1.5. CONSERVATION OF MASS AND MATERIAL BALANCES
1.5A. Conservation of Mass
One of the basic laws of physical science is the law of conservation of mass. This law, stated simply, says that mass cannot be created or destroyed (excluding, of course, nuclear or atomic reactions). Hence, the total mass (or weight) of all materials entering any process must equal the total mass of all materials leaving plus the mass of any materials accumulating or left in the process:
Equation 1.5-1
![]()
In the majority of cases there will be no accumulation of materials in a process, and then the input will simply equal the output. Stated in other words, “what goes in must come out.” We call this type of process a steady-state process:
Equation 1.5-2
![]()
1.5B. Simple Material Balances
In this section we do simple material (weight or mass) balances in various processes at steady state with no chemical reaction occurring. We can use units of kg, lbm, lb mol, g, kg mol, and so on, in our balances. The reader is cautioned to be consistent and not to mix several units in a balance. When chemical reactions occur in the balances (as discussed in Section 1.5D), one should use kg mol units, since chemical equations relate moles reacting. In Section 2.6, overall mass balances will be covered in more detail and in Section 3.6, differential mass balances.
To solve a material-balance problem, it is advisable to proceed by a series of definite steps, as listed below:
Sketch a simple diagram of the process. This can be a simple box diagram showing each stream entering by an arrow pointing in and each stream leaving by an arrow pointing out. Include on each arrow the compositions, amounts, temperatures, and so on, of that stream. All pertinent data should be on this diagram.
Write the chemical equations involved (if any).
Make a material balance. The arrows into the process will be input items and the arrows going out output items. The balance can be a total material balance in Eq. (1.5-2) or a balance on each component present (if no chemical reaction occurs).
Typical processes that do not undergo chemical reactions are drying, evaporation, dilution of solutions, distillation, extraction, and so on. These can be solved by setting up material balances containing unknowns and solving these equations for the unknowns.
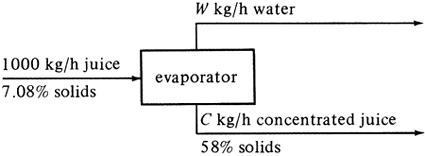
EXAMPLE 1.5-1. Concentration of Orange JuiceIn the concentration of orange juice, a fresh extracted and strained juice containing 7.08 wt % solids is fed to a vacuum evaporator. In the evaporator, water is removed and the solids content increased to 58 wt % solids. For 1000 kg/h entering, calculate the amounts of the outlet streams of concentrated juice and water. Solution: Following the four steps outlined, we make a process flow diagram (step 1) in Fig. 1.5-1. Note that the letter W represents the unknown amount of water and C the amount of concentrated juice. No chemical reactions are given (step 2). Basis: 1000 kg/h entering juice (step 3). Figure 1.5-1. Process flow diagram for Example 1.5-1.
To make the material balances (step 4), a total material balance will be made using Eq. (1.5-2): Equation 1.5-3
This gives one equation and two unknowns. Hence, a component balance on solids will be made: Equation 1.5-4
To solve these two equations, we solve Eq. (1.5-4) first for C since W drops out. We get C = 122.1 kg/h concentrated juice. Substituting the value of C into Eq. (1.5-3),
and we obtain W = 877.9 kg/h water. As a check on our calculations, we can write a balance on the water component: Equation 1.5-5
Solving,
|
In Example 1.5-1 only one unit or separate process was involved. Often, a number of processes in series are involved. Then we have a choice of making a separate balance over each separate process and/or a balance around the complete overall process.
1.5C. Material Balances and Recycle
Processes that have a recycle or feedback of part of the product into the entering feed are sometimes encountered. For example, in a sewage treatment plant, part of the activated sludge from a sedimentation tank is recycled back to the aeration tank where the liquid is treated. In some food-drying operations, the humidity of the entering air is controlled by recirculating part of the hot, wet air that leaves the dryer. In chemical reactions, the material that did not react in the reactor can be separated from the final product and fed back to the reactor.
EXAMPLE 1.5-2. Crystallization of KNO3 and RecycleIn a process producing KNO3 salt, 1000 kg/h of a feed solution containing 20 wt % KNO3 is fed to an evaporator, which evaporates some water at 422 K to produce a 50 wt % KNO3 solution. This is then fed to a crystallizer at 311 K, where crystals containing 96 wt % KNO3 are removed. The saturated solution containing 37.5 wt % KNO3 is recycled to the evaporator. Calculate the amount of recycle stream R in kg/h and the product stream of crystals P in kg/h. Solution: Figure 1.5-2 gives the process flow diagram. As a basis we shall use 1000 kg/h of fresh feed. No chemical reactions are occurring. We can make an overall balance on the entire process for KNO3 and solve for P directly: Equation 1.5-6
Figure 1.5-2. Process flow diagram for Example 1.5-2.
To calculate the recycle stream, we can make a balance around the evaporator or the crystallizer. Using a balance on the crystallizer, since it now includes only two unknowns, S and R, we get for a total balance, Equation 1.5-7
For a KNO3 balance on the crystallizer, Equation 1.5-8
Substituting S from Eq. (1.5-7) into Eq. (1.5-8) and solving, R = 766.6 kg recycle/h and S = 974.9 kg/h. |
1.5D. Material Balances and Chemical Reaction
In many cases the materials entering a process undergo chemical reactions in the process, so that the materials leaving are different from those entering. In these cases it is usually convenient to make a molar and not a weight balance on an individual component, such as kg mol H2 or kg atom H, kg mol ![]() ion, kg mol CaCO3, kg atom Na+, kg mol N2, and so on. For example, in the combustion of CH4 with air, balances can be made on kg mol of H2, C, O2, or N2.
ion, kg mol CaCO3, kg atom Na+, kg mol N2, and so on. For example, in the combustion of CH4 with air, balances can be made on kg mol of H2, C, O2, or N2.
EXAMPLE 1.5-3. Combustion of Fuel GasA fuel gas containing 3.1 mol % H2, 27.2% CO, 5.6% CO2, 0.5% O2, and 63.6% N2 is burned with 20% excess air (i.e., the air over and above that necessary for complete combustion to CO2 and H2O). The combustion of CO is only 98% complete. For 100 kg mol of fuel gas, calculate the moles of each component in the exit flue gas. Solution: First, the process flow diagram is drawn (Fig. 1.5-3). On the diagram the components in the flue gas are shown. Let A be moles of air and F be moles of flue gas. Next the chemical reactions are given: Equation 1.5-9
Equation 1.5-10
An accounting of the total moles of O2 in the fuel gas is as follows:
For all the H2 to be completely burned to H2O, we need, from Eq. (1.5-10),
For a 20% excess, we add 1.2(14.65), or 17.58 mol O2. Since air contains 79 mol % N2, the amount of N2 added is (79/21)(17.58), or 66.1 mol N2. To calculate the moles in the final flue gas, all the H2 gives H2O, or 3.1 mol H2O. For CO, 2.0% does not react. Hence, 0.02(27.2), or 0.54 mol CO will be unburned. A total carbon balance is as follows: inlet moles C = 27.2 + 5.6 = 32.8 mol C. In the outlet flue gas, 0.54 mol will be as CO and the remainder of 32.8 − 0.54, or 32.26 mol as CO2. For calculating the outlet mol O2, we make an overall O2 balance:
Equating inlet O2 to outlet, the free remaining O2 = 3.2 mol O2. For the N2 balance, the outlet = 63.6 (in fuel gas) + 66.1 (in air), or 129.70 mol N2. The outlet flue gas contains 3.10 mol H2O, 0.54 mol CO, 32.26 mol CO2, 3.20 mol O2, and 129.7 mol N2. |
In chemical reactions with several reactants, the limiting reactant component is defined as that compound which is present in an amount less than the amount necessary for it to react stoichiometrically with the other reactants. Then the percent completion of a reaction is the amount of this limiting reactant actually converted, divided by the amount originally present, times 100.