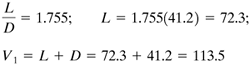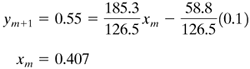11.6. FRACTIONAL DISTILLATION USING ENTHALPY–CONCENTRATION METHOD
11.6A. Enthalpy–Concentration Data
1. Introduction
In Section 11.4B the McCabe–Thiele method was used to calculate the number of theoretical steps or trays needed for a given separation of a binary mixture of A and B by rectification or fractional distillation. The main assumptions in the method are that the latent heats are equal, sensible heat differences are negligible, and constant molal overflow occurs in each section of the distillation tower. In this section we shall consider fractional distillation using enthalpy–concentration data where the molal overflow rates are not necessarily constant. The analysis will be made using enthalpy as well as material balances.
2. Enthalpy–concentration data
An enthalpy–concentration diagram for a binary vapor– liquid mixture of A and B takes into account latent heats, heats of solution or mixing, and sensible heats of the components of the mixture. The following data are needed to construct such a diagram at a constant pressure: (1) heat capacity of the liquid as a function of temperature, composition, and pressure; (2) heat of solution as a function of temperature and composition; (3) latent heats of vaporization as a function of composition and pressure or temperature; and (4) boiling point as a function of pressure, composition, and temperature.
The diagram at a given constant pressure is based on arbitrary reference states of liquid and temperature, such as 273 K (32°F). The saturated liquid line in enthalpy h kJ/kg (btu/lbm) or kJ/kg mol is calculated by
Equation 11.6-1
![]()
where xA is wt or mole fraction A, T is boiling point of the mixture in K (°F) or °C, T0 is reference temperature, K, cpA is the liquid heat capacity of the component A in kJ/kg · K (btu/lbm · °F) or kJ/kg mol · K, cpB is heat capacity of B, and ΔHsol is heat of solution at T0 in kJ/kg (btu/lbm) or kJ/kg mol. If heat is evolved on mixing, the ΔHsol will be a negative value in Eq. (11.6-1). Often, the heats of solution are small, as in hydrocarbon mixtures, and are neglected.
The saturated vapor enthalpy line of H kJ/kg (btu/lbm) or kJ/kg mol of a vapor composition yA is calculated by
Equation 11.6-2
![]()
where ![]() is the vapor heat capacity for A and
is the vapor heat capacity for A and ![]() for B. The latent heats λA and λB are the values at the reference temperature T0. Generally, the latent heat is given as λAb at the normal boiling point TbA of the pure component A and λBb for B. Then to correct this to the reference temperature T0 for use in Eq. (11.6-2),
for B. The latent heats λA and λB are the values at the reference temperature T0. Generally, the latent heat is given as λAb at the normal boiling point TbA of the pure component A and λBb for B. Then to correct this to the reference temperature T0 for use in Eq. (11.6-2),
Equation 11.6-3
![]()
Equation 11.6-4
![]()
In Eq. (11.6-3) the pure liquid is heated from T0 to TbA, vaporized at TbA, and then cooled as a vapor to T0. Similarly, Eq. (11.6-4) also holds for λB. For convenience, the reference temperature T0 is often taken as equal to the boiling point of the lower-boiling component A. This means λA = λAb. Hence, only λBb must be corrected to λB.
EXAMPLE 11.6-1. Enthalpy–Concentration Plot for Benzene–ToluenePrepare an enthalpy–concentration plot for benzene–toluene at 1 atm pressure. Equilibrium data are given in Table 11.1-1 and Figs. 11.1-1 and 11.1-2. Physical property data are given in Table 11.6-1.
Solution: A reference temperature of T0 = 80.1°C will be used for convenience so that the liquid enthalpy of pure benzene (xA = 1.0) at the boiling point will be zero. For the first point, we will select pure toluene (xA = 0). For liquid toluene at the boiling point of 110.6°C, using Eq. (11.6-1) with zero heat of solution and data from Table 11.6-1, Equation 11.6-5
For the saturated vapor enthalpy line, Eq. (11.6-2) is used. However, we must first calculate λB at the reference temperature T0 = 80.1°C, using Eq. (11.6-4): Equation 11.6-4
To calculate H, Eq. (11.6-2) is used, and yA = 0: Equation 11.6-2
For pure benzene, xA = 1.0 and yA = 1.0. Using Eq. (11.6-5), since T = T0 = 80.1, h = 0. For the saturated vapor enthalpy, using Eq. (11.6-2) and T = 80.1,
Selecting xA = 0.50, the boiling point Tb = 92°C and the temperature of saturated vapor for yA = 0.50 is 98.8°C from Fig. 11.1-1. Using Eq. (11.6-5) for the saturated liquid enthalpy at the boiling point,
Using Eq. (11.6-2) for yA = 0.5, the saturated vapor enthalpy at 98.8°C is
Selecting xA = 0.30 and yA = 0.30, h = 2920 and H = 36 268. Also, for xA = 0.80 and yA = 0.80, h = 562 and H = 32 380. These values are tabulated in Table 11.6-2 and plotted in Fig. 11.6-1. Figure 11.6-1. Enthalpy-concentration plot for benzene-toluene mixture at 1.0 atm abs.
| |||||||||||||||||||||||||||||||||||||||||||||
Some properties of the enthalpy–concentration plot are as follows. The region in between the saturated vapor line and the saturated liquid line is the two-phase liquid–vapor region. From Table 11.1-1, for xA = 0.411, the vapor in equilibrium is yA = 0.632. These two points are plotted in Fig. 11.6-1; this tie line represents the enthalpies and compositions of the liquid and vapor phases in equilibrium. Other tie lines can be drawn in a similar manner. The region below the h-versus-xA line represents liquid below the boiling point.
11.6B. Distillation in Enriching Section of Tower
To analyze the enriching section of a fractionating tower using enthalpy–concentration data, we make an overall and a component balance in Fig. 11.6-2:
Equation 11.6-6
![]()
Equation 11.6-7
![]()
Figure 11.6-2. Enriching section of distillation tower.

Equation (11.6-7) can be rearranged to give the enriching-section operating line:
Equation 11.6-8
![]()
This is the same as Eq. (11.4-7) for the McCabe–Thiele method, but now the liquid and vapor flow rates Vn+1 and Ln may vary throughout the tower, and Eq. (11.6-8) will not be a straight line on an x-y plot.
Making an enthalpy balance,
Equation 11.6-9
![]()
where qc is the condenser duty, kJ/h or kW (btu/h). An enthalpy balance can be made just around the condenser:
Equation 11.6-10
![]()
By combining Eqs. (11.6-9) and (11.6-10) to eliminate qc, an alternative form is obtained:
Equation 11.6-11
![]()
Substituting the value of Ln from Eq. (11.6-6) into (11.6-11),
Equation 11.6-12
![]()
Equations (11.6-8) and (11.6-12) are the final working equations for the enriching section.
In order to plot the operating line Eq. (11.6-8), the terms Vn+1 and Ln must be determined from Eq. (11.6-12). If the reflux ratio is set, V1 and L are known. The values H1 and hD can be determined by means of Eqs. (11.6-1) and (11.6-2) or from an enthalpy–concentration plot. If a value of xn is selected, it is a trial-and-error solution to obtain Hn+1, since yn+1 is not known. The steps to follow are given below:
Select a value of xn. Assume Vn+1 = V1 = L + D and Ln = L. Then using these values in Eq. (11.6-8), calculate an approximate value for yn+1. This assumes a straight operating line.
Using this yn+1, obtain Hn+1, and obtain hn using xn. Substitute these values into Eq. (11.6-12) and solve for Vn+1. Obtain Ln from Eq. (11.6-6).
Substitute into Eq. (11.6-8) and solve for yn+1.
If the calculated value of yn+1 does not equal the assumed value of yn+1, repeat steps 2–3. Generally, a second trial is not needed. Assume another value of xn and repeat steps 1–4.
Plot the curved operating line for the enriching section. Generally, only a few values for the flows Ln and Vn+1 are needed to determine the operating line, which is slightly curved.
11.6C. Distillation in Stripping Section of Tower
To analyze the stripping section of a distillation tower, an overall and a component material balance are made on Fig. 11.4-5a:
Equation 11.6-13
![]()
Equation 11.6-14
![]()
Equation 11.6-15
![]()
Making an enthalpy balance with qR kJ/h or kW(btu/h) entering the reboiler in Fig. 11.4-5a, and substituting (Vm+1 + W) for Lm from Eq. (11.6-13),
Equation 11.6-16
![]()
Making an overall enthalpy balance in Fig. 11.4-3,
Equation 11.6-17
![]()
The final working equations to use are Eqs. (11.6-15)–(11.6-17).
Using a method similar to that for the enriching section to solve the equations, select a value of ym+1 and calculate an approximate value for xm from Eq. (11.6-15), assuming constant molal overflow. Then calculate Vm+1 and Lm from Eqs. (11.6-16) and (11.6-13). Then use Eq. (11.6-15) to determine xm. Compare this calculated value of xm with the assumed value.
EXAMPLE 11.6-2. Distillation Using Enthalpy–Concentration MethodA liquid mixture of benzene–toluene is being distilled under the same conditions as in Example 11.4-1, except that a reflux ratio of 1.5 times the minimum reflux ratio is to be used. The value Rm = 1.17 from Example 11.4-2 will be used. Use enthalpy balances to calculate the flow rates of the liquid and vapor at various points in the tower and plot the curved operating lines. Determine the number of theoretical stages needed. Solution: The given data are as follows: F = 100 kg mol/h, xF = 0.45, xD = 0.95, xW = 0.10, R = 1.5Rm = 1.5(1.17) = 1.755, D = 41.2 kg mol/h, W = 58.8 kg mol/h. The feed enters at 54.4°C and q = 1.195. The flows at the top of the tower are calculated as follows:
From Fig. 11.1-1, the saturation temperature at the top of the tower for y1 = xD = 0.95 is 82.3°C. Using Eq. (11.6-2),
This value of 31 206 could also have been obtained from the enthalpy–concentration plot, Fig. 11.6-1. The boiling point of the distillate D is obtained from Fig. 11.1-1 and is 81.1°C. Using Eq. (11.6-5),
Again, this value could have been obtained from Fig. 11.6-1. Following the procedure outlined for the enriching section for step 1, a value of xn = 0.55 is selected. Assuming a straight operating line for Eq. (11.6-8), an approximate value of yn+1 is obtained:
Starting with step 2 and using Fig. 11.6-1, for xn = 0.55, hn = 1590, and for yn+1 = 0.695, Hn+1 = 33 240. Substituting into Eq. (11.6-12) and solving,
Using Eq. (11.6-6),
For step 3, substituting into Eq. (11.6-8),
This calculated value of yn+1 = 0.700 is sufficiently close to the approximate value of 0.695 that no further trials are needed. Selecting another value for xn = 0.70 and using Eq. (11.6-8), an approximate value of yn+1 is calculated:
Using Fig. 11.6-1 for xn = 0.70, hn = 1000, and for yn+1 = 0.791, Hn+1 = 32 500. Substituting into Eq. (11.6-12) and solving,
Using Eq. (11.6-6),
Substituting into Eq. (11.6-8),
In Fig. 11.6-3, the points for the curved operating line in the enriching section are plotted. This line is approximately straight and is very slightly above that for constant molal overflow. Figure 11.6-3. Plot of curved operating lines using enthalpy–concentration method for Example 11.6-2. Solid lines are for enthalpy–concentration method and dashed lines for constant molal overflow.
Using Eq. (11.6-10), the condenser duty is calculated:
To obtain the reboiler duty qR, values for hW and hF are needed. Using Fig. 11.6-1 for xW = 0.10, hW = 4350. The feed is at 54.5°C. Using Eq. (11.6-5),
Using Eq. (11.6-17),
Using Fig. 11.4-5 and making a material balance below the bottom tray and around the reboiler, Equation 11.6-18
Rewriting Eq. (11.6-16) for this bottom section, Equation 11.6-19
From the equilibrium diagram, Fig. 11.1-2, for xW = 0.10, yW = 0.207, which is the vapor composition leaving the reboiler. For equimolal overflow in the stripping section, using Eqs. (11.4-14) and (11.4-15), Equation 11.4-14
Equation 11.4-15
Selecting ym+1 = yW = 0.207, and using Eq. (11.6-15), an approximate value of xm = xN is obtained: Equation 11.6-15
Solving, xN = 0.174. From Fig. (11.6-1), for xN = 0.174, hN = 3800, and for yW = 0.207, HW = 37 000. Substituting into Eq. (11.6-19),
Solving, VW = 125.0. Using Eq. (11.6-18), LN = 183.8. Substituting into Eq. (11.6-15) and solving for xN,
This value of 0.173 is quite close to the approximate value of 0.174. Assuming a value of ym+1 = 0.55 and using Eq. (11.6-15), an approximate value of xm is obtained:
From Fig. (11.6-1), for xm = 0.412, hm = 2300, and for ym+1 = 0.55, Hm = 34 400. Substituting into Eq. (11.6-16),
Solving, Vm+1 = 126.5. Using Eq. (11.6-13),
Substituting into Eq. (11.6-15) and solving for xm,
This value of 0.407 is sufficiently close to the approximate value of 0.412 that no further trials are needed. The two points calculated for the stripping section are plotted in Fig. 11.6-3. This stripping line is also approximately straight and is very slightly above the operating line for constant molal overflow. Using the operating line for the enthalpy balance method, the number of theoretical steps is 10.4. Using the equimolal method, 9.9 steps are obtained. This difference would be larger if the reflux ratio of 1.5 times Rm were decreased to, say, 1.2 or 1.3. At larger reflux ratios, this difference in number of steps would be less. |
Note that in Example 11.6-2, in the stripping section the vapor flow increases slightly from 125.0 to 126.5 in going from the reboiler to near the feed tray. These values are lower than the value of 133.0 obtained assuming equimolal overflow. Similar conclusions hold for the enriching section. The enthalpy–concentration method is useful in calculating the internal vapor and liquid flows at any point in the column. These data are then used in sizing the trays. Also, calculations of qc and qR are used in designing the condenser and reboiler. This method is very applicable to design using a computer solution for binary and multicomponent mixtures to make tray-to-tray mass and enthalpy balances for the whole tower. The more restrictive Ponchon–Savarit graphical method for only binary mixtures is available (K3, T2).