3.2 Rechargeable Batteries for Energy Storage
3.2.1 Introduction
A shift toward the use of renewable energy sources is increasingly attracting attention. The renewable energy sources (i.e., solar PV, solar heat, geothermal, wind, and biomass) provide to date only a few percent of the alternative energy carriers electricity and hydrogen (Goldemberg, 2007). In particular, the decentralized conversion of solar and wind energy is being studied and engineered worldwide. Solar PV and wind energy produce electrons and can be coupled directly to the electricity grid, thereby reducing the emission of greenhouse gases (van Geenhuizen and Schoonman, 2010). However, these renewable energy sources are discontinuous and, therefore, require adequate storage systems.
While present and future energy storage technologies fall in the broad categories of fly wheel, compressed air, superconducting coil, hydrogen, supercapacitor, and rechargeable battery, advanced materials for supercapacitors and rechargeable batteries are attracting widespread research and development attention in academia and industry. This chapter will focus on rechargeable batteries and, in particular, on the rechargeable lithium-ion (Li-ion) battery. The types of Li-ion batteries will be presented, along with the Li-ion battery principle and the state-of-the-art battery anode, cathode, and electrolyte materials. A new class of soft-matter electrolytes is obtained via heterogeneous doping of non-aqueous (polymer) salt solutions. These composite electrolytes behave as a solid and are referred to as soggy sand electrolytes. Nanostructured battery components are also the focus of widespread research, and the nanoscale will give Li-ion batteries benefits in terms of capacity, power, cost, and materials sustainability. The utilization of advanced rechargeable batteries to design emission-free mobility is a challenge, next to the option of hydrogen and, in this case, the polymer electrolyte membrane fuel cell (PEMFC) for emission-free vehicles, the major problem then being the safe large-scale storage of hydrogen. The safe storage of hydrogen is possible in the form of a metal hydride, an example being the nickel–metal hydride battery.
Energy carrier hydrogen can be obtained by (photo-)electrolysis of water using renewable energy sources. In fact, hydrogen is a means of storing renewable energy and can be converted back into electrical energy (as mentioned) in a fuel cell, thereby setting the scene for a hydrogen economy.
Here, the main focus will be on rechargeable batteries, in particular rechargeable Li-ion batteries and the design criteria for electric vehicle (EV) requirements and technology comparison.
3.2.2 Rechargeable Batteries
To date, more and more devices and tools are being invented and created that require energy, and usually this energy is provided by primary or rechargeable batteries. Regarding the types of batteries, there are at least five electrochemistries available today, that is, lead–acid (Pb–acid), nickel–cadmium (Ni–Cd), nickel–metal hydride (Ni–MH), lithium-ion (Li-ion), and zinc–air (Zn–air). The Ni–Cd battery is replaced by the Ni–MH battery, because cadmium is no longer accepted in the environment.
The nominal cell voltage, specific energy, energy density, cycle life, and efficiency of selected batteries are presented in Table 3.2.1.
Table 3.2.1 Practical specifications of selected batteries; data from Flipsen (2006) and Kan (2006)
Several metals and alloys can absorb hydrogen to form a hydride as has been referred to in Section 3.2.1. In these metal hydrides the hydrogen ions (protons) are mobile and, therefore, can also participate in an electrode reaction. Here, the nickel–metal hydride battery, which contains a nickel positive electrode and a metal hydride as the negative electrode, is presented as an example. The electrode reactions are as follows:
Positive electrode reaction:
(3.2.1) ![]()
Negative electrode reaction:
(3.2.2) ![]()
Overall battery reaction:
(3.2.3) ![]()
The open circuit voltage (OCV) of this battery is 1.32 V.
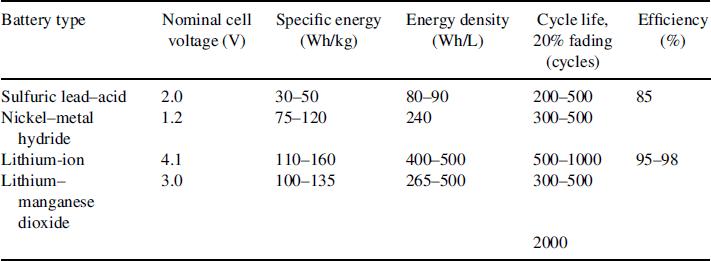
A cross-section of a nickel–metal hydride battery is presented in Figure 3.2.1.
Figure 3.2.1 Internal structure of the cylindrical Ni–MH battery
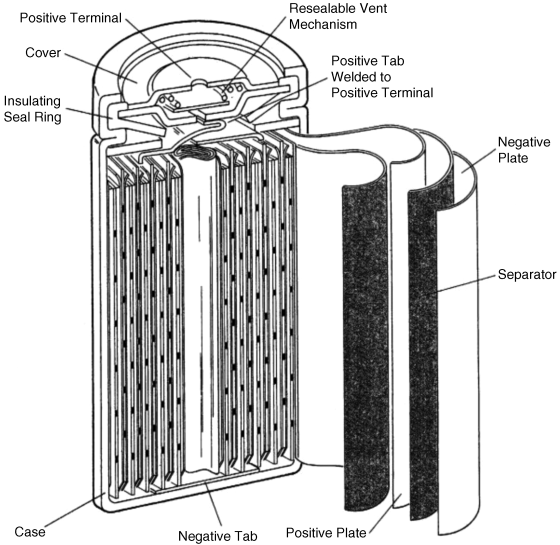
Figure 3.2.2 presents a comparison of the gravimetric and volumetric energy densities of the various rechargeable battery systems.
Figure 3.2.2 The gravimetric and volumetric energy densities of selected rechargeable battery systems
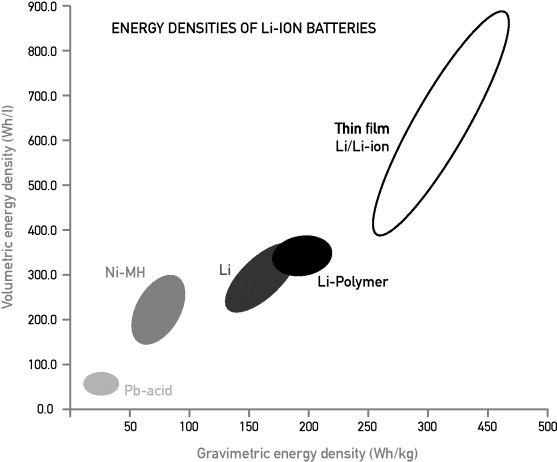
The development of the rechargeable batteries differs a lot from a few years old for metal–air systems to more than 150 years old for lead–acid technology. The properties of these batteries also differ significantly. The application, therefore, will primarily determine which battery to use. Figure 3.2.3 presents Ragone diagrams of these different applications, where the requirements for two specific applications, electric and hybrid vehicles, are indicated in detail.
Figure 3.2.3 Ragone diagrams for different types of rechargeable batteries. Requirements for emission-free electric vehicle and hybrid vehicle applications are indicated
It seems that these requirements have not yet been reached for any battery technology, but the lithium battery technology is the closest in terms of both power and energy density. Indeed, to achieve high energy and power density, a combination of a large specific capacity and current and a high output potential is required. Since lithium is one of the lightest elements in nature, lithium batteries have a very high specific capacity. Moreover, because it has the lowest electrochemical potential, EoLi+/Li = −0.3045 V/NHE (normal hydrogen electrode), the output potential will also be very high (Simonin, 2009). Therefore, the lithium battery is one of the best candidates for high-energy applications.
The sulfuric lead–acid, nickel–metal hydride, lithium-ion, and lithium–manganese dioxide batteries have different designs, and the design aspects of these batteries are presented in Table 3.2.2.
Table 3.2.2 Design aspects of different battery technologies; data from Flipsen (2006) and Kan (2006)
3.2.3 Lithium Batteries
The first lithium-ion battery was commercialized by Sony in 1991 and was based on the exchange of lithium ions between lithiated carbon (LiC6) and a layered oxide (Li1-xMO2), with M being a transition metal ion, usually cobalt, nickel, or manganese. The energy it stores is about 180 Wh/kg and only five times higher than that stored by the lead–acid battery, but it took a revolution in materials science to achieve it (Armand and Tarascon, 2008).
The primary lithium battery comprises a lithium anode (Li) and a manganese dioxide (MnO2) cathode, and has an open-circuit voltage of about 3 V. More interesting are the rechargeable lithium batteries, and these are classified as follows:
- Lithium battery: metallic lithium versus an insertion compound.
- Lithium-ion battery: insertion compound versus an insertion compound and a liquid electrolyte.
- Lithium–polymer battery: insertion compound versus an insertion compound and a polymer electrolyte.
- Lithium–ceramic battery: insertion compound versus an insertion compound and a ceramic electrolyte.
Several metal oxide electrode materials can incorporate lithium atoms during discharge of the battery and release lithium atoms during charge (positive electrode), or can release lithium atoms during discharge and incorporate lithium atoms during charge (negative electrode). Such metal oxides are referred to as insertion compounds.
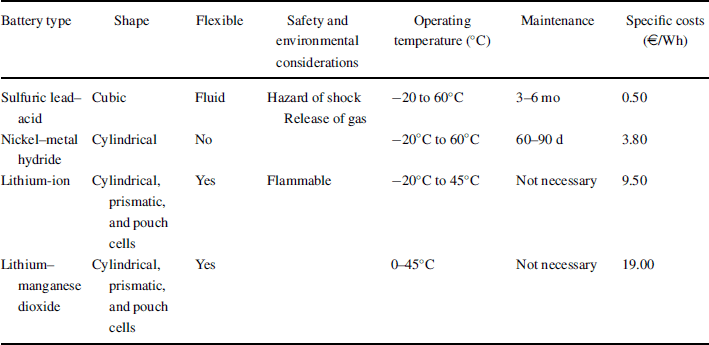
In Figure 3.2.4 the lithium-ion battery principle is shown; the anode (negative electrode) is, for example, lithiated carbon (LiC6) and the cathode (positive electrode) is a metal oxide that can incorporate lithium atoms during discharge and release lithium atoms upon charging the battery. Usually these metal oxides comprise oxides of the transition metals. Characteristic of the insertion compounds is that they are mixed ionic–electronic conductors (MIECs), that is, they exhibit lithium-ion conductivity and electronic conductivity. The liquid, polymer, or ceramic electrolytes exhibit only lithium-ion conductivity. Any electronic conductivity would internally short-circuit the battery.
Figure 3.2.4 The lithium-ion battery principle
In the Periodic Table of the Elements, there are 10 elements between Ca (Z = 20) and Ga (Z = 31), whose occurrence at this position is due to the filling of the 3D orbitals. These elements are called the transition elements or sometimes the d-block elements. Their common characteristic is that the neutral atom, some important ion it forms, or both have an incomplete set of 3D electrons and exhibit variable valence, which leads to many characteristic physical and chemical properties that are important for application in, for example, rechargeable lithium-ion batteries.
Examples of state-of-the-art battery components are as follows:
- Anode materials: Li, C6Li, Li0.5TiO2, Li7Ti5O12, LiFePO4, LiCrTiO4, and LixM (M = Si, Sn, Sb, SnSb).
- Cathode materials: LiMn2O4, doped spinels and inverse spinels, LiCoO2, LiNiO2, LiCo1-xNixO2, and LiFePO4.
- Electrolytes: commercial ethylene carbonate with LiPF6, and ceramic LixBPO4.
All inorganic battery components have a specific crystal structure. In the case of cathode materials, materials with the spinel structure have attracted widespread attention. Spinel is a mineral, MgAl2O4. The structure is based on a cubic close-packed (ccp) array of the oxide ions, with Mg2+ ions in a set of tetrahedral holes and Al3+ ions in a set of octahedral holes. Many compounds of the types M2 + M3 + 2O4 and M4 + M2 + 2O4 adopt this structure. More highly charged cations tend to prefer the octahedral holes so that in the latter compounds, the octahedral holes are occupied by all the M4+ ions and one half of the M2+ ions.
With regard to developments in electrolytes, a new class of soft-matter electrolytes is obtained via heterogeneous doping of non-aqueous (polymer) salt solutions. Typically, the composite electrolytes comprise dispersions of fine inorganic acidic oxide particles (e.g., SiO2 and TiO2) in non-aqueous Li–salt solutions; as discussed, these composite electrolytes behave as a solid and are referred to as soggy sand electrolytes. Characteristic is the occurrence of a percolation type of electrical conductivity along the ceramic–polymer interfaces in the soggy sand electrolytes (Bhattacharyya et al., 2006; Edwards et al., 2006).
To date, research and development are focused on improved anode, cathode, and electrolyte materials, as well as on interfacial problems between the electrodes and the electrolyte. While many attempts to improve the properties of lithium-ion batteries have tackled the problem at the macroscopic scale, current battery materials research is focusing on the nanoscale (Chan et al., (2007, 2008, 2009); Kim et al., 2008). Nanostructured components will give lithium-ion batteries benefits in terms of capacity, power, cost, and materials sustainability that are still far from being fully exploited.
Recent examples are reported on fast and completely reversible Li insertion in vanadium pentoxide nanoribbons (Chan et al., 2007) and on spinel LiMn2O4 nanorods as lithium-ion battery cathodes. In addition, electrode kinetic issues can be circumvented by combining nanomaterials with nanopainting, in which the nanoparticles are coated with a very thin layer of carbon to provide the required electrical conductivity to the individual particles, whose small sizes shorten the diffusion pathways of lithium ions and electrons (Armand and Tarascon, 2008). Moreover, the insertion and removal reactions of lithium in nanostructured LixM anode materials cause the volume to expand or contract several-fold, and accommodating the strains associated with the lithium insertion and removal reactions has been studied in detail with practical solutions having been reported (Chan et al., 2008, 2009; Cui et al., 2009; Simonin, 2009).
Examples of commercially available Li-ion batteries are presented in Figure 3.2.5 and were taken from the Panasonic Lithium Ion Batteries Technical Handbook (Panasonic, 2007).
Figure 3.2.5 Lithium-ion batteries, dedicated to support various types of mobile equipment that require small size, light weight, and high performance (Panasonic, 2007)
In general, lithium-ion batteries are used in battery packs that contain both lithium-ion batteries and battery safety circuits. Both items are sealed in a container made of a material such as resin, so that the battery pack cannot easily be disassembled.
The “constant voltage–constant current” method is used to charge Li-ion batteries, as is illustrated in Figure 3.2.6 for a single cell (Panasonic, 2007).
Figure 3.2.6 The diagram to illustrate the “constant–voltage current” of the charging process

3.2.4 Electric Vehicles
Ni–MH batteries are used to power cheaper electronics and are used in hybrid vehicles. Lithium-ion batteries have conquered high-end electronics and are now being used in power tools. Lithium-ion batteries are also entering the hybrid vehicle market and are serious contenders to power the electric vehicles (EVs) of the future (Armand and Tarascon, 2008). With regard to the introduction of large-scale electric vehicles into society, the most important development is focused indeed on lithium battery technology, and in particular on improved energy density and substantial reduction of costs. The issue of cost will not be addressed in detail within this section, but it is an important issue, as in a recent report of the German Bank stating that a 25 kWh battery package for a battery-powered electric vehicle will cost US$11,250. SB Limotive (Samsung and Bosch) anticipate €350 per kWh in 2015 and €250 around 2020 (van Ginneken, 2011). With regard to battery design criteria, the present EV battery requirements and technology comparison have been taken in part from Broussely (2009) of Saft, Besenhard (1999), and Balbuena and Wang (2004).
The energy consumption of an EV is proportional to its weight. The energy consumption of an EV is typically 120 Wh/ton/km. Since the allowable weight for the battery onboard a given vehicle is limited, the better the energy density, the larger the available energy and the vehicle range (Besenhard, 1999; Broussely, 2009).
The amount of electrical energy per mass or volume that a rechargeable battery can deliver is a function of the cell's voltage and capacity, which are dependent on the chemistry of the system. Power is also an important parameter, which depends partly on the battery's engineering, but crucially on the chemicals comprising the battery. Armand and Tarascon state that the stored energy content of a battery can be maximized in three ways: (1) by a large OCV, (2) by making the mass (or volume) of the active electrode materials per exchanged electrons as small as possible, and (3) by ensuring that the electrolyte is not consumed in the chemistry of the battery. This latter condition holds only for the Ni–MH and the lithium-ion batteries.
In a comparison of different technologies (energy density) of EV batteries, that is, lead–acid (33 Wh/kg), Ni–Cd (45 Wh/kg), Ni–MH (70 Wh/kg), and Li–ion (120 Wh/kg) for a typical size of 250 kg, as well as power characteristics for a 250 kg battery, that is, 75 W/kg, 120 W/kg, 170 W/kg, and 370 W/kg, respectively. Here, lithium-ion batteries exhibit superiority over traditional electrochemistries.
For pure EV applications, the battery manufacturers have developed ranges of cells with individual capacities between 25 and 100 Ah. Each EV battery maker has its own proprietary choice of negative electrode material and electrolyte solvent mixture, but the use of the lithium hexafluoro–phosphate (LiPF6) to provide lithium-ion conduction in the liquid electrolyte is standard.4
