Chapter 3. Clay Stabilization
Problems caused by shales in petroleum activities are not new. At the beginning of the 1950s, many soil mechanics experts were interested in the swelling of clays, which are important for maintaining wellbore stability during drilling, especially in water-sensitive shale and clay formations.
The rocks within these types of formations absorb the fluid used in drilling, which causes them and may lead to a wellbore collapse. The swelling of clays and the problems that may so arise have been reviewed in the literature (Durand et al., 1995a and Durand et al., 1995b; Van Oort, 1997; Zhou et al., 1995). Various additives for clay stabilization are shown in Table 3.1.
| BDBiodegradable | |
| LTLow toxicity | |
| SFWell stimulation fluid | |
| a75% Hydrolyzed, 50 k Dalton | |
| bShear-degraded, for montmorillonite clay dispersed in sand packs | |
| cInjection of unreactive gas | |
| Additive | References |
|---|---|
| Polymer latices | Stowe et al. (2002) |
| Partially hydrolyzed polyvinylacetatea | Kubena Jr., et al. (1993) |
| Polyacrylamideb | Zaitoun and Berton (1990), Zaltoun and Berton (1992) |
| Copolymer of anionic and cationic monomers: Acrylic acid (AA), methacrylic acid, 2-acrylamido-2-methyl-1-propane sulfonic acid, dimethyl diallyl ammonium chloride | Aviles-Alcantara et al. (2000), Smith and Thomas (1995a,b, 1997) |
| Nitrogenc | Sloat, 1989 and Sloat, 1991 |
| Partially hydrolyzed acrylamide-acrylate copolymer, potassium chloride, and polyanionic cellulose (PAC) | Halliday and Thielen (1987) |
| Aluminum/guanidine complexes with cationic starches and polyalkylene glycolsMA | Branch (1988) |
| Hydroxyaldehydes or hydroxyketones | Westerkamp et al. (1991) |
| Polyols and alkaline salt | Hale and van Oort (1997) |
| Tetramethylammonium chloride and methyl chloride quaternary salt of polyethyleneimineSF | Aften and Gabel (1992a,b, 1994) |
| Pyruvic aldehyde and a triamine | Crawshaw et al. (2002) |
| Quaternary ammonium compounds | |
| In situ crosslinking of epoxide resins | Coveney et al. (1999a,b) |
| Oligomer (methyl quaternary amine containing 3–6 moles of epihalohydrin) | Himes and Vinson (1989) |
| Quaternary ammonium carboxylatesBD and LT | Himes (1992) |
| Quaternized trihydroxyalkyl amineLT | Patel and McLaurine (1993) |
| Polyvinyl alcohol, potassium silicate, and potassium carbonate | Alford (1991) |
| Copolymer of styrene and substituted maleic anhydride (MA) | Smith and Balson (2000) |
| Potassium salt of carboxymethyl cellulose | Palumbo et al. (1989) |
| Water-soluble polymers with sulfosuccinate derivative-based surfactants, zwitterionic surfactantsBD and LT | Alonso-Debolt and Jarrett, 1994 and Alonso-Debolt and Jarrett, 1995 |
Properties of Clays
Clay minerals are generally crystalline in nature, and the structure of these crystals determines their properties. Typically, clays have a flaky, mica-type structure, with the flakes being made up of a number of crystal platelets stacked face to face. Each platelet is called a unit layer, and the surfaces of the unit layer are called basal surfaces. A unit layer is composed of multiple sheets. One sheet type is called the octahedral sheet. It is composed of either aluminum or magnesium atoms, octahedrally coordinated with the oxygen atoms of hydroxyl groups. Another sheet type is called the tetrahedral sheet, which consists of silicon atoms tetrahedrally coordinated with oxygen atoms. Sheets within a unit layer link together by sharing oxygen atoms.
When this linking occurs between one octahedral and one tetrahedral sheet, one basal surface contains exposed oxygen atoms, while the other has exposed hydroxyl groups. It is also quite common for two tetrahedral sheets to bond with one octahedral sheet by sharing oxygen atoms. The resulting structure, known as the Hoffmann structure, has an octahedral sheet that is sandwiched between the two tetrahedral sheets (Hoffmann and Lipscomb, 1962). As a result, both basal surfaces in a Hoffmann structure contain exposed oxygen atoms.
The unit layers stack together face-to-face and are held in place by weak attractive forces. The distance between corresponding planes in adjacent unit layers is called the c-spacing. A clay crystal structure with a unit layer consisting of three sheets typically has a c-spacing of about 9.5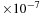 mm.
mm.
In clay mineral crystals, atoms having different valences will be commonly positioned within the sheets of the structure to create a negative potential at the crystal surface. In that case, a cation will be adsorbed onto the surface. These adsorbed cations are called exchangeable cations, because they may chemically trade places with other cations when the clay crystal is suspended in water. In addition, ions may also be adsorbed on the clay crystal edges and exchange with other ions in the water (Patel et al., 2007).
The type of substitutions occurring within the clay crystal structure and the exchangeable cations adsorbed on the crystal surface greatly affect clay swelling, a property of primary importance in the drilling fluid industry. In this phenomenon, water molecules surround a clay crystal structure and position themselves in such a way as to increase the structure's c-spacing, thus resulting in an increase in its volume.
Swelling of Clays
Two types of swelling may occur in clays (Patel et al., 2007). Surface hydration is one type, where water molecules are adsorbed on crystal surfaces. Hydrogen bonding holds a layer of water molecules to the oxygen atoms, which are exposed on the crystal surfaces. Subsequent layers of water molecules align to form a quasi-crystalline structure between unit layers, which results in an increased c-spacing. All types of clays swell in this manner.
Osmotic swelling is a second type of swelling. Where the concentration of cations between unit layers in a clay mineral is higher than that in the surrounding water, water is osmotically drawn between the unit layers and the c-spacing is increased. Osmotic swelling results in larger overall volume increases than surface hydration, but only a few clays, like sodium montmorillonite, swell in this manner (Patel et al., 2007).
Clays are naturally occurring layered minerals formed by weathering and decomposition of igneous rocks. Details of clay mineralogy can be found in the literature (Grim, 1968; Murray, 2007). Each layer is comprised of fused sheets of octahedra of Al3+, Mg2+, or Fe3+ oxides and sheets of tetrahedra of Si4+ oxides (Auerbach, 2007). If a clay mineral contains one tetrahedral and one octahedral sheet, it is known as a 1:1 clay, and if it contains two tetrahedral sheets sandwiching one central octahedral sheet, it is called a 2:1 clay. Octahedral and tetrahedral layers are illustrated in Figure 3.1.
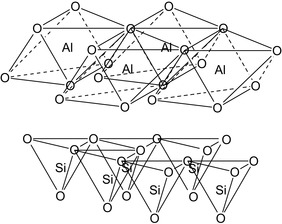 |
| Figure 3.1 Octahedral and tetrahedral layers in clays (Murray, 2007, p. 9). |
The metal atoms in the clay lattice can be substituted with others, which results in an overall negative charge on individual clay layers. This charge is compensated for by cations located in the interlayer region, which can be freely exchanged. The cation exchange capacity of the mineral depends on crystal size, pH, and the type of the cation its involved. These may not only be small ions, but poly-cations (Blachier et al., 2009) also.
Studies on the adsorption of a polycationic quaternary amine polymer onto clays have been presented. In charge scale, it can be observed that both the adsorption curve of the quaternary amine polymer and that corresponding to the released sodium are superimposed, as shown in Figure 3.2. The replacement of the counter ions by the amine polymer almost follows a 1:1 relationship at low polymer concentrations. Further, the silicate surfaces of the tetrahedral sheets of clay minerals are comparatively hydrophobic. This property may allow the intercalation of neutral organic compounds including polymers.
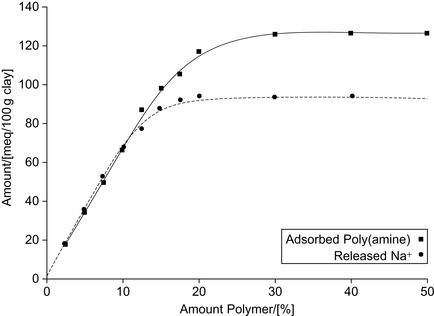 |
| Figure 3.2 Exchange of sodium cations against polyamine cations (Blachier et al., 2009). |
Smectite clays are of the type 2:1 and frequently occur in drilling situations (Anderson et al., 2010). Sodium-saturated smectite swells macroscopically, which causes in instability of shales during drilling operations. In the worst case, the wellbore may collapse as a result of clay swelling.
The type of exchangeable cations found in clay minerals is reported to have a significant impact on the amount of swelling that takes place. They compete with water molecules for the available reactive sites in the clay structure. Generally, cations with high valences are more strongly adsorbed than ones with low valences. Thus, clays with low valence exchangeable cations will swell more than those with high valences.
Water-based drilling fluids are generally considered to be more environmentally acceptable than oil-based or synthetic-based fluids. However, the former type of drilling fluid facilitates clay hydration and swelling, which can lead to significantly increased oil well construction costs (Anderson et al., 2010). For this reason, minimizing clay swelling is an important field of research. In order to reduce the extent of clay swelling effectively, its mechanism needs to be understood, so that efficient swelling inhibitors may be developed. Suitable clay swelling inhibitors must significantly reduce the hydration of the clay, and must also meet increasingly stringent environmental guidelines.
It is known that swelling takes place in a discrete fashion, in a stepwise formation of integer-layer hydrates. The transitions of the distances of the layers are thermodynamically analogous to phase transitions. Electro-osmotic swelling can occur only in clay minerals that contain exchangeable cations in the interlayer region. This type of swelling may yield significantly greater expansion than crystalline swelling.
Sodium-saturated smectites have a strong tendency to electro-osmotic swelling, but potassium-saturated smectites do not swell in this way. Thus, an appropriate ion exchange reaction may be helpful in clay stabilization (Anderson et al., 2010).
The water desorption isotherms of montmorillonite intercalated with exchangeable cations of the alkali metal group have show that for larger cations, less water is adsorbed (Mooney et al., 1952), and there is a relationship between the tendency to swell and the energy of hydration of the cation (Norrish, 1954).
Clay swelling during the drilling of a subterranean well can have a tremendous adverse impact on drilling operations. The overall increase in bulk volume impedes the removal of cuttings from beneath the drill bit, increases friction between the drill string and the sides of the borehole, and inhibits formation of the thin filter cake that seals formations. Clay swelling can also create other drilling problems, such as loss of circulation or cause pipes to stick (Patel et al., 2001).
In the North Sea and the United States Gulf Coast, drillers commonly encounter argillaceous sediments in which the predominant clay mineral is sodium montmorillonite, commonly called gumbo clay, in which sodium cations are predominately the exchangeable cations. Because the sodium cation has a low positive valence, (i.e., a 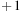 valence), it easily disperses into water. Consequently, gumbo clay is notorious for its swelling. Given the frequency in which this material is encountered in subterranean wells, the development of a substance and method for reducing clay swelling is of primary importance (Klein and Godinich, 2006).
valence), it easily disperses into water. Consequently, gumbo clay is notorious for its swelling. Given the frequency in which this material is encountered in subterranean wells, the development of a substance and method for reducing clay swelling is of primary importance (Klein and Godinich, 2006).
Montmorillonite
Montmorillonite clays, for example, bentonite and kaolinite clays, are suitable for preparing a solids-stabilized oil-in-water emulsion. Bentonite clay can be easily exfoliated (Bragg and Varadaraj, 2006). As mined, bentonite clays naturally consist of aggregates of particles that can be dispersed in water, or broken up by shearing into units with an average particle size of 2 μ or less. However, each of these particles is a laminated unit containing approximately 100 layers of fundamental silicate layers of 1 nm thickness bonded together by inclusions of atoms such as calcium in the layers.
By exchanging calcium with sodium or lithium, which are larger and have a strong attraction for water molecules in fresh water, and then exposing the bentonite to fresh water, it can be broken into individual 1 nm thick layers, called fundamental particles. The result of this delamination process is a gel consisting of a finely divided bentonite clay (Bragg and Varadaraj, 2006).
Guidelines
The literature offers several papers that may serve as guidelines for issues such as selecting a proper clay stabilizing system or completing wellbore stability analysis of practical well designs (Chen et al., 1996; Crowe, 1990 and Crowe, 1991; Evans and Ali, 1997; Scheuerman and Bergersen, 1989).
Mechanisms Causing Instability
Shale stability is an important problem faced during drilling and is most often attributed to the swelling of shales. It has been shown that several mechanisms can be involved (Gazaniol et al., 1994 and Gazaniol et al., 1995): pore pressure diffusion, plasticity, anisotropy, capillary effects, osmosis, and physicochemical alterations. Most importantly, three processes that contribute to the instability of shales must be considered (Bailey et al., 1994):
1. Movement of fluid between the wellbore and shale (limited to flow from the wellbore into the shale),
2. Changes in stress (and strain) that occur during shale-filtrate interaction, and
3. Softening and erosion caused by invasion of mud filtrate and consequent chemical changes in the shale.
The major reason for these effects is due to the hydration of clays. Borehole instabilities have been observed even with the most inhibitive fluids, that is oil-based drilling mud, which demonstrates that mechanical aspects are also important. In fact, the coupling of both chemical and mechanical mechanisms has to be considered. For this reason, it is still difficult to predict the behavior of rock at medium-to-large depth under certain loading conditions.
The stability of shales is governed by a complex relationship between transport processes (e.g., hydraulic flow, osmosis, diffusion of ions, pressure) and chemical changes (e.g., ion exchange, alteration of water content, swelling pressure).
They have the ability to absorb water, thus causing the instability of wells either because of the swelling of some mineral species or because the supporting pressure is suppressed by modification of the pore pressure. The response of a shale to a water-based fluid depends on its initial water activity and on the composition of the fluid.
The behavior of shales can be classified into either deformation mechanisms or transport mechanisms (Tshibangu et al., 1996). Optimization of mud salinity, density, and filter cake properties is important in achieving optimal shale stability and drilling efficiency with (WBM).
Kinetics of the Swelling of Clays
Basic studies on the kinetics of swelling have been performed (Suratman, 1985). Pure clays (montmorillonite, illite, and kaolinite) with polymeric inhibitors were investigated, and phenomenologic kinetic laws were established.
Hydrational Stress
Stresses caused by chemical forces, such as hydration stress, can have a considerable influence on the stability of a wellbore (Chen et al., 1995). When the total pressure and the chemical potential of water increase, water is absorbed into the clay platelets. This results either in the platelets moving farther apart (swelling) if they are free to move, or the generation of hydrational stress if the swelling is constrained (Tan et al., 1997). Hydrational stress results in an increase in pore pressure and a subsequent reduction in effective mud support, which leads to a less stable wellbore condition.
Borehole Stability Model
A borehole stability model has been developed that takes into account both the mechanical and chemical aspects of the interactions between drilling fluid and shale (Mody and Hale, 1993). Chemically induced stress alteration, based on the thermodynamics of differences in water molar free energies of the drilling fluid and shale is combined with mechanically induced stress. Based on this model, it should be possible to obtain the optimal mud weight and salt concentration for drilling fluids.
Further stability models based on surface area, equilibrium water-content–pressure relationships, and electric double-layer theory can successfully characterize borehole stability problems (Wilcox, 1990). The application of surface area, swelling pressure, and water requirements of solids can be integrated into these models, and mud process control approaches can be derived, which improve the design of WBM in active or older shales.
Shale Inhibition with Water-based Muds
One potential mechanism by which polymers may stabilize shales is by reducing the rate of water invasion into the shale. This is not the only mechanism involved in shale stabilization (Ballard et al., 1993); there is also an effect of the polymer additive.
Inhibiting Reactive Argillaceous Formations
Argillaceous formations are very reactive in the presence of water. Such formations can be stabilized by bringing them in contact with a polymer solution that contains hydrophilic and hydrophobic links (Audibert et al., 1997). The hydrophilic portion consists of polyoxyethylene, with hydrophobic end groups based on isocyanates. The polymer is capable of inhibiting the swelling or dispersion of argillaceous rock because of its adsorptive and hydrophobic capacities.
Thermal Treatment to Increase the Permeability
To increase the permeability of a certain region of the reservoir, the liquid-absorbed water is evaporated by heating the portion to above the boiling point of water, taking into account the ambient pressure (Jamaluddin and Nazarko, 1994; Reed, 1993). The liquid water is evaporated by injecting a water-undersaturated gas, such as heated nitrogen, into the reservoir.
Formation Damage by Fluids
Formation damage due to invasion by drilling fluids is a well-known problem in drilling. This is caused by the differential pressure between the hydrostatic column and the formation pressure, especially in low-pressure or depleted zones (Whitfill et al., 2005).
Invasion is also caused by openings in the rock, and the ability of fluids. When drilling depletes sands under overbalanced conditions, the mud will penetrate progressively into the formation unless there is an effective flow barrier present at the wellbore wall.
Horizontal drilling may also drill across highly fractured or permeable, low-pressure or depleted zones, which increases the probability of the drill pipe getting stuck due to it lying on the low side of the borehole. The exposure of numerous fractures or openings with low formation pressures will increase the problems of lost circulation and formation invasion (Whitfill et al., 2005).
Formation Damage in Gas Production Shut-in
Sometimes it may become necessary to shut-in a gas well when the demand for gas is low. In such instances, the well is shut-in for an indefinite period, after which it is reopened and production is resumed. It has often been found that the production rate of gas from the reopened well is substantially less than before the shut-in.
During production, the inner wall of the production tubing will be coated with a film of condensed fresh water because of the geothermal gradient. This water flows down when production is interrupted, and can cause formation damage, because clays are normally saturated with brine and not with fresh water. This swelling can be prevented with the injection of some additive, for example, sodium chloride, potassium chloride, calcium chloride, or an alcohol or a similar organic material (Wilson and Miller, 2001).
Swelling Inhibitors
Inhibitors of swelling by chemical means, rather than in a mechanical manner. They change the ionic strength and the transport behavior of the fluids into the clays. Both the cations and the anions are important for the efficiency of the inhibition of swelling of clays (Doleschall et al., 1987).
Salts
Swelling can be inhibited by the addition of KCl in relatively high amounts. Other swelling inhibitors are both uncharged polymers and polyelectrolytes (Anderson et al., 2010).
Quaternary Ammonium Salts
Choline salts are effective anti-swelling drilling fluid additives for underbalanced drilling operations (Kippie and Gatlin, 2009). Choline is a quaternary ammonium salt containing the N,N,N-trimethylethanolammonium cation, often present as the chloride.
Preparation 3–1
Triethanol amine methyl chloride can be prepared by adding methyl chloride in excess to triethanol amine in aqueous solution and heating for several hours. Upon completion of the reaction, the excess of methyl chloride is evaporated.
Choline formate is prepared from an aqueous solution of choline hydroxide by the reaction with formic acid simply by stirring.
Argillaceous formations contain clay particles. If a water-based drilling fluid is used in such formations, ion exchange, hydration, etc., will take place. These reactions cause swelling, crumbling, or dispersion of the clay particles. Ultimately, washout and even complete collapse of the borehole may occur (Eoff et al., 2006). Certain quaternized polymer additives may prevent these unfavorable reactions.
Such polymers have been shown in laboratory testing to vastly reduce shale erosion. Quaternized polymers can be synthesized by Eoff et al. (2006):
1. Quaternization of an AA based amine derivative with an alkyl halide, and subsequent polymerization, or
2. First polymerization and afterwards quaternization of the polymeric moieties.
Preparation 3–2
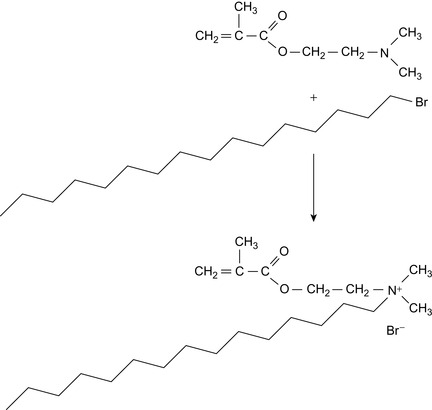
A quaternized monomer can be prepared by mixing dimethyl amino ethyl methacrylate with hexadecyl bromide. The mixture is heated to 43°C and stirred for 24 h. Then, the mixture is poured into petroleum ether, whereby the quaternized monomer precipitates (Eoff et al., 2006). The reaction is shown in Figure 3.3.
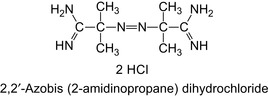
A copolymer can be prepared using the quaternized monomer described above and dimethyl amino ethyl methacrylate. The aqueous solution is neutralized with sulfuric acid and radically polymerized with 2,2′-azobis (2-amidinopropane) dihydrochloride, c.f., Figure 3.4. This initiator is water soluble. The polymerization is carried out at 43°C for 18 h (Eoff et al., 2006).
The quaternization of a polymer from dimethyl amino ethyl methacrylate has been described. To an aqueous solution of a homopolymer from dimethyl amino ethyl methacrylate sodium hydrochloride is added to adjust the pH to 8.9. Then again some water is added and hexadecyl bromide as alkylation agent, further benzylcetyldimethyl ammonium bromide as emulsifier. This mixture is then heated, with stirring, to 60°C for 24 h (Eoff et al., 2006).
Potassium Formate
Clay is stabilized in drilling and treatment operations by adding potassium formate to the drilling fluid along with a cationic formation control additive. Potassium formate can be generated in situ from potassium hydroxide and formic acid. The cationic additive is basically a polymer containing quaternized amine units, e.g., polymers of dimethyl diallyl ammonium chloride or acrylamide (Smith, 2009).
In the clay pack flow test, where the higher volumes at a given time indicate better clay stability, the addition of a small amount of potassium formate increases the volume throughput for a given polymer concentration. For example, 0.1% polydimethyl diallyl ammonium chloride added to the formulation had a volume at 10 min of 112 ml.
The same polymer, when combined with potassium formate and treated at 0.05% of the polymer, i.e., half the original polymer concentration, had a volume of 146 ml, indicating better clay stability and a possible synergistic effect from the addition of the potassium formate (Smith, 2009).
Saccharide Derivatives
The reaction product of methyl glucoside and alkylene oxides such as ethylene oxide (EO), propylene oxide (PO), or 1,2-butylene oxide is a drilling fluid additive that acts as a clay stabilizer. It is soluble in water at ambient conditions, but becomes insoluble at elevated temperatures (Clapper and Watson, 1996). Because of this insolubility, these compounds concentrate at important surfaces, such as the drill bit cutting surface, the borehole surface, and the surfaces of the drilled cuttings.
Sulfonated Asphalt
Asphalt is a solid, black-brown to black, bitumen fraction, which softens when heated and re-hardens upon cooling. It is not water soluble and difficult to disperse or emulsify in water.
Sulfonated asphalt can be obtained by reacting asphalt with sulfuric acid and sulfur trioxide. By neutralization with alkaline hydroxides, such as NaOH or NH3, sulfonate salts are formed. Only a limited portion of the sulfonated product can be extracted with hot water, but the fraction thus obtained, which is water soluble, is crucial for quality.
Sulfonated asphalt is predominantly used in water-based drilling fluids but also for those based on oil (Huber et al., 2009). It is reduced filtrate loss, improved filter cake properties, good lubrication of the drill and decreased formation damage (Huber et al., 2009).
The mechanism of action of sulfonated asphalt as a clay inhibitor in a drilling fluid is due to the electronegative sulfonated macromolecules attaching to the electropositive ends of the clay platelets. This creates a neutral barrier, which suppresses the absorption of water into the clay.
In addition, because the sulfonated asphalt is partially lipophilic, and therefore water repellent, the water influx into the clay is restricted by purely physical means. As mentioned already, the solubility in water of the sulfonated asphalt is crucial for proper application. By the introduction of a water-soluble and an anionic polymer component, the proportion of water-insoluble asphalt can be markedly reduced.
In other words, the proportion of the water-soluble fraction is increased by introducing the polymer component. Especially suitable are lignosulfonates as well as sulfonated phenol, ketone, naphthalene, acetone, and amino plasticizing resins (Huber et al., 2009).
Grafted Copolymers
The clay stabilization of copolymers of styrene and MA grafted with polyethylene glycol (PEG) has been investigated (Smith and Balson, 2004).
The amounts of shale recovery from bottle rolling tests have been used to measure the shale inhibition properties. The tests were done using Oxford Clay cuttings, a water-sensitive shale, sieved to 2–4 mm. Swelling was performed in 7.6% aqueous KCl.
The grafted copolymer used is an alternating copolymer of styrene and MA. It is grafted with polyethylene glycol (PEG) of varying molecular weights. The amount of shale recovery with various PEG types is shown in Table 3.2.
| SMAC Styrene and MA copolymer | ||
| SMAC 2:1 Styrene and MA copolymer, 2 styrene units for every MA | ||
| MPEG Polyethylene glycol monomethyl ethers, the number refers to the molecular weight | ||
| Sample | KCl/[%] | Shale Recovery/[%] |
|---|---|---|
| KCl only | 7.6 | 25 |
| PEG | 7.6 | 38 |
| SMAC MPEG 200 | 7.6 | 54 |
| SMAC MPEG 300 | 7.6 | 87 |
| SMAC MPEG 400 | 7.6 | 85 |
| SMAC MPEG 500 | 7.6 | 72 |
| SMAC MPEG 600 | 7.6 | 69 |
| SMAC MPEG 750 | 7.6 | 70 |
| SMAC MPEG 1100 | 7.6 | 66 |
| SMAC MPEG 1500 | 7.6 | 49 |
| KCl only | 12.9 | 27 |
| PEG | 12.9 | 53 |
| SMAC MPEG 500 | 12.9 | 85 |
| SMAC 2:1 MPEG 500 | 12.9 | 95 |
It seems that there is an optimum, with respect to the molecular weight of the grafted PEG. Further, the results in the lower part of Table 3.2 indicate that increasing the amount of styrene in the backbone increases the amount of shale that is recovered.
Polyoxyalkylene Amines
One method for reducing clay swelling is to use salts in drilling fluids. Salts generally reduce the swelling of clays, but they flocculate the clays resulting in both high fluid losses and an almost complete loss of thixotropy. Further, increasing the salinity often decreases the functional characteristics of drilling fluid additives (Patel et al., 2007).
Another method for controlling clay swelling is to use organic shale inhibitor compounds. It is believed that they are adsorbed onto the surfaces of clays where they compete with water molecules for clay reactive sites and thus serve to reduce clay swelling.
Polyoxyalkylene amines are a class of compound that contains primary amino groups attached to a polyether backbone. They are also known as polyether amines. They are available in a variety of molecular weights, ranging up to 5 k Dalton.
They are synthesized by the ring opening polymerization of oxirane compounds in the presence of amino compounds. Such compounds were made by reacting Jeffamine® with 2 equivalents of EO. Alternatively, PO is reacted with an oxyalkyldiamine (Patel et al., 2007). The polyether backbone is based either on EO, or PO, or a mixture of these oxirane compounds (Patel et al., 2007).
A typical polyether amine is shown in Figure 3.5. Such products belong to the Jeffamine® product family. A related shale hydration inhibition agent is based on an N-alkylated 2,2′-diaminoethylether.
 |
| Figure 3.5 Polyether amine (Klein and Godinich, 2006). |
Anionic Polymers
Anionic polymers may act by the long chain with negative ions attaching to the positive sites on the clay particles, or to the hydrated clay surface through hydrogen bonding (Halliday and Thielen, 1987). Surface hydration is reduced as the polymer coats the surface of the clay.
The protective coating also seals, or restricts the surface fractures or pores, thereby reducing or preventing the capillary movement of filtrate into the shale. This stabilizing process is supplemented by PAC. Potassium chloride enhances the rate of polymer absorption onto the clay.
Amine Salts of Maleic Imide
Compositions containing amine salts of the imides of MA polymers are useful for clay stabilization. These types of salts are formed by the reaction of MA with a diamine such as dimethyl aminopropylamine, in ethylene glycol (EG) solution (Poelker et al., 2009). The primary nitrogen dimethyl aminopropylamine forms the imide bond.
In addition, it may add to the double bond of MA. Further, the EG may add to the double bond, but also may condense with the anhydride itself. On repetition of these reactions, oligomeric compounds may be formed. The elementary reactions are shown in Figure 3.6. Finally, the product is neutralized with acetic acid or methanesulfonic acid to a pH of 4.
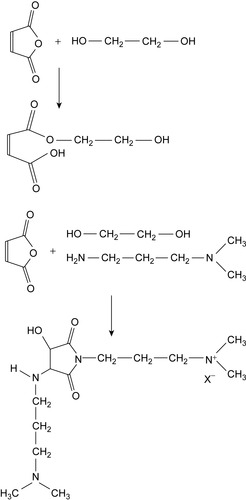 |
| Figure 3.6 Start of condensation with ethylene glycol (top) and formation of amine salts of imides (bottom) (Poelker et al., 2009). |
The performance of this compound was tested in Bandera sandstone, where it was found that the material neutralized with methanesulfonic acid performed somewhat less well then that neutralized with acetic acid. The compositions are particularly suitable for water-based hydraulic fracturing fluids.
Comparative Study
Three different clay inhibitors, a Performatrol® drilling fluid, a Claygrabber® shale stabilizer, and a Clay Sync™ shale stabilizer were compared to 4% glycol, a standard clay inhibitor, for their ability to inhibit the uptake of water by shale cuttings (Valenziano et al., 2009).
Clay Sync is a low molecular-weight, non-ionic polyacrylamide (PAM); Claygrabber Clay Sync is a highmolecular-weight, non-ionic PAM; and Performatrol® is polyN-vinyl-2-pyrrolidone, a water-soluble polymer. All of these compounds are commercially available from Baroid Fluid Services.
For each clay inhibitor, two types of shale cuttings were used. Each cutting was then placed in a 350 ml solution containing water and the indicated clay inhibitor for 4 h at 27°C. 0.5% Claygrabber®, 2 pounds per barrel Clay Sync, and 14 pounds per barrel Performatrol were used. The swelling S in Eq. 3.1 is the relative increase of the volume before Vb and after treatment Va.
(3.1)
A summary of the results is shown in Table 3.3.
| Clay Inhibitor | Cutting | Swelling/[% v/v] |
|---|---|---|
| Pure water | ||
| PERFORMATROL drilling fluid | 1 | 48.3 |
| PERFORMATROL drilling fluid | 2 | 95.5 |
| CLAYGRABBER shale stabilizer | 1 | 60.6 |
| CLAYGRABBER shale stabilizer | 2 | |
| CLAY SYNC shale stabilizer | 1 | 85.0 |
| CLAY SYNC shale stabilizer | 2 | 72.7 |
| 4% Glycol | 1 | 52.4 |
| Water | ||
| PERFORMATROL drilling fluid | 1 | 33.3 |
| PERFORMATROL drilling fluid | 2 | 13.3 |
| CLAYGRABBER shale stabilizer | 1 | 39.3 |
| CLAYGRABBER shale stabilizer | 2 | 18.8 |
| CLAY SYNC shale stabilizer | 1 | 30.0 |
| CLAY SYNC shale stabilizer | 2 | 18.5 |
| 4% Glycol | 1 | 35.0 |
| 4% Glycol | 2 | 26.5 |
| Water | ||
| PERFORMATROL drilling fluid | 1 | 9.5 |
| PERFORMATROL drilling fluid | 2 | 20.0 |
| CLAYGRABBER shale stabilizer | 1 | 16.7 |
| CLAYGRABBER shale stabilizer | 2 | 17.9 |
| CLAY SYNC shale stabilizer | 1 | 13.3 |
| CLAY SYNC shale stabilizer | 2 | 13.3 |
| 4% Glycol | 1 | 0.0 |
| 4% Glycol | 2 | 6.8 |
This demonstrates that the addition of either KCl or NaCl to solutions of clay inhibitors improves their ability to reduce the absorption of aqueous fluid by shale (Valenziano et al., 2009).
Test Methods
Shale Erosion Test
A shale erosion test is commonly employed to determine the ability of a drilling fluid plus additives to prevent a shale from eroding in the presence of an aqueous medium such as a drilling fluid (Eoff et al., 2006).
Such erosion, when encountered in actual field conditions in a borehole, and as noted above, can lead to problems ranging from a washout to a complete collapse of the borehole. Various shale erosion tests have been developed based on (Eoff et al., 2006; Reed, 1977):
• Time of total disintegration of the particles, and
• The change of particle size during rolling.
Disintegration of Particles
A shale erosion test has been developed that consists of compressing a known unstable oil field shale into a 0.5 in. diameter by 1 in. The cylinder is then placed into a capped round pint jar, which is two-thirds filled with the test fluid.
This jar is put onto motor driven rollers, which cause the shale cylinder to smoothly roll through the test fluid on the side of the jar. The time taken for the shale pellets to totally disintegrate is recorded (Reed, 1977).
Change of Mesh Size
A typical shale erosion test is conducted by rolling a weighed portion of sized shale particles in an aqueous medium, and then screening the particles to determine the amount of shale that eroded to the point of passing through a selected sized screen.
The shale is crushed and ground into particles that can pass through a 6 mesh screen but are retained on a 14 mesh screen. Thus, particles of a mesh size from 6 to 14 are used for the erosion test.
Portions of 40 g of the shale are placed in a laboratory barrel containing 350 ml of the test fluid and rolled for 16 hrs at the desired temperature, 65°C.
Afterwards, the drilling fluids are again screened through the 14 mesh screen. The retained solids are washed, dried, and weighed. Finally, the percent of erosion is calculated on the basis of the weight loss, corrected for the moisture content of the original sample (Eoff et al., 2006).
Hassler Cell
The effect of drilling fluid additives on reactive shales can be assessed by the Hassler Cell test, which basically measures permeability. For this reason, all effects that cause a change in permeability can be assessed. For example, a shale inhibition can be measured, as the permeability of a core is reduced. Furthermore the change in wettability by chemical treatment has been measured (Fleury et al., 1999). Hassler developed his method in the 1940 (Hassler, 1944; Hassler and Brunner, 1945).
The cores under investigation are centrifuged and saturated with liquid to establish a pressure gradient. The effluent fluids from the samples are collected in glass tubes. A strobed light source is used to determine the amounts of fluids collected.
The relative permeability of the core to a given fluid 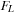 can be expressed as
can be expressed as
(3.2)
Measuring the capillary pressure requires increasing the speed of the centrifuge in increments and measuring the amount of fluid produced from the core sample when the flow has ceased for that particular centrifuge speed (Vinegar et al., 1987).
It has been pointed out that for two-phase flow some problems in the application of this method may arise (Rose, 1980). It is confined to a drainage mode of flow for a water-wet core initially filled with a wetting fluid, which is then invaded by a non-wetting fluid, i.e., oil invading a water-wet core, the method hence, is not useful when a wetting fluid invades a water-wet core containing a non-wetting fluid as the equilibrium level of production of the non-wetting fluid is dependent upon imbibition and not centrifuge speed. However, such measurements are needed in order to design waterflood recovery methods, where the invading fluid is wetting (Vinegar et al., 1987).
Even when developed in 1945, the method was readily automated, and methods have since been developed to determine the saturation of the fluid inside the core. Electromagnetic radiation is used to image a region while it is being centrifuged. From the attenuation coefficients, the fluid saturation may be calculated at several of points within the core. These saturations may be used to calculate capillary pressure or relative permeability (Vinegar et al., 1987).
Nowadays, pressure buildup can be readily established by chromatographic pumps instead of centrifugal forces (Buckley et al., 2007). In this design, a Hassler cell resembles a short chromatographic column.
Aften, C.W., Gabel, R.K., 1994. Clay stabilizer. US Patent 5 342 530, August 30 1994.
Alford, S.E., North Sea field application of an environmentally responsible water-base shale stabilizing system, In: Proceedings Volume, SPE/IADC Drilling Conf.Amsterdam, the Neth, 3/11–14/91. (1991), pp. 341–355.
Alonso-Debolt, M.A.; Jarrett, M.A., New polymer/surfactant systems for stabilizing troublesome gumbo shale, In: Proceedings Volume, SPE Int. Petrol. Conf. of MexVeracruz, Mex, 10/10–13/94. (1994), pp. 699–708.
Alonso-Debolt, M.; Jarrett, M., Synergistic effects of sulfosuccinate/polymer system for clay stabilization, In: Proceedings Volume, Vol. PD-65, Asme Energy-Sources Technol. Conf. Drilling Technol. Symp.Houston, 1/29/95–2/1/95. (1995), pp. 311–315.
Anderson, R.L.; Ratcliffe, I.; Greenwell, H.C.; Williams, P.A.; Cliffe, S.; Coveney, P.V., Clay swelling – A challenge in the oilfield, Earth-Sci. Rev. 98 (3–4) (2010) 201–216.
Audibert, A., Lecourtier, J., Bailey, L., Maitland, G., 1997. Method for inhibiting reactive argillaceous formations and use thereof in a drilling fluid. US Patent 5 677 266, assigned to Inst. Francais Du Petrole, October 14 1997.
In: (Editor: Auerbach, S.M.) Handbook of Layered Materials (2007) CRC Press, Boca Raton; reprint from 2004 Edition.
Aviles-Alcantara, C.; Guzman, C.C.; Rodriguez, M.A., Characterization and synthesis of synthetic drilling fluid shale stabilizer, In: Proceedings Volume, SPE Int. Petrol. Conf. in MexVillahermosa, 2/1–3/2000. (2000).
Bailey, L.; Reid, P.I.; Sherwood, J.D., Mechanisms and solutions for chemical inhibition of shale swelling and failure, In: Proceedings Volume, Recent Advances in Oilfield Chemistry, 5th Royal Soc. Chem. Int. Symp.Ambleside, Engl, 4/13–15/94. (1994), pp. 13–27.
Ballard, T.; Beare, S.; Lawless, T., Mechanisms of shale inhibition with water based muds, In: Proceedings Volume, IBC Tech. Serv. Ltd Prev. Oil Discharge from Drilling Oper. The Options Conf.Aberdeen, Scot, 6/23–24/93. (1993).
Blachier, C.; Michot, L.; Bihannic, I.; Barrès, O.; Jacquet, A.; Mosquet, M., Adsorption of polyamine on clay minerals, J. Colloid Interface Sci. 336 (2) (2009) 599–606.
Bragg, J.R., Varadaraj, R., 2006. Solids-stabilized oil-in-water emulsion and a method for preparing same. US Patent 7 121 339, assigned to ExxonMobil Upstream Research Company (Houston, TX), October 17 2006.
Branch, H.I., 1988. Shale-stabilizing drilling fluids and method for producing same. US Patent 4 719 021, January 12 1988.
Buckley, L.J.; Carter, M.A.; Wilson, M.A.; Scantlebury, J.D., Methods of obtaining pore solution from cement pastes and mortars for chloride analysis, Cement Concr. Res. 37 (11) (2007) 1544–1550.
Chen, M.; Chen, Z.; Huang, R., Hydration stress on wellbore stability, In: Proceedings Volume, 35th US Rock Mech Symp.Reno, NV, 6/5–7/95. (1995), pp. 885–888.
Chen, X.; Tan, C.P.; Haberfield, C.M., Wellbore stability analysis guidelines for practical well design, In: Proceedings Volume, SPE Asia Pacific Oil & Gas Conf.Adelaide, Australia, 10/28–31/96. (1996), pp. 117–126.
Clapper, D.K., Watson, S.K., 1996. Shale stabilising drilling fluid employing saccharide derivatives. EP Patent 702 073, assigned to Baker Hughes Inc., March 20 1996.
Coveney, P.V., Watkinson, M., Whiting, A., Boek, E.S., 1999. Stabilizing clayey formations. WO Patent 9 931 353, assigned to Sofitech NV, Dowell Schlumberger SA, and Schlumberger Canada Ltd., June 24 1999.
Crawshaw, J.P., Way, P.W., Thiercelin, M., 2002. A method of stabilizing a wellbore wall. GB Patent 2 363 810, assigned to Sofitech NV, January 09 2002.
Crowe, C.W., Laboratory study provides guidelines for selecting clay stabilizers, In: Proceedings Volume, Vol. 1, Cim. Petrol. Soc/SPE Int. Tech. Mtg.Calgary, Can, 6/10–13/90. (1990).
Crowe, C.W., 1991. Laboratory study provides guidelines for selecting clay stabilizers, SPE Unsolicited Pap SPE-21556, Dowell Schlumberger (January 1991).
Doleschall, S.; Milley, G.; Paal, T., Control of clays in fluid reservoirs, In: Proceedings Volume, 4th BASF AG et al Enhanced Oil Recovery Europe Symp.Hamburg, Ger, 10/27–29/87. (1987), pp. 803–812.
Durand, C.; Onaisi, A.; Audibert, A.; Forsans, T.; Ruffet, C., Influence of clays on borehole stability: A literature survey: Pt.1: Occurrence of drilling problems physico-chemical description of clays and of their interaction with fluids, Rev. Inst. Franc. Pet. 50 (2) (1995) 187–218.
Durand, C.; Onaisi, A.; Audibert, A.; Forsans, T.; Ruffet, C., Influence of clays on borehole stability: A literature survey: Pt.2: Mechanical description and modelling of clays and shales drilling practices versus laboratory simulations, Rev. Inst. Franc. Pet. 50 (3) (1995) 353–369.
Eoff, L.S., Reddy, B.R., Wilson, J.M., 2006. Compositions for and methods of stabilizing subterranean formations containing clays. US Patent 7 091 159, assigned to Halliburton Energy Services, Inc. (Duncan, OK), August 15 2006.
Evans, B.; Ali, S., Selecting brines and clay stabilizers to prevent formation damage, World Oil 218 (5) (1997) 65–68.
Fleury, M.; Branlard, P.; Lenormand, R.; Zarcone, C., Intermediate wettability by chemical treatment, J. Pet. Sci. Eng. 24 (2–4) (1999) 123–130.
Gazaniol, D.; Forsans, T.; Boisson, M.J.F.; Piau, J.M., Wellbore failure mechanisms in shales: Prediction and prevention, In: Proceedings Volume, Vol. 1, SPE Europe Petrol. Conf.London, UK, 10/25–27/94. (1994), pp. 459–471.
Gazaniol, D.; Forsans, T.; Boisson, M.J.F.; Piau, J.M., Wellbore failure mechanisms in shales: Prediction and prevention, J. Pet. Technol. 47 (7) (1995) 589–595.
Grim, R.E., In: Clay Mineralogysecond ed. (1968) McGraw-Hill, New York.
Hale, A.H., van Oort, E., 1997. Efficiency of ethoxylated/propoxylated polyols with other additives to remove water from shale. US Patent 5 602 082, February 11 1997.
Halliday, W.S., Thielen, V.M., 1987. Drilling mud additive. US Patent 4 664 818, assigned to Newpark Drilling Fluid In, May 12 1987.
Hassler, G.L., 1944. Method and apparatus for permeability measurements. US Patent 2 345 935, assigned to Dev., April 04 1944.
Hassler, G.L.; Brunner, E., Measurement of capillary pressures in small samples, Pet. Trans. AIME 160 (1945) 114–123.
Himes, R.E., 1992. Method for clay stabilization with quaternary amines. US Patent 5 097 904, assigned to Halliburton Co., March 24 1992.
Himes, R.E., Vinson, E.F., 1989. Stabilizing clay-containing formations. EP Patent 308 138, assigned to Halliburton Co., March 22 1989.
Hoffmann, R.; Lipscomb, W.N., Theory of polyhedral molecules. i. physical factorizations of the secular equation, J. Chem. Phys. 36 (8) (1962) 2179–2189.
Huber, J., Plank, J., Heidlas, J., Keilhofer, G., Lange, P., 2009. Additive for drilling fluids. US Patent 7 576 039, assigned to BASF Construction Polymers GmbH (Trostberg, DE), August 18 2009.
Jamaluddin, A.K.M., Nazarko, T.W., 1994. Process for increasing near-wellbore permeability of porous formations. US Patent 5 361 845, November 08 1994.
Kippie, D.P., Gatlin, L.W., 2009. Shale inhibition additive for oil/gas down hole fluids and methods for making and using same. US Patent 7 566 686, assigned to Clearwater International, LLC (Houston, TX), July 28 2009.
Klein, H.P., Godinich, C.E., 2006. Drilling fluids. US Patent 7 012 043, assigned to Huntsman Petrochemical Corporation (The Woodlands, TX), March 14 2006.
Kubena Jr., E., Whitebay, L.E., Wingrave, J.A., 1993. Method for stabilizing boreholes. US Patent 5 211 250, assigned to Conoco Inc., May 18 1993.
Mody, F.K.; Hale, A.H., A borehole stability model to couple the mechanics and chemistry of drilling fluid shale interaction, In: Proceedings Volume, SPE/IADC Drilling Conf.Amsterdam, Neth, 2/23–25/93. (1993), pp. 473–490.
Mooney, R.W.; Keenan, A.G.; Wood, L.A., Adsorption of water vapor by montmorillonite. II. Effect of exchangeable ions and lattice swelling as measured by X-ray diffraction, J. Am. Chem. Soc. 74 (6) (1952) 1371–1374.
Murray, H.H., In: Applied Clay Mineralogy: Occurrences, Processing, and Application of Kaolins, Bentonites, Palygorskite-Sepiolite, and Common Clays, Vol. 2 (2007) Elsevier, Amsterdam.
Norrish, K., The swelling of montmorillonite, Discuss. Faraday Soc. 18 (1954) 120–134.
Palumbo, S.; Giacca, D.; Ferrari, M.; Pirovano, P., The development of potassium cellulosic polymers and their contribution to the inhibition of hydratable clays, In: Proceedings Volume, SPE Oilfield Chem. Int. Symp.Houston, 2/8–10/89. (1989), pp. 173–182.
Patel, A.D., McLaurine, H.C., 1993. Drilling fluid additive and method for inhibiting hydration. CA Patent 2 088 344, assigned to M I Drilling Fluids Co., October 11 1993.
Patel, A.D., Stamatakis, E., Davis, E., 2001. Shale hydration inhibition agent and method of use. US Patent 6 247 543, assigned to M I Llc., June 19 2001.
Patel, A.D., Stamatakis, E., Davis, E., Friedheim, J., 2007. High performance water based drilling fluids and method of use. US Patent 7 250 390, assigned to M-I L.L.C. (Houston, TX), July 31 2007.
Poelker, D.J., McMahon, J., Schield, J.A., 2009. Polyamine salts as clay stabilizing agents. US Patent 7 601 675, assigned to Baker Hughes Incorporated (Houston, TX), October 13 2009.
Reed, M.G., 1977. Hydroxy-aluminum based drilling fluid. US Patent 4 045 357, assigned to Chevron Research Company (San Francisco, CA), August 30 1977.
Reed, M.G., 1993. Permeability of fines-containing earthen formations by removing liquid water. CA Patent 2 046 792, January 12 1993.
Rose, W., Some problems in applying the hassler relative permeability method, J. Pet. Technol. 32 (7) (1980) 1161–1163.
Scheuerman, R.F.; Bergersen, B.M., Injection water salinity, formation pretreatment, and well operations fluid selection guidelines, In: Proceedings Volume, SPE Oilfield Chem. Int. Symp.Houston, 2/8–10/89. (1989), pp. 33–49.
Sloat, B.F., 1989. Nitrogen stimulation of a potassium hydroxide wellbore treatment. US Patent 4 844 169, assigned to Marathon Oil Co., July 04 1989.
Sloat, B.F., 1991. Nitrogen stimulation of a potassium hydroxide wellbore treatment. CA Patent 1 291 419, October 29 1991.
Smith, K.W., 2009. Well drilling fluids. US Patent 7 576 038, assigned to Clearwater International, L.L.C. (Houston, TX), August 18 2009.
Smith, C.K., Balson, T.G., 2000. Shale-stabilizing additives. GB Patent 2 340 521, assigned to Sofitech NV and Dow Chemical Co., February 23 2000.
Smith, C.K., Balson, T.G., 2004. Shale-stabilizing additives. US Patent 6 706 667, March 16 2004.
Smith, K.W., Thomas, T.R., 1997. Method of treating shale and clay in hydrocarbon formation drilling. US Patent 5 607 902, assigned to Clearwater Inc., March 04 1997.
Stowe, C., Bland, R.G., Clapper, D., Xiang, T., Benaissa, S., 2002. Water-based drilling fluids using latex additives. GB Patent 2 363 622, assigned to Baker Hughes Inc., January 02 2002.
Suratman, I., 1985. A study of the laws of variation (kinetics) and the stabilization of swelling of clay (contribution a l'etude de la cinetique et de la stabilisation du gonflement des argiles), Ph.D. thesis. Malaysia.
Tan, C.P.; Richards, B.G.; Rahman, S.S.; Andika, R., Effects of swelling and hydrational stress in shales on wellbore stability, In: Proceedings Volume, SPE Asia Pacific Oil & Gas Conf.Kuala Lumpur, Malaysia, 4/14–16/97. (1997), pp. 345–349.
Tshibangu, J.P.; Sarda, J.P.; Audibert-Hayet, A., A study of the mechanical and physicochemical interactions between the clay materials and the drilling fluids: Application to the boom clay (Belgium) (etude des interactions mecaniques et physicochimiques entre les argiles et les fluides de forage: Application a l'argile de boom (Belgique)), Rev. Inst. Franc. Pet. 51 (4) (1996) 497–526.
Valenziano, R., Harris, K.L., Dixon, M.D., 2009. Servicing a wellbore with an aqueous based fluid comprising a clay inhibitor. US Patent 7 549 474, assigned to Halliburton Energy Services, Inc. (Duncan, OK), June 23 2009.
Van Oort, E., Physico-chemical stabilization of shales, In: Proceedings Volume, SPE Oilfield Chem. Int. Symp.Houston, 2/18–21/97. (1997), pp. 523–538.
Vinegar, H.J., O'Meara Jr., D.J., Rohan, J.A., 1987. Method and apparatus for determining distribution of fluids. US Patent 4 671 102, assigned to Shell Oil Company (Houston, TX), June 9 1987.
Westerkamp, A., Wegner, C., Mueller, H.P., 1991. Borehole treatment fluids with clay swelling-inhibiting properties (ii) (bohrloch- behandlungsfluessigkeiten mit tonquellungsinhibierenden eigenschaften (ii)). EP Patent 451 586, assigned to Bayer AG, October 16 1991.
Whitfill, D.L., Pober, K.W., Carlson, T.R., Tare, U.A., Fisk, J.V., Billingsley, J.L., 2005. Method for drilling depleted sands with minimal drilling fluid loss. US Patent 6 889 780, assigned to Halliburton Energy Services, Inc. (Duncan, OK), May 10 2005.
Wilcox, R.D., Surface area approach key to borehole stability, Oil Gas J. 88 (9) (1990) 66–80.
Wilson, S.J., Miller, M.E., 2001. Treatment for shut-in gas well. US Patent 6 302 206, assigned to Vastar Resources Inc. and Atlantic Richfield Co., October 16 2001.
Zaitoun, A.; Berton, N., Stabilization of montmorillonite clay in porous media by high-molecular-weight polymers, In: Proceedings Volume, 9th SPE Formation Damage Contr. Symp.Lafayette, LA, 2/22–23/90. (1990), pp. 155–164.
Zaltoun, A.; Berton, N., Stabilization of montmorillonite clay in porous media by high-molecular-weight polymers, SPE Prod. Eng. 7 (2) (1992) 160–166.
Zhou, Z.J.; Gunter, W.D.; Jonasson, R.G., Controlling formation damage using clay stabilizers: A review, In: Proceedings Volume-2, no. CIM 95-71, 46th Annu. Cim. Petrol. Soc. Tech. Mtg.Banff, Can, 5/14–17/95. (1995).
Tradenames
| Tradename Description | Supplier |
|---|---|
| Aerosil® Fumed silica (Bragg and Varadaraj, 2006) | Degussa AG |
| Barasil™ –S Shale stabilizer (Valenziano et al., 2009) | Baroid Fluid Services |
| Baromega™ Aqueous-based silicate containing resilient graphitic carbon (Whitfill et al., 2005) | Halliburton Energy Services, Inc. |
| Carbolite™ Sized ceramic proppant (Kippie and Gatlin, 2009) | Carbo Corp. |
| Clay Sync™ Shale stabilizer (Valenziano et al., 2009) | Baroid |
| ClaySeal® Shale stabilizer (Valenziano et al., 2009) | Baroid Fluid Services |
| Dacron® Polyethylene terephtthalate (Kippie and Gatlin, 2009) | DuPont |
| EZ-Mud® Shale stabilizer (Valenziano et al., 2009) | Baroid |
| GEM™ 2000 Shale stabilizer (Valenziano et al., 2009) | Baroid |
| Grabber® Flocculant (Valenziano et al., 2009) | Baroid |
| Hydro-Guard® Inhibitive water-based-fluid (Valenziano et al., 2009) | Halliburton Energy Services, Inc. |
| Jeffamine® (Series) Amine capped polyalkoxylene glycol (Patel et al., 2007) | Huntsman Petrochemical Corp. |
| Jeffamine® D-230 Polyoxypropylene diamine (Klein and Godinich, 2006) | Huntsman |
| Jeffamine® EDR-148 Triethyleneglycol diamine (Klein and Godinich, 2006) | Huntsman |
| Jeffamine® HK-511 Polyoxyalkylene amine (Klein and Godinich, 2006) | Huntsman |
| Performatrol® Shale stabilizer (Valenziano et al., 2009) | Baroid |
| Shale Guard™ NCL100 Shale anti-swelling agent (Kippie and Gatlin, 2009) | Weatherford Int. |
| Steelseal® Resilient graphitic carbon (Whitfill et al., 2005) | Halliburton Energy Services, Inc. |
..................Content has been hidden....................
You can't read the all page of ebook, please click here login for view all page.