Chapter 26: Health, Safety, and the Environment
WHAT YOU WILL LEARN
The basics of health, safety, and the environment
One goal of chemical engineering is to produce goods and services that enhance the quality of life. Chemical engineers have been at the forefront of efforts to improve health (e.g., pharmaceuticals), safety (e.g., shatterproof polymer glasses), and the environment (e.g., catalytic converters). Moreover, throughout the product life cycle, chemical engineers are concerned with potential harm to the health and safety of people and damage to the environment. This chapter focuses on assessment of these potential dangers. Chapter 27 focuses on proactive strategies to avoid them.
Although many of the chemical processing industries regard improvements of health, safety, and the environment (HSE) as one general function, the U.S. government has separated the field into distinct categories, based on the varying rights afforded to different classes and for historical purposes. The health and safety of employees, for example, is regulated by the Occupational Safety and Health Administration (OSHA), whereas the health of nonemployees and the environment are regulated by the Environmental Protection Agency (EPA). Other activities are regulated by the Department of Transportation (DOT) or the Department of Energy (DOE), among other agencies.
An exact description of applicable laws, rules, and regulations would be out of date before any textbook could be printed; rather, this chapter describes the types of regulations that are relevant to chemical engineering and provides guidance on where to find the current (and proposed) regulations. The focus is on the general concepts and strategies of risk assessment and reduction that transcend the details of regulations.
26.1 Risk Assessment
There are many ways to view risks to a person’s health or safety. Some people will knowingly accept tremendous risk (such as in rock climbing or smoking), some expose themselves to risk perhaps without knowing (such as by sunbathing), and some refuse to be exposed at all to certain risks (such as extremely low concentrations of pesticides in food). Generally, people are much more accepting of risks that they choose (voluntary exposures) than to risks that are forced upon them (involuntary exposures). Also, people are generally much more accepting of risks that they understand than they are of risks that they do not understand. For example, the population at large tends to accept that human activity often degrades rivers and lakes with biological and chemical pollution, but they do not accept any measurable radioactive emissions from power plants.
26.1.1 Accident Statistics
Engineers quantify risks to provide a rational basis for deciding what activities should be undertaken and which risks are worth the benefits provided. These decisions are frequently made by nonengineers; thus, it is essential that the measures that engineers use be understandable to the public. For this reason, several measures have been established.
The OSHA incidence rate is the number of injuries and illnesses per 200,000 hours of exposure. Any injury or work-related illness that results in a “lost workday” is counted in the ratio. Thus, minor injuries that can be treated with first aid are not counted, but counted injuries range all the way to death. The 200,000 hours is roughly equivalent to 100 worker years. The OSHA incidence rate illustrates two features of risk measures: the details of the accident are not included, and these measures can be used for non-work-related exposures.
The fatal accident rate (FAR) is the number of fatalities per 108 hours of exposure. Only fatalities are counted, and the 100,000,000 hours is roughly equivalent to 1000 worker lifetimes. Although only deaths are counted, in many fields there is a strong correlation between numbers of injuries and numbers of deaths. Thus, the reasonable presumption is that a decrease in the FAR will also decrease the OSHA incidence rate.
For some exposures, the available data are insufficient to determine the total time of exposure. For these cases, the fatality rate can be used. This measure is the number of fatalities per year divided by the total population at risk. For example, the exposure of individual smokers is extremely variable because of differences in type of cigarette, number of inhalations per cigarette, and so on. However, the fatality rate can be determined (~0.005), which gives a rough estimate of the chance that a smoker will die this year from smoking-related causes of 0.5%.
Table 26.1 shows some representative numbers of these three risk measurements. One of the possibly surprising observations is that the chemical process industries are relatively safe (for workers) compared with many other industries. There are many reasons for this, ranging from the high level of remote sensing and operation, which separates workers from the most dangerous parts of the plant, to the historical concern for the hazards of industrial chemicals. Regardless of the reasons, chemical engineers continue to try to improve the safety of chemical plants.
Table 26.1 Comparison of Three Risk Measurements
Activity |
OSHA Incident Rate (Injuries and Deaths per 200,000 h) |
Fatal Accident Rate (Deaths per 100,000,000 h) |
Fatality Rate (Deaths per Person per Year) |
Working in chemical industry |
1.9 |
2.8 |
|
Staying at home |
3 |
||
Working in construction |
2.8 |
11.1 |
|
Traveling by car |
57 |
170 × 10−6 |
|
Rock climbing |
4000 |
40 × 10−6 |
|
Smoking (1 pack per day) |
5000 × 10−6 |
||
Being struck by lightning |
|
|
1 × 10−7 |
Source: Crowl, D. A., and J. F. Louvar, Chemical Process Safety: Fundamentals with Applications, 3rd ed. (Englewood Cliffs, NJ: Prentice Hall, 2011) [1]. |
|||
Another observation is that there are differences in relative rankings if one uses the different risk measurements. Perhaps most striking is that the FAR for employees of the chemical industry is only slightly higher than the FAR for people staying in their homes. However, this is a potentially misleading comparison. The portion of the population that works is on average more healthy than the portion that does not. Also, most people take risks at home that they would not be allowed to take at work. Finally, a worker is probably more tired and prone to accidents at home after a hard day at work.
The main use of these statistics is not to compare one activity with another (unless they can be substituted for one another) but to monitor improvements to the health and safety of workers and others achieved by process modifications.
26.1.2 Worst-Case Scenarios
Another measure of risk is to imagine the worst possible consequence of an operation. Such a study is called a worst-case-scenario study, and it is required by some government agencies. These studies have some drawbacks, but they can be very useful in identifying ways to avoid serious accidents. A related strategy that is routinely used throughout the industry for identifying potential hazards is called HAZOP and is discussed in Section 26.4.
The development of worst-case scenarios is certainly subjective, but government agencies and organizations develop guidelines for this task. For example, there is certainly a chance that an asteroid will impact Earth directly in the middle of the chemical plant. Should this be the worst-case scenario? Most people would argue that this takes the worst-case scenario study beyond reason, but there are no clear-cut rules. The subjective rules that have been developed contain definitions such as the worst accident that might reasonably be assumed possible over the life of the facility. Different people would define “possible” (or “probable”) in different ways. Is sabotage by an employee possible? Is the simultaneous failure of three independent safety systems probable? Certainly the events of September 11, 2001, have indicated that a terrorist-attack scenario is not impossible. Sometimes probabilities of occurrence can be estimated, but often they cannot.
By EPA definition (40CFR68.3), “Worst-case release means the release of the largest quantity of a regulated substance from a vessel or process line failure that results in the greatest distance to an endpoint defined in § 68.22(a).” This end point is a specified concentration of a toxic substance, an overpressure of 1 psi for explosions, a radiant heat flux of 5 kW/m2 for 40 seconds for a fire, or the lower flammability limit for a flammable substance, whichever is appropriate. Thus, the worst case is defined by the size of the area adversely affected by the release. There are also definitions of how much of the material in a tank (often 100%) should be considered over what period of time (often 10 minutes) in the scenario.
All this is to say that worst-case-scenario analyses can be extremely helpful, but they are difficult to perform and potentially difficult to understand. They are most useful when specific guidelines are followed and when they are used to enhance safety by developing safeguards against the accident scenarios developed. For the risk management plan described in Section 26.2.2, worst-case-scenario analyses are required by regulation.
These guidelines are constantly changing, so they are not described in detail here. The most current guidelines should be obtained from the EPA, OSHA, or other agencies.
26.1.3 The Role of the Chemical Engineer
As the professional with the best knowledge of the risks of a chemical processing operation, the chemical engineer has a responsibility to communicate those risks to employers, employees, clients, and the public.
It can be very difficult to explain FARs in deaths per 108 hours to the general, nontechnical public. However, the consequences of failure to explain rationally and honestly to the public the risks and the steps taken to reduce those risks are tremendous. The ethical role of the chemical engineer in this communication is discussed in Chapter 25. Beyond those responsibilities, the damage from poor communication can destroy an industry. For example, the nuclear power industry in the United States has been affected in large part by the failure to communicate risks to the general public in a way that it could understand. When even relatively minor accidents occur, many feel that they were misled by engineers who seemed to have said that there were no risks, no chance of an accident.
26.2 Regulations and Agencies
Rules and regulations arise both from governmental agencies and from nongovernmental organizations (such as AIChE). These rules and regulations change constantly, and the most up-to-date rules should always be determined. The following sections describe the kinds of rules and regulations promulgated (put into effect by formal public announcement) by various organizations. The actual agency should be contacted directly for the latest rules.
Federal government rules and regulations are published in the Federal Register (FR), which is issued daily. Notices of proposed or pending regulations are also given in the FR. Annually, the regulations of a specific type are collated and published in the Code of Federal Regulations (CFR). Both the FR and the CFR are available in large libraries and in all law libraries, as well as on the Internet. In Table 26.2 the Internet addresses for the FR and CFR as of the date this book is written are provided. Because Internet addresses change frequently, the use of a search engine to find new URLs is suggested if the links given here are broken. However, note that only server addresses ending in “.gov” are official U.S. government Web sites.
Table 26.2 Internet Addresses for Federal Agencies
Code of Federal Regulations (CFR) |
|
Federal Register (FR) and U.S. Code |
|
DOT regulations (FR and 49 CFR) |
|
EPA regulations (FR and 40 CFR) |
|
MSHA regulations (FR and 30 CFR) |
|
NIOSH databases |
|
OSHA regulations (FR and 29 CFR) |
In addition to the direct government sources, numerous private firms collate federal regulations (more quickly than does the government), and they sell their compendia, often tailored to the needs of the customer.
State and local government regulations are also available in hard copy and increasingly in online form. However, it is best to contact these agencies directly to be sure that one has all the relevant regulations. Nongovernmental organizations can be contacted directly for their rules. However, sources of information on the Internet are notoriously inaccurate and out of date. Unless it is an official government Web site (with the date of the latest update), beware.
26.2.1 OSHA and NIOSH
In general, the Occupational Safety and Health Administration promulgates regulations having to do with worker safety and health in industries other than mining (Mine Safety and Health Administration, MSHA, serves a similar role there). The National Institute for Occupational Safety and Health (NIOSH) is a federal research organization that provides information to employees, employers, and OSHA to help assess health and safety risks. Regulations are not promulgated by NIOSH, although it does certify various analytical techniques and equipment (such as respirators).
One major law and five major regulations in this area are the OSHA Act [2], Hazard Communication (29CFR1910.1200), Air Contaminants (29CFR1910.1000), Occupational Exposure to Hazardous Chemicals in Laboratories (29CFR1910.1450), Hazardous Waste Operations and Emergency Response (26CFR1901.120), and Process Safety Management of Highly Hazardous Chemicals (29CFR1910.119).
OSHA Act and Air Contaminants Standard. The original act of Congress that set up OSHA, the Occupational Safety and Health Act of 1970 [2], specified certain regulations and standards and required OSHA to promulgate others. Perhaps of most importance is the so-called general duty clause of the OSHA Act stating that “each employer shall furnish to each of his employees employment and a place of employment which are free from recognized hazards that are causing or are likely to cause death or serious physical harm to his employees” [Reference 2, Section 5.a.1]. This clause has been interpreted to mean that an employer must avoid exposing employees to hazards that should have been known to the employer, whether or not that hazard is specifically regulated by OSHA. Thus, the responsibility of researching the literature to see whether anyone has identified a hazard is placed on the employer. Most chemical engineers are employees, and yet they often represent the employer and therefore assume the responsibilities under the general duty clause.
To search for published data on chemical hazards, a good place to start is the Integrated Risk Information System (IRIS), the Hazardous Substances Data Bank (HSDB), and other databases at http://www.toxnet.nlm.nih.gov.
Some specific regulations ensued from the OSHA Act, notably for the chemical industry exposure limits for certain substances in breathing air for employees (29CFR1910.1000 Air Contaminants). These limits are called permissible exposure limits (PEL) and are often given in parts per million (by volume). Related limits are updated yearly by the American Conference of Governmental and Industrial Hygienists (ACGIH, a nongovernmental association) and are called threshold limit values (TLV) [3]. In fact, the original OSHA regulations merely made these TLVs the official government limits. NIOSH also publishes recommended exposure limits (REL), but these are not legally binding regulations. Each of these exposure limits is based on the assumption that a typical worker can be exposed to that concentration of the substance for eight hours a day, five days a week, for a working lifetime, without ill effects. These assumptions are often based on sketchy data extrapolated from animal studies. All these limits (PEL, TLV, REL) are given in the convenient NIOSH Pocket Guide to Chemical Hazards [4], available in printed form from NIOSH for a small charge or available free as an HTML or PDF download from the NIOSH Web site. Respirator requirements and a description of the health effects of exposure are also given.
The exposure limits are based on a time-weighted average (TWA) over an eight-hour shift, which means that higher concentrations are allowable, as long as the average over the shift is not greater than the PEL. When even short-term exposure to higher levels is harmful, there is a separate short-term exposure limit (STEL), which is a 15-minute time-weighted average concentration that must never be exceeded, or an OSHA ceiling concentration, which is an instantaneous concentration that must never be exceeded. Also, maximum concentrations from which one could escape within 30 minutes without experiencing escape-impairing or irreversible health effects are identified as immediately dangerous to life and health (IDLH) concentrations. The IDLH limit is used under conditions in which a respirator is normally used. In the event of a respirator failure, the employee might not be able to escape if the concentration is greater than the IDLH. These limits are also given in the NIOSH handbook [4].
Hazard Communication Standard. This regulation is also known as the Worker Right to Know or simply HazCom. The reference is 29CFR1910.1200. It requires that the employer train all employees so that the employees understand the hazards of the substances that they are handling or are exposed to or potentially exposed to. It is explicitly stated that the employer has not met this standard merely by giving the employee the hazard information orally or in written form. The employer must make sure that the employee understands the hazards; thus, much effort in training goes into satisfying this requirement. Two very obvious results and requirements of this regulation are proper labeling of containers and availability of safety data sheets (SDS), which were formerly known as material safety data sheets (MSDS), with the former name still being ubiquitous on the Internet. Table 26.3 lists typical major sections of an SDS, but the precise format of an SDS is not presently defined by regulation, although some of the minimum requirements are, and these are also listed in Table 26.3. The SDS must list the substances, their known hazards, and procedures for proper handling of the material and for proper actions in an emergency. Although only hazardous materials must have these SDSs, they are available for almost all substances and mixtures in commerce. They are available directly from the supplier or manufacturer or on the Internet. It is recommended to use the Internet only as a preliminary source for SDSs. SDSs should be obtained directly from more than one manufacturer if possible, because errors in SDSs do occur and because different SDSs will contain different data.
Table 26.3 Typical Sections of a Safety Data Sheet (SDS)
1. |
Material and manufacturer identification |
2. |
Hazardous ingredients/identity information |
3. |
Physical/chemical characteristics |
4. |
Fire and explosion hazard data |
5. |
Reactivity data |
6. |
Health hazard data |
7. |
Precautions for safe handling and use |
8. |
Control measures |
From OSHA Form 1218-0072. |
|
Unfortunately, often SDSs are difficult to read quickly. They are filled with much useful information, but they are often several pages long. In addition, different chemical suppliers use different formats. (OSHA now recommends the ANSI 16-section format.) They must be studied extensively before any hazardous situation is encountered. A group of international agencies (UN Environment Programme, International Labour Office, and the World Health Organization in cooperation with NIOSH and the European Community) has developed a simpler, standard, two-page data card that can be understood quickly and used in an emergency. In addition to the required SDSs, the ready availability of this international chemical safety card wherever a hazardous material is used, stored, or transported is highly recommended. They can be found at https://www.cdc.gov/niosh/ipcs/.
On June 1, 2007, the European Community put into force a new regulation called Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH). Although it specifically applies to manufacturers within the European Union and manufacturers that import into the EU, it is becoming the de facto worldwide standard for regulation of chemical products. All firms that handle one metric tonne or more per year of any chemical product or produce any chemical product that is new to commerce are covered. For many existing chemical products, new data have been measured to determine hazards more accurately. Substances that are high-volume, mutagenic, or very toxic to aquatic organisms have already been fully phased in. Other low-volume substances will be phased in through May 2018. Details of the regulation are available at http://echa.europa.eu.
Minimum Requirements for SDS (29CFR1910.1200[g]). An SDS is required for each “hazardous chemical,” including those specifically listed in 29CFR1910 (subpart Z), any material assigned a TLV, or any material determined to be cancer causing, corrosive, toxic, an irritant, a sensitizer, or one that has damaging effects on specific body organs. There are numerous facts that may be given in an SDS; however, the minimum information required is listed below.
Written in English.
Identity used on label.
Chemical name and common name of all ingredients that are hazardous and that are present in ≥ 1% concentration or that could be released in harmful concentrations.
Chemical name and common name of all ingredients that are carcinogens and that are present in ≥ 0.1% concentration or that could be released in harmful concentrations.
Physical and chemical characteristics of the hazardous chemical (such as vapor pressure, flash point).
Physical hazards of the hazardous chemical, including the potential for fire, explosion, and reactivity.
Health hazards of the hazardous chemical, including signs and symptoms of exposure, and any medical conditions that are generally recognized as being aggravated by exposure to the chemical.
Primary routes of entry.
OSHA permissible exposure limit, ACGIH TLV, and any other exposure limit used or recommended by the chemical manufacturer, importer, or employer.
Whether the hazardous chemical is listed in the National Toxicology Program (NTP) Annual Report on Carcinogens (latest edition) or has been found to be a potential carcinogen in the International Agency for Research on Cancer (IARC) Monographs (latest editions), or by OSHA.
Any generally applicable precautions for safe handling and use that are known to the chemical manufacturer, importer, or employer preparing the SDS, including appropriate hygienic practices, protective measures during repair and maintenance of contaminated equipment, and procedures for cleanup of spills and leaks.
Any generally applicable control measures that are known to the chemical manufacturer, importer, or employer preparing the SDS, such as appropriate engineering controls, work practices, or personal protective equipment.
Emergency and first aid procedures.
Date of preparation of the SDS or the last change to it.
Name, address, and telephone number of the chemical manufacturer, importer, employer, or other responsible party preparing or distributing the material safety data sheet that can provide additional information on the hazardous chemical and appropriate emergency procedures, if necessary.
Process Safety Management of Highly Hazardous Chemicals (29CFR1910.119). This OSHA regulation applies to essentially all of the chemical processing industries and requires action in 13 different types of activities as shown in Table 26.4. Note, however, that transportation of hazardous materials is regulated by the U.S. Department of Transportation under 49CFR100 through 49CFR185 (https://www.phmsa.dot.gov/hazmat/standards-rulemaking/regulations). Similarly, the safe operation of chemical laboratories is regulated by OSHA through 29CFR1910.1450, which recognizes that laboratories and production facilities present different kinds of hazards.
Table 26.4 Process Safety Management of Highly Hazardous Chemicals (29CFR1910.119)
1. |
Employee participation |
2. |
Process safety information |
3. |
Process hazards analysis |
4. |
Operating procedures |
5. |
Training |
6. |
Contractors |
7. |
Pre-start-up safety review |
8. |
Mechanical integrity |
9. |
Hot work permit |
10. |
Management of change |
11. |
Incident investigation |
12. |
Emergency planning and response |
13. |
Compliance safety audit |
When OSHA promulgated the Process Safety Management Regulation in 1992, it used the already existing rules of the American Institute of Chemical Engineers’ Guidelines for Technical Management of Chemical Process Safety [5] and the American Petroleum Institute’s Recommended Practices 750 [6] as guides. In fact, as is often the case, OSHA essentially gave the force of law to these voluntary nongovernmental standards.
Process safety management (PSM) embraces nearly the entire safety enterprise of a chemical process organization. It requires employee training, written operating procedures, specific quality in the engineering design of components and systems, very specific procedures for some activities, investigation and reporting of accidents that do occur, and an internal audit of the safety enterprise of the company. Following is a description of each of the 13 components.
Employee Participation: The employer must actively involve the employees in the development and implementation of the safety program. Employees are more likely to understand the hazards and to follow the established safety procedures when they are involved early and continuously in the development of the safety program. This item was added to the earlier API and AIChE standards.
Process Safety Information: The employer must research the materials, process, and operation to determine the potential hazards and keep in an immediately accessible form all safety information. This includes all SDSs, as well as information on the process itself, such as up-to-date process flow diagrams and P&IDs.
Process Hazards Analysis: Before a process is started up and periodically thereafter (typically every three to five years or whenever significant modifications are made), a detailed study must be made of the process to determine potential hazards and to correct them. There are several approved procedures, and an organization can opt to use an alternative procedure if it can be shown to be as effective. In fact, most of the chemical processing industry uses the HAZOP technique, which is described in Section 26.4. This technique is a modified brainstorming process in which potential hazards are identified, their consequences are determined, and an action to deal with the hazard is identified.
Operating Procedures: Written operating procedures must be available to operators, and any deviations in the plant operation from these procedures must be noted. These procedures must include start-up, shutdown, and emergency response to process upset.
Training: The employer must train all employees in the hazards present and the procedures for mitigating them.
Contractors: The employer is responsible for the safe conduct of any contractors. Although each contractor is responsible for the safe conduct of the contractor’s employees, the owner or operator of the plant who enters into a contract with the contractor remains liable for the safe operation of the contractor. This is an OSHA addition to the earlier API and AIChE standards.
Pre-Start-up Safety Review: The regulation specifically requires that there be a review of the safety aspects of the process before any processing occurs on the site. The review must be documented, and any deviations of the plant as built from the design specifications must be addressed.
Mechanical Integrity: Vessels and other equipment must meet existing codes and be inspected during manufacture and after installation. Appropriate procedures for maintenance must be developed and followed.
Hot Work Permit: This is a very specific procedure by which a wide range of people in the plant are notified before hot work, such as welding, can occur. Many chemical plants use flammable materials, and everyone in the area needs to be informed so that no flammable vapors are released during the operation.
Management of Change: During accident investigations in the chemical process industries, it has often been found that severe incidents (involving deaths and massive destruction) occurred because equipment, processes, or procedures were changed from the original design without careful study of the consequences. Thus, the OSHA regulation requires companies to have in place a system by which any modification is reviewed by all of the appropriate people. For example, any change in the reactor design must be reviewed not only by the design engineer but also by the process engineer who can evaluate how the overall process is affected. The maintenance leader must also make sure that the modification does not adversely affect the maintenance schedule or the ability of workers to get to or to maintain the equipment.
Incident Investigation: When there is a hazardous process upset, it must be investigated and a written report must be developed indicating the details of the incident, the probable cause, and the steps taken to avoid future incidents.
Emergency Planning and Response: There must be a written plan, and employees must be trained to respond to possible emergency situations.
Compliance Safety Audit: Periodically, all of the elements of the safety system (including items 1–12 above) must be audited to make sure that the approved procedures are being followed and that they are effective.
One item that is included in the industry codes but not in the PSM regulation is the entry of workers into confined spaces. This situation—in which the environment of the space or the difficulty of egress from the space could create a hazard—is encountered frequently in the chemical process industries. OSHA regulation 29CFR1910.146 covers the required permitting procedures to ensure that workers are protected in confined spaces.
26.2.2 Environmental Protection Agency (EPA)
The role of the EPA is to protect the environment from the effects of human activity. Although this is a very broad role, in the context of the chemical processing industries it usually relates to emissions of harmful or potentially harmful materials from the plant site to the outside by air or by water. There are three classes of such emissions: (1) planned emissions, (2) fugitive emissions, and (3) emergency emissions. This section describes some of the present regulations for these classes of emissions. There are many more regulations that are not mentioned here. In any facility, one must keep constantly aware of new and modified regulations through research, use of an environmental compliance consulting firm, or communication with the local, state, and federal environmental protection agencies.
Planned Emissions. Any process plant will have emissions. These may be harmful to the environment, benign, or, in rare cases, beneficial. In any case, a permit is usually required before construction or operation of the plant. Significant modifications to the plant (especially if they change the design emissions) will likely require a modification to the permit or a new permit.
These emissions permits are normally obtained through the state environmental protection agency, but federal regulations must be met. In some regions, states, or localities, the requirements for the permit may be significantly more stringent than the federal EPA regulations. One must contact the local agencies. However, searching the EPA databases described in Section 26.2 can provide a good preliminary overview.
Permits frequently require an extensive environmental impact statement (EIS) detailing the present environment and any potential disturbances that the planned activity could produce. For process plants, these EISs are typically written by a team of chemical engineers, biologists, and others, and they deal not only with planned emissions but also with potential process upsets and emergencies. In this regard, the worst-case scenario mentioned in Section 26.1.2 is used.
Permitting is based on assessment of potential degradation of the environment, and thus both the level of emissions from the plant and the present level of contamination in the local environment are considered. There are National Ambient Air Quality Standards (NAAQS) for a few materials and National Emissions Standards for Hazardous Air Pollutants (NESHAP) and New Source Performance Standards (NSPS) for these and others. Major sources (defined as those plants that emit more than some annual threshold quantity, such as 25 tons of hazardous air pollutants) must meet the most stringent emissions criteria and require more permits. Similar standards are applied for water quality and for discharges into the water. Many of these regulations are based on the Clean Air Act and Clean Water Act, among others.
Beyond the effects on the environment after emissions are fully dispersed in the air or the water, there can be acute, short-term effects on nearby populations. Often chemical engineers perform dispersion studies to determine the range and longevity of the plume of harmful materials that flows from the point of discharge into the air or water.
The focus of the Clean Air Act Amendments of 1990, Title I, is the release of volatile organic compounds (VOCs), which are precursors to the photochemical production of ozone (smog), especially in areas that have not attained NAAQS. The definition of a VOC is any organic compound with an appreciable vapor pressure at 25°C that participates in atmospheric photochemical reactions. It does not include methane, ethane, and a number of other substances listed in 40CFR51.100(s). Hazardous air pollutants (HAP) are also regulated through Title III of the act.
An important part of planned emissions are so-called fugitive emissions. These are losses from seals in rotating equipment (e.g., pumps, agitators, compressors), losses through connections between equipment (e.g., piping connections, valves), and other losses that result from incomplete isolation of the interior of the process from the atmosphere (e.g., tanks). Although substantial progress has been made over the last several decades to reduce fugitive emissions through improved equipment design, fugitive emissions are still substantial and are, in some cases, the major source of all emissions from a process plant.
Emergency Releases. Process upsets can create catastrophic releases of hazardous materials, and regulations require that there be an effective plan to deal with these occurrences and that the consequences for affected populations not be too serious. As mentioned earlier, worst-case scenarios and dispersion modeling are used to make this assessment.
One such regulation is the Emergency Planning and Community Right to Know Act (EPCRA) of 1986, also known as SARA, Title III. This regulation requires plants to provide the local community with information about potentially hazardous or toxic materials or processes. Further, the plant must work with the local community to develop effective emergency procedures that will be implemented automatically in the event of an accident. A local emergency planning committee is formed of members of the local government, emergency response organizations, and plant personnel. Also, releases of certain hazardous substances must be reported to the EPA and a compilation of these releases made available to the public through the Toxic Release Inventory System.
The EPA provides querying and mapping functions for its databases through the EPA Envirofacts System at https://www.epa.gov/emefdata/em4ef.home. Included are the Toxic Release Inventory and databases on Superfund sites, drinking water, water discharge, hazardous waste, UV index, and air releases.
Through the DOT, regulations require all over-the-road transport vehicles to carry a manifest of hazardous materials that is immediately available to all emergency personnel in the event of an accident. Also, the DOT and the U.S. Coast Guard regulate the conditions under which hazardous cargo can be transported. For example, these regulations frequently require stabilizing additives to prevent runaway polymerization.
The National Response Center (1-800-424-8802) is operated by the U.S. Coast Guard “to serve as the sole national point of contact for reporting all oil, chemical, radiological, biological, and etiological discharges into the environment anywhere in the United States and its territories.” When a call is received, the information is relayed to the National Response Team as well as to various government agencies that maintain incident databases.
Many other EPA regulations that are beyond the scope of this book impact the operation of chemical processing facilities, including the Resource Conservation and Recovery Act (RCRA); the Comprehensive Environmental Response, Compensation, and Liability Act known as Superfund (CERCLA); the Superfund Amendments and Reauthorization Act (SARA); and the Toxic Substances Control Act (TSCA).
EPA Risk Management Program. The Clean Air Act Amendments of 1990 also “require the owner or operator of stationary sources at which a regulated substance is present to prepare and implement a Risk Management Plan (RMP) and provide emergency response in order to protect human health and the environment” [40 CFR 68]. The final rule was implemented in 1999. As with the OSHA Act, there is a general duty clause in this regulation specifying that owners and operators of plants have “a general duty...to identify hazards which may result from such releases using appropriate hazard assessment techniques, to design and maintain a safe facility taking such steps as necessary to prevent releases, and to minimize the consequences of accidental releases which do occur” [Reference 7, Section 112(r)(1)]. The RMPs must be registered with the EPA, they must be made public, and they must be periodically updated. The risk management program, which includes as a subset the risk management plan, must include three elements:
Hazard assessment
Prevention
Emergency response
This program is coordinated with OSHA’s process safety management (PSM). In fact, compliance with the PSM standard is considered equivalent to the prevention part of the RMP. The following overview of the risk management program pertains to all plants covered under the PSM standard, which includes most plants in the chemical process industries.
The hazard assessment must include a worst-case analysis, an analysis of non-worst-case accidental releases, and a five-year accident history. The worst-case release scenario is defined by the EPA [40 CFR 68] as
the release of the largest quantity of a regulated substance from a vessel or process line failure, including administrative controls and passive mitigation that limit the total quantity involved or the release rate. For most gases, the worst-case release scenario assumes that the quantity is released in 10 minutes. For liquids, the scenario assumes an instantaneous spill; the release rate to the air is the volatilization rate from a pool 1 cm deep unless passive mitigation systems contain the substance in a smaller area. For flammables, the worst case assumes an instantaneous release and a vapor cloud explosion.
The EPA rule specifies default values of wind speed, atmospheric stability class, and other parameters for the development of the offsite consequence analysis of worst-case scenarios. It also specifies the end point for the consequence analysis, based on the calculated concentration of toxic materials, the overpressure (1 psi) from vapor cloud explosions, and the radiant heat exposure for flammable releases (5 kW/m2 for 40 seconds).
The prevention program is identical to the PSM standard, except that the emergency planning and response item is covered under a separate category in the RMP.
The emergency response program portion of the risk management plan is coordinated with other federal regulations. For example, compliance with the OSHA Hazardous Waste and Emergency Operations (HAZWOPER) rule (29 CFR 1910.120), the emergency planning and response portion of the PSM standard, and EPCRA will satisfy this requirement in the RMP regulation.
The RMP must designate a qualified person or position with overall responsibility for the program, as well as show the lines of authority or responsibility for implementation of the plan.
Overall, then, the only additional RMP requirement for plants already covered by the OSHA process safety management regulation is the hazard assessment (including off-site consequence analyses of worst-case and non-worst-case accidental release scenarios). This hazard assessment must not be confused with the process hazard analysis (PHA). The hazard assessment is a study of what will happen in the event of an accidental release and usually includes, for example, air dispersion simulations. The PHA (e.g., HAZOP) studies the hazards present in the process and seeks to minimize them through redesign or modifications to operating procedures.
26.2.3 Nongovernmental Organizations
Many professional societies and industry associations develop voluntary standards, and these are often accepted by government agencies and thereby are given the force of law. Examples of such organizations and their standards are as follows:
American Petroleum Institute (API), Recommended Practices 750
American Institute of Chemical Engineers (AIChE)
Center for Chemical Process Safety (CCPS)
Design Institute for Emergency Relief Systems (DIERS)
American National Standards Institute (ANSI)
American Society for Testing and Materials (ASTM)
National Fire Protection Association (NFPA), fire diamond
American Conference of Governmental Industrial Hygienists (ACGIH), TLVs
American Chemistry Council, Responsible Care program
Synthetic Organic Chemicals Manufacturers Association (SOCMA)
American Society of Mechanical Engineers (ASME), boiler and pressure vessel code
The Responsible Care program is a chemical industry initiative started in 1988. All the members of the American Chemistry Council (about 160 companies) as well as several other industry organizations in the United States and in 50 other countries agree to operate according to this health, safety, and environment code. The details are given on the ACC Web site (http://www.americanchemistry.com). Its key areas include
Environmental impact
Employee, product, and process safety
Energy
Chemical industry security
Product stewardship: managing product safety and public communications
Accountability through management system certification
Contribution to the economy
26.3 Fires and Explosions
The most common hazards on many chemical plant sites are fires and explosions. Whenever a fuel, an oxidizer, and an ignition source are present, such a hazard exists. Detailed analyses of these hazards and their consequences are covered in other books [1, 8]. Here, the terminology of the field is introduced.
26.3.1 Terminology
Combustion is the very rapid oxidation of a fuel. Most fuels oxidize slowly at room temperature. As a fuel is heated, it oxidizes more rapidly. If the heating source is removed, the fuel cools, and its oxidation returns to its normal rate for room temperature.
However, if a certain temperature (the auto-ignition temperature) is exceeded, the heat liberated by the oxidation is sufficient to sustain the temperature, even if the external heating source is removed. Thus, above the auto-ignition temperature, the reaction zone will expand into other areas having appropriate mixtures of fuel and oxygen. The minimum energy required to heat a small region to the auto-ignition temperature is called the ignition energy and is often exceedingly small.
A gaseous mixture of fuel and air will ignite only if it is within certain concentration limits. The lower flammability (or explosive) limit (LFL or LEL) is the minimum concentration of fuel that will support combustion and is somewhat below the stoichiometric concentration. The maximum concentration of fuel that will support combustion is called the upper flammability (or explosive) limit (UFL or UEL). Above the UFL, the mixture is too “rich”—that is, it does not contain enough oxygen. These two limits (UFL and LFL) straddle the stoichiometric concentration for complete combustion of the fuel. It is because of the convenient upper flammability limit of gasoline that this fuel is not more dangerous than it is. (See Problem 26.8.) Any mixture within the flammability limits should be avoided or very carefully controlled.
The flash point of a liquid is the temperature at which the vapor in equilibrium with the standard atmosphere above a pool of the liquid is at the LFL. Thus, a low flash point indicates a potential flammability problem if the liquid is spilled. Diesel fuel, for example, is much safer than is gasoline because diesel has a higher flash point. Regulations for transportation and use of gasoline are therefore much more stringent than they are for diesel. Flash point can be measured by the open-cup or the closed-cup method. In the open-cup method, an open container of the liquid is heated while a flare-up of the vapor is intentionally attempted with an ignition source. The temperature at which flare-up occurs is the flash point. In the closed-cup method, the liquid is placed in a closed container and allowed to come to equilibrium with air at standard pressure. Ignition is attempted at increasing temperatures. Although the open-cup and closed-cup flash points for many materials are very close, materials that vaporize slowly and disperse in the atmosphere quickly can have much higher open-cup flash points than their closed-cup flash points. Although the SDS will give the flash point, the type of flash point being reported must be noted.
Explosions are very rapid combustions in which the pressure waves formed propagate the combustion. The combustion creates a local pressure increase, which heats the flammable mixture to its auto-ignition temperature. This secondary combustion causes the pressure wave to propagate through the mixture. This traveling pressure pulse is called a shock wave. Often, a strong wind accompanies the shock wave. The combination of shock wave and wind, called a blast wave, causes much of the damage from explosions. When the shock wave speed is less than the speed of sound in the ambient atmosphere, the explosion is called a deflagration. When the speed is greater than the speed of sound, the explosion is called a detonation. Detonations can cause considerably more damage from the combination of blast wave, overpressure, and concussion. The damage from an overpressure of only 1 psi on structures can be extensive. Such a pressure differential on a typical door, for example, would result in considerably more than one ton of pressure on the door—enough to break most locks.
Of special concern when flammable gaseous mixtures (or dispersions of combustible dusts in air) are present is the so-called vapor cloud explosion (VCE). If there is a natural gas leak, for example, the cloud (mostly methane) will spread and mix with air. The cloud, parts of which are within the flammable limits, can be quite large. If it ignites, the deflagration will cause a shock wave perpendicular to the ground that can cause great damage, often flattening buildings. When a liquid stored above its ambient boiling point suddenly comes in contact with the atmosphere (through a rupture in the tank, for example), the rapid release and expansion of the vapor can cause a massive shock wave. This phenomenon is called a boiling-liquid expanding-vapor explosion (BLEVE). The failure of a steam drum, for example, can cause a BLEVE. When the BLEVE is of a flammable substance, the resulting cloud can explode. This combination of BLEVE and VCE is one of the most destructive forces in chemical plant accidents. The classic example is a propane tank that ruptures when it becomes overheated in a normal fire (a BLEVE). The propane is stored as a liquid under pressure. As the tremendous quantity of propane that vaporizes mixes rapidly with the atmosphere, it creates a massive VCE.
Runaway reactions are confined, exothermic reactions that go from their normal operating temperatures to greater than the ignition temperature; that is, they liberate more heat than can be dissipated. Thus, the temperature increases, increasing the reaction rate. Although there may be a steady state at a higher temperature (as there is in combustion), often the limits of mechanical integrity of the reaction vessel are reached before that point, causing catastrophic failure of the vessel. Such a failure can cause direct injuries, release toxic material, cause a fire, or lead to a BLEVE and/or a VCE.
To reduce the chance of a runaway condition during process upsets, the temperature difference between the reacting mixture and the cooling medium should be kept small. This may seem counterintuitive. However, consider a case where the temperature driving force is 1°C. If the temperature of the reacting mixture increases by 1°C during a process upset, the driving force for cooling has doubled! If the heat-exchange system had been designed for a 10°C driving force, that same upset would result in only a 10% increase in cooling. In systems with a chance of runaway, increased heat transfer area is the cost of an inherently safer system.
A common scenario for an accident involving an exothermic reaction is the loss of coolant accident (LOCA). Unless the cooling system is backed up to the extent that it is essentially 100% reliable, one must consider this scenario in designing the vessels and the pressure-relief systems.
26.3.2 Pressure-Relief Systems
During a severe process upset, the pressure and/or temperature limits of integrity for vessels can be approached. To avoid an uncontrolled, catastrophic release of the contents or the destruction of the vessel, pressure-relief systems are installed. Usually, these are relief valves on vessels or process lines that open automatically at a certain pressure. Downstream, they are connected to f lares (for flammable or toxic materials), scrubbers (for toxic materials), or a stack directed away from workers (for materials such as steam that present physical hazards). The design of the pressure-relief system is especially important, because the worst-case scenario must be considered, which is sometimes the simultaneous failure of multiple relief systems, as was the case for the Bhopal tragedy in 1984.
The design of such systems is complicated by several factors. The devices are designed to operate under unsteady conditions. Therefore, a dynamic simulation is required. Also, the flow through the relief system may be single-phase or two-phase flow. For two-phase flow, not only are the calculations more difficult, but also more factors affect the pressure drop, such as whether the line is horizontal or vertical.
In addition to the relief valves (which are called safety valves, relief valves, pressure-relief valves, or pop valves depending on service), rupture disks are used to open the process to the discharge system. Rupture disks are specially manufactured disks that are installed in a line, similar to the metal blanks used between flanges to close a line permanently. However, the disks are designed to fail rapidly at a set pressure. Ideally, the rupture disk allows no flow when the pressure is less than the set pressure, and it ruptures immediately, offering no resistance to flow, when the pressure hits the set point.
26.4 Process Hazard Analysis
Under the “Process Hazard Analysis” requirement of the Process Safety Management of Highly Hazardous Chemicals regulation (29 CFR 1910.119), employers must complete such an analysis of all covered processes using one or more of the following techniques:
What-if
Checklist
What-if/checklist
Failure mode and effects analysis (FMEA)
Fault-tree analysis (FTA)
Hazards and operability study (HAZOP)
or “an appropriate equivalent methodology.” The OSHA regulation specifically refers to the AIChE Center for Chemical Process Safety for details of process hazard analysis methods [9], which is an excellent source for details of these techniques.
The what-if technique involves a group of engineers and others going through the flowsheet and operating procedures methodically and considering what would happen if something were not as expected. For example, what if the reactor were not at the specified temperature? The answers to these hypothetical situations can uncover potential problems. This process hazard analysis technique is normally used only for simple, small-scale processes, such as laboratory experiments. For more complicated processes, the more rigorous HAZOP technique is used. This technique, which is described in the next section, is a formalized version of what-if.
Checklists have been developed by various companies for their specific processes. These lists can include hundreds of items [1, 9]. Checklists are very specific and focused; they do not typically lead to the identification of safety problems that have never been encountered. Therefore, checklists (which are focused on areas of known concern) are often used in combination with what-if techniques (which are focused on thinking “outside the box”).
The FMEA (Failure Modes and Effects Analysis) was invented by NASA in the 1960s. The underlying principle is that failures of individual components cannot be avoided, but these component failures must not cause a catastrophic failure of the system. Therefore, this analysis begins by identifying the various ways that each individual component can fail (a failure mode). Then the effect of these failures (individually and in combination) is studied. FMEA is thus a bottom-up approach that leads to identification of critical combinations of component failures that can cause some catastrophic failure. The result is usually an attempt to improve the reliability of specific components or to design protective redundancy into the system. In principle, FMEA requires the prediction and consideration of all failure modes of all components—a very large task for a complex system.
FTA (Fault-Tree Analysis) is based on the premise that many of the component failure modes that would be studied in the FMEA technique would not contribute to any system failure. FTA is a top-down analysis of the system failures. First, the catastrophic system failures to be avoided are identified. Then contributing failures of subsystems and individual components are considered. FTA is widely used in the nuclear power industry, where catastrophic system failures are clearly defined.
In both the FTA and FMEA analyses, large logic diagrams are created to show the connections between low-level failures and higher-level failures. If a combination of failures is required to create a higher-level failure, the connection is denoted as an AND gate. If any one of several failures can create a high-level failure, the connection is denoted as an OR gate. There can be many levels of failures, dozens of systems failures, and several failure modes for each of thousands of components. This logic diagram leads directly to the probability of system failure if the reliability of the individual components is known.
26.4.1 HAZOP (Hazard and Operability Study)
The most widely used process hazards analysis technique in the chemical process industries is HAZOP. Unlike FTA and FMEA, the HAZOP technique is an outside-the-box technique. It is a modified brainstorming technique for identifying and resolving process hazards by considering seemingly unusual occurrences. Although it is a bottom-up technique, it is more efficient than the FMEA because it involves early dismissal of component failure modes that are of no consequence to system operation and focuses early on the more probable failure scenarios. A HAZOP is especially useful in identifying human factors that can contribute to system failures. For example, a HAZOP based on a sabotage scenario could consider failure modes not apt to be uncovered by the FMEA.
HAZOP consists of asking questions about possible deviations that could occur in the process (or part of a process) under consideration. A HAZOP is always done in a group, and the regulation requires that the team have “expertise in engineering and process operations,” have “experience and knowledge specific to the process being evaluated,” and be “knowledgeable” about the HAZOP methodology. As with any brainstorming process, the ideas and suggestions can come very quickly, and there must be an identified scribe ready with appropriate software to capture them. Various software packages are available to speed this process and to offer additional triggers in the brainstorming process.
The first step in a HAZOP is to identify the normal operating condition or purpose of the process or unit. This is called the intention. Next, a guide word is used to identify a possible deviation in the process. For example, the intention may be to keep the temperature in a vessel constant. The guide words are
None, no, or not
More of
Less of
More than or As well as
Part of
Reverse
Other than
In the example here, there may be no coolant flow. Once such a possible deviation is identified, the team notes any possible causes of the deviation. If there are any safety consequences of the deviation, those are noted. Suppose the coolant flow ceased because of a pump failure. The consequence may be a runaway reaction. The action to be taken is assigned by the HAZOP team. In this case, the action might be assigning the process engineer to investigate a backup pumping system. The team then goes on to the next possible deviation, until all reasonable deviations have been considered. The team does not solve the safety problem during the HAZOP; its job is to identify the problem and to assign its resolution to a specific person. An example of part of a HAZOP for the feed heater (H-101) of the hydrodealkylation of toluene process (Figure 1.5) is shown in Table 26.5. Several features of a HAZOP are shown. Several of the items are dismissed for “no probable cause.” Others are redundant. A few are outside-the-box deviations that could lead to important safeguards for rare events. Also, the result of the HAZOP is a list of action items. These action items are not themselves decisions to change the process. They are decisions to study potential changes.
Table 26.5 HAZOP for the Feed Heater of the Hydrodealkylation (HDA) Process
Process Unit: H-101, Feed Heater, Figure 1.5 |
||||
Intention: To provide feed to the reactor (Stream 6) at 600ºC |
||||
Guide Word |
Deviation |
Cause |
Consequence |
Action |
No |
No flow (Stream 4) |
Blockage in line |
Fluid in H-101 overheats |
Consider an interlock on fuel gas flow. |
⇓ |
No O2 in combustion products |
Rich fuel:air mixture |
Unburned fuel and CO in combustion products |
None. O2 analyzer with self-checking circuit controls ratio reliably. |
⇓ |
|
O2 analyzer malfunction |
Potentially rich fuel:air mixture |
|
⇓ |
No flow (Stream 6) |
Heat tubes burst |
Explosion |
Interlock with sudden pressure drop alarm and shutdown. |
⇓ |
No benzene in Stream 6 |
C-101 not working |
Hydrogen:toluene ratio off to R-101 and loss of hydrogen to fuel gas |
Maintain spare compressor C-101. |
⇓ |
No fuel gas flow |
Supply pipe rupture |
Cold shot to R-101, quenching reaction |
Interlock with process shutdown. |
⇓ |
No flame |
Momentary loss of fuel gas |
Explosive mixture |
Automatic flame detection with reignition cycle. |
More of |
More flow in Stream 4 |
Surge of C-101 |
Unstable operation |
Alarm. |
⇓ |
Higher temperature |
Sudden reduction in Stream 6 flowrate |
Reactor overheats |
Consider an interlock on fuel gas flow. |
⇓ |
Higher pressure |
Dowstream blockage |
Tube failure |
Pressure-relief system on tubes. |
⇓ |
Higher temperature in heater |
Increased temperature of Stream 4 |
Flame becomes erratic |
Robust demister design. |
⇓ |
|
Loss of furnace control |
Higher temperature in Stream 6 |
Interlock on furnace controls. |
⇓ |
Higher concentration of O2 in exhaust |
Lean fuel:air ratio |
Waste of fuel |
|
⇓ |
|
No flame |
Explosive mixture |
Automatic flame detection with reignition cycle. |
Less of |
Lower temperature |
Flameout |
Cold shot to R-101, quenching reaction |
Include automatic flame detection with reignition cycle. |
⇓ |
Less flow in Stream 6 than in Stream 4 |
Heat tubes burst |
Explosion |
Interlock with differential flow alarm and shutdown. |
⇓ |
Less pressure in tubes |
Burst pipe downstream |
Explosive and toxic release |
Alarm on low pressure or low flow. |
⇓ |
Fuel gas flow |
Process upset |
Cold shot to R-101 |
Automatic supervisory control of plant. |
⇓ |
Less atmospheric pressure |
Storm |
No consequence |
|
⇓ |
|
Tornado |
Destruction of plant |
Monitor severe weather. |
As well as |
Liquid drops in fuel gas |
Failure of V-101 demister |
Flame becomes erratic |
Robust demister design. |
⇓ |
|
Failure of V-103 demister |
|
|
⇓ |
Water in fuel gas |
No probable cause |
||
⇓ |
Benzene in fuel gas |
Unlike overflow of V-102 |
||
Part of |
Low toluene in Stream 6 |
P-101 not working |
No reaction in R-101 |
Install low-flow alarm on Stream 2. |
⇓ |
Low benzene in Stream 6 |
C-101 not working |
Hydrogen:toluene ratio off to R-101 and loss of hydrogen to fuel gas |
Maintain spare compressor C-101. |
Reverse |
Decrease in Stream 6 temperature through H-101 |
No probable cause |
||
⇓ |
Reversal of flow (from Stream 6 to 4) |
No probable cause |
||
Other than |
Impurities in Stream 6 |
Impurities in feed or overheating in tubes |
Impurities in product and/or catalyst deactivation |
Monitor concentrations and H-101 temperatures. |
⇓ |
Toluene in Stream 4 replaced by other hydrocarbon |
Wrong connection to TK-101 by sabotage |
Explosion and loss of product |
Redundant management controls on storage facilities. |
⇓ |
Fuel gas replaced by liquid fuel |
Wrong connection during plant modification |
Explosion |
Better management of change procedures. |
The OSHA process safety management regulation requires that the actions assigned be taken in a timely manner and that all process hazard analyses be updated at least every five years.
26.4.2 Dow Fire & Explosion Index and Chemical Exposure Index
The Dow Fire & Explosion Index was developed by Dow Chemical in the 1960s and is today used by many companies to identify high-risk systems. It is a form of process hazards analysis that focuses on fires or explosions, but it also goes beyond the identification of these hazards to quantifying the probable loss from a resulting fire or explosion.
The format of this index is similar in many ways to an income tax form, as seen in Figures 26.1 and 26.2, which are for a fictitious reactor in a polymer plant (provided by Dow Chemical, Inc., May 1998, at a workshop for faculty). The details of these procedures are given in the official guide, available from AIChE [10].

Figure 26.1 Dow Fire & Explosion Index (Form reproduced with permission of the American Institute of Chemical Engineers. Copyright © 1994 AIChE. All rights reserved. Example used by permission of SACHE (http://www.aiche.org/sache), a component of the Center for Chemical Process Safety providing instructional materials to member chemical engineering departments throughout the world.)
First, a specific process unit is selected. The components in the unit have the greatest impact on the hazard, so a “material factor” is determined. This factor is a measure of the energy released during a fire or explosion involving a specific material and varies from 1 (e.g., for sulfur dioxide) to 40 (e.g., for nitromethane). Material factors for 328 materials (substances or defined mixtures) are given in the official guide, as well as procedures for determining the factor for any other material based on flammability and reactivity. In the case of the hypothetical example, the reactor contains several materials, but the material factor for butadiene is the highest, so it is used as the base.
Various corrections are made for type of reaction, facility, and materials handling to arrive at a general process hazards factor. In the case of Figure 26.1, there is a polymerization (exothermic) penalty, and there is a drainage and spill control penalty because there is only a 20-minute supply of fire water available.
Then a special process hazards factor is calculated based on extreme process conditions, storage of large quantities of hazardous materials, corrosion and erosion, fired equipment, and so on. In the example, there is a toxic penalty of 0.4 for butadiene. The operation is nitrogen padded but operates in the flammable range, so a penalty of 0.3 applies. There is a 0.06 penalty based on high-pressure operation with the specified relief setting. The flammable material penalty is based on the total heat of combustion for all materials in the unit (not only the butadiene). Corrosion has been estimated at less than 0.127 mm/y, and minor leakage at pumps occurs, which lead to the respective penalties. There is an agitator, which accounts for the rotating equipment penalty.
From these three hazards indicators (materials factor, general process hazards factor, and special process hazards factor), the Fire & Explosion Index (F&EI) is calculated, 169 in the example. Table 26.6 shows the qualitative level of hazard for various values of the F&EI. This example is in the “severe” hazard class. Although the F&EI is useful in identifying process units where hazardous conditions exist, it does not estimate the damage that might result from such an event.
Table 26.6 Dow Fire & Explosion Index
Fire & Explosion Index |
Qualitative Hazard Level |
1–60 |
Light |
61–96 |
Moderate |
97–127 |
Intermediate |
128–158 |
Heavy |
159– |
Severe |
Source: Dow’s Fire & Explosion Index Hazard Classification Guide, 7th ed. (New York: American Institute of Chemical Engineers, 1994) [10]. |
|
The second part of the analysis (Figure 26.2) involves estimating the probable damage if the hazard leads to a catastrophic event. The damage from a fire or explosion depends on the area affected by the event, the value of the equipment destroyed, and the loss of production while the equipment damage is being repaired. All three of these are estimated by detailed algorithms described in the guide. Three categories of mitigating factors are considered: process control, material isolation, and fire protection. Typical values are used in the example. These allow the calculation of a loss control credit factor, which is used to correct the damage estimates.
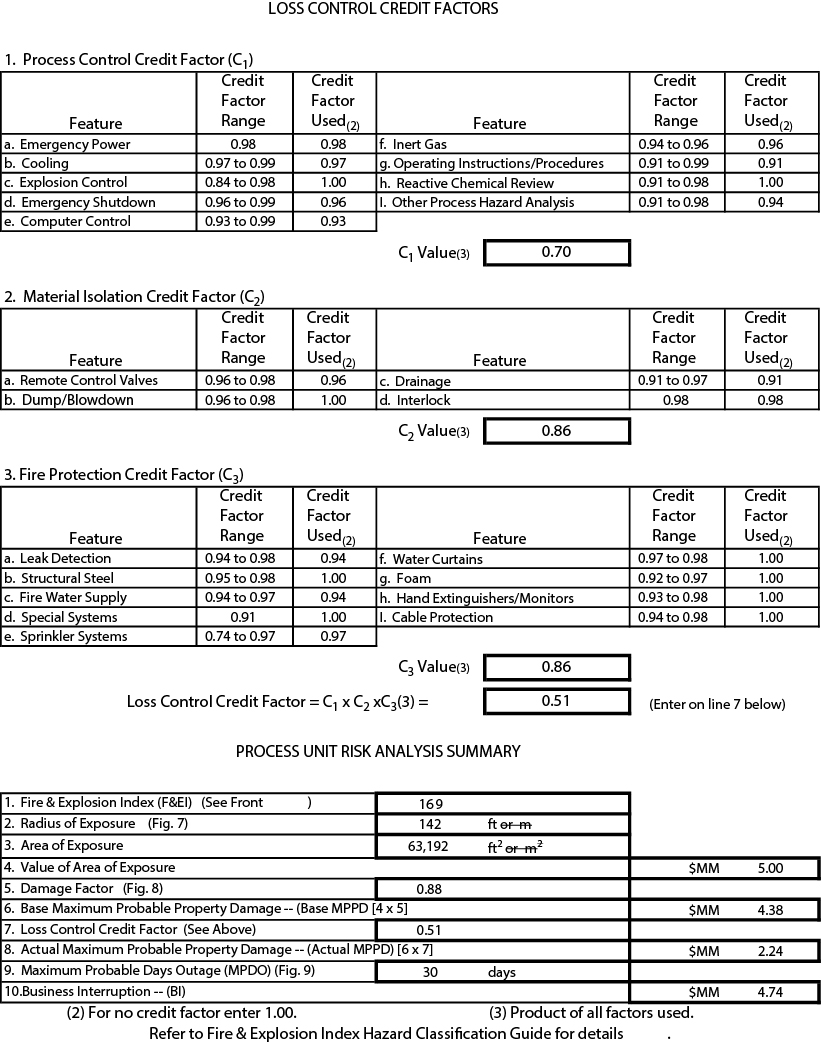
Figure 26.2 Loss Control Credit Factors (Form reproduced with permission of the American Institute of Chemical Engineers. Copyright © 1994 AIChE. All rights reserved. Example used by permission of SACHE [http://www.aiche.org/sache], a component of the Center for Chemical Process Safety providing instructional materials to member chemical engineering departments throughout the world.)
The final part of the analysis (bottom of Figure 26.2) is the calculation of probable loss of property and loss of business if a fire or explosion were to occur. The area likely to be damaged is estimated from the F&EI. The value of the equipment in this area ($5 million in the example) is used to estimate the likely property loss, which is a function of loss control credits. The business interruption loss is estimated based on (1) probable days of outage and (2) annual fixed costs plus before-tax profit.
The Dow Chemical Hazards Index is a somewhat similar index that provides an estimate of the hazard from accidental atmospheric release of toxic substances. A central factor in this analysis is the CEI, which is proportional to the square root of the ratio (toxic release flowrate):(threshold limit value). The details are available in the official guide [11].
A Dow “risk analysis package” consists of the analyses developed with the Dow F&EI and the Dow Chemical Exposure Index plus reports of loss prevention measures. This risk analysis package is used by industrial insurance carriers to predict the likelihood and size of loss from catastrophic events.
26.5 Chemical Safety and Hazard Investigation Board
The Clear Air Act Amendments of 1990 (Reference 7, Section 112[r][6]) created the Chemical Safety and Hazard Investigation Board to “investigate, determine and report to the public in writing the fact, conditions and circumstances and the cause or probable cause of any accidental release resulting in fatality, serious injury or substantial property damages.” The board is an independent scientific investigatory agency with no regulatory or enforcement duties. This board investigates chemical accidents, but it does not investigate all such accidents. Investigations are prioritized according to the likelihood that they would reduce further such accidents, either through enhanced knowledge of the causes or through stimulating a regulatory agency to consider further actions. Although the board was authorized in 1990, it was not funded until 1998. Since that time, it has produced numerous investigation reports, which are available on its Web site (http://www.csb.gov).
As with other governmental safety and accident investigation boards, the Chemical Safety and Hazard Investigation Board seeks to determine root causes of accidents, with a focus on avoiding future accidents and not on assigning blame.
26.6 Inherently Safe Design
Although safety controls can be added to existing processes, a more effective and efficient strategy is called inherently safe design [12]. The idea is to streamline the process to eliminate hazards, even if there is a major process upset. This strategy is based on a hierarchy of six approaches to process plant safety:
Substitution: Avoid using or producing hazardous materials on the plant site. If the hazardous material is an intermediate product, for example, alternative chemical reaction pathways might be used. In other words, the most inherently safe strategy is to avoid the use of hazardous materials.
Intensification: Attempt to use less of the hazardous materials. In terms of a hazardous intermediate, the two processes could be more closely coupled, reducing or eliminating the inventory of the intermediate. The inventories of hazardous feeds or products can be reduced by enhanced scheduling techniques such as just-in-time (JIT) manufacturing [13].
Attenuation: Reducing, or attenuating, the hazards of materials can often be effected by lowering the temperature or adding stabilizing additives. Any attempt to use materials under less hazardous conditions inherently reduces the potential consequences of a leak.
Containment: If the hazardous materials cannot be eliminated, they at least should be stored in vessels with mechanical integrity beyond any reasonably expected temperature or pressure excursion. This is an old but effective strategy to avoid leaks. However, it is not as inherently safe as substitution, intensification, or attenuation.
Control: If a leak of hazardous material does occur, there should be safety systems that reduce the effects. For example, chemical facilities often have emergency isolation of the site from the normal storm sewers, and large tanks for flammable liquids are surrounded by dikes that prevent any leaks from spreading to other areas of the plant. Scrubbing systems and relief systems in general are in this category. They are essential, because they allow a controlled, safe release of hazardous materials, rather than an uncontrolled, catastrophic release from a vessel rupture.
Survival: If leaks of hazardous materials do occur and they are not contained or controlled, the personnel (and the equipment) must be protected. This lowest level of the hierarchy includes firefighting, gas masks, and so on. Although essential to the total safety of the plant, the greater the reliance on survival of leaks rather than elimination of leaks, the less inherently safe the facility.
26.7 Summary
This chapter described the overall framework of health, safety, and environmental activities in the chemical process industries. The specific regulations change constantly, and the cognizant agencies must be consulted for the current rules.
26.8 Glossary
ACC: American Chemistry Council
ACGIH: American Congress of Governmental Industrial Hygienists
AIChE: American Institute of Chemical Engineers
ANSI: American National Standards Institute
API: American Petroleum Institute
ASME: American Society of Mechanical Engineers
ASTM: American Society for Testing and Materials
BLEVE: boiling-liquid expanding-vapor explosion
CAA: Clean Air Act
CCPS: Center for Chemical Process Safety
CERCLA: Comprehensive Environmental Response, Compensation, and Liability Act
CFR: Code of Federal Regulations
CMA: Chemical Manufacturers’ Association; former name of the American Chemistry Council
DIERS: Design Institute for Emergency Relief Systems
DOT: Department of Transportation
EIS: environmental impact study (or statement)
EPA: Environmental Protection Agency
EPCRA: Emergency Planning and Community Right to Know Act, also known as SARA, Title III
F&EI: Dow Fire & Explosion Index
FAR: fatal accident rate
FMEA: failure modes and effects analysis
FR: Federal Register
FTA: fault-tree analysis
HAP: hazardous air pollutant
HAZOP: hazard and operability study
IDLH: immediately dangerous to life and health
LEL: lower explosive limit
LFL: lower flammability limit
LOCA: loss of coolant accident
MSHA: Mine Safety and Health Agency
NAAQS: National Ambient Air Quality Standards
NESHAP: National Emissions Standards for Hazardous Air Pollutants
NFPA: National Fire Protection Association
NIOSH: National Institute for Occupational Health
NIOSHTIC: NIOSH Technical Information Center
NSPS: New Source Performance Standards
OSHA: Occupational Safety and Health Agency
PEL: permissible exposure limit
PHA: process hazard analysis
PSM: process safety management
RCRA: Resource Conservation and Recovery Act
REL: recommended exposure limit
RMP: risk management program (or plan)
SARA: Superfund Amendments and Reauthorization Act
SDS: safety data sheet
SOCMA: Synthetic Organic Chemical Manufacturers’ Association
STEL: short-term exposure limit
TLV: threshold limit values
TSCA: Toxic Substances Control Act
TWA: time-weighted average
UEL: upper explosive limit
UFL: upper flammability limit
VCE: vapor cloud explosion
VOC: volatile organic compound
WHAT YOU SHOULD HAVE LEARNED
There are numerous federal agencies regulating the chemical process industry.
Process safety management involves numerous activities aimed at creating an environment that anticipates potential safety problems and corrects them before incidents occur.
A HAZOP is a procedure by which potential process hazards are identified, and by which strategies to prevent incidents are built into the design.
References
1. Crowl, D. A., and J. F. Louvar, Chemical Process Safety: Fundamentals with Applications, 3rd ed. (Englewood Cliffs, NJ: Prentice Hall, 2011).
2. Occupational Safety and Health Act of 1970, Public Law 91-596, 29 U.S. Code §651 et seq., (December 29, 1970).
3. 2011 TLVs® and BEIs®: Threshold Limit Values for Chemical Substances and Physical Agents and Biological Exposure Indices (Cincinnati, OH: American Conference of Governmental Industrial Hygienists, 2011).
4. NIOSH Pocket Guide to Chemical Hazards, National Institute for Occupational Safety and Health, Cincinnati, OH, printed version: 2005. (Updated and downloadable at http://www.cdc.gov/niosh/npg.)
5. Guidelines for Technical Management of Chemical Process Safety (New York: American Institute of Chemical Engineers, 1989).
6. API Recommended Practices 750 (Washington: American Petroleum Institute, 1990).
7. Clean Air Act Amendments of 1990, Public Law 101-549, 42 U.S. Code §7401 et seq. (November 15, 1990). https://www.congress.gov/bill/101st-congress/senate-bill/1630.
8. Bodurtha, F. T., Industrial Explosion Prevention and Protection (New York: McGraw-Hill, 1980).
9. Guidelines for Hazard Evaluation Procedures, 2nd ed. with worked examples (New York: Center for Chemical Process Safety of the American Institute of Chemical Engineers, 1992).
10. Dow’s Fire & Explosion Index Hazard Classification Guide, 7th ed. (New York: American Institute of Chemical Engineers, 1994).
11. Dow’s Chemical Exposure Index Guide, 2nd ed. (New York: American Institute of Chemical Engineers, 1998).
12. Kletz, T. A., Cheaper, Safer Plants or Wealth and Safety at Work: Notes on Inherently Safer and Simpler Plants (Rugby, England: Institution of Chemical Engineers, 1984).
13. Hall, R. W., Attaining Manufacturing Excellence: Just-in-Time, Total Quality, Total People Involvement (Homewood, IL: Business One Irwin, 1987).
Problems
1. You work for a chemical company with 30,000 employees. If your company has a typical safety record for the chemical industry, what is your best estimate of how many of your employees
Will succumb to a fatal accident while on the job this year?
Will be injured but not killed on the job?
2. Locate the nearest steam plant on campus.
Develop two possible accident scenarios that should be considered when searching for worst-case scenarios.
List the safeguards that have been built into the system to mitigate some (or all) of these effects.
3. Find an SDS for each of the components listed in the HDA process.
4. Summarize all regulations (safety, environmental, transportation) that you can find for benzene.
5. Assume that the unit operations lab in your department must meet the process safety management standard. Choose two of the 13 components of PSM, and write a critical analysis of these aspects of lab operation.
6. Some paints have a closed-cup flash point near room temperature, whereas they have no measurable open-cup flash point.
Explain this apparent paradox.
Which is the more useful flash point when using a paint?
7. Assume gasoline to be 87 vol% iso-octane (2,2,4 tri-methyl pentane) and 13 vol% n-heptane. At room temperature, the air above a pool of gasoline will become saturated.
Is the air-gasoline mixture within its flammable limits?
Could there be any location where the mixture will be within its flammable limits? Explain.
8. Perform a HAZOP on R-101 of the HDA process. Be sure to perform the analysis in a team.
9. Benzene in gasoline is now limited by regulation to a maximum of 0.62 vol%. Is the benzene concentration in the air above a pool of gasoline greater than or less than the PEL? Show the effect of temperature.
10. Calculate the Dow Fire & Explosion Index for the HDA Reactor 101. Compare this with the F&EI for tank TK-101.
11. Use the various resources from this chapter to find health, safety, and environmental information about a chemical process plant in your area.
12. Obtain an environmental impact study for a facility in your area. Prepare a synopsis and lead a class discussion.
