternary amines may prevent microbiologically influenced corrosion by mechanisms other than killing bacteria and that treatments preventing colonization on the surface may persist longer than most biocides.
Periodic Change in Ionic Strengths
For effective control of microorganisms, it is necessary to take into account the mechanism of formation of bacteria and the ecologic factors affecting it. The process of vital activity of bacteria begins with their adsorption onto the enclosing rocks and their adaptation to the new habitat conditions. Pure cultures of sulfate-reducing bacteria are not active in crude oil.
Population development in oil reservoirs depends entirely on hydrocarbon-oxidizing bacteria, which are the primary cause of oil breakdown. If the ecological conditions in the reservoir change during formation of the microorganisms, the established food chains are disrupted and the active development of microflora ceases. It was experimentally established periodically injecting waters markedly differing in mineralization, taking into account the ecologic characteristics of the formation of the microorganisms, makes it possible to control the biogenic processes in an oil reservoir without disturbing the surrounding environment (Blagov et al. 1990).
Biocides
Various biocides have been used successfully in water treatment applications for many years. These include oxidizers, such as chlorine and bromine products, and non-oxidizing biocides, including isothiazolones, quats, organobromines, and glutaraldehyde.
Biocides are often misapplied in the petroleum industry, particularly if the characteristics of the biocides are not considered before use. Some guidelines for biocide selection are outlined in a review in the literature (Boivin 1994). Early detection of microbiologic problems is imperative, and reparative actions must be taken as soon as possible.
Remedial measures should include changes in operating methods to prevent degradation of the operating environment. This might include the rejection of untreated waters for cleaning deposits in vessels and lines. In general, biocides are needed to control the activity of the bacteria in a system, but biocides alone usually will not solve a microbiologic problem.
Five requirements for bactericide selection are emphasized (Zhou 1990):
1. Wide bacteria-killing ability and range,
2. Non-corrosive properties, good inhibiting ability, and convenience of transportation and application,
4. Good miscibility, with no damage or interference to the drilling fluid or its chemical agents, and
5. Bacteria killing effect that is not affected by environmental adaptation of the bacteria.
Various Biocides
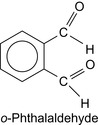
In Table 5.2 some biocides proposed for bacteria control are listed. Other aldehydes and hydroxy compounds are summarized in Table 5.3 and o -phthalaldehyde is shown in Figure 5.3.
| a)Drilling lubricant | |
| b)5–50ppm | |
| c)25–75ppm | |
| d)Waste from the production of 1-naphthol-3,6-disulfonic acid | |
| Biocide | References |
|---|---|
| Zinc slurryd | Trushevskaya et al. (1992) |
| Formaldehyde, Glutaraldehyde | Kriel et al. (1993) |
| Nitrateb | Sears et al. (1996) |
| Monochloroamine | Boivin et al. (1992) |
| 3-Diazaspiro(4,5)decane | Austin (1987) |
| o -Phthalaldehyde | Theis and Leder (1992) |
| 2-Bromo-4-hydroxyacetophenone | Oppong and King (1995) |
| Methyl tetrahydrophthalic acidc | Khanlarova et al. (1993) |
| Diammonium salts of tetrahydrophthalic acid | Khanlarova et al. (1993) |
| 2,6-Dimethyl-m -dioxan-4-ol acetate | Smith et al. (2008) |
| Bis[tetrakis(hydroxymethyl)phosphonium] sulfate | Macleod et al. (1995) |
| Thiocyanomethylthio-benzothiazolea | Oppong and Hollis (1995) |
| 1-(2-Hydroxyethyl)-2-methyl-5-nitroimidazole = (metronidazole) | Littmann and McLean (1987) |
| Di-(tri-N -butyl)-(1,4-benzodioxan-6,7-dimethyl) diammonium dichloride | Muganlinskij et al. (1995) |
| Dimethyl-tetrahydro-thiadiazine-thione | Karaseva et al. (1995) |
| Biocide | References |
|---|---|
| Glutaraldehyde | Cash et al. (1992), Eagar et al. (1988) |
| Pentanedial | Lamarre and Martin (1990) |
| 4,4-dimethyl-2-oxazolidinone, glycouril | Sweeny (1996) |
| Anthraquinones | Weimer et al. (1995), Burger et al. (2001) |
| Phenoxyethanol | Smith et al. (2008) |
| Tetrakis-(hydroxymethyl)-phosphonium salts | Bryan et al. (1990), Veale et al. (1990) |
| o -Phenylphenol | Smith et al. (2008) |
| p-Chloro-m -cresol | Smith et al. (2008) |
Formaldehyde
Coreflood experiments were used to evaluate the efficacy of periodic formaldehyde injection for the control of in situ biogenic reservoir souring. Formaldehyde treatments were demonstrated to control souring in both environments; if the formaldehyde can be transported through the reservoir, in situ biogenic souring should be mitigated.
Glutaraldehyde
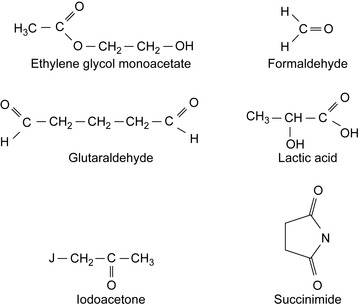
Glutaraldehyde is a useful antimicrobial agent, but it is dangerous and unpleasant to handle, and is thermally unstable. Despite these disadvantages, it is specified for use against bacteria in cooling towers of air-conditioning systems in buildings and to control anaerobic sulfate-reducing bacteria in oil wells. Some aldehydes and related compounds are shown in Figure 5.4.
Bisulfite Adduct
A bisulfite addition complex of an aldehyde or dialdehyde has been proposed for use as an antimicrobial agent (Wrench, 1990 and Wrench, 1991). The complex is less toxic than free glutaraldehyde. In oil wells, its digestion by the sulfate-reducing bacteria releases the free dialdehyde, which in turn controls the bacteria. In this way, a more economic and environmentally safer use of antimicrobial additives is possible.
Combined Chlorine-Aldehyde Treatment
A combined chlorine-aldehyde treatment that has two stages, chlorination and subsequent biocide application, has been suggested. Short-residence–time shock doses of glutaraldehyde have been applied after chlorination (Maxwell et al. 1986). It has been established that a primary chlorination is useful in overall bacterial control.
Green Biocide Enhancer
It is known that ethylene diamine tetraacetic acid (EDTA) is a synergist for biocides (Raad and Sherertz 2001), but it is only slowly biodegradable, which is a drawback for environmental reasons. For this reason, it has been recommended to replace EDTA with green chelating agents in various industrial applications (Munn et al., 2004).
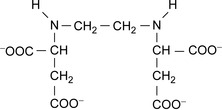
Ethylene diamine disuccinate is a biodegradable chelating agent. Its structure is shown in Figure 5.5 where it can be seen to contain two chiral carbon atoms (the CH ), and has three stereoisomers ([R,R], [R,S]/[S,R], and [S,S]). The [S,S]-isomer is rapidly and completely mineralized, in contrast to the other isomers. Thus, the stereospecificity greatly influences biodegradation and metabolite formation (Schowanek et al., 1997).
This chemical has been found to enhance the efficacy of glutaraldehyde in the treatment of sulfate-reducing bacteria. It has a similar chelation ability to EDTA, but produces no persistent metabolites during biodegradation (Schowanek et al., 1997).
It has been demonstrated that the dosage of glutaraldehyde can be considerably reduced by the addition of ethylene diamine disuccinate to inhibit the growth of sulfate-reducing bacteria (Wen et al., 2009).
Glutaraldehyde is hazardous to handle and causes environmental concerns, and it can also deleteriously affect the fluid viscosity of the well treatment fluid at elevated temperatures. This can be problematic in fracturing applications, since higher fluid viscosity downhole could hinder flowback. In addition, glutaraldehyde has been shown to negatively impact the behavior of oxygen scavengers (Starkey et al. 2008).
Chloromethyl methylisothiazolone compounds are biocides with a broad spectrum versus bacteria, algae, and fungi that have been used successfully for microbial control and preventing biofouling in industrial water treatment (Williams, 2007a and Williams, 2007b). The most frequently used product is a mixture of 5-chloro-2-methyl-4-isothiazolin-3-one and 2-methyl-4-isothiazolin-3-one in a ratio of 3:1 at a final concentration of 1.5%. 1,2-Benzisothiazolin-3-one (BIT) products have also been used in a limited range of industrial applications, which require long-term preservation for bacterial control.
Understanding their mechanism of action is important for optimizing their use, and combating resistance if encountered. Isothiazolones utilize a two-step mechanism (Williams 2006):
1. Rapid inhibition of growth and metabolism within minutes and
2. Irreversible cell damage within hours resulting in the loss of viability.
The cells are inhibited by disruption of metabolic pathways involving dehydrogenase enzymes. This means that critical physiological functions are rapidly inhibited, including growth, respiration, and ATP synthesis. The death of the cells results from the destruction of protein thiols and the production of free radicals.
The rate of action and effectiveness may be enhanced by various additives, including the use of surfactants. A technology based on microemulsions has been introduced, using 4,5-dichloro-2-n -octyl-4-isothiazolin-3-one as an algicide.
Oxidizing biocides play a key role in the control of microbial populations and biofouling in industrial cooling water systems. Bromochlorodimethylhydantoin is an oxidizing biocide (Kramer 2007), which has been evaluated in several excellent field efficacy studies. These studies reveal that its continuous application releases 1–2 ppm of free chlorine, and is effective in reducing the concentration of Legionella pneumophila to undetectable levels in recirculating water.
Bromochlorodimethylhydantoin has also been shown to be effective against a mixed bacterial biofilm under laboratory conditions. Studies in dynamic laboratory systems that had been inoculated with a natural microbial flora revealed that it was equally effective against planktonic and biofilm populations of L. pneumophila. The literature on these issues has been reviewed (Kramer 2007).
Benzotriazole and tolyl triazole are corrosion inhibitors for yellow metals. There is a controversy concerning the interaction of azoles with halogenated biocides; it is suspected that their presence may cause halogenated biocides to degrade (Ward and Glaser, 2007). Some researchers claim that the inhibitor is rendered ineffective due to this degradation, others agree that there is an interaction, but state that the products of degradation are still capable of protecting the metal. Laboratory studies have been presented on these open questions, in which it has been shown that azoles are not significantly affected by high concentrations of halogenated biocides bromine, in particular. In fact, azoles still perform well when residual inhibitor is present, even at extremely high dosages of free bromine.
However, azoles have other drawbacks. The triazole moiety is active enough to absorb to the metal, producing a protective film on the copper surface. Even when a protective film is formed, a certain residual level of azole needs to be maintained in the aqueous solution. If this is removed, the protective film starts to break down, causing an almost instantaneous increase in corrosion rates. In such cases, the presence of halogenated biocides only accelerates the rates of corrosion. Protective films formed by tolyl triazole have been found to be more resistant to breakdown in aqueous environments where the methyl group of the tolyl triazole moiety causes a steric hinderance.
2-Propenal (acrolein) is known as biocide, which is commercially available for several applications in the oil and gas industry. However, it has not been widely used on offshore oil production platforms due to safety concerns. However, recent advances allow it a lower level of risk than conventional biocides (Gregg et al., 2006). A case study assessed the risks of using 2-propenal vs. other biocides. 2-Propenal is related to conventional biocides that are applied on an offshore oil production platform with respect to its efficacy, injection hardware requirements, and associated risk elements.
The sea water as well as the sea water injection system was batch treated weekly with tetrakis hydroxyl methyl phosphonium sulfate, prior to sea water breakthrough, and glutaraldehyde, and after sea water breakthrough with 2-propenal, in order to control the biological activity. In later treatments, the biocide batch was supplemented with anthroquinone treatments to prolong the time between the application of glutaraldehyde and 2-propenal, respectively, thereby reducing the costs of treatment. In summary the results of this study looked promising.
The assumed biocidal mechanism of 2-propenal is the attack of sulfhydryl and amine groups on bacterial proteins (Penkala et al., 2004). The reactivity with sulfides renders acrolein effective as an H2S scavenger and an iron sulfide dissolver. These compounds are byproducts of the metabolism of sulfate-reducing bacteria. A number of case histories have been reviewed on the performance of 2-propenal as biocide, and the results of laboratory studies comparing the efficacy of 2-propenal with other biocides have been compiled.
2-Propenal shows superior performance as biocide against general aerobic and facultative anaerobic bacteria, as well against sulfate-reducing bacteria. It is soluble in oil and penetrates biofilms, hence it is a versatile and effective biocide for use against persistent sessile populations of bacteria. Due to its low minimum inhibitory concentration, 2-propenal is used in batch applications and also in continuous treatment programs (Penkala et al., 2004).
Quaternary Ammonium-based Biocides
Quaternary ammonium-based biocides such as alkyl dimethyl benzyl ammonium chloride and dialkyl dimethyl ammonium chloride compounds have been used for microbiological control in industrial water systems for a long time. They perform well against algae, but they can cause problems with foaming, or can react with anionic additives in an undesired way (Kramer 2006). In the 1980s, bis[tetrakis(hydroxymethyl)phosphonium] sulfate came on the market. This type of biocide overcame some of these issues: they lack surface activity and are not compatible with halogens.
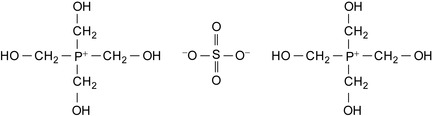
On the other hand, tributyl tetradecyl phosphonium chloride is unique in that it combines a quaternary phosphonium group with the long alkyl chain moiety of the quaternary ammonium biocides in the same molecule, making it effective at low concentrations. It is also fast acting and effective against a variety of microorganisms, including L. pneumophila (Kramer 2006). Its excellent surface activity makes tributyl tetradecyl phosphonium chloride highly effective for removing biofouling. It remains effective in combination with halogens, and it exhibits low foaming and is compatible with anionic scale and corrosion inhibitors. The structure of bis[tetrakis(hydroxmethyl)phosphonium] sulfate is shown in Figure 5.6.
Tetrakis-hydroxymethyl phosphonium salts have acceptable environmental profiles (Lloyd and Neail 1993), and they are regarded as a preferred product for bacterial control within the oil production industry.
Technical developments with respect to its use in oil production applications have been reviewed (Jones et al., 2006). Bis[tetrakis(hydroxymethyl)phosphonium] sulfate was initially applied as an industrial biocide in cooling systems, but it has been used in petroleum production since 1987. In fact, it became the leading biocide where sulfate-reducing bacteria cause problems. The product acts very fast and is effective for the control of freeswimming bacteria.
It is highly effective in downhole applications for controlling biogenic hydrogen sulfide. Treatment results in the dissolution of iron sulfide, which in turn increases oil production.
It has been found that bacteria protect themselves from the bis[tetrakis (hydroxymethyl)phosphonium] sulfate in water by producing a comparatively hydrophobic layer of polysaccharides.
In contrast to conventional quaternary biocides, bis[tetrakis(hydroxymethyl) phosphonium] sulfate does not bear long hydrophobic moieties, so the molecule is not surface active.
A variety of advanced formulations have been developed. The effectiveness of bis[tetrakis(hydroxymethyl)phosphonium] sulfate can be increased by formulating it with surfactants, such as benzalkonium chlorides (Jones et al., 2006).
Thiones for Treatment Fluids
Polymeric additives used in well treatment fluids may encounter an environment conducive to bacterial growth and oxidative degradation. If bacteria grow on these polymers, the physical characteristics of the fluids can be materially altered. For example, bacterial action can degrade the polymer, leading to loss of viscosity, so making the fluid ineffective (Starkey et al. 2008).
Fluids containing polysaccharide and synthetic polymers, such as polyacrylamides (PAM), polyglycosans, and carboxyalkyl ethers are especially susceptible to bacterial degradation. These polymers are also susceptible to oxidative degradation in the presence of free oxygen. This degradation can be directly caused by free oxygen or can be mediated by aerobic microorganisms. This means that biocides and oxygen scavengers are frequently added to well treatment fluids to control bacterial growth and oxygen degradation.
The biocide should be selected to have minimal interaction with any of the components in the well stimulation fluid. It should not affect fluid viscosity to any significant extent and should not affect the performance of oxygen scavengers contained within the fluid.
Traditionally, either glutaraldehyde or bis[tetrakis(hydroxymethyl)phosphonium] sulfate is used to control bacterial contamination in well stimulation fluids but more recently, 2,5-dimethyl-1,3,5-thiadiazinane-2-thione has been proposed as an alternative as it is less environmentally harmful (Starkey et al. 2008). Oxygen scavengers are generally chosen from bisulfite salts. Other sulfur containing compounds are summarized in Table 5.4.
| Biocide | References |
|---|---|
| n -Butyl benzisothiazolinone | Smith et al. (2008) |
| 1,2-Benzoisothiazolin-3-one | Smith et al. (2008), Morpeth and Greenhalgh (1990) |
| 2-N -Octyl-4-isothiazolin-3-one | Hsu (1995) |
| 2-Methyl-4-isothiazolin-3-one | Hsu (1994), Smith et al. (2008) |
| 3-Acetoxy-4-methylthiazol-2(3H)-thione | Austin (1987) |
| 3-Hydroxy-4-methylthiazol-2(3H)-thione | Austin (1987) |
| 3-Hydroxy-4-phenylthiazol-2(3H)-thione | Austin (1987) |
| Isothiazolin-3-one | Lein (1989), Mattox (1989), Gironda et al. (1995) |
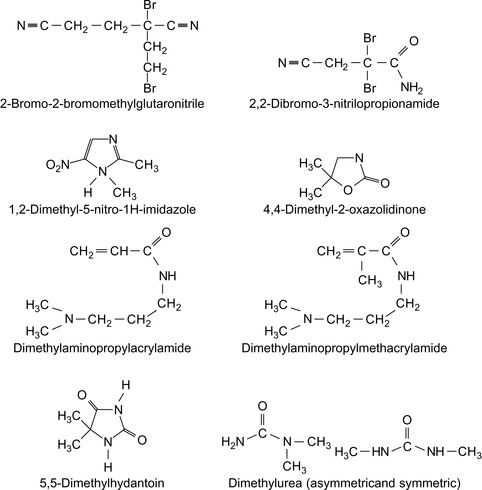
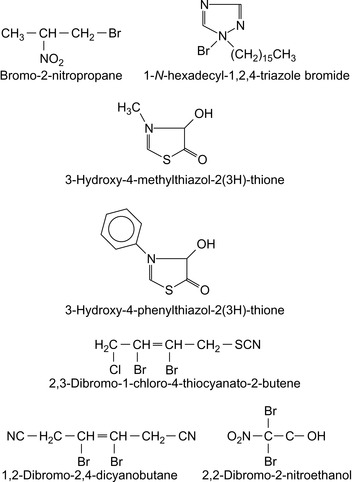
Halogen Compounds
Halogen containing compounds are summarized in Table 5.5.
| Biocide | References |
|---|---|
| Chlorine dioxide | Clark and Langley (1990) |
| Sodium chlorite | Mason (1990) |
| N,N -Dimethyl-N′-phenyl-N′-fluoro- dichloromethylthiosulfamidesulfamide | Downey et al. (1995) |
| 1-(3-chloroallyl)-3,5,7-triaza-1-azoniaadamantane chloride | Smith et al. (2008) |
| 2,3-Dibromo-1-chloro-4-thiocyanato-2-butene | Austin (1989) |
| 3-(3,4-dichlorophenyl)-1,1-dimethylurea | Morpeth and Greenhalgh (1990) |
| 4,5-Dichloro-2-N -octyl-isothiazolin-3-one | Downey et al. (1995) |
| 5-Chloro-2-methyl-4-isothiazolin-3-one | Hsu (1994), Smith et al. (2008) |
| Chlorothalonil | Smith et al. (2008) |
| Dichloro-octylisothiazolinone | Smith et al. (2008) |
| Tributyl tetradecyl phosphonium chloride | Lamarre and Martin (1990) |
| Dibromo-octylisothiazolinone | Smith et al. (2008) |
| 1,2-Dibromo-2,4-dicyanobutane | Hsu (1995) |
| 1-N -Hexadecyl-1,2,4-triazole bromide | Demikhov et al. (1992) |
| 2,2-Dibromo-2-nitroethanol | Leder (1990), Leder (1991) |
| 2,2-dibromo-3-nitrilopropionamide (DBNPA) | Smith et al. (2008) |
| 2-Bromo-2-bromomethylglutaronitrile | Jakubowski (1986) |
| 2-Bromo-2-nitro-1,3-propanediol | Smith et al. (2008) |
| Bromo-2-nitropropane-1,3-diol (Bronopol) | McLennan et al. (1987) |
| Iodine | Derr et al. (1995) |
| Iodoacetone | Rayudu and Pera (1989) |
| Iodopropynylbutylcarbamate | Smith et al. (2008) |
| Diiodomethyltolylsulfone | Smith et al. (2008) |
Bromine Chloride
Liquid biocides are popular for the control of microorganisms in industrial water systems. Concentrated formulations of stabilized bromine chloride have been developed as biocides (Nalepa and Azomia, 2006). These are generally used for the treatment of industrial water.
The activity of the formulation approaches that of a fresh bleach, while still delivering the benefits of a stabilized bromine system. In order to achieve a balance of acceptable low temperature performance, i.e., a low freezing point with a good retention of its activity, computer-designed experiments have been performed.
Chlorine Dioxide
Chlorine dioxide has been evaluated as a replacement for chlorine (Simpson et al. 1993). Gaseous chlorine is declining in use as a biocide for industrial applications because of safety, environmental, and community impact considerations. Various alternatives have been explored, for example, bromo-chorodimethyl hydantoin, non-oxidizing biocides, ozone, and chlorine dioxide. Chlorine dioxide offers some unique advantages because of its selectivity, effectiveness over a wide pH range, and speed of kill. Safety and cost considerations have restricted its use as a viable replacement.
Nitrogen Containing Compounds
Nitrogen containing compounds are summarized in Table 5.6 and some of their structures some shown in Figure 5.7 and Figure 5.8.
| Biocide | References |
|---|---|
| 1,2-Dimethyl-5-nitro-1H-imidazole | Horstmann and Jones (1990) |
| 1-Hydroxy-5-methyl-4-phenylimidazoline-2-thione | Austin (1987) |
| N,N′-Methylene-bis-morpholine | Smith et al. (2008) |
| 4-(2-nitrobutyl)-morpholine | Smith et al. (2008) |
| 4,4′-(2-ethyl-2-nitrotrimethylene)dimorpholine | Smith et al. (2008) |
| 1,3,5-Tris-(2-hydroxyethyl)-s -triazine | Smith et al. (2008) |
| 2-Methylthio-4-tert -butylamino-6-cyclopropylamino-S-triazine | Downey et al. (1995) |
| Trimethyl-1,3,5-triazine-1,3,5-triethanol | Smith et al. (2008) |
| Tetramethylol acetylene diurea | Smith et al. (2008) |
| Tris(hydroxymethyl)nitromethane | Smith et al. (2008) |
| Sodium pyrithione | Smith et al. (2008) |
| Zinc pyrithione | Smith et al. (2008) |
| 4,4-Dimethyloxazolidine | Smith et al. (2008) |
| 7-Ethyl bicyclooxazolidine | Smith et al. (2008) |
| Dimethylol-dimethyl-hydantoin | Smith et al. (2008) |
1,2-Dibromo-2,4-dicyanobutane can be prepared by reacting 2-methyleneglutaronitrile with bromine in an alcoholic solvent at 25–65°C and isolating the product without color or odor problems and in high yields (Nigam and Stiffler 2003). 2-Bromo-2-bromomethylglutaronitrile is used in compositions of personal care and nutritional and pharmaceutical products.
Sodium pyrithione is synthesized by the reaction of 2-halopyridine-N -oxide with sodium hydrosulfide and sodium carbonate (Farmer and Katz 1983). Zinc pyrithione is obtained by reacting the sodium pyrithione with a zinc salt.
Effervescent Biocide Compositions
Compositions of this kind generally include one or more biocidal ingredients delivered in the form of an effervescent tablet.
It has been discovered that effervescent tablets provide a useful delivery method for delivering biocidal agents to oil field fluids because (Smith et al. 2008):
1. They alleviate problems encountered with the application of dry biocides, i.e., water-soluble bags and
2. The effervescent action of the tablet when it dissolves in the fluid serves to disperse the biocidal agent.
Effervescent compositions are available for 2,2-dibromo-3-nitrilo-propionamide, 1,2-dibromo-2,4-dicyanobutane, 2-bromo-2-nitro-1,3-propanediol, 4,4-dimethyloxazolidine, 1-(3-chloroallyl)-3,5,7-triaza-1-azoniaadamantane chloride, and tris(hydroxymethyl)nitromethane (Smith et al. 2008).
References
Agrawal, A.; Vanbroekhoven, K.; Lal, B., Diversity of culturable sulfidogenic bacteria in two oil-water separation tanks in the north-eastern oil fields of India, Anaerobe 16 (1) (2010) 12–18.
Anonymous, 1990. Microbiologically influenced corrosion and biofouling in oilfield equipment. NACE TPC Publication TPC 3.
Austin, P., 1987. Heterocyclic thione compounds and their use as biocides. EP Patent 249 328, December 16, 1987.
Austin, P.W., 1989. Unsaturated, halogenated thiocyanates, the preparation thereof and use as a biocide. EP Patent 316 058, May 17, 1989.
In: (Editors: Barton, L.; Hamilton, W.A.) Sulphate-Reducing Bacteria: Environmental and Engineered Systems (2007) Cambridge University Press, Cambridge.
Barton, L.L.; Fauque, G.D., Biochemistry, physiology and biotechnology of sulfate-reducing bacteria, In: (Editors: Laskin, A.I.; Sariaslani, S.; Gadd, G.M.) Advances in Applied Microbiology, vol. 68 (2009) Academic Press, New York, pp. 41–98; ch. 2.
Blagov, A.V.; Prazdnikova, Z.F.; Praporshchikov, V.I., Use of ecological factors for controlling biogenic sulfate reduction, Neft Khoz (5) (1990) 48–50.
Boivin, J., Oil industry biocides, Mater. Perf. 34 (2) (1994) 65–68.
Boivin, J.W.; Costerton, J.W.; Laishley, E.J.; Bryant, R., A new rapid test for microbial corrosion detection and biocide evaluation, In: Proceedings Volume, 2nd Inst. Gas Technol. Gas, Oil, Coal, & Environmental Biotechnology International SymposiumNew Orleans, LA, December 11–13, 1989. (1989), pp. 537–547.
Boivin, J.W.; Shapka, R.; Khoury, A.E.; Blenkinsopp, S.; Costerton, J.W., An old and a new method of control for biofilm bacteria, In: Proceedings Volume, Annual NACE Corrosion Conference (Corrosion 92)Nashville, TN, April 27–May 1, 1992. (1992).
Borenstein, S.W.; Licina, G.J., An overview of monitoring techniques for the study of microbiologically influenced corrosion, In: Proceedings Volume, 49th Annual NACE Inter- national Corrosion Conference (Corrosion 94)Baltimore, MD, February 27–March 4, 1994. (1994).
Borenstein, S.W.; Lindsay, P.B., MIC (microbiologically influenced corrosion) failure analysis, Mater. Perf. 33 (4) (1994) 43–45.
Bryan, E., Veale, M.A., Talbot, R.E., Cooper, K.G., Matthews, N.S., 1990. Biocidal compositions and treatments. EP Patent 385 801, September 5, 1990.
Bryant, J.E., McMechan, D.E., McCabe, M.A., Wilson, J.M., King, K.L., 2009. Treatment fluids having biocide and friction reducing properties and associated methods. US Patent Application 20090229827, September 17, 2009.
Burger, E.D.; Crews, A.B.; Ikerd II, H.W., Inhibition of sulfate-reducing bacteria by anthraquinone in a laboratory biofilm column under dynamic conditions, In: Proceedings Volume, NACE International Corrosion Conference (Corrosion 2001)Houston, TX, March 11–16, 2001. (2001).
Cash, H.A.; Krupa, A.S.; Vance, I.; Johnson, B.V., Laboratory testing of biocides against sessile oilfield bacteria, In: Proceedings Volume, Inst. Gas Technol. Gas, Oil & Environ. Biotechnol. SymposiumChicago, IL, September 21–23, 1992. (1992).
Cheung, C.W.S.; Beech, I.B.; Campbell, S.A.; Satherley, J.; Schiffrin, D.J., The effect of industrial biocides on sulphate-reducing bacteria under high pressure, Int. Biodeterior. Biodegrad. 33 (4) (1994) 299–310.
Clark, J.B., Langley, D.E., 1990. Biofilm control. US Patent 4 929 365, May 29, 1990.
Coburn, C.E., Pohlman, J.L., Pyzowski, B.A., Brutto, P.E., Green, G.D., Swedo, R.J., 2010. Aminoalcohol and biocide compositions for aqueous based systems. US Patent Application 20100093736, assigned to Global Technologies Inc., Midland, April 15, 2010.
Cowan, J.K., Rapid enumeration of sulfate reducing bacteria. Corrosion 2005. (2005) NACE International, Houston, TX.
Demikhov, V.N., Gilyazov, M.M., Trutneva, E.K., Levin, Y.A., Rakov, A.P., Shermergorn, I.M., 1992. New 1-n-alkyl-1,2,4-triazole bromide bactericidal compounds – prepared by alkylation of 1,2,4-triazole with n-hexadecyl or n-octadecyl. SU Patent 1 776 653, November 23, 1992.
Derr, R.; Morris III, E.A.; Pope, D.H., Fate and persistence of glutaraldehyde in a natural gas storage facility, In: Proceedings Volume, 7th Inst. Gas Technol. Gas, Oil, & Environmental Biotechnology International SymposiumColorado Springs, CO, December 12–14, 1994. (1994).
Derr, R.M.; Morris, E.A.; Pope, D.H., Applicability and efficacy of iodine as a mitigation strategy for advanced microbiologically influenced souring (MIS), In: Proceedings Volume, 8th Inst. Gas Technol. Gas, Oil, & Environmental Biotechnology International SymposiumColorado Springs, CO, December 11–13, 1995. (1995).
Dowling, N.J.E.; Guezennec, J.; White, D.C., Facilitation of corrosion of stainless steel exposed to aerobic seawater by microbial biofilms containing both facultative and absolute anaerobes, In: Proceedings Volume, Inst. Petrol. Microbiol Comm Microbial Problems in the Offshore Oil Ind. International ConferenceAberdeen, Scotland, April 15–17, 1986. (1986).
Downey, A.B., Willingham, G.L., Frazier, V.S., 1995. Compositions comprising 4,5-dichloro-2-n-octyl-3-isothiazolone and certain commercial biocides. EP Patent 680 695, assigned to Rohm & Haas Co., November 8, 1995.
Eagar, R.G.; Leder, J.; Stanley, J.P.; Theis, A.B., The use of glutaraldehyde for microbiological control in waterflood systems, Mater. Perf. 27 (8) (1988) 40–45.
Elboujdaini, M.; Sastri, V.S., Field studies of microbiological corrosion in water injection plant, In: Proceedings Volume, 50th Annual NACE International Corrosion Conference (Corrosion 95)Orlando, FL, March 26–31, 1995. (1995).
Enzien, M.V.; Pope, D.H.; Wu, M.M.; Frank, J., Nonbiocidal control of microbiologically influenced corrosion using organic film-forming inhibitors, In: Proceedings Volume, 51st Annual NACE International Corrosion Conference (Corrosion 96)Denver, CO, March 24–29, 1996. (1996).
Farmer, D.A., Jr., Katz, L.E., 1983. Process for producing sodium and zinc pyrithione. US Patent 4 396 766, assigned to Olin Corporation (New Haven, CT), August 2, 1983.
Farquhar, G.B., A review of trends in MIC (microbiologically influenced corrosion), Mater. Perf. 32 (1) (1990) 53–55.
Gironda, K.F., Redlich, G.H., Petigara, R.B., 1995. Bromate stabilization of nitrate-free 3-isothiazolones at ph 4-5.1. US Patent 5 478 797, assigned to Rohm & Haas Co., December 26, 1995.
Gregg, M.; Dickinson, A.; Oates, S.; Walsh, G.G.; Mulak, K.J., A novel approach to managing a seawater injection biocide program reduces risk, improves biological control, and reduces capital and opex costs on an offshore platform, In: Corrosion 2006 (2006) NACE International, San Diego, CA.
Hamilton, W.A., Sulphate-reducing bacteria and the offshore oil industry, Trends Biotechnol. 1 (2) (1983) 36–40.
Hamilton, W.A., Mechanisms of microbial corrosion, In: Proceedings Volume, Inst. Petrol. Microbiol Comm Microbial Problems in the Offshore Oil Ind. International ConferenceAberdeen, Scotland, April 15–17, 1986. (1986), pp. 1–11.
Hegarty, B.M., Levy, R., 1996. Control of oilfield biofouling. EP Patent 706 759, April 17, 1996.
Hill, E.C.; Hill, G.C.; Robbins, D.A., An informative and practical strategy for preventing spoilage and improving preservation using a simple assay for biocides and preservatives, Int. Biodeterior. 25 (1–3) (1989) 245–252.
Hitzman, D.O.; Dennis, D.M., Sulfide removal and prevention in gas wells, In: Procee- dings Volume, SPE Prod. Oper. SymposiumOklahoma City, March 9–11, 1997. (1997), pp. 433–438.
Horstmann, D.G., Jones, D.S., 1990. Synergistic biocides of certain nitroimidazoles and aldehydes. US Patent 4 920 141, assigned to Petrolite Corp., April 24, 1990.
Hsu, J.C., 1994. Synergistic microbicidal combinations containing 3-isothiazolone and commercial biocides. US Patent 5 278 178, January 11, 1994.
Hsu, J.C., 1995. Biocidal compositions. EP Patent 685 159, December 6, 1995.
Jakubowski, J.A., 1986. Admixtures of 2-bromo-2-bromomethylglutaronitrile and 2,2-dibromo-3-nitrilopropionamide. US Patent 4 604 405, assigned to Calgon Corp., August 5, 1986.
Jones, C.R.; Diaz, R.; Hernandez, K.; Talbot, R.E.; Fidoe, S.D.; Downward, B.L., Keeping pace with the need for advanced, high performance biocide formulations of oil production applications, In: Corrosion 2006, NACE InternationalSan Diego, CA. (2006).
Karaseva, E.V., Dedyukhina, S.N., Dedyukhin, A.A., 1995. Treatment of water-based drilling solution to prevent microbial attack – by addition of dimethyl-tetrahydro-thiadiazine-thione bactericide. RU Patent 2 036 216, May 27, 1995.
Khanlarova, A.G., Musaev, M.R., Samedov, A.M., Kandinskaya, L.I., Gasanov, A.G., Alieva, L.I., et al., 1993. Inhibiting growth of sulphate-reducing bacteria-involves introducing diammonium salts of tetrahydrophthalic acid or methyl-tetrahydrophthalic acid into bacteria-containing circulating water. SU Patent 1 828 917, July 23, 1993.
Kramer, J.F., A new high performance quaternary phosphonium biocide for biofouling control in industrial water systems, In: Corrosion 2006 (2006) NACE International, San Diego, CA.
Kramer, J.F., Efficacy of bromochlorodimethylhydantoin against legionella pneumophila in industrial cooling water systems, In: Corrosion 2007 (2007) NACE International, Nashville, TN.
Kriel, B.G.; Crews, A.B.; Burger, E.D.; Vanderwende, E.; Hitzman, D.O., The efficacy of formaldehyde for the control of biogenic sulfide production in porous media, In: Proceedings Volume, SPE Oilfield Chem. International SymposiumNew Orleans, LA, March 2–5, 1993. (1993), pp. 441–448.
Lamarre, T.M., Martin, C.H., 1990. Synergistic biocide of tributyl tetradecyl phosphonium chloride and 1.5-pentanedial. CA Patent 1 269 300, May 22, 1990.
Leder, J., 1990. Antimicrobial composition and method of use. EP Patent 364 789, April 25, 1990.
Leder, J., 1991. Antimicrobial composition and method of use in oil well flooding. US Patent 5 055 493, October 8, 1991.
Lein Jr., G.M., 1989. Preparation of isothiazolones. EP Patent 318 194, May 31, 1989.
Littmann, E.S.; McLean, T.L., Chemical control of biogenic H2S in producing formations, In: Proceedings Volume, SPE Prod. Oper. SymposiumOklahoma City, March 8–10, 1987. (1987), pp. 339–342.
Lloyd, G.R.; Neail, P.W., Biocides for minimum environmental impact, In: Proceedings Volume, no. 2 III-7, 2nd Shell Co. Australia et al Australian International Oil, Gas & Petrochem Conference (Offshore Australia 93)Melbourne, Australia, November 23–26, 1993. (1993).
Macleod, N.; Bryan, E.; Buckley, A.J.; Talbot, R.E.; Veale, M.A., Control of reservoir souring by a novel biocide, In: Proceedings Volume, 50th Annual NACE International Corrosion Conference (Corrosion 95)Orlando, FL, March 26–31, 1995. (1995).
Mansfeld, F.; Little, B., The application of electrochemical techniques for the study of MIC (microbiologically influenced corrosion) – a critical review, In: Proceedings Volume, NACE International Corrosion Forum (Corrosion 90)Las Vegas, NV, April 23–27, 1990. (1990).
Mason, J.A., 1990. Use of chlorous acid in oil recovery. US Patent 4 892 148, January 9, 1990.
Mattox, J.R., 1989. Stabilized isothiazolone compositions. EP Patent 315 464, May 10, 1989.
Maxwell, S., Improved monitoring of bacterially mediated corrosion risks in offshore systems, Inst. Petrol. Quart. J. Tech. (1986) 1–25.
Maxwell, S.; McLean, K.M.; Kearns, J., Biocide application and monitoring in a waterflood system, In: Proceedings Volume, Inst. Petrol. Microbiol Comm Microbial Problems in the Offshore Oil Ind. International ConferenceAberdeen, Scotland, April 15–17, 1986. (1986), pp. 209–218.
McCabe, M.A., Wilson, J.M., Weaver, J.D., Venditto, J.J., 1991. Biocidal well treatment method. US Patent 5 016 714, assigned to Halliburton Co., May 21, 1991.
McLennan, J.M., Brunt, K.D., Guthrie, W.G., 1987. Solid antibacterial compositions. GB Patent 2 183 477, June 10, 1987.
Miranda-Tello, E.; Fardeau, M.-L.; Fernández, L.; Ramírez, F.; Cayol, J.-L.; Thomas, P.; et al., Desulfovibrio capillatus sp. nov., a novel sulfate-reducing bacterium isolated from an oil field separator located in the gulf of mexico, Anaerobe 9 (2) (2003) 97–103.
Moody, S.S., Montgomerie, H.T.R., 1996. Control of oilfield biofouling. EP Patent 706 974, April 17, 1996.
Morpeth, F.F., Greenhalgh, M., 1990. Composition and use. EP Patent 390 394, assigned to Imperial Chemical Inds Pl, October 3, 1990.
Morris III, E.A.; Dziewulski, D.M.; Pope, D.H.; Paakkonen, S.T., Field and laboratory studies into the detection and treatment of microbiologically influenced souring (MIS) in natural gas storage facilities, In: Proceedings Volume, 49th Annual NACE International Corrosion Conference (Corrosion 94)Baltimore, MD, February 27–March 4, 1994. (1994).
Morris III, E.A.; Pope, D.H., Field and laboratory investigations into the persistence of glutaraldehyde and acrolein in natural gas storage operations, In: Proceedings Volume, 49th Annual NACE International Corrosion Conference (Corrosion 94)Baltimore, MD, February 27–March 4, 1994. (1994).
Muganlinskij, F.F., Lyushin, M.M., Samedov, A.M.O., Akosta, V.K.U. Suppressing activity of sulphate-reducing bacteria on petroleum extraction – by treating flooding water with di-(tri-n-butyl)-(1,4-benzodioxan-6,7-dimethyl) diammonium dichloride. RU Patent 2 033 393, April 20, 1995.
Munn, S.J., Allanou, R., Aschberger, K., Berthault, F., Cosgrove, O., de Bruijn, J., et al., 2004. Edetic acid (EDTA). European Union Risk Assessment Report 49, Institute for Health and Consumer Protection, European Chemicals Bureau, Luxembourg, http://ecb.jrc.ec.europa.eu/documents/Existing-Chemicals/RISK_ASSESSMENT/REPORT/edtareport061.pdf.
Nalepa, C.J.; Azomia, F.D., Development of a high activity liquid biocide for industrial water treatment, In: Corrosion 2006 (2006) NACE International, San Diego, CA.
Nigam, S.C., Stiffler, C., 2003. Method for preparing 1,2-dibromo-2,4-dicyanobutane. US Patent 6 548 692, assigned to ISP Investments Inc. (Wilmington, DE), April 15, 2003.
Novikov, I.A.; Gurov, B.N.; Shtuchnaya, G.V.; Fomchenkov, V.M.; Kholodenko, V.P., Rapid assay for assessment of the potential of chemical biocides against microbial destructors of industrial materials, Appl. Biochem. Microbiol. 37 (1) (2001) 110–114.
Oblinger, J.L.; Koburger, J.A., Understanding and teaching the most probable number technique, J. Milk Food Technol. 38 (9) (1975) 540–545.
Oppong, D., Hollis, C.G., 1995. Synergistic antimicrobial compositions containing (thiocyanomethylthio) benzothiazole and an organic acid. WO Patent 9 508 267, assigned to Buckman Labs International Inc., March 30, 1995.
Oppong, D., King, V.M., 1995. Synergistic antimicrobial compositions containing a halogenated acetophenone and an organic acid. WO Patent 9 520 319, assigned to Buckman Labs International Inc., August 3, 1995.
Penkala, J.; Law, M.D.; Horaska, D.D.; Dickinson, A.L., Acrolein 2-propenal: a versatile microbiocide for control of bacteria in oilfield systems, In: Corrosion 2004 (2004) NACE International, New Orleans, LA.
Piddock, L.J.V., Techniques used for the determination of antimicrobial resistance and sensitivity in bacteria, J. Appl. Microbiol. 68 (4) (1990) 307–318.
Pope, D.H., Concern over MIC (microbiologically-influenced corrosion) expanding among corrosion engineers, Pipe Line Gas Ind. 80 (2) (1997) 23–25.
Pope, D.H.; Dziewulski, D.; Frank, J.R., Microbiologically influenced corrosion in the gas industry, Pipeline 62 (5) (1990) 8–9.
Pope, D.H.; Dziewulski, D.M.; Lockwood, S.F.; Werner, D.P.; Frank, J.R., Microbiological corrosion concerns for pipelines and tanks, In: Proceedings Volume, API Pipeline ConferenceHouston, TX, April 7–8, 1992. (1992), pp. 290–321.
Postgate, J.R., The Sulphate-Reducing Bacteria. (1979) Cambridge University Press, Cambridge.
Prasad, R., Pros and Cons of ATP (adenosine triphosphate) measurement in oil field waters, In: Proceedings Volume, NACE Corrosion 88St Louis, MO, March 21–25, 1988. (1988).
Raad, I., Sherertz, R., 2001. Chelators in combination with biocides: treatment of microbially induced biofilm and corrosion. US Patent 6 267 979, assigned to Wake Forest University (Winston-Salem, NC) Board of Regents, The University of Texas System (Austin, TX), July 31, 2001.
Rajasekar, A.; Anandkumar, B.; Maruthamuthu, S.; Ting, Y.-P.; Rahman, P., Characterization of corrosive bacterial consortia isolated from petroleum-product-transporting pipelines, Appl. Microbiol. Biotechnol. 85 (4) (2010) 1175–1188.
Rayudu, S.R., Pera, J.D., 1989. A method of inhibiting the growth of microorganisms in aqueous liquids. EP Patent 313 272, April 26, 1989.
Recommended practice for biological analysis of water-flood injection, 1975. API Standard API RP 38-3, American Petroleum Institute, Washington, DC, WITHDRAWN No Replacement.
Rodin, V.B.; Zhigletsova, S.K.; Kobelev, V.S.; Akimova, N.A.; Kholodenko, V.P., Efficacy of individual biocides and synergistic combinations, Int. Biodeterior. Biodegrad. 55 (4) (2005) 253–259.
Salanitro, J.P.; Williams, M.P.; Langston, G.C., Growth and control of sulfidogenic bacteria in a laboratory model seawater flood thermal gradient, In: Proceedings Volume, SPE Oilfield Chem. International SymposiumNew Orleans, LA, March 2–5, 1993. (1993), pp. 457–467.
Sandbeck, K.A.; Hitzman, D.O., Biocompetitive exclusion technology: a field system to control reservoir souring and increase production, In: US DOE Rep, no. CONF-9509173, 5th US DOE et al Microbial Enhanced Oil Recovery & Relat Biotechnol. for Solving Environ. Probl International ConferenceDallas, TX, September 11–14, 1995. (1995), pp. 311–319.
Sarioglu, F.; Javaherdashti, R.; Aksöz, N., Corrosion of a drilling pipe steel in an environment containing sulphate-reducing bacteria, Int. J. Pres. Ves. Pip. 73 (2) (1997) 127–131.
Schowanek, D.; Feijtel, T.C.J.; Perkins, C.M.; Hartman, F.A.; Federle, T.W.; Larson, R.J., Biodegradation of [S,S], [R,R] and mixed stereoisomers of ethylene diamine disuccinic acid (EDDS), a transition metal chelator, Chemosphere 34 (11) (1997) 2375–2391.
Sears, J.T., Mueller, R., Reinsel, M.A., 1996. Inhibition of sulfate-reducing bacteria via nitrite production. WO Patent 9 612 867, assigned to Montana State Univ., May 2, 1996.
Simpson, G.D.; Miller, R.F.; Laxton, G.D.; Clements, W.R., A focus on chlorine dioxide: the “ideal” biocide, In: Proceedings Volume, Annual NACE Corrosion Conference (Corrosion 93)New Orleans, LA, March 7–12, 1993. (1993).
Smith, K., Persinski, L.J., Wanner, M., 2008. Effervescent biocide compositions for oilfield applications. US Patent Application 20080004189, assigned to Weatherford/Lamb Inc., Houston, TX, January 3, 2008.
Starkey, R.J., Monteith, G.A., Aften, C.W., 2008. Biocide for well stimulation and treatment fluids. US Patent Application 20080032903, February 7, 2008.
Sunde, E.; Thorstenson, T.; Torsvik, T., Growth of bacteria on water injection additives, In: Proceedings Volume, 65th Annual SPE Technical ConferenceNew Orleans, LA, September 23–26, 1990. (1990), pp. 727–733.
Sunde, E.; Thorstenson, T.; Torsvik, T.; Vaag, J.E.; Espedal, M.S., Field-related mathematical model to predict and reduce reservoir souring, In: Proceedings Volume, SPE Oilfield Chem. International SymposiumNew Orleans, LA, March 2–5, 1993. (1993), pp. 449–456.
Sweeny, P.G., 1996. Hydantoin-enhanced halogen efficacy in pulp and paper applications. WO Patent 9 611 882, April 25, 1996.
Theis, A.B., Leder, J., 1992. Method for the control of biofouling. US Patent 5 128 051, July 7, 1992.
Trushevskaya, A.M., Sklyarskaya, L.B., Zhurakivskij, I.M., 1992. Suppressing growth of sulphate(s)-reducing bacteria in stratal injection water by addition of zinc slurry solutions obtained as waste from filtration stage in production of naphthol-di:sulphonic acid. SU Patent 1 730 502, April 30, 1992.
Veale, M.A.; Bryan, E.; Talbot, R.E., A new biocide with respect to industrial water treatment and oilfield applications, In: Proceedings Volume, Norwegian Soc. Chartered Eng. Oil Field Chem. ConferenceGeilo, Norway, March 19–21, 1990. (1990).
Videla, H.A.; Guiamet, P.S.; Pardini, O.R.; Echarte, E.; Trujillo, D.; Freitas, M.M.S., Monitoring biofilms and MIC (microbially induced corrosion) in an oilfield water injection system, In: Proceedings Volume, Annual NACE Corrosion Conference (Corrosion 91)Cincinnati, OH, March 11–15, 1991. (1991).
von Rège, H.; Sand, W., Evaluation of biocide efficacy by microcalorimetric determination of microbial activity in biofilms, J. Microbiol. Methods 33 (3) (1998) 227–235.
Ward, E.C.; Glaser, D.E., A new look at azoles, In: Corrosion 2007 (2007) NACE International, Nashville, TN.
Weimer, P.J., Odom, J.M., Cooling, F.B.I., Anderson, A.G., 1995. Anthraquinones as inhibitors of sulfide production from sulfate-reducing bacteria. US Patent 5 385 842, January 31, 1995.
Wen, J.; Zhao, K.; Gu, T.; Raad, I.I., A green biocide enhancer for the treatment of sulfatereducing bacteria (srb) biofilms on carbon steel surfaces using glutaraldehyde, Int. Biodeterior. Biodegrad. 63 (8) (2009) 1102–1106.
Wheat, P.F., History and development of antimicrobial susceptibility testing methodology, J. Antimicrob. Chemother. 48 (Suppl.) (2001) 1–4.
Williams, T.M., The mechanism of action of isothiazolone biocide, In: Corrosion 2006 (2006) NACE International, San Diego, CA.
Williams, T.M., Efficacy of chloromethyl-methylisothiazolone (CMIT/MIT) biocide versus legionella and protozoa, In: Corrosion 2007 (2007) NACE International, Nashville, TN.
Williams, T.M., Methylisothiazolone: a new biocide product for closed loop systems, In: Corrosion 2007 (2007) NACE International, Nashville, TN.
Wrench, E., 1990. Anti-microbial agent. WO Patent 9 006 054, June 14, 1990.
Wrench, E., 1991. Anti-microbial agent. GB Patent 2 244 216, November 27, 1991.
Youssef, N.; Elshahed, M.S.; McInerney, M.J., Microbial processes in oil fields: culprits, problems, and opportunities, In: (Editors: Laskin, A.I.; Sariaslani, S.; Gadd, G.M.) Advances in Applied Microbiology, vol. 66 (2009) Academic Press, New York, pp. 141–251; ch. 6.
Zhou, X.; King, V.M., A rapid bactometer method for screening of biocides against sulfate-reducing bacteria, Appl. Microbiol. Biotechnol. 43 (2) (1995) 336–340.
Zhou, Y., Bactericide for drilling fluid, Drill. Fluid Completion Fluid 7 (3) (1990) 10–12; 2A.
TRADENAMES
| Tradename | |
|---|---|
| Description | Supplier |
| Disotate | Forest Pharmaceuticals |
| EDTA compound (Raad and Sherertz 2001) | |
| Endtrate | Abbott |
| EDTA compound (Raad and Sherertz 2001) | |
| Etidronate™ | Various manufacturers |
| Etidronic acid salt, 1-hydroxyethane 1,1-diphosphonic acid salt (Raad and Sherertz 2001) |
..................Content has been hidden....................
You can't read the all page of ebook, please click here login for view all page.