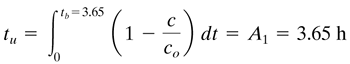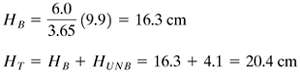12.3. DESIGN OF FIXED-BED ADSORPTION COLUMNS
12.3A. Introduction and Concentration Profiles
A widely used method for adsorption of solutes from liquid or gases employs a fixed bed of granular particles. The fluid to be treated is usually passed down through the packed bed at a constant flow rate. The situation is more complex than that for a simple stirred-tank batch process which reaches equilibrium. Mass-transfer resistances are important in the fixed-bed process, and the process is unsteady state. The overall dynamics of the system determine the efficiency of the process, rather than just the equilibrium considerations.
The concentrations of the solute in the fluid phase and of the solid adsorbent phase change with time and with position in the fixed bed as adsorption proceeds. At the inlet to the bed, the solid is assumed to contain no solute at the start of the process. As the fluid first comes in contact with the inlet of the bed, most of the mass transfer and adsorption takes place here. As the fluid passes through the bed, the concentration in this fluid drops very rapidly with distance in the bed and reaches zero well before the end of the bed is reached. The concentration profile at the start at time t1 is shown in Fig. 12.3-1a, where the concentration ratio c/co is plotted versus bed length. The fluid concentration co is the feed concentration and c is the fluid concentration at a point in the bed.
Figure 12.3-1. Concentration profiles for adsorption in a fixed bed: (a) profiles at various positions and times in the bed, (b) breakthrough concentration profile in the fluid at outlet of bed.
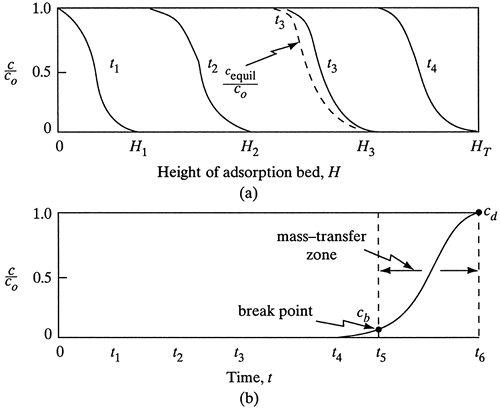
After a short time, the solid near the entrance to the tower is almost saturated, and most of the mass transfer and adsorption now takes place at a point slightly farther from the inlet. At a later time t2, the profile or mass-transfer zone where most of the concentration change takes place has moved farther down the bed. The concentration profiles shown are for the fluid phase. Concentration profiles for the concentration of adsorbates on the solid would be similar. The solid at the entrance would be nearly saturated, and this concentration would remain almost constant down to the mass-transfer zone, where it would drop off rapidly to almost zero. The dashed line for time t3 shows the concentration in the fluid phase in equilibrium with the solid. The difference in concentrations is the driving force for mass transfer.
12.3B. Breakthrough Concentration Curve
As seen in Fig. 12.3-1a, the major part of the adsorption at any time takes place in a relatively narrow adsorption or mass-transfer zone. As the solution continues to flow, this mass-transfer zone, which is S-shaped, moves down the column. At a given time t3 in Fig. 12.3-1a, when almost half of the bed is saturated with solute, the outlet concentration is still approximately zero, as shown in Fig. 12.3-1b. This outlet concentration remains near zero until the mass-transfer zone starts to reach the tower outlet at time t4. Then the outlet concentration starts to rise, and at t5 the outlet concentration has risen to cb, which is called the break point.
After the break-point time is reached, the concentration c rises very rapidly up to point cd, which is the end of the breakthrough curve, where the bed is judged ineffective. The break-point concentration represents the maximum that can be discarded and is often taken as 0.01 to 0.05 for cb/co. The value cd/co is taken as the point where cd is approximately equal to co.
For a narrow mass-transfer zone, the breakthrough curve is very steep and most of the bed capacity is used at the break point. This makes efficient use of the adsorbent and lowers energy costs for regeneration.
If the mass-transfer rate were infinitely fast and if no axial dispersion were present, the mass-transfer-zone width would be zero and the breakthrough curve would be a vertical line from c/co = 0 to c/co = 1.0.
12.3C. Mass-Transfer Zone
As shown in Fig. 12.3-1a, on entering the bed the concentration profile in the fluid starts to develop. For systems with a favorable isotherm, similar to the Freundlich and Langmuir isotherms (Fig. 12.1-1), which are concave downward (L1), the concentration profile in the mass-transfer zone soon acquires the typical S shape of the mass-transfer zone. Then the mass-transfer zone is constant in height as it moves through the column.
This constant height of the mass-transfer zone can then be used in scale-up when the height of the overall bed is large relative to the mass-transfer zone (L1, T1). Many industrial applications fall within these restrictions, which are applicable to adsorption from gases and liquids. Note that if the isotherm is linear or an unfavorable isotherm, the mass-transfer-zone width increases with bed length. A favorable isotherm for adsorption is unfavorable for effective regeneration (P4).
12.3D. Capacity of Column and Scale-Up Design Method
The mass-transfer-zone width and shape depend on the adsorption isotherm, flow rate, mass-transfer rate to the particles, and diffusion in the pores. A number of theoretical methods have been published which predict the mass-transfer zone and concentration profiles in the bed. The predicted results may be inaccurate because of many uncertainties due to flow patterns and correlations for predicting diffusion and mass transfer. Hence, experiments in laboratory scale are needed in order to scale up the results.
The total or stoichiometric capacity of the packed-bed tower, if the entire bed comes to equilibrium with the feed, can be shown to be proportional to the area between the curve and a line at c/co = 1.0, as shown in Fig. 12.3-2. The total shaded area represents the total or stoichiometric capacity of the bed as follows (R6):
Equation 12.3-1
![]()
Figure 12.3-2. Determination of capacity of column from breakthrough curve.
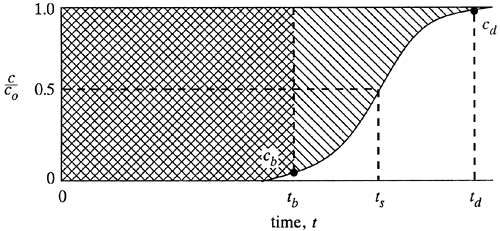
where tt is the time equivalent to the total or stoichiometric capacity. The usable capacity of the bed up to the break-point time tb is the crosshatched area:
Equation 12.3-2
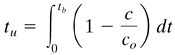
where tu is the time equivalent to the usable capacity or the time at which the effluent concentration reaches its maximum permissible level. The value of tu is usually very close to that of tb. Numerical integration of Eqs. (12.3-1) and (12.3-2) can be done using a spreadsheet.
The ratio tu/tt is the fraction of the total bed capacity or length utilized up to the break point (C3, L1, M1). Hence, for a total bed length of HT m, HB is the length of bed used up to the break point,
Equation 12.3-3
![]()
The length of unused bed HUNB in m is then the unused fraction times the total length:
Equation 12.3-4
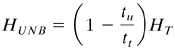
The HUNB represents the mass-transfer section or zone. It depends on the fluid velocity and is essentially independent of total length of the column. The value of HUNB may, there fore, be measured at the design velocity in a small-diameter laboratory column packed with the desired adsorbent. Then the full-scale adsorber bed can be designed simply by first calculating the length of bed needed to achieve the required usable capacity, HB, at the break point. The value of HB is directly proportional to tb.Then the length HUNB of the mass-transfer section is simply added to the length HB needed to obtain the total length, HT:
Equation 12.3-5
![]()
This design procedure is widely used; its validity depends on the conditions in the laboratory column being similar to those for the full-scale unit. The small-diameter unit must be well insulated to be similar to the large-diameter tower, which operates adiabatically. The mass velocity in both units must be the same and the bed must be of sufficient length to contain a steady-state mass-transfer zone (L1). Axial dispersion or axial mixing may not be exactly the same in both towers, but if caution is exercised, this is a useful design method.
An approximate alternative procedure to use instead of integrating and obtaining areas is to assume that the breakthrough curve in Fig. 12.3-2 is symmetrical at c/co = 0.5 and ts. Then the value of tt in Eq. (12.3-1) is simply ts. This assumes that the area below the curve between tb and ts is equal to the area above the curve between ts and td.
EXAMPLE 12.3-1. Scale-Up of Laboratory Adsorption ColumnA waste stream of alcohol vapor in air from a process was adsorbed by activated carbon particles in a packed bed having a diameter of 4 cm and length of 14 cm containing 79.2 g of carbon. The inlet gas stream having a concentration co of 600 ppm and a density of 0.00115 g/cm3 entered the bed at a flow rate of 754 cm3/s. Data in Table 12.3-1 give the concentrations of the breakthrough curve. The break-point concentration is set at c/co = 0.01. Do as follows:
Solution: The data from Table 12.3-1 are plotted in Fig. 12.3-3.For part (a), for c/co = 0.01, the break-point time is tb = 3.65 h from the graph.The value of td is approximately 6.95 h.Numerically or graphically integrating, the areas are A1 = 3.65 h and A2 = 1.51 h.Then from Eq. (12.3-1), the time equivalent to the total or stoichiometric capacity of the bed is
Figure 12.3-3. Breakthrough curve for Example 12.3-1.
The time equivalent to the usable capacity of the bed up to the break-point time is, using Eq. (12.3-2),
Hence, the fraction of total capacity used up to the break point is tu/tt = 3.65/5.16 = 0.707. From Eq. (12.3-3) the length of the used bed is HB = 0.707(14) = 9.9 cm. To calculate the length of the unused bed from Eq. (12.3-4),
For part (b), for a new tb of 6.0 h, the new HB is obtained simply from the ratio of the break-point times multiplied by the old HB:
We determine the saturation capacity of the carbon:
The fraction of the new bed used up to the break point is now 16.3/20.4, or 0.799. |
In the scale-up, not only may it be necessary to change the column height, but also the actual throughput of fluid might be different from that used in the experimental laboratory unit.Since the mass velocity in the bed must remain constant for scale-up, the diameter of the bed should be adjusted to keep it constant.
Typical gas adsorption systems use heights of fixed beds of about 0.3 to 1.5 m with downflow of the gas. Low superficial gas velocities of 15–50 cm/s (0.5–1.7 ft/s) are used. Adsorbent particle sizes range from about 4 to 50 mesh (0.3 to 5 mm). Pressure drops are low, only a few inches of water per foot of bed. The adsorption time is about 0.5 h up to 8 h. For liquids the superficial velocity of the liquid in the bed is about 0.3 to 0.7 cm/s (4 to 10 gpm/ft2).
Practical bed depths usually need to be 5–10 times the length of bed of the mass-transfer zone to be economical (W1). The adsorption step usually uses downward flow in the bed. For desorption the flow is usually upward for greater efficiency (S3).
12.3E. Basic Models for Predicting Adsorption
Adsorption in fixed beds is the most important method used for this process. A fixed or packed bed consists of a vertical cylindrical pipe filled or packed with the adsorbent particles. Adsorbers are mainly designed using laboratory data and the methods described in Section 12.3D. In this section, the basic equations for isothermal adsorption are described so that the fundamentals involved in this process can be better understood.
An unsteady-state solute material balance in the fluid is as follows for a section dz length of bed:
Equation 12.3-6
![]()
where ε is the external void fraction of the bed; ν is superficial velocity in the empty bed, m/s; ρp is density of particle, kg/m3; and E is an axial dispersion coefficient, m2/s. The first term represents accumulation of solute in the liquid. The second term is accumulation of solute in the solid. The third term represents the amount of solute flowing in by convection to the section dz of the bed minus that flowing out. The last term represents axial dispersion of solute in the bed, which leads to mixing of the solute and solvent.
The second differential equation needed to describe this process relates the second term of Eq. (12.3-6) for accumulation of solute in the solid to the rate of external mass transfer of the solute from the bulk solution to the particle and the diffusion and adsorption on the internal surface area. The actual physical adsorption is very rapid. The third equation is the equilibrium isotherm.
There are many solutions for these three equations which are nonlinear and coupled. These solutions frequently do not fit experimental results very well and will not be discussed here.
12.3F. Processing Variables and Adsorption Cycles
Large-scale adsorption processes can be divided into two broad classes. The first and most important is the cyclic batch system, in which the adsorption fixed bed is alternately saturated and then regenerated in a cyclic manner. The second is a continuous flow system, which involves a continuous flow of adsorbent countercurrent to a flow of feed.
There are four basic methods in common use for the cyclic batch adsorption system using fixed beds. These methods differ from each other mainly in the means used to regenerate the adsorbent after the adsorption cycle. In general, these four basic methods operate with two or sometimes three fixed beds in parallel, one in the adsorption cycle and the other one or two in a desorbing cycle, to provide continuity of flow. After a bed has completed the adsorption cycle, the flow is switched to the second newly regenerated bed for adsorption. The first bed is then regenerated by any of the following methods.
Temperature-swing cycle. This is also called the thermal-swing cycle. The spent adsorption bed is regenerated by heating it with embedded stream coils or with a hot purge gas stream to remove the adsorbate. The elevation in temperature is used to shift the adsorption equilibrium curve and affect regeneration of the adsorbent. Finally, the bed must be cooled so that it can be used for adsorption in the next cycle. The time for regeneration is generally a few hours or more.
Pressure-swing cycle. In this case the bed is desorbed by reducing the pressure at essentially constant temperature and then purging the bed at this low pressure with a small fraction of the product stream. Reduction in pressure shifts the adsorption equilibrium and affects the regeneration of the adsorbent. This process for gases uses a very short cycle time for regeneration compared to that for the temperature-swing cycle.
Inert-purge gas stripping cycle. In this cycle the adsorbate is removed by passing a nonadsorbing or inert gas through the bed. This lowers the partial pressure or concentration around the particles and desorption occurs. Regeneration cycle times are usually only a few minutes.
Displacement-purge cycle. The pressure and temperature are kept essentially constant as in purge-gas stripping, but a gas or liquid is used that is adsorbed more strongly than the adsorbate and displaces the adsorbate. Again, cycle times are usually only a few minutes.
Steam stripping is often used in regeneration of solvent-recovery systems using activated-carbon adsorbent. This can be considered a combination of the temperature-swing cycle and the displacement-purge cycle.