Chapter 17. Fracturing Fluids
Hydraulic fracturing is a technique used to stimulate the productivity of a well. A hydraulic fracture is a superimposed structure that remains undisturbed outside the fracture, so the effective permeability of a reservoir remains unchanged by this process. The increased wellbore radius increases its productivity, because a large contact surface between the well and the reservoir is created.
Stresses and Fractures
Hydraulic fracturing is one of the newer techniques in petroleum sciences, not being used for more than approximately 50 years. The classic treatment (Hubbert and Willis, 1957) of hydraulic fracturing states that the fractures are approximately perpendicular to the axis of the least stress. For most deep reservoirs, the minimal stresses are horizontal, hence vertical stresses will occur in fracturing.
The actual stress can be calculated by balancing the (vertical) geostatic stress and the horizontal stress by the common tools of the theory of elasticity. For example, the geostatic stress must be corrected in a porous medium filled with a liquid having a poroelastic constant and hydrostatic pressure. The horizontal stress can be calculated from the corrected vertical stress by using the Poisson's ratio. Under some circumstances, in particular in shallow reservoirs, horizontal stresses can be created, as well as vertical stresses. The possible stress modes are summarized in Table 17.1.
Knowledge of the stresses in a reservoir is essential to find the pressure at which initiation of a fracture can take place. The upper bound of this pressure can be estimated using a formula given by Terzaghi (von Terzaghi, 1923), which states that:
(17.1)
The closure pressure indicates the pressure at which the width of the fracture becomes zero. This is normally the minimal horizontal stress.
The pressure response during fracturing provides important information about the success of the operation. The fluid efficiency can be estimated from the closure time.
Comparison of Stimulation Techniques
In addition to hydraulic fracturing, there are other stimulation techniques, such as acid fracturing or matrix stimulation, and hydraulic fracturing is also used in coal seams to stimulate the flow of methane.
Fracturing fluids are often divided into water-based, oil-based, alcohol-based, emulsion, or foam-based fluids. Several reviews are available in the literature dealing with the basic principles of hydraulic fracturing, and the guidelines that are used to select a formulation for a specific job (Ebinger and Hunt, 1989; Ely, 1989; Lemanczyk, 1991).
Polymer hydration, crosslinking, and degradation are the key processes that these materials undergo. Technological improvements over the years have focused primarily on improved rheological performance, thermal stability, and clean-up of crosslinked gels.
Action of a Fracturing Fluid
Fracturing fluids must meet a number of conditions simultaneously. They must be stable at high temperatures, high pumping rates, and shear rates, which can cause the fluids to degrade and prematurely settle out the proppant before the fracturing operation is complete.
Most commercially used fracturing fluids are aqueous liquids that have been either gelled or foamed. Typically, the fluids are gelled by a polymeric gelling agent. The thickened or gelled fluid helps keep the proppants within the fluid during the fracturing operation. Fracturing fluids are injected into a subterranean formation for the following purposes (Kelly et al., 2007):
• To create a conductive path from the wellbore extending into the formation and
• To carry proppant material into the fracture to create a conductive path for produced fluids.
Stages in a Fracturing Job
A fracturing job has several stages, including injecting a prepad, a pad, a proppant containing fracturing fluid, and finally, a treatment with flush fluids. A prepad is a low viscosity fluid used to condition the formation, which may contain fluid loss additives, surfactants, and have a defined salinity to prevent formation damage. The generation of the fractures takes place by injecting the pad, a viscous fluid, but without proppants.
After the fractures develop, a proppant must be injected to keep them permeable. When the fracture closes, the proppant left there creates a large flow area and a highly conductive pathway for hydrocarbons to flow into the wellbore. Thus, the proppant is utilized to maintain an open fracture. Viscous fluids are utilized to transport, suspend, and eventually allow the proppant to be trapped inside the fracture. These fluids typically exhibit a power law behavior for the range of shear rates encountered in hydraulic fracturing treatments.
A uniform proppant distribution is needed in order to get a uniformly conductive fracture along the wellbore height and fracture half-length, but the complicated nature of proppant settling in non-Newtonian fluids often leads to a higher concentration of proppant in the lower part of the fracture. This often leads to a lack of adequate proppant coverage of the upper portion of the fracture and the wellbore. Clustering of proppant, encapsulation, bridging, and embedment are all phenomena that lower the potential conductivity of the proppant pack (Watters et al., 2010).
The job ends eventually with a clean-up stage, in which flush fluids and other clean-up agents are applied. The actual detailed time schedule depends on the particular system used.
After the completion the fluid viscosity should decrease to allow the placement of the proppant and a rapid fluid return through the fracture. It is important to control the time at which the viscosity break occurs. In addition, the degraded polymer should produce little residue to restrict the flow of fluids through the fracture.
Types of Hydraulic Fracturing Fluids
Generally, a hydraulic fracturing treatment involves pumping a proppant-free viscous fluid, or pad, which is usually water with some fluid additives, in order to generate high viscosity, into a well faster than the fluid can escape into the formation. This causes the pressure to rise and the rock to break, creating artificial fractures or enlarging existing ones.
After fracturing the formation, a propping agent such as sand is added to the fluid. This forms a slurry that is pumped into the newly formed fractures in the formation to prevent them from closing when the pumping pressure is released. The proppant transportability of a base fluid depends on the type of viscosifying additives that have been added to the water base (Lukocs et al., 2007).
Since the late 1950s, more than half of fracturing treatments have been conducted with fluids comprising guar gums, or guar derivatives such as hydropropyl guar (HPG), hydroxypropyl cellulose (HPC), carboxymethyl guar, and carboxymethyl hydropropyl guar.
Crosslinking agents based on boron, titanium, zirconium, or aluminum complexes are typically used to increase the effective molecular weight of the polymers and make them better suited for use in high-temperature wells.
Cellulose derivatives, such as hydroxyethyl cellulose (HEC) or HPC and carboxymethylhydroxyethyl cellulose are also used, with or without crosslinkers. Xanthan and scleroglucan have also been shown to have excellent proppant-suspension ability, but they are more expensive than guar derivatives and therefore used less frequently.
Polyacrylamide (PAM) and polyacrylate polymers and copolymers are typically used for high-temperature applications or as friction reducers at low concentrations for all temperatures ranges (Lukocs et al., 2007).
Polymer-free, water-based fracturing fluids can be obtained by using viscoelastic surfactants (VES). These fluids are normally prepared by mixing appropriate amounts of suitable surfactants such as anionic, cationic, non-ionic, and zwitterionic surfactants. Their viscosity is attributed to the three-dimensional structure formed by the components in the fluids. The viscosity increases when the surfactant concentration exceeds a critical concentration. Then the surfactant molecules aggregate into micelles, which can interact to form a network that exhibits viscous and elastic behavior.
Cationic VESs – typically consisting of long chain quaternary ammonium salts such as cetyltrimethylammonium bromide (CTAB) – have so far been the type attracting most commercial interest. Other common reagents that generate viscoelasticity in surfactant solutions include salts, such as ammonium chloride, potassium chloride, sodium chloride, sodium salicylate, and sodium isocyanate, and also non-ionic organic molecules such as chloroform. The electrolyte content of surfactant solutions is also important for controlling their viscoelastic behavior (Lukocs et al., 2007).
Fluids showing this type of cationic VESs tend to lose their viscosity at high brine concentrations, hence they have seen limited use as gravel-packing or drilling fluids. Anionic VESs are also used.
Amphoteric/zwitterionic surfactants (Allan et al., 2008) and an organic acid, salt, or an inorganic salt can also impart viscoelastic properties. The surfactants could be, for dihydroxyl alkyl glycinate, alkyl ampho acetate or VESs propionate, alkyl betaine, alkyl amidopropyl betaine, and alkylamino mono- or dipropionates derived from certain waxes, fats, and oils. They are used in conjunction with an inorganic water-soluble salt or organic additives such as phthalic acid, salicylic acid, or their salts.
Amphoteric/zwitterionic surfactants, in particular those comprising a betaine moiety, are useful for temperatures up to about 150°C and are therefore of particular interest for medium to high temperature wells. Betaine is shown in Figure 17.1. However, like the cationic VES mentioned above, anionic surfactants are usually not compatible with high brine concentrations.
Proppants can be sand, intermediate strength ceramic proppants, or sintered bauxites, which can be coated with a resin to improve their clustering ability. They can be coated with resin or a proppant flowback control agent such as fibers. By selecting proppants having a contrast a property such as density, size, or concentration, different settling rates will be achieved.
Waterfrac treatments combine low-cost, low-viscosity fluids to stimulate very low permeability reservoirs. The treatments rely on the mechanisms of asperity creation (rock spalling), shear displacement of rock, and localized high concentration of proppant to create adequate conductivity, with the last mechanism being mostly responsible for the success of the treatment. The mechanism can be described as analogous to a wedge splitting wood.
A viscous well treatment fluid is generally composed of a polysaccharide or synthetic polymer in an aqueous solution, which is crosslinked by an organometallic compound. Examples of well treatments in which metal crosslinked polymers are used are hydraulic fracturing, gravel packing operations, water blocking, and other well completion operations.
In order for the treatment to be successful, the fluid viscosity should eventually diminish to levels approaching that of water after the proppant is placed. This allows a portion of the treating fluid to be recovered without producing excessive amounts of proppant after the well is opened and returned to production. If the viscosity of the fluid is low, it will flow naturally from the formation under the influence of formation fluids. This viscosity reduction or conversion is referred to as breaking, and is accomplished by incorporating chemical agents, referred to as breakers, into the initial gel.
Some fracturing fluids, such as those based upon guar polymers, break naturally without the intervention of a breaking agent, but their breaking time is generally somewhere in the range from greater than 24 hours, to weeks, months, or years depending on the conditions in the reservoir.
To decrease this break time, chemical agents are usually incorporated into the gel. These are typically either oxidants or enzymes that degrade the polymeric gel structure. Oxidizing agents, such as persulfate salts, chromous salts, organic peroxides or alkaline earth or zinc peroxide salts, or enzymes are the most effective.
The timing of the break is also of great importance. Gels that break prematurely can cause suspended proppant material to settle out they penetrate before a sufficient distance into the produced fracture. Premature breaking can also lead to a premature reduction in the fluid viscosity, resulting in an inadequate fracture width. On the other hand, gelled fluids that break too slowly can cause slow recovery of the fracturing fluid, with attendant delay in resuming production.
Additional problems may occur, such as the tendency of proppant to become dislodged from the fracture, resulting in at least partial closing and decreased efficiency of the fracturing operation. The fracturing gel should preferably, begin to break when the pumping operations are finished, and be completely broken within about 24 hours after completion of the treatment.
Fracturing fluid compositions comprise a solvent, a polymer-soluble or hydratable in the solvent, a crosslinking agent, an inorganic breaking agent, an optional ester compound, and a choline carboxylate. The solvent may be an aqueous potassium chloride solution, and the inorganic breaking agent may be a metal-based oxidizing agent, such as an alkaline earth metal or a transition metal, or it may be magnesium, calcium, or zinc peroxide. The ester compound may be an ester of a polycarboxylic acid, such as an ester of oxalate, citrate, or ethylene diamine tetraacetate. Those having hydroxyl groups can also be acetylated, for instance, citric acid can be acetylated to form acetyl triethyl citrate, which is a preferred ester.
The hydratable polymer can be a water-soluble polysaccharide, such as galactomannan or cellulose, and the crosslinking agent may be a borate, titanate, or zirconium-containing compound, such as Na3BO3 × n H2O.
A general review of commercially available additives for fracturing fluids is given in the literature (Anonymous, 1999). Possible components in a fracturing fluid are listed in Table 17.2, which indicates the complexity of a fracturing fluid formulation. Some additives may not be used together, such as oil-gelling additives in a water-based system. More than 90% of the fluids are based on water. Aqueous fluids are economical and can provide control of a broad range of physical properties if used with additives. Additives for fracturing fluids serve two purposes (Harris, 1988):
1. They enhance fracture creation and proppant-carrying capability and
2. They minimize formation damage.
Viscosifiers, such as polymers and crosslinking agents, temperature stabilizers, pH control agents, and fluid loss control materials assist the creation of a fracture. Formation damage is reduced by gel breakers, biocides, surfactants, clay stabilizers, and gases. Table 17.3 summarizes the various types of fluids and techniques used in hydraulic fracturing.
| Type | Remarks |
|---|---|
| Water-based fluids | Predominant |
| Oil-based fluids | Water sensitive; increased fire hazard |
| Alcohol-based fluids | Rarely used |
| Emulsion fluids | High pressure, low temperature |
| Foam-based fluids | Low pressure, low temperature |
| Noncomplex gelled water fracture | Simple technology |
| Nitrogen-foam fracture | Rapid clean-up |
| Complexed gelled water fracture | Often the best solution |
| Premixed gel concentrates | Improve process logistics |
| In situ precipitation technique | Reduces scale-forming ingredients Hrachovy (1994) |
Comparison of Different Techniques
The optimal technique to be used depends on the type of reservoir. Reports that compare the techniques in a related environment are available. In the Kansas Hugoton field (Mesa Limited Partnership), several hydraulic fracturing methods were tested (Cottrell et al., 1988).
Expert Systems for Assessment
A PC-based, interactive computer model has been developed to help engineers choose the best fluid and additives and the most suitable propping agent for a given set of reservoir properties (Holditch et al., 1993; Xiong et al., 1996). The model also optimizes treatment volume, based on reservoir performance and economics. To select the fluids, additives, and propping agents, the expert system surveys stimulation experts from different companies, reviews the literature, and then incorporates the knowledge so gained into rules, using an expert system shell.
In addition, the fluid leak-off during hydraulic fracturing can be modeled, calculated, and measured experimentally. Procedures for converting laboratory data to an estimate of the leak-off under field conditions have been given in the literature (Penny and Conway, 1989).
Water-Based Systems
Thickeners and Gelling Agents
A gelling agent is also known as a viscosifying agent, and refers to a material that can make the fracturing fluid into a gel, thereby increasing its viscosity (Welton et al., 2010).
Suitable gelling agents include guar gum, xanthan gum, welan gum, locust bean gum, gum ghatti, gum karaya, tamarind gum, and tragacanth gum. Guar gum can be functionalized to hydroxyethyl guar, hydroxypropyl guar, or carboxymethyl guar. Examples of water-soluble cellulose ethers include methyl cellulose, carboxymethyl cellulose (CMC), HEC, and hydroxyethyl carboxymethyl cellulose (Welton et al., 2010).
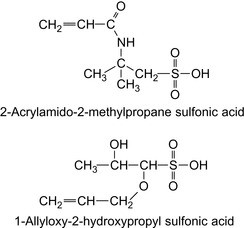
Artificial polymers, such as copolymers from acrylamide, methacrylamide, acrylic acid (AA), or methacrylic acid, or those from 2-acrylamido-2-methyl-1-propane sulfonic acid (AMPS) derivates and N-vinylpyridine have all been used (Welton et al., 2010), but naturally occurring polysaccharides and their derivatives (Lemanczyk, 1992). They can increase the viscosity of the fluid when used in comparatively small amounts. Table 17.4 summarizes suitable polymers.
| aGeneral purpose eightfold power of thickening in comparison to starch | |
| bIncreased temperature stability, used with boron-based crosslinkers | |
| cHigh-temperature stability | |
| dSuperior fluid performance | |
| eImparts high viscosity | |
| Thickener | References |
|---|---|
| Hydroxypropyl guara | |
| Galactomannansb | Mondshine (1987) |
| HEC -modified vinylphosphonic acid | Holtmyer and Hunt (1992) |
| Carboxymethyl cellulose | |
| Polymer from N-vinyl lactam monomers, vinylsulfonatesc | Bharat (1990) |
| Reticulated bacterial cellulosed | Westland et al. (1993) |
| Bacterial xanthane | Hodge (1997) |
Guar is shown in Figure 17.2. In hydroxypropyl guar, some of the hydroxyl groups are etherified with oxopropyl units. Compositions for gelling a hydrocarbon fracturing fluid are basically different from those for aqueous fluids. A possible formulation consists of a gelling agent, a phosphate ester, a crosslinking agent, a multivalent metal ion, and a catalyst, a fatty quaternized amine (Lawrence and Warrender, 2010).
Guar
Guar is a branched polysaccharide from the guar plant Cyamopsis tetragonolobus, which originated in India, and is now found in the southern United States. It has a molar mass of approximately 220 k Dalton, and it consists of mannose in the main chain and galactose in the side chain. The ratio of mannose to galactose is 2:1.
Polysaccharides having this structure are referred to as heteromannans, and in particular as galactomannans. Derivatives of guar are therefore sometimes called galactomannans.
Guar-based gelling agents, typically hydroxypropyl guar, are widely used to viscosify fracturing fluids because of their desirable rheological properties, economics, and ease of hydration. Nonacetylated xanthan is a variant of xanthan gum, which interacts synergistically with guar to give superior viscosity and particle transport at lower polymer concentrations.
Static leak-off experiments with borate crosslinked and zirconate crosslinked hydroxypropyl guar fluids showed practically the same leak-off coefficients (Zeilinger et al., 1991). An investigation of their stress-sensitive properties showed that zirconate filter cakes have viscoelastic properties, but borate filter cakes are merely elastic. Non-crosslinked fluids show no filter cake-type behavior for a large range of core permeabilities, but rather, a viscous flow that is dependent on characteristics of the porous medium.
The addition of glycols, such as ethylene glycol (EG), to aqueous fluids gelled with guar gum can increase the viscosity of the fluid and stabilize the fluid brines. Such fluids are more stable at high temperatures from 27–177°C (80–350°F). The formation damage is minimized after hydraulic fracturing operations, as less of the guar polymer can be used, but the same viscosity is achieved by the addition of a glycol (Kelly et al., 2007).
The crosslinker can be a borate, a titanate, or a zirconate. The stability of the gel is improved by the addition of sodium thiosulfate. The development of the viscosity at 93°C (200°F) of brine fluids with 2.4 kg m−3 guar and 5% KCl, with varying amounts of EG, is shown in Figure 17.3.
 |
| Figure 17.3 Viscosity of guar brines with varying amounts of ethylene glycol (EG) (Kelly et al., 2007). |
By using the sodium derivative of ethylene diamine tetraacetic acid (EDTA) as gel breaker in these compositions with EG, the decay of viscosity with time can be adjusted accordingly (Kelly et al., 2007).
Anionic galactomannans, which are derived from guar gum by partially esterifying hydroxyl groups with sulfonate groups that result from AMPS and 1-allyloxy-2-hydroxypropyl sulfonic acid (Yeh, 1995), have been claimed to be suitable as thickeners. The composition is capable of producing enhanced viscosities, when used alone, or in combination with a cationic polymer and distributed in a solvent.
Polyhydroxy compounds can be modified by various reactions. Etherification, exemplified with dextrose as the model compound, is shown in Figure 17.4. Vinyl compounds used for the modification of guar are shown in Figure 17.5.
Hydroxyethyl Cellulose
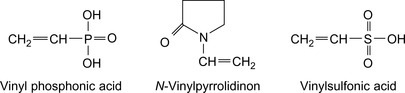
HEC can be chemically modified by reaction with vinylphosphonic acid in the presence of the reaction product of hydrogen peroxide and a ferrous salt. The HEC forms a graft copolymer with the vinylphosphonic acid.
Amylose and cellulose are shown in Figure 17.6. Amylose is a linear polymer of glucose, and is water-soluble. The difference between amylose and cellulose is the way in which the glucose units are linked; amylose has α-linkages whereas cellulose contains, β-linkages. Because of this difference, amylose is soluble in water and cellulose is not. Chemical modification allows cellulose to become water-soluble.
Modified HEC has been proposed as a thickener for hydraulic fracturing fluids (Holtmyer and Hunt, 1992). Polyvalent metal cations may be employed to crosslink the polymer molecules to further increase the viscosity of the aqueous fluid.
Biotechnological Products
Gellan Gum and Wellan Gum
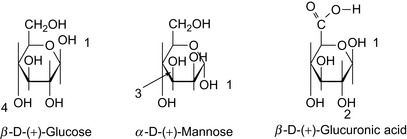
Gellan gum is the generic name for an extracellular polysaccharide produced by the bacterium Pseudomonas elodea. It is a linear anionic polysaccharide with a molecular mass of 500 k Dalton, consisting of 1,3-β-D-glucose, 1,4-β-D-glucuronic acid, 1,4-β-D-glucose, and 1,4-α-L-rhamnose.
Wellan gum is produced by aerobic fermentation. The backbone of wellan gum is identical to gellan gum, but it has a side chain of L-mannose or L-rhamnose. It is used in fluid loss additives and is extremely compatible with calcium ions in alkaline solutions.
Reticulated Bacterial Cellulose
A cellulose produced from bacteria, with an intertwined reticulated structure, has unique properties and functionalities unlike conventional cellulose. It improves fluid rheology and particle suspension over a wide range of conditions in aqueous systems (Westland et al., 1993).
Xanthan Gum
Xanthan gum is produced by the bacterium Xanthomonas campestris, and has been used commercially since 1964. Xanthans are water-soluble polysaccharide polymers with the repeating units (Doherty et al., 1992) shown in Table 17.5 and Figure 17.7.
| Number | Repeating Units | Ratio |
|---|---|---|
| Pentamer | D-glucose: D-mannose: D-glucuronic acid | 2:2:1 |
| Tetramer | D-glucose: D-mannose: D-glucuronic acid | 2:1:1 |
The D-glucose moieties are linked in a β-(1,4) configuration, and the inner D-mannose moieties are linked in an α-(1,3) configuration, generally alternating with glucose moieties. The D-glucuronic acid moieties are linked in a β-(1,2) configuration to the inner mannose moieties. The outer mannose moieties are linked to the glucuronic acid moieties in a β-(1,4) configuration.
Xanthan gum is used in oil field applications in the form of a fermentation broth containing 8–15% of the polymer. The viscosity is less dependent on the temperature than for other polysaccharides.
Viscoelastic Formulations
VES fluids have the following advantages over conventional polymer formulations (Li et al., 2010):
• Higher permeability in the oil-bearing zone,
• Lower formation or subterranean damage,
• Higher viscosifier recovery after fracturing,
• No need for enzymes or oxidizers to break down viscosity, and
• Easier hydration and faster build up to optimum viscosity.
Disadvantages and drawbacks of VES fluids are their high costs, their low tolerance to salts, and stability against high temperatures as found in deep well applications. However there are recent formulations that overcome these difficulties, at least to some extent.
The components of a viscoelastic fluid are a zwitterionic surfactant, erucyl amidopropyl betaine, an anionic polymer, or N-erucyl-N,N-bis-(2-hydroxyethyl)-N-methyl ammonium chloride, poly(napthalene sulfonate), and cationic surfactants, methyl poly(oxyethylene) octadecanammonium chloride, and poly(oxyethylene) cocoalkylamines (Couillet and Hughes, 2008; Li et al., 2010). The corresponding fluids exhibit a good viscosity performance.
Typical VESs are N-erucyl-N,N-bis(2-hydroxyethyl)-N-methyl ammonium chloride and potassium oleate, solutions of which form gels when mixed with corresponding activators such as sodium salicylate and potassium chloride (Jones and Tustin, 2010).
The cationic surfactant should be soluble in both organic and inorganic solvents. Solubility in hydrocarbon solvents is promoted by attaching multiple long chain alkyl groups to the active surfactant unit (Jones and Tustin, 2010). Examples are hexadecyltributylphosphonium and trioctylmethylammonium ions. In contrast, cationic surfactants have a single, long, linear hydrocarbon moiety attached to the surfactant group.
Obviously, there is a conflict between the structural requirements for achieving solubility in hydrocarbons and for the formation of viscoelastic solutions. As a compromise, surfactant compounds that are suitable for reversibly thickening water-based wellbore fluids and also soluble in both organic and aqueous fluids have been designed.
Tallow amido propylamine oxide (McElfresh and Williams, 2007) is a suitable, non-ionic surfactant gelling agent. Non-ionic fluids are inherently less damaging to the producing formations than cationic fluid types, and are more efficacious than anionic gelling agents.
The synthesis of branched oleates have been described. 2-methyl oleic acid methyl ester, or 2-methyl oleate, can be prepared from methyl oleate and methyl iodide in presence of a pyrimidine-based catalyst (Jones and Tustin, 2010). The methyl ester is then hydrolyzed to obtain 2-methyl oleic acid.
It is sometimes believed that contact with a VES-gelled fluid instantaneously reduces the viscosity of the gel, but it has been discovered that mineral oil can be used as an internal breaker for VES-gelled fluid systems (Crews et al., 2010). The rate of viscosity breaking at a given temperature is influenced by the type and amount of salts present. In the case of low molecular weight mineral oils, it is important to add them after the VES component is added to the aqueous fluid.
By using combinations of internal breakers, both the initial and final break of the VES fluid may be customized. Fatty acid compounds or bacteria may be used in addition to mineral oil (Crews, 2006; Crews et al., 2010).
Miscellaneous Polymers
A copolymer of 2-ethylhexyl acrylate and AA is not soluble in water or in hydrocarbons. The ester units are hydrophobic and the acid units are hydrophilic. An aqueous suspension with a particle size smaller than 10 μ can be useful in preparing aqueous hydraulic fracturing fluids (Harms and Norman, 1988). 2-Ethylhexyl acrylate is shown in Figure 17.8.
A water-soluble polymer of N-vinyl lactam or vinyl-containing sulfonate monomers reduces the water loss and enhances other properties of well-treating fluids in high-temperature subterranean environments (Bharat, 1990). Lignites, tannins, and asphaltic materials are added as dispersants. Vinyl monomers are shown in Figure 17.9.
Lactide Polymers
Degradable thermoplastic lactide polymers are used for fracturing fluids. Hydrolysis is the primary mechanism used for the degradation of the lactide polymer (Cooke, 2009).
Biodegradable Formulations
Biodegradable drilling fluid formulations have been suggested, which consist of a polysaccharide in a concentration that is insufficient to permit contamination by bacteria. The polymer is a high-viscosity CMC that is sensitive to bacterial enzymes produced by the degradation of the polysaccharide (Pelissier and Biasini, 1991).
The biodegradability of seven kinds of mud additives was studied by determining the content of dissolved oxygen in water, a simple biochemical oxygen demand testing method. The biodegradability is high for starch but lower for polymers of allyl monomers and additives containing an aromatic group (Guo et al., 1996).
Concentrates
Historically, fracture stimulation treatments have been performed by using conventional batch mix techniques, which involves premixing chemicals into tanks and circulating the fluids until a desired gelled fluid rheology is obtained. This method is time-consuming and burdens the oil company with disposal of the fluid if the treatment ends prematurely.
Environmental damage during spillage or disposal can be avoided if the fluid is capable of being gelled as needed. Thus gelling-as-needed technology has been developed with water, methanol, and oil (Gregory et al., 1991). This procedure eliminates batch mixing and minimizes handling of chemicals and base fluid. The customer is charged only for products used, and environmental concerns regarding disposal are virtually eliminated. Computerized chemical addition and monitoring, combined with on-site procedures, ensure quality control throughout treatment. Fluid rheologies can be accurately varied during the treatment by varying polymer loading.
The use of a diesel-based concentrate with hydroxypropyl guar gum has been evolved from batch-mixed dry powder procedures (Harms et al., 1988). The application of such a concentrate reduces system requirements, and companies can benefit from the reduced logistic burden that comes from using the diesel hydroxypropyl guar gum concentrate.
A fracturing fluid slurry concentrate has been proposed (Brannon, 1988) that consists of the components shown in Table 17.6. Such a polymer slurry concentrate will readily disperse and hydrate when admixed with water at the proper pH, thus producing a high-viscosity aqueous fracturing fluid. This concentrate is useful for producing large volumes of high-viscosity treating fluids at the well site on a continuous basis. Suitable surfactants are shown in Figure 17.10.
| Component | Example |
|---|---|
| Hydrophobic solvent base | Diesel |
| Suspension agent | Organophilic clay |
| Surfactant | Ethoxylated nonyl phenol |
| Hydratable polymer | Hydroxypropyl guar gum |
Fluidized aqueous suspensions of 15% or more of HEC, hydrophobically modified cellulose ether, hydrophobically modified HEC, methyl cellulose, hydroxypropylmethyl cellulose, and polyethylene oxide (PEO) are prepared by adding the polymer to a concentrated sodium formate solution containing xanthan gum as a stabilizer (Burdick and Pullig, 1993).
The xanthan gum is dissolved in water before sodium formate is added. Then the polymer is added to the solution to form a fluid suspension of the polymers. The polymer suspension can serve as an aqueous concentrate for further use.
Friction Reducers
Low pumping friction pressures are achieved by delaying crosslinking, but specific additives are also available to reduce the drag in the tubings. Gaur was first applied as a drag reducer in oil well fracturing, which is now a routine practice.
Relatively small quantities of a bacterial cellulose (0.60–1.8 g l−1) in hydraulic fracturing fluids enhance their rheologic properties (Penny et al., 1991). The suspension of the proppant is enhanced and friction loss through well casings is reduced.
Fluid Loss Additives
Fluid loss additives are widely used additives for drilling fluids. High-permeability fracturing zones can easily be damaged by deeply penetrating fluid leak-off along the fracture, or by the materials in the fluid to minimize the amount of leak-off.
Several fracturing treatments in high-permeability formations exhibit positive post-treatment skin effects. This is the result of fracture face-damage (Aggour and Economides, 1996). If the invasion of the fracturing fluid is minimized, the degree of damage is of secondary importance. So if the fluid leak-off penetration is small, even severe permeability impairments can be tolerated without exhibiting positive skin effects. The first priority in designing fracture treatments should be to maximize the conductivity of the fracture. In high-permeability fracturing, the use of high concentrations of polymer crosslinked fracturing fluids with fluid loss additives and breakers is recommended.
Materials used to minimize leak-off can also damage the conductivity of the proppant pack. High shear rates at the tip of the fracture may prevent the formation of external filter cakes, hence increasing the magnitude of spurt losses in highly permeable formations, so non-damaging additives are needed. Enzymatically degradable fluid loss additives are available. Table 17.7 summarizes some fluid loss additives suitable for hydraulic fracturing fluids.
| aWellan or xanthan gum polymer can be added to keep the calcium carbonate and lignosulfonate in suspension | |
| bSynergistic effect, see text | |
| c500 mD permeability | |
| Chemical | References |
|---|---|
| Calcium carbonate and lignosulfonatea | Johnson (1996) and Johnson and Smejkal (1993) |
| Natural starch | Elbel et al. (1995), Navarrete et al. (1996) and Navarrete and Mitchell (1995) |
| Carboxymethyl starch | Elbel et al. (1995), Navarrete et al. (1996) and Navarrete and Mitchell (1995) |
| Hydroxypropyl starchb | Elbel et al. (1995), Navarrete et al. (1996) and Navarrete and Mitchell (1995) |
| HEC with crosslinked guar gumsc | Cawiezel et al. (1999) |
| Granular starch and particulate mica | Cawiezel et al. (1999) |
Degradation of Fluid Loss Additives
A mixture of natural starch (cornstarch) and chemically modified starches (carboxymethyl and hydroxypropyl derivatives) plus an enzyme, has been described as a fluid loss additive for fracturing fluids (Williamson and Allenson, 1989; Williamson et al., 1991b). The enzyme degrades the α-linkage of starch but not the β-linkage of guar and modified guar gums.
The starches can be coated with a surfactant, such as sorbitan monooleate, ethoxylated butanol, or ethoxylated nonyl phenol, to facilitate dispersion in the fracturing fluid. Modified starches or blends of modified and natural starches with a broad particulate size distribution have been found to maintain the injected fluid within the created fracture more effectively than natural starches (Williamson et al., 1991a). The starches can be degraded by oxidation or by bacterial attack.
pH Control Additives
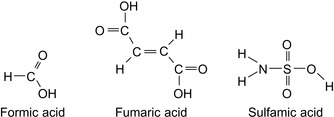
Buffers, necessary to adjust and maintain the pH, can be salts of a weak acid and a weak base, such as carbonates, bicarbonates, and hydrogen phosphates (Nimerick, 1996), or weak acids such as formic acid, fumaric acid, and sulfamic acid. Common aqueous buffers are shown in Table 17.8 and in Figure 17.11 and Figure 17.12.
Increased temperature stability of various gums can be achieved by adding sodium bicarbonate to the fracturing fluid and thus raising its pH to 9.2–10.4.
Clay Stabilizers
Advances in treating clay-bearing formations have led to the development of numerous clay-stabilizing treatments and additives. Most additives are high molecular weight cationic organic polymers, but it has been shown that these stabilizers are less effective in low-permeability formations (Himes et al., 1989).
The use of salts such as potassium chloride and sodium chloride, as temporary clay stabilizers during oil well drilling, completion, and servicing, has been practiced for many years. Because of the bulk and potential environmental hazards associated with the salts, many operators have looked for alternatives. Recent research has shown a relationship between the physical properties of various cations (e.g., K+, Na+) and their efficiency as temporary clay stabilizers. These properties were used to synthesize an organic cation (Table 17.9) with a higher efficiency as a clay stabilizer.
| aAdded to a gel concentrate with a diesel base | |
| bMinimum 0.05% to prevent swelling of clays | |
| cAlkyl equals methyl, ethyl, propyl, and butyl | |
| dSynergistically retards water absorption by the clay formation | |
| eHydroxyl-substituted alkyl radials | |
| Compound | References |
|---|---|
| Ammonium chloride | |
| Potassium chloridea | Yeager and Bailey (1988) |
| Dimethyl diallyl ammonium saltb | Thomas and Smith (1993) |
| N-Alkyl pyridinium halides | |
| N,N,N-Trialkylphenylammonium halides | |
| N,N,N-Trialkylbenzylammonium halides | |
| N,N-Dialkylmorpholinium halidesc | Himes (1992) and Himes and Vinson (1989) |
| Reaction product of a homopolymer of maleic anhydride and an alkyl diamined | Schield et al. (1991) |
| Tetramethylammonium chloride and methyl chloride quaternary salt of ethylene-ammonia condensation polymerd | Aften and Gabel (1992) |
| Quaternary ammonium compoundse | Hall and Szememyei (1992) |
These additives provide additional benefits when used in conjunction with acidizing and fracturing treatments, since a much lower salt concentration can be used (Himes et al., 1990; Himes and Vinson, 1991), and the liquid product is much easier to handle and transport. It is also environmentally compatible and biodegradable in its diluted form.
Biocides
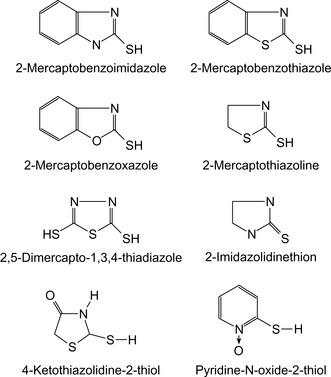
A hydraulic fracturing fluid containing guar gum or other natural polymers can be stabilized against bacterial attack by adding heterocyclic sulfur compounds, thus preventing any undesired degradation of the fracturing fluid, such as reduction of its rheological properties at high temperatures. Biocides suitable for fracturing fluids are shown in Table 17.10 and Figure 17.13.
| aFor Guar gum | |
| bFor Xanthan gum | |
| Compound | References |
|---|---|
| Mercaptobenzimidazolea | Kanda et al. (1986) |
| 1,3,4-Thiadiazole-2,5-dithiola and b | Kanda and Kawamura, 1988 and Kanda and Kawamura, 1989 and Kanda et al. (1988) |
| 2-Mercaptobenzothiazole | |
| 2-Mercaptothiazoline | |
| 2-Mercaptobenzoxazole | |
| 2-Mercaptothiazoline | |
| 2-Thioimidazolidone | |
| 2-Thioimidazoline | |
| 4-Ketothiazolidine-2-thiol | |
| N-Pyridineoxide-2-thiol | |
Surfactants
Surface active agents are included in most aqueous treating fluids to improve their compatibility with the hydrocarbon-containing reservoir. The formation must be water-wet to achieve maximal conductivity of hydrocarbons.
Alkylamino phosphonic acids and fluorinated alkylamino phosphonic acids adsorb onto solid surfaces, particularly of carbonate materials in subterranean hydrocarbon-containing formations, in a layer only one molecule thick. This is significantly thinner than a layer of water or a water-surfactant mixture on water-wetted surfaces (Penny, 1987; Penny and Briscoe, 1987). These compounds resist or substantially reduce the wetting of the surfaces by water and hydrocarbons, and provide high interfacial tensions between the surfaces and water and hydrocarbons. The hydrocarbons displace injected water, leaving a lower water saturation and an increased flow of hydrocarbons through capillaries and flow channels in the formation.
A methyl quaternized erucyl amine is useful for aqueous VES-based fracturing fluids in high-temperature and high-permeability formations (Gadberry et al., 1999).
Crosslinkers
Kinetics of Crosslinking
The rheology of hydroxypropyl guar is greatly complicated by crosslinking reactions with titanium ions. A study of the rheology of this system has been performed (Barkat, 1987). Continuous flow and dynamic data suggest a crosslinking reaction order of approximately 4/3 and 2/3, with respect to the crosslinker and hydroxypropyl guar concentration. Dynamic tests have shown that the shearing time is important in determining the final gel properties. Continued steady shear and dynamic tests show that high shear irreversibly destroys the gel structure, and the extent of the crosslinking reaction decreases with increasing shear. Studies at shear rates below 100 s−1 suggest a shear-induced structural change in the polymer that affects the chemistry of the reaction and the nature of the product molecule.
Delayed Crosslinking
Delayed crosslinking is desirable because the fluid can be pumped more easily. A delay is a retarded reaction rate of crosslinking, which can be achieved with the methods explained in the following section.
Borate Systems
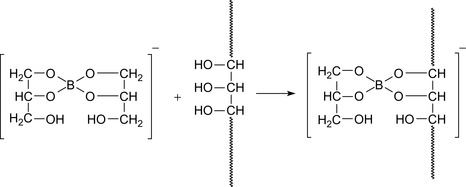
Boric acid can form complexes with hydroxyl compounds as shown in Figure 17.14. Three hydroxyl units form an ester and one unit forms a complex bond, releasing a proton that lowers the pH. The scheme is valid also for polyhydroxy compounds, where two polymer chains are connected via such a link.
The control of the delay time requires the pH, or the availability of borate ions, or both to be controlled. Control of pH can be effective in fresh water systems (Ainley et al., 1993), but the control of borate is effective in both fresh water and sea water. This may be accomplished by using sparingly soluble borate species or by complexing the borate with a variety of organic species.
Borate-crosslinked fracturing fluids have been used successfully in fracturing operations. These fluids provide excellent rheological, fluid loss, and fracture conductivity properties over fluid temperatures up to 105°C. The mechanism of borate crosslinking is an equilibrium process that can produce very high fluid viscosities under conditions of low shear (Cawiezel and Elbel, 1990). A fracturing fluid containing borate is prepared in the following way (Harris et al., 1994):
1. Adding a polysaccharide (sea) water to produce a gel,
2. Adding an alkaline agent to the gel to obtain a pH of at least 9.5, and
3. Adding a borate crosslinking agent to the gel to crosslink the polymer.
A dry granular composition can be prepared in the following way (Harris and Heath, 1994):
1. Dissolving 0.2–1.0% of a water-soluble polysaccharide in aqueous solution,
2. Admixing a borate source with the aqueous gel formed in step 1,
3. Drying the borate crosslinked polysaccharide formed in step 2, and
4. Granulating the product.
The crosslinking agent can be boric acid, borax, an alkaline earth metal borate, or an alkali metal alkaline earth metal borate. The borate source must be present at 5–30% calculated as boric oxide.
Borated starch compositions are useful for controlling the rate of crosslinking of hydratable polymers in aqueous media. They are prepared by reacting starch and a borate source in an aqueous medium. The complex provides a source of borate ions, which cause crosslinking of hydratable polymers in aqueous media (Sanner et al., 1996). Delayed crosslinking takes place at low temperatures.
Glyoxal (Dawson, 1992 and Dawson, 1995) shown in Figure 17.15 is effective as a delay additive within a certain pH range. It bonds chemically with both boric acid and borate ions to limit the number of borate ions that are initially available in solution for subsequent crosslinking. The subsequent rate of crosslinking can be controlled by adjusting the pH of the solution. The mechanism of delayed crosslinking is shown in Figure 17.16. If two hydroxyl compounds with low molecular weight are exchanged with high molecular weight compounds, and the hydroxyl units belonging to different molecules, then a crosslink is formed.
Other dialdehydes, keto aldehydes, hydroxyl aldehydes, ortho-substituted aromatic dialdehydes, and ortho-substituted aromatic hydroxyl aldehydes are claimed to be active in a similar way.
Borate-crosslinked, guar-fracturing fluids have been reformulated to allow use at higher temperatures in both fresh water and sea water. The temporary temperature range is extended for the use of magnesium oxide-delayed borate crosslinking of a galactomannan gum fracturing fluid by adding fluoride ions that precipitate insoluble magnesium fluoride (Nimerick et al., 1993). Alternatively, a chelating agent for the magnesium ion may be added. With the precipitation of magnesium fluoride or the chelation of the magnesium ion, insoluble magnesium hydroxide cannot form at elevated temperatures, which would otherwise lower the pH and reverse the borate crosslinking reaction. The addition effectively extends the use of such fracturing fluids to temperatures of 135–150°C.
Polyols, such as glycols or glycerol, can delay the crosslinking of borate in hydraulic fracturing fluids based on galactomannan gum (Ainley and McConnell, 1993). This is suitable for high-temperature applications up to 150°C. In this case, low molecular weight borate complexes initially are formed but exchange slowly with the hydroxyl groups of the gum.
Titanium Compounds
Organic titanium compounds are useful as crosslinkers (Putzig and Smeltz, 1986). Aqueous titanium compositions often consist of mixtures of compounds.
Zirconium Compounds
Various zirconium compounds are used as delayed crosslinkers, as shown in Table 17.11. The complexes initially form with low molecular weight compounds, and are then exchanged with intermolecular polysaccharide complexes, which cause delayed crosslinking.
| aGood high-temperature stability | |
| bHigh-temperature application, enhanced stability | |
| Zirconium Crosslinker/Chelate | References |
|---|---|
| Hydroxyethyl-tris-(hydroxypropyl) ethylene diaminea | Putzig (1988) |
| Zirconium halide chelates | Ridland and Brown (1990) |
| Boron zirconium chelatesb | Dawson and Le (1998) and Sharif, 1993 and Sharif, 1995 |
A diamine-based compound for complex forming is shown in Figure 17.17 and hydroxy acids are shown in Figure 17.18. Polyhydroxy compounds suitable for complex formation with zirconium compounds are shown in Figure 17.19.
Borozirconate complexes can be prepared by the reaction of tetra-n-propyl zirconate with triethanol amine and boric acid (Putzig, 2010). They can be used at a pH of 8–11.
Gel Breaking in Water-based Systems
In general, there are two methods for combining a fracturing fluid and a breaker (Carpenter, 2009):
1. Mixing the breaker with the fracturing fluid prior to sending the fracturing fluid downhole or
2. Sending the fracturing downhole first, followed by the breaker.
The first method is favored because of convenience, but a disadvantage of this method is that the breaker can act to decrease the viscosity of the fracturing fluid before the desired time.
In the second method, the fracturing fluid is dispatched first, and the breaker is sent downhole later. While inconvenient, this prevents premature decrease in the viscosity (Carpenter, 2009).
The properties of the formation should be restored after fracturing. Maximal well production can be achieved only when the solution viscosity and the molecular weight of the gelling agent are significantly reduced after the treatment, that is, the fluid is degraded.
Basic Studies
Comprehensive research on the degradation kinetics of a hydroxypropyl guar fracturing fluid by enzyme, oxidative, and catalyzed oxidative breakers has been performed (Craig, 1991; Craig and Holditch, 1993a and Craig and Holditch, 1993b). Changes in viscosity were measured as a function of time. The studies revealed that enzyme breakers are effective only in acid media at temperatures of 60°C or below. In an alkaline medium and at temperatures below 50°C, a catalyzed oxidative breaker system was the most effective breaker. At temperatures of 50°C or higher, hydroxypropyl guar fracturing fluids can be degraded by an oxidative breaker without a catalyst.
Oxidative Breakers
Alkali metal hypochlorites and inorganic and organic peroxides have been described in literature. These materials degrade the polymer chains by oxidative mechanisms. CMC, guar gum, or partially hydrolyzed polyacrylamides were used for testing a series of oxidative gel breakers in a laboratory study (Bielewicz and Kraj, 1998).
Hypochlorite Salts
Hypochlorites are powerful oxidants and therefore may degrade polymeric chains. They are often used in combination with tertiary amines (Williams et al., 1987), which increases the reaction rate above that achievable with the application of a hypochlorite alone. A tertiary amino galactomannan may serve as an amine source (Langemeier et al., 1989), which also serves as a thickener before breaking. Hypochlorites are also effective for breaking stabilized fluids (Walker and Shuchart, 1995). Sodium thiosulfate has been proposed as a stabilizer for high-temperature applications.
Peroxide Breakers
Alkaline earth metal peroxides have been described as delayed gel breakers in alkaline aqueous fluids containing hydroxypropyl guar (Mondshine, 1993). The peroxides are activated by increasing the temperature of the fluid.
Perphosphate esters or amides can be used for oxidative gel breaking (Laramay et al., 1995). The salts of the perphosphate ion interfere with the action of the crosslinkers, but the esters and amides do not. Fracturing fluids that contain these breakers and using metal ion crosslinkers are useful for fracturing deeper wells that operate at temperatures of 90–120°C such as titanium and zirconium. Breaker systems based on persulfates have also been described (Harms, 1992).
Organic peroxides are also suitable for gel breaking (Dawson and Le, 1995). They need not be completely soluble in water. The time needed to break is kept in the range 4–24 h by adjusting the amount of breaker added to the fluid.
Redox Gel Breakers
Gel breakers act basically, according to a redox reaction. Copper (II) ions and amines can degrade various polysaccharides (Shuchart et al., 1999).
Delayed Release of Acid
Regained permeability studies with HEC polymer in high-permeability cores revealed that persulfate-type oxidizing breakers and enzyme breakers do not adequately degrade the polymer. Sodium persulfate breakers were found to be thermally decomposed, and the decomposition was accelerated by minerals present in the formation.
The enzyme breaker adsorbed onto the formation but still partly functioned as a breaker. Dynamic fluid loss tests at reduced pH with borate crosslinked gels suggest that accelerated leak-off away from the wellbore could be obtained through the use of a delayed release acid. Rheological measurements confirmed that a soluble, delayed release acid could be used to convert a borate crosslinked fluid into a linear gel (Noran et al., 1995).
A condensation product of hydroxyacetic acid can be used as a fluid loss material in a fracturing fluid where another hydrolyzable aqueous gel is used (Cantu and Boyd, 1989; Cantu et al., 1990a, Cantu et al., 1990b and Cantu et al., 1993). The hydroxyacetic acid condensation product degrades under formation conditions to release hydroxyacetic acid, which breaks the aqueous gel. This mechanism may be used for delayed gel breaking, as shown in Figure 17.20. Here the permeability is restored without the need for the separate addition of a gel breaker, and the condensation product acts a fluid loss additive.
Enzyme Gel Breakers
Enzymes cleave the backbone structure of the thickeners specifically and eventually that of the fluid loss additive. They offer several advantages over other breaker systems, because of their inherent specificity and the infinite polymer-degrading activity. Initially their application has been limited to low-temperature fracturing treatments, because of pH and temperature constraints, but recently, extreme temperature-stable and polymer-specific enzymes have been developed (Brannon and Tjon-Joe-Pin, 1994).
Basic Studies
Basic studies have been performed to investigate the performance of enzymes. The products and kinetics of degradation, and limits of application, such as temperature and pH, have been analyzed (Craig et al., 1992; Slodki and Cadmus, 1991). Enzymes degrade chemical linkages highly selectively, hence no general-purpose enzyme exists, but one must be selected for each thickener. Enzymes suitable for particular systems are shown in Table 17.12.
| aElevated temperatures and salt concentrations | |
| bHigh alkalinity and elevated temperature | |
| Polymer | References |
|---|---|
| Xanthana | Ahlgren (1993) |
| Mannan-containin hemicelluloseb | Fodge (1996) |
Enzymes break the chains of the thickener directly or degrade polymers into organic acid molecules (Harris and Hodgson, 1995).
Interactions
Despite their advantages over conventional oxidative breakers, enzyme breakers have limitations because of interferences and incompatibilities with other additives. Interactions between enzyme breakers and fracturing fluid additives including biocides, clay stabilizers, and certain types of resin-coated proppants have been reported (Prasek, 1996).
Encapsulated Gel Breakers
The chemical in encapsulated gel breakers is contained with in a membrane that is impermeable, or is only slightly permeable to the breaker that does not come in initial contact with the polymer to be degraded. The breaker diffuses, slowly out from the capsulation, or the capsulation is destroyed so that the breaker can act successfully.
Encapsulated gel breakers are widely applied for delayed gel breaking. The breaker is encapsulated by a water-resistant coating, which shields the fluid from the breaker, so that a high concentration can be added without causing premature loss of fluid properties.
The barrier properties of the coating, release mechanisms, and the properties of the reactive chemicals are critical factors in the design of encapsulated breakers. For example, a hydrolytically degradable polymer can be used as the membrane (Muir and Irwin, 1999). This method of delayed gel breaking has been reported both for oxidative breaking and for enzyme gel breaking. Formulations of encapsulated gel breakers are shown in Table 17.13 and membranes for encapsulators are shown in Table 17.14.
| aGuar or cellulose derivatives | |
| bOpen cellular coating | |
| cFor titanium and zirconium; wood resin encapsulated | |
| Breaker system | References |
|---|---|
| Ammonium persulfatea | Gulbis et al., 1990a, Gulbis et al., 1990b and Gulbis et al., 1992 and King et al. (1990) |
| Enzyme breakerb | Gupta and Prasek (1995) |
| Complexing agentsc | Boles et al. (1996) |
| aFor peroxide particle sizes 50–420 μ | |
| bEnzyme coated on cellulose derivative | |
| Membrane Material | References |
|---|---|
| Polyamide (PA) a | Satyanarayana Gupta and Cooney (1992) |
| Crosslinked elastomer | Manalastas et al. (1992) |
| Partially hydrolyzed acrylics crosslinked with aziridine prepolymer or carbodiimideb | Hunt et al. (1997), Norman and Laramay (1994) and Norman et al. (2001) |
| 7% Asphalt and 93% neutralized sulfonated ionomer | Swarup et al. (1996) |
Gel Breaking of Guar
Maximal well production can be achieved only when the solution viscosity and the molecular weight of the gelling agent are significantly reduced after the treatment. However, the reduction of the fracturing fluid viscosity, the traditional method of evaluating these materials, does not necessarily indicate that the gelling agent has also been thoroughly degraded.
The reaction between hydroxypropyl guar and ammonium peroxydisulfate in an aqueous potassium chloride solution was studied (Hawkins, 1986) under controlled conditions to determine changes in solution viscosity and the weight average of the molecular mass of hydroxypropyl guar.
Bromine compositions used for gel breaking can be stabilized with sodium sulfamate (Carpenter, 2009). The sulfamate used in the production of such breakers stabilizes the active bromine species over long periods of time, especially at a pH of 13. For example, a WELLGUARD™ 7137 gel breaker is stable for over a year if protected from sunlight. The halogen source of the breaker is an interhalogen compound, bromine chloride, or mixtures of bromine and chlorine.
Unlike hypobromites (−OBr), these breakers do not oxidize or otherwise destroy the organic phosphonates typically used as corrosion or scale inhibitors, and they exhibit a low corrosivity against metals, especially ferrous alloys because of their low oxidation-reduction potential (Carpenter, 2007 and Carpenter, 2009). The effect of the breakers on guar is shown in Figure 17.21. The composition was prepared and studied at 50°C (120°F).
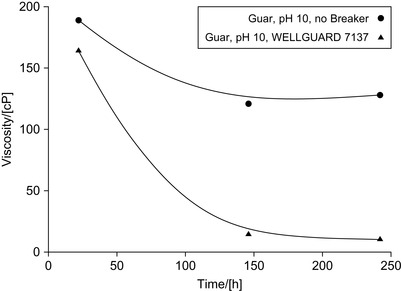 |
| Figure 17.21 Effect of halogen-based breakers on guar (Carpenter, 2009). |
Borate-crosslinked guar polymer gels can be broken with EDTA compounds (Crews, 2007a). Examples are shown in Table 17.15. It is believed these breakers act directly on the polymer itself and not on any crosslinker that may be present. Polyhydroxy compounds can also break guar gels, and these are formed by polysaccharides. They include mannitol and sorbitol, and can be used in combination with enzyme breakers (Crews, 2007b).
| Complex Compound |
|---|
| Tetrasodium propylenediamine tetraacetic acid |
| Trisodium hydroxyethylene diamine tetraacetic acid |
| Trisodium nitrilo triacetic acid |
| Trisodium ethylene diaminetriacetic acid |
| Disodium ethylene diamine diacetic acid |
| Disodium calcium dihydrate ethylene diamine diacetic acid |
| Tetraammonium ethylene diamine tetraacetic acid |
Gel Breaking of VES-gelled Fluids
The viscosity of fluids viscosified with VESs can be controlled by fatty acid salts. For example, a brine fluid gelled with an amine oxide surfactant may have its viscosity broken with a composition containing naturally occurring fatty acid salts from canola oil or corn oil (Crews, 2010).
The alteration of the fatty acid or the saponification reaction may occur during mixing and pumping of the fluid downhole. The method may also be used where most of the saponification occurs within the reservoir shortly after the treatment is over. Alternatively, the components may be preformed and added later as an external breaker solution to remove the VES-gelled fluids that have been already placed downhole.
It may be possible that the viscosity initially increases and then decreases. When canola oil is saponified with CaOH, initially a slight increase of the VES fluid is observed, followed by a breaking reaction (Crews, 2010). The increase in viscosity occurs because the saponified fatty acids may act as viscosity-enhancing cosurfactants for the fluid containing VESs.
Granules
Granules containing 40–90% of sodium or ammonium persulfate breaker and 10–60% of an inorganic powdered binder, such as clay, have been described (McDougall et al., 1993) as acting as a delayed breaker.
Controlled solubility compounds or clean-up additives, including poly-phosphates, that slowly release certain salts act as delayed breakers (Mitchell et al., 2001).
Granules composed of a particulate breaker dispersed in a wax matrix are used in fracturing operations to break hydrocarbon liquids gelled with salts of alkyl phosphate esters. The wax granules are solid at surface temperatures, but melt or disperse in the hydrocarbon liquid at formation temperature, releasing the breaker to react with the gelling agent (Acker and Malekahmadi, 2001).
Scale Inhibitors
The formation of calcium carbonate (CaCO3), calcium sulfate (CaSO4), and barium sulfate (BaSO4) scales in brine may create permeability problems, so newly made fractures need a scale inhibitor in place. Formulations of hydraulic fracturing fluids containing a scale inhibitor have been described in the literature (Watkins et al., 1993).
Interference of Chelate Formers
Trace amounts of metal chelate-forming additives have been shown to have a debilitating effect on the performance of widely used barium sulfate scale inhibitors. Ethylene diamine tetraacetic acid, citric acid, and gluconic acid render some scale inhibitors, such as phosphonates, polycarboxylates, and phosphate esters, completely ineffective at concentrations as low as 0.1 mg l−1. Such low concentrations may be expected to return from formation stimulation treatments for many months and would appear to jeopardize any scale inhibitor program in place.
This conclusion follows from experiments with a simulated North Sea scaling system at pH 4 and 6. The scale inhibitor concentrations studied were 50 and 100 mg l−1. The large negative effect of the organic chelating agents was observed at pH 4 and 6. The only scale inhibitors studied that remained unaffected by these interferences were polyvinyl sulfonates (Barthorpe, 1993).
Encapsulated Scale Inhibitors
A solid, encapsulated scale inhibitor (calcium-magnesium polyphosphate) has been developed and extensively tested for use in fracturing treatments (Powell et al., 1995a, Powell et al., 1995b and Powell et al., 1996). The inhibitor is compatible with borate and zirconium crosslinked fracturing fluids and foamed fluids because of its coating which exhibits a short-term effect on the release rate profile. The composition of the solid derivative has the greatest effect on its long-term release rate profile.
Oil-Based Systems
One advantage of fracturing with hydrocarbon-based gels compared to their aqueous equivalents is that some formations may imbibe large quantities of water, whereas others are water sensitive and will swell if water is introduced.
Organic Gel Aluminum Phosphate Ester
A gel of diesel or crude oil can be produced using a phosphate diester or an aluminum compound with phosphate diester (Gross, 1987). The metal phosphate diester may be prepared by reacting a triester with phosphorous pentoxide to produce a polyphosphate, which is then reacted with hexanol to produce a phosphate diester (Huddleston, 1989).
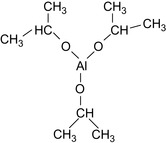
The latter diester is then added to the organic liquid along with a nonaqueous source of aluminum, such as aluminum isopropoxide, c.f., Figure 17.22, in diesel oil, to produce the metal phosphate diester. The conditions in the previous reaction steps are controlled to provide a gel with good viscosity versus temperature and time characteristics. All the reagents are substantially free of water and will not affect the pH. The synthesis of phosphate diesters goes via. triethyl phosphate, using phosphorous pentoxide and the esterification reaction with hexanol. It is shown in Figure 17.23.
Amino compounds are enhancers for phosphate esters (Geib, 2002). The 2-ethylhexanoic acid trialuminum salt has been suggested with fatty acids as an activator (Subramanian et al., 2001).
Another method of producing oil-based hydrocarbon gels uses ferric salts (Smith and Persinski, 1995) rather than aluminum compounds for combination with orthophosphate esters. This can be done in the presence of large amounts of water, up to 20%. Ferric salts can be applied over wide pH ranges. The linkages that are formed can still be broken with conventional gel breaking additives.
Increasing the Viscosity of Diesel
A copolymer of N,N-dimethylacrylamide and N,N-dimethyl aminopropyl methacrylamide, a monocarboxylic acid, and ethanolamine increases the viscosity of diesel or kerosene (Holtmyer and Hunt, 1988). These compounds are shown in Figure 17.24.
Gel Breakers
Gel breakers used in nonaqueous systems have a completely different chemistry from those used in aqueous systems. A mixture of hydrated lime and sodium bicarbonate is useful in breaking nonaqueous gels (Syrinek and Lyon, 1989). Sodium bicarbonate used by itself is totally ineffective for breaking the fracturing fluid for aluminum phosphate-based or aluminum phosphate ester-based gellants. Alternatively, sodium acetate can be used as a gel breaker for nonaqueous gels.
Foam-Based Fracturing Fluids
Foam fluids can be used in many fracturing jobs, especially when environmental sensitivity is a concern (Stacy and Weber, 1995). Foam-fluid formulations are reusable, shear stable, and form stable foams over a wide temperature range, exhibiting high viscosities even at relatively high temperatures (Bonekamp et al., 1993).
A foamed fracturing fluid contains a relatively large volume of gas dispersed in a relatively small volume of liquid, and includes a surfactant for foaming, and stabilization of the foam produced when the gas is mixed with the liquid (Welton et al., 2010).
A coarse foamed fluid has a relatively nonuniform bubble size distribution, i.e., a combination of large and small gas bubbles, whereas a fine-textured foam has relatively uniform bubble size distribution and most of the bubbles are relatively small (Middaugh et al., 2007). In coarse foamed fracturing fluids, there may be regions of fine-textured foam. Such foams are able to support proppant in the fine textured regions even at very high foam quality levels.
The most commonly used gases for foam fluids are nitrogen and carbon dioxide, because they are noncombustible, readily available, and relatively cheap (Welton et al., 2010).
The content of the gas is called quality, therefore a 70 quality foam contains 70% gas. Recently, foams with 95% gas have been examined, but only foam prepared from 2% of an anionic surfactant with plain water had uniform, fine-bubble structure (Harris and Heath, 1996).
Surfactants are available that can change their power of foaming. For example, a tertiary alkyl amine ethoxylate can be changed from a foaming to a nonfoaming surfactant by lowering the pH of the environment. It can then be changed back to a foaming surfactant by the addition of a basic material, e.g., hydroxide ions. At low pH the amine group is quaternized, as shown in Figure 17.25.
 |
| Figure 17.25 Changing the foaming ability by changing the pH. Top: Foaming modification. Bottom: Nonfoaming modification (Welton et al., 2010). |
Cocobetaine and α-olefin sulfonate have also been proposed as foamers (Pakulski and Hlidek, 1992), and a mixture of laurylamine and myristylamine oxide performs well as a surfactant. Lauryl betaine is shown in Figure 17.26.
Recyclable foamed fracturing fluids are available (Chatterji et al., 2007). After use, the pH of the fracturing fluid is changed so that the foam is destroyed. At this stage, the fracturing fluid also releases its proppant. Afterwards, the fracturing fluid is allowed to flow back to the surface, where it can be recycled by restoring the initial pH and adding a gas to the fluid, causing it to foam again.
Defoamers
A defoamer and an antifoamer composition are described for defoaming aqueous fluid systems (Zychal, 1986). The composition of a typical defoamer for hydraulic fracturing fluids is shown in Table 17.16.
| Compound | Amount/[%] |
|---|---|
| C6–C12 mixture of polar compounds | 50 to 90 |
| Sorbitan monooleate | 10 to 50 |
| Polyglycol M = 3.8 k Dalton | 10 |
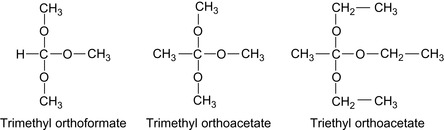
Orthoesters such as trimethyl orthoacetate, triethyl orthoacetate, and the corresponding orthoformates will generate acids in order to degrade the foam. Polyorthoesters are also important in medical applications (Heller et al., 2002). Some simple orthoesters are shown in Figure 17.27.
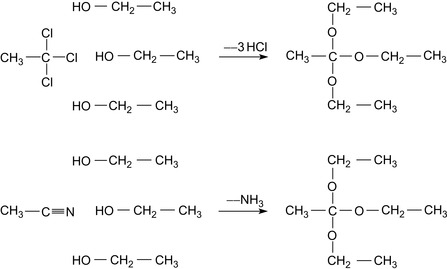
The synthesis of orthoesters may proceed either by a Williamson synthesis or by the addition of alcohols to a cyanide. The respective reactions are shown in Figure 17.28.
Orthoesters are stable to alkalis, but not to acids and water. The orthoester decreases the pH of the foamed fracturing fluid by enough to convert the foaming surfactant to a nonfoaming surfactant. To allow the orthoester to hydrolyze and produce an acid, a source of water is needed, whether from the formation or introduced. The water should be present in an amount of 2 mol of water per mol of orthoester (Welton et al., 2010).
The orthoester compositions may also contain an inhibitor, to delay acid generation, and they may neutralize any generated acid during the delay period. Suitable inhibitors include bases, e.g., alkali hydroxides, sodium carbonate, or hexamethylenetetramine.
Sometimes a small amount of a strong base as opposed to a large amount of a relatively weak base is preferred (Welton et al., 2010). A foamed fracturing composition may additionally contain other usual ingredients, such as (Welton et al., 2010).
• Gelling agents,
• Bactericides, and
• Proppants.
Fracturing in Coal-Beds
The production of natural gas from coal typically requires stimulation by hydraulic fracturing. Basic studies on the effectiveness of various treatment methods for coal-beds have been presented in the literature (Conway and Schraufnagel, 1995; Penny and Conway, 1995).
Treating a coal seam with a well treatment fluid containing a dewatering agent will enhance the methane production through a well. This additive enhances the permeability of the formation to water production and binds tenaciously to the coal surface, so that the permeability-enhancement benefits are realized over a long production term.
Polyoxyethylene, polyoxypropylene, and polyethylene carbonates (Nimerick and Hinkel, 1991), or p-tert-amylphenol condensed with formaldehyde, or copolymers of 80–100% alkyl methacrylate monomers and hydrophilic monomers have all been suggested as surfactants (Harms and Scott, 1993). Selected compounds for this purpose are shown in Figure 17.29.
Propping Agents
The best proppant and fluids have to be combined with a good design plan and the right equipment to give optimum performance. To select the best proppant for each well, a general understanding of available proppants is imperative.
They should have high permeability at the respective formation pressures, high resistance to compression, low density, and good resistance to acids. Some propping agents are listed in Table 17.17.
| Material | Description/Property | References |
|---|---|---|
| Bauxite | Standard | Andrews (1986) and Fitzgibbon (1986) |
| Bauxite + ZrO2 | Stress corrosion resistant | Khaund, 1987a and Khaund, 1987c |
| Sand | Low permeability at higher pressures | |
| Light weights | Specific gravity control | Bienvenu (1996a) |
| Ceramic | Gibb et al., 1988 and Gibb et al., 1990 | |
| Clay | Fitzgibbon, 1988 and Fitzgibbon, 1989 and Khaund (1987b) |
Sand
Sand is the simplest proppant material. It is cheap, but shows a comparatively strong reduction in permeability at higher stresses.
Ceramic Particles
Fired ceramic spheroids have been described for use as a well proppant (Laird and Beck, 1989). Each spheroid has a core made from mineral particulates, silicium carbide, and a binder. The mixture includes a mineral with chemically bound water or sulfur, which blows the mixture during firing, giving the core a number of closed air cells. Each spheroid has an outer shell of a metal oxide selected from aluminum oxide and magnesium oxide. The fired ceramic spheroids have a density of less than 2.2 g cm−3.
Bauxite
Sintered bauxite spheres containing silica are standard proppant materials. The particles have a size range from 0.02–0.3 μ. They are enhanced to resist stress corrosion by inclusion of 2% zirconia in the mix before firing. A process for manufacturing a suitable material is characterized by the following steps (Andrews, 1988).
A fine fraction is separated from naturally occurring bauxite, which will contain mostly monomineralic particles of gibbsite, boehmite, and kaolinite. The kaolinite represents no more than 25% of the total. The separated fine fraction is pelletized in the presence of water, which are then treated to remove water.
Light-weight Proppants
Light-weight propping agents have a specific gravity of less than 2.60 g cm−3. They are made from kaolin clay and a light-weight aggregate. Special conditions of calcination are necessary (Lemieux and Rumpf, 1994). Their alumina content is between 25% and 40% (Sweet, 1993). A high-strength proppant has been described (Bienvenu, 1996b) with a specific gravity of less than 1.3 g cm−3.
Porous Pack with Fibers
It is possible to build a porous pack within the formation that is a mixture of fibers and the proppant. The fibrous material may be natural or synthetic organic fibers, glass fibers, ceramic fibers, or carbon fibers.
A porous pack filters out unwanted particles, proppant, and fines, while still allowing the production of oil. Using fibers to make a porous pack of fibers and a proppant within the formation reduces energy consumption by equipment. Pumping the fibers together with the proppant provides significant reductions in the frictional forces that otherwise limit the pumping of fluids containing a proppant (Card et al., 2001).
Coated Proppants
Typically, particulates, such as graded sand, suspended in a portion of the fracturing fluid are deposited in the fractures when the fracturing fluid is converted to a thin fluid to be returned to the surface. These particulate solids, or proppant particulates, serve to prevent the fractures from fully closing, and form conductive channels through which produced hydrocarbons can flow (Dusterhoft et al., 2008).
To prevent the subsequent flowback, the proppant may be coated with a curable resin or tackifying agent, which facilitates the consolidation of particles in the fracture. The partially closed fractures apply pressure to the coated proppant particulates, which forces them into contact with each other, at which point the resin or tackifying agent enhances the grain-to-grain contact between them. This ensures the consolidation of the proppant particles into a permeable mass with compressive and tensile strength, while still allowing small amounts of deformation at the surface of the proppant packs, to reduce the effects of point loading or to reduce proppant crushing (Dusterhoft et al., 2008).
An epoxy resin composition typically includes an oligomeric bisphenol-A epichlorohydrin resin, a 4,4′-diaminodiphenyl sulfone curing agent, a solvent, a silane coupling agent, and a surfactant (Nguyen et al., 2007).
In a series of experiments, three different types of proppant particulates were assessed using a two-component high-temperature epoxy resin system (Dusterhoft et al., 2008). In each experiment, 3% resin was used with bauxite, an intermediate strength proppant, and a lightweight proppant. These proppants are known to withstand pressures from 40 M Pa to 80 M Pa without substantial crushing. The test temperature was 120°C for all tests (Dusterhoft et al., 2008).
The stress was continuously increased from 14 M Pa to 80 M Pa over several days. Uncoated proppant particulates were also tested. The effects of the closure stresses and flow rates on resin-treated proppant were evaluated by using an API linear conductivity cell.
The conductivity and permeability of each proppant pack was continuously monitored at 14 M Pa (2000 psi) and 120°C (250°F) for 25–30 h.
For all the three proppants, the fracture conductivity and proppant pack permeability were greater for the coated proppants than the uncoated proppants. The improvement was pronounced under lower stress conditions. There was an evidence for the coated proppants, that a much more stable interface was created between the proppant and the formation material.
The propping particles can be individually coated with a curable thermoset coating, which enhances the chemical resistance of the proppants. This modification is necessary if a proppant is not stable against the additives in the fracturing fluid, such as an acid gel breaker. Resole-type phenolic resins are recommended as coating materials in the presence of oxidative gel breakers (Dewprashad, 1995). Polymer coatings for propping agents are listed in Table 17.18.
| aChemically resistant | |
| bFlowback prevention | |
| Material | References |
|---|---|
| Phenolic/furan resin or furan resina | Armbruster (1987) |
| Novolak epoxide resina | Gibb et al. (1989) |
| Pyrolytic carbon coatinga | Hudson and Martin (1989) |
| Bisphenolic resinb | |
| Phenolic resin | Johnson et al., 1993a and Johnson et al., 1993b |
| Furfuryl alcohol resinb | Ellis and Surles (1997) |
| Bisphenol-A resin (curable) b | Johnson and Tse (1996) |
| Epoxide resin with N-β-(aminoethyl)-δ-aminopropyltrimethoxysilane crosslinker | Nguyen et al. (2001) |
| PA and others | Nguyen and Weaver (2001) |
Coating reduct also reduces friction between the proppant particles (de Grood and Baycroft, 2010). Coating materials are summarized in Table 17.19. Not all of these materials are economically viable. Multiple coatings of particulate material result in a final coated product that has a smooth, uniform surface.
| Material |
|---|
| Antimony trioxide |
| Bismuth |
| Boric acid |
| Calcium barium fluoride |
| Copper |
| Graphite |
| Indium |
| Lead oxide |
| Lead sulfide |
| Molybdenum disulfide |
| Niobium diselenide |
| Fluoropolymers |
| Polytetrafluoroethylene |
| Silver |
| Tin |
| Tungsten disulfide |
| Zinc oxide |
Anti-settling Additives
Proppant transport inside a hydraulic fracture has two components when the fracture is being generated. The horizontal component is dictated by the fluid velocity and by the associated streamlines, which help to carry the proppant to the tip of the fracture. The vertical component is dictated by the terminal particle settling velocity, and is a function of proppant diameter and density and fluid viscosity and density (Watters et al., 2010).
An additive with a lower density than the fluid medium can be used to vary the density gradients inside the fracture. The upward movement of the low-density additive will interfere with the downward movement of high-density proppant and vice versa, and this mutual interference will significantly hinder the settling of the high-density proppant. This can be used to control the settling time of the proppant.
The low-density material should have a particle size distribution similar to that of a standard proppant. Examples of such materials are polylactic acid particles or glass beads. The influence of such a low density additive on the settling time is summarized in Table 17.20.
| Distance/[ ft] | Time to Settle/[ s] | |
|---|---|---|
| Additive Added→ | [0%] | [5%] |
| 1 | 8 | 11 |
| 2 | 22 | 42 |
| 3 | 47 | 61 |
| 4 | 56 | 77 |
The tests were conduced in a 15 lb Mgal−1 (1.8 g m−3) guar-based linear gel with an apparent viscosity of 8 c P at 511 s−1. The gel contained a proppant concentration of 240 kg m−3 with a diameter of 30/50 mesh.
Proppant Flowback
The flowback of a proppant following fracture stimulation treatment is a major concern because of potential damage to equipment and loss in well production. The mechanisms involved and the methods to control it have been discussed in the literature (Nguyen et al., 1996a). A curable resin-coated proppant (Nimerick et al., 1990), which must be placed across the producing interval can be applied to prevent or reduce the proppant flowback.
Thermoplastic Films
Thermoplastic film (Nguyen et al., 1996b and Nguyen et al., 1996a) materials have been developed. A heat-shrinkable film cut into thin slivers provides flowback reduction over broad temperature ranges and closure stress ranges, and was found to cause little impairment to fracture conductivity with some dependency on use concentration, temperature, and closure stress.
Adhesive-coated Material
The addition of an adhesive-coated material to proppants decreases the flowback of the particulates (Caveny et al., 1996). The adhesive-coated material interacts mechanically with the proppant particles to prevent the flowback of particulates to the wellbore. The materials can be inorganic or organic fibers.
The consolidation of a proppant also may occur with a polyurethane coating, which will slowly polymerize after the fracturing treatment because of a polyaddition process (Wiser-Halladay, 1990).
Magnetized Material
Magnetized beads, fibers, strips, or particles can be placed with a proppant. The magnetized material moves to voids or channels where it forms clusters held together by magnetic attraction, which in turn facilitate the formation of permeable proppant bridges.
The magnetized material/proppant bridges retard and ultimately prevent the flowback of proppant and formation solids, but still allow production of oil and gas through the fracture at sufficiently high rates (Clark et al., 2000). In a similar way, fibrous bundles placed with the proppant may act in a fracture as a flowback preventer (Nguyen and Schreiner, 1999).
Acid Fracturing
A difference exists between acid fracturing and matrix acidizing. Acid fracturing is used for low-permeability, acid-soluble rocks, whereas matrix acidizing is a technique used for high-permeability reservoirs. Formations such as limestones (CaCO3) or dolomites (CaCO3 × MgCO3) are candidates for acid fracturing. These materials react easily with hydrochloric acid to form chlorides and carbon dioxide, and this technique has the advantage that no problem with proppant clean-out will appear. The acid etches the fracture faces unevenly, which on closure forms a highly conductive channel for the reservoir fluid to flow into the wellbore (Mukherjee and Cudney, 1993).
On the other hand, shorter, fractures are formed, because the acid reacts with the formation and therefore is spent. Also, if traces of fluoride are present in the hydrochloric acid, then insoluble calcium fluoride precipitates out, and plugging by the precipitate can jeopardize the desired effect of stimulation.
Encapsulated Acids
Acids and etching agents in general, may be mixed with a gelling agent and encapsulated with oils and polymers (Gonzalez and Looney, 2000 and Gonzalez and Looney, 2001).
The In Situ Formation of Acids
The acid fracturing of sandstone formations has not been common practice due to the low rock solubility of mud acid, but useful methods and compositions have been developed. Such a method consists of (Qu and Wang, 2010):
1. Injecting an acid fracturing fluid into the formation at a pressure sufficient to form fractures within the formation. The fluid comprises a sulfonate ester, a fluoride salt, a proppant, and water; the sulfonate ester is hydrolyzed to produce sulfonic acid.
2. Producing hydrofluoric acid in situ in the formation by reacting the sulfonic acid with the fluoride salt after injection of the acid fracturing fluid into the formation.
Generating hydrofluoric acid in situ makes it possible to perform acid fracturing of sandstone formations with the assistance of partial monolayers of effectively placed propping agents and to create enlarged, propped fractures. Increased fracture width will lead to higher fracture conductivity and enhanced hydrocarbon production than is generally achievable by a conventional propped fracturing treatment.
Fluid Loss
Fluid loss limits the effectiveness of acid fracturing treatments, so formulations to control this have been developed and characterized (Sanford et al., 1992; White et al., 1992). It was discovered that viscosifying the acid improved acid fluid loss control. The enhancement was most pronounced in very low permeability limestone cores. The nature of the viscosifying agent also influenced the success, with polymeric materials being more effective than surfactant type viscosifiers (Gdanski, 1993).
A viscosity-controlled acid contains gels that break back to their original viscosity one day after being pumped. These acids have been used both for matrix acidizing and for fracture acidizing to obtain longer fractures. The pH of the fluid controls the gel formation and breaking. The gels are limited to formation temperatures of 50–135°C (Yeager and Shuchart, 1997).
Gel Breaker for Acid Fracturing
A particulate gel breaker for acid fracturing for gels crosslinked with titanium or zirconium compounds is composed of complexing materials such as fluoride, phosphate, sulfate anions, and multicarboxylated compounds. The particles are coated with a water-insoluble resin coating, which reduces the rate of release of the breaker materials of the particles so that the viscosity of the gel is reduced at a slower rate (Boles et al., 1996).
Special Problems
Corrosion Inhibitors
Water-soluble 1,2-dithiol-3-thiones for fracturing fluids and other workover fluids have been described as corrosion inhibitors for aqueous environments (Oude Alink, 1993). These compounds are prepared by reacting a PEO capped with isopropylphenol with elemental sulfur.
The compounds perform better in aqueous systems than their nonoxylated analogs. The concentration range is usually in the 10–500 ppm range, based on the weight of the water in the system.
The Problem of Iron Control in Fracturing
Results from laboratory tests and field work show that iron presents a significant and complex problem in stimulation operations (Smolarchuk and Dill, 1986). The problem differs with acidizing and nonacidic or weakly acidic fracturing fluids.
In acidic situations, the fluid dissolves iron compounds from the equipment and the flow lines as it is mixed and pumped into the formation.
The acid may dissolve additional iron as it reacts with the formation, which could precipitate if the fluid does not contain an effective iron control system. This precipitate may then accumulate as it is carried toward the wellbore during flowback, which could decrease the natural and created permeability and so have a detrimental effect on the recovery of the treating fluid and on production.
Iron can be controlled by complexing agents, such as glucono-δ-lactone, citric acid, ethylene diamine tetraacetic acid, nitrilo triacetic acid, hydroxyethylethylene diaminetriacetic acid, hydroxyethyliminodiacetic acid, and the salts of these compounds. They must be added together with nitrogen-containing compounds, such as hydroxylamine salts or hydrazine salts (Dill et al., 1988; Frenier, 2001; Walker et al., 1987). These complexing agents are shown in Figure 17.30. In general, chelating agents possess some unique chemical characteristics, the most significant of which is the high solubility of the free acids in aqueous solutions.
Linear coreflood tests were used to study the formation of wormholes. Both hydroxyethylethylene diaminetriacetic acid and hydroxyethyliminodiacetic acid produced wormholes in limestone cores when tested at 65°C (150°F), but their efficiency and capacities differ. Because these chemicals have high solubility in the acidic pH range, it was possible to test acidic formulations with pH of less than 3.5 (Frenier et al., 2001).
To control the iron in an aqueous fracturing fluid with a pH below 7.5, a thioalkyl acid may be added (Brezinski et al., 1994). This is a reducing agent for ferric iron, in contrast to the complexants described in the previous paragraph.
Enhanced Temperature Stability
During the initial fracturing process, degradation resulting in a decrease of viscosity is undesirable. The polymer in fracturing fluids will degrade at elevated temperatures.
One method for preventing premature degradation is to cool down the formation with large volumes of pad solution before fracturing. The temperature stability of the fracturing fluid is extended by adding quantities of unhydrated, particulate guar or guar-derivative polymers before pumping it into the formation (Nimerick and Boney, 1992). Adjustment of the pH to moderate alkaline conditions can also improve stability.
The preferred crosslinkers for high-temperature applications are zirconium compounds. A formulation for a high-temperature guar-based fracturing fluid is given in Table 17.21. The fracturing fluid exhibits good viscosity and is stable at moderate to high temperatures, that is, 80–120°C.
| Component | Action |
|---|---|
| Guar gum | Thickener |
| Zirconium or hafnium compound | Crosslinking agent |
| Bicarbonate salt | Buffer |
Chemical Blowing
The efficiency of a fracturing fluid recovered from a formation can be increased by adding blowing agents (Abou-Sayed and Hazlett, 1989; Jennings, 1995). After placing the blowing agent, for example, agglomerated particles and granules containing the blowing agent of dinitrosopentamethylenetetramine, sodium hydrogen carbonate and p-toluene sulfonyl hydrazide, azodicarbonamide, and p,p-oxybis(benzenesulfonyl hydrazide) and fracturing the formation, the blowing agent decomposes. The filter cake hence becomes more porous or provides a driving force for the removal of fluid load from the matrix.
Increased porosity enhances the communication between the formation and the fracture, thus increasing the efficiency of the production of the fracturing fluid. The gas liberation within the matrix establishes communication pathways between subsequent fractures and the well.
Frost-resistant Formulation
A frost-resistant formulation is given in Table 17.22, which is stable from −35 to −45°C (Barsukov et al., 1993).
| aGas condensate, oil, or benzene | |
| bSlows down filtration and increases sand-holding capability, frost resistance, and stability | |
| cSurfactant-emulsifier | |
| Component | % |
|---|---|
| Hydrocarbon phasea | 2 to 20 |
| Surfactant | |
| Mineralized water | |
| Sludge from production of sulfonate additives (hydrocarbons 10–30%, calcium sulfonate 20–30%, calcium carbonate and hydroxide 18–40%) b | 10 to 35 |
| Emultalc | 0.5 to 2.0 |
Formation Damage in Gas Wells
Studies (Gall et al., 1988) on formation damage using artificially fractured, low permeability sandstone cores indicated that viscosified fracturing fluids can severely restrict the gas flow through narrow fractures. Polysaccharide polymers, such as hydroxypropyl guar, HEC, and xanthan gum caused a significant reduction in gas flow through the cracked cores, by up to 95%.
In contrast, PAM gels caused little or no reduction in the gas flow through cracked cores after a liquid clean-up. Other components of fracturing fluids, such as surfactants and breakers, caused less damage to gas flows.
Characterization of Fracturing Fluids
Historically, viscosity measurements have been the single most important method for characterizing fluids in petroleum-producing applications. The ability to measure a fluid's resistance to flow has been available in the laboratory for a long time, but the need to measure fluid properties at the well site has prompted the development of more portable and less sophisticated viscosity-measurement devices (Parks et al., 1986). These instruments must be durable and simple enough to be used by persons with a wide range of technical skills. As a result, the Marsh funnel and the Fann concentric cylinder, both variable-speed viscometers, have found wide use, and in some instances, the Brookfield viscometer has also been used.
It has been established that an intense control of certain variables may improve the execution of a hydraulic fracturing job and the success of a stimulation, meaning that intense quality control is recommended (Ely, 1996; Ely et al., 1990). This includes monitoring the breaker performance at low temperatures and measuring the sensitivity of fracturing fluids to variations in crosslinker loading, temperature stabilizers, and other additives at higher temperatures.
Rheological Characterization
To design a successful hydraulic fracturing treatment employing crosslinked gels, accurate measurements of rheological properties of these fluids are required. This turned out to be difficult with a rotational viscometer. In a laboratory apparatus, field pumping conditions (i.e., crosslinking the fluid on the fly) and fluid flow down tubing or casing and in the fracture could be simulated (Shah et al., 1988), and the effects of the pH and temperature of the fluid and the type and concentration of the gelling agent could be determined.
These parameters have a significant effect on the final viscosity of the gel in the fracture. Correlations to estimate friction pressures in field size tubulars have been developed from laboratory test data. In conjunction with field calibrations, these correlations can aid in the accurate prediction of the friction pressure of borate crosslinked fluids.
Zirconium-based Crosslinking Agent
The concentration of a crosslinking agent containing zirconium in a gel is determined by first adding an acid to break the gel, and convert the zirconium into the ionic, noncomplexed form (Chakrabarti and Marczewski, 1990). This is followed by the addition of Arsenazo (III) to produce a colored complex, which can be determined with standard colorimetric methods. Arsenic compounds are highly toxic. The colorimetric reagent to measure zirconium in gels is shown in Figure 17.31.
Oxidative Gel Breaker
The concentration of an oxidative gel breaker can be measured by colorimetric methods, by periodically or continuously sampling the gel (Chakrabarti et al., 1988). The colorimetric reagent is sensitive to oxidizing agents. It contains iron ions and thiocyanate. Thus the quantity of breaker added to the fracturing fluid can be controlled. The method is based on the oxidation of ferrous ions to ferric ions:
Size Exclusion Chromatography
Size exclusion chromatography (Brannon and Tjon, 1995; Gall and Raible, 1986) has been used to monitor the degradation of the thickeners initiated by various oxidative and enzymatic breakers.
Assessment of Proppants
There are standardized methods for the characterization of the effect of proppants (API Standard API RP 19C, 2008; ISO Standard ISO-13503-5, 2006). The general methods of assessment have been exemplified (Wen et al., 2007).
For some proppants, the experiments reveal polynomial relationships between the long-term fracture conductivity and the closure pressure (Wen et al., 2007)
(17.2)
(17.3)
References
Abou-Sayed, I.S., Hazlett, R.D., 1989. Removing fracture fluid via chemical blowing agents. US Patent 4 832 123, assigned to Mobil Oil Corp., May 23, 1989.
Acker, D.B., Malekahmadi, F., 2001. Delayed release breakers in gelled hydrocarbons. US Patent 6 187 720, February 13, 2001.
Aften, C.W., Gabel, R.K., 1992. Clay stabilizing method for oil and gas well treatment. US Patent 5 099 923, assigned to Nalco Chemical Co., March 31, 1992.
Aggour, T.M.; Economides, M.J., Impact of fluid selection on high-permeability fracturing, In: Proceedings Volume, Vol. 2, SPE Europe Petroleum ConferenceMilan, Italy, October 22–24, 1996. (1996), pp. 281–287.
Ahlgren, J.A., Enzymatic hydrolysis of xanthan gum at elevated temperatures and salt concentrations, In: Proceedings Volume, 6th Institute of Gas Technology, Gas, Oil, & Environmental Biotechnology International SymposiumColorado Springs, CO, November 29–December 1, 1993. (1993).
Ainley, B.R., McConnell, S.B., 1993. Delayed borate crosslinked fracturing fluid. EP Patent 528 461, assigned to Pumptech NV and Dowell Schlumberger SA, February 24, 1993.
Ainley, B.R.; Nimerick, K.H.; Card, R.J., High-temperature, borate-crosslinked fracturing fluids: A comparison of delay methodology, In: Proceedings Volume, SPE Production Operations SymposiumOklahoma City, March 21–23, 1993. (1993), pp. 517–520.
Allan, T.L., Amin, J., Olson, A.K., Pierce, R.G., 2008. Fracturing fluid containing amphoteric glycinate surfactant. US Patent 7 399 732, assigned to Calfrac Well Services Ltd. (Calgary, Alberta, CA) Chemergy Ltd. (Calgary, Alberta, CA), July 15, 2008.
Andrews, W.H., 1986. Process for making a new type of propping agent derived from bauxite for use in hydraulic fracturing, and material for use as a propping agent (procede pour la production d'un nouveau type d'agent de soutenement derive de la bauxite pour l'utilisation dans la fracturation hydraulique, et matiere propre a etre utilisee comme agent de soutenement). FR Patent 2 582 346, November 28, 1986.
Andrews, W.H., 1988. Sintered bauxite pellets and their application as proppants in hydraulic fracturing. AU Patent 579 242, November 17, 1988.
Anonymous, Fracturing products and additives, World Oil 220 (8) (1999) 135; 137,139–145.
API Standard API RP 19C, Recommended practice for measuring the long-term conductivity of proppants. (2008) American Petroleum Institute, Washington, DC.
Armbruster, D.R., 1987. Precured coated particulate material. US Patent 4 694 905, September 22, 1987.
Barkat, O., 1987. Rheology of flowing, reacting systems: The crosslinking reaction of hydroxy- propyl guar with titanium chelates. Ph.D. thesis, Tulsa Univ.
Barsukov, K.A., Ismikhanov, V.Y., Akhmetov, A.A., Pop, G.S., Lanchakov, G.A., Sidorenko, V.M., 1993. Composition for hydro-bursting of oil and gas strata – consists of hydrocarbon phase, sludge from production of sulphonate additives to lubricating oils, surfactant-emulsifier and mineralised water. SU Patent 1 794 082, assigned to Urengoi Prod. Assoc., February 7, 1993.
Barthorpe, R.T., The impairment of scale inhibitor function by commonly used organic anions, In: Proceedings Volume, SPE Oilfield Chemistry International SymposiumNew Orleans, March 2–5, 1993. (1993), pp. 69–76.
Bharat, P., 1990. Well treating fluids and additives therefor. EP Patent 372 469, June 13, 1990.
Bielewicz, V.D.; Kraj, L., Laboratory data on the effectivity of chemical breakers in mud and filtercake (Untersuchungen zur Effektivität von Degradationsmitteln in Spülungen), Erdöl Erdgas Kohle 114 (2) (1998) 76–79.
Bienvenu Jr., R.L., 1996a. Lightweight proppants and their use in hydraulic fracturing. US Patent 5 531 274, July 2, 1996.
Bienvenu Jr., R.L., 1996b. Lightweight proppants and their use in hydraulic fracturing. WO Patent 9 604 464, February 15, 1996.
Boles, J.L., Metcalf, A.S., Dawson, J.C., 1996. Coated breaker for crosslinked acid. US Patent 5 497 830, assigned to BJ Services Co., March 12, 1996.
Bonekamp, J.E., Rose, G.D., Schmidt, D.L., Teot, A.S., Watkins, E.K., 1993. Viscoelastic surfactant based foam fluids. US Patent 5 258 137, assigned to Dow Chemical Co., November 2, 1993.
Brannon, H.D., 1988. Fracturing fluid slurry concentrate and method of use. EP Patent 280 341, August 31, 1988.
Brannon, H.D., Hodge, R.M., England, K.W., 1989. High temperature guar-based fracturing fluid. US Patent 4 801 389, assigned to Dowell Schlumberger Inc., January 31, 1989.
Brannon, H.D.; Tjon-Joe-Pin, R.M., Biotechnological breakthrough improves performance of moderate to high-temperature fracturing applications, In: Proceedings Volume, Vol. 1, 69th Annual SPE Technical ConferenceNew Orleans, September 25–28, 1994. (1994), pp. 515–530.
Brannon, H.D.; Tjon, J.P.R.M., Characterization of breaker efficiency based upon size distribution of polymeric fragments, In: Proceedings Volume, Annual SPE Technical Conference and ExhibitionDallas, TX, October 22–25, 1995. (1995), pp. 415–429.
Brezinski, M., Gardner, T.R., Harms, W.M., Lane Jr., J.L., King, K.L., 1994. Controlling iron in aqueous well fracturing fluids. EP Patent 599 474, assigned to Halliburton Co., June 1, 1994.
Burdick, C.L., Pullig, J.N., 1993. Sodium formate fluidized polymer suspensions process. US Patent 5 228 908, assigned to Aqualon Co., July 20, 1993.
Cantu, L.A.; Boyd, P.A., Laboratory and field evaluation of a combined fluid-loss control additive and gel breaker for fracturing fluids, In: Proceedings Volume, SPE Oilfield Chemistry International SymposiumHouston, TX, February 8–10, 1989. (1989), pp. 7–16.
Cantu, L.A., McBride, E.F., Osborne, M., 1990a. Formation fracturing process. EP Patent 401 431, assigned to Conoco Inc. and Du Pont De Nemours & Co., December 12, 1990.
Cantu, L.A., McBride, E.F., Osborne, M., 1990b. Well treatment process. EP Patent 404 489, assigned to Conoco Inc. and Du Pont De Nemours & Co., December 27, 1990.
Cantu, L.A., McBride, E.F., Osborne, M.W., 1993. Formation fracturing process. CA Patent 1 319 819, assigned to Conoco Inc., July 6, 1993.
Card, R.J., Howard, P.R., Feraud, J.P., Constien, V.G., 2001. Control of particulate flowback in subterranean wells. US Patent 6 172 011, assigned to Schlumberger Technol. Corp., January 9, 2001.
Carpenter, J.F., 2007. Breaker composition and process. US Patent 7 223 719, assigned to Albemarle Corporation, Richmond, VA, May 29, 2007.
Carpenter, J.F., 2009. Bromine-based sulfamate stabilized breaker composition and process. US Patent 7 576 041, assigned to Albemarle Corporation, Baton Rouge, LA, August 18, 2009.
Caveny, W.J., Weaver, J.D., Nguyen, P.D., 1996. Control of particulate flowback in subterranean wells. US Patent 5 582 249, December 10, 1996.
Cawiezel, K.E.; Elbel, J.L., A new system for controlling the crosslinking rate of borate fracturing fluids, In: Proceedings Volume, 60th Annual SPE California Regional MeetingVentura, California, April 4–6, 1990. (1990), pp. 547–552.
Cawiezel, K.E., Navarrete, R.C., Constien, V.G., 1999. Fluid loss control. US Patent 5 948 733, assigned to Dowell Schlumberger Inc., September 7, 1999.
Chakrabarti, S., Marczewski, C.Z., 1990. Determining the concentration of a cross-linking agent containing zirconium. GB Patent 2 228 996, assigned to British Petroleum Co. Ltd., September 12, 1990.
Chakrabarti, S., Martins, J.P., Mealor, D., 1988. Method for controlling the viscosity of a fluid. GB Patent 2 199 408, assigned to British Petroleum Co. Ltd., July 6, 1988.
Chatterji, J., King, B.J., King, K.L., 2007. Recyclable foamed fracturing fluids and methods of using the same. US Patent 7 205 263, assigned to Halliburton energy Services, Inc., Duncan, OK, April 17, 2007.
Clark, M.D., Walker, P.L., Schreiner, K.L., Nguyen, P.D., 2000. Methods of preventing well fracture proppant flow-back. US Patent 6 116 342, assigned to Halliburton Energy Service, September 12, 2000.
Conway, M.W.; Schraufnagel, R.A., The effect of fracturing fluid damage on production from hydraulically fractured wells, In: Proceedings Volume, Ala Univ. et al Int. Unconven. Gas Symposiu, Intergas 95Tuscaloosa, AL, May 14–20, 1995. (1995), pp. 229–236.
Cooke Jr., C.E., 2009. Method and materials for hydraulic fracturing of wells using a liquid degradable thermoplastic polymer. US Patent 7 569 523, August 4, 2009.
Cottrell, T.L.; Spronz, W.D.; Weeks III, W.C., Hugoton infill program uses optimum stimulation technique, Oil Gas J. 86 (28) (1988) 88–90.
Couillet, I., Hughes, T., 2008. Aqueous fracturing fluid. US Patent 7 427 583, assigned to Schlumberger Technology Corporation, Ridgefield, CT, September 23, 2008.
Craig, D.P., 1991. The degradation of hydroxypropyl guar fracturing fluids by enzyme, oxidative, and catalyzed oxidative breakers, Ph.D. thesis, Texas A & M Univ.
Craig, D., Holditch, S.A., 1993a. The degradation of hydroxypropyl guar fracturing fluids by enzyme, oxidative, and catalyzed oxidative breakers: Pt.1: Linear hydroxypropyl guar solutions: Topical report, February-December 1991, Gas Res Inst Rep GRI-93/04191, Gas Res Inst., December 1993.
Craig, D., Holditch, S.A., 1993b. The degradation of hydroxypropyl guar fracturing fluids by enzyme, oxidative, and catalyzed oxidative breakers: Pt.2: Crosslinked hydroxypropyl guar gels: Topical report, January-April 1992, Gas Res Inst Rep GRI-93/04192, Gas Res Inst, December 1993.
Craig, D.; Holditch, S.A.; Howard, B., The degradation of hydroxypropyl guar fracturing fluids by enzyme, oxidative, and catalyzed oxidative breakers, In: Proceedings Volume, 39th Annual Southwestern Petroleum Short Course Association, Inc. et al Mtg.Lubbock, TX, April 22–23, 1992. (1992), pp. 1–19.
Crews, J.B., 2006. Bacteria-based and enzyme-based mechanisms and products for viscosity reduction breaking of viscoelastic fluids. US Patent 7 052 901, assigned to Baker Hughes Incorporated, Houston, TX, May 30, 2006.
Crews, J.B., 2007a. Aminocarboxylic acid breaker compositions for fracturing fluids. US Patent 7 208 529, assigned to Baker Hughes Incorporated, Houston, TX, April 24, 2007.
Crews, J.B., 2007b. Polyols for breaking of fracturing fluid. US Patent 7 160 842, assigned to Baker Hughes Incorporated, Houston, TX, January 9, 2007.
Crews, J.B., 2010. Saponified fatty acids as breakers for viscoelastic surfactant-gelled fluids. US Patent 7 728 044, assigned to Baker Hughes Incorporated, Houston, TX, June 1, 2010.
Crews, J.B., Huang, T., Gabrysch, A.D., Treadway, J.H., Willingham, J.R., Kelly, P.A., Wood, W.R., 2010. Methods and compositions for fracturing subterranean formations. US Patent 7 723 272, assigned to Baker Hughes Incorporated, Houston, TX, May 25, 2010.
Dawson, J.C., 1992. Method for delaying the gellation of borated galactomannans with a delay additive such as glyoxal. US Patent 5 160 643, assigned to BJ Services Co., November 3, 1992.
Dawson, J.C., 1995. Method and composition for delaying the gellation of borated gallactomannans. GB Patent 2 253 868, assigned to BJ Services Co., April 5, 1995.
Dawson, J.C., Le, H.V., 1995. Controlled degradation of polymer based aqueous gels. US Patent 5 447 199, assigned to BJ Services Co., September 5, 1995.
Dawson, J.C., Le, H.V., 1998. Gelation additive for hydraulic fracturing fluids. US Patent 5 798 320, assigned to BJ Services Co., August 25, 1998.
de Grood, R.J.C., Baycroft, P.D., 2010. Use of coated proppant to minimize abrasive erosion in high rate fracturing operations. US Patent 7 730 948, assigned to Baker Hughes Incorporated, Houston, TX, June 8, 2010.
Dewprashad, B., 1995. Method of producing coated proppants compatible with oxidizing gel breakers. US Patent 5 420 174, assigned to Halliburton Co., May 30, 1995.
Dill, W.R., Ford, W.G.F., Walker, M.L., Gdanski, R.D., 1988. Treatment of iron-containing subterranean formations. EP Patent 258 968, March 9, 1988.
Doherty, D.H., Ferber, D.M., Marrelli, J.D., Vanderslice, R.W., Hassler, R.A., 1992. Genetic control of acetylation and pyruvylation of xanthan based polysaccharide polymers. WO Patent 9 219 753, assigned to Getty Scientific Dev Co., November 12, 1992.
Dusterhoft, R.G., Fitzpatrick, H.J., Adams, D., Glover, W.F., Nguyen, P.D., 2008. Methods of stabilizing surfaces of subterranean formations. US Patent 7 343 973, assigned to Halliburton Energy Services, Inc., Duncan, OK, March 18, 2008.
Ebinger, C.D.; Hunt, E., Keys to good fracturing: Pt.6: New fluids help increase effectiveness of hydraulic fracturing, Oil Gas J. 87 (23) (1989) 52–55.
Elbel, J.L.; Navarrete, R.C.; Poe Jr., B.D., Production effects of fluid loss in fracturing high-permeability formations, In: Proceedings Volume, SPE Europe Formation Damage Control ConferenceThe Hague, Netherland, May 15–16, 1995. (1995), pp. 201–211.
Ellis, P.D., Surles, B.W., 1997. Chemically inert resin coated proppant system for control of proppant flowback in hydraulically fractured wells. US Patent 5 604 184, assigned to Texaco Inc., February 18, 1997.
Ely, J.W., Fracturing Fluids and Additives, Vol. 12 of Recent Advances in Hydraulic Fracturing (SPE Henry L Doherty Monogr Ser).. (1989) SPE, Richardson, Texas.
Ely, J.W., How intense quality control improves hydraulic fracturing, World Oil 217 (11) (1996) 59–60; 62–65, 68.
Ely, J.W.; Wolters, B.C.; Holditch, S.A., Improved job execution and stimulation success using intense quality control, In: Proceedings Volume, 37th Annual Southwestern Petroleum Short Course Association, et al Mtg.Lubbock, Texas, April 18–19, 1990. (1990), pp. 101–114.
Fitzgibbon, J.J., 1986. Use of uncalcined/partially calcined ingredients in the manufacture of sintered pellets useful for gas and oil well proppants. US Patent 4 623 630, November 18, 1986.
Fitzgibbon, J.J., 1988. Sintered, spherical, composite pellets prepared from clay as a major ingredient useful for oil and gas well proppants. CA Patent 1 232 751, February 16, 1988.
Fitzgibbon, J.J., 1989. Sintered spherical pellets containing clay as a major component useful for gas and oil well proppants. US Patent 4 879 181, November 7, 1989.
Fodge, D.W., Anderson, D.M., Pettey, T.M., 1996. Hemicellulase active at extremes of pH and temperature and utilizing the enzyme in oil wells. US Patent 5 551 515, assigned to Chemgen Corp., September 3, 1996.
Frenier, W.W., 2001. Well treatment fluids comprising chelating agents. WO Patent 0 183 639, assigned to Sofitech NV, Schlumberger Serv. Petrol, Schlumberger Canada Ltd., Schlumberger Technol. BV, and Schlumberger Holdings Ltd., November 8, 2001.
Frenier, W.W.; Fredd, C.N.; Chang, F., Hydroxyaminocarboxylic acids produce superior formulations for matrix stimulation of carbonates, In: Proceedings Volume, SPE Europe Formation Damage Control ConferenceThe Hague, Netherlands, May 21–22, 2001. (2001).
Gadberry, J.F., Hoey, M.D., Franklin, R., Del Carmen Vale, G., Mozayeni, F., 1999. Surfactants for hydraulic fracturing compositions. US Patent 5 979 555, assigned to Akzo Nobel NV, November 9, 1999.
Gall, B.L.; Maloney, D.R.; Raible, C.J.; Sattler, A.R., Permeability damage to natural fractures caused by fracturing fluid polymers, In: Proceedings Volume, SPE Rocky Mountain Regional MeetingCasper, Wyoming, May 11–13, 1988. (1988), pp. 551–560.
Gall, B.L.; Raible, C.J., The use of size exclusion chromatography to study the degradation of water-soluble polymers used in hydraulic fracturing fluids, In: Proceedings 192nd ACS Nat Mtg, Vol. 55, Amer. Chem. Soc. Polymeric Mater Sci Eng. Div Tech. ProgramAnaheim, California, September 7–12, 1986. (1986), pp. 572–576.
Gdanski, R.D., Fluid properties and particle size requirements for effective acid fluid-loss control, In: Proceedings Volume, SPE Rocky Mountain Regional Meeting: Low Permeability Reservoirs SymposiumDenver, April 26–28, 1993. (1993), pp. 81–94.
Geib, G.G., 2002. Hydrocarbon gelling compositions useful in fracturing formations. US Patent 6 342 468, assigned to Ethox Chemicals LLC, January 29, 2002.
Gibb, J.L., Laird, J.A., Berntson, L.G., 1989. Novolac coated ceramic particulate. EP Patent 308 257, March 22, 1989.
Gibb, J.L., Laird, J.A., Lee, G.W., Whitcomb, W.C., 1988. Particulate ceramic useful as a proppant. CA Patent 1 232 921, February 16, 1988.
Gibb, J.L., Laird, J.A., Lee, G.W., Whitcomb, W.C., 1990. Particulate ceramic useful as a proppant. US Patent 4 944 905, July 31, 1990.
Gonzalez, M.E., Looney, M.D., 2000. The use of encapsulated acid in acid fracturing treatments. WO Patent 0 075 486, assigned to Texaco Development Corp., Gonzalez, Manuel E., and Looney, Mark D., December 14, 2000.
Gonzalez, M.E., Looney, M.D., 2001. Use of encapsulated acid in acid fracturing treatments. US Patent 6 207 620, assigned to Texaco Inc., March 27, 2001.
Gregory, G.; Shuell, D.; Thompson Sr., J.E., Overview of contemporary LFC (liquid frac concentrate) fracture treatment systems and techniques, In: Proceedings Volume, no. 91-01, 4th Cade/caodc Spring Drilling Conf.Calgary, Can, April 10–12, 1991. (1991).
Gross, J.M., 1987. Gelling organic liquids. EP Patent 225 661, assigned to Dowell Schlumberger Inc., June 16, 1987.
Gulbis, J.; King, M.T.; Hawkins, G.W.; Brannon, H.D., Encapsulated breaker for aqueous polymeric fluids, In: Proceedings Volume, 9th SPE Formation Damage Control SymposiumLafayette, LA, February 22–23, 1990. (1990), pp. 245–254.
Gulbis, J., Williamson, T.D.A., King, M.T., Constien, V.G., 1990b. Method of controlling release of encapsulated breakers. EP Patent 404 211, assigned to Pumptech NV and Dowell Schlumberger SA, December 27, 1990.
Gulbis, J.; King, M.T.; Hawkins, G.W.; Brannon, H.D., Encapsulated breaker for aqueous polymeric fluids, SPE Prod. Eng. 7 (1) (1992) 9–14.
Guo, D.R.; Gao, J.P.; Lu, K.H.; Sun, M.B.; Wang, W., Study on the biodegradability of mud additives, Drill. Fluid Completion Fluid 13 (1) (1996) 10–12.
Gupta, D.V.S., Prasek, B.B., 1995. Method for fracturing subterranean formations using controlled release breakers and compositions useful therein. US Patent 5 437 331, assigned to Western Co. North America, August 1, 1995.
Hall, B.E., Szememyei, C.A., 1992. Fluid additive and method for treatment of subterranean formations. US Patent 5 089 151, assigned to Western Co. North America, February 18, 1992.
Harris, P.C., Fracturing-fluid additives, J. Pet. Technol. 40 (10) (1988) 1277–1279.
Harris, P.C., Heath, S.J., 1994. Delayed release borate crosslinking agent. EP Patent 594 364, assigned to Halliburton Co., April 27, 1994.
Harris, P.C.; Heath, S.J., High-quality foam fracturing fluids, In: Proceedings Volume, SPE Gas Technology SymposiumCalgary, Canada, April 28–May 1, 1996. (1996), pp. 265–273.
Harris, R.E., Hodgson, R.J., 1995. Delayed acid for gel breaking. WO Patent 9 533 914, December 14, 1995.
Harris, P.C., Norman, L.R., Hollenbeak, K.H., 1994. Borate crosslinked fracturing fluids. EP Patent 594 363, assigned to Halliburton Co., April 27, 1994.
Harms, W.M., 1992. Catalyst for breaker system for high viscosity fluids. US Patent 5 143 157, assigned to Halliburton Co., September 1, 1992.
Harms, W.M., Norman, L.R., 1988. Concentrated hydrophilic polymer suspensions. US Patent 4 772 646, September 20, 1988.
Harms, W.M., Scott, E., 1993. Method for stimulating methane production from coal seams. US Patent 5 249 627, assigned to Halliburton Co., October 5, 1993.
Harms, W.M.; Watts, M.; Venditto, J.; Chisholm, P., Diesel-based HPG (hydroxypropyl guar) concentrate is product of evolution, Pet. Eng. Int. 60 (4) (1988) 51–54.
Hawkins, G.W., Molecular weight reduction and physical consequences of chemical degradation of hydroxypropylguar in aqueous brine solutions, In: Proceedings 192nd ACS Nat Mtg, Vol. 55, Amer. Chem. Soc. Polymeric Mater Sci Eng. Div Tech. ProgramAnaheim, California, September 7–12, 1986. (1986), pp. 588–593.
Heller, J.; Barr, J.; Ng, S.Y.; Abdellauoi, K.S.; Gurny, R., Polyortho esters: Synthesis, characterization, properties and uses, Adv. Drug Deliv. Rev. 54 (7) (2002) 1015–1039.
Himes, R.E., 1992. Method for clay stabilization with quaternary amines. US Patent 5 097 904, assigned to Halliburton Co., March 24, 1992.
Himes, R.E.; Parker, M.A.; Schmelzl, E.G., Environmentally safe temporary clay stabilizer for use in well service fluids, In: Proceedings Volume, Vol. 3, CIM Petrol. Soc/SPE International Technical MeetingCalgary, Canada, June 10–13, 1990. (1990).
Himes, R.E., Vinson, E.F., 1989. Fluid additive and method for treatment of subterranean formations. US Patent 4 842 073, June 27, 1989.
Himes, R.E.; Vinson, E.F., Environmentally safe salt replacement for fracturing fluids, In: Proceedings Volume, SPE East Regional ConferenceLexington, KY, October 23–25, 1991. (1991), pp. 237–248.
Himes, R.E.; Vinson, E.F.; Simon, D.E., Clay stabilization in low-permeability formations, In: Proceedings Volume, SPE Production Operations SymposiumOklahoma City, March 12–14, 1989. (1989), pp. 507–516.
Hodge, R.M., 1997. Particle transport fluids thickened with acetylate free xanthan heteropolysac-charide biopolymer plus guar gum. US Patent 5 591 699, assigned to Du Pont De Nemours & Co., January 7, 1997.
Holditch, S.A.; Xiong, H.; Rahim, Z.; Rueda, J., Using an expert system to select the optimal fracturing fluid and treatment volume, In: Proceedings Volume, SPE Gas Technology SymposiumCalgary, Canada, June 28–30, 1993. (1993), pp. 515–527.
Holtmyer, M.D., Hunt, C.V., 1988. Method and composition for viscosifying hydrocarbons. US Patent 4 780 221, October 25, 1988.
Holtmyer, M.D., Hunt, C.V., 1992. Crosslinkable cellulose derivatives. EP Patent 479 606, assigned to Halliburton Co., April 8, 1992.
Hrachovy, M.J., 1994. Hydraulic fracturing technique employing in situ precipitation. US Patent 5 322 121, assigned to Union Oil Co. California, June 21, 1994.
Hubbert, M.K.; Willis, D.G., Mechanics of hydraulic fracturing, Trans. AIME 210 (1957) 153–168.
Huddleston, D.A., 1989. Hydrocarbon geller and method for making the same. US Patent 4 877 894, assigned to Nalco Chemical Co., October 31, 1989.
Hudson, T.E., Martin, J.W., 1989. Pyrolytic carbon coating of media improves gravel packing and fracturing capabilities. US Patent 4 796 701, January 10, 1989.
Hunt, C.V., Powell, R.J., Carter, M.L., Pelley, S.D., Norman, L.R., 1997. Encapsulated enzyme breaker and method for use in treating subterranean formations. US Patent 5 604 186, assigned to Halliburton Co., February 18, 1997.
ISO Standard ISO-13503-5, 2006. Petroleum and natural gas industries – Completion fluids and materials – Part 5: Procedures for measuring the long-term conductivity of proppants. International Organization for Standardization, Geneva, Switzerland.
Jennings Jr., A.R., 1995. Method of enhancing stimulation load fluid recovery. US Patent 5 411 093, assigned to Mobil Oil Corp., May 2, 1995.
Johnson, M., 1996. Fluid systems for controlling fluid losses during hydrocarbon recovery operations. EP Patent 691 454, assigned to Baker Hughes Inc., January 10, 1996.
Johnson, M.H., Smejkal, K.D., 1993. Fluid system for controlling fluid losses during hydrocarbon recovery operations. US Patent 5 228 524, assigned to Baker Hughes Inc., July 20, 1993.
Johnson, C.K., Tse, K.T., 1996. Bisphenol-containing resin coating articles and methods of using same. EP Patent 735 234, assigned to Borden Inc., October 2, 1996.
Johnson, C.K., Tse, K.T., Korpics, C.J., 1993a. Improved phenolic resin coated proppants with reduced hydraulic fluid interaction. EP Patent 542 397, May 19, 1993.
Johnson, C.K., Tse, K.T., Korpics, C.J., 1993b. Phenolic coated proppants with reduced hydraulic fluid interaction. CA Patent 2 067 261, May 15, 1993.
Jones, T.G.J., Tustin, G.J., 2010. Process of hydraulic fracturing using a viscoelastic wellbore fluid. US Patent 7 655 604, assigned to Schlumberger Technology Corporation, Ridgefield, CT, February 2, 2010.
Kanda, S., Kawamura, Z., 1988. Stabilization of xanthan gum in aqueous solution. GB Patent 2 192 402, January 13, 1988.
Kanda, S., Kawamura, Z., 1989. Stabilization of xanthan gum in aqueous solution. US Patent 4 810 786, March 7, 1989.
Kanda, S., Yanagita, M., Sekimoto, Y., 1986. A method of stabilizing a fracturing fluid and a stabilized fracturing fluid. GB Patent 2 172 007, September 10, 1986.
Kanda, S., Yanagita, M., Sekimoto, Y., 1988. Stabilized fracturing fluid and method of stabilizing fracturing fluid. US Patent 4 721 577, January 26, 1988.
Kelly, P.A., Gabrysch, A.D., Horner, D.N., 2007. Stabilizing crosslinked polymer guars and modified guar derivatives. US Patent 7 195 065, assigned to Baker Hughes Incorporated, Houston, TX, March 27, 2007.
Khaund, A.K., 1987a. Improved stress-corrosion resistant proppant for oil and gas wells. EP Patent 207 427, January 7, 1987.
Khaund, A., 1987b. Sintered low density gas and oil well proppants from a low cost unblended clay material of selected composition. US Patent 4 668 645, May 26, 1987.
Khaund, A.K., 1987c. Stress-corrosion resistant proppant for oil and gas wells. US Patent 4 639 427, January 27, 1987.
King, M.T.; Gulbis, J.; Hawkins, G.W.; Brannon, H.D., Encapsulated breaker for aqueous polymeric fluids, In: Proceedings Volume, Vol. 2., CIM Petrol. Soc/SPE International Technical Meeting.Calgary, Canada, June 10–13, 1990. (1990).
Laird, J.A., Beck, W.R., 1989. Ceramic spheroids having low density and high crush resistance. EP Patent 207 668, April 5, 1989.
Langemeier, P.W., Phelps, M.A., Morgan, M.E., 1989. Method for reducing the viscosity of aqueous fluids. EP Patent 330 489, August 30, 1989.
Laramay, S.B., Powell, R.J., Pelley, S.D., 1995. Perphosphate viscosity breakers in well fracture fluids. US Patent 5 386 874, assigned to Halliburton Co., February 7, 1995.
Lawrence, S., Warrender, N., 2010. Crosslinking composition for fracturing fluids. US Patent 7 749 946, assigned to Sanjel Corporation, Calgary, Alberta, CA, July 6, 2010.
Lemanczyk, Z.R., The use of polymers in well stimulation: Performance, availability and economics, In: Proceedings Volume, Plast Rubber Inst. Use of Polymers in Drilling & Oilfield Fluids ConferenceLondon, England, December 9, 1991. (1991).
Lemanczyk, Z.R., The use of polymers in well stimulation: An overview of application, performance and economics, Oil Gas Europe Mag. 18 (3) (1992) 20–26.
Lemieux, P.R., Rumpf, D.S., 1994. Lightweight proppants for oil and gas wells and methods for making and using same. CA Patent 1 330 255, June 21, 1994.
Li, F., Dahanayake, M., Colaco, A., 2010. Multicomponent viscoelastic surfactant fluid and method of using as a fracturing fluid. US Patent 7 772 164, assigned to Rhodia, Inc., Cranbury, NJ, August 10, 2010.
Lukocs, B., Mesher, S., Wilson, T.P.J., Garza, T., Mueller, W., Zamora, F., Gatlin, L.W., 2007. Non-volatile phosphorus hydrocarbon gelling agent. US Patent Application 20070173413, assigned to Clearwater International, LLC, July 26, 2007.
Manalastas, P.V., Drake, E.N., Kresge, E.N., Thaler, W.A., McDougall, L.A., Newlove, J.C., Swarup, V., Geiger, A.J., 1992. Breaker chemical encapsulated with a crosslinked elastomer coating. US Patent 5 110 486, assigned to Exxon Research & Eng. Co., May 5, 1992.
McDougall, L.A., Malekahmadi, F., Williams, D.A., 1993. Method of fracturing formations. EP Patent 540 204, assigned to Exxon Chemical Patents In, May 5, 1993.
McElfresh, P.M., Williams, C.F., 2007. Hydraulic fracturing using non-ionic surfactant gelling agent. US Patent 7 216 709, assigned to Akzo Nobel NV, Arnhem, NL, May 15, 2007.
Middaugh, R.L., Harris, P.C., Heath, S.J., Taylor, R.S., Hoch, O.F., Phillippi, M.L., Slabaugh, B.F., Terracina, J.M., 2007. Coarse-foamed fracturing fluids and associated methods. US Patent 7 261 158, assigned to Halliburton Energy Services, Inc., Duncan, OK, August 28, 2007.
Mitchell, T.O., Card, R.J., Gomtsyan, A., 2001. Cleanup additive. US Patent 6 242 390, assigned to Schlumberger Technol. Corp., June 5, 2001.
Mondshine, T.C., 1987. Crosslinked fracturing fluids. WO Patent 8 700 236, assigned to Texas United Chemical Corp., January 15, 1987.
Mondshine, T.C., 1993. Process for decomposing polysaccharides in alkaline aqueous systems. EP Patent 559 418, assigned to Texas United Chemical Corp., September 8, 1993.
Muir, D.J., Irwin, M.J., 1999. Encapsulated breakers, compositions and methods of use. WO Patent 9 961 747, assigned to 3m Innovative Propertie C, December 2, 1999.
Mukherjee, H.; Cudney, G., Extension of acid fracture penetration by drastic fluid-loss control, J. Pet. Tech. 45 (2) (1993) 102–105.
Navarrete, R.C.; Brown, J.E.; Marcinew, R.P., Application of new bridging technology and particulate chemistry for fluid-loss control during fracturing highly permeable formations, In: Proceedings Volume, Vol. 2, SPE Europe Petroleum ConferenceMilan, Italy, October 22–24, 1996. (1996), pp. 321–325.
Navarrete, R.C.; Mitchell, J.P., Fluid-loss control for high-permeability rocks in hydraulic fracturing under realistic shear conditions, In: Proceedings Volume, SPE Production Operations SymposiumOklahoma City, April 2–4, 1995. (1995), pp. 579–591.
Nguyen, P.D., Barton, J.A., Isenberg, O.M., 2007. Methods and compositions for consolidating proppant in fractures. US Patent 7 264 052, assigned to Halliburton Energy Services, Inc., Duncan, OK, September 4, 2007.
Nguyen, P.D., Schreiner, K.L., 1999. Preventing well fracture proppant flow-back. US Patent 5 908 073, assigned to Halliburton Energy Serv., June 1, 1999.
Nguyen, P.D., Weaver, J.D., 2001. Method of controlling particulate flowback in subterranean wells and introducing treatment chemicals. US Patent 6 209 643, assigned to Halliburton Energy Serv., April 3, 2001.
Nguyen, P.D., Weaver, J.D., Brumley, J.L., 2001. Stimulating fluid production from unconsolidated formations. US Patent 6 257 335, assigned to Halliburton Energy Serv., July 10, 2001.
Nguyen, P.D.; Weaver, J.D.; Parker, M.A.; King, D.G., Thermoplastic film prevents proppant flowback, Oil Gas J. 94 (6) (1996) 60–62.
Nguyen, P.D.; Weaver, J.D.; Parker, M.A.; King, D.G.; Gillstrom, R.L.; Van Batenburg, D.W., Proppant flowback control additives, In: Proceedings Volume, Annual SPE Technical ConferenceDenver, October 6–9, 1996. (1996), pp. 119–131.
Nimerick, K., 1996. Fracturing fluid and method. GB Patent 2 291 907, assigned to Sofitech NV, February 7, 1996.
Nimerick, K.H., Boney, C.L., 1992. Method of fracturing high temperature wells and fracturing fluid therefore. US Patent 5 103 913, assigned to Dowell Schlumberger Inc., April 14, 1992.
Nimerick, K.H., Crown, C.W., McConnell, S.B., Ainley, B., 1993. Method of using borate crosslinked fracturing fluid having increased temperature range. US Patent 5 259 455, November 9, 1993.
Nimerick, K.H., Hinkel, J.J., 1991. Enhanced methane production from coal seams by dewatering. EP Patent 444 760, assigned to Pumptech NV and Dowell Schlumberger SA, September 4, 1991.
Nimerick, K.H.; McConnell, S.B.; Samuelson, M.L., Compatibility of resin-coated proppants with crosslinked fracturing fluids, In: Proceedings Volume, 65th Annual SPE Technical ConferenceNew Orleans, September 23–26, 1990. (1990), pp. 245–250.
Noran, L.; Vitthal, S.; Terracina, J., New breaker technology for fracturing high-permeability formations, In: Proceedings Volume, SPE Europe Formation Damage Control ConferenceThe Hague, Netherland, May 15–16, 1995. (1995), pp. 187–199.
Norman, L.R., Laramay, S.B., 1994. Encapsulated breakers and method for use in treating subterranean formations. US Patent 5 373 901, assigned to Halliburton Co., December 20, 1994.
Norman, L.R., Turton, R., Bhatia, A.L., 2001. Breaking fracturing fluid in subterranean formation. EP Patent 1 152 121, assigned to Halliburton Energy Serv., November 7, 2001.
Oude Alink, B.A., 1993. Water soluble 1,2-dithio-3-thiones. US Patent 5 252 289, assigned to Petrolite Corp., October 12, 1993.
Pakulski, M.K., Hlidek, B.T., 1992. Slurried polymer foam system and method for the use thereof. WO Patent 9 214 907, assigned to Western Co. North America, September 3, 1992.
Parks, C.F.; Clark, P.E.; Barkat, O.; Halvaci, J., Characterizing polymer solutions by viscosity and functional testing, In: Proceedings 192nd ACS Nat. Mtg., Vol. 55, Amer. Chem. Soc. Polymeric Mater Sci Eng. Div Tech. ProgramAnaheim, California, September 7–12, 1986. (1986), pp. 880–888.
Pelissier, J.J.M., Biasini, S., 1991. Biodegradable drilling mud (boue de forage biodegradable). FR Patent 2 649 988, January 25, 1991.
Penny, G.S., 1987. Method of increasing hydrocarbon production from subterranean formations. US Patent 4 702 849, October 27, 1987.
Penny, G.S., Briscoe, J.E., 1987. Method of increasing hydrocarbon production by remedial well treatment. CA Patent 1 216 416, January 13, 1987.
Penny, G.S.; Conway, M.W., Fluid Leakoff, Vol. 12 of Recent Advances in Hydraulic Fracturing (SPE Henry L. Doherty Monogr Ser). (1989) SPE, Richardson, Texas; pp. 147–176.
Penny, G.S., Conway, M.W., 1995. Coordinated studies in support of hydraulic fracturing of coalbed methane: Final report (July 1990–May 1995), Gas Res Inst Rep GRI-95/0283, Gas Res Inst, September 1995.
Penny, G.S., Stephens, R.S., Winslow, A.R., 1991. Method of supporting fractures in geologic formations and hydraulic fluid composition for same. US Patent 5 009 797, assigned to Weyerhaeuser Co., April 23, 1991.
Powell, R.J.; Fischer, A.R.; Gdanski, R.D.; McCabe, M.A.; Pelley, S.D., Encapsulated scale inhibitor for use in fracturing treatments, In: Proceedings Volume, Annual SPE Technical ConferenceDallas, October 22–25, 1995. (1995), pp. 557–563.
Powell, R.J.; Fischer, A.R.; Gdanski, R.D.; McCabe, M.A.; Pelley, S.D., Encapsulated scale inhibitor for use in fracturing treatments, In: Proceedings Volume, SPE Permian Basin Oil & Gas Recovery ConferenceMidland, TX, March 27–29, 1996. (1996), pp. 107–113.
Powell, P.J.; Gdanski, R.D.; McCabe, M.A.; Buster, D.C., Controlled-release scale inhibitor for use in fracturing treatments, In: Proceedings Volume, SPE Oilfield Chemistry International SymposiumSan Antonio, February 14–17, 1995. (1995), pp. 571–579.
Prasek, B.B., Interactions between fracturing fluid additives and currently used enzyme breakers, In: Proceedings Volume, 43rd Annual Southwestern Petroleum Short Course Association, Inc. et al Mtg.Lubbock, Texas, April 17–18, 1996. (1996), pp. 265–279.
Putzig, D.E., 1988. Zirconium chelates and their use for cross-linking. EP Patent 278 684, assigned to Du Pont De Nemours & Co., August 17, 1988.
Putzig, D.E., 2010. Process to prepare borozirconate solution and use as cross-linker in hydraulic fracturing fluids. US Patent 7 683 011, March 23, 2010.
Putzig, D.E., Smeltz, K.C., 1986. Organic titanium compositions useful as cross-linkers. EP Patent 195 531, September 24, 1986.
Qu, Q., Wang, X., 2010. Method of acid fracturing a sandstone formation. US Patent 7 704 927, assigned to BJ Services Company, Houston, TX, April 27, 2010.
Ridland, J., Brown, D.A., 1990. Organo-metallic compounds. CA Patent 2 002 792, June 16, 1990.
Sanford, B.D.; Dacar, C.R.; Sears, S.M., Acid fracturing with new fluid-loss control mechanisms increases production, little knife field, north dakota, In: Proceedings Volume, SPE Rocky Mountain Regional MeetingCasper, Wyoming, May 18–21, 1992. (1992), pp. 317–324.
Sanner, T., Kightlinger, A.P., Davis, J.R., 1996. Borate-starch compositions for use in oil field and other industrial applications. US Patent 5 559 082, assigned to Grain Processing Corp., September 24, 1996.
Satyanarayana Gupta, D.V., Cooney, A., 1992. Encapsulations for treating subterranean formations and methods for the use thereof. WO Patent 9 210 640, assigned to Western Co. North America, June 25, 1992.
Schield, J.A., Naiman, M.I., Scherubel, G.A., 1991. Polyimide quaternary salts as clay stabilization agents. GB Patent 2 244 270, assigned to Petrolite Corp., November 27, 1991.
Shah, S.N.; Harris, P.C.; Tan, H.C., Rheological characterization of borate crosslinked fracturing fluids employing a simulated field procedure, In: Proceedings Volume, SPE Production Technology SymposiumHobbs, NM, November 7–8, 1988. (1988).
Sharif, S., 1993. Process for preparation and composition of stable aqueous solutions of boron zirconium chelates for high temperature frac fluids. US Patent 5 217 632, assigned to Zirconium Technology Corp., June 8, 1993.
Sharif, S., 1995. Process for preparation of stable aqueous solutions of zirconium chelates. US Patent 5 466 846, assigned to Benchmark Res. & Technl In, November 14, 1995.
Shuchart, C.E., Terracina, J.M., Slabaugh, B.F., McCabe, M.A., 1999. Method of treating subterranean formation. EP Patent 916 806, assigned to Halliburton Energy Serv., May 19, 1999.
Slodki, M.E.; Cadmus, M.C., High-temperature, salt-tolerant enzymic breaker of xanthan gum viscosity, In: (Editor: Donaldson, E.C.) Microbial Enhancement of Oil Recovery: Recent Advances: Proceedings of the 1990 International Conference on Microbial Enhancement of Oil Recovery, Vol. 31 of Developments in Petroleum Science (1991) Elsevier Science Ltd., pp. 247–255.
Smith, K.W., Persinski, L.J., 1995. Hydrocarbon gels useful in formation fracturing. US Patent 5 417 287, assigned to Clearwater Inc., May 23, 1995.
Smolarchuk, P.; Dill, W., Iron control in fracturing and acidizing operations, In: Proceedings Volume, Vol. 1, 37th Annu. Cim. Petrol. Soc. Tech. Mtg.Calgary, Canada, June 8–11, 1986. (1986), pp. 391–397.
Stacy, A.L., Weber, R.B., 1995. Method for reducing deleterious environmental impact of subterranean fracturing processes. US Patent 5 424 285, assigned to Western Co. North America, June 13 1995.
Subramanian, S., Zhu, Y.P., Bunting, C.R., Stewart, R.E., 2001. Gelling system for hydrocarbon fluids. WO Patent 0 109 482, assigned to Crompton Corp., February 8, 2001.
Swarup, V., Peiffer, D.G., Gorbaty, M.L., 1996. Encapsulated breaker chemical. US Patent 5 580 844, assigned to Exxon Research & Eng. Co., December 3, 1996.
Sweet, L., 1993. Method of fracturing a subterranean formation with a lightweight propping agent. US Patent 5 188 175, February 23, 1993.
Syrinek, A.R., Lyon, L.B., 1989. Low temperature breakers for gelled fracturing fluids. US Patent 4 795 574, assigned to Nalco Chemical Co., January 3, 1989.
Thomas, T.R., Smith, K.W., 1993. Method of maintaining subterranean formation permeability and inhibiting clay swelling. US Patent 5 211 239, assigned to Clearwater Inc., May 18, 1993.
von Terzaghi, K., 1923. Die Berechnung der Durchlässigkeitsziffer des Tones aus dem Ver-lauf der hydrodynamischen Spannungserscheinungen, Sitzungsberichte der Akademie der Wissenschaften in Wien, Mathematisch-Naturwissenschaftliche Klasse, Abteilung 2a.
Walker, M.L., Ford, W.G.F., Dill, W.R., Gdanski, R.D., 1987. Composition and method of stimulating subterranean formations. US Patent 4 683 954, August 4, 1987.
Walker, M.L., Shuchart, C.E., 1995. Method for breaking stabilized viscosified fluids. US Patent 5 413 178, assigned to Halliburton Co., May 9, 1995.
Watkins, D.R., Clemens, J.J., Smith, J.C., Sharma, S.N., Edwards, H.G., 1993. Use of scale inhibitors in hydraulic fracture fluids to prevent scale build-up. US Patent 5 224 543, assigned to Union Oil Co. California, July 6, 1993.
Watters, J.T., Ammachathram, M., Watters, L.T., 2010. Method to enhance proppant conductivity from hydraulically fractured wells. US Patent 7 708 069, assigned to Superior Energy Services, LLC, New Orleans, LA, May 4, 2010.
Welton, T.D., Todd, B.L., McMechan, D., 2010. Methods for effecting controlled break in pH dependent foamed fracturing fluid. US Patent 7 662 756, assigned to Halliburton Energy Services, Inc., Duncan, OK, February 16, 2010.
Wen, Q.; Zhang, S.; Wang, L.; Liu, Y.; Li, X., The effect of proppant embedment upon the long-term conductivity of fractures, J. Pet. Sci. Eng. 55 (3–4) (2007) 221–227.
Westland, J.A.; Lenk, D.A.; Penny, G.S., Rheological characteristics of reticulated bacterial cellulose as a performance additive to fracturing and drilling fluids, In: Proceedings Volume, SPE Oilfield Chemistry International SymposiumNew Orleans, March 2–5, 1993. (1993), pp. 501–514.
White, D.J.; Holms, B.A.; Hoover, R.S., Using a unique acid-fracturing fluid to control fluid loss improves stimulation results in carbonate formations, In: Proceedings Volume, SPE Permian Basin Oil & Gas Recovery ConferenceMidland, Texas, March 18–20, 1992. (1992), pp. 601–610.
Williamson, C.D.; Allenson, S.J., A new nondamaging particulate fluid-loss additive, In: Proceedings Volume, SPE Oilfield Chemistry International SymposiumHouston, TX, February 8–10, 1989. (1989), pp. 147–158.
Williamson, C.D., Allenson, S.J., Gabel, R.K., 1991a. Additive and method for temporarily reducing permeability of subterranean formations. US Patent 4 997 581, assigned to Nalco Chemical Co., March 5, 1991.
Williamson, C.D., Allenson, S.J., Gabel, R.K., Huddleston, D.A., 1991b. Enzymatically degradable fluid loss additive. US Patent 5 032 297, assigned to Nalco Chemical Co., July 16, 1991.
Williams, M.M., Phelps, M.A., Zody, G.M., 1987. Reduction of viscosity of aqueous fluids. EP Patent 222 615, May 20, 1987.
Wiser-Halladay, R., 1990. Polyurethane quasi prepolymer for proppant consolidation. US Patent 4 920 192, April 24, 1990.
Xiong, H.; Davidson, B.; Saunders, B.; Holditch, S.A., A comprehensive approach to select fracturing fluids and additives for fracture treatments, In: Proceedings Volume, Annual SPE Technical ConferenceDenver, October 6–9, 1996. (1996), pp. 293–301.
Yeager, R.R.; Bailey, D.E., Diesel-based gel concentrate improves rocky mountain region fracture treatments, In: Proceedings Volume, SPE Rocky Mountain Regional MeetingCasper, Wyoming, May 11–13, 1988. (1988), pp. 493–497.
Yeager, V.; Shuchart, C., In situ gels improve formation acidizing, Oil Gas J. 95 (3) (1997) 70–72.
Yeh, M.H., 1995. Anionic sulfonated thickening compositions. EP Patent 632 057, assigned to Rhone Poulenc Spec. Chem. C, January 4, 1995.
Zeilinger, S.C.; Mayerhofer, M.J.; Economides, M.J., A comparison of the fluid-loss properties of borate-zirconate-crosslinked and noncrosslinked fracturing fluids, In: Proceedings Volume, SPE East Regional ConferenceLexington, KY, October 23–25, 1991. (1991), pp. 201–209.
Zychal, C., 1986. Defoamer and antifoamer composition and method for defoaming aqueous fluid systems. US Patent 4 631 145, assigned to Amoco Corp., December 23, 1986.
Tradenames
| Tradename Description | Supplier |
|---|---|
| ClearFRAC™ Stimulating fluid (Crews, 2010) | Schlumberger Technology Corp. |
| DiamondFRAQ™ VES System (Crews, 2010) | Baker Oil Tools |
| Injectrol® (Series) Selants (Dusterhoft et al., 2008) | Halliburton Energy Services, Inc. |
| Jordapon® ACl Sodium cocoyl isothionate surfactant (Crews, 2010) | BASF |
| Jordapon® Cl Ammonium cocoyl isothionate surfactant (Crews, 2010) | BASF |
| Microsponge™ Porous solid substrate (Crews, 2010) | Advanced Polymer Systems |
| Poly-S.RTM Polymer encapsulation coating (Crews, 2010) | Scotts Comp. |
| Wellguard™ 7137 Interhalogen gel breaker (Carpenter, 2009 and Carpenter, 2007) | Albemarle Corp. |
| WS-44 Emulsifier (Welton et al., 2010) | Halliburton Energy Services, Inc. |
..................Content has been hidden....................
You can't read the all page of ebook, please click here login for view all page.