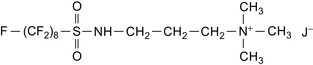Chapter 9. Filter Cake Removal
Well drill-in and servicing fluids are employed in the course of drilling, which typically include fluid loss control fluids. These fluids make up a comparatively small portion of the total, but sufficient to form a filter cake so as to plug off thief zones.
In general, well drill-in and servicing fluids are formulated to form a fast and efficient filter cake on the walls of a wellbore within a producing formation, in order to minimize leak-off and damage, which often contains an inorganic and an organic portion. This arises because drill-in fluids are typically composed of either starch or cellulose polymers, xanthan polymers, and sized calcium carbonate or salt particulates.
Before the production starts, the filter cake must be removed (Munoz, 2009). Insufficient degradation of the filter cake can significantly impede the flow capacity at the wellbore wall. Partially dehydrated, gelled drilling fluid and filter cake must be displaced from the wellbore annulus to achieve a successful primary cement job.
Conventional methods of filter cake removal consist of contacting and washing with suitable fluids, or it can be removed by special formulations of the drilling fluid, which often contains an acid-soluble particulate solid bridging agent. The filter cake formed by such a fluid is contacted with a strong acid to dissolve the bridging agent. This method is somewhat dangerous, because the strong acid may corrode the metallic surfaces of the completion equipment, thereby causing premature damage.
Another method of filter cake removal utilizes a water-soluble particulate solid bridging agent in the fluid. The bridging agent is contacted with an aqueous salt solution that is undersaturated with respect to the bridging agent. If this procedure is used, the bridging agents may require a comparatively long period of time to dissolve. The presence of a gelling agent may prevent the salt solution from contacting the water-soluble bridging agents.
A further method for filter cake removal is to contact it with a combination of an acid and an oxidizer. The acid may be used to degrade the inorganic portion of the filter cake, while the oxidizer may be employed to degrade the organic portion (Munoz, 2009).
Bridging Agents
Magnesium oxide, manganese oxide, calcium oxide, lanthanum oxide, cupric oxide, and zinc oxide can be used in combination with hydroxyethyl cellulose as fluid loss agents, and xanthan as suspension aid for solid particle bridging agents.
In addition to the bridging agent, the drilling or servicing fluid may also include an oxidizer, which is deposited in the filter cake and activated by ammonium chloride (NH4Cl) in the cleaning solution to degrade the polymer in the filter cake. Magnesium peroxide in an encapsulated form is the most suitable oxidizer.
These compounds should be soluble in a clean-up solution containing a quaternary organic ammonium salt, or simply ammonium chloride (Todd et al., 2002). The solubilities of some selected particulate bridging agents are shown in Table 9.1. A chelating agent such as citric acid or its salts is also included in the clean-up solution.
| Particulate Bridging Agent | Aqueous Ammonium Salt Clean-up Solution | Solubility/[g/100 ml] |
|---|---|---|
| Magnesium oxide | 4 M ammonium chloride | 1.6 |
| Magnesium oxide | 8 M ammonium acetate | 2.8 |
| Magnesium oxide | 1.3 M ammonium chloride plus 1 M sodium citrate | 2.8 |
| Magnesium carbonate | 8 M ammonium acetate | 2.2 |
| Magnesium carbonate | 4 M ammonium chloride plus 0.4 M trisodium salt of nitrilotriacetic acid | 2.9 |
| Anhydrite (CaSO4) | 4 M ammonium chloride | 1.7 |
| Anhydrite (CaSO4) | 8 M ammonium acetate | 2.9 |
| Lime (Ca(OH)2) | 1.3 M ammonium chloride | 3.0 |
| Zinc oxide | 4 M ammonium chloride | 3.0 |
| Zinc oxide | 1.3 M ammonium chloride plus 0.8 M sodium citrate | 2.9 |
| Zinc carbonate | 4 M ammonium chloride | 2.4 |
| Lanthanum oxide | 0.36 M diammonium salt of ethylene diamine tetraacetic acid (EDTA) | 2.2 |
| Manganese hydroxide | 4 M ammonium chloride | 1.5 |
Production engineers have been reluctant to use particle bridging because of the possibility of particle transport into the formation, resulting in formation damage, or costly and often ineffective stimulation treatments, but an example has been developed that quickly and effectively controls the fluid loss in a wide range of permeabilities and pore diameters (Johnson, 1994).
Water-soluble organic polymers, such as hydroxethyl cellulose, have been used to slow down the leak-off rate of clear brines into permeable formations. Fluid loss or leak-off, however, can be effectively controlled only by bridging the pore openings with rigid or semirigid particles of sufficient size and number.
The filter cake formed in this process is highly dispersible in the produced fluid and thus is effectively removed by putting the well into production. No acid treatment or other removal techniques are required. The primary bridging agent in this fluid is a sized calcium carbonate with particle sizes capable of initiating bridging pore diameters in excess of 100 μ.
Degradable Bridging Agents
Bridging agents made up from a degradable material can enhance filter cake removal. In this way, a self-degrading filter cake is formed. The bridging agent is suspended in a treatment fluid, and, as it begins to form a filter cake within the subterranean formation, the bridging agent becomes distributed throughout the resulting filter cake. After a certain period of time, the material degrades, which in turn causes the degradable material to be removed from the filter cake. As a result, voids are created in the filter cake that allow the produced fluids to flow more freely (Munoz and Eoff, 2010).
A polymeric bridging agent consists of polylactides, commonly synthesized by a ring opening polymerization of cyclic lactide monomers. Lactic acid belongs to the group of hydroxy acids, which are shown in Figure 17.18.
Lactide units are chiral, which allows degradation rates and physical and mechanical properties to be adjusted. Poly(L-lactide) is a semicrystalline polymer with a relatively slow hydrolysis rate, which could be desirable in applications where a slow degradation of the degradable material is desired. In contrast, poly(D,L-lactide) is a more amorphous polymer with a correspondingly faster hydrolysis rate. This may be suitable for applications where a more rapid degradation may be appropriate.
The various stereoisomers of lactic acid may be used as they are, or they can be combined. It may also be copolymerized with ε-caprolactone, 1,5-dioxepan-2-one, or trimethylene carbonate to tailor the desired properties. Yet another possibility is to control the desired properties via the molecular weight or to use blends of different molecular weights. Oligomeric lactic acid can be used as plasticizer.
Other types of degradable polymers include polyanhydrides, such as polyadipic anhydride, polysuberic anhydride, polysebacic anhydride, and polydodecanedioic anhydride.
The bridging agents are contacted with an acidic breaker solution, e.g., dilute aqueous acetic acid (Munoz and Eoff, 2010) so that they can be destroyed by hydrolysis. Another possibility is to use delayed-release acid compositions, or enzymatic degradation, which may be achieved by the use of lactate oxidase (Munoz, 2010).
Dissolvable Bridging Agents
Similarly to degradable bridging agents, dissolvable bridging agents are helpful in removing filter cakes. They are based on salts of hydroxy acids (Todd, 2009b). Suitable examples are listed in Table 9.2.
| Compound |
|---|
| Magnesium citrate |
| Magnesium tartrate |
| Calcium citrate |
| Calcium tartrate |
| Calcium succinate |
| Calcium malate |
| Bismuth citrate |
The particle size of the bridging agent is 1–200 μ, and they should have a specific gravity sufficiently different from that of the drill solids in order to permit separation on the basis of gravity (Todd, 2009b). Alternatively, they can be dissolved EDTA compounds.
Degradation by Acids
In permeable carbonate formations, hydrochloric acid treatments are usually utilized to remove formation damage and the mud filter cake produced by drilling operations.
Citric Acid
An aqueous solution of citric acid and potassium chloride, alkali metal formate, acid tetraphosphate, alkaline earth chloride, and alkali metal thiophosphate has been reported as a composition for dissolving filter cake deposits left by the drilling mud in wellbores (Kristiansen, 1994).
The composition is useful as an additive for clearing stuck pipe in wellbores and as a fixer spacer for cementing pipe in wellbores. It can also be used as a well stimulation fluid in oil and gas production wells, where it is effective for dissolving filter cake that blocks pores in the production formation.
Horizontal Well Acid Breaker
Horizontal completions in unconsolidated formations can be enhanced by a hydrochloric acid (HCl) breaker system for well clean-up. Typically, the use of HCl in open-hole environments is avoided because of wellbore stability concerns, but it successfully removes salt fluid loss control materials in wells without noticeable hole collapse (Ali et al., 1993).
Acetic Acid
A case study was reported regarding the use of acetic acid (Nasr-El-Din et al., 2001). A large-scale acetic acid-based stimulation treatment was developed to remove drilling mud filter cake in vertical wells in a carbonate reservoir in Saudi Arabia. The wellbore stability of this weakly consolidated carbonate formation can be easily reduced by contact with HCl-based compositions. Laboratory testing indicated that the formation was mechanically weak, became brittle upon contact with acids, and produced large amounts of fine particles that can cause severe damage.
A chloride-free acid formula was therefore required to minimize the interference with the pulsed-neutron logs. A special acid treatment was needed to reduce the damage and maintain the integrity of the formation. Laboratory tests were performed with both regular and emulsified acetic acid and an auxiliary chemical, mainly EDTA. Pressure buildup tests after the treatment indicated that the acid was successful in removing the filter cake in all cases investigated. No fine particles nor any type of emulsion was observed in the well flowback samples.
Acid Generating Coatings
For sand control operations, gravel particles can be coated with an acid-releasing degradable material. This procedure is helpful during subsequent clean up. Coating is a straightforward procedure (Todd and Powell, 2006).
Preparation 9–1
Polylactic acid is dissolved in methylene chloride. This solution is then mixed with particulate sand. Afterwards, the solvent is stripped from the coated sand in vacuo in order to create a free flowing coated sand. The coated sand contains a 2% coating of polylactic acid.
As an alternative, polyglycolic acid has been used for coating of sand by coating as a melt (Lee, 2006).
Preparation 9–2
1. A mixture consisting of 380 g of 20–40 mesh industrial quartz sand from Unimin Corporation, and 190 g of technical grade 65–70% glycolic acid solution from J. T. Baker was mixed together in a 2–liter crystallizing dish.
2. The dish was placed on a hot plate and heated under a ventilated hood. A temperature of at least 210–220°F was maintained for about 8–10 hours.
3. The mixture was stirred frequently until it turned into a light-brown colored, somewhat viscous and sticky mixture.
4. At this point, heating was stopped.
5. The mixture was cooled to room temperature while stirring. Large aggregates formed during cooling were broken up into individual grains using a mortar and pestle.
6. The loose polyglycolic acid coated sand grains were sieved through a 60-mesh screen to remove fine-grained, uncoated material. The product was used for the filter cake clean-up test.
Table 9.3 illustrates the generation of acidic components from a coated sand from procedure Preparation 9–2. Each fluid was exposed to brine at 60°C for 4 d. The acid release was characterized by the change in pH. As can readily be seen from the table, the use of polyglycolic acid coated sand with divalent brines is more beneficial than freshwater.
Acidic Foam
Foams tend to inhibit fluid flow and end the loss of fluids into the subterranean formation even if some portion of the filter cake mass has been prematurely broken. This allows continued dissolution of the rest of the filter cake. Hence, even if an acid acts more effectively at a specific section of the horizontal wellbore, fluid loss will still be arrested by the presence of the foam in these areas. Removal of filter cake from a horizontal wellbore is performed as follows (Chan, 2009):
1. Forming a stable aqueous acidic foam, the foam should be stable at 95°C,
2. Positioning the aqueous acidic foam in the horizontal wellbore, and
3. Retaining the aqueous acidic foam in the horizontal wellbore for 2–4 h.
An example of such a surfactant foam composition is shown in Table 9.4. Cationic fluorocarbon surfactants consist of an insoluble fluorocarbon tail and a water-soluble moiety (Chan, 2009). For example, FLUORAD™ FC 754 is N,N,N-trimethyl[3-(perfluorooctanesulfonylamino)propyl]ammoniumiodide, which is shown in Figure 9.1.
| Component | % |
|---|---|
| Hydrochloric acid | 3–10 |
| Citric acid | 5–20 |
| Cationic fluorocarbon surfactant | 0.1–0.5 |
| n-Hexanol | 0.1–2 |
| Cocoamido propyl betaine | 0.5–2 |
| Alkyl polyglycoside | 0.5–2 |
The alcohol promotes the formation of microemulsions once the surfactant foam has been spent, and it becomes mixed with oily fluids, such as crude oil and condensate from the formation. The foam may be produced in situ by alternately injecting slugs of air and the composition through the horizontal wellbore.
Orthoesters
Orthoesters find use in various oil field applications, including the delayed delivery of acids that eventually degrade filter cakes (Schriener and Munoz, 2009). Orthoester compositions generate acids that are capable of degrading the acid-soluble portion of a filter cake.
Tripropyl orthoformate is a commonly used example, however, a variety of orthoesters and orthoester polymers have been claimed to be usable. These are summarized in Table 9.5.
| Ortoester Compound | Boiling Point/[°C] |
|---|---|
| Trimethyl orthoacetate | 107 |
| Triethyl orthoacetate | 142 |
| Tripropyl orthoacetate | |
| Triisopropyl orthoacetate | |
| Polyorthoacetates | |
| Trimethyl orthoformate | 101 |
| Triethyl orthoformate | 146 |
| Tripropyl orthoformate | 106 (40 mmHg) |
| Triisopropyl orthoformate | 65 (18 mmHg) |
| Polyorthoformates | |
| Trimethyl orthopropionate | 121 |
| Triethyl orthopropionate | 155 |
The presence of water is required to allow the orthoester to hydrolyze and produce an acid. Inhibitor bases, including sodium hydroxide, potassium hydroxide, amines, e.g., hexamethylenetetramine, and sodium carbonate are added to the orthoester to delay the generation of the acid (Schriener and Munoz, 2009).
Enzymatic Degradation
Enzymes are promising candidates for clean-up operations because they can degrade the polymeric components of a filter cake. Thus the permeability of the rock is re-established. The particular properties of the enzymes are (Battistel et al., 2010):
1. High specificity, which allows activity to be accurately controlled with respect to the polymeric substrate,
2. Catalytic efficiency, which allows a high reaction rate per mole of reacted product to be obtained, under optimum conditions, and
3. Activity under bland conditions.
Damaging materials such as filter cakes and very viscous fluids within a subterranean formation of a wellbore can be removed by enzyme treatment (Tjon-joe Pin et al., 1994), which degrades polysaccharide-containing filter cakes and their damaging fluids by reducing their viscosity. The degraded filter cake and damaging fluid can then be removed from the formation back to the well surface.
The enzymes used act specifically on a given type of polysaccharide and are active at low to moderate temperatures. The enzymes attack only specific linkages in filter cakes and damaging fluids and are active in the pH range of 2 to 10.
Enzymes are available to degrade crosslinked hydroxypropylated starch and xanthan gum polymer systems (Beall et al., 1997; Moore et al., 1996). They are efficient in reducing the near-wellbore damage induced by the starch polymer, eventually returning permeabilities to 80–98% without the use of acid systems.
The use of enzymes as breakers allows the optimization well completion operations, so reducing the damage caused by fracturing during drilling. Unlike acids and other chemical oxidants, enzymes do not interact with the formation rock and the metals present, thus making undesirable secondary reactions impossible (Battistel et al., 2010).
An enhanced process for the degradation of scleroglucan or xanthan gums has been developed. Cellulase enzymes obtained specifically from Trichoderma reesei and glucosidase obtained from Aspergillus niger are used (Battistel et al., 2010).
Peroxides
Hydrogen Peroxide
A reagent for removing clay deposits based on an aqueous solution of H2O2 and Na2CO3 in a concentration range of 15–30 g l−1 and 75–150 g l−1, or a solution of sodium bicarbonate and HCl in a concentration range of 60–80 g l−1 and 3.5–4.0 g l−1 has been reported (Bulanov et al., 1992). Injection is followed by a holding time of preferably 2–5 h. Clay layer breakup products are washed out with a wash solution such as petroleum and circulating water.
Metal Peroxides
Mixture containing polysaccharide polymers and certain bridging particles and alkaline earth metal peroxides and zinc peroxide in an acidic aqueous solution successfully removes filter cake (Dobson and Mondshine, 1995; Mondshine and Benta, 1993). On soaking, a loosely adherent mass is left behind on the walls of the borehole that can be removed with a wash solution in which the bridging particles are soluble.
Magnesium Peroxide in Filter Cake
Magnesium peroxide is very stable in an alkaline environment, and remains inactive when added to polymer-based drilling fluids, completion fluids, or workover fluids. Because it is a powdered solid, it becomes an integral part of the deposited filter cake (Dobson and Kayga, 1995), which can be activated by a mild acid soak. This treatment produces hydrogen peroxide, which decomposes into oxygen and hydroxyl radicals (OHċ) when catalyzed by a transition metal.
These highly reactive OHċ species attack positions at the polymers that are resistant to acid alone, thus realizing significant improvements in filter cake removal. Magnesium peroxide is used as a breaker in alkaline water-based systems, especially in wells with a bottom hole temperature of 150°F (65°C) or less, when drilling into a pay-zone, underreaming, and in lost circulation pills, and fluid loss pills for gravel prepacks.
Degradation by Oligosaccharides
A gelled and dehydrated drilling fluid or filter cake can be removed from the walls of wellbores by injecting an aqueous sugar solution (Weaver et al., 1996). This solution is kept in contact with the filter cake for long enough to cause the disintegration of the gelled drilling fluid and the filter cake. The whole composition is then displaced from the wellbore. Monosaccharide, disaccharide, and trisaccharide sugars can be used, and surface active agents, such as a blend of non-ionic ethoxylated alcohols or a mixture of aromatic sulfonates, can be added.
Breaking by Emulsions
Some water-in-oil emulsions are highly efficient in breaking the residual emulsion inside the filter cake, decreasing cake cohesion and reducing cake adherence to the formation face (Javora et al., 2009).
They act like a demulsifier to break oil-based drilling mud (OBM) or synthetic OBM water-in-oil emulsions and thus changes the adherence of the filter cake to the wellbore and formation.
When the emulsion is specifically formulated, the emulsion may pass the no-sheen requirement for use in Gulf of Mexico applications, where the emulsion must not produce a silvery or iridescent sheen on the surface of sea water (Javora et al., 2009).
In particular, the emulsion used should provide excellent particle suspension capacity in order to prevent particulates from redepositing within the well, e.g., on tubings, casings, or the formation surface.
The internal (or discontinuous) phase of the water-in-oil emulsion is water, and the external phase is a hydrophobic organic solvent. D-Limonene is a preferred organic solvent. Preferred surfactants are fatty acids, e.g., caprylic or capric acids, but several other types have been considered (Javora et al., 2009).
Suitable dispersing agents include organophosphate esters, such as (2-ethylhexyl) orthophosphate. The effectiveness of the concept of weakening the filter cakes has been tested muds in laboratory experiments (Javora et al., 2009).
Surfactant Nanotechnology
The removal of residues from OBMs in a wellbore is important, since brines contaminated with an OBM can adversely affect the productivity of a reservoir. All OBM residues can be removed by a series of solvent and surfactant treatments, but such treatments result in large disposal volumes, and incomplete water-wetting of the casing or formation by the surfactant may emerge. A recently developed method relies on surfactant nanotechnology in a high-density brine in order to form a microemulsion upon contact with oil (Zanten and Ezzat, 2010).
OBM filter cake with an acid-degradable weighting agent can also be removed and the reservoir becomes water-wet if the surfactant is used in conjunction with an acid precursor. Microemulsion technology is also suitable for remediation, as emulsion blockages can be removed (Zanten and Ezzat, 2010).
The issues of microemulsions have been described in detail (Fanun, 2008; Harrison, 2004), and their classification goes back to Winsor (1948). It is defined as a system of oil, water, and amphiphile that is a single phase, optically isotropic and thermodynamically stable liquid solution.
Single phase microemulsions are used to improve the removal of filter cakes formed during drilling with OBMs. The microemulsion removes oil and solids from the deposited filter cake (Jones et al., 2010). In addition, microemulsions find use in enhanced oil recovery operations (Santanna et al., 2009).
Special Issues
Manganese tetroxide
Manganese tetraoxide (Mn3O4) has been recently used as a weighting material for water-based drilling fluids. It has a specific gravity of 4.8 g cm−3, making it suitable in muds for drilling deep gas wells. The filter cake formed by this mud also contains Mn3O4 (Moajil et al., 2008).
Several articles concerning the use of manganese tetroxide with other additives in drilling fluid formulations have reported negative effects on the reservoir performance. The permeability of reservoirs is reduced when they are contacted with such drilling fluids, meaning special and expensive stimulation techniques have been proven to be necessary.
Unlike CaCO3, Mn3O4 is a strong oxidant (Moajil et al., 2008), hence the use of HCl is not recommended for the removal of the filter cake. Various organic acids, chelating agents, and enzymes, have been tested at temperatures up to 150°C.
Research has been presented which indicates that a drilling fluid formulation containing manganese tetroxide causes a minimal reduction of the permeability of the reservoir formation with respect to hydrocarbon flow (Al-Yami, 2009). These formulations are particularly useful for wells that are otherwise difficult to stimulate. A return permeability of 90% or greater was achieved without the need for acidizing treatments.
In order to achieve these performance levels, the formulation must possess certain rheological, density, temperature, and fluid loss properties. Such a formulation is shown in Table 9.6. Recall that manganese tetroxide has a density of 4.7 g cm−3.
| Ingredient | lbs bbl−1 | kg m −3 |
|---|---|---|
| Fresh water (95.2%) | 0.952 | 2.7 |
| Bentonite | 4.0 | 11.4 |
| Xanthan biopolymer | 1.5 | 4.3 |
| Starch with biocide | 6.0 | 17.1 |
| Hydrated lime | 0.25 | 0.7 |
| Manganese tetroxide | 80.0 | 228.2 |
In comparison to a synthetic mud based on alkalis salts of formic acid, where a return permeability is 66% of the initial volume of oil injected, in a manganese tetroxide based mud a return permeability is 93% (Al-Yami, 2009).
Multiply Active Compositions
In many oil field applications, fluid loss additives and filter cakes are both needed during a treatment, but after the treatment they need to be removed entirely (Willberg and Dismuke, 2009). To degrade the acid-soluble particulate portion of the drill-in fluid filter cake, a conventional delayed-release acid system is usually used.
Oxidizers are used to degrade the polymeric portions of filter cakes, but they are not able to degrade the acid-soluble portion of a filter cake, so this usefulness is limited to cases where the bridging particles that comprise the particulate portion of the filter cake are small enough to flowback.
Filter cake degradation compositions comprise a delayed-release oxidizer component, which will release an acid-consuming component, and a delayed-release acid component that will release an acid derivative (Todd, 2009a). When a filter cake degradation composition is added to a wellbore, the acid-consuming component interacts with acids in such a way that the acids do not in turn interact with the acid-soluble portion of the filter cake for a period of time. This delays degradation of the acid-soluble portion of the filter cake. Thus, the integrity of the filter cake may not be jeopardized for a desired delay period. In addition, the reaction between the acid-consuming component and the acid derivative generates a peroxide that ultimately can degrade the polymeric portion of the filter cake.
Calcium peroxide CaO2 is a solid with a yellowish color. It is insoluble in water, but it dissolves in acids, e.g., acetic acid, to form hydrogen peroxide. The hydrogen peroxide can then interact with the polymer in the filter cake to ultimately degrade at least a portion of its polymeric portion.
Orthoesters or polymers of hydroxy acids are used as delayed-release acid components. Compounds suitable as delayed-release oxidizers are magnesium peroxide, MgO2 or calcium peroxide, CaO2. The delayed-release oxidizer components may be encapsulated (Todd, 2009a).
Self-destructing Filter Cake
Self-destructing filter cake compositions are formulated from a mixture of particulate solid acid-precursors, and particulate solid acid-reactive materials. The solid acid-precursors hydrolyze and dissolve in the presence of water, generating acids that then dissolve the solid acid-reactive materials (Willberg and Dismuke, 2009).
The cyclic dimer of lactic acid, which has a melting point of 95–125°C or the cyclic dimer of glycolic acid are examples of suitable solid acid-precursors. Variants are polymers of these compounds. Particulate solid acid-reactive materials include calcium carbonate, aluminum hydroxide, or magnesia, which can be coated with a hydrolysis-delaying material (Willberg and Dismuke, 2009).
The compositions are used in oil field treatments such as drilling, completion, and stimulation. Here they disappear when no longer needed without the use of mechanical means or the injection of additional fluids.
Oscillatory Flow
A physical method to remove filter cake can be applied wherein a fluid is oscillated in the annulus prior to cementing (Keller, 1986 and Keller, 1987), the direction of flow of the fluid in the annulus being changed at least twice. This removes the drilling mud and the filter cake from the annulus. After this oscillatory flow treatment, the cement slurry is pumped into the annulus.
Al-Yami, A.S.H.A.-B., 2009. Non-damaging manganese tetroxide water-based drilling fluids. US Patent 7 618 924, assigned to Saudi Arabian Oil Company (Dhahran, SA), November 17 2009.
Ali, S.A.; Sanclemente, L.W.; Sketchler, B.C.; Lafontaine-McLarty, J.M., Acid breakers enhance open-hole horizontal completions, Pet. Eng. Int. 65 (11) (1993) 20–23.
Battistel, E., Bianchi, D., Cobianco, S., Fornaroli, M., 2010. Process for the enzymatic removal of filter-cakes produced by water-based drilling and completion fluids. US Patent Application 20100069266, assigned to ENI S.P.A., Rome IT, March 18 2010.
Beall, B.B.; Brannon, H.D.; Tjon-Joe-Pin, R.M.; O'Driscoll, K., Evaluation of a new technique for removing horizontal wellbore damage attributable to drill-in filter cake, In: Proceedings Volume, 4th Annu. India Oil & Nat. Gas Corp India Oil & Gas Rev Symp.Mumbai, India, 8/18–19/97. (1997), pp. 53–65.
Bulanov, N.I., Monastyrev, V.A., Balakin, V.V., Pavlenko, A.N., Voropanov, V.E., 1992. Well bottom zone treatment with improved efficiency – includes injection of reagent breaking up clay crust for removal by flush solution stream. SU Patent 1 761 944, assigned to Oil Gas Res. Inst., September 15 1992.
Chan, A.F., 2009. Method and composition for removing filter cake from a horizontal wellbore using a stable acid foam. US Patent 7 514 391, assigned to Conocophillips Company (Houston, TX), April 7 2009.
Dobson Jr., J.W.; Kayga, P.D., Magnesium peroxide breaker system improves filter cake removal, Pet. Eng. Int. 68 (10) (1995) 49–50.
Dobson, J.W., Mondshine, T.C., 1995. Well drilling and servicing fluids which deposit an easily removable filter cake. EP Patent 672 740, assigned to Texas United Chem. Co. Llc., September 20 1995.
In: (Editor: Fanun, M.) Microemulsions: Properties and Applications, Vol. 144 of Surfactant Science Series (2008) CRC Press, Boca Raton, FL.
Harrison, J., Microemulsion technology for surfactants, Speciality Chemicals Mag. 24 (10) (2004) 32–36; [electronic:] http://surfaceactive.squarespace.com/storage/2006prsas_stl_specialitychemicals_magazine_nov2004.pdf..
Javora, P.H., Beall, B.B., Vorderburggen, M.A., Qu, Q., Berry, S.L., 2009. Method of using water-inoil emulsion to remove oil base or synthetic oil base filter cake. US Patent 7 481 273, assigned to BJ Services Company (Houston, TX), January 27 2009.
Johnson, M.H., Completion fluid-loss control using particulates, In: Proceedings Volume, SPE Formation Damage Contr. Int. Symp.Lafayette, LA, 2/9–10/94. (1994), pp. 319–320.
Jones, T.A., Clark, D.E., Quintero, L., 2010. Microemulsions to convert OBM filter cakes to wbm filter cakes having filtration control. US Patent 7 709 421, assigned to Baker Hughes Incorporated (Houston, TX), May 4 2010.
Keller, S.R., 1986. Flow method and apparatus for well cementing. GB Patent 2 172 629, assigned to Exxon Production Research Co., September 24 1986.
Keller, S.R., 1987. Oscillatory flow method for improved well cementing. CA Patent 1 225 018, assigned to Exxon Production Research Co., August 04 1987.
Kristiansen, K., 1994. Composition for use in well drilling and maintenance. WO Patent 9 409 253, assigned to Gait Products Ltd. and Kristiansen, Kastholm, April 28 1994.
Lee, L.-J., 2006. Acid-coated sand for gravel pack and filter cake clean-up. US Patent 7 132 389, assigned to M-I LLC (Houston, TX), November 7 2006.
Moajil, A.M.A.; Nasr-El-Din, H.A.; Al-Yami, A.S.; Al-Aamri, A.D.; Al-Agil, A.K., Removal of filter cake formed by manganese tetraoxide-based drilling fluids, In: SPE International Symposium and Exhibition on Formation Damage ControlSociety of Petroleum Engineers, Lafayette, Louisiana. (2008).
Mondshine, T.C., Benta, G.R., 1993. Process and composition to enhance removal of polymercontaining filter cakes from wellbores. US Patent 5 238 065, assigned to Texas United Chemical Corp., August 24 1993.
Moore, W.R.; Beall, B.B.; Ali, S.A., Formation damage removal through the application of enzyme breaker technology, In: Proceedings Volume, SPE Formation Damage Contr. Int. Symp.Lafayette, LA, 2/14–15/96. (1996), pp. 135–141.
Munoz Jr., T., 2009. In-situ filter cake degradation compositions and methods of use in subterranean formations. US Patent 7 553 800, assigned to Halliburton Energy Services, Inc. (Duncan, OK), June 30 2009.
Munoz Jr., T., 2010. Methods of degrading filter cakes in subterranean formations. US Patent 7 648 946, assigned to Halliburton Energy Services, Inc. (Duncan, OK), January 19 2010.
Munoz Jr., T., Eoff, L.S., 2010. Treatment fluids and methods of forming degradable filter cakes comprising aliphatic polyester and their use in subterranean formations. US Patent 7 674 753, assigned to Halliburton Energy Services, Inc. (Duncan, OK), March 9 2010.
Nasr-El-Din, H.A.; Lynn, J.D.; Taylor, K.C., Lab testing and field application of a large-scale acetic acid-based treatment in a newly developed carbonate reservoir, In: Proceedings Volume, SPE Oilfield Chem. Int. Symp.Houston, TX, 2/13–16/2001. (2001).
Santanna, V.; Curbelo, F.; Castro Dantas, T.; Dantas Neto, A.; Albuquerque, H.; Garnica, A., Microemulsion flooding for enhanced oil recovery, J. Pet. Sci. Eng. 66 (3–4) (2009) 117–120.
Schriener, K., Munoz Jr., T., 2009. Methods of degrading filter cakes in a subterranean formation. US Patent 7 497 278, assigned to Halliburton Energy Services, Inc. (Duncan, OK), March 3 2009.
Tjon-joe Pin, R.M., Brannon, H.D., Rickards, A.R., 1994. Method of dissolving organic filter cake obtained in drilling and completion of oil and gas wells. WO Patent 9 401 654, assigned to BJ Services Co., January 20 1994.
Todd, B.L., 2009a. Filter cake degradation compositions and methods of use in subterranean operations. US Patent 7 598 208, assigned to Halliburton Energy Services, Inc. (Duncan, OK), October 6 2009.
Todd, B.L., 2009b. Methods and fluid compositions for depositing and removing filter cake in a well bore. US Patent 7 632 786, assigned to Halliburton Energy Services, Inc. (Duncan, OK), December 15 2009.
Todd, B.L., Powell, R.J., 2006. Compositions and methods for degrading filter cake. US Patent 7 080 688, assigned to Halliburton Energy Services, Inc. (Duncan, OK), July 25 2006.
Todd, B.L., Reddy, B.R., Fisk Jr., J.V., Kercheville, J.D., 2002. Well drilling and servicing fluids and methods of removing filter cake deposited thereby. US Patent 6 422 314, assigned to Halliburton Energy Serv. Inc., July 23 2002.
Weaver, J., Ravi, K.M., Eoff, L.S., Gdanski, R., Wilson, J.M., 1996. Drilling fluid and filter cake removal methods and compositions. US Patent 5 501 276, assigned to Halliburton Co., March 26 1996.
Willberg, D., Dismuke, K., 2009. Self-destructing filter cake. US Patent 7 482 311, assigned to Schlumberger Technology Corporation (Sugar Land, TX), January 27 2009.
Winsor, P.A., Hydrotropy, solubilisation and related emulsification processes, Trans. Faraday Soc. 44 (1948) 451–471.
Zanten, R.V.; Ezzat, D., Surfactant nanotechnology offers new method for removing oil-based mud residue to achieve fast, effective wellbore cleaning and remediation, In: SPE International Symposium and Exhibiton on Formation Damage ControlSociety of Petroleum Engineers, Lafayette, Louisiana. (2010).
..................Content has been hidden....................
You can't read the all page of ebook, please click here login for view all page.