13
CT-SPECT/CT-PET
R. Glenn Wells
CONTENTS
13.2 Advantages of Localization
13.3 Attenuation with CT versus Transmission Scans
13.7 Creating an Attenuation Map from the CT Scan
13.1 Introduction
Nuclear medicine has been available as an imaging technology first in planar form in the 1950s and since the late 1960s as a tomographic technique. It is an extremely sensitive technique capable of measuring concentrations in the nanomolar to picomolar range. Using tracer technology, it provides functional information about a large number of different organs and systems within the human body. However, its poor spatial resolution and the often specific nature of the tracer uptake made the images difficult to understand for the untrained eye and earned nuclear medicine the moniker “unclear medicine” (von Schulthess 2004, Patel et al. 2009). The value of an anatomical reference had long been recognized and transmission/emission imaging had been proposed as early as 1966 (Kuhl et al. 1966). Many nuclear medicine studies like ventilation–perfusion scans and sentinel-node lymphoscintigra-phy are frequently interpreted with side-by-side reference to an anatomical image, be it a chest x-ray or a crude body outline formed by the shadow of a flood transmission source.
The notion of acquiring both a nuclear medicine tomograph and a CT scan on the same camera, with a single patient bed, was first suggested in 1987 (Mirshanov 1987) but not developed. SPECT/CT imaging was further investigated by Hasegawa and colleagues at the University of California San Francisco starting in the late 1980s (Hasegawa et al. 1989, 1990), and produced the first modern configuration of a SPECT/CT camera in the mid-1990s (Blankespoor et al. 1996). They combined a 9800 Quick CT scanner with a single-head XR/T SPECT gamma camera (both devices from GE Healthcare) and integrated a patient bed that could be slid through gantries. They also demonstrated the feasibility of using the CT image to create an attenuation coefficient map that could be used for attenuation correction (Blankespoor et al. 1996, Hasegawa et al. 1999, Seo et al. 2008, Patton et al. 2009). This technology was taken up and first offered commercially as the Hawkeye CT system attached to a Millennium VG gamma camera (GE Healthcare) in 1999. The CT component was a single-slice, slow-rotation device, which led to long acquisition times and CT images that were not of diagnostic quality, but still sufficient for both attenuation correction and anatomical localization.
Meanwhile, Beyer et al. at the University of Pittsburgh also suggested the use of CT in combination with PET scanning (Beyer et al. 1994). Their work led to the first PET/CT prototype scanner in 1998 (Beyer et al. 2000, Patton et al. 2009). This unit combined both a diagnostic single-slice CT scanner (Somatom AR.SP, Siemens) and an ECAT-ART PET system (CTI PET Systems). At this time, CT technology was undergoing a giant boost in speed as multislice scanners were starting to be introduced. This made whole-body CT scanning considerably faster than the transmission-based options and reduced whole-body FDG study times by almost a factor of two. In addition, the localization aspect of overlaying the FDG oncology study on top of a diagnostic quality CT scan was a powerful draw for oncology. With reimbursement available for PET oncology in North America, this technology took off. Commercial PET/CT systems became available in 2001 and the additional benefit of the combined scanner became rapidly apparent. Within about 4 years, PET-only scanners were no longer being sold in North America and every PET system came combined with a CT scanner.
The success of PET-CT spurred further development in SPECT-CT. Though SPECT-only cameras have not been replaced by SPECT-CT to the same extent that PET-CT replaced PET, there are now a very large number of SPECT-CT cameras in use and a variety of configurations available. Traditional dual-head gamma cameras are available with everything from a Hawkeye CT scanner (upgraded to a 4-slice CT) to a high-speed 64-slice diagnostic quality CT (Patton et al. 2009). New developments in dedicated cardiac systems also have the option of a SPECT-CT configuration (NM 570c, GE Healthcare (Bocher et al. 2010); Cardius XPO, Digirad Inc. (Bai et al. 2010)).
Side-by-side use of anatomical x-ray/CT and function nuclear medicine images has been done for some time. However, the combined modality camera has many advantages for both the physician and the patient. This chapter addresses the advantages and disadvantages of combining nuclear medicine (NM) and CT and some of the methodology required to implement it.
13.2 Advantages of Localization
Nuclear medicine (NM) is a very sensitive technology allowing measurement of picomolar concentrations of tracer (Levin 2005, Seo et al. 2008). Accumulation of tracer at focal sites of cancer can allow us to detect very small lesions, but these lesions may appear as a bright focal source with little other background uptake in structures nearby. It is like a bright light-bulb in a dark room—easy to see, but difficult to tell exactly where it is. In the case of cancer detection, location can be extremely important. Accurate localization is essential for guiding biopsies, surgery, and radiation therapy. It provides information about the stage of the disease, indicating, for example, if the tumor has penetrated the adjacent bone or is contained in the surrounding soft tissue. Many studies have shown the benefits of having both nuclear medicine study and the CT study fused together as was highlighted in an excellent series of articles published in 2009 (Bockisch et al. 2009, Delbeke et al. 2009, Evan-Sapir 2009, Gnanasegaran et al. 2009, Kaufmann and Di Carli 2009).
In cancer imaging, the use of fused imaging in FDG-PET/CT improves the staging of cancer patients, and hence changes management compared to either modality alone or even both modalities evaluated side-by-side. The difference is 10%–15% or more and statistically significant (Czernin et al. 2007, Bockisch et al. 2009, Delbeke et al. 2009, Czernin et al. 2010). It has been shown for a variety of cancers including those of the head and neck, breast, lung, gastrointestinal tract, thyroid, and lymphoma (Delbeke et al. 2009). Combination of PET and CT also leads to improved lesion detection, improved diagnostic accuracy, and confirmation of small or subtle lesions.
Hybrid imaging improves the distinction between normal uptake and that representing true disease by accurately placing the activity within the correct anatomical structures. The combination has proven useful in guiding radiation therapy planning (Delbeke et al. 2009, Evan-Sapir 2009, Roach et al. 2010). It allows for a better definition of tumor boundaries and metastatic spread, which can help guide the definition of planned radiation fields during therapy and consequently leads to a sparing of normal tissues. Fused imaging is also useful in the follow-up of disease, particularly in the case of residual CT abnormalities. Whereas evaluation with CT alone is based on changes in tumor size, successful treatment may lead to tumor necrosis or halting of disease progression without immediate loss in tumor size. Functional imaging can more rapidly assess response to treatment by displaying a loss in metabolic activity in the treatment bed and distinguishing it from sites of activity outside the treated area (Evan-Sapir 2009). Finally, fused images can help guide biopsies by clarifying which potential tumors are most metabolically active and even within tumors, distinguishing areas that are less aggressive or even necrotic (Evan-Sapir 2009).
For SPECT/CT, the story is similar. One of the more popular uses for SPECT is a bone scan to evaluate for metastases based on Tc99m-labeled methylene diphosphonate (MDP). While sensitivity is very good for this test, it is complicated by tracer uptake due to a number of other processes such as degenerative joint diseases and osteomyelitis. The addition of CT can help to clarify diagnosis and improve the specificity of this test (Bockisch et al. 2009, Gnanasegaran et al. 2009, Mohan et al. 2010). Like FDG-PET, I-131, somatostatin receptor SPECT imaging, Ga67-white blood cell infection imaging and many other SPECT studies also benefit from localization (Delbeke et al. 2009).
In cardiac imaging, we know where the heart is so the benefit of CT is not to locate the organ, but rather to associate CT anatomy with function signifi-cance. A number of studies (Flotats et al. 2011) have demonstrated that there is a mismatch between the degree of stenosis and its functional significance. While <50% lesions are known to not degrade the function of the heart, and >90% lesions are known to definitely affect performance, in between there is a great deal more ambiguity. The DEFER trial (Pijls et al. 2007) showed that there was certainly a benefit to stenting lesions if they were functionally significant, as measured by a reduced fraction flow reserve by flow-wire. There was no benefit to stenting if the lesion was not functionally significant. CT-angiography (CTA) on its own shows that <50% of lesions with a 50% stenosis are functionally significant (Di Carli and Hachamovitch 2007). By registering the CTA with a perfusion study, it is possible to identify which lesions (Flotats et al. 2011), if any, have a functional significance and thus would benefit from intervention.
13.3 Attenuation with CT versus Transmission Scans
There are a number of factors that degrade the accuracy and quality of nuclear medicine images of which attenuation is the most important. The half-value thickness (HVT) for PET (511 keV) photons in water is 7.2 cm, but because a pair of photons is detected, the attenuation path length is the entire width of the patient, making the total attenuation much higher. For SPECT, the path lengths are shorter on average and the HVT is 4.5 cm, but the effects remain significant as is evidenced by the change in specificity of cardiac imaging with the application of attenuation correction (Garcia 2007).
Accurate correction for attenuation requires a patient-specific map of the linear attenuation coefficients of the tissues in the body. This was previously acquired by radioisotope transmission sources, but with the advent of PET/CT and SPECT/CT is now frequently obtained from a CT scan instead. There are a number of advantages to the use of CT for AC and these have bolstered its rise in popularity. There are also, though, a number of drawbacks. The advantages are a much shorter acquisition time, lower image noise, reduced staff exposure, improved consistency, and greater patient comfort. The disadvantages are increased patient exposure; larger, more complicated and more expensive equipment; a need for sequential acquisition of the emission and transmission images; and the speed of the CT acquisition.
13.4 CTAC: Advantages
Modern CT scanners rotate at speeds as high as 0.35 s/rotation. The slice thickness and spacing typically used for attenuation maps are on the order of 2.5 mm. The axial coverage of the CT scanner is 2–8 cm. The time required to obtain a transmission scan varies depending on the speed of the CT scanner and the field-of-view (FOV) that is to be acquired. It can range from a couple of seconds for a high-speed CT scan of a cardiac FOV to several minutes for a low-speed whole-body bone SPECT. In all cases though, the time required is a small fraction of the time needed for a radioisotope transmission scan. Radioisotope transmission images for PET required 3–5 min per bed position or axial FOV of the scanner. On a typical 6-bed whole-body study, the time for transmission imaging was 20–30 min. As emission imaging alone was generally 5 min per bed position, radioisotope transmission imaging almost doubled the total time required. Replacing the radioisotope scan with a high-speed CT reduces scan time by about 40% for whole-body studies (Even-Sapir et al. 2009).
The much greater intensity of the photon flux from an x-ray source compared to a radioisotope source also means that the CT images have much higher signal-to-noise. The noise in a reconstructed nuclear medicine image is a combination of the noise in the emission and transmission data (King et al. 1995, Kinahan et al. 1998). Compared to both radioisotope transmission scans and the emission scans themselves, the noise in the CT images is negligible and so using a CT scan for AC effectively eliminates the noise contribution from the transmission imaging (Figure 13.1).
The radioactive sources used for transmission imaging require periodic handling by the nuclear medicine staff to perform wipe tests to check for radioactive leakage. The sources also decay over time and so require replacement on a regular basis. For example, the half-lives of 153-Gd and 68-Ge, two sources typically used for transmission imaging, are 242 and 275 days respectively, about 2/3 of a year. Sources thus require replacement roughly every 2 years. During this time, the noise levels in the transmission scan and the amount of interference between the transmission and the emission data will vary considerably and result in a continually changing accuracy in the transmission map. The handling of the radioactive sources results in additional exposure to the nuclear medicine staff that is eliminated with CT-AC.

FIGURE 13.1
(See color insert.) Example images from an FDG PET/CT whole-body study. Shown is the same patient without attenuation correction (a), with correction using a Ge-68 attenuation map (b), with correction using a CT-based attenuation map (c), an example transverse slice through a Ge-68 transmission map (d), and the same slice through a CT map (e). For the PET images, white indicates highest amount of FDG activity.
Finally, the speed of the CT scan greatly reduces the total scan time and thus increases patient comfort and patient throughput. Increased patient comfort reduces the amount of patient motion and thereby the amount of motion-related artifacts in the images. The greater throughput increases the cost-effectiveness of the scanner and potentially reduces wait times as well.
13.5 CTAC: Disadvantages
The improvements in image quality of the transmission scan do not, unfortunately, come for free. The cost is an increase in the radiation exposure of the patient. While the exposure from a radioisotope transmission source was almost negligible next to the dose received from the injected emission tracer, the dose from the CT-scan can be a sizeable fraction of the total study dose (Patton et al. 2009). For example, a rest/stress cardiac SPECT scan with 99 mTc-tetrofosmin delivers a patient dose of 10 mSv. The exposure from transmission sources can be as low as 4 μSv (0.04%) (Perisinakis et al. 2002), but the exposure from a CT scan for attenuation correction would be about 0.8 mSv, about 200× higher. For a Rb82-PET rest/stress cardiac exam, the radiation dose is only 1.5 mSv and so the addition of the CT adds almost 50% to the total dose for the study. For whole body studies, transmission times are much longer due to the increased FOV, whereas the dose from the nuclear medicine test is constant resulting in an even greater percent contribution from the CT scan to the total study dose.
The addition of a diagnostic CT scanner to the PET or SPECT camera greatly increases its size, complexity, and cost. The increased cost is balanced by an improvement in diagnostic accuracy but can still be a barrier, particularly for small centers. The increase in complexity leads to more things that can go wrong and make the camera unavailable for use until it is repaired. The complexity also means a larger amount of time needs to be spent each morning on quality assurance tests by the technologists operating the scanner, further increasing operation costs. The increased bore length of the camera can be a problem for patients with issues like claustrophobia as the patient must be moved much deeper into the camera to obtain the images. Finally, the increased size, and need for x-ray shielding, may be a problem at some sites and require extensive renovations prior to being able to install the system.
A further disadvantage is that the CT and NM scans are acquired sequentially. The CT scanner typically uses a different set of detectors than the nuclear medicine camera, and consequently the transmission imaging is done either prior to or following the nuclear medicine acquisition. For a whole-body PET study, this can introduce a delay of 20 min or more between transmission and end of the emission scan. This delay is larger than would have occurred with radioisotope transmission imaging, wherein the transmission and emission data are interleaved at each bed position. This delay increases the likelihood of patient motion, leading to misregistration of the data sets.
13.6 Types of CT Used
There is a wide range of CT scanners used as part of an integrated PET/CT or SPECT/CT system. This is caused by both the rapidly changing environment of CT hardware and the desire by the manufacturers to offer cost-effective solutions, particularly for SPECT/CT. Early on in the development of PET/CT scanners, 1- and 2-slice CT scanners were offered commercially. Rapid improvements in CT technology have seen a leap from single-slice scanners to 320 slice scanners with dual-energy capabilities such that CT systems offered with early PET scanners are now considered obsolete. Presently, a 16-slice or better, high-speed diagnostic-quality CT is commonplace for PET/CT systems.
For SPECT/CT systems, there is a greater diversity of CT options available. The first unit introduced commercially also had a single-slice scanner (Seo et al. 2008, Patton et al. 2009). SPECT/CT systems with up to 64-slice CT scanners attached are now available, though lower-end units with only a few slices also remain on the market. The SPECT cameras also come with x-ray systems built on the SPECT gantry itself, rather than requiring a separate CT gantry. This reduces the cost of the system, but limits the speed at which the CT component can operate. These slow-rotation CTs spin at 2–5 rpm, but reduce the tube current and consequently the x-ray flux at which they operate down to 1–5 mA such that the radiation exposure to the patient is similar to that from a high-speed CT. The slow-rotation cameras are prone to CT artifacts caused by inconsistencies in the projection data introduced by patient motion such as breathing. The CT scans produced are thus not of diagnostic quality, but they are sufficient for tracer localization and attenuation correction.
Recent advances in CT acquisition protocols and data processing have allowed a reduction in the dose associated with CT scans, off-setting the increase in dose associated with using a CT scan for attenuation correction. These include approaches such as dose modulation as the tube rotates around the patient to alter the tube current in response to changing patient thickness, with the aim of maintaining similar signal to noise at all projection angles (Brisse et al. 2007). Prospective gating has been introduced for cardiac scans wherein the x-ray beam is targeted at specific phases of cardiac cycle through ECG gating. Finally, the introduction of iterative reconstruction for CT has led to an improvement in the signal to noise, or equivalently a 30%–50% reduction in patient radiation exposure for the same quality image (Marin et al. 2010, Fleischmann and Boas 2011, Park et al. 2012).
13.7 Creating an Attenuation Map from the CT Scan
To create an attenuation map from a CT scan requires several steps. The first step is the conversion from Hounsefield units into linear attenuation coefficients appropriate to the energy of the gamma ray(s) emitted by the nuclear medicine tracer. This is complicated by the fact that the x-ray beam is polyen-ergetic. X-rays are produced by accelerating electrons across a potential difference, typically 80–140 kV, and colliding them with a target. The x-rays are produced by Bremsstrahlung interactions in the target, generating photons with a range of energies up to a maximum equal to the accelerating potential. In the patient, the total mass attenuation coefficient varies with both the atomic number of the attenuating material (Z) and the energy of the incident photon (E). Thus, the relative attenuation of materials at different energies is different for different materials (Figure 13.2) and a single scaling factor cannot be used to convert a CT-map into an attenuation coefficient map. For example, at 70 keV, the mass attenuation of bone is greater than water, while at 511 keV the relationship is reversed.

FIGURE 13.2
(See color insert.) Mass attenuation coefficients for water (similar to soft tissues), bone, and iodine. The range of energies for an x-ray beam (centered at 70 keV) is indicated by the dashed blue lines at left. The energy for PET imaging (511 keV) is shown with the dashed blue line at right.
Fortunately, the composition of most soft tissues is similar and it is reasonable to scale all of these in a similar manner. Bone, however, behaves differently and so a standard approach is to segment the CT-map into bone and soft tissues and scale each separately to the appropriate gamma-ray energy. The bone and soft-tissue maps are then recombined into a single attenuation map. The scaling factors are dependent on the spectral composition of the x-ray beam and so will be different for different accelerating potentials and may vary between scanners as well.
An additional complication is that the resolution of the CT scan is much higher than that of the nuclear medicine image. The difference in spatial resolution can lead to over- and undercorrection of the emission signal at sharp boundaries like the edges of organs (Meikle et al. 1993, King et al. 1995). To compensate for this, the CT image is filtered to remove high frequencies and match the resolution of the emission data. This blurred image is used for attenuation correction, but the full-resolution CT is still retained for the purpose of localization and the presentation of fused displays.
As the attenuation correction is based on the CT map, any artifacts in the CT can lead to artifacts in the nuclear medicine image. CT artifacts include beam-hardening, metal implants, CT contrast, truncation, and patient motion. Most CT scans will include at least a first-order beam-hardening correction that accounts for spectral changes through differing amounts of water. This corrects well for the soft-tissue component of beam-hardening, but does not correct for contributions from bone. The lack of bone correction leads to residual beam-hardening artifacts, particularly between boney structures such as inside of the skull or pelvic girdle. Beam hardening decreases the apparent attenuation and thus leads to an undercorrection of the nuclear medicine image. Advanced beam-hardening algorithms have been explored to reduce these effects, and new dual-energy CT scanners have the capability of generating mono-energy-equivalent CT images, which would remove this problem (Kyriakou et al. 2010, So and Lee 2011).
Truncation of the CT image can also be a problem. The field-of-view of the CT scanner is often smaller than that of the nuclear medicine device. For example, a typical CT field of view in a PET/CT system is 50 cm in diameter, but the bore size (and hence PET FOV) is 70–80 cm. This difference can lead to truncation of the CT image, particularly in the shoulders and arms of the patient. The truncation, and in particular bilateral truncation, can introduce severe streak artifacts into the CT and an increase in the Hounsfield unit (HU) of the CT image at the edge of the FOV. These inaccuracies are then propagated into the attenuation map and from there to the attenuation-corrected NM image. To compensate for this, algorithms have been developed to extend the FOV of the CT in software by making basic assumptions about the nature of the truncated tissues and employing consistency conditions regarding the total attenuation seen by the CT at different projection angles (Mawlawi et al. 2006).
Metal objects can also be problematic for the CT image. They can completely attenuate an x-ray beam, but still be partially transparent to higher-energy gamma-rays. The CT image then contains streak artifacts and when converted to an attenuation map, the attenuation coefficients may be inappropriately large. Due to the very large attenuation, metal objects can often be identified automatically in the image and then software techniques can be used to compensate. A variety of approaches have been developed similar to the techniques used for higher-order beam-hardening correction such as separation and reprojection of the separate components, use of dual-energy CT, and interpolation of the projection data through the affected regions (Bamberg et al. 2011, Zhang et al. 2011, Joemai et al. 2012).
An additional problem is CT contrast. Typically iodinated, the attenuation from contrast scales very differently from either bone or soft tissues. At x-ray energies, it is very opaque, but it is almost completely transparent at higher energies like 511 keV. As contrast is frequently used for clinical CT, this complicates diagnostic CT procedures being performed in conjunction with a nuclear medicine exam, such as CT-angiography (CTA) combined with myocardial perfusion imaging. Fortunately, IV contrast disperses fairly rapidly, and studies have shown that it does not have a significant impact on the quality of PET images (Berthelsen et al. 2005). Oral contrasts, like barium, are more problematic as they can remain concentrated in the gastrointestinal tract for several days. An example is shown in Figure 13.3. The patient had undergone a CT procedure with oral contrast 3 days prior to the PET study. The inappropriate scaling of the attenuation map leads to artifactual uptake in the large intestine. The non-AC PET image clearly shows no such uptake present. The attenuation map can be corrected by proper segmentation and rescaling of the contrast within the CT and dual-energy CT may also be useful in mitigating this problem (Rehfeld et al. 2008). A good understanding of the source of the artifact and its appearance can also prevent misinterpretation (Groves et al. 2005).
Patient movement, both gross voluntary motion and physiological motion such as breathing or cardiac contractions, can lead to artifacts in the CT image. As the patient is moved through the CT scanner, one slice from the CT volume will be acquired with the patient in one position and another slice from a different position. For example, a mixture of slices acquired at expiration and inspiration can result in the appearance of a disjoint piece of liver apparently suspended in the lung. Another common respiratory artifact is a banana-shaped region of reduced apparent activity just above the diaphragm (Figure 13.4). In this case, the CT has been captured at a point of greater inspiration (and hence a lower diaphragm) than the mean position during PET acquisition resulting in an under correction of PET activity just above the diaphragm. Respiratory artifacts are avoided in diagnostic CT studies by having the patient hold their breath for the duration of the scan (usually only a few seconds with modern CT scanners). A breath-hold acquired with full inspiration is a poor representation of the attenuation experienced during a PET study with regular breathing averaged over several minutes.
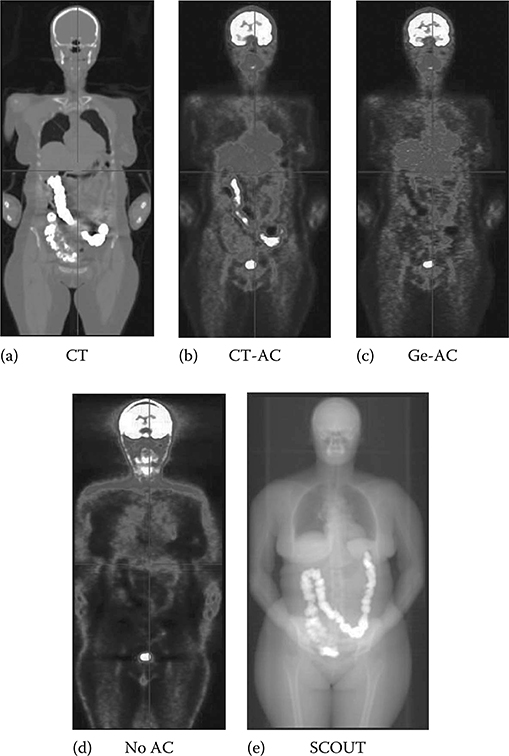
FIGURE 13.3
(See color insert.) Example of a whole-body FDG PET study in the presence of oral contrast. A coronal slice through the patient image volume is shown: CT (a), CT-based attenuation correction (b), Ge-based attenuation correction (c), and no attenuation correction (d). An x-ray through the patient is given in (e) clearly showing the oral contrast remaining in the large intestine. For the PET images, white indicates highest amount of FDG activity.

FIGURE 13.4
A common respiratory artifact in PET/CT. A coronal slice through the patient is shown for Ge-based attenuation correction (a) and CT-based attenuation correction (b). The area of reduced uptake indicated by the arrows in (b) is caused by an inconsistency in the position of the diaphragm between CT and PET imaging. For the PET images, black indicates high amounts of tracer activity.
Attempting to have the patient hold their breath at a representative moment is also unreliable. Respiratory motion has proven to be problematic, particularly for lung and cardiac studies (Pan et al. 2004, 2005, Gould et al. 2007, Bettinardi et al. 2010, Roach et al. 2010), and several different solutions have been proposed. The simplest solution is to capture a CT scan near end-expiration when patient movement is minimal—with a high-speed multi-slice scanner, it is possible to cover a small FOV such as is needed for cardiac studies in just a few seconds. This scan can then be aligned, using manual rigid-body registration tools, to the mean position of the NM scan (Khurshid et al. 2008, Wells et al. 2010). While successful in many cases, this approach cannot correct for deformations in the chest as the diaphragm moves and the lungs expand and contract (Gould et al. 2007, McQuaid and Hutton 2008). In this case, an average CT can be created that is more consistent with the time-averaged NM acquisition. This is done by acquiring a 4DCT—that is multiple CT volumes of the patient over the respiratory cycle—and then averaging them together (Pan et al. 2004). The acquisition of multiple CT volumes increases the patient radiation exposure, but the total exposure can be kept reasonably low, 1/4 mSv per bed position, by using low tube currents (Koepfli et al. 2004, Pan et al. 2006). Additionally, it is not necessary to acquire the entire whole-body CT as a 4DCT. Instead, one can focus on those portions of the patient that have the most motion, i.e., near the diaphragm, and patch in a time-average CT for that portion only (Pan et al. 2004). A final approach is a max-intensity CT (Alessio et al. 2007). This too uses a 4DCT, but instead of averaging the CT over time frames, it takes the maximum attenuation at each voxel in the volume. This increases the total attenuation in object, but has been shown to produce good results in clinical cardiac studies.
13.8 Additional Information from Diagnostic CT
One of the benefits of the combined scanner is that it provides a “one-stop shop” (Bockisch et al. 2009, Gnanasegaran et al. 2009, Czernin et al. 2010). CT is a powerful imaging modality in its own right; and if the CT on the hybrid scanner is of diagnostic quality, it is possible to perform the full suite of procedures of which CT is capable. Because the patient does not have to move from one scanner to another, there is exact concordance in the positioning and orientation of the patient. This makes image fusion and comparison of the two sets of data much easier. It also improves patient comfort and patient throughput as the time required to perform the tests is greatly reduced—there is only one patient visit.
CT provides better spatial resolution allowing detection of smaller abnormalities than might be found with PET. CT can also help to clarify sites of false-positive uptake on PET. The result is that PET/CT, compared to PET or CT alone, more accurately stages cancer and is superior for monitoring response to therapy for many different cancers such as in the lung, breast, colon, and lymphatic system (Czernin et al. 2010). CT can provide additional information in non-oncologic studies, for example, clarifying the cause of matched or mismatched defects in lung ventilation/perfusion studies (Roach et al. 2010).
With respect to cardiac evaluation, CT can add significantly to cardiac evaluation by PET or SPECT alone. Calcium scoring—a measure of the total amount of calcification in a patient's coronary arteries—and CT-angiography (CTA) have been shown to be independent indicators of patient prognosis (Schenker et al. 2008, Schwaiger et al. 2010, Chow et al. 2011, Hacker and Becker 2011), and having this information available leads to better assessment and risk stratification of patients. CTA also provides an excellent view of the coronary artery anatomy and coronary stenoses. CTA has very strong negative predictive value and fused with perfusion information can be used to help identify culprit lesions, those lesions that result in significant functional loss in the heart (Kaufman 2009, Santana 2009, Schwaiger et al. 2010).
The presence of an accurately coregistered high-resolution anatomical map also opens the door to using this information to enhance image quality in nuclear medicine. This can be done through the application of anatomical priors as part of a maximum a posteriori (MAP) reconstruction approach (Comtat et al. 2002, Lehovich et al. 2009, Vanhove et al. 2011, Cheng-Liao and Qi 2011). Radiotracers are expected to distribute fairly homogeneously through different compartments such as an organ and frequently are confined to those compartments. A sharp definition of the location of the anatomical boundaries restricts where the radiotracer may be within the body and thus provides information about the unknown tracer distribution that one is attempting to recover through image reconstruction.
13.9 Image Registration
Accurate localization, accurate attenuation correction, the use of anatomical priors, the fusing of anatomical CT information with functional NM images, indeed virtually all of the gains of a multimodal camera hinge on the accuracy of the coregistration of the images. Although hardware approaches min-imize the source of some of the errors in registration, they cannot completely eliminate them (Gould et al. 2007, Goetze and Wahl 2007, Goetze et al. 2007) and further registration is often required. This final registration is often a rigid-body registration and is performed manually as a quality assurance procedure (Goetze et al. 2007). Manual intervention introduces uncertainty and variability into the accuracy of the final registration and can be a time-consuming process. The alternative is automated approaches to image registration. Semi-automated methods might take in user-designated landmarks to assist in registration. More automated approaches also exist that segment and align the organ of interest (Khurshid et al. 2008) or make use of consistency conditions to aid in registration (Alessio et al. 2010). For example, the total amount of activity seen in a patient should be the same, regardless of the angle of view, once you have compensated for attenuation. Thus, it is possible to adjust the position and orientation of the attenuation map until the difference between projections is minimized.
Even with hardware coregistered images from a hybrid camera, it is still necessary to perform some software registration following acquisition. One might then wonder about performing the entire registration by software and simply acquiring the images on separate machines. Indeed, some have argued to do just that. A number of sophisticated software registration packages have been developed and a recent review on the topic is given by Slomka and Baum (2009). The central advantage of software registration is flexibility. Images acquired previously, either at the same site or even among different sites, can be aligned. This reduces costs as it avoids repeating studies that have already been performed. It similarly saves time for the patient and avoids repeated radiation exposure in the case of tests like CT that use ionizing radiation. Use of software registration improves the efficiency of camera use. A CT scanner that takes a minute to acquire a whole-body attenuation map does not then sit idle during the 20 min PET acquisition or the 15 min SPECT study. A single CT scanner could potentially provide the attenuation map needs of multiple NM systems, greatly reducing the cost of the installation. Alternatively, it could be used for purely CT procedures, while the PET study is being performed. Likewise, particularly with SPECT/CT systems that use slow-rotation CT scanners, the transmission portion of the study can increase the time required for each exam, and thus reduce the number of SPECT studies that might be performed in a standard work day.
By allowing the alignment of any studies available, the physician is not restricted to the modalities available on a particular machine. Thus, one can combine SPECT and PET studies, incorporate MRI or ultrasound information, and use whatever images are available and relevant to the case at hand. This also allows for integration of images acquired at a later date based on the outcomes of the current test. Thus it is not necessary to decide upfront all the tests or procedures that need to be performed on a given patient. In addition, software registration allows for intramodality registration and thereby accurate serial evaluation of a patient during progression of disease or treatment. Software registration can also be used to accurately compare a patient's study with that of a population template. This can assist in identifi-cation of regions of interest and evaluation of the extent of disease.
A complication for software registration is the differences in the configuration of patient during the acquisition of the two images. Different days and different cameras mean rigid-body registration is not sufficient and so warping algorithms are often used to deform the volumes in a constrained manner so that they match. The problem is worse the longer the interval between scans, but even images acquired of the same patient on the same day on the same camera can be quite different unless great care is taken to ensure the patient is in exactly the same position. For example, differences are seen with cardiac SPECT rest/stress imaging during which two images of the patient are taken a few hours apart. Small movements of the arms between the two acquisitions can shift the distribution of soft tissues on the thorax and lead to variations in the amount of breast attenuation (Corbett and Ficaro 2000). Different protocols can also have different preferred patient positions such as arms above the head or arms resting at the patient's sides, which can be extremely difficult to correct for using software techniques. Different cameras have bed shapes that alter how a patient lies. An example is the flat table common for radiation therapy compared to the curved table that is typical for diagnostic imaging. For this reason, diagnostic images that are intending for integration into radiation therapy planning are often acquired on a flat table. A more extreme difference can occur with cardiac imaging; although supine imaging is most common, some cardiac cameras are now configured for upright imaging (e.g., Cardius XPO, Digirad). Finally, the longer the time delay between acquisitions, the greater the chance that the patient's morphology will change whether it is simple weight loss or gain or changes due to disease progression or treatment such as tumor shrinkage due to radiation therapy or surgery.
To correct for these changes, nonlinear registration algorithms are used. These “warping” algorithms modify the morphology of the patient to improve the alignment of one image with the other. A concern is that the warping could also distort the data of interest in the image and so great care must be taken to appropriately constrain the changes applied. Many different approaches have been developed from landmark registration, to mutual information, finite element models, and beyond. The interested reader is referred to (Slomka and Baum 2009) for a more complete introduction to this topic.
Another important application of registration algorithms has been in the area of motion compensation. Whether it is from voluntary motion or involuntary action such as breathing and cardiac contraction, patient motion leads to a blurring of the image data and a consequent change in the standard uptake value of tumors, variation in the size and intensity of lesions, and ambiguity in the position of the measured tracer uptake. Incorporated into pure list-mode reconstruction algorithms or working with data gated based on external motion measurement or physiologic signals, the registration algorithms can serve to reduce or remove the effects of motion. Efforts along these lines are being applied to both oncology and cardiology studies (Slomka et al. 2004, Gravier et al. 2006, Mair et al. 2006, Qiao et al. 2007, Dawood et al. 2008).
Many of the software registration algorithms are complicated and are not widely employed outside research centers. Registration is easiest when the two patient studies are acquired within a very short time of each other and with the patient in the same position. For this reason, most multimodal studies are presently acquired on multimodal cameras such as PET/CT and SPECT/CT systems. The large advantage of the combined camera is that it provides a registration that is approximately correct and that can be easily fine-tuned manually or with simple registration software. The great strength of software registration, though, is its flexibility and it will always remain a valuable tool in multimodal imaging.
13.10 MRI: PET/SPECT
Recently, there has been a surge in interest in combining nuclear medicine imaging with magnetic resonance imaging (MRI) (Cherry 2009). MRI is a powerful imaging technology that has many advantages over CT as a means of providing the anatomical information in a combined scanner. MRI uses radiofrequency radiation and is thus nonionizing, avoiding the radiation dose delivered by CT. This addresses one of the concerns of PET/SPECT-CT imaging, which is the increased dose from the CT component. MRI provides very high-resolution images with excellent soft-tissue contrast—an aspect poorly defined by CT. Different MRI acquisition pulse sequences allow probing of many different attributes within the body tissues such as differences in magnetic relaxation times (T1-weighted imaging), chemical composition (magnetic resonance spectroscopy), or diffusion properties of water (diffusion imaging). These properties can be quite complementary to the tracer-based imaging of nuclear medicine, and thus there can be seen great potential in the combination of these two techniques (Cherry 2009, Bouchelouche et al. 2010, Chen and Kinahan 2010, Czernin et al. 2010).
Unlike the typical PET-SPECT/CT configuration, the scanners combined with MRI are being designed for simultaneous acquisition of both the nuclear medicine and the MRI images. This addresses another issue with the current NM-CT systems, which is the delay between the CT and NM studies brought on by a serial acquisition strategy that can increase the risk of mis-registration due to patient motion or produce other inconsistencies between the two data sets. Simultaneous acquisition is possible because of changes in the design of the PET detector elements. Photomultiplier tubes are very sensitive to the magnetic field environment and thus do not function well in the high magnetic field and rapidly changing gradient magnetic fields of the MRI scanner. Switching to solid-state amplification such as avalanche photodiodes and careful design of the detector electronics has made it possible to build PET-MR systems that do not interfere with each other (Cherry 2009). Though initially focused on small-animal and brain-only cameras, whole-body human systems have recently become commercially available (Biograph mMR, Siemens). Simultaneous acquisition provides exact coreg-istration and thus real-time evaluation of (and potentially correction for) patient voluntary and physiologic motion. It also ensures accurate localization of transient signals.
This technology is very new, however, and there remain several hurdles that must be overcome. The first of which is use of the MRI for attenuation correction of the PET/SPECT data set. Attenuation correction is essential to obtaining quantitatively accurate NM images. Absolute quantification is one of the strengths of PET imaging and needs to be maintained. The attenuation of photon signal from PET and SPECT is due to the interaction of photons with the patient soft tissues. The same interactions, albeit at different photon energies, generate the signal seen with a CT scanner and thus the CT directly measures the attenuation and can provide the map of attenuation coefficients needed for attenuation correction. The MRI signal strength is based on the relaxation properties of hydrogen and is not directly related to the effects causing attenuation in NM. The attenuation coefficient map must be inferred indirectly from the MRI. Different approaches have been explored to achieve this goal (Hofmann et al. 2009, 2011). One approach is to segment the image based on anatomical structures and then assign previously determined population attenuation coefficients. This works well in some areas, but is unable to capture the variable patient-specific attenuation in others such as the lungs. Another approach is nonlinear registration of a predetermined atlas (such as a CT scan), but this similarly does not address variable patient-specific attenuation. A final approach would be to register a previously acquired CT scan of the patient to a simultaneously acquired MRI image, though this reintroduces the radiation dose of the CT (Schreibmann et al. 2010). Some initial efforts at MRI-based attenuation correction have, nevertheless, had some success and work in this area is ongoing.
Additional problems for NM-MRI are those already associated with MRI. Metal implants are often a contraindication and might prevent use of this technology due to the high-magnetic fields. Metal can cause problems for CT, but algorithms have been developed that mitigate these effects and the residual impact is both small and local. Finally, cost of the equipment is also a consideration. MRI is an expensive technology and the cost-benefit analysis will need to be done, as it has been with PET-CT, to demonstrate its cost-effectiveness.
13.11 Summary
The use of SPECT/CT and PET/CT has seen tremendous growth over the past decade. The modalities are very complementary: SPECT and PET provide a very sensitive measure of the function of organs and systems in the body while CT provides structural anatomy with exquisite spatial resolution and excellent bone to soft tissue contrast. Integrating the two technologies is not without difficulty and can lead to artifacts. Nevertheless, the combination of anatomy and function has proven to have a greater diagnostic performance than either modality alone or even the two side by side. The success of the merger of these two imaging techniques has given strong support to the combination of other modalities as well, such as MRI which can provide superb soft-tissue contrast and a reduced radiation burden, making the future of multimodality imaging in medicine look very promising.
References
Alessio A.M., Kinahan P.E., Champley K.M. Attenuation-emission alignment in cardiac PET/CT based on consistency conditions. Med Phys. 2010 Mar;37(3):1191–1200.
Alessio A.M., Kohlmyer S., Branch K. Cine CT for attenuation correction in cardiac PET/CT. J Nucl Med. 2007 May; 48(5):794–801.
Bai C., Conwell R., Kindem J. Phantom evaluation of a cardiac SPECT/VCT system that uses a common set of solid-state detectors for both emission and transmission scans. J Nucl Cardiol. 2010 Jun;17(3):459–469.
Bamberg F., Dierks A., Nikolaou K. Metal artifact reduction by dual energy computed tomography using monoenergetic extrapolation. Eur Radiol. 2011 Jul;21(7):1424–1429.
Berthelsen A.K., Holm S., Loft A. PET/CT with intravenous contrast can be used for PET attenuation correction in cancer patients. Eur J Nucl Med Mol Imaging. 2005 Oct;32(10):1167–1175.
Bettinardi V., Picchio M., Di Muzio N Detection and compensation of organ/lesion motion using 4D-PET/CT respiratory gated acquisition techniques. Radiother Oncol. 2010 Sep;96(3):311–316.
Beyer T., Kinahan P.E., Townsend D.W. The use of X-ray CT for attenuation correction of PET data. Nucl Sci Symp Med Imaging Conf Rec. 1994;4:1573–1577.
Beyer T., Townsend D.W., Brun T. A combined PET/CT scanner for clinical oncology. J Nucl Med. 2000;41:1369–1379.
Blankespoor S.C., Wu X., Kalki K. Attenuation correction of SPECT using X-ray CT on an emission-transmission CT system: Myocardial perfusion assessment. IEEE Trans Nucl Sci. 1996;41:2263–2274.
Bocher M., Blevis I.M., Tsukerman L. A fast cardiac gamma camera with dynamic SPECT capabilities: Design, system validation and future potential. Eur J Nucl Med Mol Imaging. 2010 Oct;37(10):1887–1902.
Bockisch A., Freudenberg L.S., Schmidt D. Hybrid imaging by SPECT/CT and PET/CT: Proven outcomes in cancer imaging. Semin Nucl Med. 2009 Jul;39(4):276–289.
Bouchelouche K., Turkbey B., Choyke P. Imaging prostate cancer: An update on positron emission tomography and magnetic resonance imaging. Curr Urol Rep. 2010 May;11(3):180–190.
Brisse H.J., Madec L., Gaboriaud G. Automatic exposure control in multichannel CT with tube current modulation to achieve a constant level of image noise: Experimental assessment on pediatric phantoms. Med Phys. 2007 Jul;34(7):3018–3033.
Chen D.L., Kinahan P.E.. Multimodality molecular imaging of the lung. J Magn Reson Imaging. 2010 Dec;32(6):1409–1420.
Cheng-Liao J., Qi J. PET image reconstruction with anatomical edge guided level set prior. Phys Med Biol. 2011 Nov 7;56(21):68996918.
Cherry S.R.. Multimodality imaging: Beyond PET/CT and SPECT/CT. Semin Nucl Med. 2009 Sep;39(5):348–353.
Chow B.J., Small G., Yam Y. CONFIRM Investigators. Incremental prognostic value of cardiac computed tomography in coronary artery disease using CONFIRM: COroNary computed tomography angiography evaluation for clinical outcomes: An InteRnational Multicenter registry. Circ Cardiovasc Imaging. 2011 Sep;4(5):463–472.
Comtat C., Kinahan P.E., Fessler J.A. Clinically feasible reconstruction of 3D whole-body PET/CT data using blurred anatomical labels. Phys Med Biol. 2002;47:1–20.
Corbett J.R., Ficaro E.P.. Attenuation corrected cardiac perfusion SPECT. Curr Opin Cardiol. 2000 Sep;15(5):330–306.
Czernin J., Allen-Auerbach M., Schelbert H.R.. Improvements in cancer staging with PET/CT: Literature-based evidence as of September 2006. J Nucl Med. 2007;48:78S–88S.
Czernin J., Benz M.R., Allen-Auerbach M.S. PET/CT imaging: The incremental value of assessing the glucose metabolic phenotype and the structure of cancers in a single examination. Eur J Radiol. 2010 Mar;73(3):470–480.
Dawood M., Buther F., Jiang X. Respiratory motion correction in 3-D PET data with advanced optical flow algorithms. IEEE Trans Med Imaging. 2008 Aug;27(8):1164–1175.
Delbeke D., Schöder H., Martin W.H. Hybrid imaging (SPECT/CT and PET/CT): Improving therapeutic decisions. Semin Nucl Med. 2009 Sep;39(5):308–340.
Di Carli M.F., Hachamovitch R. New technology for noninvasive evaluation of coronary artery disease. Circulation. 2007 Mar 20;115(11):1464–1480.
Even-Sapir E., Keidar Z., Bar-Shalom R. Hybrid imaging (SPECT/CT and PET/CT)—improving the diagnostic accuracy of functional/metabolic and anatomic imaging. Semin Nucl Med. 2009 Jul;39(4):264–275.
Fleischmann D., Boas F.E.. Computed tomography—old ideas and new technology. Eur Radiol. 2011 Mar;21(3):510–517.
Flotats A., Knuuti J., Gutberlet M. Cardiovascular Committee of the EANM, the ESCR and the ECNC. Hybrid cardiac imaging: SPECT/CT and PET/CT. A joint position statement by the European Association of Nuclear Medicine (EANM), the European Society of Cardiac Radiology (ESCR) and the European Council of Nuclear Cardiology (ECNC). Eur J Nucl Med Mol Imaging. 2011 Jan;38(1):201–212.
Garcia E.V.. SPECT attenuation correction: An essential tool to realize nuclear cardiology's manifest destiny. J Nucl Cardiol. 2007 Jan;14(1):16–24.
Gnanasegaran G., Barwick T., Adamson K. Multislice SPECT/CT in benign and malignant bone disease: When the ordinary turns into the extraordinary. Semin Nucl Med. 2009 Nov;39(6):431–442.
Goetze S., Brown T.L., Lavely W.C. Attenuation correction in myocardial perfusion SPECT/CT: Effects of misregistration and value of reregistration. J Nucl Med. 2007 Jul;48(7):1090–1095.
Goetze S., Wahl R.L.. Prevalence of misregistration between SPECT and CT for attenuation-corrected myocardial perfusion SPECT. J Nucl Cardiol. 2007 Apr;14(2):200–206.
Gould K.L., Pan T., Loghin C. Frequent diagnostic errors in cardiac PET/CT due to misregistration of CT attenuation and emission PET images: A definitive analysis of causes, consequences, and corrections. J Nucl Med. 2007 Jul;48(7):1112–1121.
Gravier E., Yang Y., King M.A. Fully 4D motion-compensated reconstruction of cardiac SPECT images. Phys Med Biol. 2006 Sep 21;51(18):4603–4619.
Groves A.M., Kayani I., Dickson J.C. Oral contrast medium in PET/CT: Should you or shouldn't you? Eur J Nucl Med Mol Imaging. 2005 Oct;32(10):1160–1166.
Hacker M., Becker C.. The incremental value of coronary artery calcium scores to myocardial single photon emission computer tomography in risk assessment. J Nucl Cardiol. 2011 Aug;18(4):700–711.
Hasegawa B.H., Gingold E.L., Reilly S.M. Description of a simultaneous emission-transmission CT system. Proc SPIE. 1990;1231:50–60.
Hasegawa B.H., Reilly S.M., Gingold E.L. Design considerations for a simultaneous emission-transmission CT scanner. Radiology. 1989;173:414.
Hasegawa B.H., Tang H.R., Da Silva A.J. Implementation and applications of a combined CT/SPECT system. IEEE Nucl Sci Symp Med Imaging Conf Rec. 1999;3:1373–1377.
Hofmann M., Bezrukov I., Mantlik F. MRI-based attenuation correction for whole-body PET/MRI: Quantitative evaluation of segmentation- and atlas-based methods. J Nucl Med. 2011 Sep;52(9):1392–1399.
Hofmann M., Pichler B., Schölkopf B. Towards quantitative PET/MRI: A review of MR-based attenuation correction techniques. Eur J Nucl Med Mol Imaging. 2009 Mar;36 Suppl 1:S93–S104.
Joemai R.M., de Bruin P.W., Veldkamp W.J. Metal artifact reduction for CT: Development, implementation, and clinical comparison of a generic and a scanner-specific technique. Med Phys. 2012 Feb;39(2):1125–1132.
Kaufmann P.A., Di Carli M.F.. Hybrid SPECT/CT and PET/CT imaging: The next step in noninvasive cardiac imaging. Semin Nucl Med. 2009 Sep;39(5):341–347.
Khurshid K., McGough R.J., Berger K.. Automated cardiac motion compensation in PET/CT for accurate reconstruction of PET myocardial perfusion images. Phys Med Biol. 2008 Oct 21;53(20):5705–5718.
Kinahan P.E., Townsend D.W., Beyer T. Attenuation correction for a combined 3D PET/CT scanner. Med Phys. 1998 Oct;25(10):2046–2053.
King M.A., Tsui B.M.W., Pan T.. Attenuation compensation for cardiac single-photon emission computed tomographic imaging: Part 1. Impact of attenuation and methods of estimating attenuation maps. J Nucl Cardiol. 1995;2:513–524.
Koepfli P., Hany T.F., Wyss C.A. CT attenuation correction for myocardial perfusion quantification using a PET/CT hybrid scanner. J Nucl Med. 2004 Apr;45(4):537–542.
Kuhl D.E., Hale J., Eaton W.L.. Transmission scanning: A useful adjunct to conventional emission scanning for accurately keying isotope deposition to radiographic anatomy. Radiology. 1966;87:278–284.
Kyriakou Y., Meyer E., Prell D. Empirical beam hardening correction (EBHC) for CT. Med Phys. 2010 Oct;37(10):5179–5187.
Lehovich A., Bruyant P.P., Gifford H.S. Impact on reader performance for lesion-detection/localization tasks of anatomical priors in SPECT reconstruction. IEEE Trans Med Imaging. 2009 Sep;28(9):1459–1467.
Levin C.S.. Primer on molecular imaging technology. Eur J Nucl Med Mol Imaging. 2005;32:S325–S345.
Mair B.A., Gilland D.R., Sun J.. Estimation of images and nonrigid deformations in gated emission CT. IEEE Trans Med Imaging. 2006 Sep;25(9):1130–1144.
Marin D., Nelson R.C., Schindera S.T. Low-tube-voltage, high-tube-current mul-tidetector abdominal CT: Improved image quality and decreased radiation dose with adaptive statistical iterative reconstruction algorithm—initial clinical experience. Radiology. 2010 Jan;254(1):145–153.
Mawlawi O., Erasmus J.J., Pan T. Truncation artifact on PET/CT: Impact on measurements of activity concentration and assessment of a correction algorithm. AJR Am J Roentgenol. 2006 May;186(5):1458–1467.
McQuaid S.J., Hutton B.F.. Sources of attenuation-correction artefacts in cardiac PET/CT and SPECT/CT. Eur J Nucl Med Mol Imaging. 2008 Jun;35(6):1117–1123.
Meikle S.R., Dahlbom M., Cherry S.R.. Attenuation correction using count-limited transmission data in positron emission tomography. J Nucl Med. 1993 Jan;34(1):143–150.
Mirshanov D.M.. Transmission-Emission Computer Tomograph. Tashkent Branch. All-Union Research Surgery Center, USSR Academy of Medical Science, USSR, 1987.
Mohan H.K., Gnanasegaran G., Vijayanathan S. SPECT/CT in imaging foot and ankle pathology-the demise of other coregistration techniques. Semin Nucl Med. 2010 Jan;40(1):41–51.
Pan T., Lee T.Y., Rietzel E. 4D-CT imaging of a volume influenced by respiratory motion on multi-slice CT. Med Phys. 2004 Feb;31(2):333–340.
Pan T., Mawlawi O., Luo D. Attenuation correction of PET cardiac data with low-dose average CT in PET/CT. Med Phys. 2006 Oct;33(10):3931–3938.
Pan T., Mawlawi O., Nehmeh S.A. Attenuation correction of PET images with respiration-averaged CT images in PET/CT. J Nucl Med. 2005 Sep;46(9):1481–1487.
Park E.A., Lee W., Kim K.W. Iterative reconstruction of dual-source coronary CT angiography: Assessment of image quality and radiation dose. Int J Cardiovasc Imaging. 2012 Oct;28(7): 1775–1786.
Patel C.N., Chowdhury F.U., Scarsbrook A.F.. Hybrid SPECT/CT: The end of “unclear” medicine. Postgrad Med J. 2009 Nov;85(1009):606–613.
Patton J.A., Townsend D.W., Hutton B.F.. Hybrid imaging technology: From dreams and vision to clinical devices. Semin Nucl Med. 2009 Jul;39(4):247–263.
Perisinakis K., Theocharopoulos N., Karkavitsas N. Patient effective radiation dose and associated risk from transmission scans using 153Gd line sources in cardiac spect studies. Health Phys. 2002 Jul;83(1):66–74.
Pijls N.H., van Schaardenburgh P., Manoharan G. Percutaneous coronary intervention of functionally nonsignificant stenosis: 5-year follow-up of the DEFER Study. J Am Coll Cardiol. 2007 May 29;49(21):2105–2111.
Qiao F., Pan T., Clark J.W. Jr Joint model of motion and anatomy for PET image reconstruction. Med Phys. 2007 Dec;34(12):4626–4639.
Rehfeld N.S., Heismann B.J., Kupferschläger J. Single and dual energy attenuation correction in PET/CT in the presence of iodine based contrast agents. Med Phys. 2008 May;35(5):1959–1969.
Roach P.J., Gradinscak D.J., Schembri G.P. SPECT/CT in V/Q scanning. Semin Nucl Med. 2010 Nov;40(6):455–466.
Santana C.A., Garcia E.V., Faber T.L. Diagnostic performance of fusion of myocardial perfusion imaging (MPI) and computed tomography coronary angiogra-phy. J Nucl Cardiol. 2009 Mar–Apr;16(2):201–211.
Schenker M.P., Dorbala S., Hong E.C. Interrelation of coronary calcification, myocardial ischemia, and outcomes in patients with intermediate likelihood of coronary artery disease: A combined positron emission tomography/computed tomography study. Circulation. 2008 Apr 1;117(13):1693–1700.
Schreibmann E., Nye J.A., Schuster D.M. MR-based attenuation correction for hybrid PET-MR brain imaging systems using deformable image registration. Med Phys. 2010 May;37(5):2101–2109.
Schwaiger M., Ziegler S.I., Nekolla S.G.. PET/CT challenge for the non-invasive diagnosis of coronary artery disease. Eur J Radiol. 2010 Mar;73(3):494–503.
Seo T., Mari C., Hasegawa B.H.. Technological development and advances in single-photon emission computed tomography/computed tomography. Semin Nucl Med. 2008;38:177–198.
Slomka P.J. and Baum R.P.. Multimodality image registration with software: State-of-the-art. Eur J Nucl Med Mol Imaging. 2009 Mar;36 Suppl 1:S44–S55.
Slomka P.J., Nishina H., Berman D.S. “Motion-frozen” display and quantification of myocardial perfusion. J Nucl Med. 2004 Jul;45(7):1128–1134.
So A., Lee T.Y.. Quantitative myocardial CT perfusion: A pictorial review and the current state of technology development. J Cardiovasc Comput Tomogr. 2011 Nov–Dec;5(6):467–481.
Vanhove C., Defrise M., Bossuyt A. Improved quantification in multiple-pinhole SPECT by anatomy-based reconstruction using microCT information. Eur J Nucl Med Mol Imaging. 2011 Jan;38(1):153–165.
von Schulthess G.K.. Positron emission tomography versus positron emission tomography/computed tomography: From “unclear” to “new-clear” medicine. Mol Imaging Biol. 2004 Jul–Aug;6(4):183–187.
Wells R.G., Ruddy T.D., DeKemp R.A. Single-phase CT aligned to gated PET for respiratory motion correction in cardiac PET/CT. J Nucl Med. 2010 Aug;51(8):1182–1190.
Zhang X., Wang J., Xing L.. Metal artifact reduction in X-ray computed tomography (CT) by constrained optimization. Med Phys. 2011 Feb;38(2):701–711.