Chapter 16. Enhanced Oil Recovery
Approximately 60–70% of oil reserves cannot be recovered by conventional methods (Al-Khafaji, 1999), and so enhanced oil recovery methods become increasingly important with respect to the limited worldwide resources of crude oil. The estimated worldwide production from enhanced oil recovery and heavy oil projects at the beginning of 1996 was approximately 2.2 million barrels per day (bpd) compared to 1.9 million bpd at the beginning of 1994 (Moritis, 1996). This is approximately 3.6% of the world's oil production. Some production data by enhanced oil recovery (EOR) are summarized in Table 16.1.
| aBarrels of oil per day; includes in situ thermal heavy oil projects and primary heavy recovery oil projects | |||
| Region | BPDa (1996) (Moritis, 1996) | BPDa (1998) (Moritis, 1998) | BPDa (2006) (Sandrea and Sandrea, 2007) |
|---|---|---|---|
| United States | 724,000 | 760,000 | 649,000 |
| Canada | 515,000 | 400,000 | – |
| China | 166,000 | 280,000 | – |
| Former Soviet Union | 200,000 | 200,000 | – |
| Others | 593,000 | 700,000 | – |
| Total | 2,198,000 | 2,340,000 | |
Enhanced oil recovery processes include chemical and gas floods, steam, combustion, and electric heating. Gas floods, including immiscible and miscible processes, are usually defined by injected fluids used (carbon dioxide, flue gas, nitrogen, or hydrocarbon). Steam projects involve cyclic steam (huff and puff) or steam drive. Combustion technologies can be subdivided into those that autoignite and those that require a heat source at injectors (Duncan, 1994).
Chemical floods are identified by the specific chemical that is injected. The most commonly used are polymers, surfactants, and alkalis, but chemicals are often combined. For example, polymer slugs usually follow surfactant or alkaline slugs to improve the sweep efficiency. Injection of materials that plug permeable channels may be required for injection profile control and to prevent or mitigate premature water or gas breakthrough.
Crosslinked or gelled polymers are pumped into injectors or producers for water shutoff or fluid diversion. Cement squeezes can often fix near wellbore water channeling problems. The design of chemical injection-EOR projects can be more complicated than that of waterflood projects. Downhole conditions are more severe than those for primary or secondary recovery production.
Well injectivity is complicated by chemicals in injected waters, so in addition to precautions used in waterfloods, chemical interactions, reduced injectivity, deleterious mixtures at producers, potential for accelerated corrosion, and possible well stimulations to cause reduced injectivity must be considered (Duncan and Bulkowski, 1995). Monographs on EOR technologies are available (Alvarado and Manrique, 2010; Green and Willhite, 2008; Littmann, 1988; Sorbie, 1991).
Waterflooding
The surfactants described or characterized for waterflooding are summarized in Table 16.2. Commercial alkene sulfonates are a mixture of alkene sulfonate, hydroxyalkane sulfonate, and olefin disulfonate (Borchardt and Strycker, 1997).
| aNatural precursor | |
| bNon-ionic | |
| Surfactant | References |
|---|---|
| Ethoxylated methylcarboxylates | Strycker (1990) |
| Propoxyethoxy glyceryl sulfonate | Jenneman and Clark (1994b) |
| Alkylpropoxyethoxy sulfate as surfactant, xanthan, and a copolymer of acrylamide (AAm) and sodium 2-acrylamido-2-methylpropane sulfonate | Austad et al. (1997) |
| Carboxymethylated ethoxylated surfactants (CME) | Gall |
| Polyethylene oxide as a sacrificial adsorbate | Austad et al. (1992) |
| Polyethylene glycols, propoxylated/ethoxylated alkyl sulfates | Osterloh and Jante (1992) |
| Mixtures of sulfonates and non-ionic alcohols | Austad et al. (1991) and Shpakoff and Raney (2009) |
| Combination of lignosulfonates and fatty amines | Debons and Whittington (1991) |
| Alkyl xylene sulfonates, polyethoxylated alkyl phenols, octaethylene glycol mono n-decyl ether, and tetradecyl trimethyl ammonium chloride | Campbell and Sinquin (2008) and Somasundaran (1994) |
| Anionic sodium dodecyl sulfate, cationic cationic tetradecyl trimethyl ammonium chloride, non-ionic pentadecylethoxylated nonyl phenol (NP–15), and non-ionic octaethylene glycol N-dodecyl ether | Somasundaran (1995) |
| Dimethylalkyl amine oxides as cosurfactants and viscosifiers | Olsen (1989) |
| N-Dodecyltrimethylammonium bromide | Austad et al. (1998) |
| Petrochemical sulfonate | Ashrawi et al. (1992) |
| α-Olefin sulfonate | Ashrawi et al. (1992) and Sanz and Pope (1995) |
| Sugar-based surfactants (sorbitan monolaurate) | Shpakoff and Raney (2009) |
| Cocoamidopropyl betaine | Thompson et al. (2001) |
| 1-Phenylalkane sulfonates | Zhang et al. (2003) |
| Heels of vegetable oil | Chen et al. (2003) |
| Linoleic acid | Thibodeau et al. (2003) |
| Naphthenic acida | Horvath-Szabo et al. (2002) |
| Dodecyl benzene sulfonates | Elkamel et al. (2002) |
| Lauric acid | Amaya et al. (2002) |
| Naphthyl sulfonates | Berger and Lee (2002) |
| Gemini type aryl sulfonates | Berger and Lee (2002) |
| Dodecyltrimethylammonium bromide | Austad et al. (1998) |
| Ethoxylated nonyl phenolsb | Lakatos-Szabo and Lakatos (1989) |
| Hybrid ionic non-ionic surfactants | Wang et al., 2001a and Wang et al., 2001b |
Surfactants
Alkyl-aryl Sulfonates
Alkyl-aryl sulfonates have been recognized as being promising for EOR by surfactant flooding. They can be manufactured in large quantities and can generate low interfacial tensions (IFTs) in oils under favorable conditions.
While pure alkyl-aryl sulfonates, such as hexadecyl benzene sulfonate, can generate adequate phase behavior and low IFT with light alkanes, they are unsatisfactory when dealing with heavier crude oils, particularly those with a high wax content. They do not follow normal phase behavior when mixed with crude oil and brines of varying salinity. At low salinity, the surfactant stays predominantly in the aqueous phase, forming a lower-phase microemulsion, whereas at high salinity the surfactant stays predominantly in the oil phase, forming an upper-phase microemulsion.
Normally, a surfactant-oil-brine system with high oil recovery potential exhibits a lower-phase to middle-phase to upper-phase microemulsion transition as the salinity increases. Near the mid-range salinity, often termed optimal salinity, a middle-phase microemulsion forms with appreciable amounts of oil and brine solubilized in the microemulsion phase. However, if the oil contains a significant fraction of wax, the above phase transition often does not occur and the solubilization capacity is low, resulting in high IFT and poor oil recovery capability (Chou and Campbell, 2001).
A series of homologous 1-phenylalkane sulfonates, i.e., 1-phenyldodecane sulfonate, 1-phenyltetradecane sulfonate, and 1-phenylhexadecane sulfonate were synthesized, and their IFT of 1-phenylalkane sulfonates was investigated against crude oil from the Daqing oil field (China).
Minima in the IFTs were observed with respect to changes of the concentrations of surfactant and with time. Alkali concentrations at which the lowest IFTs were achieved moved to low regions by increasing the chain length of the aliphatic chain in the sulfonate. The lowest concentration needed was observed with 1-phenylhexadecane sulfonate at about 0.3%.
On the other hand, no minima were observed when IFTs of the 1-phenylalkane sulfonates against alkanes themselves were measured. This behavior is quite distinct from that of the 1-phenylalkane sulfonates against crude oil (Zhang et al., 2003).
For waxy crudes, the use of a broad distribution of α-olefins greater than C10 are used for the alkylation of xylene sulfonate or toluene sulfonate, in contrast to conventionally used alkyl-aryl sulfonates, which generally have a narrow range of olefin carbon number, such as C12 -xylene sulfonate (Chou and Campbell, 2001).
Surfactants of High Activity
Various surfactants have been developed that can be used at very low concentrations to produce ultra-low IFTs for sandstone and limestone formations. These surfactants can be used for alkaline surfactant polymer floods, surfactant floods, and as an adjuvant for waterfloods (Berger and Lee, 2002).
The new surfactants differ from conventional alkyl-aryl sulfonic acids, as the sulfonate group is attached to the end of the alkyl chain, as opposed to being attached directly to the aromatic ring. In general, the surface tensions and critical micelle concentrations are similar, but the solubility of the new alkyl-aryl sulfonic acids and their salts in water is generally greater than the corresponding conventional alkyl-aryl sulfonic acids and their salts. Because the sulfonate group is attached to the alkyl group instead of to the ring, more positions are available on the aromatic ring for additional substitution.
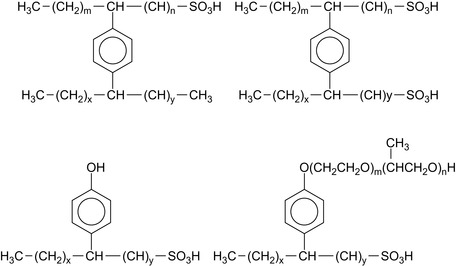
An olefinic sulfonic acid is used to simultaneously alkylate and sulfonate the aromatic compound. The aromatics that can be used include benzene, toluene, xylenes, naphthalene, phenol, and diphenylether, as well as substituted derivatives of these compounds. The olefinic sulfonic acid can be linear or branched with a range of chain lengths. Examples are shown in Figure 16.1. Several laboratory case studies have been performed for the evaluation and application of these compounds.
Sulfoalkylated nonyl phenol can be condensed with formaldehyde into oligomers (Zaitoun et al., 2003). The use of these oligomers, in combination with other surfactants reduces the adsorption of the surfactants.
Interactions between Crude Oil and Alkaline Solutions
The effectiveness of alkaline flooding for the recovery of an Arabian heavy crude oil has been studied. The alkaline reagents react with the acidic species in crude oil to form surface-active soaps in situ, leading to a lowering of the IFT and subsequent mobilization of the residual oil.
The equilibrium IFTs obtained through alkaline flooding have been compared with the IFTs when a synthetic surfactant, dodecyl benzene sulfonic acid sodium salt is used (Elkamel et al., 2002).
Effects of Connate Water in Alkaline Flooding
The effects of connate water on caustic flooding processes in porous media, by employing acidified paraffin oil as the oil phase and aqueous sodium hydroxide as the water phase, were studied. Displacement processes were performed in basic solution and with linoleic acid, in the absence and presence of connate water.
The results indicate that connate water has a greater effect on the displacement pattern for systems with higher IFTs. For lower IFTs, the patterns are similar both with and without connate water. Reducing the acid concentration has a considerable effect on the displacement, indicating that the organic acid is the limiting reagent. Generally, systems containing connate water increase the oil recovery. As the IFT decreases, the number of fingers increases and the finger width decreases (Thibodeau et al., 2003).
Combination of Primary and Secondary Surfactant Systems
The recovery of crude oil by an improved surfactant flooding process has been described. An alkaline polymer surfactant was used, which results in ultra-low IFTs with brine against crude oil even while the surfactant present is at or below its critical micelle concentration. This process is used in oil reservoirs; a primary surfactant system is diluted with brine and pumped downhole, where the alkali, which is usually sodium hydroxide or sodium carbonate, reacts with the residual acidic organic components in the oil to form a secondary surfactant system. This secondary surfactant helps the primary surfactant to further reduce the IFT between the residual oil and the injected fluid, thereby allowing the removal of residual oil from the pores of the reservoir.
The process utilizes a primary surfactant system (anionic surfactants, non-ionic cosurfactants), solvents, and a strong base. An improved, concentrated surfactant formulation primarily containing a mixture of anionic surfactants demonstrated ultra-low (<10−2 mNm−1) IFT against crude oils containing acidic organic components over a broad range of external parameters, such as surfactant, electrolyte and alkali concentrations, temperature, etc. The primary concentrated surfactant solution is a combination of a linear alkyl benzene sulfonate, a branched alkyl benzene sulfonate, and nonyl phenol (Hsu and Hsu, 2000).
Lignosulfonate Acrylic Acid Graft Copolymers as Sacrificial Agents
One of the most difficult problems in the use of surfactant flooding for EOR is the frequent, substantial loss of surfactant due to adsorption on the formation matrix and precipitation by polyvalent cations such as calcium and magnesium. A significant percentage of surfactants become physically entrapped within the pore spaces of the rock matrix. Surfactant adsorption on the formation matrix significantly decreases its efficiency, making greater quantities necessary, and hence increasing operational costs.
Most surfactants are only satisfactory for surfactant flooding if the calcium and magnesium concentrations of the formation water are less than about 500 ppm. Petroleum sulfonates, the most popular type of surfactants, precipitate where divalent ion concentrations exceed about 500 ppm. Such precipitation renders the sulfonates inoperative for recovering oil and in some instances, causes formation plugging.
The main cause of surfactant loss is adsorption due to physical contact with the formation matrix, or entrapment within its pores. Carbonate or sandstone matrices contain a range of adsorptive sites hence, adsorption is a particularly vexing problem here.
The most promising way of reducing this problem has been to use sacrificial agent compounds, either in a preflush solution injected before the surfactant-containing solution, or in the surfactant solution itself. The compounds are sacrificial because their adsorption and entrapment reduces the loss of the more expensive surfactants, solubilizers, and polymers contained within the surfactant solutions.
Various chemicals have been employed, including lignosulfonates, which are economically attractive because they are unwanted by-products of the pulp industry. A lignosulfonate acrylic acid graft copolymer has been used as such a sacrificial agent. It is believed that sacrificial agents generally work by several chemical mechanisms (Kalfoglou and Paulett, 1993):
• The sacrificial agent complexes with polyvalent cations in the formation fluids, so there will be less of these left for the surfactant to interact with;
• Electrostatic attraction of the matrix and the sacrificial agent for each other; and
• Blocking access to other sites onto which injected surfactants, solubilizers, and polymers could adsorb.
Silicone Compounds with Surfactants
Gas production from gas fields and underground gas storage is usually accompanied by unwanted water production, which often negatively affects the gas flow and recovery efficiency in wells operating in gas fields and hamper the environmental compatibility of the operation. Silicone compounds, such as silanes, siloxanes, silicone oils, and resins have been examined for their ability to restrict water production in gas wells.
Research has therefore been directed at developing a viable method to cure the problem. The methods can be categorized (Lakatos et al., 2003a) as:
1. Application of chain-like polymers,
2. Injection or in situ generation of weak polymer gels,
3. Application of alcohol-containing polymer solutions,
4. Treatment of wells with surfactant-stabilized oil-in-water emulsions,
5. Injection of silicone microemulsions, and
6. Hydrophobization of the formation rock.
The injection of a silicone microemulsion has been developed for the restriction of water production in gas wells. The treating solution was a surfactant-stabilized siloxane emulsion, which was driven into the formation by water and nitrogen.
According to the laboratory studies, the water retention is caused by disproportional permeability modification. This phenomenon is attributed to the inversion of the microemulsion into a macroemulsion initiated by spontaneous dilution by water and then entrapping siloxane droplets so formed by the pores. Field tests showed the beneficial effect of the silicone injection on gas production and gas/water ratio; the gas production tripled and was maintained for at least six months (Lakatos et al., 2002).
Non-ionic Tensides
The first non-ionic tensides were synthesized by C. Schöller at BASF by condensing oleic and stearic acid with polyoxyethylene glycol in 1930. Non-ionic surfactants are better than ionic surfactants in many respects, and their industrial application has been quite widespread during the past century.
The interfacial rheological properties of different Hungarian crude oil/water systems were determined over wide temperature and shear rate ranges, and in the presence of inorganic electrolytes, water-soluble polymers, non-ionic tensides and alkaline materials (Lakatos and Lakatos-Szabò, 2001a; Lakatos et al., 2003b).
Ethoxylated Nonyl Phenols
Ethoxylated nonyl phenols significantly reduce both the interfacial viscosity and the non-Newtonian character of the flow. Ethoxylated nonyl phenols with ethoxy groups of 10–40 were screened. The efficiency of ethoxylated nonyl phenols decreases with increasing ethoxy units and increasing concentration. This phenomenon can be explained by the formation of a closely packed adsorption layer between the phases (Lakatos and Lakatos-Szabò, 1997; Lakatos-Szabò and Lakatos, 1989). The activation energy of viscous flow in NaOH-containing oil-water systems is similar to those calculated for surfactant-containing systems (Lakatos-Szabò and Lakatos, 1999).
Interactions between Ethoxy Nonyl Phenol and Polyacrylamide
Micellization experiments on an ethoxylated nonyl phenol in the presence of partially hydrolyzed polyacrylamide (PHPA) showed that the presence of a highly hydrophilic polymer in an aqueous solution of non-ionic surfactants has only a negligible effect on the micelle structure and the mechanism of micelle formation. On the other hand, above the critical polymer concentration, the network structure is stabilized by the tenside. Therefore, the tenside exhibits positive effects on the performance of the polymer (Bedo et al., 1997).
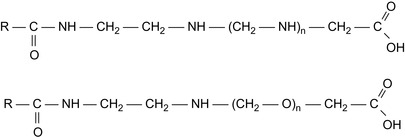
Hybrid Ionic Non-ionic Surfactants
In high salinity formations, the common non-ionic and anionic surfactants are inefficient because of salting out or cloud point phenomena. A hybrid ionic/non-ionic surfactant is shown in Figure 16.2. Such a surfactant has been synthesized, and found to be soluble in 30% NaCl brine, and to show good surface activity in brine. The IFT is particularly low if the surfactant is combined with petroleum sulfonate (Wang et al., 2001a and Wang et al., 2001b).
Interphase Structure
To elucidate whether the phase behavior or the IFT is the governing criterion for an alkaline surfactant polymer flooding formulation, an experimental study has been performed (Li et al., 2000a). The volume and the color of the middle-phase liquid were observed, and the transient IFT at different salt and alkaline concentrations was measured. Finally, a coreflood test was performed in the laboratory. It was concluded that the primary and most important phenomenon affecting the oil recovery is the low or ultra-low minimum IFT at the crude oil/soluble phase interface.
The phase behavior and the IFT were investigated in the course of alkaline surfactant polymer flooding. The size distribution and structure analysis of the middle mixed layer were studied by a size analyzer and freeze-fracture transmission electron microscopy. Some correlations between the volume of the middle mixed layer and the concentration of each component could be established. The IFT between the middle mixed layer and the oil phase or water phase can reach a very low value (Mu et al., 2002).
Several systems of surfactant and alkaline combination flooding were studied by microscopy, polarizing microscopy, microcalorimetry, laser particles analysis instrument, and IFT meter.
Liquid crystals distributed on the surface of the small particles, which were formed, were observed. The small particles and the liquid crystals are responsible for the ultra low IFT (Li et al., 1999).
Sandwich Structures
Experimental evidence on the appearance of a third liquid crystal phase between the oil and water forming a sandwich like structure has been presented. The presence of this structure modifies both the equilibrium and the transport properties of oil-water systems.
Polarization microscopy was used to observe the sandwich structures. Naphthenic acid was used because it is the most important precursor of natural surfactants and its phase behavior is well known (Horvath-Szabó et al., 2001a and Horvath-Szabó et al., 2001b). An equilibrium liquid crystal (LC) layer on an interface between crude oils and water was observed at high pH. This layer is composed mainly of sodium naphthenates formed in situ at the water/oil interface. The transient LC layer was also generated at the interface between aqueous sodium hydroxide solution and oleic naphthenic acid solution as result of a salt formation between NaOH and naphthenic acid. The chemical reaction causes a transport process resulting in a disturbance of the interface. The optical observation of this interfacial disturbance revealed that the interface covered with LC shows a considerably lower flexibility in comparison to an LC-free interface. The LC layer eventually dissolves in the water phase at low oil-to-water ratios, while at high oil-to-water ratio it can form an equilibrium phase, which spreads spontaneously at the oil-water interface (Horvath-Szabò et al., 2002).
Dynamic Interfacial Tension Behavior with In Situ Formed Surfactants
The time-dependent IFT has been investigated for an interfacially reactive immiscible system, composed of acidified oil and alkaline water. The acidified oil was composed of either lauric acid or linoleic acid dissolved in n-dodecane. Drop volume tensiometry was used to measure the IFT.
The rate of formation of the interfacial area depends on the alkali concentration. For lauric acid, the IFT value was found to decrease sharply with increasing alkali concentration, even at low drop formation times. In the case of linoleic acid, the decrease of the IFT with the drop formation time was more gradual, in particular at low alkali concentration (Amaya et al., 2002).
Interfacial Rheological Properties
In hydrocarbon reservoirs, interfacial phenomena play a fundamental role in displacement processes and phase-exchange mechanisms. Interfacial rheology is an efficient and powerful detection technique for these phenomena.
The positive effect of alkalis on microscopic displacement efficiency is attributed to Lakatos-Szabò and Lakatos (1999):
1. The alteration of the wettability,
2. The lowering of the IFT,
3. The restriction of rigid films at the oil-water interfaces,
4. The initiation of phase inversion in dispersed systems,
5. The formation of oil external emulsions,
6. Chemical reactions with some constituents of formation water and rocks, and
7. The sorption of naturally occurring surfactants.
Microemulsion Phase Diagrams
• The ability of a microemulsion to dissolve oil and water, and
• The attainment of very low IFTs.
So the understanding of chemical flooding processes for EOR relies on the knowledge of phase equilibria for such systems, which are composed of brine, oil, surfactant, and cosurfactant.
A thermodynamic analytical representation of the phase diagram of microemulsion systems similar to those used in EOR has been developed (Garcia-Sanchez et al., 2001). Since the system is basically a four component system, the data can be represented in Gibbs tetrahedral coordinates, and multiphase liquid equilibria can be estimated by excess Gibbs energy interaction parameters. An empirical expression was introduced into the selected excess Gibbs energy model to account for the specific role of the surfactant in these complex systems.
The results have been successfully tested for an oil-brine-surfactant-alcohol model system consisting of a sodium alkyl benzene sulfonate and n-butanol as co-surfactant.
Interfacial Tension
The oil-water IFT is one of the most important parameters for chemical-EOR. It has a strong time dependency, especially under alkaline conditions, so a knowledge of this behavior is necessary for the prediction of oil recovery.
The addition of sodium hydroxide to floodwater improves the oil or bitumen production because the alkaline additive activates the natural surfactants precursors, most likely acidic components, present in crude oil (Horvath-Szabò et al., 2002).
The IFT varies strongly with temperature and pressure and thus influences the transport of the fluid in a reservoir. It is probably the dominant factor that renders one-third of the total oil in place unrecoverable by gas drive or waterflooding (Amin and Smith, 1998).
Imbibition Experiments
Spontaneous imbibition experiments in nearly oil-wet chalk material of low permeability saturated with oil has been performed at ambient conditions with and without the cationic surfactant dodecyltrimethylammonium bromide present in the aqueous solution.
Without surfactant present the rate of imbibition is very small, and only approximately 13% of the oil could be expelled from the core within 90 days. After that time, a sudden increase in the oil production was observed by a 1.0% surfactant solution to the water. However, if the surfactant is present during the whole experiment, an oil production plateau of approximately to 65% recovery was obtained within 90 days (Austad et al., 1998).
Caustic Waterflooding
Injection Strategies
To develop improved alkali surfactant flooding methods, several different injection strategies were tested for recovering heavy oils. Oil recovery was compared for four different injection strategies (French and Josephson, 1992):
• Surfactant followed by polymer,
• Surfactant followed by alkaline polymer,
• Alkaline surfactant followed by polymer, and
• Alkali, surfactant, and polymer mixed in a single formulation.
The effect of alkaline preflush was also studied under two different conditions. All of the oil recovery experiments were conducted under optimal conditions with a viscous, nonacidic oil and with Berea sandstone cores.
Foam-enhanced Caustic Waterflooding
The alkaline waterflooding process is enhanced by the injection of aqueous solutions of foam-forming surfactant and gases, or preformed foams, either ahead of or behind conventional alkaline slugs. A slug of an aqueous solution containing an alkaline agent, followed by a driving fluid, is injected into the formation, and this displaces oil through the relatively high-permeability zones of the formation, and this is recovered via the production well. Thereafter, a slug of an aqueous solution with a foaming agent is coinjected into the formation with a gas and creates a foam upon mixing with the gas. The foam will preferentially go into the formation zones that have a relatively high-permeability and low oil saturation, substantially plugging them. Then a slug of an aqueous alkaline agent is injected, followed by a driving fluid that displaces the alkaline solution and oil through the less permeable zones toward the production well (Hurd, 1991).
Alkaline Surfactant Polymer Flooding
Polymers can be used for mobility control. The interaction between polymers and surfactants is shown to be affected by pH, ionic strength, crude oil type, and the properties of the polymers and surfactants (French and Josephson, 1993).
Surfactants, whose major components are natural mixed carboxylates from the heels of vegetable oil and fats such as soybean oil, vegetable oil, animal oil, and tea oil, etc., have been developed. Optimal formulations were obtained using an orthogonal-test-design method to screen the alkaline surfactant polymer flooding systems. The transient IFTs at the oil/aqueous interface were measured. The oil recovery can be increased by 26.8% of the original oil in place in a coreflood experiment. The waste water resulting from the production of the natural mixed carboxylates also exhibits a high surface activity (Chen et al., 2003; Li et al., 2000b; Mu et al., 2001).
Interphase Properties
Alkaline agents can reduce surfactant losses and permit the use of low concentrations of surfactants. Laboratory tests show that alkali and synthetic surfactants produce interfacial properties that are more favorable for increased oil mobilization than either alkali or surfactant alone (French, 1990; French and Josephson, 1991).
Clay Dissolution
During caustic waterflooding, the alkali can be consumed by the dissolution of clay and is lost. The loss depends on the kinetics of the particular reaction. Several studies have been performed with kaolinite, using quartz as a yardstick, and the kinetic data are documented in the literature. The initial reaction rate has been found to be pH independent in the pH range of 11–13 (Drillet and Defives, 1991). The kinetics of silica dissolution could be quantitatively described in terms of pH, salinity, ion exchange properties, temperature, and contact time (Saneie, 1992).
Acid Flooding
Hydrochloric Acid
Acid flooding can be successful in formations that are soluble in the particular acid mixture, thus opening the pores. Hydrochloric acid is commonly used, at a concentration of 6–30%, sometimes also with hydrofluoric acid and surfactants added (e.g., isononyl phenol) (Balakirov et al., 1992; Gorodnov et al., 1993). The acidic environment converts the sulfonates into sulfonic acid, which has a lower IFT toward oil, so giving greater efficiency in forcing out oil than is obtained from neutral, aqueous solutions of sulfonates. Cyclic injection can be applied (Abdulmazitov et al., 1997; Diyashev et al., 1996a), and sulfuric acid has also been used for acid treatment (Aleev, 1996; Aleev et al., 1996; Glumov et al., 1994). Injecting additional aqueous lignosulfonate increases the efficiency of a sulfuric acid treatment (Verderevskij et al., 1996).
Hydrochloric acid in combination with chlorine dioxide can be used as a treatment fluid in water injection wells that get impaired by the deposition of solid residues (Cavallaro et al., 2000 and Cavallaro et al., 2001). The treatment seems to be more effective than the conventional acidizing system when the plugging material contains iron sulfide and bacterial agents, because of the strongly oxidative effect of chlorine dioxide. Mixtures of chlorine dioxide, lactic acid, and other organic acids (Mason, 1991a and Mason, 1991b) also have been described.
Iron control chemicals are used during acid stimulation to prevent the precipitation of iron-containing compounds, since this can decrease well productivity or injectivity. Acetic acid, citric acid, nitrilotriacetic acid, ethylene diamine tetraacetic acid, and erythorbic acid have all been used (Taylor and Nasr-El-Din, 1999; Taylor et al., 1998). A time dependence of iron (III) hydroxide precipitation was observed. Acetic acid can prevent the precipitation of iron (III) at high concentrations at low temperatures.
If the injected acid itself contains iron (III), a precipitation of asphaltic products can occur if it comes in contact with certain crude oils, which leads to practically irreversible damage to the treated zone. The amount of precipitate generally increases with the strength and concentration of the acid. Certain organic sulfur compounds, such as ammonium thioglycolate, mercaptoethanol, cysteamine, thioglycerol, cysteine, and thiolactic acid can reduce iron (III) (Feraud et al., 2001).
Sulfuric Acid
In contrast to hydrochloric acid, sulfuric acid, in particular in a thermal treatment, reacts with the crude oil itself and causes a reduction in the viscosity of the oil (Varadaraj, 2008). In laboratory experiments, crude oil was placed in an autoclave with 10–50 ppm sulfuric acid. After mixing and deaeration, the mixture was heated to 360°C for 2–6 h at elevated pressure. No significant changes were observed in the total acid number, distribution of naphthenic acids, toluene equivalence, and n-heptane insolubles between the thermally treated and sulfuric-acid-catalyzed, thermally treated samples. These data indicate that the chemistry of the crude oil is not significantly altered by sulfuric acid addition prior to thermal treatment.
A decrease in energy of activation of flow (viscosity) is observed, suggesting that the sulfuric acid catalyzed thermal treatment alters the fundamental aggregation properties of the species that are responsible for high viscosities of heavy crude oils.
Emulsion Flooding
Optimizing the formulation of micellar surfactant solutions used for EOR aims to obtain IFTs as low as possible in multiphase systems, which can be achieved by mixing the injected solution with formation fluids. The solubilization of hydrocarbons by the micellar phases of such systems is directly linked to the interfacial efficiency of surfactants. Numerous research projects have shown that the amount of hydrocarbons that are solubilized by the surfactant is generally as great as the IFT between the micellar phase and the hydrocarbons. The solubilization of crude oils depends strongly on their chemical composition (Baviere and Rouaud, 1990).
Micellar flooding is a promising tertiary oil recovery method, perhaps the only method that has been shown to be successful in the field for depleted light oil reservoirs. As a tertiary recovery method, this process has desirable features of several chemical methods (e.g., miscible-type displacement) and is less susceptible to some of their drawbacks, such as adsorption.
It has been shown that a suitable preflush can considerably curtail the surfactant loss to the rock matrix. In addition, the use of multiple micellar solutions, selected on the basis of their phase behavior, can increase the oil recovery with respect to the amount of surfactant, in comparison with a single solution. Laboratory tests showed that volume ratios of oil recovery to slug as high as 15 can be achieved (Daharu et al., 1991).
A solids-stabilized water-in-oil emulsion may be used either as a drive fluid for displacing hydrocarbons from the formation, or to produce a barrier for diverting the flow of fluids in the formation. The solid particles may be formation or non-formation solid particles (Bragg, 1998 and Bragg, 1999).
Micellar Polymer Flooding
The factors affecting the equilibrium IFT at the oil/water interface were studied. The effect of parameters including reservoir temperature, pressure, surfactant concentration, and salinity were investigated. The pendant drop technique, enhanced by video imaging was employed for measuring the IFT.
The IFT decreases with temperature and salinity and decreases exponentially with surfactant concentration, but increases with pressure (Al-Sahhaf et al., 2002). The oil recovery performance of micellar floods is the highest, followed by polymer floods. Alkaline floods have been largely unsuccessful (Thomas and Ali, 1999).
Micellar and Alkaline Surfactant Polymer Flooding
The results for micellar flooding and alkaline surfactant polymer flooding processes were compared. Laboratory experiments on micellar floods in consolidated sandstone cores and in unconsolidated sand packs were performed using combinations of an alkali, a surfactant, and a polymer. Slugs were injected sequentially in a series of experiments, while the three components were mixed and injected as a single slug in other experiments. The oil recoveries in the two series of experiments were similar. Micellar flooding was found to be the superior process, with oil recoveries ranging from 50–80% (Thomas and Ali, 2001).
Scale-up Methods for Micellar Flooding
The design of micellar floods is largely based on laboratory experiments, which are usually unscaled. Dimensional and inspectional analysis is helpful for scaling up the design. General scaling criteria can be simplified for corefloods, and were verified by micellar floods in scaled models. Good agreement was obtained in most cases between the actual and predicted oil production histories, showing the validity of the scale-up. The scaling criteria that were derived can be also used for a micellar flood (Thomas et al., 2000).
Chemical Injection
The state of the art in chemical oil recovery has been reviewed (Thomas and Farouq, 1999). More than two-thirds of the original oil remains unrecovered in an oil reservoir after primary and secondary recovery methods have been exhausted. Many chemically based oil recovery methods have been proposed and tested in the laboratory and field. Indeed, chemical oil recovery methods offer a real challenge in view of their success in the laboratory and lack of success in the field.
The problem lies in the inadequacy of laboratory experiments and the limited knowledge of reservoir characteristics. Field test performances of polymer, alkaline, and micellar flooding methods have been examined for nearly 50 field tests. The oil recovery performance of micellar floods is the best, followed by polymer floods. Alkaline floods have been largely unsuccessful. The reasons underlying success or failure are examined in the literature (Thomas and Farouq, 1999).
Ammonium Carbonate
Ammonium carbonate decomposes in an acid medium into ammonium salts and carbon dioxide. It is thus valuable for the in situ generation of carbon dioxide (Diyashev et al., 1996b; Stepanova et al., 1994a and Stepanova et al., 1994b).
Hydrogen Peroxide
The physical properties of hydrogen peroxide indicate that hydrogen peroxide injection has the potential to combine the more favorable aspects of many enhanced oil recovery processes, namely:
1. Steam,
2. Combustion,
3. Oxygen-water combustion, and
4. Carbon dioxide injection.
Hydrogen peroxide decomposes to form water and oxygen. Both products are environmentally desirable and effective in recovering oil. Heat is generated in the oil reservoir when the decomposition reaction occurs, which supports steam and hot waterflooding operations, among others. Continued injection of liquid hydrogen peroxide advances the heat bank, steam zone, hot-water zone, oxygen-burning front, and CO2 bank through the formation, effectively displacing oil (Moss and Moss, 1994).
Combinations of hydrogen peroxide, sulfuric acid, and urea have been proposed (Abasov et al., 1993). The temperature influences the urea decomposition into ammonia and carbon dioxide, which causes pressure buildup in a formation model and a 19% increase in oil-displacement efficiency in comparison with water.
Reactions of hydrogen peroxide with near-wellbore formation and liquids create high temperatures, which lower the oil viscosity and remove formation damage. The application of this chemical technique for heat-bank-type flooding is noted as being technically superior, but it is probably not economically viable (Bayless, 1998). There is a wide potential field for the application of hydrogen peroxide, including pressure generation, hydrate melting in subsea equipment, and metal cutting for offshore structure decommissioning (Bayless, 2000).
Alcohol–Waterflooding
Butanol
n-Butanol and other C4 alcohols are suitable for hot waterflooding in medium to heavy oil reservoirs at depths greater than 1500 m (Richardson and Kibodeaux, 2001).
Isopropanol and Ammonia
A composition that includes ammonia and a low molecular weight alcohol, e.g., isopropanol, in an aqueous carrier solution has been proposed to be cost-effective for EOR (Cobb, 2010). The composition can be recovered and recycled to further decrease costs. Apparently, there is no reaction with oil nor is there a significant amount remaining trapped in the formation, so the mixture can be separated from the oil and recycled.
Residue from the Production of Glycerol or Ethylene glycol
Waste water-soluble alcohols are useful for miscible waterflooding (Ignateva et al., 1996).
Chemical Injection of Waste Gases
Waste gas produced from hydrocarbons can be safely disposed by reinjecting it into a formation. It is mixed with a surfactant to form a foam that is then placed within a disposal zone of a subterranean formation. The waste gas is trapped within the foam, thereby reducing its mobility, which in turn restricts its ability to flow out of the disposal zone and into the producing zone of the formation. The foam can be placed in the formation by coinjecting a surfactant and the waste gas together, or it can be formed in situ by first injecting the surfactant and then injecting the waste gas (Northrop, 1993).
Thermal conversion of organic waste material, such as plastics, or of biomass under the influence of oxygen, into crude synthesis gas yields a hydrogen product. The crude mixture can be injected into a depleted crude oil well, which still contains high molecular organic material. Hydrogen will crack the long chains of the sticking organics in situ and will make them more able to flow. In this way, improved oil recovery and plastics waste disposal by oxidative pyrolysis can be achieved, followed by in situ degradative hydrogenation of geopolymers. Thus more organic material can be recovered than was initially put into the well (Fink and Fink, 1998).
Polymer Waterflooding
The polymer in a polymer waterflooding process acts primarily as a thickener. It decreases the permeability of the reservoir and thus improves the vertical and lateral sweep efficiency.
Associative copolymers of AAm with N-alkylacrylamides, terpolymers of AAm, N-decylacrylamide, and sodium acrylate, or sodium-3-acrylamido-3-methylbutanoate have all been shown to possess the required rheological behavior for enhanced oil recovery processes (McCormick and Hester, 1990). Other copolymers of AAm with the zwitterionic 3-(2-acrylamido-2-methylpropyldimethyl ammonio)-1-propane sulfonate monomer also have been examined. Polymers used in polymer waterflooding are shown in Table 16.3.
| Polymer | References |
|---|---|
| Polyacrylamide | Ma et al., 1996 and Ma et al., 1999; Putz et al. (1994) and Ren et al. (1998) |
| Partially hydrolyzed polyacrylamide | Chen et al. (1997) |
| Polyacrylamide, bentonite clay | Gorodnov et al. (1992) |
| Polydimethyl diallyl ammonium chloride, biopolymers | Mamleev et al. (1997) |
| Exopolysaccharide produced by Acinetobacter | Starukhina et al. (1991) |
| Xanthan | Han et al. (1999) and Nashawi (1991) |
| Wellan | Hoskin et al. (1991) |
Low-tension Polymer Flood Technique
This technique consists of combining low levels of polymer-compatible surfactants and a polymer with a waterflood. This affects mobility control and reduces front-end and total costs (Kalpakci et al., 1993). The synergy of surfactant polymer complex formation has been studied by gel permeation chromatography (Austad et al., 1993).
Influence of Viscosity on Ionic Strength
The viscosity and non-Newtonian characteristics of polymer solutions decrease significantly in the presence of inorganic salts, alkali silicates, and multivalent cations. The effect is due to the decrease in dissociation of polyelectrolytes, to the formation of a badly dissociating polyelectrolyte metal complex, and to the separation of such a complex from the polymer solution (Lakatos and Lakatosne, 1991).
Modified Acrylics
A hydrophobically associating modified AAm polymer remarkably improved salt resistance and temperature resistance properties, compared with high molecular weight polyacrylamide (PAM) (Niu et al., 2001).
Biopolymers
Pseudozan
Pseudozan is an exopolysaccharide produced by a Pseudomonas species. It has high viscosity at low concentrations in formation brines, forms stable solutions over a wide pH range, and is relatively stable at temperatures up to 65°C. The polymer is not shear-degradable, and has a pseudoplastic behavior. The polymer has been proposed for enhanced oil recovery processes for mobility control (Lazar et al., 1993).
Xanthan
Xanthan interacts with anionic surfactants, which is a beneficial synergistic effect for mobility control in chemical-enhanced oil recovery processes (Liu and Zhang, 1995).
Combination Flooding
Combination flooding combines at least two of the basic techniques of gas flooding, caustic flooding, surfactant flooding, polymer flooding, or foam flooding. There may be synergy between the various chemical reagents used. There are specific terms that clarify the individual combination of the basic methods, such as surfactant-enhanced alkaline flooding, alkaline-assisted thermal oil recovery, and others. Methods for combined flooding are summarized in Table 16.4.
Low-tension Polymer Flood
Coinjecting a surfactant and a biopolymer, followed by a polymer buffer for mobility control, leads to reduced chemical consumption and high oil recovery. There may be synergistic effects between the surfactant and the polymer in a dynamic flood situation. The chromatographic separation of surfactant and polymer is important for obtaining good oil recovery and low surfactant retention (Taugbol et al., 1994).
In buffered, surfactant-enhanced, alkaline flooding, it was found that the minimum in IFT and the region of spontaneous emulsification correspond to a particular pH range. Hence, buffering the aqueous pH against changes in alkali concentration allows a low IFT to be maintained when the amount of alkali decreases because of acids, rock consumption, and dispersion (Wason, 1990).
Effect of Alkaline Agents on the Retention
The effectiveness of any alkaline additives tends to increase with increasing pH. However, for most reservoirs, the reaction of these additives with minerals is a serious problem for strong alkalis, and a flood needs to be operated at the lowest effective pH, approximately 10. The ideal process by which alkaline agents reduce losses of surfactants and polymers in oil recovery by chemical injection has been detailed in the literature (Lorenz, 1991).
Alkaline Steamflooding
The performance of steamflooding often suffers from channeling and gravity segregation. Alkaline additives may be used with steam for certain types of crude oils to improve the steamflood performance. Experimental results show that sodium orthosilicate outperforms sodium hydroxide and sodium metasilicate (Mohanty and Khataniar, 1995).
Sediment-forming Materials
Aluminum trichloride and trisodium phosphate can be injected as sediment-forming material (Gorodilov et al., 1997).
Water-alternating Gas Technology
The oil production from thin under-gas cap zones with an active aquifer is not efficient because of the rapid breakthrough of gas or water. The water-alternating gas technology, based on the injection of a water solution with oil-and water-soluble polymers seems to be promising for the stimulation of such wells. For heavy oils, this technology can be considered as an alternative to thermal EOR (Stepanova et al., 1997).
Hydrocarbon-assisted Steam Injection
In steam injection, the mobility of the hydrocarbons is greater if a C1–C25 hydrocarbon is added than if steam is used alone, under substantially similar formation conditions (Frolov et al., 1998; Nasr and Isaacs, 2001a and Nasr and Isaacs, 2001b).
Foam Flooding
Earlier reviews on the state of the art of foam flooding can be found in the literature (Schramm, 2000).
Basic Principles of Foam Flooding
Injection of a foam with oil-imbibing and transporting properties enhances the recovery of oil from a subterranean formation. The foam is selected either by determination of the lamella number or by micro visualization techniques. A suitable surfactant is selected by the following steps (Wang, 1999):
1. Determining the surface tension of the foaming solution,
2. Measuring the radius of a foam lamella plateau border where it initially contacts the oil or of an emulsified drop,
3. Determining the IFT between the foaming solution and the oil, and
4. Correlating these measurements with a mathematical model to obtain a value indicative of the oil-imbibing properties of the foam.
The foam, having a viscosity greater than the displacing medium, will preferentially accumulate in the well-swept and higher permeability zones of the formation. The displacing medium is thus forced to move into the unswept or underswept areas of the formation. It is from these latter areas that the additional oil is recovered. However, when a foam is used to fill a low oil-content area of the reservoir, the oil contained therein is, for all practical purposes, lost. This is because the foam diverts the displacement fluid from such areas (Schramm et al., 1991a, Schramm et al., 1991b and Schramm et al., 1991c).
Foam stability in the presence of oil can be described by thermodynamics in terms of the spreading and entering coefficients S and E, respectively. These coefficients are defined as follows:
(16.1)
(16.2)
However, experimental results have not borne out these predictions. The theory was developed on the basis that the oil droplets are readily imbibed into the foam lamellae, but experimental results show that some foams, particularly those of type A, do not readily imbibe oil.
Therefore, there exists a need to distinguish between foams that are stable to oil but do not significantly imbibe oil, as in type A, foams that are stable to oil and do imbibe oil as in the second type above and finally, foams that are unstable to oil as in the third predicted type (Wang, 1999).
A foam drive method comprises the following steps (Wang, 1999):
1. Injecting into the reservoir an aqueous polymer solution as preceding slug;
2. Periodically injecting simultaneously or alternately a non-condensable gas and a foaming composition solution containing alkalis, surfactants, and polymers to form combined foam or periodically injecting the gas and the foam previously formed from the solution; and
3. Injecting a polymer solution as a protecting slug and then continuing with waterflooding.
Ambient Pressure Foam Tests
Several surfactants were studied by using ambient pressure foam tests, including alcohol ethoxylates, alcohol ethoxy sulfates, alcohol ethoxyethylsulfonates, and alcohol ethoxyglycerylsulfonates (Borchardt et al., 1987). The surfactants that performed well in the 1 atm foaming experiment were also good foaming agents in the site cell and core flood experiments that were performed in the presence of CO2 and reservoir fluids under realistic reservoir temperature and pressure conditions.
Laboratory studies of foam flow in porous media suggest that the relative foam mobility is approximately inversely proportional to the permeability. This means that foam has potential as a flow-diverting agent, and could potentially sweep low-permeability regions as effectively as high-permeability regions (Goodyear and Jones, 1995).
Sand Pack Model
A one-dimensional sand pack model has been used to investigate the behavior of four anionic sulfonate surfactants of varying chemical structure in the presence of steam. The study was performed with a crude oil at a residual oil saturation of approximately 12% of the pore volume. The observed pressure drop across various sections of the pack was used to study the behavior of the surfactant. The tested surfactants varied in chain length, aromatic structure, and number of ionic charges.
A linear toluene sulfonate produced the strongest foam in the presence of oil at residual saturations, in comparison with α-olefin sulfonates. This contrasts with the behavior of the surfactants in the absence of oil, where the α-olefin sulfonates perform better. The reason for this change is the relative propagation rate of the foams produced by the surfactants (Razzaq and Castanier, 1992).
Foaming Agents
When an oil reservoir is subjected to steam injection, the steam tends to move up in the formation, whereas condensate and oil tend to move down due to the density difference between the fluids. Gradually, a steam override condition develops, in which the injected steam sweeps the upper portion of the formation but leaves the lower portion untouched. Injected steam will tend to follow the path of least resistance from the injection well to a production well (Osterloh, 1994).
Thus, areas of high permeability will receive more and more of the injected steam, which in turn raises the permeability of such areas. This phenomenon exists to an even larger degree with low injection rates and thick formations, and the problem worsens at greater radial distances from the injection well, because the steam flux decreases with increasing steam zone radius.
Although residual oil saturation in the steam-swept region can be as low as 10%, the average residual oil saturation in the formation remains much higher due to poor vertical conformance. This it is because of the creation of steam override zones.
A similar conformance problem exists with carbon dioxide flooding. CO2 has a large tendency to channel through oil, since its viscosity may be 10–50 times lower than that of the oil. This channeling problem is exacerbated by the inherent tendency of a highly mobile fluid such as carbon dioxide to preferentially flow through more permeable rock sections.
These two factors, namely unfavorable mobility ratios between carbon dioxide and the oil in place and the tendency of carbon dioxide to take advantage of permeability variations, often make carbon dioxide flooding uneconomical. Conformance problems increase as the miscibility of the carbon dioxide with the oil in place decreases.
Although not much attention has been devoted to carbon dioxide conformance, it has long been the intention of the oil industry to improve the conformance of a steamflood by reducing the permeability of the steam swept zone by various means. The injection of numerous chemicals such as foams, foaming solutions, gelling solutions, or plugging or precipitating solutions have all been tried.
Because of the danger of damaging the reservoir, it is considered important to have a non-permanent method of lowering the permeability in the steam override zones. For this reason, certain plugging agents are deemed unacceptable. In order to successfully divert steam and improve the vertical conformance, the injected chemical should be:
1. Stable at high steam temperatures (150–315°C),
2. Effective in reducing permeability in steam swept zones,
3. Non-damaging to the oil reservoir, and
4. Economical.
The literature is replete with references to various foaming agents that are employed to lower permeability in steam swept zones, the vast majority of which require the injection of a non-condensable gas to generate the foam in conjunction with the injection of steam and the foaming agent (Osterloh, 1994).
C12 to C15 alcohols and α-olefin sulfonate are highly effective when used with steam or carbon dioxide foaming agents in reducing the permeability of flood-swept zones (Osterloh, 1994). The sodium salt of tall oil acid is suitable as a foam surfactant. Experimental results show that sodium tallates are effective foaming agents that can produce pressure gradients of hundreds of pounds per square inch per foot in a sand pack (Osterloh and Jante, 1995).
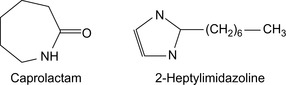
The foam-holding characteristics of foam from surfactants in oil field jobs can be tailored by adding an imidazoline-based amphoacetate surfactant, which are a special class of amphoteric tensides (Figure 16.3). Imidazoles, such as 2-heptylimidazoline, c.f., Figure 16.4, are reacted with fatty acids by ring opening. For alkylation, the imidazoline is reacted with, for example, chloroacetate (Dino and Homack, 1997). Residues from the production of caprolactam have been proposed as surfactants (Tulbovich et al., 1996).
Fluorocarbon Surfactant
A foam can be generated by using an inert gas and a fluorocarbon surfactant solution in admixture with an amphoteric or anionic hydrocarbon surfactant solution. A relatively small amount of the fluorocarbon surfactant is needed when mixed with the hydrocarbon surfactant and foamed. The foam has a better stability than a foam made with hydrocarbon surfactant alone when in contact with oil (Rendall et al., 1991).
Polymer-enhanced Foams
Polymer concentration, the chemical nature of the surfactants and their concentration, aqueous phase salinity and pH, and shear rate affect foam performance (Zhu et al., 1998). The performance of polymer-enhanced foams was shown to be much better than conventional foams. PAM polymers were used as an additive.
Higher foam resistance and longer persistence were achieved by using relatively low concentrations of polymers. Studies also showed that the foam performance was significantly improved over a broad range of polymer concentrations. Foams are severely affected by the presence of oil, but polymer-enhanced foam reduced the negative impact of oils on foam mobility. Polymer-enhanced foams are suitable for plugging fracture reservoirs (Sydansk, 1992).
Carbon Dioxide Flooding
In the 1990s certain research groups focused on the development of CO2-soluble polymers that could be used as direct thickeners, in particular, ionomers (Kovarik and Heller, 1990).
Sandstone rock surfaces are normally highly water-wet, but can be altered by treatment with solutions of chemical surfactants or by asphaltenes. Increasing the pH of the treating solution decreases the water wettability of the sandstone surface and, in some cases, makes the surface medium oil-wet (Smith and Comberiati, 1990). Thus, the chemical treatment of sandstone cores can increase the oil production when flooded with carbon dioxide.
A cosolvent used as a miscible additive for CO2 changed the properties of the supercritical gas phase resulting in increased viscosity and density of the gas mixture and enhanced extraction of the oil compounds into the CO2-rich phase. Gas phase properties were measured in an equilibrium cell with a capillary viscometer and a high-pressure densitometer. Cosolvent miscibility with CO2, brine solubility, cosolvent volatility, and relative quantity of the cosolvent partitioning into the oil phase must all be considered for the successful application of cosolvents. The results indicate that additives with low molecular weight, such as propane, are the most effective cosolvents for increasing oil recovery (Raible, 1992).
By adding common solvents as chemical modifiers, the flooding fluid shows a marked improvement in solvent ability for heavy components of crudes because of its increased density and polarity (Hwang and Ortiz, 1999). Miscible or immiscible carbon dioxide injection is considered to be one of the most effective technologies for improving oil recovery from complicated formations and hard-to-recover oil reserves. Application of this technology can increase the ultimate oil recovery by 10–15%, and it can be applied in a wide range of geological conditions, for producing both light and heavy oils.
The main factors that restrict its application are the dependence on natural CO2 sources, transportation of CO2, safety and environmental problems, breakthrough of CO2 to the production wells, and corrosion of well and field equipment.
A technology for in situ CO2 generation has been developed and described. It is based on an exothermic chemical reaction between a gas-forming water solution and active acids at low concentrations (Dzhafarov et al., 1999).
Hydrocarbons and other fluids are recovered at a production well by mixing CO2 and 0.1–20% trichloroethane at a temperature and pressure above the bubble point of the mixture, which ensures that the mixture will be in a single phase (Hsu, 1992).
Steamflooding
Carbon Dioxide
When the temperature of a carbonate reservoir that is saturated with high-viscosity oil and water increases to 200°C or more, chemical reactions occur in the formation, resulting in the formation of considerable amounts of CO2. This results from the dealkylation of aromatic hydrocarbons in the presence of water vapor, catalytic conversion of hydrocarbons by water vapor, and oxidation of organic materials.
Clay material and metals of variable valence, such as nickel, cobalt, or iron in the carbonate rock can serve as a catalyst. There is optimal amount of CO2 for which maximal oil recovery is achieved (Ruzin et al., 1990). The performance of a steamflooding process can be improved by the addition of CO2 or methane (Metwally, 1990).
Air Injection
Air used as a steam additive results in an increased rate of oil recovery because of low temperature oxidation reactions (Ivory et al., 1989).
Chemical Reactions
The reactivity of steam can be reduced via pH control. The injection or addition of a buffer, such as ammonium chloride, inhibits the dissolution of certain mineral groups, controls the migration of fines, inhibits the swelling of clays, controls chemical reactions in which new clay minerals are formed, and helps to prevent the precipitation of asphaltenes and the formation of emulsions (Wyganowski, 1991).
The reaction of sulfate with sulfide is strongly pH dependent, and the oxidation potential of sulfate at neutral pH is very low. At atmospheric pressure and temperatures up to the boiling points of the inorganic and organic media, no reaction takes place within 100 h. However, the reaction may proceed very slowly over geochemical time periods.
Large amounts of H2S are produced, together with CO2 and small amounts of elemental hydrogen in the steamflooding process. In the producing zones, the temperatures lie in the range of 250–270°C, which is significantly below the conditions described in the literature. H2S production rises from 50 ppm to up to 300,000 ppm, causing enormous corrosion and health and safety risks (Hoffmann and Steinfatt, 1993). Addition of 2–5% urea with respect to water is claimed to reduce the viscosity of the heavy hydrocarbons by at least 50% (Campos and Hernandez, 1993).
In Situ Combustion
A significant increase in light oil production can be achieved by air injection. A total consumption of 5–10% of the remaining oil is expected to maintain a propagation of the in situ oxidation process. The flue gas and steam generated at the combustion front strip, swell, and heat the contacted oil. The light oil is displaced at near-miscible conditions with complete utilization of the injected oxygen (Surguchev et al., 1999).
Special Techniques
Viscous Oil Recovery
Special techniques, particularly thermal methods, have been developed for the recovery of viscous oils.
Low Temperature Oxidation
Cap Gas
Both crude and asphaltene-free oil were used to determine the consequences of low temperature oxidation. It was found that the oxygen contained in an artificial gas cap was completely consumed by chemical reactions, i.e., oxidation, condensation, and water formation, before the asphaltene content had reached equilibrium.
The application of a pillow (cap) gas containing air and oxygen for improving the gravitational segregation in offshore production technology may offer an appropriate alternative for increasing the recovery factor in heavy oil–bearing reservoirs (Lakatos et al., 1997).
Special Surfactant Formulations
An alkaline PAM solution in liquid hydrocarbons has been suggested for EOR (Almaev et al., 1996). Special surfactant formulations have been tried to recover heavy crude oils. Ternary surfactant formulations, so called mixed-surfactant-enhanced alkaline systems, were successful in reversing the trend of increasing IFT with time that is typical in additive-free alkaline crude oil systems.
At higher temperatures (65°C), these ternary surfactant formulations were capable of generating very low IFT values against the crude oil, which suggests that they could be suitable candidates for commercial heavy oil recovery processes (Chiwetelu et al., 1994).
Visbreaking
In situ visbreaking with steam and a catalyst can produce crude oils with reduced viscosity (Higuerey et al., 2001). A special variety of visbreaking that involves partial steam reforming, which produces smaller hydrocarbon components and additional hydrogen free radicals and carbon dioxide, has been described.
Low-permeability Flooding
Oil recovery from diatomaceous formations is usually quite limited because a significant portion of oil saturation may be bypassed using conventional production techniques such as primary, waterflooding, cyclic, or drive steaming. Significant improvement of oil recovery would require that a method of displacing oil from the interior of the diatoms into the flow channels between the diatoms be provided (Burcham et al., 1995; Northrop, 1995).
It would also be necessary to improve the permeability in the natural flow channels so that the oil can be recovered. A combination of chemical additives is used to increase the water wetness of a rock and so increase the capillary pressure that forces oil and water from the diatomaceous formation. The additives used include wetting agents such as mono-, di-, and tri-basic forms of sodium or potassium phosphate and sodium silicate.
Surfactants, including sulfonates, ammonium salts of linear alcohol, ethoxy sulfates, or calcium phenol ethoxylated alkyl sulfonates are also added to lower the IFT between oil and water, and allow oil to flow more freely through the diatomaceous matrix. Imbibition experiments with up to 3% of an active surfactant concentration indicate a 31% improvement in oil recovery over that obtainable with brine alone (Burcham et al., 1995; Northrop, 1995).
Injecting a solvent (Davis, 1992), for example, jet fuel, petroleum naphtha, aromatic hydrocarbons, or naphthenic hydrocarbons, before injecting the surfactant solution has also been proposed.
Microbial-Enhanced Oil Recovery Techniques
Microbiologists initially laid the foundations for microbial-enhanced oil recovery (MEOR), which increased after the petroleum crisis in 1973 (Lazar, 1993; Momeni et al., 1990; Zekri, 2001).
MEOR was first proposed in 1926 when Vadie (2002), Zobell (1937) and Zobell and Johnson (1979) laid down the foundations of the technique between 1943 and 1953. The results were then largely dismissed in the United States because there was little interest in finding methods to enhance the recovery of oil.
However, in some European countries, the interest for MEOR increased and several field trials were conducted. The first MEOR waterflood field project in the United States was initiated in 1986 in the Mink Unit of Delaware-Childers Field in Nowata County, Oklahoma (Bryant et al., 1991).
Basic Principles and Methods
The injection of microbes into the formation is a common MEOR technique. This should stimulate the in situ microflora, resulting in the production of certain compounds that increase the oil recovery of exhausted reservoirs. The following basic effects can be achieved by microbes (Vadie, 2002):
• In situ production of gels for selective water shutoff;
• In situ production of biosurfactants for surfactant flooding,
• In situ production of acids for dissolving carbonate rocks;
• In situ production of CO2;
• In situ degradation of long chain molecules to reduce viscosity and paraffin content;
• Displacement of oil by metabolites of inoculated bacteria, grown in situ; and
• Huff and puff technique:
1. Huff: Migration of cells and synthesis of metabolic products following inoculation and closing of injection well;
2. Puff: Production and recovery of oil after incubation period.
It is often stressed that the technology is environmentally friendly. The stimulation of oil production by in situ bacterial fermentation is thought to be initialized by one or a combination of the following mechanisms:
1. Improvement of the relative mobility of oil to water by biosurfactants and biopolymers;
2. Partial repressurization of the reservoir by methane and CO2 gases;
4. Increase of reservoir permeability and widening of the fissures and channels through the etching of carbonaceous rocks in limestone reservoirs by organic acids produced by anaerobic bacteria;
5. Cleaning of the wellbore region by the acids and gas produced from in situ fermentation: the gas serves to push oil from dead space and dislodge debris that plugs the pores, the average pore size is increased, and, as a result, the capillary pressure near the wellbore is made more favorable for the flow of oil; and
6. Selective plugging of highly permeable zones by injecting slime-forming bacteria followed by sucrose solution that turns on the production of extracellular slimes.
Successful microbial MEOR requires (Sheehy, 1990):
1. The selection, injection, dispersion, metabolism, and persistence of organisms with properties that facilitate the release of residual oil and
2. The coinjection of growth-effective nutrients into the extreme environments that characterize petroleum reservoirs.
Economics
The most widely practiced technique for applying MEOR involves cyclic stimulation treatments of producing wells. Improvements in oil production can result from removal of paraffinic or asphaltic deposits from the near wellbore region, or from the mobilization of residual oil in the limited volume of the treated reservoir.
An alternative method involves applying microbes in an ongoing waterflood to improve oil recovery (Bryant et al., 1989). Microorganisms have been shown in the laboratory to produce chemicals such as surfactants, acids, solvents (alcohols and ketones), and gases, primarily CO2, all of which could be effective in mobilizing crude oil under reservoir conditions. Microbial growth and polymer production in porous media have been shown to improve the sweep efficiency by permeability modification. In general, cost-effective MEOR methods are best applied in shallow, sandstone reservoirs in mature producing fields.
The function of aerobic MEOR is based on the ability of oil-degrading bacteria to reduce the IFT between oil and water. The process involves pumping water that contains oxygen and mineral nutrients into the oil reservoir to stimulate the growth of aerobic, oil-degrading bacteria. Based on coreflood experiments, the amount of bacterial biomass responsible for dislodging the oil can be calculated. The process is limited by the amount of oxygen available to the bacteria to degrade the oil. The bacterial biomass is more efficient than synthetic surfactants in dislodging the oil (Sunde et al., 1992).
Experiments have shown that bacterial cells may penetrate a solid porous medium with at least 140 mD permeability, and that a bacterial population can become established in such a medium if suitable substrates are supplied. The suitability of an organism for enhanced oil recovery is governed by parameters such as its capacity to produce a surfactant or cosurfactant, cell morphology and relationship of bacterial size to pore size, and pore size distribution of the porous rock. The activity of the organism is directly affected by conditions in the reservoir, such as oxygen availability, temperature, pressure, and substrate availability (Bubela, 1983).
A physical model to predict the results of large-scale application for MEOR has been developed. This model simulates both the radial flow of fluids toward the wellbore and bacteria transport through porous media (Momeni et al., 1988).
Field studies of MEOR processes require routine monitoring to determine the effects that microorganisms exert in the release of oil from petroleum-bearing formations. Careful monitoring of oil production, flow rates, oil/water ratios, temperature, pH, viscosity, ionic strength, and other factors allows observation of the real changes that occur as a result of microbial activities after selected microbes are injected. Simple microbial counts can be used to determine the viability and transport of injected microbes. The effect of injected energy sources, such as molasses, on indigenous microbes inhabiting a reservoir can also be detected (Cruze and Hitzman, 1987).
An example process for recovering hydrocarbons from a subterranean, hydrocarbon-bearing formation consists of the following:
1. Introducing microbes into the formation, the microbes being effective to render at least a portion of the hydrocarbons in the formation more easily recoverable;
2. Passing electrical energy through at least a portion of the formation to increase the mobility of the microbes in the formation; and
3. Recovering hydrocarbons from the formation.
The specific microbes chosen depends on many factors, for example, the particular formation involved, the specific hydrocarbons in the formation, and the desired action of the microbes. They may be aerobic or anaerobic and may or may not require one or more additional nutrients, either naturally occurring or injected, to be included in the formation. Highly mobile microbes, such as flagellated or ciliated bacilli, are useful. The microbes are sized so that they are mobile in the connate water of the formation (Killough, 1987).
Bacillus licheniformis produces a water-insoluble levan that has a potential application as a selective plugging agent in MEOR. The microorganisms grow on sucrose, glucose, and fructose but produce levan only on sucrose. Thus plugging may be selectively controlled in the reservoir by substrate manipulation. Oil reservoirs having a temperature of less than 55°C, a pH between 6 and 9, a pressure less than 500 atm, and a salt concentration of 4% or less are potentially suitable (Ramsay et al., 1989) for this treatment.
A possible approach to MEOR consists of the additional aeration of the water injected into the formation, together with the addition of mineral salts of nitrogen and phosphorus. The result is the activation of the aerobic microorganisms and the oxidation of the residual oil. The metabolic products of the petroleum-oxidizing bacteria are CO2 and water-soluble organic compounds. These compounds enter the non-oxygenated zone of the formation and can act as oil recovery agents. The compounds also may serve as additional substrates for anaerobic bacteria, particularly for methanogens. The methane so formed can be easily recovered. It increases the mobility of the oil in place (Ivanov and Belyaev, 1989; Sorokin, 1989).
Potential Health Hazards of Bacteria
Practically all life forms may be infected by one or more kinds of microorganism, some of which confer a mutual advantage, such as in symbiosis, whereas some result in a disease of the host. The use of bacteria in MEOR operations necessitates a consideration of possible untoward effects against man and other living creatures. Because large numbers of bacteria are going to be placed into the ground and possibly come into direct contact with oil field workers who know little about them, it is necessary to closely examine possible hazards that may be associated with their use (Grula et al., 1989b).
Metabolism
MEOR methods mainly utilize the metabolites (biosurfactant, biopolymer, organic acid, and biogas) generated in situ or ex situ by bacteria to improve the mobility of the oil phase. In situ MEOR is mainly targeted toward the residual oil left after primary or secondary production, and its success depends strongly on the penetration and the stability of recovering agents. To contact the trapped oil with appropriate bacteria, the microbes must be transported from a wellbore to locations deep within the reservoir (Jang et al., 1989).
When microbial activity develops in a subsurface geologic environment, the geologic, mineralogic, hydrologic, and geochemical aspects of the environment will have a profound effect on the microorganisms and, in turn, the microbial population will have some effect on the rocks and fluids. The most significant geologic changes are (Bubela, 1989):
1. The precipitation of dissolved minerals, especially carbonates;
2. The change of permeability caused by precipitation in pore throats; and
3. A change of porosity, either an increase or decrease, depending on the equilibria of dissolved salts and products of organic acids.
Various bacterial species have proven useful in MEOR, depending on the biochemical materials produced by the the species, such as gases, surfactants, solvents, acids, swelling agents, and cosurfactants, which facilitate the displacement of oil. In field experiments, in situ fermentation is often desirable for producing a great quantity of gases. Clostridium hydrosulfuricum 39E was found to have surface active properties during simulated EOR experiments (Grula et al., 1989a; Yen et al., 1991).
Key mechanisms important for improved oil mobilization by microbial formulations have been identified, including wettability alteration, emulsification, oil solubilization, alteration in interfacial forces, lowering of the mobility ratio, and permeability modification. Aggregation of the bacteria at the oil-water-rock interface may produce locally high concentrations of metabolic chemicals that result in oil mobilization. A decrease in relative permeability to water and an increase in relative permeability to oil was usually observed in microbial-flooded cores, causing an apparent curve shift toward a more water-wet condition. Cores preflushed with sodium bicarbonate showed increased oil recovery efficiency (Chase et al., 1991).
Microorganisms inhabiting petroleum-bearing formations or that are introduced into subterranean environments are subject to extremes of redox potential, pH, salinity, temperature, pressure, ecologic pressure, geochemistry, and nutrient availability. Successful MEOR requires the selection, injection, dispersion, metabolism, and persistence of organisms that have the right properties to facilitate the release of residual oil (Sheehy, 1991).
Microbial Control of the Production of Sulfide
A microbial process was developed for controlling the production of hydrogen sulfide by sulfate-reducing bacteria, using mutant strains of Thiobacillus denitrificans. T. denitrificans oxidizes sulfide to sulfate, using oxygen or nitrate as the electron acceptor, but is inhibited by sulfide concentrations above 100–200μ. A mutant of T. denitrificans that is resistant to glutaraldehyde (40 mgl−1) and sulfide (1500μ) was obtained by repeated subculturing at increasing concentrations of the inhibitors.
This strain prevented the accumulation of sulfide by Desulfovibrio desulfuricans when both organisms were grown in a liquid medium, or in Berea sandstone cores, which the wild-type strain did not. The mutant also prevented the accumulation of sulfide by a mixed population of sulfate-reducing bacteria enriched from an oil field brine.
Fermentation balances showed that this strain stoichiometrically oxidized the biogenically produced sulfide to sulfate. The mutant grew at temperatures up to 40°C, in salinities up to 2%, and at pressures up to 120 atm (McInerney et al., 1991). C. hydrosulfuricum 39E was found to have surface active properties during simulated enhanced oil recovery experiments (Yen et al., 1991).
Bacillus licheniformis
The B. licheniformis JF-2 strain produces a very effective surfactant under conditions typical of oil reservoirs. The partially purified biosurfactant from JF-2 was shown to be the most active microbial surfactant found, and it gave an IFT against decane of 0.016mNm−1. An optimal production of the surfactant was obtained in cultures grown in the presence of 5% NaCl at a temperature of 45°C and pH of 7.
The major end-products of fermentation were lactic acid and acetic acid, with smaller amounts of formic acid and acetoin. The growth and biosurfactant formation were also observed in anaerobic cultures supplemented with a suitable electron acceptor, such as NaNO3 (Lin et al., 1991).
Microbial Ecology of Corrosion
The sulfur bacteria, which use sulfur compounds in their metabolism, are among the bacteria that can inhabit an oil reservoir. They produce hydrogen sulfide, which is responsible for extensive corrosion in the oil field, so the exclusion of these bacteria from MEOR is highly desirable. The net effect of souring a reservoir is a decrease in its economic value (Westlake, 1991).
B. licheniformis JF-2 and Clostridium acetogutylicum were investigated under simulated reservoir conditions. Sandstone cores were equilibrated to the desired conditions, saturated with oil and brine, and flooded to residual oil saturation. The waterflood brine was displaced with a nutrient solution. The MEOR efficiency was found to be directly related to the dissolved gas/oil ratio. The principal MEOR mechanism observed in this work was solution gas drive (Donaldson and Obeida, 1991).
Strict Anaerobic Bacteria
Several strict anaerobic bacteria belonging to different phylogenetic taxons were isolated from the Tatar and Siberian oil fields under different physicochemical conditions. All isolated strains are capable of producing oil-releasing compounds, such as: biopolymers, organic acids, or gases. Methanogenic bacteria were shown to produce polysaccharides. The polysaccharide of Methanococcoides euhalobius was partly purified and characterized. Some acetogenic strains capable of producing volatile fatty acids from CO2 and H2 were also isolated from stratal waters of the Tatar and Siberian oil fields (Belyaev et al., 1991).
Diverse populations of anaerobic, heterotrophic bacteria were present in highly saline brines collected from the Vassar Vertz Sand Unit, Payne County, Oklahoma. All strains grew in a mineral salts medium containing glucose, yeast extract, and casamino acids in the presence of NaCl concentrations of up to 20% (Bhupathiraju et al., 1991).
Shewanella putrefaciens
Many S. putrefaciens strains can reduce metal oxides, and Shewanella species that can utilize butane have been proposed for a method of bioremediation of petroleum contaminants. Biofilms of Shewanella species can sequester gases, in particular CO2, in underground geological formations and prevent the release into the atmosphere (Cunningham et al., 2006).
S. putrefaciens grows under denitrifying anaerobic conditions on crude oil as the sole carbon source. This organism can assist the release of oil from a substrate when grown on either lactate or peptone as a carbon source, as shown by in vitro experiments. Thus, this strain can be used to improve oil recovery (Keeler et al., 2010).
Thauera Strains
Particular strains of denitrifying bacteria belonging to the genera Azoarcus and Thauera have been shown to grow on oil and or oil constituents under anaerobic conditions, without the need for nutrient supplementation (Anders et al., 1995). Enzymes that catalyze the metabolism of simple aromatic compounds have been identified in these species.
Enzymes from Thauera aromatica metabolize toluene and all cresols, but no xylene isomers. Most of the aromatic compounds are converted to the central intermediate benzoyl-CoA via different metabolic pathways. These strains may be useful in the maintenance of oil pipelines as well as in EOR (Hendrickson et al., 2010).
Methanohalophilus
A methanogenic bacterium was isolated from oil reservoir brines by enrichment with trimethylamine. Methane production occurred only with trimethylamine compounds or methanol as substrates. Sodium ions, magnesium ions, and potassium ions were all required for growth. This organism was considered to be a member of the genus Methanohalophilus on the basis of its substrate utilization and general growth characteristics (Gevertz et al., 1991).
Sulfate-reducing Desulfovibrio
A sulfate-reducing bacterium was isolated by enrichment with a lactate-sulfate medium containing 3% NaCl. The isolate utilized lactate as an electron donor for sulfate reduction and contained desulfoviridin, typical of the genus Desulfovibrio (Gevertz et al., 1991).
Ultramicrobacteria
Selective plugging of high-permeability areas in a reservoir rock will increase the oil recovery during waterflooding. The injected water flows through the low-permeability, oil-bearing zones, pushing oil along its path. Many plugging agents that have been developed do not penetrate the reservoir deeply and often wash out when the injection pressure is reduced. Ultramicrobacteria, however, can penetrate deep into formations and grow exponentially with nutrient stimulation to preferentially plug high-permeability zones (Cusack et al., 1991).
The microorganisms reduce the nitrate and produce sulfuric acid, which eventually dissolves the rock formation, thus releasing oil. The microorganisms can be denitrifying thiobacilli, such as T. denitrificans (Sperl and Sperl, 1991).
Lactic Acid Bacteria
Particularly preferred bacteria are those of the genera Lactobacillus or Pediococcus. Lactic acid produced by these bacteria may be used for removal of carbonate or iron scale in oil field equipment (Coleman et al., 1992).
Scale Inhibitors as a Microbial Nutrient
Organic phosphates and phosphonates are known to be scale inhibitors. Substances in this class can also be nutrients for certain bacteria, so a phosphorous nutrient injection system can both prevent scales and act as a nutrient in certain cases (Jenneman and Clark, 1994a and Jenneman and Clark, 1994b).
Interfacial Properties
Interfacial Tension
The IFT plays an important role in the success of enhanced oil recovery methods, but additional complications arise when the components undergo a chemical reaction. The dynamic IFT behavior of reacting acidic oil-alkaline solutions has been studied for both an artificially acidified synthetic oil, and a real crude oil, at various concentrations, with either a drop volume or a spinning drop tensiometer (Ball, 1995; Ball et al., 1996).
The spinning drop technique measures the shape of an oil drop in the flooding solution in a capillary tube. An automatic measuring system has been developed that combines a video-image analysis, an automatic recording system, and a computer for calculation of the IFT (Yamazaki et al., 2000).
Interfacial Rheologic Properties
The interfacial rheology is very sensitive to the chemical composition of immiscible formation liquids (Lakatos and Lakatos-Szabò, 2001), hence an understanding of these factors may contribute significantly to an extension reservoir characterization, a better understanding of displacement mechanisms, development of more profitable enhanced and improved oil recovery methods, intensification of the surface technologies, optimization of the pipeline transportation, and improvement of refinery operations (Lakatos-Szabò et al., 1997).
Interfacial rheologic properties of different crude oil water systems were determined over wide ranges of temperature and shear rate, and in the presence of inorganic electrolytes, surfactants, alkaline materials, and polymers (Lakatos-Szabò et al., 1997).
Caustic Waterflooding
In caustic waterflooding, the interfacial rheological properties of a model crude oil-water system were studied in the presence of sodium hydroxide. The interfacial viscosity, non-Newtonian flow behavior, and activation energy of viscous flow were determined as a function of shear rate, alkali concentration, and aging time.
The interfacial viscosity drastically decreases in the presence of sodium hydroxide by up to three or four orders of magnitude. Sodium hydroxide also effectively suppresses the non-Newtonian flow behavior of the interfacial layer (Lakatos-Szabò and Lakatos, 1997).
Tracers
The addition of tracer chemicals to an injection fluid provides information about the permeability of a reservoir. Small amounts of a tracer are added to the injected fluid, and the distribution of the material at the production well is monitored with respect to time. Radioactive or nonradioactive tracers can be used.
Isotopically labeled tracers behave like the standard components in the fluid of interest; for example, tritium-labeled water behaves exactly like water. If less similar chemicals are used as tracers, their selective adsorption, chemical reaction, and liquid-liquid distribution must be considered. The tracer must be chosen so that the analytical method is sufficiently sensitive to detect it at the desired levels.
Tritiated or 14C-tagged hydrocarbons (including tritium gas) can be measured by a liquid scintillation counter or a gas proportional counter (Tang and Harker, 1992a and Tang and Harker, 1992b). Isotopic tracers are not exclusively radioactive; for instance, 13C is often used.
Application of Tracers
Sensitive analytical procedures enable the detection and measurement of very low levels of tracer. In studies, an identifiable tracer material is injected through one or more injection wells into the reservoir being studied. Water is then injected to push the tracer to one or more recovery wells in the reservoir. The output of the recovery wells is then monitored to determine the tracer breakthrough and flow through the recovery wells. Analysis of breakthrough times and flows yields important information regarding how to perform the secondary or enhanced recovery processes. A sharp breakthrough of tracers in a two-well tracer test is achieved by the following method (Stegemeier and Perry, 1992):
1. A solution of a water-soluble tracer and a partitioning tracer that distributes between the formation oil and water into the formation is injected through a temporary injection well
2. This is stopped after a slug of the tracer solution has been injected,
3. Formation fluids are produced from the production well,
4. Monitoring the concentration of each tracer and the volumes of fluids produced from the producing wellborehole, and
5. Determining the formation of residual oil saturation from the chromatographic separation of the water-soluble tracer and the partitionable tracer.
Retention of the Tracer
To improve evaluation techniques in a water and gas pilot, tracers were injected in the gas phase at the beginning of the first two-gas injection periods. Perfluoromethylcyclopentane and perfluoromethylcyclohexane were used. In laboratory studies, these compounds were shown to have a higher partitioning to the oil phase than did tritiated methane. This caused a minor retention of the tracer (Dugstad et al., 1992; Ljosland et al., 1993).
Radioactive Tracers
Seven water tracers, namely tritiated water (HTO) and the ions S14CN−, 36Cl−, 131I−, 35SO42−, H14CO3−, and 22Na+ were tested for use in carbonate reservoirs. HTO is the ideal water tracer, although S14CN− and 36Cl− were found to be near-ideal tracers for water flow in chalk. 131I− and 35SO42− show a more complicated behavior because of ion exclusion, adsorption, desorption, and chemical reactions (Bjornstad et al., 1994).
Nonradioactive Tracers
Analysis of halohydrocarbons, halocarbons, and sulfur hexafluoride is usually performed by gas chromatography with an electron capture detector. Complex metal anions, such as cobalt hexacyanide, are used as nonradioactive tracers in reservoir studies. The cobalt in the tracer compound must be in the complex anion portion of the molecule, because cationic cobalt tends to react with materials in the reservoir, leading to inaccurate analytic information (Miller et al., 1993).
In most production reservoirs, the brines produced are injected into the formation to maintain reservoir pressure and avoid subsidence and environmental pollution (Hutchins and Saunders, 1993), and, in the case of geothermal fields, to recharge the formation. These brines can, however, adversely affect the fluids produced from the reservoir. For example, in geothermal fields, the injected brine can lower the temperature of the produced fluids by mixing with the hotter formation fluids. In order to mitigate this problem, the subsurface paths of the injected fluids must be known.
Tracers have been used to label fluids in order to track their movement and monitor chemical changes in the injected fluid. Radioactive materials are one class of commonly used tracers, but they have several drawbacks.
They require special handling because of the danger posed to personnel and the environment. They also alter the natural isotope ratio that is indigenous to the reservoir. In addition, the half-life of radioactive tracers tends to be either too long or too short for practical use.
A number of organic compounds are suitable for use as tracers in a process for monitoring the flow of subterranean fluids. The following have been proposed: benzene tetracarboxylic acid, methylbenzoic acid, naphthalene sulfonic acid, naphthalene disulfonic acid, naphthalene trisulfonic acid, alkyl benzene sulfonic acid, alkyl toluene sulfonic acid, alkyl xylene sulfonic acid, α-olefin sulfonic acid, salts of the foregoing acids, naphthalenediol, aniline, substituted aniline, pyridine, and substituted pyridines (Hutchins and Saunders, 1993).
Thermal Stability of Alkyl Benzene Sulfonate
The thermal degradation of alkyl benzene sulfonates in alkaline media is important because of its potential application at elevated temperatures. The half-lives, with respect to thermal degradation, of several commercially available sulfonates have been estimated at hundreds to thousands of years at 204°C. The degradation mechanism is predominately a clipping of the alkyl chain to yield an alkyl benzene sulfonate with the phenyl group attached to the α-carbon; however, desulfonation also occurred (Shupe and Baugh, 1991).
Asphaltene Deposition
Asphaltenes are components of crude oils that contain numerous individual compounds, particularly high molecular weight condensed aromatic components including heteroatoms. Because of the complexity of their chemistry, asphaltenes are summarized as the oil fraction that is soluble in benzene, but not in n-pentane. In crude oil, asphaltenes are normally present as a colloidal dispersion stabilized by oleoresins. During the production, refining, transportation, and storage of crude oil, asphaltenes may precipitate.
Any precipitation caused by a temperature drop, or composition change in the porous media near the wellbore will reduce the permeability. Asphaltenes may also precipitate during flow through porous media, which may in particular, be stimulated by CO2 flooding during the production process.
Partial esters of phosphoric acid with carboxylic acids are dispersants for asphaltenes (Miller et al., 1999), or the injection of organic aromatic solvents, and soaking is a feasible method for removing the precipitates (Ju et al., 2001). Alternatively, the precipitation of asphalt can be reduced by adding an N,N-dialkylamide of a fatty acid (Romocki, 1994 and Romocki, 1995). When asphaltenes are precipitated out, they can be removed from the walls of a well or a pipeline by washing with a hydrocarbon solvent. It has been shown that isopropyl benzoate is exceptionally useful as a solvent for asphaltene removal (Scovell et al., 2001).
Stabilizer Dispersant
The addition of hydrogenated castor oil to a copolymer of AAm and sodium acrylate formulation will suspend the copolymer and retard the settling process (Sommese and Nagarajan, 1995).
Reservoir Properties
Reservoir Models
There are several simulators for modeling the processes in EOR, which are indicated in Table 16.5.
| Simulator | References |
|---|---|
| Second-order Godunov-type finite difference for 2-dimensional, 3-component incompressible polymer floods | Holing et al. (1990) |
| Alkaline/surfactant/polymer compositional reservoir simulator, 3-dimensional compositional reservoir simulator, for alkaline chemical flooding processes | Bhuyan (1989) |
| New flux correcting (NFC) | Song (1992) |
| PC-GEL 3-dimensional, 3-phase (oil, water, and gas) permeability modification simulator | Chang and Gao (1993) |
| Fully implicit total variation diminishing high-order algorithm for compositional simulation and chemical flooding simulator | Liu et al. (1995) |
| Compositional chemical flooding simulator (UTCHEM) | Dakhlia (1995) and Rame and Delshad (1995) |
| Front-tracking model for in situ combustion oil recovery | Rocha et al. (1997) |
| Caustic waterflooding | Bhuyan et al. (1991), Islam and Chakma (1991) and Saneie and Yortsos (1993) |
Profile Control
Profile control occurs by artificially changing the permeability as is done in water shutoff. For more information, see Chapter 18.
AAm Polymers
The permeability of a high-permeability zone in a high-temperature oil can be reduced by placing an aqueous solution of a gellable polymeric and gelling this solution in situ (Lockhart and Burrafato, 1990b and Lockhart and Burrafato, 1990c). The gel is made of a copolymer or a mixture of a synthetic polymer and a biopolymer (e.g., xanthan gum) (Lockhart and Burrafato, 1990a). Crosslinking agents are trivalent chromium ions or aldehydes (Sydansk, 1995). The pH is adjusted to 1.5–5.5, depending on the desired gelling time.
Anti-syneresis properties can be obtained with various organic acids and their alkali metal or ammonium salts. A delay in gelation also occurs when malonic acid is used (Albonico and Lockhart, 1993). AAms also can be crosslinked in situ by o-hydroxyphenylmethanol (Moradi-Araghi and Stahl, 1991b) or furfuryl alcohol and formaldehyde (Moradi-Araghi and Stahl, 1991a).
Melamine and Phenol-formaldehyde Resins
Melamine resins (Shu, 1990) and phenol-formaldehyde resins (Shu and Shu, 1991) can be gelled in situ to reduce the permeability of a formation. Various classes of polymers can be gelled by similar principles (Hutchins and Dovan, 1992).
Latex
Latex particles may flocculate when injected in to a reservoir at high formation temperatures. When the particles flocculate, shrink, and harden, they form a more effective blocking agent than the dispersed, expanded, and softer particles (Snowden et al., 1993).
In Situ Carbonate Precipitation
Carbon dioxide flooding is one of the most promising enhanced oil recovery method. To overcome the tendency of CO2 to bypass the smaller pores containing residual oil, one approach is to plug the larger pores by chemical precipitation. Several, relatively inexpensive, water-soluble salts of alkaline earth metals react with CO2 to form a precipitate.
Laboratory experiments have indicated that carbonate precipitation can alter the permeability of the core samples under reservoir conditions. The precipitation reduces the gas permeability in favor of the liquid permeability indicating that precipitation occurs preferentially in the larger pores.
Once the precipitate is formed, subsequent fluid flow will be diverted to smaller pores, thereby increasing the sweep efficiency. Additional experimental work with a series of connected cores suggested that the permeability profile can be modified successfully, but pH control plays a critical role in the propagation of the chemical precipitation reaction (Ameri et al., 1991).
In Situ Silica Cementation
The permeability profile of a formation where temperatures over 90°C are encountered can be modified by the following process: an aqueous solution of an alkali-metal hydroxide, ammonium hydroxide, or organo-ammonium hydroxide is injected into a zone of greater permeability in a formation. After this, a spacer volume of a water-miscible organic solvent is injected, followed by a water-miscible organic solvent containing an alkylpolysilicate in the greater permeability zone. A silica cement is formed in situ, which substantially closes off the higher permeability zone to fluid flow. Finally, a steamflooding, waterflooding, carbon dioxide stimulation, or fireflooding enhanced oil recovery operation is commenced in the lower permeability zone (Shu et al., 1993a and Shu et al., 1993b) .
Hydratable Clay
Hydratable clays may be used as plugging agents for profile control. The swelling of the clay is desirable, unlike when formation damage occurs (Zhou, 2000). First, an aqueous solution of the salts of cations such as K+, Ca2+, or Mg2+ that inhibit clay swelling is prepared.
The clay slurry is introduced into the formation, where it enters the channels of high permeability where it contacts the NaCl solution already present in natural or injected drive fluids. The inhibitive cations that are bound to the clay particles are replaced by Na+ ions, which attract water molecules and promote clay swelling. The Na+ clay swells up to 10 times its original volume, causing the slurry to acquire a gel-like consistency, which then blocks the flow of water. The gel can resist a differential pressure gradient of up to 500 kPam−1.
Silicate Gel
Silicate gel enhances the sweep efficiency of a waterflood, gasflood, or steamflood operation by reducing the permeability of the high-permeability zones. Weak acids may be added to control gel generation rate (Chou and Bae, 1994).
Formation Damage
The in situ release of fine particles in a porous medium resulting from changes in the colloidal character of the fines has been studied. Changes in the electrolytic condition of the permeating fluid induce damage. The results showed that high pH and low salinity cause the fines to be released.
This release causes a drastic decline in the permeability of the medium. These findings establish the interplay between salinity changes, cation exchange, and pH during a water shock, and elucidate the vital role of the ion exchange process in formation damage (Vaidya and Fogler, 1990).
Formation damage caused by clay migration may be observed when the injected brine replaces the connate water during operations such as waterflooding, chemical flooding including alkaline, and surfactant and polymer processes. These effects can be predicted by a physicochemical flow model based on cationic exchange reactions that occur when the salinity decreases (Souto et al., 1993). Other models have also been presented (Chang and Civan, 1997; Moore, 2001).
The pH variation of the flowing fluid suggests that chemical reactions are occurring in the formation. A high pH promotes formation damage by particle deposition within porous media. The permeability reduction is minimized by using brines and high oil recoveries. Suspended solid particles are released and moved with the injection water when the salt concentration drops below critical levels, causing a reduction of permeability and eventually formation damage (Bagci et al., 2000).
Wettability
The wettability of the rock is responsible for the behavior of a reservoir subjected to any oil recovery process. Because the chemical composition of a mineral surface is mostly responsible for its wetting behavior, the relationship between wettability and chemical composition of the surface is key information.
X-ray photoelectron spectroscopy is a suitable technique for examining surfaces, whereas a detailed interpretation of coreflooding experiments and wettability index measurements gives the wettability. The results of such studies show that the organic carbon content of the surface correlates well with the wetting behavior of the material characterized by petrophysical measurements (Quet et al., 1991 and Quet et al., 1992).
Flooding of Oil in Chalk
Chalk reservoirs encounter specific problems during secondary recovery of oil by waterflooding. Displacement experiments in several formations indicated that the shape of the leaching front depends not only on the nature of the fluids used, but also on the morphology of the formations. The following must be distinguished from each other:
• Voids filled with unrecoverable oil,
• Easily accessible pores, and
• Preferential paths.
Injecting water into oil-saturated chalk produces a regular front of leaching in some formations with a high percentage of preferential paths, especially when chalk contains a high proportion of rounded grains (Monjoie and Schroeder, 1997).
Treatment of Produced Water
The liquid produced from an oil well is a mixture of oil and water. After dehydration, most of the crude oil is separated and a water phase that consists of oily waste water is formed. This needs to be treated, and can then be injected into the stratum again.
The amount of produced water can be appreciable. Experiments with a crude oil from the Daqing oil field (China) with a water content of less than 0.5%, a density of 850 kgm−3 and a viscosity of 60.89 mPa, at 45°C, and a PHPA with a degree of hydrolysis of about 25–30% were performed (Deng et al., 2002). The surfactant was an alkyl benzene sulfonate. The surfactant can decrease the ζ-potential of an oil droplet greatly, especially when its concentration is less than 200 mg l−1 The surfactant and the polymer increase interfacial elasticity between aqueous phase and oil phase. This indicates that the coalescence of the oil droplets is hindered.
Soil Remediation
A chemically enhanced oil recovery technology can be used to remove oily contaminants from soil. Laboratory studies have demonstrated that a variety of alkaline surfactant combinations can be used with a polymer to reduce the residual oil saturation in waterflooding (Pitts et al., 1993).
Polyaromatic hydrocarbons absorb strongly to humus and other soil components, making them difficult to remove by thermal, physical, or chemical means, and unavailable for biodegradation. To desorb polyaromatic hydrocarbons from soil, surfactant flooding and soil-washing processes, or treatments to enhance the biodegradation of polyaromatic hydrocarbons have been considered.
Surfactant flooding may contaminate ground water, and soil washing requires excavation and biodegradation of polyaromatic hydrocarbons and is incomplete even with surfactants. Biodegradable surfactants that can form reasonably stable foams in the presence of up to 50% ethanol have been developed. These ethanol-based foams can readily desorb the polyaromatic hydrocarbons from gas plant soils and move well through soils at pressure gradients of 1.5 psift−1 or less (Kilbane et al., 1996 and Kilbane et al., 1997). A partially hydrolyzed copolymer of AAm and n-octylacrylamide together with sodium alkyl sulfates has also been described for in situ decontamination by flooding operations (Varadaraj, 1997).
Abasov, M.T.; Khismetov, T.V.; Strekov, A.S.; Mamalov, E.N.; Bernshtein, A.M., New EOR methods based on hydrogen peroxide and urea solutions application, In: Proceedings Volume, vol. 1, 7th Eapg Impr. Oil Recovery Europe SymposiumMoscow, Russia, October 27–29, 1993. (1993), pp. 272–276.
Abdulmazitov, R.G., Muslimov, R.K., Zakirov, A.F., Khajretdinov, F.M., 1997. Oil recovery from carbonate oil-bearing seam – involves cyclic injection of acid solutions of increasing concentrations after reduction of seam pressure. RU Patent 2 073 791, assigned to Tartar Oil Inst., February 20, 1997.
Albonico, P., Lockhart, T.P., 1993. Aqueous gellable composition containing an anti- syneresis agent. EP Patent 544 377, assigned to Eniricerche SPA and Agip SPA, June 02, 1993.
Aleev, F.I., 1996. Oil displacement from heterogeneous seam by pumping sulphuric acid and water to seam - until sulpho-acids appear in liquid removed from wells. RU Patent 2 055 166, assigned to Orenburgneft Assoc. Cent. Lab, February 27, 1996.
Aleev, F.I., Andreev, V.V., Ivanov, S.V., Kivilev, P.P., Talalaev, N.A., Khodyrev, V.A., 1996. Chernoshtanov, I.F., Oil deposit water flooding with improved efficiency – by pumping of sulphuric acid and water into seam prior to periodic pumping of water into seam through injection well and liquid removal from producing well. RU Patent 2 055 165, assigned to Orenburgneft Assoc. Cent. Lab, February 27, 1996.
Al-Khafaji, A.H., Implementations of enhanced oil recovery techniques in the Arab world are questioned? In: Proceedings Volume, 6th International Energy Found et al Mediter Petroleum ConferenceTripoli, Libya, November 23–25, 1999. (1999), pp. 124–133.
Almaev, R.K., Gabdrakhmanov, A.G., Kashapov, O.S., Bazekina, L.V., Kostilevskij, S.E., 1996. Extraction of viscous oil from oil strata – involves pumping-in aqueous alkalipolymericsolution containing fraction of liquid hydrocarbon(s) from oil processing and using polyacrylamide. RU Patent 2 068 084, assigned to Yuzharlanneft Oil Gas Conference, October 20, 1996.
Al-Sahhaf, T.; Ahmed, A.S.; Elkamel, A., Producing ultralow interfacial tension at the oil/water interface, Pet. Sci. Technol. 20 (7–8) (2002) 773–788.
Alvarado, V.; Manrique, E., Enhanced Oil Recovery: Field Planning and Development Strategies. (2010) Elsevier Gulf Professional, Amsterdam.
Amaya, J.; Rana, D.; Hornof, V., Dynamic interfacial tension behavior of water/oil systems containing in situ-formed surfactants, J. Solut. Chem. 31 (2) (2002) 139–148.
Ameri, S., Aminian, K., Wasson, J.A., Durham, D.L., 1991. Improved CO2 enhanced oil recovery – mobility control by in-situ chemical precipitation: Final report, US DOE Rep DOE/MC/22044-15, West Virginia Univ, June 1991.
Amin, R.; Smith, T.N., Measurement of interfacial tension and spreading coefficient under reservoir conditions: experimental investigation, Colloid Surf. A 137 (1–3) (1998) 35–43.
Anders, H.J.; Kaetzke, A.; Kampfer, P.; Ludwig, W.; Fuchs, G., Taxonomic position of aromatic-degrading denitrifying pseudomonad strains K 172 and KB 740 and their descriptionas new members of the genera thauera, as thauera aromatica sp. nov., and azoarcus, as azoarcus evansii sp. nov., respectively, members of the beta subclass of the proteobacteria, Int. J. Syst. Evol. Microbiol. 45 (1995) 327–333.
Ashrawi, S.S., 1992. Hot water, surfactant, and polymer flooding process for heavy oil. US Patent 5 083 612, assigned to Texaco Inc., January 28, 1992.
Austad, T.; Bjorkum, P.A.; Rolfsvag, T.A.; Oysaed, K.B., Adsorption: Pt.3: Nonequilibrium adsorption of surfactants onto reservoir cores from the North Sea. The effects of oil and clay minerals., J. Pet. Sci. Eng. 6 (2) (1991) 137–148.
Austad, T.; Ekrann, S.; Fjelde, I.; Taugbol, K., Chemical flooding of oil reservoirs: Pt 9: Dynamic adsorption of surfactant onto sandstone cores from injection water with and without polymer present, Colloids Surf. 127 (1–3) (1997) 69–82.
Austad, T.; Matre, B.; Milter, J.; Saevareid, A.; Oyno, L., Chemical flooding of oil reservoirs: Pt 8: Spontaneous oil expulsion from oil- and water-wet low permeable chalk material by imbibition of aqueous surfactant solutions, Colloids Surf. 137 (1–3) (1998) 117–129.
Austad, T.; Rorvik, O.; Rolfsvag, T.A.; Oysaed, K.B., Adsorption: Pt.4: An evaluation of polyethylene glycol as a sacrificial adsorbate towards ethoxylated sulfonates in chemical flooding, J. Pet. Sci. Eng. 4 (6) (1992) 265–276.
Austad, T.; Veggeland, K.; Fjelde, I.; Taugbol, K., Physicochemical principles of low tension polymer flood, In: Proceedings Volume, vol. 1, 7th Eapg Impr. Oil Recovery Europe SymposiumMoscow, Russia, October 27–29, 1993. (1993), pp. 208–219.
Bagci, S.; Kok, M.V.; Turksoy, U., Determination of formation damage in limestone reservoirs and its effect on production, J. Pet. Sci. Eng. 28 (1–2) (2000) 1–12.
Balakirov, Y.A., Chernorubashkin, A.I., Makeev, G.A., Korolev, I.P., Glushchenko, V.N., 1992. Composition for treatment of stratum head zone – contains oxyethylated isononyl phenol, aqueous solution of hydrofluoric acid and aqueous solution of hydrochloric acid. SU Patent 1 770 555, assigned to Ukrgoproniineft Oil Ind. and Assoc. Ukr. Bioorg Chem. & Petrol., October 23, 1992.
Ball, S.D., 1995. Comparison of transient interfacial tension behaviours of oil/alkaline systems as measured by the drop volume and spinning drop tensiometers, Ph.D. thesis, Ottawa Univ.
Ball, S.D.; Hornof, V.; Neale, G.H., Transient interfacial tension behavior between acidic oils and alkaline solutions, Chem. Eng. Commun. 147 (1996) 145–156.
Baviere, M.; Rouaud, T., Solubilization of hydrocarbons in micellar solutions, Influence of structure and molecular weight (solubilisation des hydrocarbures dans les solutions micellaires. Influence de la structure et de la masse moleculaire). Rev. Inst. Franc. Pet. 45 (5) (1990) 605–620.
Bayless, J.H., Hydrogen peroxide: A new thermal stimulation technique, World Oil 219 (5) (1998) 75–78.
Bayless, J.H., Hydrogen peroxide applications for the oil industry, World Oil 221 (5) (2000) 50–53.
Bedo, Z.; Lakatos, I.; Lakatos-Szabó, J., Interactions between an ethoxy-nonyl-phenol and a polyacrylamide in aqueous solutions, ACH-Models in Chemistry 134 (6) (1997) 735–752.
Belyaev, S.S.; Charakchian, I.A.; Kuznetsova, V.G., Strict anaerobic bacteria and their possible contribution to the enhancement of oil recovery, In: (Editor: Donaldson, E.C.) Microbial Enhancement of Oil Recovery: Recent Advances: Proceedings of the 1990 International Conference on Microbial Enhancement of Oil Recovery, vol. 31 of Developments in Petroleum Science (1991) Elsevier Science Ltd., pp. 163–172.
Berger, P.D.; Lee, C.H., Ultra-low concentration surfactants for sandstone and limestone floods, In: Proceedings Volume, SPE/DOE Thirteenth Improved Oil Recovery Symposium held in TulsaOklahoma, April 13–17, 2002. (2002).
Bhupathiraju, V.K.; Sharma, P.K.; McInerney, M.J.; Knapp, R.M.; Fowler, K.; Jenkins, W., Isolation and characterization of novel halophilic anaerobic bacteria from oil field brines, In: (Editor: Donaldson, E.C.) Microbial Enhancement of Oil Recovery: Recent 1990 International Conference on Microbial Enhancement of Oil Re Advances: Proceedings of the 1990 International Conference on Microbial Enhancement of Oil Recovery, vol. 31 of Developments in Petroleum Science (1991) Elsevier Science Ltd., pp. 131–143.
Bhuyan, D., 1989. Development of an alkaline/surfactant/polymer compositional reservoir simulator, Ph.D. thesis, Texas Univ, Austin.
Bhuyan, D.; Pope, G.A.; Lake, L.W., Simulation of high-pH coreflood experiments using a compositional chemical flood simulator, In: Proceedings Volume, SPE Oilfield Chemicals International SymposiumAnaheim, Calif, February 20–22, 1991. (1991), pp. 307–316.
Bjornstad, T.; Haugen, O.B.; Hundere, I.A., Dynamic behavior of radio-labelled water tracer candidates for chalk reservoirs, J. Pet. Sci. Eng. 10 (3) (1994) 223–238.
Borchardt, J.K.; Bright, D.B.; Dickson, M.K.; Wellington, S.L., Surfactants for carbon dioxide foam flooding: Effects of surfactant chemical structure on one-atmosphere foaming properties, In: Proceedings Volume, no. 373, 61st Annual ACS Colloid & Surface Sci SymposiumAnn Arbor, MI, June 21–24, 1987. (1987), pp. 163–180.
Borchardt, J.K.; Strycker, A.R., Olefin sulfonates for high temperature steam mobility control: Structure-property correlations, In: Proceedings Volume, SPE Oilfield Chemicals International SymposiumHouston, February 18–21, 1997. (1997), pp. 91–102.
Bragg, J.R., 1998. Oil recovery method using an emulsion. WO Patent 9 853 181, assigned to Exxon Production Res. Co., November 26, 1998.
Bragg, J.R., 1999. Oil recovery method using an emulsion. US Patent 5 855 243, assigned to Exxon Production Res. Co., January 05, 1999.
Bryant, R.S.; Burchfield, T.E., Review of microbial technology for improving oil recovery, In: Proceedings Volume, Nat. Inst. Petrol. Energy Res. Microbial Enhanced Oil Recovery Short CourseBartlesville, Okla, May 23, 1989. (1989).
Bryant, R.S.; Burchfield, T.E.; Porter, R.E.; Dennis, D.M.; Hitzman, D.O., Microbial enhanced waterflooding: A pilot study, In: (Editor: Donaldson, E.C.) Microbial Enhancement of Oil Recovery: Recent Advances: Proceedings of the 1990 International Conference on Microbial Enhancement of Oil Recovery, vol. 31 of Developments in Petroleum Science (1991) Elsevier Science Ltd., pp. 399–419.
Bubela, B., Geobiology and microbiologically enhanced oil recovery, In: (Editors: Donaldson, E.C.; Chilingarian, G.V.; Yen, T.F.) Microbial Enhanced Oil Recovery, vol. 22 of Developments in Petroleum Science (1989) Elsevier Science Ltd., pp. 75–97.
Bubela, B., 1983. In situ biological production of surfactants for enhanced oil recovery, Australia Dep Resources Energy End of Grant Rep 151, March 1983.
Burcham, C., Fast, R.E., Murer, A.S., Northrop, P.S., 1995. Oil recovery by enhanced imbibition in low permeability reservoirs. US Patent 5 411 086, assigned to Mobil Oil Corp., May 02, 1995.
Campbell, C.B., Sinquin, G.P., 2008. Alkylxylene sulfonates for enhanced oil recovery processes. US Patent 7 468 343, assigned to Chevron Oronite Company LLC, San Ramon, CA, December 23, 2008.
Campos, R.E., Hernandez, J.A., 1993. In-situ reduction of oil viscosity during steam injection process in eor. US Patent 5 209 295, assigned to Intevep, May 11, 1993.
Cavallaro, A.N.; Baigorria, R.; Curci, E., Design of an acid stimulation system with chlorine dioxide for the treatment of water-injection wells, In: Proceedings Volume, 51st Annual Cim Petroleum Society Technology MeetingCalgary, Canada, June 4–8, 2000. (2000).
Cavallaro, A.; Curci, E.; Galliano, G.; Vicente, M.; Crosta, D.; Leanza, H., Design of an acid stimulation system with chlorine dioxide for the treatment of water-injection wells, In: Proceedings Volume, 7th SPE Latin Amer. & Caribbean Petrol. Eng. ConferenceBuenos Aires, Argentina, March 25–28, 2001. (2001).
Chang, F.F.; Civan, F., Practical model for chemically induced formation damage, J. Pet. Sci. Eng. 17 (1–2) (1997) 123–137.
Chang, M.M., Gao, H.W., 1993. User's guide and documentation manual for “PCGel” simulator: Topical report, US DOE Fossil Energy Rep NIPER-705, NIPER, October 1993.
Chase, K.L.; Bryant, R.S.; Burchfield, T.E.; Bertus, K.M.; Stepp, A.K., Investigations of microbial mechanisms for oil mobilization in porous media, In: (Editor: Donaldson, E.C.) Microbial Enhancement of Oil Recovery: Recent Advances: Proceedings of the 1990 International Conference on Microbial Enhancement of Oil Recovery, vol. 31 of Developments in Petroleum Science (1991) Elsevier Science Ltd., pp. 79–94.
Chen, T.; Pu, W.; Ping, K.; Ye, Z., The rheological behavior of partially hydrolyzed polyacrylamide (HPAM) solutions in reservoir, J. Southwest Pet. Inst. 19 (3) (1997) 2A–3A; 28–34.
Chen, W.J.; Li, G.Z.; Mu, J.H.; Zhai, L.M.; Zhou, G.W., Effects of modified natural mixed carboxylates in ASP-flooding systems, J. Dispersion Sci. Technol. 24 (2) (2003) 197–202.
Chiwetelu, C.I.; Hornof, V.; Neale, G.H.; George, A.E., Use of mixed surfactants to improve the transient interfacial tension behaviour of heavy oil/alkaline systems, Can. J. Chem. Eng. 72 (3) (1994) 534–540.
Chou, S., Bae, J., 1994. Method for silica gel emplacement for enhanced oil recovery. US Patent 5 351 757, assigned to Chevron Res. & Technol. Co., October 04, 1994.
Chou, S., Campbell, C.B., 2001. Oil recovery method for waxy crude oil using alkylaryl sulfonate surfactants derived from alpha-olefins and the alpha-olefin compositions. US Patent 6 269 881, assigned to Chevron U.S.A. Inc., San Francisco, CA, Chevron Chemical Company LLC, San Francisco, CA, August 7, 2001.
Cobb, H.G., 2010. Composition and process for enhanced oil recovery. US Patent 7 691 790, assigned to Coriba Technologies, L.L.C., North Little Rock, AR, April 6, 2010.
Coleman, J.K., Brown, M.J., Moses, V., Burton, C.C., 1992. Enhanced oil recovery. WO Patent 9 215 771, September 17, 1992.
Cruze, J.A., Hitzman, D.O., 1987. Microbial field sampling techniques for MEOR (microbial enhanced oil recovery) processes, US DOE Fossilenergy Rep NIPER-351 CONF-870858, US DOE, September 1987.
Cunningham, A.B., Spangler, L.H., Gerlach, R., Phillips, A.J., 2006. Use of bacteria to prevent gas leakage. US Patent Application 20060216811, September 28, 2006.
Cusack, F.M.; Costerton, J.W.; Novosad, J., Ultramicrobacteria enhance oil recovery, In: Proceedings Volume, 4th Inst. Gas Technol. Gas, Oil, & Environ. Biotechnol. International SymposiumColorado Springs, CO, December 9–11, 1991. (1991), pp. 491–504.
Daharu, R.; Thomas, S.; Farouq, A.S.M., Micellar flooding for tertiary recovery: Recent advances and potential, In: Proceedings Volume, 10th SPE Trinidad & Tobago Sect. Tech. ConferencePort of Spain, Trinidad, June 26–28, 1991. (1991), pp. 414–428.
Dakhlia, H., 1995. A simulation study of polymer flooding and surfactant flooding using horizontal wells, Ph.D. thesis, Texas Univ, Austin.
Davis, B.W., 1992. In situ chemical stimulation of diatomite formations. CA Patent 1 308 550. Assigned to Chevron Res., October 13, 1992.
Debons, F.E.; Whittington, L.E., Improved oil recovery surfactants based on lignin, J. Pet. Sci. Eng. 7 (1–2) (1991) 131–138.
Deng, S.B.; Bai, R.B.; Chen, J.P.; Yu, G.; Jiang, Z.P.; Zhou, F.S., Effects of alkaline/ surfactant/polymer on stability of oil droplets in produced water from ASP flooding, Colloid Surf. A 211 (2–3) (2002) 275–284.
Dino, D.J., Homack, A., 1997. Use of high purity imidazoline based amphoacetate surfactant as foaming agent in oil wells. US Patent 5 614 473, assigned to Rhone Poulenc Inc., March 25, 1997.
Diyashev, R.N., Sattarova, F.M., Mazitov, K.G., Khusainov, V.M., Mannanov, F.N., Diyashev, I.R., et al., 1996a. Extraction of oil from lens-shaped deposits – involves cyclic and portion-wise pumping-in of solutions of potassium carbonate and inhibited hydrochloric acid. RU Patent 2 065 942, assigned to Tartar Oil Inst., August 27, 1996.
Diyashev, R.N., Sattarova, F.M., Mazitov, K.G., Khusainov, V.M., Sulejmanov, K.I., Karimov, G.S., et al., 1996b. Recovering oil not exploited from reservoir – by injecting alternating portions of ammonium carbonate and hydrochloric acid and displacing formed carbon dioxide with water. RU Patent 2 065 940, assigned to Tartar Oil Inst., August 27, 1996.
Donaldson, E.C.; Obeida, T., Enhanced oil recovery at simulated reservoir conditions, 3rd US DOE Microbial Enhancement of Oil Recovery International Conference (Norman, Okla, 5/27/90–6/1/90), In: (Editor: Donaldson, E.C.) Microbial Enhancement of Oil Recovery: Recent Advances: Proceedings of the 1990 International Conference on Microbial Enhancement of Oil Recovery, vol. 31 of Developments in Petroleum Science (1991) Elsevier Science Ltd., pp. 227–245.
Drillet, V.; Defives, D., Clay dissolution kinetics in relation to alkaline flooding, In: Proceedings Volume, SPE Oilfield Chemicals International SymposiumAnaheim, Calif, February 20–22, 1991, SPE Number: 21030. (1991), pp. 317–326.
Dugstad, O.; Bjornstad, T.; Hundere, I., Measurements and application of partition coefficients of compounds suitable for tracing gas injected into oil reservoirs, Rev. Inst. Franc. Pet. 47 (2) (1992) 205–215.
Duncan, G., Enhanced recovery engineering: Pt.1, World Oil 215 (9) (1994) 97–100; 95.
Duncan, G.; Bulkowski, P., Enhanced recovery engineering: Pt.7, World Oil 216 (9) (1995) 77–84.
Dzhafarov, I.S.; Brezitsky, S.V.; Shakhverdiev, A.K.; Panakhov, G.M.; Suleimanov, B.A., New in-situ carbon dioxide generation enhanced oil recovery technology, In: Proceedings Volume, no. 106, 10th EAGE Improvement of Oil Recovery Europe Symposium, Brighton, UK, August 18–20, 1999. (1999).
Elkamel, A.; Al-Sahhaf, T.; Ahmed, A.S., Studying the interactions between an arabian heavy crude oil and alkaline solutions, Pet. Sci. Technol. 20 (7–8) (2002) 789–807.
Feraud, J.P., Perthuis, H., Dejeux, P., 2001. Compositions for iron control in acid treatments for oil wells. US Patent 6 306 799, assigned to Schlumberger Technology Corporation, Sugar Land, TX, October 23, 2001.
Fink, M.; Fink, J., Usage of pyrolysis products from organic materials to improve recovery of crude oil, In: Extended Abstr Volume, vol. 2, 60th EAGE ConferenceLeipzig, Ger, June 8–12, 1998. (1998), p. P553.
French, T.R., 1990. Design and optimization of phosphate-containing alkaline flooding formulations: Topical report, US DOE Fossil Energy Rep NIPER-446, NIPER, February 1990.
French, T.R., Josephson, C.B., 1991. Surfactant-enhanced alkaline flooding with weak alkalis, US DOE Rep NIPER-507, NIPER, February 1991.
French, T.R., Josephson, C.B., 1992. Alkaline flooding injection strategy, US DOE Fossil Energy Rep NIPER-563, NIPER, March 1992.
French, T.R., Josephson, C.B., 1993. The effect of polymer-surfactant interaction on the rheological properties of surfactant-enhanced alkaline flooding formulations: Topical report, US DOE Fossil Energy Rep NIPER-635, NIPER, February 1993.
Frolov, A.I., Khisamov, R.S., Rjabov, I.I., Taziev, M.Z., 1998. Recovery of oil from reservoir - by injection of water and surfactant solution also additionally of wide hydrocarbon(s) fraction and of surfactant solution. RU Patent 2 103 492, assigned to Tatneft Stock Co., January 27, 1998.
Gall, B., 1989. Use of sacrificial agents to reduce carboxymethylated ethoxylated surfactant loss during chemical flooding: Topical report, US DOE Fossil Energy Rep.
Garcia-Sanchez, F.; Eliosa-Jimenez, G.; Salas-Padron, A.; Hernandez-Garduza, O.; Apam-Martinez, D., Modeling of microemulsion phase diagrams from excess gibbs energy models, Chem. Eng. J. 84 (3) (2001) 257–274.
Gevertz, D.; Paterek, J.R.; Davey, M.E.; Wood, W.A., Isolation and characterization of anaerobic halophilic bacteria from oil reservoir brines, In: Proceedings Volume, no. 31, 3rd US DOE Microbial Enhancement of Oil Recovery International ConferenceNorman, Okla, May 27, 90–6, 1, 90, 1991. (1991), pp. 115–129.
Glumov, I.F., Ibatullin, R.R., Abdulkhairov, R.M., Slesareva, V.V., Kochetkov, V.D., Roshchektaeva, N.A., 1994. Working out of oil stratum – comprises pumping sulphuric acid into stratum through pressing-in wells, pressing-in with water and collecting oil through extraction wells. RU Patent 1 480 411, assigned to Tartar Oil Inst. and Tatnipineft, October 30, 1994.
Goodyear, S.G.; Jones, P.I.R., Assessment of foam for deep flow diversion, In: Proceedings Volume, vol. 2, 8th Eapg Impr. Oil Recovery Europe SymposiumVienna, Austria, May 15–17, 1995. (1995), pp. 174–182.
Gorodilov, V.A., Shevchenko, V.N., Tipikin, S.I., Makurov, A.D., Makeev, G.A., Fomichev, V.F., 1997. Method for high temperature seam oil deposit development – by pumping aluminium chloride and tri- sodium phosphate as sediment forming material. RU Patent 2 094 599, assigned to Noyabrskneftegeo Stock Co., October 27, 1997.
Gorodnov, V.P., Ryskin, A.Y., Kharlanov, G.P., Belov, A.A., Shein, A.V., 1992. Enhancing oil recovery from boreholes – by injecting into seam aqueous solution of polyacrylamide, chrome alum and bentonite clay for improved flow characteristics. SU Patent 1 731 942, assigned to Giprovostokneft Des. Inst., May 07, 1992.
Gorodnov, V.P., Ryskin, A.Y., Pavlov, M.V., 1993. Increasing oil extraction from stratum – by pumping- in hydrochloric acid solution or its mixture with hydrofluoric acid or ammonium fluoride, followed by pumping aqueous solution or surfactant. SU Patent 1 795 092, assigned to Giprovostokneft Des. Inst., February 15, 1993.
Green, D.W.; Willhite, G.P., Enhanced Oil Recovery, In: vol. 8. of SPE Textbook Seriesfourth ed. (2008) Society of Petroleum Engineers, Richardson, TX.
Grula, E.A.; Russell, H.H.; Bryant, D.; Kenaga, M., Oil displacement by anaerobic and facultatively anaerobic bacteria, In: (Editors: Donaldson, E.C.; Chilingarian, G.V.; Yen, T.F.) Microbial Enhanced Oil Recovery, vol. 22 of Developments in Petroleum Science (1989) Elsevier Science Ltd., pp. 113–123.
Grula, E.A.; Russell, H.H.; Grula, M.M., Potential health hazard of bacteria to be used in microbial enhanced oil recovery, In: (Editors: Donaldson, E.C.; Chilingarian, G.V.; Yen, T.F.) Microbial Enhanced Oil Recovery, vol. 22 of Developments in Petroleum Science (1989) Elsevier Science Ltd, pp. 209–213.
Han, D.K.; Yang, C.Z.; Lou, Z.H.; Zhang, Z.Q.; Chang, Y.I., Recent development of enhanced oil recovery in china, J. Pet. Sci. Eng. 22 (1–3) (1999) 181–188.
Hendrickson, E.R., Jackson, R.E., Keeler, S.J., Luckring, A.K., Perry, M.P., Wolstenholme, S., 2010. Identification, characterization, and application of thauera sp. al9:8 useful in microbially enhanced oil recovery. US Patent 7 708 065, assigned to E.I. du Pont de Nemours and Company, Wilmington, DE, May 4, 2010.
Higuerey, I.; Pereira, P.; Leon, V., Comparative study of compositional changes between thermal cracking and aquaconversion(r) process, ACS Pet. Chem. Div. Preprints 46 (1) (2001) 64–65.
Hoffmann, G.G.; Steinfatt, I., Thermochemical sulfate reduction at steam flooding processes – a chemical approach, In: ACS Pet. Chem. Div. Preprints, vol. 38, 205th ACS Nat. Mtg. Enhanced Oil Recovery SymposiumDenver, March 28, 93–4, 2, 1993. (1993), pp. 181–184.
Holing, K.; Alvestad, J.; Trangenstein, J.A., The use of second-order godunov-type methods for simulating EOR processes in realistic reservoir models, In: Proceedings Volume, 2nd Inst. Franc Du Petrol. Math. of Oil Recovery Europe ConferenceArles, Fr, September 11–14, 1990. (1990), pp. 101–111.
Horvath-Szabó, G.; Czarnecki, J.; Masliyah, J., Liquid crystals in aqueous solutions of sodium naphthenates, J. Colloid Interface Sci. 236 (2) (2001) 233–241.
Horvath-Szabó, G.; Masliyah, J.H.; Czarnecki, J., Phase behavior of sodium naphthenates, toluene, and water, J. Colloid Interface Sci. 242 (1) (2001) 247–254.
Horvath-Szabó, G.; Czarnecki, J.; Masliyah, J.H., Sandwich structures at oil-water interfaces under alkaline conditions, J. Colloid Interface Sci. 253 (2) (2002) 427–434.
Hoskin, D.H., Mitchell, T.O., Shu, P., 1991. Oil reservoir permeability profile control with crosslinked welan gum biopolymers. US Patent 4 981 520, assigned to Mobil Oil Corp., January 01, 1991.
Hsu, J.J.C., 1992. Recovering hydrocarbons with a mixture of carbon dioxide and trichloroethane. US Patent 5 117 907, June 02, 1992.
Hsu, O.Y., Hsu, N.S., 2000. Alkaline surfactant polymer flooding composition and process. US Patent 6 022 834, assigned to Oil Chemicals Technologies, Inc., Sugarland, TX, February 8, 2000.
Hurd, B.G., 1991. Method for using foams to improve alkaline flooding oil recovery. US Patent 4 981 176, assigned to Mobil Oil Corp., January 01, 1991.
Hutchins, R.D., Dovan, H.T., 1992. Method for reducing water production from wells. US Patent 5 161 615, assigned to Union Oil Co. California, November 10, 1992.
Hutchins, R.D., Saunders, D.L., 1993. Tracer chemicals for use in monitoring subterranean fluids. US Patent 5 246 860, assigned to Union Oil Co. California, September 21, 1993.
Hwang, R.J.; Ortiz, J., Mitigation of asphaltics deposition during CO2 flood by enhancing CO2 solvency with chemical modifiers, Organic Geochem 31 (12) (1999) 1451–1462.
Ignateva, V.E., Telin, A.G., Khisamutdinov, N.I., Safronov, S.V., Artemev, V.N., Ermilov, Y.A., 1996. Composition for oil extraction – contains hydrocarbon- or alcohol-containing solvent, water and vat residue from production of glycerine or ethylene glycol. RU Patent 2 065 941, assigned to Neftegaztek Res. Eng. Cent., August 27, 1996.
Islam, M.R.; Chakma, A., Mathematical modelling of enhanced oil recovery by alkali solutions in the presence of cosurfactant and polymer, J. Pet. Sci. Eng. 5 (2) (1991) 105–126.
Ivanov, M.V.; Belyaev, S.S., Biotechnology and enhanced oil recovery, Neft Khoz 10 (1989) 28–32.
Ivory, J.; Derocco, M.; Paradis, N., Investigation of the mechanisms involved in the steamair injection process, In: Preprints, no. 21, 3rd Cim Petroleum Society Technology MeetingRegina, Canada, September 25–27, 1989. (1989).
Jang, L.K.; Yen, T.F.; Chilingarian, G.V.; Donaldson, E.C., Bacterial migration through nutrient-enriched sandpack columns for in-situ recovery of oil, In: (Editors: Donaldson, E.C.; Chilingarian, G.V.; Yen, T.F.) Microbial Enhanced Oil Recovery, vol. 22 of Developments in Petroleum Science (1989) Elsevier Science Ltd., pp. 151–164.
Jenneman, G.E., Clark, J.B., 1994a. Injection of scale inhibitors for subterranean microbial processes. US Patent 5 337 820, assigned to Phillips Petroleum Co., August 16, 1994.
Jenneman, G.E., Clark, J.B., 1994b. Utilization of phosphite salts as nutrients for subterranean microbial processes. US Patent 5 327 967, assigned to Phillips Petroleum Co., July 12, 1994.
Ju, B.; Luan, Z.; Wu, Z.; Lu, G., A study of removal of organic formation damage by experiments and modeling approaches, In: Proceedings Volume, SPE Asia Pacific Oil & Gas ConferenceJakarta, Indonesia, April 17–19, 2001. (2001).
Kalfoglou, G., Paulett, G.S., 1993. Method of using lignosulfonate-acrylic acid graft copolymers as sacrificial agents for surfactant flooding. US Patent 5 251 698, assigned to Texaco Inc., White Plains, NY, October 12, 1993.
Kalpakci, B.; Arf, T.G.; Grist, D.M.; Hyde, S.B.; Vikane, O.; Espedal, S., A preliminary evaluation of an LTPF (low tension polymer flood) process for Statfjord field, Norway, In: Proceedings Volume, vol. 1, 7th Eapg Impr. Oil Recovery Europe SymposiumMoscow, Russia, October 27–29, 1993. (1993), pp. 193–207.
Keeler, S.J., Hendrickson, E.R., Hnatow, L.L., Jackson, S.C., 2010. Identification, characterization, and application of shewanella putrefaciens (lh4:18), useful in microbially enhanced oil release. US Patent 7 776 795, assigned to E.I. du Pont de Nemours and Company, Wilmington, DE, August 17, 2010.
Kilbane II, J.J.; Chowdiah, P.; Kayser, K.J.; Misra, B.; Jackowski, K.A.; Srivastava, V.J.; et al., In-situ remediation of contaminated soils using foams as carriers for chemicals, nutrients, and other amendments, In: Proceedings Volume, 9th Inst. Gas Technol. Gas, Oil, & Environ. Biotechnol. International SymposiumColorado Springs, CO, December 9–11, 1996. (1996).
Kilbane II, J.J.; Chowdiah, P.; Kayser, K.J.; Misra, B.; Jackowski, K.A.; Srivastava, V.J.; et al., Remediation of contaminated soils using foams, In: Proceedings Volume, 10th Inst. Gas Technol. Gas, Oil & Environ. Biotechnol. & Site Remediation Technol. International SymposiumOrlando, Florida, December 8–10, 1997. (1997).
Killough, J.E., 1987. Hydrocarbon recovery process. US Patent 4 678 033, July 07, 1987.
Kovarik, F.S., Heller, J.P., 1990. Improvement of CO2 flood performance, US DOE Rep DOE/MC/ 21136-24, New Mex Inst Mining Techn, August 1990.
Lakatos, I.; Bauer, K.; Lakatos-Szabó, J., Potential application of oxygen containing gases to enhance gravity drainage in heavy oil bearing reservoirs, Erdöl Erdgas Kohle 113 (6) (1997) 260–263.
Lakatos, I.; Lakatosne, S.J., Dynamical characteristics of the polymer flooding: Pt.2 (a polimeres elarasztas kinamikus jellemzoi: 2. resz), Koolaj Foldgaz 24 (6) (1991) 170–178.
Lakatos, I.; Lakatos-Szabó, J., Effect of ethoxylated nonyl-phenols on interfacial rheological properties of oil/water systems, Colloid Polym. Sci. 275 (5) (1997) 493–501.
Lakatos, I.; Lakatos-Szabó, J., Effect of IOR/EOR [improved oil recovery/enhanced oil recovery] chemicals on interfacial rheological properties of crude oil/water systems, In: Proceedings Volume, SPE Oilfield Chemicals International SymposiumHouston, TX, February 13–16, 2001. (2001); SPE Number: 65391.
Lakatos, I.; Lakatos-Szabó, J., Effect of IOR/EOR chemicals on interfacial rheological properties of crude oil/water systems, In: Proceedings Volume, SPE International Symposium on Oilfield Chemistry held in HoustonTexas, February 13–16, 2001. (2001).
Lakatos, I.; Lakatos-Szabó, J.; Bedo, Z., Application of nonionic tenside homologues in IOR/EOR and oilfield chemistry: Fundamental and engineering aspects, In: Proceedings Volume, SPE International Symposium on Oilfield ChemistryHouston, Texas, U.S.A., February 5–7, 2003. (2003).
Lakatos, I.; Tóth, J.; Bauer, K.; Lakatos-Szabó, J.; Kosztin, B.; Palásthy, G.; et al., Comparative study of different silicone compounds as candidates for restriction of water production in gas wells, In: Proceedings Volume, SPE International Symposium on Oilfield ChemistryHouston, Texas, U.S.A., February 5-7, 2003. (2003).
Lakatos, I.; Tóth, J.; Lakatos-Szabó, J.; Kosztin, B.; Palásthy, G.; Wöltje, H., Application of silicone microemulsion for restriction of water production in gas wells, In: Proceedings Volume, SPE 13th European Petroleum ConferenceAberdeen, Scotland, U.K. October 29–31, 2002. (2002).
Lakatos-Szabó, J.; Lakatos, I., Effect of non-ionic surfactants on interfacial rheological properties of water/oil systems, Erdöl Erdgas Kohle 105 (10) (1989) 406–410.
Lakatos-Szabó, J.; Lakatos, I., Effect of sodium hydroxide on interfacial rheological properties of oil-water systems, In: Colloids and Surfaces, vol. 149, 9th Surface & Colloid Sci International ConferenceSofia, Bulgaria, July 6–12, 1997. (1997), pp. 507–513.
Lakatos-Szabó, J.; Lakatos, I., Effect of sodium hydroxide on interfacial rheological properties of oil-water systems, Colloid Surf. A 149 (1–3) (1999) 507–513.
Lakatos-Szabó, J.; Lakatos, I.; Kosztin, B., Role of interfacial rheological properties of oil/water systems in mechanism and design of EOR/IOR technologies, In: Proceedings Volume, no. 057, 9th EAGE Impr. Oil Recovery Europe SymposiumThe Hague, Neth, December 20–22, 1997. (1997); Proc.
Lazar, I., The microbiology of MEOR: (microbial enhanced oil recovery): Practical experience in europe, In: Proceedings Volume, vol. 2, Minerals, Metals & Mater Soc. et al Biohydromet Technol. International SymposiumJackson Hole, WY, August 22–25, 1993. (1993), pp. 329–338.
Lazar, I.; Voicu, A.; Archir, G.; Toma, T.; Lazar, I.G.; Blanck, L.; et al., Investigations on a new Romanian biopolymer (pseudozan) for use in enhanced oil recovery (EOR), In: Proceedings Volume, vol. 2, Minerals, Metals & Mater Soc. et al Biohydromet Technology International SymposiumJackson Hole, WY, August 22–25, 1993. (1993), pp. 357–364.
Li, G.Z.; Mu, J.H.; Li, Y.; Xiao, H.D.; Gu, Q., What is the criterion for selecting alkaline/ surfactant/polymer flooding formulation: Phase behavior or interfacial tension, J. Dispersion Sci. Technol. 21 (3) (2000) 305–314.
Li, G.Z.; Mu, J.H.; Li, Y.; Yuan, S.L., An experimental study on alkaline/surfactant/polymer flooding systems using nature mixed carboxylate, Colloid Surf. A 173 (1–3) (2000) 219–229.
Li, Z.P.; Zeng, H.X.; Zhao, R.B.; Jiao, L.M., The nature of colloid and interface of miscible phase system in alkaline-surfactant combination flooding, J. Dispersion Sci. Technol. 20 (4) (1999) 1143–1162.
Lin, S.C.; Goursaud, J.C.; Kramer, P.J.; Georgiou, G.; Sharma, M.M., Production of biosurfactant by bacillus licheniformis strain jf-2, In: (Editor: Donaldson, E.C.) Microbial Enhancement of Oil Recovery: Recent Advances: Proceedings of the 1990 International Conference on Microbial Enhancement of Oil Recovery, vol. 31 of Developments in Petroleum Science (1991) Elsevier Science Ltd., pp. 219–226.
Littmann, W., Polymer Flooding, vol. 24. of Developments in Petroleum Science. (1998) Elsevier Science, New York.
Liu, H.; Zhang, Y., Rheological property of the xanthan biopolymer flooding systems, J, Univ. Pet., China 19 (4) (1995) 41–44.
Liu, J.; Pope, G.A.; Sepehrnoori, K., A high-resolution, fully implicit method for enhanced oil recovery simulation, In: Proceedings Volume, 13th SPE Reservoir Simulation SymposiumSan Antonio, February 12–15, 1995. (1995), pp. 35–50.
Ljosland, E.; Bjornstad, T.; Dugstad, O.; Hundere, I., Perfluorocarbon tracer studies at the Gullfaks field in the North Sea, J. Pet. Sci. Eng. 10 (1) (1993) 27–38.
Lockhart, T.P., Burrafato, G., 1990a. Gellable buffered aqueous composition and its use inenhanced petroleum recovery. EP Patent 390 281, assigned to Eniricerche SPA and Agip SPA, October 03, 1990.
Lockhart, T.P., Burrafato, G., 1990b. Method and composition for reducing the permeability of a high-permeability zone in an oil reservoir. CA Patent 2 013 468, assigned to Eniricerche SPA and Agip SPA, September 30, 1990.
Lockhart, T.P., Burrafato, G., 1990c. Method and composition for reducing the permeability of a high permeability zone in an oil reservoir. EP Patent 390 280, assigned to Eniricerche SPA and Agip SPA, October 03, 1990.
Lorenz, P.B., 1991. The effect of alkaline agents on retention of EOR chemicals, US DOE Rep NIPER-535, NIPER.
Ma, G., Laboratory study on polymer flooding in oil reservoir with high salinity, Oil Gas Recovery Technol. 3 (2) (1996); I, 1–4, 33.
Ma, G.; Zuo, K.; Xu, Z., Polymer flooding test in low permeability and high salinity reservoir of maling oilfield, Oil Drilling Prod. Technol. 21 (1) (1999) 89–93; 109–110.
Mamleev, R.A., Yulbarisov, E.M., Fakhretdinov, R.N., Zagidullina, L.N., Kulikov, A.N., Kutushev, Z.R., 1997. Composition for pumping into oil stratum – contains specified biopolymer, polydimethyldiallyl ammonium chloride, water and formaldehyde. SU Patent 1 828 161, assigned to Oil Strata Geolog. Phys., January 10, 1997.
Mason, J.A., 1991a. Use of chlorous acid in oil recovery. CA Patent 2 007 218, July 05, 1991.
Mason, J.A., 1991b. Use of chlorous acid in oil recovery. GB Patent 2 239 867, July 17, 1991.
McCormick, C.L., Hester, R.D., 1990. Polymers formobility control in enhanced oil recovery: Final report, US DOE Fossil Energy Rep DOE/BC/10844-20, Southern Mississippi Univ.
McInerney, M.J.; Montgomery, A.D.; Sublette, K.L., Microbial control of the production of sulfide, In: (Editor: Donaldson, E.C.) Microbial Enhancement of Oil Recovery: Recent Advances: Proceedings of the 1990 International Conference on Microbial Enhancement of Oil Recovery, vol. 31 of Developments in Petroleum Science (1991) Elsevier Science Ltd., pp. 441–449.
Metwally, M., Effect of gaseous additives on steam processes for lindbergh field, alberta, J. Can. Pet. Technol. 29 (6) (1990) 26–30.
Miller, D., Vollmer, A., Feustel, M., Klug, P., 1999. Synergistic mixtures of phosphoric acid esters with carboxylic acid derivatives as dipersants for asphaltenes. EP Patent 0 967 361, assigned to Clariant GmbH, December 29, 1999.
Miller, J.F., Sheely, C.O., Wimberley, J.W., Howard, R.A., 1993. Use of nonradioactive complex metal anion as tracer in subterranean reservoirs. US Patent 5 246 861, assigned to Conoco Inc., September 21, 1993.
Mohanty, S.; Khataniar, S., Sodium orthosilicate: An effective additive for alkaline steamflood, J. Pet. Sci. Eng. 14 (1–2) (1995) 45–49.
Momeni, D.; Chen, J.R.; Yen, T.F., MEOR (microbial enhanced oil recovery) studies in a radial flow system – the research outlook, In: Proceedings Volume, no. NIPER-351 CONF-870858, US Dep Energy et al Appl of Microorganisms to Petrol. Technol. SymposiumBartlesville, Okla, August 12–13, 1987, 1988. (1988); XVII–1–XVII–7.
Momeni, D.; Yen, T.F.; Jang, L.K.; McDavid, R.; Kuo, J.F.; Huang, V.; et al., Microbial Enhanced Oil Recovery: Principle and Practice. (1990) CRC Press, Inc, Boca Raton, FL.
Monjoie, A.; Schroeder, C., Flooding of oil in chalk (deplacement des hydrocarbures dans la craie), Soc. Geol. Nord. Ann. 5 (4) (1997) 325–329.
Moore, C.H., Computer simulation of formation damage resulting from thermal recovery, In: Proceedings Volume, SPE International Therm Oper. & Heavy Oil SymposiumMargarita Island, Venezuela, March 12–14, 2001. (2001).
Moradi-Araghi, A., Stahl, G.A., 1991a. Gelation of acrylamide-containing polymers with furfuryl alcohol and water dispersible aldehydes. EP Patent 447 967, assigned to Phillips Petroleum Co., September 25, 1991.
Moradi-Araghi, A., Stahl, G.A., 1991b. Gelation of acrylamide-containing polymers with hydroxyphenyl alkanols. EP Patent 446 865, assigned to Phillips Petroleum Co., September 18, 1991.
Moritis, G., EOR survey and analysis, Oil Gas J. 94 (16) (1996) 39–42; 44–61.
Moritis, G., EOR oil production up slightly, Oil Gas J. 96 (16) (1998) 49–77.
Moss Jr., J.T., Moss, J.T., 1994. Enhanced oil recovery using hydrogen peroxide injection, US DOE Fossil Energy Rep NIPER/BDM-0086, Tejas Petrol. Engineers In.
Mu, J.H.; Li, G.Z.; Li, Y., An experimental study on alkaline/surfactant/polymer flooding systems using natural mixed carboxylate, Chin. J. Chem. Eng. 9 (2) (2001) 162–166.
Mu, J.H.; Li, G.Z.; Liao, G.Z.; Huang, L.; Zhao, K.S., Phase behavior and structure analysis of the middle mixed layer for ASP flooding system at low surfactant concentration, Sci. China Ser. B 45 (2) (2002) 184–190.
Nashawi, I.S., 1991. Laboratory investigation of the effect of brine composition on polymer solutions: Pt.2: Xanthan gum (XG) case, SPE Unsolicited Pap SPE-23534, United Arab Emirates Univ, August 1991.
Nasr, T.N., Isaacs, E.E., 2001a. Process for enhancing hydrocarbon mobility using a steam additive. US Patent 6 230 814, assigned to Alta Oil Sand Tech. Res. Aut, May 15, 2001.
Nasr, T.N., Isaacs, E.E., 2001b. Process for enhancing hydrocarbon mobility using a steam additive. WO Patent 0 127 439, assigned to Alberta Sci Res. Tech. Auth, April 19, 2001.
Niu, Y.; Ouyang, J.; Zhu, Z.; Wang, G.; Sun, G.; Shi, J., Research on hydrophobically associating water-soluble polymer used for EOR, In: Proceedings Volume, SPE Oilfield Chemicals International SymposiumHouston, TX, February 13–16, 2001. (2001).
Northrop, P.S., 1993. Method for disposing of waste gas in subterranean formations. US Patent 5 267 614, assigned to Mobil Oil Corp., December 07, 1993.
Northrop, P.S., 1995. Imbibition process using a horizontal well for oil production from low permeability reservoirs. US Patent 5 411 094, assigned to Mobil Oil Corp., May 02, 1995.
Olsen, D.K., 1989. Use of amine oxide surfactants for chemical flooding EOR: Topical report, US DOE Fossil Energy Rep NIPER-417, National Inst. for Petroleum and Energy Research, Bartlesville, OK, November 1989.
Osterloh, W.T., 1994. Long chain alcohol additives for surfactant foaming agents. US Patent 5 333 687, assigned to Texaco Inc., August 02, 1994.
Osterloh, W.T.; Jante Jr., M.J., Surfactant-polymer flooding with anionic po/eo surfactant microemulsions containing polyethylene glycol additives, In: Proceedings Volume, vol. 1, 8th SPE/DOE Enhanced Oil Recovery SymposiumTulsa, April 22–24, 1992. (1992), pp. 485–494.
Osterloh, W.T.; Jante, M.J., Low-cost foam surfactant from wood pulping by-products, In: Proceedings Volume, vol. 2, 8th Eapg Impr. Oil Recovery Europe SymposiumVienna, Austria, May 15–17, 1995. (1995), pp. 191–199.
Pitts, M.J.; Wyatt, K.; Sale, T.C.; Piontek, K.R., Utilization of chemical-enhanced oil recovery technology to remove hazardous oily waste from alluvium, In: Proceedings Volume, SPE Oilfield Chemicals International SymposiumNew Orleans, March 2–5, 1993. (1993), pp. 33–44.
Putz, A.G.; Pedron, B.M.; Bazin, B., Commercial polymer injection in the Courtenay field, 1994 update, In: Proceedings Volume, Vol. 1, 69th Annual SPE Technical ConferenceNew Orleans, September 25–28, 1994. (1994), pp. 403–413.
Quet, C.; Cheneviere, P.; Bourrel, M.; Glotin, G., Core surface analysis for wettability assessment, In: Proceedings Volume, Advances in Core Evaluation II: Reservoir Appraisal (2nd Soc. Core Anal et al Europe Core Anal Symposium, (Eurocas II)London, England, May 20–22, 1991. (1991) Gordon & Breach Science Publishers, pp. 119–131.
Quet, C.; Cheneviere, P.; Glotin, G.; Bourrel, M., Pore surface chemistry and wettability, In: Proceedings Volume, 6th Inst. Francais Du Petrole Explor. & Prod. Res. ConferenceSaint-Raphael, France, September 4–6, 1991. (1992), pp. 81–88.
Raible, C., 1992. Improvement in oil recovery using cosolvents with CO2 gas floods, US DOE Fossil Energy Rep NIPER-559, NIPER, January 1992.
Rame, M.; Delshad, M., A compositional reservoir simulator on distributed memory parallel computers, In: Proceedings Volume, 13th SPE Reservoir Simulation SymposiumSan Antonio, February 12–15, 1995. (1995), pp. 89–100.
Ramsay, J.A.; Cooper, D.G.; Neufeld, R.J., Effects of oil reservoir conditions on the production of water-insoluble levan by bacillus licheniformis, Geomicrobiol. J. 7 (3) (1989) 155–165.
Razzaq, A., 1992. Characterization of surfactants in the presence of oil for steam foam applications, Ph.D. thesis, Stanford Univ.
Razzaq, A., Castanier, L.M., 1992. Characterization of surfactants in the presence of oil for steam foam application, US DOE Fossil Energy Rep DOE/BC/14600-37, Stanford Univ.
Ren, Z.C.; Shi, A.P.; Leng, S.L.; Zhang, W.S.; Qin, L.P., Formulation of crosslinking polyacrylamide solution for EOR, Oilfield Chem. 15 (2) (1998) 146–149.
Rendall, W.A., Ayasse, C., Novosad, J., 1991. Surfactant-stabilized foams for enhanced oil recovery. US Patent 5 074 358, assigned to Alta Oil Sand Tech. Res. Aut, December 24, 1991.
Richardson, W.C., Kibodeaux, K.R., 2001. Chemically assisted thermal flood process. US Patent 6 305 472, assigned to Texaco Inc., October 23, 2001.
Rocha, P.S.; Miller, M.A.; Sepehrnoori, K., A succession-of-states front-tracking model for the in-situ combustion recovery process, In Situ 21 (1) (1997) 65–100.
Romocki, J., 1994. Application of N, N-dialkylamides to reduce precipitation of asphalt from crude oil. WO Patent 9 418 430, assigned to Buckman Labs Internat. Inc., August 18, 1994.
Romocki, J., 1995. Application of N, N-dialkylamides to reduce precipitation of asphalt from crude oil. US Patent 5 388 644, assigned to Buckman Labs Internat. Inc., February 14, 1995.
Ruzin, L.M.; Pleshkova, O.E.; Konovalova, L.V., Generation of carbon dioxide during thermal steam treatment of carbonate reservoirs containing high- viscosity oil, Neft Khoz (11) (1990) 59–62.
Sandrea, I.; Sandrea, R., Global oil reserves – recovery factors leave vast target for EOR technologies, Oil & Gas J. 105 (41) (2007) 44.
Saneie, S., 1992. Alkaline assisted thermal oil recovery: Kinetic and displacement studies, Ph.D. thesis, Southern California Univ.
Saneie, S., Yortsos, Y.C., 1993. Alkaline assisted thermal oil recovery: Kinetic and displacement studies: Topical report, US DOE Rep DOE/BC/14600-45, Southern California Univ, June 1993.
Sanz, C.A.; Pope, G.A., Alcohol-free chemical flooding: From surfactant screening to coreflood design, In: Proceedings Volume, SPE Oilfield Chemicals International SymposiumSan Antonio, February 14–17, 1995. (1995), pp. 117–128.
In: (Editor: Schramm, L.L.) Surfactants: Fundamentals and Applications in the Petroleum Industry (2000) Cambridge University Press, Cambridge.
Schramm, L.L., Ayasse, C., Mannhardt, K., Novosad, J., 1991a. Method for improving enhanced recovery of oil using surfactant-stabilized foams. CA Patent 2 006 482, June 20, 1991.
Schramm, L.L., Ayasse, C., Mannhardt, K., Novosad, J., 1991b. Method for improving enhanced recovery of oil using surfactant-stabilized foams. US Patent 5 060 727, assigned to Alta Oil Sand Tech. Res. Aut, October 29, 1991.
Schramm, L.L., Ayasse, C., Mannhardt, K., Novosad, J., 1991c. Recovery of oil using surfactantstabilized foams. GB Patent 2 239 278, assigned to Alta Oil Sand Tech. Res. Aut, June 26, 1991.
Scovell, E.G., Grainger, N., Cox, T., 2001. Maintenance of oil production and refining equipment. WO Patent 0 174 966, assigned to Imperial Chemical Inds Pl, October 11, 2001.
Sheehy, A.J., Microbial physiology and enhanced oil recovery, In: Proceedings Volume, 3rd US DOE Microbial Enhancement of Oil Recovery International ConferenceNorman, Okla, May 27, 90–6, 1, 1990. (1990).
Sheehy, A.J., Microbial physiology and enhanced oil recovery, In: (Editor: Donaldson, E.C.) Microbial Enhancement of Oil Recovery: Recent Advances: Proceedings of the 1990 International Conference on Microbial Enhancement of Oil Recovery, vol. 31 of Developments in Petroleum Science (1991) Elsevier Science Ltd., pp. 37–44.
Shpakoff, P.G., Raney, K.H., 2009. Method and composition for enhanced hydrocarbons recovery. US Patent 7 612 022, assigned to Shell Oil Company, Houston, TX, November 3, 2009.
Shu, P., 1990. Programmed gelation of polymers using melamine resins. US Patent 4 964 461, assigned to Mobil Oil Corp., October 23, 1990.
Shu, P., Ng, R.C., Phelps, C.H., 1993a. In-situ cementation for profile control. US Patent 5 211 231, assigned to Mobil Oil Corp., May 18, 1993.
Shu, P., Phelps, C.H., Ng, R.C., 1993b. In-situ silica cementation for profile control during steam injection. US Patent 5 211 232, assigned to Mobil Oil Corp., May 18, 1993.
Shu, W.R., Shu, P., 1991. Method for steam flooding profile control. CA Patent 2 041 584, November 03, 1991.
Shupe, R.D.; Baugh, T.D., Thermal stability and degradation mechanism of alkylbenzene sulfonates in alkaline media, J. Colloid Interface Sci. 145 (1) (1991) 235–254.
Smith, D.H.; Comberiati, J.R., Chemical alteration of the rock surfaces by asphaltenes or surfactants, and its effect on oil recovery by CO2 flooding, In: Proceedings Volume, Annual Aiche MeetingChicago, Ill, November 11–16, 1990. (1990).
Snowden, M.J., Vincent, B., Morgan, J.C., 1993. Conformance control in underground reservoirs. GB Patent 2 262 117, assigned to British Petroleum Co. Ltd., June 09, 1993.
Somasundaran, P., 1994. Surfactant loss control in chemical flooding: Spectroscopic and calorimetric study of adsorption and precipitation on reservoir minerals – annual report for the reporting period September 30, 1992 to September 30, 1993, US DOE Fossil Energy Rep DOE/BC/14884-5, Columbia Univ.
Somasundaran, P., 1995. Surfactant loss control in chemical flooding spectroscopic and calorimetric study of adsorption and precipitation on reservoir minerals: Annual report, September 30, 1993–September 30, 1994, US DOE Fossil Energy Rep DOE/BC/14884-12, Columbia Univ.
Sommese, A.G., Nagarajan, R., 1995. Settling stabilization of polymer containing particle dispersions in oil. US Patent 5 438 088, assigned to Nalco Chemical Co., August 01, 1995.
Song, J., 1992. A new flux correcting method for reducing numerical dispersion – application to EOR (enhanced oil recovery) by chemical processes (reduction de la dispersion numerique par correction des flux massiques – application au probleme de la recuperation d'hydrocarbures par procedes chimiques), Ph.D. thesis, Paris VI Univ.
Sorbie, K.S., Polymer-Improved Oil Recovery. (1991) CRC Press, Inc., Boca Raton, FL.
Sorokin, V.A., Microbiological technology of oil recovery, Neft Khoz (6) (1989) 49–50.
Souto, E.; Bazin, B.; Sardin, M., Ion exchange between hydrogen and homoionic brines related to permeability reduction, In: Proceedings Volume, SPE Oilfield Chemicals International SymposiumNew Orleans, March 2–5, 1993. (1993), pp. 491–500.
Sperl, G.T., Sperl, P.L., 1991. Enhanced oil recovery using denitrifying microorganisms. US Patent 5 044 435, assigned to Injectech Inc., September 03, 1991.
Starukhina, L.A.; Deriabin, V.V.; Titov, V.J., New biopolymer for EOR Moscow, In: Proceedings Volume, vol. 1, 6th Europe Impr. Oil Recovery SymposiumStavanger, Norway, May 21–23, 1991. (1991), pp. 371–393.
Stegemeier, G.L., Perry, G.E., 1992. Method utilizing spot tracer injection and production induced transport for measurement of residual oil saturation. US Patent 5 168 927, assigned to Shell Oil Co., December 08, 1992.
Stepanova, G.S.; Mamedov, Y.G.; Babayeva, I.A.; Mosina, A.A.; Nenartovich, T.L.; Li, A.A., Thin under gas oil rims – new technology for efficient oil production using foam generating oil/water polymers, In: Proceedings Volume, no. 050, 9th EAGE Impr. Oil Recovery Europe SymposiumThe Hague, Neth, October 20–22, 1997, Proc.. (1997).
Stepanova, G.S., Rozenberg, M.D., Boksha, O.A., Gubkina, G.F., Nenartovich, T.L., Safronov, S.V., 1994a. Development of oil field – comprises pumping down well portion of aqueous solution of ammonium carbonate and displacing solution into seam with water, giving increased oil recovery. RU Patent 2 021 495, assigned to Oil Gas Res. Inst., October 15, 1994.
Stepanova, G.S., Rozenberg, M.D., Boksha, O.A., Gubkina, G.F., Nenartovich, T.L., 1994b. Chemical treatment of oil strata – comprises pumping-in ammonium carbonate and nitrate solution followed by aqueous solution of ammonium nitrate-sulphuric acid mixture, and water. RU Patent 2 021 496, assigned to Oil Gas Res. Inst., October 15, 1994.
Strycker, A., 1990. Selection and design of ethoxylated carboxylates for chemical flooding: Topical report, Government Report NIPER-449, US Dept Energy.
Sunde, E.; Beeder, J.; Nilsen, R.K.; Torsvik, T., Aerobic microbial enhanced oil recovery for offshore use, In: Proceedings Volume, vol. 2, 8th SPE/DOE Enhanced Oil Recovery SymposiumTulsa, April 22–24, 1992. (1992), pp. 497–502.
Surguchev, L.M.; Koundin, A.; Yannimaras, D., Air injection – cost effective IOR method to improve oil recovery from depleted and waterflooded fields, In: Proceedings Volume, SPE Asia Pacific Improvement of Oil Recovery Conference (Apiorc 99)Kuala Lumpur, Malaysia, October 25–26, 1999. (1999); SPE Number: 57296.
Sydansk, R.D., 1992. Enhanced liquid hydrocarbon recovery process. US Patent 5 129 457, assigned to Marathon Oil Co., July 14, 1992.
Sydansk, R.D., 1995. Hydrocarbon recovery process utilizing a gel prepared from a polymer and a preformed crosslinking agent. US Patent 5 415 229, assigned to Marathon Oil Co., May 16, 1995.
Tang, J.S., Harker, B.C., 1992a. Use of tracers to monitor in situ miscibility of solvent in oil reservoirs during eor. CA Patent 1 310 140, assigned to Esso Resources Canada Ltd., November 10, 1992.
Tang, J.S., Harker, B.C., 1992b. Use of tracers to monitor in situ miscibility of solvent in oil reservoirs during eor. US Patent 5 111 882, assigned to Exxon Production Res. Co., May 12, 1992.
Taugbol, K.; Zhou, H.H.; Austad, T., Low tension polymer flood. the influence of surfactant-polymer interaction, In: Proceedings Volume, Recent Advances in Oilfield Chemistry, 5th Royal Soc. Chemicals International SymposiumAmbleside, England, April 13–15, 1994. (1994), pp. 281–294.
Taylor, K.C.; Nasr-El-Din, H.A., A systematic study of iron control chemicals: Pt 2, In: Proceedings Volume, SPE Oilfield Chemicals International SymposiumHouston, February 16–19, 1999. (1999), pp. 649–656.
Taylor, K.C.; Nasr-El-Din, H.A.; Al-Alawi, M.J., A systematic study of iron control chemi- cals used during well stimulation, In: Proceedings Volume, SPE Formation Damage Contr. International SymposiumLafayette, LA, February 18–19, 1998. (1998), pp. 19–25.
Thibodeau, L.; Sakanoko, M.; Neale, G.H., Alkaline flooding processes in porous media in the presence of connate water, Powder Technol. 132 (2–3) (2003) 101–111.
Thomas, S.; Ali, S.M.F., Status and assessment of chemical oil recovery methods, Energy Sources 21 (1–2) (1999) 177–189.
Thomas, S.; Ali, S.M.F., Micellar flooding and ASP-chemical methods for enhanced oil recovery, J. Can. Pet. Technol. 40 (2) (2001) 46–52.
Thomas, S.; Ali, S.M.F.; Thomas, N.H., Scale-up methods for micellar flooding and their verification, J. Can. Pet. Technol. 39 (2) (2000) 18–27.
Thomas, S.; Farouq, A.S.M., Status and assessment of chemical oil recovery methods, Energy Sources 21 (1–2) (1999) 177–189.
Thompson Sr., J.E., Brannon, H.D., Woo, G.T.-C., Wood, W.R., Dawson, J.C., Ault, M.G., 2001. Surfactant compositions and uses therefor. US Patent 6 302 209, assigned to BJ Services Company, Houston, TX, October 16, 2001.
Tulbovich, B.I., Kazakova, L.V., Kozhevskikh, V.I., 1996. Foam-forming composition – contains surfactant, in form of alkali effluent from caprolactam production, and solvent, in form of e.g. stratal water. RU Patent 2 061 859, assigned to Perm Oil Ind. Res. Des. Inst., June 10, 1996.
Vadie, A., 2002. Microbial enhanced oil recovery, [electronic] http://www.msstate.edu/dept/wrri/meor/.
Vaidya, R.N.; Fogler, H.S., Formation damage due to colloidally induced fines migration, Colloids Surf 50 (1990) 215–229.
Varadaraj, R., 1997. Polymer-surfactant fluids for decontamination of earth formations. US Patent 5 614 474, assigned to Exxon Research & Eng. Co., March 25, 1997.
Varadaraj, R., 2008. Mineral acid enhanced thermal treatment for viscosity reduction of oils (ecb-0002). US Patent 7 419 939, assigned to ExxonMobil Upstream Research Company, Houston, TX, September 2, 2008.
Verderevskij, Y.L., Valeeva, T.G., Khabirov, R.A., Gusev, V.I., Arefev, Y.N., Golovko, S.N., et al., 1996. Development of oil-bearing strata – comprises pumping in spent sulphuric acid, preceded by additional aqueous lignosulphonate(s) to increase efficiency. SU Patent 1 448 783, assigned to Oil Ind. Chem. Process Asso and Perm Paper Res. Inst., November 20, 1996.
Wang, D., 1999. A foam drive method. WO Patent 9 951 854, assigned to Daqing Petroleum Admin. Bu, October 14, 1999.
Wang, Y.; Li, J.; Zhao, F., Surfactants oil displacement system in high salinity formations, In: Proceedings Volume, SPE Asia Pacific Oil and Gas Conference and ExhibitionJakarta, Indonesia, April 17–19, 2001. (2001).
Wang, Y.; Wang, L.; Li, J.; Zhao, F., Surfactants oil displacement system in high salinity formations: Research and application, In: Proceedings Volume, SPE Permian Basin Oil and Gas Recovery ConferenceMidland, Texas, May 15–16, 2001. (2001).
Wason, D.T., 1990. Enhanced oil recovery through in-situ generated surfactants augmented by chemical injection, US DOE Rep DOE/BC/10847-20, Inst Gas Technol, Chicago, August 1990.
Westlake, D.W.S., Microbial ecology of corrosion and reservoir souring, In: (Editor: Donaldson, E.C.) Microbial Enhancement of Oil Recovery: Recent Advances: Proceedings of the 1990 International Conference on Microbial Enhancement of Oil Recovery, vol. 31 of Developments in Petroleum Science (1991) Elsevier Science Ltd., pp. 257–263.
Wyganowski, M.W., 1991. Method of reducing the reactivity of steam and condensate mixtures in enhanced oil recovery. US Patent 5 036 915, assigned to Alberta Energy Co. Ltd., Amoco Canada Petroleum Co., and Diminex Ltd., Canada, August 06, 1991.
Yamazaki, T.; Aso, K.; Okabe, H.; Akita, Y., Automatic continuous-measuring system of interfacial tension by spinning drop technique, In: Proceedings Volume, SPE Asia Pacific ConferenceYokohama, Japan, April 25–26, 2000. (2000).
Yen, T.F.; Park, J.K.; Lee, K.I.; Li, Y., Fate of surfactant vesicles surviving from thermophilic, halotolerant, spore forming, clostridium thermohydrosulfuricum, In: (Editor: Donaldson, E.C.) Microbial Enhancement of Oil Recovery: Recent Advances: Proceedings of the 1990 International Conference on Microbial Enhancement of Oil Recovery, vol. 31 of Developments in Petroleum Science (1991) Elsevier Science Ltd., pp. 297–309.
Zaitoun, A.; Fonseca, C.; Berger, P.; Bazin, B.; Monin, N., New surfactant for chemical flood in high-salinity reservoir, In: Proceedings Volume, SPE International Symposium on Oilfield ChemistryHouston, Texas, February 5–7, 2003. (2003).
Zekri, A.Y., Microbial enhanced oil recovery – a short review, Oil Gas Europe Mag. 27 (1) (2001) 22–25.
Zhang, S.B.; Qiao, W.H.; Li, Z.S.; Cheng, L.B.; Hu, J.Z., 1-Phenylalkane sulfonates for studying interfacial tensions, Pet. Sci. Technol. 21 (7–8) (2003) 1043–1054.
Zhou, Z., 2000. Process for reducing permeability in a subterranean formation. US Patent 6 143 699, assigned to Alta Oil Sand Tech. Res. Auth, November 07, 2000.
Zhu, T.; Strycker, A.; Raible, C.J.; Vineyard, K., Foams for mobility control and improved sweep efficiency in gas flooding, In: Proceedings Volume, vol. 2, 11th SPE/DOE Improvement of Oil Recovery SymposiumTulsa, April 19–22, 1998. (1998), pp. 277–286.
Zobell, C.E., Bacterial action in seawater, Proc. Soc. Exp. Biol. Med. (34) (1937) 113–116.
Zobell, C.E.; Johnson, F.H., The influence of hydrostatic pressure on the growth and viability of terestrial and marine bacteria, J. Bacteriol. Bioeng. (57) (1979) 1979.
..................Content has been hidden....................
You can't read the all page of ebook, please click here login for view all page.