Chapter 2. Fluid Loss Additives
Comparative tables of fluid loss additives, also called filtrate-reducing agents, can be found on the internet (Petrochem 2009). Losses may occur when the fluid comes in contact with a porous formation. This is relevant for drilling and completion fluids, fracturing fluids, and cement slurries.
The extent of fluid loss is dependent on the porosity and thus the permeability of the formation, and it may reach approximately 10 t/h. Because the fluids used in petroleum technology are in some cases quite expensive, an extensive fluid loss may not be tolerable. Of course there are also environmental reasons to prevent fluid loss.
Mechanism of Action of Fluid Loss Agents
Reduced fluid loss is achieved by plugging a porous rock in some way. The basic mechanisms are shown in Table 2.1.
Action of Macroscopic Particles
A monograph concerning the mechanism of invasion of particles into the formation is given by Chin (1995).
One of the basic mechanisms used in fluid loss prevention is shown in Figure 2.1. The fluid contains suspended particles. These particles move with the lateral flow out of the drill hole into the porous formation, which acts like a sieve for the suspended particles. The particles will therefore be captured near the surface, and accumulate as a filter cake.
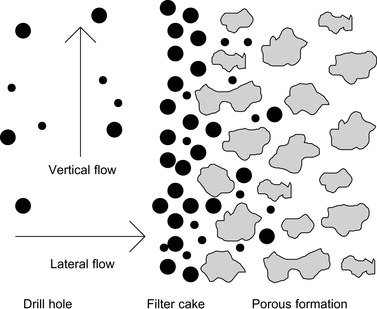 |
| Figure 2.1 |
The hydrodynamic forces acting on the suspended colloids determine the rate of cake buildup and therefore the fluid loss rate. A simple model has been proposed in the literature, which predicts a power law relationship between the filtration rate and the shear stress at the cake surface (Jiao and Sharma 1994).
The model shows that the cake formed will be inhomogeneous, with the particle size being deposited decreasing as the filtration proceeds. An equilibrium cake thickness is achieved when no particles small enough to be deposited are available in the suspension. The cake thickness can be computed as a function of time from the model.
For a given suspension rheology and flow rate there is a critical permeability of the filter, below which no cake will be formed. The model also suggests that the equilibrium cake thickness can be precisely controlled by an appropriate choice of suspension flow rate and filter permeability.
Action of Cement Fluid Loss Additives
Two stages are considered with respect to the fluid loss behavior of a cement slurry (Baret 1988):
1. A dynamic stage corresponding to placement; and
2. A static stage, awaiting the setting of the cement.
During the first period, the slurry flow erodes the filter cake as it is growing; thus a steady state, in which the filtration occurs through a cake of constant thickness, is rapidly reached. At the same time, because the slurry is losing water but not solid particles, its density increases in line with the fluid loss rate.
During the second period, the cake grows because of the absence of flow. It may grow to a point at which it locally but completely fills the annulus: Bridging takes place and the hydrostatic pressure is no longer transmitted to the deeper zones. From the typical mud cake resistance values it can be estimated that under both dynamic and static conditions, the fluid loss could require reduction to an American Petroleum Institutue (API) value lower than what is generally considered a fair control of fluid loss.
Testing of Fluid Loss Additives
Fluid loss prevention is a key performance attribute of drilling fluids. For water-based drilling fluids, significant loss of water or fluid into the formation can cause irreversible changes in the drilling fluid properties, such as density and rheology, which can create instability in the borehole. Fluid loss control is measured in the laboratory according to a standard procedure for testing drilling fluids (Recommended practice for field testing water-based drilling fluids (API) 2009).
Predictions of the effectiveness of a formulation can be made on a laboratory scale, by characterizing the properties of the filter cake that is formed by appropriate experiments. Most of the fluids containing fluid loss additives are thixotropic, so the apparent viscosity will change when a shear stress in a vertical direction is applied, as is very normal in a circulating drilling fluid. For this reason, the results from static filtering experiments are expected to differ from dynamic experiments.
Static fluid loss measurements provide inadequate results for comparing fracturing fluid materials, or for understanding the complex mechanisms of viscous fluid invasion, filter cake formation, and filter cake erosion (Vitthal and McGowen 1996). On the other hand, proper laboratory methods and dynamic fluid loss studies have not been developed for, which has led to erroneous and conflicting results.
Results from a large-scale, highworddash temperature, highworddash pressure simulator were compared with laboratory data, and significant differences in spurt loss values were found (Lord et al. 1995).
Static experiments with piston-like filtering can be reliable, however, for obtaining information on the fluid loss behavior at certain stages of a cementation process, in particular when the slurry is at rest.
Formation Damage
The damage to the formation that results from the use of a filtration loss agent can be a serious problem in certain fields of application. Providing effective fluid loss control without damaging formation permeability in completion operations has been a prime requirement for the ideal fluid loss control pill.
Filter cakes are hard to remove, and thus can cause considerable formation damage. Cakes with very low permeability can be broken up by reverse flow. No high-pressure spike occurs during the removal of the filter cake.
Typically, a high-pressure spike indicates damage to the formation and wellbore surface, because damage typically reduces the overall permeability of the formation. Often formation damage results from the incomplete back-production of viscous, fluid loss control pills, but there may be other reasons.
Reversible Gels
Another mechanism for fluid loss prevention is used by other additives, which are able to form gels via a molecular mechanism.
Bacteria
Instead of using polymers, the addition of bacterial cultures, which may form natural polymers that could then prevent fluid loss, has been suggested.
In one study, a bacterial culture selected for its abundant exopolymer production was added to drilling mud, to determine whether the polyanionic cellulose (PAC) component could be replaced without sacrificing viscosity or fluid retention (Anderson et al. 1991). The performance of the drilling mud was tested using a standard API test series. The bacterial inoculum was not as effective in maintaining viscosity or preventing fluid loss as was the PAC. However, the inoculum was capable of reducing the amount of PAC that was required in the drilling mud.
The combination of the bacterial inoculum with less expensive sources, for example carboxylated methyl cornstarch, crosslinked hydroxypropyl cornstarch, or amine-derivatized potato starch, gave viscosity and fluid loss control that was as good as or better than PAC alone. The bacterial strain tested was effective over a wide range of drilling mud conditions with growth occuring at varying pHs (3–11), varying salinities (0–15%), and a wide range of temperatures.
Inorganic Additives
Bentonite
Bentonite is an impure clay that is formed by weathering of volcanic tuffs. It contains a high content of montmorillonite. Bentonites exhibit properties such as the ability to swell, a capacity for ion exchange, and thixotropy. Their properties can be modified by ion exchange, for example, exchange of earth alkali metals with alkali metals. The specific surface can be modified by acid treatment, and their organophilic properties can be increased by treatment with quaternary ammonia compounds.
Sodium Metasilicate
Sodium silicate has been successfully used as a chemical grouting material for many years. It is used in particular during the drilling of very permeable formations (Xiang 2007). When an aqueous mixture of sodium silicate and an activating agent, such as an ester, is injected into the ground, the silicate solution reacts to form a colloid, which polymerizes further to form a gel. The gel provides increased strength, stiffness, and reduced permeability in predominantly granular soils.
These properties have been utilized in water-based drilling fluid systems, particularly during drill-in and completion operations (Xiang 2009). The gel produced by the silicate reaction is soluble in both acids and bases.
The upper limit on amount of alkali metal silicate depends on the gel strength necessary and the pore size of the formation. The bigger the pore size, the higher the gel strength that is needed and, generally, the higher the desired concentration of alkaline silicate. As a practical matter, the concentration of alkaline silicate generally is about 40% because most commercial silicates are available at this concentration.
The drilling fluid system also contains activating agents, which becomes effective as it hydrolyzes, thereby decreasing the pH. Examples are formamide and water-soluble esters; accelerating agents may also be added to accelerate the gel formation such as sodium aluminate (Xiang, 2007 and Xiang, 2009). Examples of the formulation of drilling mud systems that use silicate for fluid loss control have been given in detail (Xiang 2009).
Ultra-fine Filtrate-Reducing Agents
Methods are available for reducing the fluid loss and for reducing the concentration of polymer required to do this for a drilling fluid and to a well servicing fluid, respectively (Dobson et al. 1998). The fluids contain polymeric viscosifiers, a polymeric fluid loss additive and a water-soluble bridging agent suspended in a liquid in which the bridging agent is not soluble.
It is important to add a particulate, water-soluble, ultra-fine filtrate-reducing agent to the fluids. The particle size distribution should be such that approximately 90% of the particles are less than 10 μ, the average particle size being between 3 μ and 5 μ and the ultra-fine filtrate-reducing agent being insoluble in the liquid.
Bridging Agents for Fluid Loss Control
Common bridging agents include calcium carbonate, suspended salts, or oil soluble resins. For lost circulation treatments outside the production interval, any suitably sized product may be used, including mica, nutshells, and fibers (Munoz and Todd 2008).
The selection of an appropriate bridging material is more critical in the production interval and during workover operations, since the barrier should be completely removed in preparation for placing the well back into production.
Chemically bonded ceramic particulates are useful as an alternative to conventional bridging agents, because they are customizable. These particulates are made via a process similar to that of mixing a cementitious material, so their composition and properties can be varied and they can be impregnated with desirable additives. Another advantageous feature of these particular bridging agents is that they are soluble in ammonium salts and chelating agents.
Starches derived from corn, wheat, oats, rice, potatoes, yucca, etc., are often used in conjunction with bridging agents. Most starches usually comprise about 27% amylose and about 73% amylopectin. These two polymers are intertwined within the starch granules. These granules are generally insoluble in cold water, but soaking in hot water or under steam pressure ruptures their covering and the polymers hydrate into a colloidal suspension. Amylose and amylopectin are non-ionic polymers that do not interact with electrolytes. Derivatized starches such as hydroxypropyl and carboxylmethyl starch are used in drill-in fluids, completion fluids, and various brine systems as well as in drilling fluid systems.
When conventional starches are added to fluids that consist of chemically bonded ceramic particulates, problems may arise. When combined, the fluid gels to a point where it ultimately has the consistency of paste, which makes it unusable in downhole applications. This is unfortunate because the starch provides an added means of ensuring fluid loss control in a process using the desirable chemically bonded ceramic particulates. However, modified starches can overcome these problems.
Ceramic particulate bridging agents are chemically bonded particulates. Chemically bonded particulates are preferred because they have an inherent flexibility in their composition, properties, and in their ability to act as carriers for desirable additives such as breakers.
The bridging agents are substantially insoluble in water, but soluble in aqueous ammonium salt clean-up solutions. An example of such a magnesium-based ceramic particulate bridging agent is:
(2.1)
(2.2)
Modified starch compositions provide enhanced fluid loss control when used in conjunction with ceramic bridging agents. These starches may be crosslinked ether derivatives of a partially depolymerized starch. The molecular weight of the crosslinked starch derivative is decreased by the partial depolymerization of the starch polymer.
Formulations are shown in Table 2.2. The starch in formulation of #1 is N-DRIL HT PLUS, a commonly used starch available from Halliburton Energy Services. N-DRIL HT PLUS is a stabilized non-ionic starch derivative (waxy maize) that seeks to control high-pressure, high-temperature filtrate loss.
| Component | Unit | Amount #1 | Amount #2 |
|---|---|---|---|
| Water | ml | – | 317 |
| NaCl | g | – | 90.4 |
| 10% NaCl | g | 336 | – |
| NaOH | g | 0.1 | – |
| Xanthan | g | 0.85 | 1.25 |
| N-DRIL HT PLUS | g | 7.4 | – |
| BROMA FLA™ | g | – | 5.0 |
| Newberyite | g | 25.0 | 25.0 |
In combination with other polymers, such as xanthan, N-DRIL HT PLUS is synergistic and yields improved suspension. However, when used in combination with ceramic bridging agents, the combination will form a poor filter cake and will become a thick gel.
In contrast, a modified starch is used in formulation #2. This composition forms a tight filter cake, and does not form an unusable thick gel, even over a period of 24–48 h. The starch used in this recipe is BROMA FLA™, which is commercially available from TBC Brinadd of Houston, Texas.
Organic Additives
Some polymers and copolymers used for fluid loss additives are shown in Table 2.3.
Tall Oil Pitch
Air-blown tall oil pitch, which has a softening point (ring and ball) of 100–165∘C is a useful fluid loss additive for well drilling fluids. Tall oil pitch is available as the residue from the distillation of tall oil. It is largely insoluble in fatty acids, and soluble in fatty esters, higher alcohols, and sterols.
Blowing air through tall oil pitch at an elevated temperature partially oxidizes and polymerizes the material and drives off volatiles. Blowing reduces the volume of the pitch by 30% and increases its viscosity and softening point. The softening point of the resultant blown pitch is therefore a measure of the degree of oxidation-polymerization that has occurred. It has been found that optimal properties as a fluid loss additive are given by blown tall oil pitches that have a softening point between 125∘C and 130∘C (Williamson 1987).
Mercaptans for Iron Control
Acid treatments are accompanied by very familiar problems, which are linked with the presence of iron in the acid, essentially because the acid dissolves the rust in the casings during pumping, and possibly also iron-containing minerals in the formation.
The presence of Fe3+ in the injected acid may cause, in contact with certain crude oils, the precipitation of the asphaltic products contained in the oil in the form of vitreous deposits, known as sludges. These cause practically irreversible damage to the zone treated. The scale of precipitation generally increases with the strength and concentration of the acid. The dispersibility of customary additives, such as surfactants, is also affected by the presence of Fe3+ through the formation of complexes.
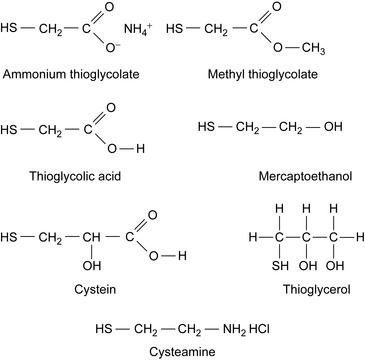
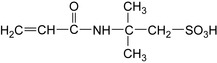
When the injected acid is consumed by the dissolution of the minerals of the formation, the presence of Fe3+ leads to the precipitation of a colloidal precipitate of ferric hydroxide, which damages the formation. For this reason, the use of iron control additives is necessary in most acid treatments (Feraud et al. 2001). Suitable reducing agents are compounds with a mercaptan functionality, as shown in Figure 2.2.
The efficiency of a copper catalyst reducing agent was tested with regard to the reduction of Fe3+ to Fe2+ in media of varying acidities. The concentration of the reducing agent was 3× 10−3moll−1. Copper chloride is used as catalyst. The results are shown in Table 2.4.
| Fe3+ Reduced/[10−3 mol] | |||
|---|---|---|---|
| Reducing Agent | T/[∘C] | 5% HCl | 15% HCl |
| Ammonium thioglycolate | 20 | 2.9 | 2.7 |
| 90 | 2.9 | 2.6 | |
| Thioglycolic acid | 20 | 2.6 | 2.3 |
| 90 | 2.4 | 2.3 | |
| Methyl thioglycolate | 20 | 2.8 | – |
| 90 | 2.7 | – | |
| Mercaptoethanol | 20 | 2.7 | 2.8 |
| 90 | 2.9 | 2.9 | |
| Cysteamine | 20 | 3.1 | 2.6 |
| 90 | 3.4 | 2.2 | |
| Thioglycerol | 20 | 3 | 2.6 |
| 90 | 2.9 | 2.5 | |
Polysaccharides
Cellulose-based Fluid Loss Additives
Polyanionic Cellulose
A composition containing PAC and a synthetic sulfonate polymer has been tested for fluid loss reduction and thermal stabilization of a water-based drilling fluid for extended periods at deep well drilling temperatures (Hen 1991).
Improved fluid loss is obtained when PAC and the sulfonate-containing polymer, which has a molecular weight of 300–10,000 kDalton, are combined in a (WBM), after prolonged aging at 300∘F (150∘C).
Carboxymethyl Cellulose
Certain admixtures of carboxymethyl hydroxyethyl cellulose or copolymers and copolymer salts of N,N -dimethylacrylamide and 2-acrylamido-2-methyl-1-propane sulfonic acid (AMPS), together with a copolymer of acrylic acid (AA), may provide fluid loss control to cement compositions under elevated temperature conditions.
Hydroxyethyl Cellulose
Hydroxyethyl cellulose (HEC) with a degree of substitution of 1.1–1.6 has been tested for fluid loss control in water-based drilling fluids (Raines 1986). An apparent viscosity in water of at least 15 cP is needed to achieve an API fluid loss of less than 50 ml/30 min. Crosslinked HEC is suitable for high-permeability formations (Chang et al. 1998; Chang and Parlar 1999).
A derivatized HEC polymer gel exhibited excellent fluid loss control over a wide range of conditions in most common completion fluids. This particular grated gel was compatible with the formation material, and caused little or no damage to its original permeability (Nguyen et al. 1996).
Detailed measurements of fluid loss, injection, and regained permeability were taken to determine the polymer particulate's effectiveness in controlling fluid loss and to assess its ease of removal. HEC can be etherified or esterified with long chain alcohols or esters. An ether bond is more stable in aqueous solution than is an ester bond (Audibert et al. 1997).
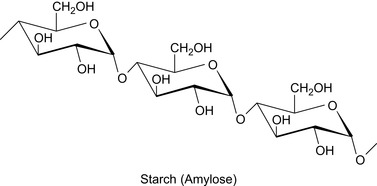
Starch
Starch, (c.f., Figure 2.3) has been traditionally used to control the fluid loss properties of a drilling mud. The characteristics of the fluid loss of several newly developed starch types with different amylose contents have been assessed. Details are shown in Table 2.5.
| Moisture/ | Amylose/ | Molecular Weight/ | |
|---|---|---|---|
| Starch Type | [%] | [%] | [kDalton] |
| Waxy | 12.9 | 0 | 20,787 |
| Low amylose | 12.7 | 26 | 13,000 |
| Intermediate amylose | 12.3 | 50 | 5115 |
| High amylose | 12.2 | 80 | 673 |
| Crosslinked high amylose | – | 80 | – |
The products are manufactured by a gelatinization process during reactive extrusion. The extrusion was carried out at 80 bar and 140∘C with a residence time of 3 min. For the crosslinked, high amylose type a chemical was introduced in the course of the extrusion process.
The starches have negligible impurities. No solvent is needed during gelatinization, and further, no waste water is produced as a by-product. Thus, these types are suitable for environmentally sensitive areas.
The presence of most of the starches in a bentonite mud reduce its API filtration at room temperature. However, the presence of the chemically modified, crosslinked high amylose did not confer any improvement to the filtration behavior of a bentonite mud.
The static fluid loss properties have been measured after thermal treatment at different temperatures. These indicate that the new starch products can be used as fluid loss additives for drilling boreholes having a bottom hole temperature of up to 150∘C (Amanullah and Yu, 2005).
The results indicate that some of the starches have static and dynamic fluid loss characteristics that are similar to, or better than, those of a widely used modified starch.
Crosslinked Starch
A crosslinked starch was described as a fluid loss additive for drilling fluids (Francis et al. 1987; Sifferman et al. 1999). The additive resists degradation and functions satisfactorily after exposure to temperatures of 250∘F (120∘C) for periods of up to 32 hours. To obtain crosslinked starch, a crosslinking agent is reacted with granular starch in an aqueous slurry. The crosslinking reaction is controlled by a Brabender viscometer test. Typical crosslinked starches are obtained when the initial rise of the viscosity of the product is between 104∘C and 144∘C, and the viscosity of the product does not rise above 200 Brabender units at temperatures less than 130∘C.
The crosslinked starch slurry is then drum-dried and milled to obtain a dry product. The effectiveness of the product is checked by the API Fluid Loss Test or other standards after static aging of sample drilling fluids containing the starch at elevated temperatures. The milled dry product can then be incorporated into the oil well drilling fluid of the drill site (Recommended practice for laboratory testing of drilling fluids (API) 2009; Standard test method for fluid loss of clay component of geosynthetic clay liners 2009).
Pregelatinized Starch
The properties of the filter cake formed by macroscopic particles can be significantly influenced by certain organic additives. The overall mechanism of water-soluble fluid loss additives has been studied by determining the electrophoretic mobility of filter cake fines. Water-soluble fluid loss additives are divided into four types according to their different effects on the negative electrical charge density of filter cake fines (Zhang et al. 1995):
1. Electrical charge density is reduced by polyethylene, glycol, and pregelatinized starch,
2. Electrical charge density is not changed by carboxymethyl starch, and
3. Electrical charge density is increased by a sulfonated phenolic resin, carboxymethyl cellulose (CMC), and hydrolyzed polyacrylonitrile.
The properties of filtrate reducers are governed by their different molecular structures. Non-ionic filtrate reducers work by completely blocking the filter cake pore, and anionic ones work by increasing the negative charge density of filter cakes and decreasing pore size. Anionic species cause further clay dispersion, but non-ionic species do not, and both of them are beneficial to colloid stability (Zhang et al. 1996).
The change of properties of the filter cake that occured as a result of salinity and polymeric additives has been studied by scanning electron microscope (SEM) photography (Plank and Gossen 1989). Fresh water muds with and without polymers such as starch, PAC, and a synthetic, high-temperature-stable polymer, were prepared, contaminated with electrolytes (NaCl, CaCl2, MgCl2), and aged at 200–350∘F (90–189∘C).
Static API filtrates before and after contamination and aging were measured. The freeze-dried API filter cakes were used for SEM studies. The filter cake structure was influenced by electrolytes, temperature, and polymers.
In an unaged, uncontaminated mud, bentonite forms a card-house structure with low porosity. Electrolyte addition increases the average filter cake pore size. Temperature causes coagulation and dehydration of clay platelets. Polymers protect bentonite from such negative effects.
Granular Starch and Mica
A fluid loss additive consisting of granular starch composition and fine particulate mica has been described (Cawiezel et al. 1996). It has been applied in a method for fracturing a subterranean formation penetrated by a borehole. The method comprises injecting the additive into the borehole and into contact with the formation, at a rate and pressure sufficient to fracture the formation, in an amount sufficient to provide fluid loss control.
Depolymerized Starch
Partially depolymerized starch provides decreased fluid losses at much lower viscosities than the corresponding starch derivatives that have not been partially depolymerized (Dobson and Mondshine 1997).
Controlled Degradable Fluid Loss Additives
A fluid loss additive for a fracturing fluid comprises a mixture of natural and modified starches plus an enzyme (Williamson et al. 1991b). The enzyme degrades the α-linkage of starch but does not degrade the β-linkage of guar and modified guar gums when used as a thickener.
Natural or modified starches are utilized in a preferred ratio of 3:7 to 7:3, with optimum at 1:1, and the mix is used in the dry form for application from the surface down the well. The preferred modified starches are carboxymethyl and hydroxypropyl derivatives. Natural starches may be those of corn, potatoes, wheat, or soy, with cornstarch the most preferred.
Blends include two or more modified starches, as well as blends of natural and modified starches. The starches can be coated with a surfactant, such as sorbitan monooleate, ethoxylated butanol, or ethoxylated nonyl phenol, which act to aid dispersion into the fracturing fluid.
A fluid loss additive is described (Williamson et al. 1991a) that helps achieve a desired fracture geometry by lowering the spurt loss and leak-off rate of the fracturing fluid into the surrounding formation by rapidly forming a filter cake with low permeability. The fluid loss additive is readily degraded after the completion of the fracturing process. The additive has a broad particulate size distribution that is ideal for use in effectively treating a wide range of formation porosities and is easily dispersed in the fracturing fluid.
This fluid loss additive comprises a blend of modified starches or blends of one or more modified starches and one or more natural starches. They have been found to maintain injected fluid within the created fracture more effectively than natural starches. The additive is subject to controlled degradation into soluble products by a naturally proceeding oxidation reaction, or by bacterial attack by bacteria that are naturally present in the formation. The oxidation may be accelerated by adding oxidizing agents, such as persulfates and peroxides.
Multimodal Distributed Polymers
A polymeric material with a multimodal distribution refers to a material that contains at least two pluralities of polymer molecules, which have different average molecular weights.
Polymeric materials that are found in nature are generally monomodal with a rather narrow polydispersity (P) (Weaver et al. 2010). The polydispersity is in general defined as the ratio of two different averages of molecular weight, i.e., the ratio of weight average Mw to the number average, Mn. These averages are defined as:
(2.3)
Thus the polydispersity is:
(2.4)
Polydisperse materials can be obtained by mixing chemically equivalent materials that have different molecular weights. The latter can be obtained by synthesis of artificial materials under different conditions, or by controlled degradation of naturally occurring polymers.
Polymeric materials with small polydispersities may not be able to fill the pore spaces sufficiently to prevent fluid loss into the formation. For example, if the polymer molecules are all relatively large, all of them may be unable to fit within certain pore throats in the formation to plug the pore spaces therein.
On the other hand, polymers with a multimodal distribution contain a number of molecules that will fill and plug small pores, a number of molecules that will plug medium-sized pores, etc. The principle developed above is suitable for polymers as fluid loss additives in general, i.e., it is independent of the particular chemical nature of the polymer.
A wide variety of examples of polymers have been listed that operate according to this principle. Natural polymers include polysaccharides, while synthetic polymers include 2,2′-azobis(2,4-dimethyl valeronitril) (Weaver et al. 2010,pp. 5–15).
Borate Crosslinkers
Organic polyhydroxy compounds with hydroxyl moieties positioned in the cis-position on adjacent carbon atoms, or on carbon atoms that have a 1,3-relationship can react with borates to form five or six membered ring complexes. The reaction is fully reversible with changes in pH.
Depending on concentration of the polymer and the borate anion, the crosslinking reaction may produce useful gels. Aqueous borate concentrates that provide a controllable crosslink time are very useful. Sparingly soluble borate suspensions are suitable for hydraulic fracturing operations, since they adjust the time of crosslinking more consistently (Dobson et al. 2005).
Examples of borate minerals are shown in Table 2.6.
| Mineral | Formula |
|---|---|
| Probertite | NaCaB5O9 × 5H2O |
| Ulexite | NaCaB5O9 × 8H2O |
| Nobleite | CaB6O10 × 4H2O |
| Gowerite | CaB6O10 × 5H2O |
| Frolovite | Ca2B4O8 × 7H2O |
| Colemanite | Ca2B6O11 × 5H2O |
| Meyerhofferite | Ca2B6O11 × 7H2O |
| Inyoite | Ca2B6O11 × 13H2O |
| Priceite | Ca4B10O19 × 7H2O |
| Tertschite | Ca4B10O19 × 20H2O |
| Ginorite | Ca2B14O23 × 8H2O |
| Pinnoite | MgB2O4 × 3H2O |
| Paternoite | MgB2O13 × 4H2O |
| Kurnakovite | Mg1B6O11 × 15H2O |
| Inderite | Mg2B6O11 × 15H2O |
| Preobrazhenskite | Mg3B10O18 × 4½H2O |
| Hydroboracite | CaMgB6O11 × 6H2O |
| Inderborite | CaMgB6O11 × 11H2O |
| Kaliborite (Heintzite) | KMg2B11O19 × 9H2O |
| Veatchite | SrB6O10 × 2H2O |
Guar
A hydrophobically modified guar gum can be used as an additive for drilling, completion, or servicing fluids (Audibert and Argillier, 1996 and Audibert and Argillier, 1998). The modified gum is used together with polymers or reactive clay.
Hydroxypropyl Guar Gum
Hydroxypropyl guar gum gel can be crosslinked with borates (Miller et al. 1996), titanates, or zirconates. Borate crosslinked fluids and linear HEC gels are the most commonly used fluids for highworddash permeability fracture treatments. They are used for hydraulic fracturing fluid under high temperature and high shear stress.
Succinoglycan
Succinoglycan is a biopolymer, which has been shown to possess a combination of desirable properties for fluid loss control (Lau 1994). These include ease of mixing, cleanliness, shear thinning rheology, temperature-insensitive viscosity below its transition temperature (Tm), and an adjustable transition temperature (Tm) over a wide range of temperatures. Succinoglycan fluids rely solely on viscosity to reduce fluid loss. It does not form a hard-to-remove filter cake, so avoiding considerable formation damage.
Based on these findings, succinoglycan has been used successfully as a fluid loss pill before and after gravel packing in more than 100 offshore wells. Calculations based on laboratory-measured rheology and field experience have shown it to be effective in situations in which HEC is not. Fluid loss, even over 40 barrels/h, was reduced to several barrels per hour after application of a properly designed succinoglycan pill. Most wells experienced no problem in production after completion.
Succinoglycan can be degraded with an internal acid breaker (Bouts et al. 1997). The formation damage that results from the incomplete back-production of viscous fluid loss control pills can be minimized if a slow-acting internal breaker is employed. In particular, core-flow tests have indicated that combining a succinoglycan-based pill with a hydrochloric acid internal breaker enables a fluid loss system with sustained control, followed by delayed break back that creates only low levels of impairment. To describe the delayed breaking of the succinoglycan/hydrochloric acid system, a model, based on bond breaking rate, has been used.
With this model, it is possible to predict the change in the rheological properties of the polymer as a function of time, for various formation temperatures, transition temperatures of the succinoglycan, and acid concentrations. The model can be used to identify optimal formulations of succinoglycan and acid breaker on the basis of field requirements, such as the time interval over which fluid loss control is needed, the overbalance pressure a pill should be able to withstand, and the brine density required.
Polyether-modified Polysaccharides
Some cellulose compounds are shown in Figure 2.4. Compositions that contain mixtures of metal hydroxides and a polysaccharide, partially etherified with hydroxyethyl and hydroxypropyl groups, are used as fluid loss additives for aqueous, clay-mineral-based drilling muds (Plank 1993).
Scleroglucan
A combination of graded calcium carbonate particle sizes, a non-ionic polysaccharide of the scleroglucan type, and a modified starch, has been claimed for use in fluid loss formulations (Johnson 1996). It is important that the calcium carbonate particles have a wide size range distribution to prevent filtration or fluid loss into the formation. Because the filter cake particles do not invade the wellbore due to the action of the biopolymer and the starch, no high-pressure spike occurs during the removal of the filter cake.
The rheological properties of the fluid allow it to be used in a number of applications where protection of the original permeable formation is desirable. These include drilling, fracturing, and controlling fluid losses during completion operations, such as gravel packing or well workovers.
Gellan
It has been found that gellan has good characteristics as a filtrate reducer in water-based drilling fluids (Dreveton et al., 1995 and Dreveton et al., 1998). Preferential use is made of native gellan, which has a considerable gelling capacity and good solubility. It should be noted that native gellan contains cellular debris or other insoluble residue. Xanthan gum has been used extensively in the oil industry as a viscosifier for various applications (Navarrete et al. 2000). Deacetylated xanthan gum is used in guar-free compositions instead of guar (Langlois 1999).
Humic Acid Derivates
Polysulfonated humic acid is a drilling fluid filtrate loss additive that is composed of three mud additives: sulfonated chromium humate, sulfonated phenolic resin, and hydrolytic ammonium polyacrylate (Tan 1990).
The field application and the effectiveness of this material, especially in extra-deep wells, in sylvite and undersaturated salt muds, have been described. It resists high temperature, salt concentration, and calcium contamination. This type of drilling fluid has stable properties and good rheological characteristics, and it can improve cementing quality.
Oil-based Well Working Fluids
Adducts of aminoethylethanol amine and polyethylene amines with humic acid and fatty acids (Patel and McLaurine 1992) are useful as fluid loss additives in oil-based drilling muds (OBMs) (House and Granquist 1986).
In addition, a fluid loss additive for OBM consisting of fatty acid compounds and lignite or humic acid, an oil-soluble or oil-dispersible amine with phosphoric acid, or an aliphatic amide or hydroxyamide (Coates et al. 1990), has been described.
Lignosulfonates
Grafted Lignin or Lignite
In hydraulic cement slurries, fluid loss additives that are based on sulfonated or sulfomethylated lignins have been described.
Sulfonated or sulfomethylated lignins are reacted with phenolworddash blocking reagents, such as ethylene oxide, propylene oxide, or 1,2-butylene oxide (Schilling 1990). The fluid loss and thickening time characteristics of the cement slurry so produced is altered, either by increasing the molecular weight of the lignin through crosslinking with formaldehyde or epichlorohydrin, or by adding agents such as sodium sulfite, sodium metasilicate, sodium phosphate, and sodium naphthalene sulfonate.
Another method of altering lignins is amination with a polyamine and an aldehyde (Schilling 1991). The formulation also contains sodium carbonate, sodium phosphate, sodium sulfite, sodium metasilicate, or naphthalene sulfonate. The sulfonated or sulfomethylated aminated lignin shows less retardation (shorter thickening time) than a sulfonated or sulfomethylated lignin without the attached amine.
Lignite can be grafted with synthetic comonomers to obtain lignite fluid loss additives (Huddleston and Williamson 1990). The comonomers can be AMPS, N,N -dimethylacrylamide, acrylamide (AAm), vinylpyrrolidone, vinylacetate, acrylonitrile, dimethyl amino ethyl methacrylate, styrene sulfonate, vinylsulfonate, dimethyl amino ethyl methacrylate methyl chloride quaternary, or AA and its salts.
Various polymers, for example, lignin, lignite, derivatized cellulose polyvinyl alcohol (PVA), polyethylene oxide, polypropylene oxide, and polyethyleneimine, can be used as the backbone polymer onto which the other groups are grafted (Fry et al. 1987). The grafted pendant groups can be AMPS, acrylonitrile, N,N -dimethylacrylamide, AA, N,N -dialkylaminoethylmethacrylate, and their salts. One commercial example of such compounds is HALAD™ 413 (Lewis et al. 2008; Morgan et al. 2008).
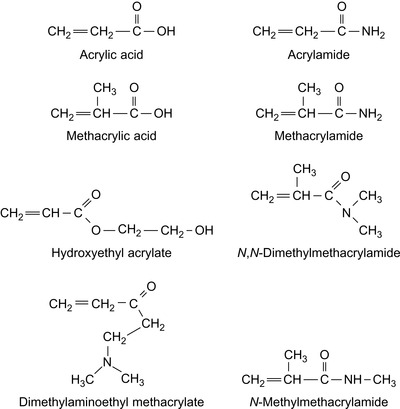
A polymeric composition for reducing fluid loss in drilling muds and well cement compositions is obtained by the free radical polymerization of a water-soluble vinyl monomer in an aqueous suspension of lignin, modified lignins, lignite, and brown coal (Giddings and Williamson 1987; Williamson 1989). The vinyl monomers can be methacrylic acid, methacrylamide, hydroxyethyl acrylate, hydroxypropyl acrylate, vinylacetate, methylvinylether, ethylvinylether, N -methylmethacrylamide, N,N -dimethylmethacrylamide, vinylsulfonate, and additional AMPS. In this process, a grafting process to the coals by chain transfer may occur. Vinyl ethers are shown in Figure 2.5 and some acrylics are shown in Figure 2.6.
The polymers are prepared by common polymerization techniques (Zhang et al. 1990). For example, they are made by providing a foamed, aqueous solution of water-soluble monomeric material, in which polymerization is started by adding an initiator. The monomeric material is exothermically polymerizing to form a foamed gel, which is then comminuted.
Preferably, the polymerization temperature is held below 60∘C for at least the first 10 min of the reaction and it then rises due to its exothermic nature. Graft copolymers that can be made by this technique and that are of particular value as fluid loss additives are formed from a polyhydroxy polymer, a sulfonate monomer, further AAm and AA.
Vinyl-grafted wattle tannin comprises a wattle tannin grafted with AMPS and small amounts of AAm (Huddleston et al. 1992). The wattle tannin is present at levels of 2–14%, and the AMPS is present at 98–84% accordingly.
Greek Lignites
Lignites that originated from Greece have been tested for their ability to control the filtration characteristics of water-bentonite suspensions (Kelessidis et al., 2009 and Kelessidis et al., 2007). The properties of a series of lignite samples from various peat/lignite deposits in Greece were compared with a commercial lignite product.
The samples were characterized with respect to their contents of humic and fulvic acids, humins, oxygen, ash, and their cation exchange capacity.
Most samples show a good filtration control when used in water-bentonite suspensions, even after exposure to temperatures of 177∘C, and some were found to be superior to the commercial product. The fluid loss was dependent on the humic and fulvic acid content and thus also on the total organic content of the samples. The correlation is shown in Figure 2.7.
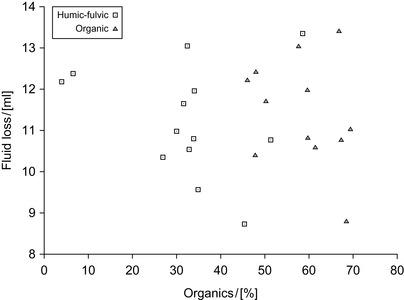 |
| Figure 2.7 Fluid loss and content of organics (Kelessidis et al., 2007). |
(2.5)
Here, Va is the volume of fluid loss after aging and Vb is the fluid loss of the base fluid under standardized conditions.
Better performance was observed after addition of 3% w/v lignite. Total humic and fulvic acids as percentage of dry lignite matter and the organic matter as lignite percentage showed a weak inverse correlation with the fluid loss volumes.
The rheograms of bentonite suspensions in its hydrated and thermally aged states has been measured. Three percent lignite of varying origin has been added to the aged suspensions. The resulting rheograms are given in Figure 2.8.
 |
| Figure 2.8 Rheograms of bentonite-lignite formulations (Kelessidis et al., 2009). |
The addition of lignite reduces the shear stress. In the high shear rate range, the rheograms exhibit a linear behavior (Kelessidis et al., 2009). The reduction in the shear stress is very similar for all the lignite types tested.
Synthetic Polymers
Polyorthoesters
Aliphatic polyesters degrade chemically by hydrolytic cleavage. The process of hydrolysis can be catalyzed by both acids and bases. During the hydrolysis, carboxylic end groups are formed during chain scission, and this may enhance the rate of further hydrolysis. This mechanism is known as autocatalysis, and it is thought to make polyester matrices more bulk-eroding.
Among the esters, polyorthoesters and aliphatic polyesters, i.e., polylactides are preferred. They can be synthesized either from lactic acid by a condensation reaction, or more commonly by ring opening polymerization of a cyclic lactide monomer.
The degradation by hydrolysis should proceed rather slowly over time. In fracturing operations, the material should not begin to degrade until after the proppant has been placed in the fracture, because slow degradation helps to provide fluid loss control during its placement (Todd et al. 2006). An example of a fracturing fluid composition is given in Table 2.7.
| a)Halliburton Energy Services, Inc. | ||
| Compound | Tradenamea | % |
|---|---|---|
| Water | ||
| Potassium chloride | 1 | |
| De-emulsifier | LO-SURF 300 | 0.05 |
| Polyester | 0.15 | |
| Guar | 0.2 | |
| Buffer (CH3COOH) | BA-20 | 0.005 |
| Caustic | MO-67 | 0.1 |
| Borate crosslinking agent | CL-28M | 0.05 |
| Gel breaker (NaClO3) | VICON NF | 0.1 |
| Bactericide (2,2-dibromo-3-nitrilopropionamide) | BE-3S | 0.001 |
| Bactericide (2-Bromo-2-nitro-1,3-propanediol) | BE-6 | 0.001 |
| Fracturing sand | 50 | |
Polyhydroxyacetic Acid
A low molecular weight condensation product of hydroxyacetic acid with itself, or with compounds containing other hydroxy acid, carboxylic acid, or hydroxy carboxylic acid moieties has been suggested as a fluid loss additive (Bellis and McBride 1987). Production methods of the polymer have been described.
The reaction products are ground to a particle size of 0.1–1500 μ. The condensation product can be used as a fluid loss material in a hydraulic fracturing process in which the fracturing fluid comprises a hydrolyzable, aqueous gel.
The hydroxyacetic acid condensation product hydrolyzes under formation conditions to produce hydroxyacetic acid, which breaks the aqueous gel autocatalytically and eventually provides the restored formation permeability without the need for the separate addition of a gel breaker (Cantu et al., 1989, Cantu et al., 1990 and Cantu et al., 1993; Casad et al. 1991).
Polydrill
Polydrill is a sulfonated polymer, used for filtration control in water-based drilling fluids (Ujma and Plank 1987). Tests have demonstrated the product's thermal stability up to 200∘C and its outstanding electrolyte tolerance. It can be used in NaCl -saturated drilling fluids, as well as in muds containing up to 75,000 ppm of calcium or 100,000 ppm of magnesium. A combination of starch with Polydrill was used successfully while drilling several wells. The deepest hole was drilled with 11–22 kgm−3 of pregelatinized starch and 2.5–5.5 kgm−3 of Polydrill to a depth of 4800m. Field experience with the calcium tolerant starch/Polydrill system useful up to 145∘C has been discussed in detail (Ujma et al. 1987).
In dispersed muds (e.g., lignite or lignosulfonate), minor Polydrill addition results in a significantly improved high-temperature, high-pressure filtrate. Major benefits come from a synergism of the polymer with starch and polysaccharides, since it exerts a thermally stabilizing effect on those polymers.
In conventional or clay-free drilling and completion fluids, Polydrill can be used by itself or in combination with other filtrate reducers for various purposes (Plank 1990). Handling and discharge of the product, as well as the waste mud was found to create no problem in the field.
Polymer of Monoallylamine
A water-soluble polymer of monoallylamine, c.f., Figure 2.9, can be used in conjunction with a sulfonated polymer such as a water-soluble lignosulfonate, condensed naphthalene sulfonate, or sulfonated vinyl aromatic polymer to minimize fluid loss from the slurry during well cementing operations (Roark et al., 1986 and Roark et al., 1987). The polymer may be a homopolymer or a copolymer, and may be crosslinked or not.
These components react with each other in the presence of water to produce a gelatinous material that tends to plug porous zones and to minimize premature water loss from the cement slurry into the formation.
Polyphenolics
Organophilic polyphenolic materials for OBMs have been described (Cowan et al. 1988). The additives are prepared from a polyphenolic material and one or more phosphatides, these being phosphoglycerides obtained from vegetable oils, preferably commercial lecithin.
The oxidized, sulfonated, or sulfomethylated derivatives of humic acids, lignosulfonic acid, lignins, phenolic condensates, or tannins may serve as polyphenolic materials.
A fluid loss additive is described that uses graded calcium carbonate particle sizes and a modified lignosulfonate (Johnson and Smejkal 1993). Optionally, a thixotropic polymer, such as a wellan or xanthan gum polymer is used to keep the CaCO3 and lignosulfonate in suspension.
In this application, it is important that the calcium carbonate particles are distributed across a wide size range to prevent filtration or fluid loss into the formation. Furthermore, the lignosulfonate must be polymerized enough to reduce its water solubility. The modified lignosulfonate is necessary for the formation of a filter cake essentially on the surface of the wellbore.
Because the modified lignosulfonate, prevents the filter cake particles from invading the wellbore no high-pressure spike occurs during the removal of the filter cake, which would indicate damage of the formation and wellbore surface. The additive is useful in fracturing fluids, completion fluids, and workover fluids.
Tests have shown that a fluid loss additive based on a sulfonated tannic-phenolic resin is effective for fluid loss control at high temperatures and pressures, and it exhibits good resistance to salt and acid (Huang 1996).
Latex
Polymeric latex, when added to a water-based drilling fluid, can reduce the rate at which the drilling fluid invades through the borehole wall during drilling, by providing a deformable latex film or seal on the borehole wall.
The permeability of the seal is sufficient to at least partially block the transmission of the fluid, which gives a great improvement in osmotic efficiency (Halliday et al., 2007 and Halliday et al., 2008). Latexes may include the following components (Reddy and Palmer 2009):
• Vulcanizable groups, e.g., butadiene,
• Vulcanizing agents such as sulfur, 2,2′-dithiobisbenzothiazole, organic peroxides, azo compounds, alkylthiuram disulfides, and selenium phenolic derivatives,
• Vulcanization accelerators, including fatty acids such as stearic acid, metallic oxides such as zinc oxide, aldehyde amine compounds, guanidine derivates, and disulfide thiuram compounds,
• Vulcanization retarders such as salicylic acid, sodium acetate, phthalic anhydride, and N -cyclohexyl thiophthalimide,
• Defoamers, and
• Fillers to increase or decrease the treatment density as required.
Latex emulsions are also used in cement compositions to reduce fluid loss (Reddy and Palmer 2009), and they can be also used to reduce the brittleness of the sealant compositions and thus improve their flexibility.
Further, latex emulsions prevent gas migration. This property is useful when the sealant starts curing, i.e., the sealant composition changes from a non-viscous fluid to a highly viscous mass. During this transition phase, the sealant mass can no longer transmit hydrostatic pressure.
When the pressure exerted on the formation by the sealant composition falls below the pressure of the gas in the formation, the gas initially migrates into and through the composition. This migration causes flow channels to form in the sealant composition, which permit further migration of the gas after the sealant composition sets (Reddy and Palmer 2009).
Polymeric latexes can also be incorporated into OBMs. In these fluids, the polymer latex seals can be formed without the need for a precipitating agent, a surfactant or any salt in the water phase (Halliday et al. 2007).
Where water is present at the continuous phase, the latex is in turn suspended in a hydrocarbon base fluid, which contains some emulsifier. Under optimal conditions, the polymer latex can be simply mixed with the hydrocarbon base fluid without the need for adding any emulsifier.
Some latex products exhibit a synergistic effect with aluminum complexes, with regard to their sealing properties.
Latex is a carboxylated styrene/butadiene copolymer or a sulfonated styrene/ butadiene copolymer. Examples are commercially available, e.g., Gencal 7463 (Halliday et al. 2008).
Sulfonated latexes can be often used in the absence of a surfactant, which may simplify the formulation and transportation of the drilling fluid additives to the production sites. A precipitating agent, such as sodium aluminate is preferably used (Halliday et al. 2008).
In the course of testing these materials, photomicrographs showed an accumulation of latex along microfractures in the shale. Since the volume and the velocity of filtration flow into these cracks is very small, filtration alone cannot account for this accumulation. It seems that precipitation effects are responsible for these findings.
When sufficient latex is deposited to bridge the crack opening, the fracture is sealed and differential pressure is established across the latex. The differential pressure consolidates the latex deposit into a solid seal.
Optionally, a surfactant that behaves as an emulsifier and a wetting agent and a weighting agent, e.g., calcium carbonate, barite, or hematite, is included. Suitable emulsifiers and wetting agents include surfactants; ionic surfactants such as fatty acids, amines, amides, and organic sulfonates; and mixtures of any of these with non-ionic surfactants such as ethoxylated surfactants. The water-in-oil emulsion may consist of an oil phase, a water phase (salt or fresh), a surfactant, a weighting agent, and salts or electrolytes (Bailey 2001; Hernandez et al., 1999a and Hernandez et al., 1999b).
Stable drilling fluid systems have been formulated with latex that remains dispersed and flexible in highly saline fluids. The stabilization is caused by two factors (Halliday et al. 2007):
1. The ultra-fine, deformable latex particles with a diameter of 0.2 μ mechanically seal shale microfractures and physically prevent further intrusion of drilling fluids into sensitive shale zones.
2. The co-precipitation of the latex with precipitating agents such as aluminum complexes produces a semi-permeable membrane on shale surfaces that chemically improves the osmotic efficiency between the fluid and the borehole.
Colloidally Stabilized Latex
Latex emulsions that are prepared by conventional emulsion polymerization become unstable in the presence of salt. In order to improve the salt tolerance of latex emulsions in sealant compositions, it is usual to add surfactants, such as ethoxylated nonyl phenol sulfates. Alternatively, a cheaper solution is to use a colloidally stabilized latex, which contains a protective colloid. Suitable examples include partially and fully hydrolyzed PVA, cellulose ethers, starch derivatives, natural and synthetic gums, and synthetic copolymers (Reddy and Palmer 2009).
As another alternative, the backbone of the latex may be modified by incorporating moieties with surfactants properties. Since the surfactant is then chemically bound to the polymer, it cannot be easily removed. An example of such a material is a carboxylated butadiene acrylonitrile latex. The carboxyl groups confer electrical charge to the polymeric backbone.
Alternatively, functionalized silane can be introduced into the polymer. This moiety is capable of adsorbing the protective colloid. A suitable functionalized silane is γ-mercaptopropylspacetrimethoxyspacesilane.
It has been demonstrated that a carboxyl modified latex can produce slurries with satisfactory properties without needing latex-stabilizing surfactants (Reddy and Palmer 2009).
Polyvinyl Alcohol
Partially hydrolyzed (PVAc), a crosslinker for the polymer, and other additives such as calcium sulfate can be used in cementing casing strings (Moran and Murray 1991). PVAc is not totally water-soluble below 50∘C, but is instead water swellable.
It is believed that the individual PVAc particles swell and soften to form small gel-balls in the slurry. These gel-balls deform by flattening and become a part of the filter cake, hence greatly reducing its permeability, thus giving good fluid loss control. Because PVAc is not totally water soluble, it does not significantly increase the viscosity of the slurry, and it does not delay the setting of the cement. It also has high-temperature properties that are relatively insensitive to external conditions.
PVAc can be crosslinked with a crosslinker that is present in a molar concentration, relative to monomer residues, of 0.01–1.0%. This may be formaldehyde, acetaldehyde, glyoxal, glutaraldehyde, maleic acid, oxalic acid, dimethylurea, polyacrolein, diisocyanate, divinylsulfonate, or a chloride of a diacid (Audebert et al., 1994, Audebert et al., 1996 and Audebert et al., 1998).
Polyethyleneimine
A reported liquid fluid loss-reducing additive for well cementing compositions consists of water, polyethyleneimine, an alkali metal salt of alkyl benzene sulfonic acid, and an alkali metal salt of naphthalene sulfonic acid, condensed with formaldehyde (Brake and Chatterji 1994). The polyethyleneimine has a molecular weight of 40–60 kDalton and is present at 50–55% by weight of the additive. The alkali metal salt of the alkyl benzene sulfonic acid is sodium dodecyl benzene sulfonate, and this is present at 3–4% of the additive. The alkali metal salt of the naphthalene sulfonic acid that is condensed with formaldehyde is sodium naphthalene sulfonate.
The sodium naphthalene sulfonate -formaldehyde condensation product has a molecular weight of 1.4–2.4 kDalton, and the condensation product is present in an amount of 3–4% of the additive. The alkyl group of the alkyl benzene sulfonic acid salt contains 8–16 carbon atoms.
Acrylics
Permeability Control
The water permeability of a formation can be controlled by the addition of a water-soluble copolymer, formed from a hydrophobic or hydrophobically modified hydrophilic monomer and a hydrophilic monomer (Zamora et al. 2007). Suitable monomers are shown in Table 2.8.
| Hydrophobic Monomers |
|---|
| Alkyl acrylates |
| Alkyl methacrylates |
| Alkyl acrylamides |
| Alkyl methacrylamides |
| Alkyl dimethyl ammonium ethyl methacrylate halides |
| Alkyl dimethyl ammonium propyl methacrylamide halides |
| C-16 alkyl(n -hexadecyl)dimethyl ammonium ethyl methacrylate bromide |
| Octadecyl methacrylate |
| Hydrophilic Monomers |
| Acrylamide |
| 2-Acrylamido-2-methyl-1-propane sulfonic acid |
| N,N -Dimethylacrylamide |
| Vinylpyrrolidone |
| Acrylic acid |
| Dimethyl aminopropyl methacrylamide |
| Trimethylammonium ethyl methacrylate chloride |
| Methacrylamide |
| Hydroxyethyl acrylate dimethyl amino ethyl methacrylate |
The polymerization proceeds in an aqueous solution or emulsion. 2,2′-Azo bis(2-amidinopropane)dihydrochloride is added as radical initiator (Zamora et al. 2007). This azo-type initiator is water soluble. In order to quaternize the polymers, if desired, benzylcetyldimethyl ammonium bromide can be added.
Copolymers
Homopolymers and copolymers from amido sulfonic acid- or salt-containing monomers can be prepared by reactive extrusion, preferably in a twin screw extruder (Sopko and Lorentz 1991). The process produces a solid polymer. Copolymers of AAm, N -vinyl-2-pyrrolidone, and (NaAMPS) are proposed to be active as fluid loss agents. Another component of the formulations is the sodium salt of naphthalene formaldehyde sulfonate (Boncan and Gandy 1986). The fluid loss additive is mixed with hydraulic cements in suitable amounts.
A fluid loss additive for hard brine environments has been developed (Stewart et al. 1988), consisting of a hydrocarbon, an anionic surfactant, an alcohol, a sulfonated asphalt, a biopolymer, and optionally an organophilic clay, a copolymer of N -vinyl-2-pyrrolidone and NaAMPS. Methylenebisacrylamide can be used as a crosslinker (Patel 1998). Crosslinking imparts thermal stability and resistance to alkaline hydrolysis.
Terpolymers and tetrapolymers have been proposed as fluid loss additives for drilling fluids (Stephens 1988; Stephens and Swanson 1992). The constituent monomers are a combination of non-ionic monomers and ionic monomers. The non-ionic monomer can be AAm>, N,N -dimethylacrylamide, N -vinyl-2-pyrrolidone, N -vinylacetamide, or dimethyl amino ethyl methacrylate. Ionic monomers are AMPS, sodium vinylsulfonate, and vinylbenzene sulfonate. The terpolymer should have a molecular weight between 0.2 MDalton and 1 MDalton.
A formulation consisting of AMPS, AAm, and itaconic acid has been proposed (Garvey et al. 1988). Such polymers are used as fluid loss control additives for aqueous drilling fluids, and are advantageous when used with lime or gypsum-based drilling muds containing soluble calcium ions.
For sea water muds, another example (Bardoliwalla 1986b) is a copolymer of 10% AMPS and 90% AA in its sodium salt form. The polymers have an average molecular weight of 50–1000 kDalton.
A terpolymer from a family of intramolecular polymeric complexes (i.e., polyampholytes), which are terpolymers of AAm–methyl styrene sulfonate-methacrylamido propyltrimethyl ammonium chloride has been reported (Peiffer et al. 1986; Audibert-Hayet et al. 1998).
A terpolymer formed from ionic monomers AMPS, sodium vinylsulfonate, or vinylbenzene sulfonate itaconic acid, and a non-ionic monomer, for example, AAm, N,N -dimethylacrylamide, N -vinylpyrrolidone, N -vinylacetamide, and dimethyl amino ethyl methacrylate, is used as a fluid loss agent in oil well cements (Savoly et al. 1987).
The terpolymer should have a molecular weight of 0.2–1 M Dalton, and comprises AMPS, AAm, and itaconic acid. Such copolymers also serve in drilling fluids (Zhang and Ye 1998).
A tetrapolymer consisting of 40–80 molmathchar45% of AMPS, 10–30 molmathchar45% of vinylpyrrolidone, 0–30 molmathchar45% of AAm, and 0–15 molmathchar45% of acrylonitrile was also suggested as a fluid loss additive (Lange and Boehmer 1988). Even at high salt concentrations, these polymers yield high-temperature-stable, protective colloids that provide minimal fluid loss under pressure.
For water-based drilling fluids, water-soluble polymers are adequate, typically polyacrylamide, but they have a limited temperature stability.
Polymers based on AAm have been developed that exhibit effective rheological properties and high-temperature/high-pressure (HTHP) filtration control at temperatures of 260∘C or more (Jarrett and Clapper 2010). They are terpolymers composed of AAm, AMPS, or alkali metal salts, and a third monommer that can be an acrylate, N -vinyl lactam, or N-vinylpyridine (Jarrett and Clapper 2010). Examples of copolymers are listed in Table 2.9.
| AMPS 2-Acrylamido-2-methyl-1-propane sulfonic acid | |||||||||||
| AAm Acrylamide | |||||||||||
| NVP N-Vinyl-2-pyrrolidone | |||||||||||
| NaAMPS Sodium AMPS | |||||||||||
| DMAAm N,N-Dimethylacrylamide | |||||||||||
| Monomer 1 | mol-% | Monomer 2 | mol-% | Monomer 3 | mol-% | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| AMPS | 10 | AAm | 90 | – | – | ||||||
| AMPS | 20 | AAm | 80 | – | – | ||||||
| AMPS | 40 | AAm | 60 | – | – | ||||||
| AMPS | 37.5 | AAm | 50 | Acrylate | 12.5 | ||||||
| AMPS | 55 | AAm | 15 | NVP | 30 | ||||||
| Monomer 1 | % w/w | Monomer 2 | % w/w | Monomer 3 | % w/w | ||||||
| NaAMPS | 90 | DMAAm | 10 | – | – | ||||||
In order to assist effective seal forming for filtration control, a plugging agent is added (Jarrett and Clapper 2010). Examples of suitable plugging agents include the following: sized sulfonated asphalt, limestone, marble, mica, graphite, cellulosics and lignins, and cellophanes.
Other additives may be used in the drilling fluid system, including shale stabilizers, further filtration control additives, suspending agents, dispersants, anti-balling additives, lubricants, weighting agents, seepage control additives, lost circulation additives, drilling enhancers, penetration rate enhancers, corrosion inhibitors, buffers, gelling agents, crosslinking agents, salts, biocides, and bridging agents (Jarrett and Clapper 2010).
After static aging up to 260∘C for 16 h, the HTHP filtrate was measured extensively, with results of less than 25 cm3min−1 (Jarrett and Clapper 2010).
Similar copolymers with N -vinyl-N -methylacetamide as a comonomer have been proposed for hydraulic cement compositions (Ganguli 1992).
The polymers are effective at well bottom hole temperatures ranging from 93–260∘C (200–500∘F) and are not adversely affected by brine. Terpolymers of 30–90 molmathchar45% AMPS, 5–60 molmathchar45% of styrene, and residual AA are also suitable for well cementing operations.
A fluid loss additive useful for cementing oil and gas wells is a blend of a copolymer of AAm/vinyl imidazole (Crema et al., 1991 and Crema et al., 1993; Kucera et al. 1989). The second component in the blend is a copolymer of vinylpyrrolidone and the sodium salt of vinylsulfonate. Details are given in Table 2.10. The copolymers are mixed together in proportions between 20:80 and 80:20. Sodium or potassium salts or a sulfonated naphthalene formaldehyde condensate can be used as a dispersant.
| Copolymer | Molecular Weight/[ kDalton ] |
|---|---|
| Acrylamide/vinyl imidazole | 100 to 3000 |
| Vinylpyrrolidone/sodium vinylsulfonate | 100 to 3000 |
An N -vinylpyrrolidone/AAm random copolymer (0.05–5.0%) is used for cementing compositions (Cheung 1993; Le et al. 1998). Furthermore, a sulfonate-containing cement dispersant is necessary. The additive can be used in wells with a bottom hole temperature of 80–300∘F (30–150∘C).
The fluid loss additive mixture is especially effective at low temperatures, for example, below 100∘F (40∘C) and in sodium silicate–extended slurries. For aqueous cement slurries a copolymer of N -vinylpyrrolidone and a salt of styrene sulfonic acid has been proposed (Sedillo et al. 1987). A naphthalene sulfonic acid salt condensed with formaldehyde serves as a dispersant.
The fluid loss control of aqueous, clay-based drilling mud compositions is enhanced by the addition of a hydrolyzed copolymer of AAm and an N -vinylamide (Costello et al. 1990). The copolymer, which is effective over a broad range of molecular weights, contains at least 5 molmathchar45% of the N -vinylamide units, which are hydrolyzed to N -vinylamine units. The copolymers can be made from various ratios of N -vinylamide and AAm by using common radical polymerization techniques.
N -Vinylamide can be polymerized by the inverse emulsion polymerization technique (Lai and Vijayendran 1989). The polymers so produced are used for cementing compositions for oil and gas wells. The method for preparing the inverse, or water-in-oil, emulsion involves colloidally dispersing an aqueous solution containing 10–90% water-soluble N -vinylamide in a hydrocarbon liquid, using a surfactant with a hydrophilic-lipophilic balance value between 4 and 9. The weight ratio of monomer-containing aqueous solution to hydrocarbon liquid is preferably from 1:2 to 2:1. To initiate the polymerization, an azo-type free radical initiator is used. The resultant high molecular weight polymer emulsion has a low viscosity, ranging from 2 to less than 10 cP at 15% solids (60 rpm Brookfield and 20∘C), thus eliminating problems of solution viscosity that arise when it is prepared by a solution polymerization process.
Copolymers of styrene with AMPS that have fluid loss capabilities suitable for use in well cementing operations have been described (Brothers 1989). The styrene is present at 15–60 molmathchar45%, and the AMPS at 40–85 molmathchar45%. The polystyrene units are not hydrophilic, so AMPS will affect the solubility in water. AMPS is shown in Figure 2.10.
Copolymers of mainly AA with 2–20% of itaconic acid are described as fluid loss additives for aqueous drilling fluids (Bardoliwalla 1986a). The polymers have an average molecular weight of 100–500 kDalton and are water-dispersible. They are advantageous when used with muds containing soluble calcium and muds containing chloride ions, such as sea water muds.
Copolymers from the monomers AMPS, diallyldimethylammonium chloride (DADMAC), N-vinyl-N-methylacetamide, AAms, and acrylates are particularly useful as fluid loss additives (Hille et al. 1996). The molecular weights of the copolymers range from 200–1000 kDalton, and are used in suspensions of solids in aqueous systems, including saline, as water binders. In these systems, the water release to a formation is substantially reduced by the addition of one or more of these copolymers.
A copolymer of AMPS and other vinyl monomers produces a suitable formulation for filtration reducers, which has good temperature resistance (over 200∘C), and good tolerance to salts and calcium compounds (Li et al. 1996).
Polymers or copolymers of N -vinyl lactam monomers or vinyl-containing sulfonate monomers produce an additive for reducing the water loss and enhancing other properties of well-treating fluids in high-temperature subterranean environments. Organic compounds like lignites, tannins, and asphaltic materials are added as dispersants (Bharat 1990).
Oil-soluble Styrene Acrylate Copolymers
Oil-soluble polymers in the form of a gel can be used as a fluid loss additive for drilling WBM compositions to improve the high-temperature stability. Also, improved shear resistance can be obtained (Guichard et al., 2007 and Guichard et al., 2008). Such oil-soluble polymers are copolymers based on acrylates, styrene, vinyl toluene, butadiene, and others (Braden 2006).
Crosslinking monomers include allyl maleate, divinylbenzene, and multifunctional acrylates, as well as methacrylates. The monomers are detailed in the literature (Guichard et al. 2006). These acrylic copolymers are also used as binders for masonry applications, concrete and metal protection, intumescent coatings (Magnet et al. 2008), and as modifiers for toner compositions.
Oil-soluble polymers are highly efficient in small proportions as a fluid loss reducers, and may be incorporated in the mud at levels of only 0.5–2.5%. The polymer is added to a WBM prepared by conventional methods, either to replace the conventional fluid loss reducers, or in addition (Guichard et al. 2008). Mud formulations have been given in detail elsewhere (Guichard et al. 2006).
In addition, the filtration value is significantly reduced after high-temperature aging when a crosslinked copolymer is used in a standard water-based drilling fluid formulation (Guichard et al. 2008).
AMPS Terpolymer
Fluid loss can be reduced by adding a terpolymer. The monomers and the composition are given in Table 2.11.
| Monomer | % |
|---|---|
| Na-2-acrylamido-2-methyl-1-propane sulfonic acid | 80 |
| N-Vinyl-2-pyrrolidone | 10 |
| Acrylamide | 10 |
Silicones
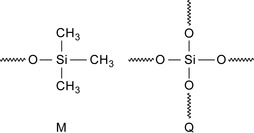
Compositions based on silicone MQ resins have been proposed for fluid loss control for WBMs. These compositions are non-damaging (Berry et al. 2008). An MQ resin contains only monovalent trimethylsiloxane units (CH3)3 SiO1/2, abbreviated as M, and tetravalent siloxane units Q, SiO4/2, c.f., Figure 2.11.
The terms 1/2 and 4/2 after the oxygen indicates that the oxygen atom is shared between groups M and Q. The resin does not contain more than 15 molmathchar45% hydroxyl units.
Such silicone resins remain soluble in hydrocarbons across a wide range of molecular weights. Silicone resins with a large proportion of Q units also show a high glass transition temperature(Tg) thus allowing wellbore fluids to be used at temperatures far higher than comparable fluids without silicone moieties.
The silicone resin is used as an emulsion, e.g., Dow Corning 1430 Antifoam (Andrea 2000). The formulations also contain conventional ingredients, such as surfactants, viscosity modifying agents, and biocides.
Phthalimide as a Diverting Material
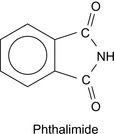
Phthalimide, c.f., Figure 2.12, has been described as a diverting material, or fluid loss additive, for diverting aqueous treating fluids, including acids, into progressively less permeable portions of a subterranean formation (Dill 1987).
This additive also reduces the fluid loss to the formation of an aqueous or hydrocarbon treating fluid utilized, for example, in fracturing treatments. Its use depends on the particle size of the material that is present.
Phthalimide will withstand high formation temperatures and can be readily removed from the formation by dissolution in the produced fluids, or by sublimation at elevated temperatures. The material is compatible either with other formation permeability-reducing materials, or formation permeability-increasing materials. The phthalimide particles act by sealing off portions of a subterranean formation by blocking off the fissures, pores, channels, and vugs that give access to the formation from the wellbore.
Special Applications
Coal-bed Methane Drilling
A series of mud systems and additives that have been in common use in coal-bed methane drilling have been evaluated with respect to their impact on the permeability of the coal matrix. Laboratory tests using both artificially cleated gypstone rock, as well as large-diameter coal cores, were performed as an assessment. In particular, various mud systems have been tested, including polyanionic and non-ionic cellulose and starch.
Muds based on xanthan gum, HEC, and Na-CMC did not have a negative impact on coal permeability. FLC™ 2000 and Q-Stop are very effective in building a thin filter cake on the coal surface almost instantaneously.
FLC™ 2000 is a micelle surfactant that very quickly forms a deformable barrier across coal cleats. Q-Stop consists of cellulose fibers. During production simulation experiments, a small pressure drop was sufficient to remove the filter cake.
It was shown that the permeability of the coal returned to its original value. This indicates that no permanent permeability damage is caused by the use of these additives. On the other hand, using the same coal type, the near-wellbore coal permeability was reduced by 87.5% by the addition of coal fines. Based on these laboratory studies, successful field applications in horizontal drilling could be achieved (Gentzis et al., 2009). The damage is illustrated in Table 2.12.
Sand Control
The rate of hydrocarbon flow declines when the bottom hole pressure falls below the dew point. When this occurs, a liquid aqueous phase accumulates near the well, sometimes termed condensate blocking. This effect reduces the relative permeability of the hydrocarbons and thus the productivity of the well (Nguyen et al. 2010b).
Also, if water swellable clays are present in this region, swelling is likely to occur, which reduces the permeability of the formation further. Well treatment fluids sometimes decrease the relative permeability of hydrocarbons. Capillary forces can tightly hold these treatment fluids. The rate of hydrocarbon flow can be also reduced by other factors, namely (Nguyen et al. 2010b):
1. The production of fines,
2. Sand migration in the formation, or
3. Precipitation.
The high velocity in the porous medium near the wellbore is sometimes sufficient to mobilize fines that subsequently can plug channels in the formation. Formation sand and fines often become unstable and migrate due to water movement through the formation. This is most likely to occur when the water phase is mobile because most of the fines are water-wet.
The presence of a mobile water phase can cause the migration of fines and subsequent formation damage. This needs to be minimized, since fines block flow paths, choking the potential production of the well, and also cause damage to downhole and surface equipment (Nguyen et al. 2010b).
Unconsolidated subterranean zones include those that contain loose particulates and those where the bonded particulates have insufficient bond strength to withstand the forces produced by the production of fluids through the zones. Support devices, such as screens and slotted liners, are often used to provide support for these unconsolidated formations in order to prevent a collapse of the formation.
In some instances, the annulus around the support device is gravel packed to reduce the voids between the device and the wellbore wall. Gravel packing forms a filtration bed near the wellbore, which acts as a physical barrier to the transport of unconsolidated formation fines with the production of hydrocarbons.
One common type of gravel packing operation involves placing a gravel pack screen in the wellbore, and packing the surrounding annulus with gravel of a specific mesh size, designed to prevent the passage of formation sand. Basically, the gravel pack screen is a filter, designed to retain the gravel placed during a gravel pack operation. Gravel packs may be time-consuming and expensive to install. Due to the time and expense needed it is sometimes advantageous to place a screen without the gravel.
An expandable screen is often installed to maintain the diameter of the wellbore for ease of access at a later time by eliminating installation of conventional screens, gravel placement, and other equipment.
Consolidation of a subterranean formation zone is often performed by pumping in a sequence of a resin, a spacer fluid and a catalyst. Such a resin application may be problematic when an insufficient amount of the spacer fluid is used, since the resin may contact the external catalyst too early.
An extensive review of literature concerning the methods for controlling unconsolidated particulates has been presented (Nguyen et al. 2010b). In addition, treatment fluids for controlling the migration of unconsolidated particulates in subterranean formations have been developed, which use a diluted epoxy resin. As diluent, a fluid that is miscible with water is used, i.e., methanol (Nguyen et al. 2010a). The addition of this solvent adjusts the viscosity of the resin composite so that a high degree of penetration into the subterranean formation can occur.
Fracturing
Hydrocarbon-producing wells are often stimulated by hydraulic fracturing operations, wherein a viscous fracturing fluid is introduced into the hydrocarbon-producing zone at a hydraulic pressure that is enough to create or enhance a fracture.
Generally, the fracturing fluid contains suspended proppant particles that are to be placed in the fractures to prevent them from fully closing (once the hydraulic pressure is released). This process forms conductive channels within the formation through which hydrocarbons can flow. Once at least one fracture is created, and at least a portion of the proppant is substantially in place, the viscosity of the fracturing fluid may be reduced, to remove it from the formation.
In certain circumstances, a portion of the fracturing fluid may be lost, e.g., through undesirable leak-off into natural fractures present in the formation. This is problematic because such natural fractures often have higher stresses than those created by a fracturing operation. These higher stresses may damage the proppant and cause it to form an impermeable plug in the natural fractures, which may prevent hydrocarbons from flowing through the natural fractures.
Conventionally, operators have attempted to solve this problem by including a fluid loss control additive in the fracturing fluid. These are generally rigid particles having a spheroid shape, but their use can be problematic in itself, since they may require particles that have a distinct particle size distribution in order to achieve efficient fluid loss control. For example, when used to block the pore throats in the formation, enough large particles will be required to obstruct most of the pore throat, and a sufficient proportion of relatively small particles will also be required to obstruct the interstices between the large particles. Such a particle size distribution may be difficult to obtain without incurring the added expense of reprocessing the materials, for example, by cryogenic grinding (Todd et al. 2006).
Cement Compositions
Polymers have been used as fluid loss control additives in cementing operations, including HEC and carboxymethylhydroxyethyl cellulose; copolymers of AMPS and AAm or N,N -dimethylacrylamide; and lignin or lignite grafted with AMPS, acrylonitrile, and N,N -dimethylacrylamide.
They may not provide the desired level of fluid loss control at high temperatures, however, i.e., at least about 260∘C (Lewis et al. 2009). Alternatively, a backbone of a humic acid salt has been proposed that has the moieties mentioned above grafted onto it. These graft copolymers are particularly suitable for use as fluid loss control additives in high-temperature applications.
Humic acids are produced by the decomposition of organic matter, such as dead plants, and may contain allomelanins found in soils, coals, and peat. The backbone may also contain PVA, PEO, polypropylene oxide, polyethyleneimine, and combinations of these moieties. Humic acid may be treated with KOH, NaOH, or NH4OH to make it soluble in water.
The solution so produced is further concentrated to increase its humic acid content, or it may be used directly in the grafting process. The graft copolymers may be prepared by free radical polymerization techniques. The initiator employed is usually a redox reagent, capable of generating a free radical in the humic acid.
Preparation 2–1
Usually the grafting is done in aqueous solution. To a reactor vessel, sodium humate, water, a defoamer, and ethylene diamine tetraacetic acid are added. Then AMPS, AAm, DADMAC, and AA are added. Then this mixture is heated and kept at 70∘C for one hour while purging with nitrogen.
Afterwards, ammonium persulfate is added to initiate the polymerization. After two hours, sodium metabisulfite is added, and the mixture is allowed to cool. When the mixture reaches room temperature, a 50% solution of sodium hydroxide is added to adjust the pH to 7–8 (Lewis et al. 2009). The amounts of the individual compounds are summarized in Table 2.13.
| Component → Formulation | #1/[%] | #2/[%] |
|---|---|---|
| Sodium humate | 4.7 | 4.7 |
| Water | 42.84 | 41.01 |
| NaOH, 50% solution | 1.69 | 1.28 |
| Defoamer | 0.01 | 0.01 |
| NaAMPS, 58% solution | 31.48 | 31.48 |
| Acrylamide, 48% solution | 5.92 | 5.92 |
| AA | 1.44 | – |
| Vinylphosphonic acid | – | 1.08 |
| DADMAC, 62% solution | 5.22 | 7.82 |
| NaEDTA | 0.1 | 0.1 |
| Ammonium persulfate, 27% solution | 3.3 | 3.3 |
| Sodium metabisulfite, 27% solution | 3.3 | 3.3 |
The performance of these additives has been tested with regard to cementing. Compressive strength and thickening time tests were performed to compare the performance of a range of cement compositions. The fluid loss of these compositions was tested at high temperatures (Lewis et al. 2009). The formulations provide desirable thickening times and compressive strengths.
Viscoelasticity
Viscoelastic materials exhibit both viscous and elastic properties under mechanical deformation. Such materials exhibit a hysteresis in their stress strain curves. Further, a relaxation of stress occurs under constant strain, i.e., the stress is diminished. Moreover, viscoelastic materials exhibit creep.
A simple model for such materials was developed by James Clerk Maxwell in 1867 (Maxwell 1867). A Maxwell fluid or Maxwell body can be modeled by an idealized viscous damper and an idealized elastic spring connected in series. The basic device is shown in Figure 2.13.
The Maxwell model can hence explain both the elastic and viscous properties of a body. Some basic issues of the Maxwell model have been revisited (Rao and Rajagopal, 2007). The basic Maxwell model can be represented by:
(2.6)
The change in total elongation εt in time t is the sum of the change of elongation of the damper εD and the change of elongation of the spring εt. The right hand side of Eq. 2.6 is simply Newton's law of flow together with Hooke's law of the elongation of an ideal spring, i.e.,
The latter is given in the more uncommon differential form. Thus, η is the Newtonian viscosity, σ is the stress, and E is the elastic modulus. (By the way, if the spring and the damper are coupled in parallel instead of consecutively, the Kelvin-Voigt model emerges, which may be experienced in self-closing doors.)
The property of viscoelasticity has been well investigated (Rehage and Hoffmann, 1988). Aqueous solutions of cationic surfactants with strong binding forces of the ions show properties similar to gels.
Microstructural transitions and rheological properties of viscoelastic solutions formed in a catanionic surfactant system were studied using a combination of rheology and dynamic light scattering (Yin et al., 2009). The rheological behavior of such systems can be very complicated. In a study of a surfactant system based on dodecyltriethylammonium bromide and sodium dodecyl sulfate, worm-like micelles began to form above a certain surfactant concentration. In an intermediate concentration range, the system exhibits a linear viscoelasticity with the characteristics of a Maxwell fluid. Eventually, at higher surfactant concentrations, a transition from linear micelles to branched structures may take place.
The changes of the viscosity at zero shear rate with the total concentration of surfactant with a ratio of dodecyltriethylammonium bromide to sodium dodecyl sulfate of 27/73 are shown in Figure 2.14.
 |
| Figure 2.14 Viscosity viz. concentration of surfactant (Yin et al., 2009). |
Viscoelastic Surfactants
Viscoelasticity is caused by a different type of micelle formation to the usual spherical micelles, which are formed by most surfactants (VESs) are believed to impart viscosity to an aqueous fluid by the molecules organizing themselves into micelles, and when the micelles have an elongated configuration, for instance, rod-shaped or worm-shaped, they become entangled with one another, thereby increasing the viscosity of the fluid (Crews and Huang 2010a).
Elongated VES structures are referred to as living, because there is a continuous exchange between surfactants leaving the micelle structures to enter the aqueous solution and those leaving the aqueous solution to entering or reentering the micelles.
VES solutions exhibit shear thinning behavior, but they remain stable despite repeated high shear applications. By comparison, a typical polymeric thickener will irreversibly degrade under high shear (Colaco et al. 2007).
Internal breakers work by the rearranging the VES micelle from rod-shaped or worm-shaped elongated structures to spherical structures. In other words, they perform the collapse or rearrangement of the viscous elongated micelle structures to nonviscous, more spherical, micelle structures (Crews and Huang 2010a).
Enhanced Shear Recovery Agents
Some VESs exhibit low shear recovery when subjected to high shear. However, unacceptably long shear recovery times hinder deep well operations.
Enhanced shear recovery agents reduce the shear recovery time of a VES fluid. They are based on alkylated polyglucosides, polyglucamides, or on copolymers based on ethylene glycol ethyl ether acrylate. Glucamides consist of cyclic forms of glucose in which the hydrogen of the hemiacetal group has been replaced with an alkyl or aryl moiety, as shown in Figure 2.15.
 |
| Figure 2.15 Glucoside and glucamide structures (Colaco et al. 2007). |
Enzyme-based Gel Breaking
The viscosities of fluids viscosified with VES may be reduced by the direct or indirect action of a biochemical agent, such as bacteria, fungi, or enzymes. This agent may directly attack the VES itself, or some other component in the fluid that produces a by-product that then causes viscosity reduction. The biochemical agent may disaggregate or otherwise attack the micellar structure of the VES-gelled fluid. The biochemical agent may produce an enzyme that reduces viscosity by one of these mechanisms.
A single biochemical agent may operate simultaneously by two different mechanisms, such as by degrading the VES directly, as well as another component, such as a glycol, the latter mechanism in turn producing a by-product, e.g. an alcohol that causes viscosity reduction.
Alternatively, two or more different biochemical agents may be used simultaneously. In a specific, non-limiting instance, a brine fluid gelled with an amine oxide surfactant can have its viscosity broken with bacteria such as Enterobacter cloacae, Pseudomonas fluorescens, Pseudomonas aeruginosa, etc (Crews 2006).
Breaker Enhancers for VES
Oil-soluble surfactants may be used as breaker enhancers for internal breakers for VES gelled aqueous fluids (Crews and Huang 2010a). The oil-soluble surfactant breaker enhancers can overcome the rate-slowing effect that salinity has on the internal breakers, particularly at lower temperatures. Oil-soluble surfactant breaker enhancers may also allow lower internal breaker concentrations to be used to achieve quick and complete VES gelled fluid breaks.
Oil-soluble surfactant breaker enhancers include various sorbitan (unsaturated) fatty acid esters Crews and Huang, 2010a and Crews and Huang, 2010b. These esters are mixed with mineral oils. Unsaturated fatty acids have been found to break down by autooxidation into VES breaking products or compositions.
Each oil with various monoenoic and polyenoic acids uniquely shows the breakdown of the VES surfactant micelle structure by the presence of these autooxidation generated by-products.
Various hydroperoxides may be formed in the course of these autooxidation reactions, The end-products of these reactions typically include carbonyl compounds, alcohols, acids, and hydrocarbons. The rate of autooxidation of various fatty acids is shown in Table 2.14.
| Fatty Acid | Double Bonds | Relative Oxidation Rate |
|---|---|---|
| Stearic | 0 | 1 |
| Oleic | 1 | 100 |
| Linoleic | 2 | 1200 |
| Linolenic | 3 | 2500 |
Surfactant Polymer Compositions
The stability of VES-based fluids can be enhanced by using monomeric VESs, together with an oligomeric or polymeric compound with a thermally stable backbone. On this structure, VES functional groups are pending (Horton et al. 2009).
A VES solution can be synthesized from N -dodecene-1-yl-N,N -bis (2-hydroxyethyl)-N -methylammonium chloride in aqueous ammonium chloride. The aqueous solution is purged with nitrogen to remove residual oxygen and then oligomerized with 2,2′-azo(bis-amidinopropane)dihydrochloride as radical initiator. The oligomerization is shown schematically in Figure 2.16.
 |
| Figure 2.16 Oligomerization of unsaturated quaternary compounds (Horton et al. 2009). |
In the same way, potassium octadeceneoate,
The oligomerization of surfactant monomers in micelles causes the viscosity of the gel to be comparatively insensitive to contact with hydrocarbons. Further, the viscosity of the surfactant gel is little altered by oligomerization of the surfactant monomers.
The preparation of several other oligomeric surfactants has been described in detail, including the use of comonomers (Horton et al., 2007 and Horton et al., 2009). An example for a cooligomer is shown in Figure 2.17.
 |
| Figure 2.17 Cooligomeric surfactant (Horton et al. 2007). |
The vicinal diol functionality renders the oligomers readily crosslinkable with polyvalent metal ions or complexes. Such formulations have been characterized with respect to fluid loss control by an API standard (Recommended practice for field testing water-based drilling fluids (API) 2009).
Additives to Reduce Fluid Loss
Viscoelastic surfactant fluids are made by mixing suitable surfactants. When the surfactant concentration significantly exceeds a critical level, and subject to the presence of an electrolyte, the surfactant molecules aggregate and form structures such as micelles that can interact to form a network, which exhibits viscoelastic behavior (Sullivan et al. 2006).
These solutions can be formed by the addition of certain reagents to concentrated surfactant solution. The surfactants are long chain quaternary ammonium salts such as cetyltrimethyl ammonium bromide.
Salts, e.g., ammonium chloride, potassium chloride, sodium salicylate, and sodium isocyanate generate viscoelasticity in surfactant solutions. In addition, non-ionic organic molecules, such as chloroform are active in generating viscoelasticity. The electrolyte content of surfactant solutions is also an important parameter for its viscoelasticity (Sullivan et al. 2006). Aqueous fluids gelled with VESs have been used for hydraulic fracturing operations.
However, the property that makes VES fluids less damaging tends to cause significantly higher fluid leakage into the reservoir matrix, which reduces the efficiency of the fluid, especially during VES fracturing treatments. Thus, it is important to use fluid loss agents for VES fracturing treatments in high-permeability formations (Huang and Crews 2009).
The fluid loss properties of such fluids can be improved by the addition of mineral oil with a viscosity greater than 20 mPas at ambient temperature. The mineral oil may initially be dispersed as oil droplets in an internal, discontinuous phase of the fluid, and is added to the fluid after it has been substantially gelled.
In the experiments shown below, tallow amido propylamine oxide available from Akzo Nobel has been used as VES surfactant (Podwysocki 2004). It has been demonstrated that the viscosity of aqueous fluids containing 3% KCl and gelled with 6% VES at 66∘C with and without 2% mineral oil is adversely affected. This behavior contrasts with other observations, since larger amounts of hydrocarbons and mineral oils tend to inhibit or break the gel of VES-gelled fluids (Huang and Crews 2009).
On the other hand, fluid loss is improved, as shown in model experiments. The fluid loss as a function of testing time is shown in Figure 2.18. The test is conducted at 0.7 MPa with 400 mD ceramic disks at 66∘C.
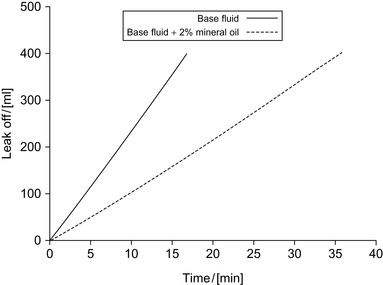 |
| Figure 2.18 Fluid loss viz. time (Huang and Crews 2009). |
It has been discovered that the addition of magnesium oxide, or calcium hydroxide, to an aqueous fluid gelled with a VES improves the fluid loss of these brines (Huang et al. 2009).
It is useful that these fluid loss control agents dissolve slowly, since this permits their easy removal from the formation, so sustaining little or no damage to the formation.
The introduction of these additives to a VES-gelled aqueous system will limit and reduce the amount of VES fluid that leaks into the pores of a reservoir during a fracturing or frac-packing treatment, thus minimizing the formation damage that may occur by the VES fluid within the reservoir pores.
Moreover, differences in reservoir permeability do not significantly change the rate of fluid loss. Thus, the rate of leak-off in 2000 mD reservoirs will be comparable that of 100 mD reservoirs. This behavior expands the range in reservoir permeability to which the VES fluid may be applied.
It is believed that fluid loss agents associate with the VES micelles. As the VES fluid is leaked-off into the reservoir, a viscous layer of micelles and fluid loss control particles accumulate on the formation face, thus reducing the rate of VES fluid leak-off.
Particulate plugging of the reservoir pores is not the mechanism of leak-off control. Tests with nanometer-sized fluid loss agents that definitely cannot bridge or plug reservoir pores of 1 mD or higher reservoir permeability, still develop a viscous micelle layer. Thus, the size of the fluid loss agent is not a controlling or primary factor for controlling the leak-off rate (Huang et al. 2009).
Amanullah, M.; Yu, L., Environment friendly fluid loss additives to protect the marine environment from the detrimental effect of mud additives, J. Pet. Sci. Eng. 48 (3–4) (2005) 199–208.
Anderson, C.P.; Blenkinsopp, S.A.; Cusack, F.M.; Costerton, J.W., Drilling mud fluid loss – an alternative to expensive bulk polymers, In: Proceedings Volume, 4th Inst. Gas Technol. Gas, Oil, & Environ. Biotechnol. Int. Symp.Colorado Springs, CO, 12/9–11/91. (1991), pp. 481–489.
Andrea, 2000. Dow Corning® 1430 Antifoam, Product Information 22-030G-01, FPH22781, Dow Corning Corporation, Midland, OH [electronic:] http://www1.dowcorning.com/DataFiles/090007c880002832.pdf.
Audebert, R., Janca, J., Maroy, P., Hendriks, H., 1994. Chemically crosslinked polyvinyl alcohol (PVA), process for synthesizing same and its applications as a fluid loss control agent in oil fluids. GB Patent 2 278 359, assigned to Sofitech NV, November 30, 1994.
Audebert, R., Janca, J., Maroy, P., Hendriks, H., 1996. Chemically crosslinked polyvinyl alcohol (PVA), process for synthesizing same and its applications as a fluid loss control agent in oil fluids. CA Patent 2 118 070, assigned to Schlumberger Canada Ltd., April 14, 1996.
Audebert, R., Maroy, P., Janca, J., Hendriks, H., 1998. Chemically crosslinked polyvinyl alcohol (PVA), and its applications as a fluid loss control agent in oil fluids. EP Patent 705 850, assigned to Sofitech NV, September 02, 1998.
Audibert, A., Argillier, J.F., 1996. Process and water-based fluid utilizing hydrophobically modified guar gums as filtrate (loss) reducer (procede et fluide a base d'eau utilisant des guars modifiees hydrophobiquement comme reducteur de filtrat). EP Patent 722 036, assigned to Inst. Francais Du Petrole, July 17, 1996.
Audibert, A., Argillier, J.F., 1998. Process and water-base fluid utilizing hydrophobically modified guars as filtrate reducers. US Patent 5 720 347, assigned to Inst. Francais Du Petrole, February 24, 1998.
Audibert, A., Argillier, J.F., Bailey, L., Reid, P.I., 1997. Process and water-base fluid utilizing hydrophobically modified cellulose derivatives as filtrate reducers. US Patent 5 669 456, assigned to Inst. Francais Du Petrole and Dowell Schlumberger Inc., September 23, 1997.
Audibert-Hayet, A., Argillier, J.F., Rousseau, L., 1998. Filtrate reducing additive and well fluid (additif reducteur de filtrat et fluide de puits). WO Patent 9 859 014, assigned to Inst. Francais Du Petrole, December 30, 1998.
Audibert, A.; Rousseau, L.; Kieffer, J., Novel high-pressure/high temperature fluid loss reducer for water-based formulation, In: Proceedings Volume, SPE Oilfield Chem. Int. Symp.Houston, 2/16–19/1999. (1999), pp. 235–242.
Bailey, L., 2001. Latex additive for water-based drilling fluids. GB Patent 2 351 986, assigned to Sofitech NV, January 17, 2001.
Bardoliwalla, D.F., 1986a. Aqueous drilling fluids containing fluid loss additives. US Patent 4 622 370, November 11, 1986.
Bardoliwalla, D.F., 1986b. Fluid loss control additives from AMPS (2-acrylamido-2-methylpropane sulfonic acid) polymers. US Patent 4 622 373, November 11, 1986.
Baret, J.F., Why cement fluid loss additives are necessary, In: Proceedings Volume, SPE Petrol. Eng. Int. Mtg.Tianjin, China, 11/1–4/88. (1988), pp. 853–860.
Bellis, H.E., McBride, E.F., 1987. Composition and method for temporarily reducing the permeability of subterranean formations. EP Patent 228 196, assigned to Du Pont De Nemours & Co., July 08, 1987.
Berry, V.L., Cook, J.L., Gelderbloom, S.J., Kosal, D.M., Liles, D.T., Olsen Jr., C.W., Rome, C.F.C., 2008. Silicone resin for drilling fluid loss control. US Patent 7 452 849, assigned to Dow Corning Corporation (Midland, MI), November 18, 2008.
Bharat, P., 1990. Well treating fluids and additives therefor. EP Patent 372 469, June 13, 1990.
Boncan, V.G., Gandy, R., 1986. Well cementing method using an AM/AMPS fluid loss additive blend. US Patent 4 632 186, December 30, 1986.
Bouts, M.N.; Trompert, R.A.; Samuel, A.J., Time delayed and low-impairment fluid-loss control using a succinoglycan biopolymer with an internal acid breaker, SPE J. 2 (4) (1997) 417–426.
Braden, J., 2006. PLIOLITE®VT/VTL Resin, Product Data Sheet PDS-PLEVT-2006-04/1, Eliokem, Inc., Akron, OH, [electronic:] http://www.eliokem.com/pdf/PLEVTL.pdf.
Brake, B.G., Chatterji, J., 1994. Fluid loss reducing additive for cement compositions. EP Patent 595 660, assigned to Halliburton Co., May 04, 1994.
Brothers, L.E., 1989. Method of reducing fluid loss in cement compositions. US Patent 4 806 164, February 21, 1989.
Cantu, L.A., McBride, E.F., Osborne, M.W., 1989. Formation fracturing process. US Patent 4 848 467, assigned to Conoco Inc. and Du Pont De Nemours & Co., July 18, 1989.
Cantu, L.A., McBride, E.F., Osborne, M., 1990. Well treatment process. EP Patent 404 489, assigned to Conoco Inc. and Du Pont De Nemours & Co., December 27, 1990.
Cantu, L.A., McBride, E.F., Osborne, M.W., 1993. Formation fracturing process. CA Patent 1 319 819, assigned to Conoco Inc., July 06, 1993.
Casad, B.M., Clark, C.R., Cantu, L.A., Cords, D.P., McBride, E.F., 1991. Process for the preparation of fluid loss additive and gel breaker. US Patent 4 986 355, assigned to Conoco Inc., January 22, 1991.
Cawiezel, K.E., Navarrete, R., Constien, V., 1996. Fluid loss control. GB Patent 2 291 906, assigned to Sofitech NV, February 07, 1996.
Chang, F.F.; Bowman, M.; Parlar, M.; Ali, S.A.; Cromb, J., Development of a new crosslinked-hec (hydroxyethylcellulose) fluid loss control pill for highly-overbalanced, high-permeability and/or high temperature formations, In: Proceedings Volume, SPE Formation Damage Contr. Int. Symp.Lafayette, LA, 2/18–19/98. (1998), pp. 215–227.
Chang, F.F., Parlar, M., 1999. Method and composition for controlling fluid loss in high permeability hydrocarbon bearing formations. US Patent 5 981 447, assigned to Schlumberger Technol. Corp., November 09, 1999.
Cheung, P.S.R., 1993. Fluid loss additives for cementing compositions. US Patent 5 217 531, assigned to Western Co. North America, June 08, 1993.
Chin, W.C., Formation Invasion: With Applications To Measurement-While-Drilling, Time Lapse Analysis, and Formation Damage. (1995) Gulf Publishing Co., Houston, TX.
Coates, J.A., Farrar, J.M., Graham, M.H., 1990. Fluid loss-reducing additives for oil-based well working fluid. US Patent 4 941 983, July 17, 1990.
Colaco, A., Marchand, J.-P., Li, F., Dahanayake, M.S., 2007. Viscoelastic surfactant fluids having enhanced shear recovery, rheology and stability performance. US Patent 7 279 446, assigned to Rhodia Inc. (Cranbury, NJ) Schlumberger Technology Corporation (Sugarland, TX), October 9, 2007.
Costello, C.A., Lai, T.W., Pinschmidt Jr., R.K., 1990. Aqueous, clay-based drilling mud. GB Patent 2 225 364, May 30, 1990.
Cowan, J.C., Granquist, V.M., House, R.F., 1988. Organophilic polyphenolic acid adducts. US Patent 4 737 295, assigned to Venture Chemicals Inc., April 12, 1988.
Crawford, D., 2000. High pressure high temperature (hpht) fluid loss control aid for drilling fluids. WO Patent 0 026 322, assigned to Sun Drilling Products Inc., May 11, 2000.
Crema, S.C., Kucera, C.H., Konrad, G., Hartmann, H., 1991. Fluid loss control additives for oil well cementing compositions. US Patent 5 025 040, assigned to BASF Corp., June 18, 1991.
Crema, S.C., Kucera, C.H., Konrad, G., Hartmann, H., 1993. Fluid loss control additives for oil well cementing compositions. US Patent 5 228 915, assigned to BASF Corp., July 20, 1993.
Crews, J.B., 2006. Bacteria-based and enzyme-based mechanisms and products for viscosity reduction breaking of viscoelastic fluids. US Patent 7 052 901, assigned to Baker Hughes Incorporated (Houston, TX), May 30, 2006.
Crews, J.B., Huang, T., 2010a. Use of oil-soluble surfactants as breaker enhancers for ves-gelled fluids. US Patent 7 696 135, assigned to Baker Hughes Incorporated (Houston, TX), April 13, 2010.
Crews, J.B., Huang, T., 2010b. Unsaturated fatty acids and mineral oils as internal breakers for ves-gelled fluids. US Patent 7 696 134, assigned to Baker Hughes Incorporated (Houston, TX), April 13, 2010.
Dill, W.R., 1987. Diverting material and method of use for well treatment. CA Patent 1 217 320, February 03, 1987.
Dobson Jr., J.W., Harrison, J.C.I., Kayga, P.D., 1998. Methods of reducing fluid loss and polymer concentration of well drilling and servicing fluids. AU Patent 697 559, assigned to Texas United Chem. Co. LLC., October 08, 1998.
Dobson Jr., J.W., Hayden, S.L., Hinojosa, B.E., 2005. Borate crosslinker suspensions with more consistent crosslink times. US Patent 6 936 575, assigned to Texas United Chemical Company, LLC. (Houston, TX), August 30, 2005.
Dobson, J.W., Mondshine, K.B., 1997. Method of reducing fluid loss of well drilling and servicing fluids. EP Patent 758 011, assigned to Texas United Chem. Co. LLC., February 12, 1997.
Dreveton, E., Lecourtier, J., Ballerini, D., Choplin, L., 1995. Process utilizing gellan as filtrate reducer for water-based drilling fluids (procede utilisant le gellane comme reducteur de filtrat pour les fluides de forage a base d'eau). EP Patent 662 563, assigned to Inst. Francais Du Petrole, July 12, 1995.
Dreveton, E., Lecourtier, J., Ballerini, D., Choplin, L., 1998. Process using gellan as a filtrate reducer for water-based drilling fluids. US Patent 5 744 428, assigned to Inst. Francais Du Petrole, April 28, 1998.
Feraud, J.P., Perthuis, H., Dejeux, P., 2001. Compositions for iron control in acid treatments for oil wells. US Patent 6 306 799, assigned to Schlumberger Technology Corporation (Sugar Land, TX), October 23, 2001.
Forrest, G.T., 1998. Drilling, completion, and workover fluid comprising ground peanut hulls. WO Patent 9 821 290, May 22, 1998.
Francis, H.P., Deboer, E.D., Wermers, V.L., 1987. High temperature drilling fluid component. US Patent 4 652 384, March 24, 1987.
Fry, S.E., Childs, J.D., Brothers, L.E., Lindsey, D.W., 1987. Method of reducing fluid loss in cement compositions which may contain substantial salt concentrations. US Patent 4 676 317, assigned to Halliburton Company (Duncan, OK), June 30, 1987.
Ganguli, K.K., 1992. High temperature fluid loss additive for cement slurry and method of cementing. US Patent 5 116 421, assigned to Western Co. North America, May 26, 1992.
Garvey, C.M., Savoly, A., Resnick, A.L., 1988. Fluid loss control additives and drilling fluids containing same. US Patent 4 741 843, May 03, 1988.
Gentzis, T.; Deisman, N.; Chalaturnyk, R.J., Effect of drilling fluids on coal permeability: Impact on horizontal wellbore stability, Int. J. Coal Geol. 78 (3) (2009) 177–191.
Giddings, D.M., Williamson, C.D., 1987. Terpolymer composition for aqueous drilling fluids. US Patent 4 678 591, July 07, 1987.
Growcock, F.B., Simon, G.A., 2006. Stabilized colloidal and colloidal-like systems. US Patent 7 037 881, May 2, 2006.
Guichard, B., Wood, B., Vongphouthone, P., 2006. Fluid loss reducer for high temperature and high pressure water-based mud application. US Patent 7 101 829, assigned to Eliokem S.A.S. (Villejust, FR), September 5, 2006.
Guichard, B., Wood, B., Vongphouthone, P., 2007. Fluid loss reducer for high temperature high pressure water based-mud application. US Patent 7 256 159, assigned to Eliokem S.A.S. (Villejust, FR), August 14, 2007.
Guichard, B., Wood, B., Vongphouthone, P., 2008. Fluid loss reducer for high temperature high pressure water based-mud application. US Patent 7 449 430, assigned to Eliokem S.A.S. (Villejust, FR), November 11, 2008.
Halliday, W.S., Schwertner, D., Xiang, T., Clapper, D.K., 2007. Fluid loss control and sealing agent for drilling depleted sand formations. US Patent 7 271 131, assigned to Baker Hughes Incorporated (Houston, TX), September 18, 2007.
Halliday, W.S., Schwertner, D., Xiang, T., Clapper, D.K., 2008. Water-based drilling fluids using latex additives. US Patent 7 393 813, assigned to Baker Hughes Incorporated (Houston, TX), July 1, 2008.
Hen, J., 1991. Sulfonate-containing polymer/polyanionic cellulose combination for high temperature/high pressure filtration control in water base drilling fluids. US Patent 5 008 025, assigned to Mobil Oil Corp., April 16, 1991.
Hernandez, M.I., Mas, M., Gabay, R.J., Quintero, L., 1999a. Thermally stable drilling fluid. US Patent 5 883 054, assigned to Intevep, March 16, 1999.
Hernandez, M.I., Mas, M., Gabay, R.J., Quintero, L., 1999b. Thermally stable drilling fluid which includes styrene-butadiene copolymers. GB Patent 2 329 657, assigned to Intevep, March 31, 1999.
Hille, M., Wittkus, H., Tonhauser, J., Engelhardt, F., Riegel, U., 1996. Water-soluble copolymers useful in drilling fluids. US Patent 5 510 436, assigned to Hoechst AG, April 23, 1996.
Horton, R.L., Prasek, B., Growcock, F.B., Kippie, D., Vian, J.W., Abdur-Rahman, K.B., Arvie Jr., M., 2007. Surfactant-polymer compositions for enhancing the stability of viscoelastic-surfactant based fluid. US Patent 7 157 409, assigned to M-I LLC (Houston, TX), January 2, 2007.
Horton, R.L., Prasek, B.B., Growcock, F.B., Kippie, D.P., Vian, J.W., Abdur-Rahman, K.B., Arvie, M., 2009. Surfactant-polymer compositions for enhancing the stability of viscoelastic-fluid surfactant based. US Patent 7 517 835, assigned to M-I LLC (Houston, TX), April 14, 2009.
Huang, N., Synthesis of fluid loss additive of sulfonate tannic-phenolic resin, Oil Drill. Prod. Technol. 18 (2) (1996) 39–42; 106–107.
Huang, T., Crews, J.B., 2009. Use of mineral oils to reduce fluid loss for viscoelastic surfactant gelled fluids. US Patent 7 615 517, assigned to Baker Hughes Incorporated (Houston, TX), November 10, 2009.
Huang, T., Crews, J.B., Treadway Jr., J.H., 2009. Fluid loss control agents for viscoelastic surfactant fluids. US Patent 7 550 413, assigned to Baker Hughes Incorporated (Houston, TX), June 23, 2009.
Huddleston, D.A., Gabel, R.K., Williamson, C.D., 1992. Method for reducing fluid loss from oilfield cement slurries using vinyl grafted wattle tannin. US Patent 5 134 215, assigned to Nalco Chemical Co., July 28, 1992.
Huddleston, D.A., Williamson, C.D., 1990. Vinyl grafted lignite fluid loss additives. US Patent 4 938 803, assigned to Nalco Chemical Co., July 03, 1990.
House, R.F., Granquist, V.M., 1986. Polyphenolic acid adducts. US Patent 4 597 878, assigned to Venture Innovations Inc., July 01, 1986.
Jarrett, M., Clapper, D., 2010. High temperature filtration control using water based drilling fluid systems comprising water soluble polymers. US Patent 7 651 980, assigned to Baker Hughes Incorporated (Houston, TX), January 26, 2010.
Jiao, D.; Sharma, M.M., Mechanism of cake buildup in crossflow filtration of colloidal suspensions, J. Colloid Interface Sci. 162 (2) (1994) 454–462.
Johnson, M., 1996. Fluid systems for controlling fluid losses during hydrocarbon recovery operations. EP Patent 691 454, assigned to Baker Hughes Inc., January 10, 1996.
Johnson, M.H., Smejkal, K.D., 1993. Fluid system for controlling fluid losses during hydrocarbon recovery operations. US Patent 5 228 524, assigned to Baker Hughes Inc., July 20, 1993.
Kelessidis, V.C.; Papanicolaou, C.; Foscolos, A., Application of greek lignite as an additive for controlling rheological and filtration properties of water-bentonite suspensions at high temperatures: A review, Int. J. Coal Geol. 77 (3–4) (2009) 394–400.
Kelessidis, V.C.; Tsamantaki, C.; Michalakis, A.; Christidis, G.E.; Makri, P.; Papanicolaou, K.; Foscolos, A., Greek lignites as additives for controlling filtration properties of water-bentonite suspensions at high temperatures, Fuel 86 (7–8) (2007) 1112–1121.
Kucera, C.H., Crema, S.C., Roznowski, M.D., Konrad, G., Hartmann, H., 1989. Fluid loss control additives for oil well cementing compositions. EP Patent 342 500, November 23, 1989.
Lai, T.W., Vijayendran, B.R., 1989. Cement composition for oil well drilling holes containing high molecular weight polyvinylamines. EP Patent 331 045, September 06, 1989.
Lau, H.C., Laboratory development and field testing of succinoglycan as a fluid-loss-control fluid. SPE, Shell Development Co., J. SPE Drill. Completion 9 (4) (1994) 221–226.
Lange, W., Boehmer, B., 1988. Water-soluble polymers and their use as flushing liquid additives for drilling. US Patent 4 749 498, June 07, 1988.
Langlois, B., 1999. Fluids useful for oil mining comprising de- acetylated xanthane gum and at least one compound increasing the medium ionic strength (fluides a utilisables dans l'exploitation du petrole comprenant de la gomme xanthane desacetylee et au moins un compose augmentant la force ionique du milieu). WO Patent 9 903 948, assigned to Rhodia Chimie, January 28, 1999.
Le, H.V., Kesavan, S., Dawson, J.C., Mack, D.J., Nelson, S.G., 1998. Compositions and methods for hydraulic fracturing. CA Patent 2 239 599, assigned to BJ Services Co., December 05, 1998.
Lewis, S., Chatterji, J., King, B., Brennels, C., 2008. Cement compositions comprising lignite grafted fluid loss control additives. US Patent 7 388 045, assigned to Halliburton Energy Services, Inc. (Duncan, OK), June 17, 2008.
Lewis, S., Chatterji, J., King, B., Brenneis, D.C., 2009. Cement compositions comprising humic acid grafted fluid loss control additives. US Patent 7 576 040, assigned to Halliburton Energy Services, Inc. (Duncan, OK), August 18, 2009.
Li, Y.G.; Li, S.L.; Wang, Z.L., New high temperature filtration reducer fla for drilling fluid, Drill. Fluid Completion Fluid 13 (3) (1996) 33–35.
Lord, D.L.; Vinod, P.S.; Shah, S.; Bishop, M.L., An investigation of fluid leakoff phenomena employing a high-pressure simulator, In: Proceedings Volume, Annu. SPE Tech. Conf.Dallas, 10/22–25/95. (1995), pp. 465–474.
Magnet, S., Duquesne, S., Delobel, R., Jama, C., 2008. Polymer binder for intumescent coatings. US Patent 7 417 091, assigned to Eliokem SAS (Villejust, FR), August 26, 2008.
Maxwell, J.C., On the dynamical theory of gases, Phil. Trans. R. Soc. Lond. 157 (1) (1867) 49–88.
Miller, W.K.; Roberts, G.A.; Carnell, S.J., Fracturing fluid loss and treatment design under high shear conditions in a partially depleted, moderate permeability gas reservoir, In: Proceedings Volume, SPE Asia Pacific Oil & Gas Conf.Adelaide, Australia, 10/28–31/96. (1996), pp. 451–460.
Mondshine, T.C., 1986. Crosslinked fracturing fluids. US Patent 4 619 776, assigned to Texas United Chemical Corp. (Houston, TX), October 28, 1986.
Moran, L.K., Murray, T.R., 1991. Well cement fluid loss additive and method. US Patent 5 009 269, assigned to Conoco Inc., April 23, 1991.
Morgan, R.L., Caveny, W.J., Koch, R.R., 2008. Cement compositions with improved fluid loss characteristics and methods of cementing in surface and subterranean applications. US Patent 7 384 893, assigned to Halliburton Energy Services, Inc. (Duncan, OK), June 10, 2008.
Munoz Jr., T., Todd, B.L., 2008. Treatment fluids comprising starch and ceramic particulate bridging agents and methods of using these fluids to provide fluid loss control. US Patent 7 462 581, assigned to Halliburton Energy Services, Inc. (Duncan, OK), December 9, 2008.
Navarrete, R.C.; Seheult, J.M.; Himes, R.E., Applications of xanthan gum in fluid-loss control and related formation damage, In: Proceedings Volume, SPE Permian Basin Oil & Gas Recovery Conf.Midland, TX, 3/21–23/2000. (2000).
Nguyen, P.D., Dusterhoft, R.G., Barton, J.A., 2010a. Methods for controlling particulate migration. US Patent 7 712 531, assigned to Halliburton Energy Services, Inc. (Duncan, OK), May 11, 2010.
Nguyen, P.D., Rickman, R.D., Dusterhoft, R.G., 2010b. Method of stabilizing unconsolidated formation for sand control. US Patent 7 673 686, assigned to Halliburton Energy Services, Inc. (Duncan, OK), March 9, 2010.
Nguyen, P.D.; Weaver, J.D.; Cole, R.C.; Schulze, C.R., Development and field application of a new fluid-loss control material, In: Proceedings Volume, Annu. SPE Tech. Conf.Denver, 10/6–9/96. (1996), pp. 933–941.
Oswald, R.J., Morschhaeuser, R., Heier, K.H., Tardi, A., Tonhauser, J., Kayser, C., Patterson, D., 2000. Water-soluble copolymers and their use for the exploration and recuperation of oil and gas. EP Patent 1 059 316, assigned to Clariant GmbH, December 13, 2000.
Patel, A.D., 1998. Water-based drilling fluids with high temperature fluid loss control additive. US Patent 5 789 349, assigned to M I Drilling Fluids Llc., August 04, 1998.
Patel, A.D., McLaurine, H.C., 1992. Drilling fluid additive and method for inhibiting hydration. US Patent 5 149 690, assigned to M I Drilling Fluids Co., September 22, 1992.
Peiffer, D.G., Lundberg, R.D., Sedillo, L., Newlove, J.C., 1986. Fluid loss control in oil field cements. US Patent 4 626 285, December 02, 1986.
Petrochem, 2009. Fluid-loss control additives, [electronic:] http://www.petrochem-usa.com/.
Plank, J., Field results with a novel fluid loss polymer for drilling muds, Oil Gas Europe Mag. 16 (3) (1990) 20–23.
Plank, J., 1993. Drilling mud composition and process for reducing the filtrate of metal hydroxide mixtures containing drilling mud compositions. WO Patent 9 312 194, assigned to Skw Trostberg AG, June 24, 1993.
Plank, J.P.; Gossen, F.A., Visualization of fluid-loss polymers in drilling mud filter cakes, In: Proceedings Volume, 64th Annu. SPE Tech. Conf.San Antonio, 10/8–11/89. (1989), pp. 165–176.
Podwysocki, M., 2004. Akzo nobel surfactants, Technical Bulletin SC05-0707, Akzo Nobel Surface Chemistry LLC, 525 W. Van Buren Street Chicago, IL 60607-3823, http://www.surface.akzonobel.com/bulletins/Aromox%20APA%20T.pdf.
Raines, R.H., 1986. Use of low m.s. (molar substitution) hydroxyethyl cellulose for fluid loss control in oil well applications. US Patent 4 629 573, assigned to Union Carbide Corp., December 16, 1986.
Rao, I.J.; Rajagopal, K.R., On a new interpretation of the classical Maxwell model, Mech. Res. Commun. 34 (7–8) (2007) 509–514.
Recommended practice for field testing water-based drilling fluids (API), 2009. Standard API RP 13B-1, American Petroleum Institute, Washington, DC.
Recommended practice for laboratory testing of drilling fluids (API), 2009. Standard API RP 13I, American Petroleum Institute, Washington, DC.
Reddy, B.R., Palmer, A.V., 2009. Sealant compositions comprising colloidally stabilized latex and methods of using the same. US Patent 7 607 483, assigned to Halliburton Energy Services, Inc. (Duncan, OK), October 27, 2009.
Rehage, H.; Hoffmann, H., Rheological properties of viscoelastic surfactant systems, J. Phys. Chem. 92 (16) (1988) 4712–4719.
Roark, D.N., Nugent Jr., A., Bandlish, B.K., 1986. Fluid loss control and compositions for use therein. EP Patent 201 355, November 12, 1986.
Roark, D.N., Nugent Jr., A., Bandlish, B.K., 1987. Fluid loss control in well cement slurries. US Patent 4 698 380, assigned to Ethyl Corp., October 06, 1987.
Savoly, A., Villa, J.L., Garvey, C.M., Resnick, A.L., 1987. Fluid loss agents for oil well cementing composition. US Patent 4 674 574, June 23, 1987.
Schilling, S., 1990. Lignin-based cement fluid loss control additive. US Patent 4 926 944, May 22, 1990.
Schilling, P., 1991. Aminated sulfonated or sulfomethylated lignins as cement fluid loss control additives. US Patent 4 990 191, assigned to Westvaco Corp., February 05, 1991.
Sebba, F., 1984. Preparation of biliquid foam compositions. US Patent 4 486 333, December 4, 1984.
Sedillo, L.P., Newlove, J.C., Portnoy, R.C., 1987. Fluid loss control in oil field cements. US Patent 4 659 750, April 21, 1987.
Sifferman, T.R., Swazey, J.M., Skaggs, C.B., Nguyen, N., Solarek, D.B., 1999. Fluid loss control additives and subterranean treatment fluids containing the same. WO Patent 9 905 235, assigned to Monsanto Co. and Natl. Starch Chem. Inv. Corp., February 04, 1999.
Sopko, T.M., Lorentz, R.E., 1991. Method of using polymers of amido-sulfonic acid containing monomers and salts as drilling additive. US Patent 5 039 433, assigned to Lubrizol Corp., August 13, 1991.
Standard test method for fluid loss of clay component of geosynthetic clay liners, 2009. ASTM Standard, Book of Standards, Vol. 04.13 ASTM D5891-02. ASTM International, West Conshohocken, PA.
Stephens, M., 1988. Fluid loss additives for well cementing compositions. GB Patent 2 202 526, September 28, 1988.
Stephens, M., Swanson, B.L., 1992. Drilling mud comprising tetrapolymer consisting of N-vinyl-2-pyrrolidone, acrylamidopropanesulfonic acid, acrylamide, and acrylic acid. US Patent 5 135 909, assigned to Phillips Petroleum Co., August 04, 1992.
Stewart, W.S., Dixon, G.G., Elsen, J.M., Swanson, B.L., 1988. Drilling fluid additives for use in hard brine environments. US Patent 4 743 383, May 10, 1988.
Sullivan, P., Christanti, Y., Couillet, I., Davies, S., Hughes, T., Wilson, A., 2006. Methods for controlling the fluid loss properties of viscoelastic surfactant based fluids. US Patent 7 081 439, assigned to Schlumberger Technology Corporation (Sugar Land, TX), July 25, 2006.
Tan, D., Test and application of drilling fluid filtrate reducer polysulfonated humic acid resin, Oil Drilling Prod. Technol. 12 (1) (1990) 27–32; 97–98.
Todd, B.L., Slabaugh, B.F., Munoz Jr., T., Parker, M.A., 2006. Fluid loss control additives for use in fracturing subterranean formations. US Patent 7 096 947, assigned to Halliburton Energy Services, Inc. (Duncan, OK), August 29, 2006.
Ujma, K.H.W.; Plank, J.P., A new calcium-tolerant polymer helps to improve drilling mud performance and reduce costs, In: Proceedings Volume, 62nd Annu. SPE Tech. Conf.Dallas, 9/27–30/87. (1987), pp. 327–334.
Ujma, K.H.; Sahr, M.; Plank, J.; Schoenlinner, J., Cost reduction and improvement of drilling mud properties by using polydrill (Kostenreduzierung und Verbesserung der Spülungseigenschaften mit Polydrill), Erdöl Erdgas Kohle 103 (5) (1987) 219–222.
Vitthal, S.; McGowen, J.M., Fracturing fluid leakoff under dynamic conditions: Pt.2: Effect of shear rate, permeability, and pressure, In: Proceedings Volume, Annu. SPE Tech. Conf.Denver, 10/6–9/96. (1996), pp. 821–835.
Weaver, J.D., Slabaugh, B.F., Walters, H.G., 2010. Subterranean treatment fluids with improved fluid loss control. US Patent 7 645 725, assigned to Halliburton Energy Services, Inc. (Duncan, OK), January 12, 2010.
Williamson, C.D., 1989. Chemically modified lignin materials and their use in controlling fluid loss. GB Patent 2 210 888, June 21, 1989.
Williamson, C.D., Allenson, S.J., Gabel, R.K., 1991a. Additive and method for temporarily reducing permeability of subterranean formations. US Patent 4 997 581, assigned to Nalco Chemical Co., March 05, 1991.
Williamson, C.D., Allenson, S.J., Gabel, R.K., Huddleston, D.A., 1991b. Enzymatically degradable fluid loss additive. US Patent 5 032 297, assigned to Nalco Chemical Co., July 16, 1991.
Williamson, R., 1987. Well drilling fluids, fluid loss additives therefor and preparation of such additives. GB Patent 2 178 785, February 18, 1987.
Xiang, T., 2007. Drilling fluid systems for reducing circulation losses. US Patent 7 226 895, assigned to Baker Hughes Incorporated (Houston, TX), June 5, 2007.
Xiang, T., 2009. Methods for reducing circulation loss during drilling operations. US Patent 7 507 692, assigned to Baker Hughes Incorporated (Houston, TX), March 24, 2009.
Yin, H.; Lin, Y.; Huang, J., Microstructures and rheological dynamics of viscoelastic solutions in a catanionic surfactant system, J. Colloid Interface Sci. 338 (1) (2009) 177–183.
Zamora, F., Eoff, L.S., Dalrymple, E.D., Reddy, B.R., 2007. Drilling fluid component. US Patent 7 220 708, assigned to Halliburton Energy Services, Inc. (Duncan, OK), May 22, 2007.
Zhang, C.G.; Sun, M.B.; Hou, W.G.; Sun, D., Study on function mechanism of filtration reducer: The influence of fluid loss additive on electrical charge density of filter cake fines, Drill. Fluid Completion Fluid 12 (4) (1995) 1–5.
Zhang, C.G.; Sun, M.B.; Hou, W.G.; Liu, Y.Y.; Sun, D.J., Study on function mechanism of filtration reducer: Comparison, Drill. Fluid Completion Fluid 13 (3) (1996) 11–17.
Zhang, L.S., Wang, Y.Q., Farrar, D., 1990. Polymerisation processes and products. EP Patent 356 242, February 28, 1990.
Zhang, G.P.; Ye, H.C., AM/MA/AMPS terpolymer as non-viscosifying filtrate loss reducer for drilling fulids, Oilfield Chem. 15 (3) (1998) 269–271.
Tradenames
| Tradename | |
|---|---|
| Description | Supplier |
| Activator™ I | MASI Technologies L.L.C. |
| 90% oligosaccharide, 10% magnesium oxide (Growcock and Simon 2006) | |
| Airflex® (Series) | Air Products and Chemicals, Inc. |
| Vinyl acetate/ethylene copolymer emulsions (Halliday et al. 2007) | |
| ALL-TEMP® | Baker Hughes Drilling Fluids |
| Acrylate tetrapolymer (Jarrett and Clapper 2010) | |
| Aquacol-S® | Baker Hughes |
| Poly(ether glycol) (Halliday et al. 2007) | |
| BARACARB® | Halliburton Energy Services, Inc. |
| Ground marble (Reddy and Palmer 2009) | |
| Barodense® | Halliburton Energy Services, Inc. |
| Ground hematite (Reddy and Palmer 2009) | |
| Benol® | Sonneborn Refined Products |
| White mineral oil (Crews and Huang, 2010a and Crews and Huang, 2010b) | |
| BIO-LOSE™ | Baker Hughes |
| Complexed polysaccharide, filtration control agent (Halliday et al., 2007 and Halliday et al., 2008; Xiang 2007) | |
| BIO-PAQ™ | Baker Hughes INTEQ |
| Water-soluble polymer (Xiang 2007) | |
| Blue Streak™ | MASI Technologies L.L.C. |
| Compostion containing alcohol ether sulfate, cocobetaine, and hydroxypropylguar (surfactant) (Growcock and Simon 2006) | |
| BORE-DRILL™ | Borden Chemicals |
| Anionic polymer (Jarrett and Clapper 2010) | |
| Britolo® 35 USP | Sonneborn Refined Products |
| High viscosity mineral oil (Huang and Crews 2009) | |
| BROMA™ FLA | TBC Brinadd |
| Starch (Munoz and Todd 2008) | |
| Captivates® liquid | ISP Hallcrest |
| Fish gelatin and gum acacia encapsulation coating (Crews and Huang, 2010a and Crews and Huang, 2010b) | |
| Carbo-Gel® | Baker Hughes |
| Amine modified, gel-forming organophilic clay (Halliday et al., 2007 and Halliday et al., 2008) | |
| Carbo-Mul™ | Baker Hughes |
| Emulsifier (Halliday et al., 2007 and Halliday et al., 2008) | |
| Carbotec® -S | Baker Hughes |
| Poly(fatty acids), emulsifier (Halliday et al. 2007) | |
| Carbotron™ | Dow |
| Cellulose derivative (Morgan et al. 2008) | |
| Carnation® | Sonneborn Refined Products |
| White mineral oil (Crews and Huang, 2010a and Crews and Huang, 2010b) | |
| Ceramicrete® | Argon National Labs. |
| Magnesium-based ceramic particulate bridging agent (Munoz and Todd 2008) | |
| CFR™ 3 | Halliburton Energy Services, Inc. |
| Cement friction reducer dispersant (Morgan et al. 2008; Reddy and Palmer 2009) | |
| CFR™(Series) | Halliburton Energy Services, Inc. |
| Formaldehyde acetone condensate, dispersant (Morgan et al. 2008) | |
| Chek-Loss® PLUS | Baker Hughes |
| Ultra-fine lignin (Jarrett and Clapper 2010; Xiang 2007) | |
| CHEMTROL® X | Baker Hughes |
| Blend of ground lignitic earth and synthetic maleic anhydride copolymers (Jarrett and Clapper 2010) | |
| Claytone® II | Claytone |
| Organophilic bentonite (Reddy and Palmer 2009) | |
| ClearFRAC™ | Schlumberger Technology Corp. |
| Stimulating fluid (Crews 2006; Crews and Huang, 2010a and Crews and Huang, 2010b; Huang and Crews 2009; Huang et al. 2009) | |
| Corning® 1430 | Dow Corning Corp. |
| Water dilutable silicone emulsion (Berry et al. 2008) | |
| DFE-129™ | Baker Hughes Drilling Fluids |
| Acrylamide/AMPS copolymer (Jarrett and Clapper 2010) | |
| DFE-243® | Baker Hughes INTEQ |
| Partially hydrolyzed polyacrylamide/ trimethylaminoethyl acrylate (Xiang 2007) | |
| Diamond FRAQ™ | Baker Oil Tools |
| VES breaker (Crews and Huang 2010a; Huang and Crews 2009) | |
| Diamond FRAQ™ | Baker Oil Tools |
| VES System ((Crews and Huang, 2010a and Crews and Huang, 2010b; Huang and Crews 2009) | |
| Disponil® | Henkel |
| Ether sulfonates (Emulsifyer) (Guichard et al., 2006, Guichard et al., 2007 and Guichard et al., 2008) | |
| DrillAhead® | Halliburton Energy Services, Inc. |
| Software (Reddy and Palmer 2009) | |
| Driltreat™ | Halliburton Energy Services, Inc. |
| Wetting agent (Reddy and Palmer 2009) | |
| Driscal® D | Drilling Specialties Comp. |
| Water-soluble polymer (Jarrett and Clapper 2010) | |
| Elvace (Series) | Reichhold |
| Vinylacetate/ethylene copolymer latex (Halliday et al. 2007) | |
| Escaid® (Series) | Crompton Corp. |
| Mineral oils (Crews and Huang, 2010a and Crews and Huang, 2010b) | |
| EZ MUL ®NT | Halliburton Energy Services, Inc. |
| Emulsifier (Reddy and Palmer 2009) | |
| FlexPlug® OBM | Halliburton Energy Services, Inc. |
| Reactive, nonparticulate lost-circulation materia (Reddy and Palmer 2009) | |
| FlexPlug® W | Halliburton Energy Services, Inc. |
| Reactive, nonparticulate lost-circulation materia (Reddy and Palmer 2009) | |
| Flo-Chek® | Halliburton Energy Services, Inc. |
| Lost circulation additive (Reddy and Palmer 2009) | |
| Germall® II | Sutton Laboratories, Chatham, N.J. |
| Biocid compostion (Berry et al. 2008) | |
| Gloria® | Sonneborn Refined Products |
| High viscosity mineral oil (Huang and Crews 2009) | |
| Glycacil® L | Lonza Inc. |
| Iodopropynl butyl carbamate (biocide) (Berry et al. 2008) | |
| Go Devil™ II | MASI Technologies L.L.C. |
| Xanthan gum-based blend, 70% xanthan gum, 20% starch, 9% oligosaccharide, 1% magnesium oxide (Growcock and Simon 2006) | |
| Halad® (Series) | Halliburton Energy Services, Inc. |
| Fluid loss control additive (Lewis et al., 2008 and Lewis et al., 2009; Morgan et al. 2008) | |
| HR® (Series) | Halliburton Energy Services, Inc. |
| Organic acids (cement set retarder) (Morgan et al. 2008) | |
| Hydrobrite® 200 | Sonneborn Inc. |
| White mineral oil (Crews and Huang, 2010a and Crews and Huang, 2010b; Huang and Crews 2009) | |
| HYPERDRILL™ CP-904L | Hychem, Inc. |
| Acrylamide copolymer (Xiang, 2007 and Xiang, 2009) | |
| Invermul® | Halliburton Energy Services, Inc. |
| Blends of oxidized tall oil and polyaminated fatty acids (Reddy and Palmer 2009) | |
| Isopar® (Series) | Exxon |
| Isoparaffinic solvent (Crews and Huang, 2010a and Crews and Huang, 2010b) | |
| Isoteq™ | Baker Hughes |
| Drilling fluid (Halliday et al. 2007) | |
| Kathon® CG | Rohm & Haas |
| 5-Chloro-2-methyl4-isothiazolin-3-one (biocide) (Berry et al. 2008) | |
| Kaydol® oil | Witco Corp. |
| Mineral oil (Huang and Crews 2009) | |
| KEM-SEAL® PLUS | Baker Hughes Drilling Fluids |
| NaAMPS/N,N -dimethylacrylamide copolymer (Jarrett and Clapper 2010) | |
| Kemseal® | Baker Hughes Norge |
| Fluid loss additive (Jarrett and Clapper 2010) | |
| Kleemul® | BW Group |
| Emulsifier (Guichard et al., 2006, Guichard et al., 2007 and Guichard et al., 2008) | |
| Kraton® | Shell |
| Styrenic block copolymer (Guichard et al., 2006, Guichard et al., 2007 and Guichard et al., 2008) | |
| Ligco® | Baker Hughes |
| Lignite (Jarrett and Clapper 2010) | |
| Ligcon® | Milchem Inc. |
| Causticized lignite (Jarrett and Clapper 2010) | |
| MAX-PLEX® | Baker Hughes |
| Resin and aluminate (Halliday et al. 2008) | |
| MAX-SEAL™ | Baker Hughes |
| Poly(olefin) hydrocarbon base fluid (Halliday et al. 2007) | |
| MAX-TROL® | Baker Hughes Drilling Fluids |
| Sulfonated resin (Jarrett and Clapper 2010) | |
| Microbond™ | Halliburton Energy Services, Inc. |
| Cement expanding additive (Lewis et al., 2008 and Lewis et al., 2009) | |
| MicroPolymer™ | Halliburton Energy Services, Inc. |
| Multimodal polymer compostion (Weaver et al. 2010) | |
| Microsponge™ | Advanced Polymer Systems |
| Porous solid substrate (Crews 2006; Crews and Huang, 2010a and Crews and Huang, 2010b) | |
| Mil-Bar® | Baker Hughes |
| Barite weighting agent (Halliday et al., 2007 and Halliday et al., 2008; Jarrett and Clapper 2010) | |
| Mil-Carb® | Baker Hughes Drilling Fluids |
| Ground marble (Halliday et al., 2007 and Halliday et al., 2008; Jarrett and Clapper 2010; Xiang, 2007 and Xiang, 2009) | |
| Mil-Gel™ | Baker Hughes |
| Ground montmorillonite (Jarrett and Clapper 2010) | |
| Mil-Gel-NT® | Baker Hughes |
| Bentonite quartz mixture (Jarrett and Clapper 2010) | |
| Mil-Pac® LV | Baker Hughes |
| Low viscosity polyamine cellulose (Halliday et al. 2008) | |
| Mil-Temp® | Baker Hughes |
| Maleic anhydride copolymer (Jarrett and Clapper 2010) | |
| Mranol® | Rhone-Poulenc, Inc. |
| Imidazoline and imidazoline derivatives (cationic surfactants) (Berry et al. 2008) | |
| Newdrill® Plus | Baker Hughes |
| Partially hydrolyzed poly(acrylamide) (Halliday et al. 2007) | |
| NEW-DRILL® PLUS | Baker Hughes INTEQ |
| Partially hydrolyzed poly(acrylamide) (Halliday et al. 2008; Xiang 2007) | |
| OMNI-MUL™ | Baker Hughes |
| Non-ionic emulsifier (Halliday et al. 2007) | |
| Performance® 225N | ConocoPhillips |
| Base oil (Huang and Crews 2009) | |
| Plex® | Rohm & Haas |
| Acrylate resin (Guichard et al., 2006, Guichard et al., 2007 and Guichard et al., 2008) | |
| Plioflex® | Goodyear Chemicals |
| Styrene butadiene rubber (Guichard et al., 2006, Guichard et al., 2007 and Guichard et al., 2008) | |
| Pliolite® DF01 | Goodyear Tire & Rubber Co. |
| Styrene-butadiene copolymer (Guichard et al., 2006, Guichard et al., 2007 and Guichard et al., 2008) | |
| Plioway® EC1 | Goodyear Tire & Rubber Co. |
| p-Methylstyrene copolymer (Magnet et al. 2008) | |
| Plioway® Ultra 200 | Goodyear Tire & Rubber Co. |
| p-tert -Butylstyrene/italic-methylstyrene/ 2-ethylhexylacrylate/isobutyl methacrylate copolymer (Magnet et al. 2008) | |
| Polydrill® | Degussa AG |
| Anionic polymer (Jarrett and Clapper 2010) | |
| Poly-S.RTM | Scotts Comp. |
| Polymer encapsulation coating (Crews 2006; Crews and Huang, 2010a and Crews and Huang, 2010b) | |
| Protecto-Magic™ | Baker Hughes |
| Ground asphalt (Jarrett and Clapper 2010) | |
| PYRO-TROL® | Baker Hughes |
| Acrylamide/AMPS copolymer (Jarrett and Clapper 2010) | |
| Rev® Dust | Milwhite, Inc. |
| Artificial drill solids (Jarrett and Clapper 2010) | |
| Silicalite® | Halliburton Energy Services, Inc. |
| High surface area amorphous silica (Lewis et al., 2008 and Lewis et al., 2009; Morgan et al. 2008) | |
| Soltex® | Chevron Phillips Chemical Comp. |
| Sulfonated asphalt (Jarrett and Clapper 2010) | |
| Span® 20 | Uniqema |
| Sorbitan monolaurate (Crews and Huang 2010a) | |
| Span® 40 | Uniqema |
| Sorbitan monopalmitate (Crews and Huang 2010a) | |
| Span® 61 | Uniqema |
| Sorbitan monostearate (Crews and Huang 2010a) | |
| Span® 65 | Uniqema |
| Sorbitan tristearate (Crews and Huang 2010a) | |
| Span® 80 | Uniqema |
| Sorbitan monooleate (Crews and Huang 2010a) | |
| Span® 85 | Uniqema |
| Sorbitan trioleate (Crews and Huang 2010a) | |
| SULFA-TROL® | Baker Hughes Drilling Fluids |
| Sulfonated asphalt (Jarrett and Clapper 2010) | |
| Superfloc™ | Cytec Industries, Inc. |
| Acrylamide copolymer (Xiang, 2007 and Xiang, 2009) | |
| SurFRAQ™ VES | Baker Oil Tools |
| Tallow amido propylamine oxide (Huang and Crews 2009; Huang et al. 2009) | |
| Synthemul® (Series) | Reichold |
| Carboxylated acrylic copolymer (Halliday et al. 2007) | |
| Teflon® | DuPont |
| Tetrafluoro polymer (Weaver et al. 2010) | |
| Tergitol® 15-S (Series) | Union Carbide Corp. |
| Ethoxylated C11-15-secondary alcohols, surfactant (Sebba 1984) | |
| Tween® 20 | Uniqema |
| Sorbitan monolaurate (Crews and Huang 2010a) | |
| Tween® 21 | Uniqema |
| Sorbitan monolaurate (Crews and Huang 2010a) | |
| Tween® 40 | Uniqema |
| Sorbitan monopalmitate (Crews and Huang 2010a) | |
| Tween® 60 | Uniqema |
| Sorbitan monostearate (Crews and Huang 2010a) | |
| Tween® 61 | Uniqema |
| Sorbitan monostearate (Crews and Huang 2010a) | |
| Tween® 65 | Uniqema |
| Sorbitan tristearate (Crews and Huang 2010a) | |
| Tween® 81 | Uniqema |
| Sorbitan monooleate (Crews and Huang 2010a) | |
| Tween® 85 | Uniqema |
| Sorbitan monooleate (Crews and Huang 2010a) | |
| Tychem® 68710 | Reichhold |
| Carboxylated styrene/butadiene copolymer (Reddy and Palmer 2009) | |
| Tylac® CPS 812 | Reichhold |
| Carboxylated styrene/butadiene copolymer (Reddy and Palmer 2009) | |
| VES-STA 1 | Baker Oil Tools |
| Gel stabilizer (Crews and Huang, 2010a and Crews and Huang, 2010b) | |
| WG-3L VES-AROMOX® APA-T | Akzo Nobel |
| Viscoelastic surfactant (Crews and Huang 2010b) | |
| XAN-PLEX™ D | Baker Hughes INTEQ |
| Polysaccharide viscosifying polymer (Halliday et al., 2007 and Halliday et al., 2008; Xiang 2007) | |
| XANVIS™ | Baker Hughes INTEQ |
| Polysaccharide viscosifying polymer (Xiang, 2007 and Xiang, 2009) |
..................Content has been hidden....................
You can't read the all page of ebook, please click here login for view all page.