8.1. INTRODUCTION
8.1A. Purpose
In Section 4.8 we discussed the case of heat transfer to a boiling liquid. An important instance of this type of heat transfer occurs quite often in the process industries and is given the general name evaporation. In evaporation the vapor from a boiling liquid solution is removed and a more concentrated solution remains. In the majority of cases the separation process called evaporation refers to the removal of water from an aqueous solution.
Typical examples of evaporation are concentration of aqueous solutions of sugar, sodium chloride, sodium hydroxide, glycerol, glue, milk, and orange juice. In these cases the concentrated solution is the desired product and the evaporated water is normally discarded. In a few cases, water, which contains a small amount of minerals, is evaporated to give a solids-free water to be used as boiler feed, for special chemical processes, or for other purposes. Evaporation processes to evaporate seawater to provide drinking water have been developed and used. In some cases, the primary purpose of evaporation is to concentrate a solution so that upon cooling, salt crystals will form and be separated. This special evaporation process, termed crystallization, is discussed in Chapter 12.
8.1B. Processing Factors
The physical and chemical properties of the solution being concentrated and of the vapor being removed bear greatly on the type of evaporator used and the pressure and temperature of the process. Some of the properties which affect the processing methods are discussed next.
1. Concentration in the liquid
Usually, the liquid feed to an evaporator is relatively dilute, so its viscosity is low, similar to that of water, and relatively high heat-transfer coefficients are obtained. As evaporation proceeds, the solution may become very concentrated and quite viscous, causing the heat-transfer coefficient to drop markedly. Adequate circulation and/or turbulence must be present to keep the coefficient from becoming too low.
2. Solubility
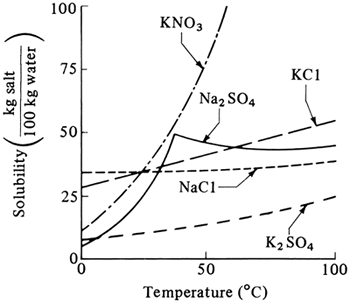
As solutions are heated and the concentration of the solute or salt increases, the solubility limit of the material in solution may be exceeded and crystals may form. This may limit the maximum concentration in solution which can be obtained by evaporation. In Fig. 8.1-1 some solubilities of typical salts in water are shown as a function of temperature. In most cases the solubility of the salt increases with temperature. This means that when a hot, concentrated solution from an evaporator is cooled to room temperature, crystallization may occur.
Figure 8.1-1. Solubility curves for some typical salts in water.
3. Temperature sensitivity of materials
Many products, especially food and other biological materials, may be temperature-sensitive and degrade at higher temperatures or after prolonged heating. Such products include pharmaceutical products; food products such as milk, orange juice, and vegetable extracts; and fine organic chemicals. The amount of degradation is a function of the temperature and the length of time.
4. Foaming or frothing
In some cases materials composed of caustic solutions, food solutions such as skim milk, and some fatty-acid solutions form a foam or froth during boiling. This foam accompanies the vapor coming out of the evaporator and entrainment losses occur.
5. Pressure and temperature
The boiling point of the solution is related to the pressure of the system. The higher the operating pressure of the evaporator, the higher the temperature at boiling. Also, as the concentration of the dissolved material in solution increases by evaporation, the temperature of boiling may rise. This phenomenon is called boiling-point rise or elevation and is discussed in Section 8.4. To keep the temperatures low in heat-sensitive materials, it is often necessary to operate under 1 atm pressure, that is, under vacuum.
6. Scale deposition and materials of construction
Some solutions deposit solid materials called scale on the heating surfaces. These could be formed by decomposition products or by decreases in solubility. The result is that the overall heat-transfer coefficient decreases, and the evaporator must eventually be cleaned. The materials used in construction of the evaporator should be chosen to minimize corrosion.
