Chapter 8
Emerging 2D Materials
Ken Sakaushi1,2
1Center for Green Research on Energy and Environmental Materials, National Institute for Materials Science, 1-1 Namiki, Tsukuba, 305-0044, Japan
2Global Research Center for Environment and Energy based on Nanomaterials Science, National Institute for Materials Science, 1-1 Namiki, Tsukuba, 305-0044, Japan
8.1 Introduction
Two-dimensional (2D) materials are attracting great interest since they are suitable model systems to play with theoretically predicted anomalous physical and chemical phenomena [1]. If we look at the history of science, some of these predictions have been constructed even at the dawn of quantum mechanics. For a long time, those “fascinating” but at the same time “unusual” physical predictions were sometimes just models for thought experiments for novel theory in order to consider the origin of the universe or even to be shown in courses for students. This can be called the pre-graphene era. Now we are living in the post-graphene era: we, human beings, observed massless Dirac fermion and related anomalous quantum phenomena using graphene [1p]. Therefore, although this material required “one small step” to prepare, exfoliating using Scotch tape, it is actually “one giant leap” for general science. Although cerebrated in 2016, quantum mechanics married with topology in the late twentieth century [1e]; thus, those predictions of unique physical and chemical phenomena in 2D systems are getting even more attractive than before. In this chapter, recent developments of 2D materials systems are discussed and unique functions originated by those systems are shown.
8.2 Revisiting Uniqueness of Graphene as the Archetype of 2D Materials Systems
In order to explain the emerging properties of 2D materials systems, we have to visit graphene since it is a suitable starting point. As many wonderful reviews have been already written [1u, v], just the key features of graphene as an archetype of 2D systems are summarized in this part. Readers can have a look at more details on basic physics and mathematical descriptions of graphene in already-published sophisticated materials.
So why is graphene interesting? This is because we can observe phenomena which can be described by the Dirac's equation as being a key to understand modern physics in solid states (and therefore these materials' anomalous functions can bring in chemistry). As written by Novoselov, Geim et al. that “quantum relativistic effects are usually minute in the known experimental systems that can be described accurately by the non-relativistic Schrödinger equation,” [1r] but graphene's many electronic properties, for example, Dirac fermions (Figure 8.1), can be described by the relativistic Schrödinger equation, that is, the Dirac's equation.
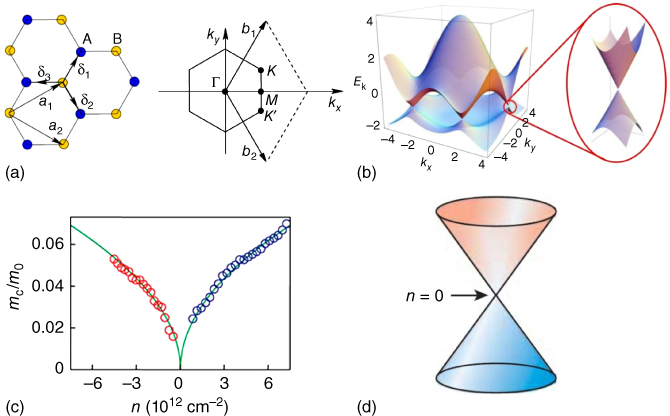
Figure 8.1 Crystal and electronic structures, and basic quantum properties of graphene. (a) Left: crystal structure of graphene and right: corresponding Brillouin zone. The Dirac cones are located at the K and K′ points. (b) Energy dispersion in the honeycomb lattice showing a zoom in of the energy band close to a Dirac point.
((a,b) Castro Neto et al. 2009 [1u]. Reproduced with permission of American Physical Society.)
(c) Cyclotron mass of charge carriers in graphene as a function of their concentration n. Positive and negative n correspond to electrons and holes, respectively. (d) Electronic spectrum of graphene as a zero-gap 2D semiconductor.((c,d) Novoselov et al. 2005 [1r]. Reproduced with permission of Nature Publishing Group.)
We can observe many unique quantum phenomena from graphene, but one of the most important findings is an anomalous quantum Hall effect (Figure 8.2a,b). In the case of this study, an external magnetic field was applied in order to induce additional discrete energy levels, the so-called Landau levels. This finding led to the design of a quantum Hall effect at zero magnetic field and the Haldane model was described as a quantum Hall effect without Landau levels based on parity anomaly of (2+1)-dimensional field theory [1j] (Figure 8.2c,d). Later, Kane and Mele showed by a theoretical study that an effect of spin–orbit interactions can induce spin and charge conductances at the edge of 2D condensed matters [1q].

Figure 8.2 (a) Shubnikov-de Haas oscillations in graphene. (b) Quantum Hall effect of graphene.
((a,b) Novoselov et al. 2005 [1r]. Reproduced with permission of Nature Publishing Group.)
(c) Haldane model showing arrows on second neighbor bonds, marking the directions of positive phase hopping in the state with broken time-reversal invariance. (d) Phase diagram of the spinless electron model showing the zero-field quantum Hall effect.((c,d) Reprinted from [1j]. Copyright 1988 American Physical Society.)
Since graphene's spin–orbit interaction is weak, this theory was applied to HgTe/(Hg, Cd)Te quantum wells, which is a 2D electron system constituted of elements heavier than the carbon atom, and confirmed that the Kane–Mele model is true [1t]. The author notes that the Haldane and Kane–Mele models are based on a combination of the Dirac's equation and topology, the origin of which can be traced to the Thouless–Kohmoto–Nightingale–Nijs equation [1e], and these studies are key to the present intensive researches on topological phase, such as topological insulator.
As such, graphene is an ideal theoretical platform to study quantum phenomena and is a key starting point to find new science. The abovementioned studies are expanding quite rapidly; for example, topological insulator was immediately expanded from 2D to three-dimensional (3D) in both theory and experiment [2]. In 3D topological insulators, novel physics is predicted [3], such as the Majorana fermion which is considered to be related to superconductors. Furthermore, as an alternative to the Dirac equation, Wyle's equation can be applied to “classical” 2D electron system and can be combined with topology (topological field theory). Therefore, this physics can also describe unique quantum phenomena and these are already found, for instance, in the materials of the so-called Wyle semimetals. Last but not the least, these studies have already given a huge impact to basic science but also could develop technology, for example, magnetic memory and quantum computing. Therefore, basic studies on 2D systems in theory and experiment were the trigger for the further understanding of fundamental laws in our world, and beautiful harmonies in nature were found. One wonderful aspect of the beautifulness written by the language of physics and mathematics is indeed the laws in our universe so that these can be translated to other fields, for example, chemistry, informatics, and biology.
8.3 Emerging 2D Materials
As the finding of graphene paved the way for studying novel physics, novel materials can contribute to further understand the basic principles existing in our universe. In this context, 2D materials system is a good starting point since many theoretical predictions were applied to systems in this dimension. In this part, several newly developing 2D materials are surveyed: one is a carbon-, nitrogen-framework (CNF), [4] which is one system in covalent organic frameworks (COFs), [5] and another in 2D metal–organic frameworks (MOFs), [6] which are also called porous coordination polymers (PCPs).
CNFs are an interesting materials system since they can be considered as porous, therefore geometrically modified, and nitrogen-containing graphene. This modification in geometrical and chemical structures of frameworks can tune the electronic structure of graphene. From another point of view, as synthesis of layered COFs (and, of course, layered MOFs/PCPs) are well-developed enough to design a specific periodicity, therefore, in principle, a specific electronic structure can be obtained if we can control the synthetic process of the abovementioned material system in 2D form.
Although graphitic carbon nitride (g-C3N4) is the best-known system among CNFs and, therefore, a wide spectrum of theoretical and experimental studies can be found [7], the structure itself is complicated. Hence, in order to study electronic structures of CNFs, a first-principle calculation was performed to covalent triazine-based frameworks (CTFs) [4c, 8]. CTFs are a unique materials system showing high thermal and chemical stabilities. Having a high surface area due to regular pore structure, CTFs can be applied to energy conversion/storage reactions [9]. Even though those reactions are strongly correlated to electronic properties of materials [10], CTFs were not considered as an electronic material. Therefore, it was compared to theoretical electronic structures of CTFs having different periodicities (Figure 8.3). This study pointed out that, as a main feature of CTF systems, the density of state (DOS) has a large peak close to the Fermi level, which suggests the existence of flat bands, being a key for nontrivial quantum properties, and charge localization being a possible a reason for low electronic conductivity. The most important indication of this study is that the ratio of the aromatic rings can tune the electronic structures. This reveals the following two points: (i) a particular electronic structure of CTFs can be emerged from a specific structural periodicity and (ii) their electronic properties are controllable by changing the number of layers and the ratio of different aromatic rings. Therefore, CTFs can lead to further development of organic semiconductors with tunable electronic properties. These results indicate that g-C3N4 is not the exceptional material to be an electronic active CNF and various numbers of CNFs can have electronic properties. Following this materials system, other new CNFs had been successfully synthesized, for instance, poly(triazine imide) incorporated with Li+ and Cl− [11], and triazine-based graphitic carbon nitride [12].
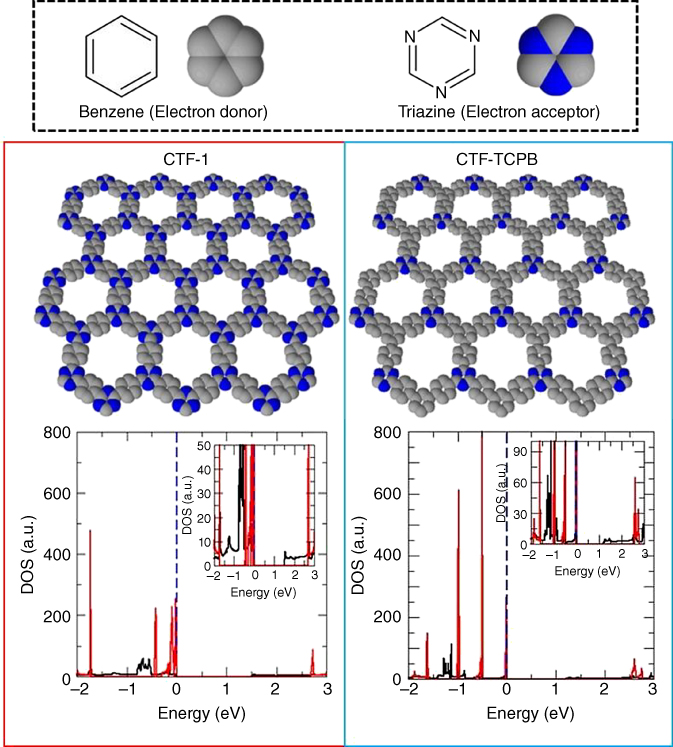
Figure 8.3 Structures of CTF-1 and CTF-TCPB. Light and dark gray balls represent carbon and nitrogen atoms, respectively. Densities of states (DOSs) of CTF-1 and CTF-TCPB are calculated on the first-principle basis.
(Copyright 2013 American Chemical Society.)
As formation of 2D frameworks is highly attractive even in simple metal-free COF systems, it is attracting a huge interest to synthesize 2D MOFs. Due to many synthetic techniques developed by huge efforts of chemists [5b, 6d], a wide spectrum of stable, electronic active 2D MOFs are reported [13]. In order to obtain 2D MOFs, often liquid–liquid or gas–liquid interfacial reactions are applied. For example, a series of π-conjugated nanosheet complexes were synthesized [13c, d, h, 14]. As the first example of this system, a 2D system comprising planar nickel bis(dithiolene) complexes was synthesized (Figure 8.4a,b) [13c], which is redox active and shows a high electronic conductivity. Following this, several nanosheets were obtained such as nanosheets based on metal bis(terpyridine) complexes and a bis(dipyrrinato) zinc(II) complex [14a]. Nickel bis(dithiolene) framework shows a high electronic conductivity of 1.6 × 102 S cm−1. Theoretical calculation and photoelectron emission spectroscopy supports that the metallic nature of these nanosheets can be the origin of this high electronic conductivity (Figure 8.4b). Dincă and coworkers synthesized Ni3(HITP)2 (HITP = 2,3,6,7,10,11-hexaiminotriphenylene) (Figure 8.4c,d) [13f], which also shows extremely high electronic conductivity of 2 and 4 × 10 S cm−1 in bulk (pellet) and surface (film) forms, respectively. In addition to a high electronic conductivity, this 2D MOF shows considerable properties as an electrocatalyst and an electrode for electric double-layer capacitors [15]. Of course these developments are on-going in the field of CNFs.

Figure 8.4 (a) Schematic illustration of a 2D MOF based on planar nickel bis(dithiolene) complex.
(Kambe et al. 2013 [13c]. Reproduced with permission of American Chemical Society.) and (b) its conductivity measurement.
(Kambe et al. 2014 [13d]. Reproduced with permission of American Chemical Society.) (c) Ni3(HITP)2, which is another 2D MOF, showing a high conductivity.
(Sheberla et al. 2014 [13f]. Reproduced with permission of American Chemical Society.) (d) Oxygen reduction reaction performance of Ni3(HITP)2.
(Miner et al. 2016 [15a], https://www.nature.com/articles/ncomms10942. Licensed under CC BY 4.0.) (e) Inactive-active transition by heterojunction based on CNFs. The inert Au surface becomes ORR active by formation of heterojunction (Sakaushi et al. 2017. Adapted with permission from [9d]. Copyright American Chemical Society.).
In addition to these promising experimental studies, theoretical works have unveiled several advanced functions in 2D MOF systems. It was identified that a 2D MOF comprising planar nickel bis(dithiolene) complexes [16], which could exhibit nontrivial topological states in both a Dirac band and a flat band, was a topological insulator (TI). The authors claimed that this materials system should be less sensitive against oxidation; thus, it could simplify the fabrication processes of devices based on TI. Indeed, there is a wide spectrum of metal ions and organic moieties to organize structures and lead to specific electronic properties of TI. Another example, trans-Au-THTAP (THTAP, trihydroxytriaminophenalenyl) [17], was predicted to exhibit a half-filled flat band of the kagome lattice, which is one of a family of lattices that shows Lieb–Mielke–Tasaki's flat-band ferromagnetism. This system has a flat band near the Fermi energy; therefore, we could expect both ferromagnetic and topologically nontrivial phases. Although the proposed system does not have a quantized Hall current due to its metallicity, a topologically nontrivial phase, the so-called Chern metallic phase, might be realized in the proposed material.
8.4 Remarks
Two-dimensional materials have entered into a new era by means of both experiment and theory, and many novel properties in basic physics had been observed. In the present stage, the frameworks shown in this discussion are expected to bridge physics and chemistry: 2D frameworks can emerge with electronic properties which are tunable and therefore can satisfy the requirements of important reactions such as energy storage/conversion reactions toward next-generation batteries and electrocatalysts to produce fuels, for instance, hydrogen and methanol, from sustainable resources, such as seawater, methane, and CO2. Even more important is that these 2D systems can be model materials to study modern quantum physics, which are currently just predicted in pure theories and could emerge as novel technology into this world.
Acknowledgment
K.S. thanks National Institute for Materials Science (NIMS) and Ministry of Education, Culture, Sports, Science and Technology, Japan (MEXT) for providing the opportunity to focus on doing basic science from various aspects, especially financially. This work was supported by JSPS KAKENHI Grant-in-Aid for Research Activity Start-up Grant Number JP15H06850.
References
- 1 (a) Wallace, P.R. (1947) Phys. Rev., 71, 622–634;(b) Lomer, W.M. (1955) Proc. R. Soc. London, Ser. A, 227, 330–349;(c) McClure, J.W. (1956) Phys. Rev., 104, 666–671;(d) Slonczewski, J.C. and Weiss, P.R. (1958) Phys. Rev., 109, 272–279;(e) Thouless, D.J., Kohmoto, M., Nightingale, M.P., and den Nijs, M. (1982) Phys. Rev. Lett., 49, 405–408;(f) Haldane, F.D.M. (1983) Phys. Rev. Lett., 51, 605–608;(g) Kohmoto, M., Kadanoff, L.P., and Tang, C. (1983) Phys. Rev. Lett., 50, 1870–1872;(h) Kohmoto, M. (1985) Ann. Phys., 160, 343–354;(i) Affleck, I., Kennedy, T., Lieb, E.H., and Tasaki, H. (1987) Phys. Rev. Lett., 59, 799–802;(j) Haldane, F.D.M. (1988) Phys. Rev. Lett., 61, 2015–2018;(k) Kennedy, T. and Tasaki, H. (1992) Phys. Rev. B, 45, 304–307;(l) Oshikawa, M. (1992) J. Phys. Condens. Matter, 4, 7469;(m) Oshikawa, M., Yamanaka, M., and Affleck, I. (1997) Phys. Rev. Lett., 78, 1984–1987;(n) Taguchi, Y., Oohara, Y., Yoshizawa, H., Nagaosa, N., and Tokura, Y. (2001) Science, 291, 2573–2576;(o) Affleck, I., Kennedy, T., Lieb, E.H., and Tasaki, H. (2004) in Condensed Matter Physics and Exactly Soluble Models: Selecta of Elliott H. Lieb (eds B. Nachtergaele, J.P. Solovej, and J. Yngvason), Springer, Berlin, Heidelberg, pp. 253–304;(p) Novoselov, K.S., Geim, A.K., Morozov, S.V., Jiang, D., Zhang, Y., Dubonos, S.V., Grigorieva, I.V., and Firsov, A.A. (2004) Science, 306, 666–669;(q) Kane, C.L. and Mele, E.J. (2005) Phys. Rev. Lett., 95, 146802;(r) Novoselov, K.S., Geim, A.K., Morozov, S.V., Jiang, D., Katsnelson, M.I., Grigorieva, I.V., Dubonos, S.V., and Firsov, A.A. (2005) Nature, 438, 197–200;(s) Zhang, Y., Tan, Y.-W., Stormer, H.L., and Kim, P. (2005) Nature, 438, 201–204;(t) König, M., Wiedmann, S., Brüne, C., Roth, A., Buhmann, H., Molenkamp, L.W., Qi, X.-L., and Zhang, S.-C. (2007) Science, 318, 766–770;(u) Castro Neto, A.H., Guinea, F., Peres, N.M.R., Novoselov, K.S., and Geim, A.K. (2009) Rev. Mod. Phys., 81, 109–162;(v) Abergel, D.S.L., Apalkov, V., Berashevich, J., Ziegler, K., and Chakraborty, T. (2010) Adv. Phys., 59, 261–482.
- 2 Fu, L., Kane, C.L., and Mele, E.J. (2007) Phys. Rev. Lett., 98, 106803.
- 3 Nayak, C., Simon, S.H., Stern, A., Freedman, M., and Das Sarma, S. (2008) Rev. Mod. Phys., 80, 1083–1159.
- 4 (a) Kroke, E. and Schwarz, M. (2004) Coord. Chem. Rev., 248, 493–532;(b) Goettmann, F., Fischer, A., Antonietti, M., and Thomas, A. (2006) Angew. Chem. Int. Ed., 45, 4467–4471;(c) Kuhn, P., Antonietti, M., and Thomas, A. (2008) Angew. Chem. Int. Ed., 47, 3450–3453;(d) Thomas, A., Fischer, A., Goettmann, F., Antonietti, M., Muller, J.-O., Schlogl, R., and Carlsson, J.M. (2008) J. Mater. Chem., 18, 4893–4908;(e) Sakaushi, K. and Antonietti, M. (2015) Bull. Chem. Soc. Jpn., 88, 386–398;(f) Sakaushi, K. and Antonietti, M. (2015) Acc. Chem. Res., 48, 1591–1600.
- 5 (a) Côté, A.P., Benin, A.I., Ockwig, N.W., O'Keeffe, M., Matzger, A.J., and Yaghi, O.M. (2005) Science, 310, 1166–1170;(b) Waller, P.J., Gándara, F., and Yaghi, O.M. (2015) Acc. Chem. Res., 48, 3053–3063.
- 6 (a) Kondo, M., Yoshitomi, T., Matsuzaka, H., Kitagawa, S., and Seki, K. (1997) Angew. Chem. Int. Ed. Engl., 36, 1725–1727;(b) Kitagawa, S. and Kondo, M. (1998) Bull. Chem. Soc. Jpn., 71, 1739–1753;(c) Yaghi, O.M., O'Keeffe, M., Ockwig, N.W., Chae, H.K., Eddaoudi, M., and Kim, J. (2003) Nature, 423, 705–714;(d) Kitagawa, S., Kitaura, R., and Noro, S.-i. (2004) Angew. Chem. Int. Ed., 43, 2334–2375.
- 7 (a) Wang, X., Maeda, K., Chen, X., Takanabe, K., Domen, K., Hou, Y., Fu, X., and Antonietti, M. (2009) J. Am. Chem. Soc., 131, 1680–1681;(b) Wang, X., Maeda, K., Thomas, A., Takanabe, K., Xin, G., Carlsson, J.M., Domen, K., and Antonietti, M. (2009) Nat. Mater., 8, 76–80;(c) Wang, Y., Wang, X., and Antonietti, M. (2012) Angew. Chem. Int. Ed., 51, 68–89;(d) Zheng, Y., Jiao, Y., Chen, J., Liu, J., Liang, J., Du, A., Zhang, W., Zhu, Z., Smith, S.C., Jaroniec, M., Lu, G.Q., and Qiao, S.Z. (2011) J. Am. Chem. Soc., 133, 20116–20119;(e) Zheng, Y., Liu, J., Liang, J., Jaroniec, M., and Qiao, S.Z. (2012) Energy Environ. Sci., 5, 6717–6731;(f) Shalom, M., Gimenez, S., Schipper, F., Herraiz-Cardona, I., Bisquert, J., and Antonietti, M. (2014) Angew. Chem. Int. Ed., 53, 3654–3658;(g) Xu, J., Brenner, T.J.K., Chabanne, L., Neher, D., Antonietti, M., and Shalom, M. (2014) J. Am. Chem. Soc., 136, 13486–13489;(h) Vilé, G., Albani, D., Nachtegaal, M., Chen, Z., Dontsova, D., Antonietti, M., López, N., and Pérez-Ramírez, J. (2015) Angew. Chem. Int. Ed., 54, 11265–11269.
- 8 Sakaushi, K., Nickerl, G., Kandpal, H.C., Cano-Cortés, L., Gemming, T., Eckert, J., Kaskel, S., and van den Brink, J. (2013) J. Phys. Chem. Lett., 4, 2977–2981.
- 9 (a) Sakaushi, K., Nickerl, G., Wisser, F.M., Nishio-Hamane, D., Hosono, E., Zhou, H., Kaskel, S., and Eckert, J. (2012) Angew. Chem. Int. Ed., 51, 7850–7854;(b) Sakaushi, K., Hosono, E., Nickerl, G., Gemming, T., Zhou, H., Kaskel, S., and Eckert, J. (2013) Nat. Commun., 4, 1485;(c) Sakaushi, K., Hosono, E., Nickerl, G., Zhou, H., Kaskel, S., and Eckert, J. (2014) J. Power Sources, 245, 553–556;(d) Sakaushi, K., Lyalin, A., Tominaka, S., Taketsugu, T., and Uosaki, K. (2017) ACS Nano, 11, 1770–1779.
- 10 (a) Vol'kenshtein, F.F. (1966) Russ. Chem. Rev., 35, 537;(b) Bockris, J.M. and Reddy, A.K. (1970) Modern Electrochemistry: An Introduction to an Interdisciplinary Area, vol. 2, Plenum Press;(c) Trasatti, S. (1972) J. Electroanal. Chem. Interfacial Electrochem., 39, 163–184.
- 11 (a) Ham, Y., Maeda, K., Cha, D., Takanabe, K., and Domen, K. (2013) Chem. Asian J., 8, 218–224;(b) Schwinghammer, K., Mesch, M.B., Duppel, V., Ziegler, C., Senker, J., and Lotsch, B.V. (2014) J. Am. Chem. Soc., 136, 1730–1733.
- 12 Algara-Siller, G., Severin, N., Chong, S.Y., Björkman, T., Palgrave, R.G., Laybourn, A., Antonietti, M., Khimyak, Y.Z., Krasheninnikov, A.V., Rabe, J.P., Kaiser, U., Cooper, A.I., Thomas, A., and Bojdys, M.J. (2014) Angew. Chem. Int. Ed., 53, 7450–7455.
- 13 (a) Makiura, R. and Kitagawa, H. (2010) Eur. J. Inorg. Chem., 2010, 3715–3724;(b) Makiura, R., Motoyama, S., Umemura, Y., Yamanaka, H., Sakata, O., and Kitagawa, H. (2010) Nat. Mater., 9, 565–571;(c) Kambe, T., Sakamoto, R., Hoshiko, K., Takada, K., Miyachi, M., Ryu, J.-H., Sasaki, S., Kim, J., Nakazato, K., Takata, M., and Nishihara, H. (2013) J. Am. Chem. Soc., 135, 2462–2465;(d) Kambe, T., Sakamoto, R., Kusamoto, T., Pal, T., Fukui, N., Hoshiko, K., Shimojima, T., Wang, Z., Hirahara, T., Ishizaka, K., Hasegawa, S., Liu, F., and Nishihara, H. (2014) J. Am. Chem. Soc., 136, 14357–14360;(e) Rodenas, T., Luz, I., Prieto, G., Seoane, B., Miro, H., Corma, A., Kapteijn, F., Llabrés, F.X., Xamena, I., and Gascon, J. (2015) Nat. Mater., 14, 48–55;(f) Sheberla, D., Sun, L., Blood-Forsythe, M.A., Er, S., Wade, C.R., Brozek, C.K., Aspuru-Guzik, A., and Dincă, M. (2014) J. Am. Chem. Soc., 136, 8859–8862;(g) Campbell, M.G., Sheberla, D., Liu, S.F., Swager, T.M., and Dincă, M. (2015) Angew. Chem. Int. Ed., 54, 4349–4352;(h) Maeda, H., Sakamoto, R., and Nishihara, H. (2016) Langmuir, 32, 2527–2538;(i) Lahiri, N., Lotfizadeh, N., Tsuchikawa, R., Deshpande, V.V., and Louie, J. (2017) J. Am. Chem. Soc., 139, 19–22.
- 14 (a) Sakamoto, R., Hoshiko, K., Liu, Q., Yagi, T., Nagayama, T., Kusaka, S., Tsuchiya, M., Kitagawa, Y., Wong, W.-Y., and Nishihara, H. (2015) Nat. Commun., 6, 6713;(b) Takada, K., Sakamoto, R., Yi, S.-T., Katagiri, S., Kambe, T., and Nishihara, H. (2015) J. Am. Chem. Soc., 137, 4681–4689.
- 15 (a) Miner, E.M., Fukushima, T., Sheberla, D., Sun, L., Surendranath, Y., and Dincă, M. (2016) Nat. Commun., 7, 10942;(b) Sheberla, D., Bachman, J.C., Elias, J.S., Sun, C.-J., Shao-Horn, Y., and Dincă, M. (2017) Nat. Mater., 16, 220–224.
- 16 Wang, Z.F., Su, N., and Liu, F. (2013) Nano Lett., 13, 2842–2845.
- 17 Yamada, M.G., Soejima, T., Tsuji, N., Hirai, D., Dincă, M., and Aoki, H. (2016) Phys. Rev. B, 94, 081102.
