Chapter 4
Tubular Nanocontainers for Drug Delivery
Yusuf Darrat1, Ekaterina Naumenko2, Giuseppe Cavallaro3, Giuseppe Lazzara3, Yuri Lvov1,2 and Rawil Fakhrullin1,2
1Louisiana Tech University, Institute for Micro manufacturing, 911 Hergot Ave., Ruston, LA, 71272, USA
2Kazan Federal University, Institute of Fundamental Medicine and Biology, Department of Microbiology, Bionanotechnology Lab, Kreml uramı 18, 420008, Kazan, Republic of Tatarstan, Russian Federation
3Università degli Studi di Palermo Vialedelle Scienze, Dipartimento di Fisica e Chimica, pad. 17, 90128 Palermo, Italy
4.1 Introduction
Efficient design and fabrication of smart and stimuli-responsive nanosized materials for biomedical applications is among the most important challenges of nanotechnology [1]. Among numerous nanoscale platforms for drug delivery [2, 3], special attention has been paid to nanotubular particles due to their morphological and chemical properties [4]. The paradigm of nanoarchitectonics [5] implies the synergistic use of several types of materials arranged into complex supraparticles, uniting and enhancing the original functionalities of the precursor materials. In this sense, nanosized tubes are an excellent candidate to be a versatile platform for fabrication of smart nanocontainers. In general, nanotubular materials exhibit a similar primary structure (shown in Figure 4.1), where a tubelike particle has an internal cavity (lumen) and external walls along with openings at both ends of the tube. In the simplest case, the tube's walls are not layered; in other words, the tube consists of a single layer of rolled material (i.e., graphene in case of single-walled carbon nanotubes (SWCNTs)). If the tubes are multilayered, the internal structure also contains the interlayer spaces, in addition to the central cavity (lumen). The geometry of nanotubes greatly depends on their chemical nature; certain kinds of nanotubes demonstrate irregular curly geometry and form bundles of varying size and shape, while other nanotubes possess regular rodlike geometry with little or no spatial defects. What makes nanotubes excellent candidates for fabrication of nanoarchitectonic drug delivery vehicles? What makes nanotubes more suitable for drug delivery if compared with other types of nanoscale particles for drug delivery, such as mesoporous silica nanoparticles [6] or graphene-based materials [7]? The major advantages of nanotubular structures are directly associated with the nanoarchitectonic paradigm, that is, nanotubes of any chemical origin possess a similar architecture (a tube having walls, lumen, and open endings), which enables researchers to do the following:
- 1. Load cargo (drugs, therapeutic nucleic acids, etc.) into the lumen, within the interwall spaces in multiwalled tubes or onto the walls (or using all of these sites simultaneously) [8];
- 2. Modify the tubes' endings with “smart” stoppers, preferably stimuli-responsive, to modulate the release of the cargo (i.e., designing the stoppers to make them permeable only within certain regions of cells or tissues) [9];
- 3. Functionalize the tubes' walls with either polymer (multi)layers or nanosized particles (or a combination of both) to enable the external triggering or spatial manipulation with the drug-loaded nanotubes [10].

Figure 4.1 A sketch demonstrating a typical nanotube-based drug delivery container carrying the drug cargo inside the lumen or on the outside surface and having stimuli-responsive end stoppers.
These features of nanotubes can be hardly reproduced using other non-tubular nanosized particles. Apparently, the best advantage of nanotubes of any kind is the large loading capacity, if compared with nanospheres or nanoporous particles. As a result, numerous reports have been published in the past decades demonstrating the use of nanotubes for drug delivery [11]. In this chapter we overview the use of two types of nanotubular materials, namely, CNTs and halloysite clay nanotubes (HCTs) for fabrication of nanoarchitectonic drug delivery platforms. We also discuss the uptake mechanisms of intracellular uptake of nanocontainers having tubular geometry.
4.2 Carbon Nanotubes for Drug Delivery
4.2.1 Characteristics of Carbon Nanotubes
CNTs are made up of carbon atoms that form a tubular structure. They can be multiwalled with diameter of 5–30 nm or single/double walled with diameter of 2–3 nm. Multiwalled nanotubes (MWNTs) consist of several concentric graphene layers, while SWCNTs consist of only one layer. CNTs have unique physical and chemical characteristics: they may be low or highly conductive, exceeding copper. They have very high Young's modulus and tensile strength. However, all these excellent characteristics are typical for SWCNTs, while the advantages of MWNTs are less impressive. These characteristics have led to the development of electrical devices, such as single electron transistors and sensors, and composite materials which incorporate CNTs for enhanced mechanical properties. CNTs can also be used for controlled drug delivery. However, pristine CNTs are hydrophobic and chemically inert, and they have been functionalized in order to bind to different pharmacological molecules and to form stable aqueous, (blood/plasma) colloids. Functionalized CNTs can be useful for vaccine treatment, gene therapy, drug delivery, and other pharmaceutical applications [12–15].
4.2.2 Functionalization of CNTs for Drug Delivery
CNTs can be functionalized in a variety of different ways for use in drug delivery applications. They can be functionalized either internally or externally. An example of external functionalization is shown in Figure 4.2a–c. This method of functionalization incorporates the external loading of magnetite nanoparticles, poly(acrylate acid) (PAA), as well as the anticancer drug gemcitabine [16]. Figure 4.2d shows an example of platinum(IV) prodrug inside rather than outside of the tubes [17].
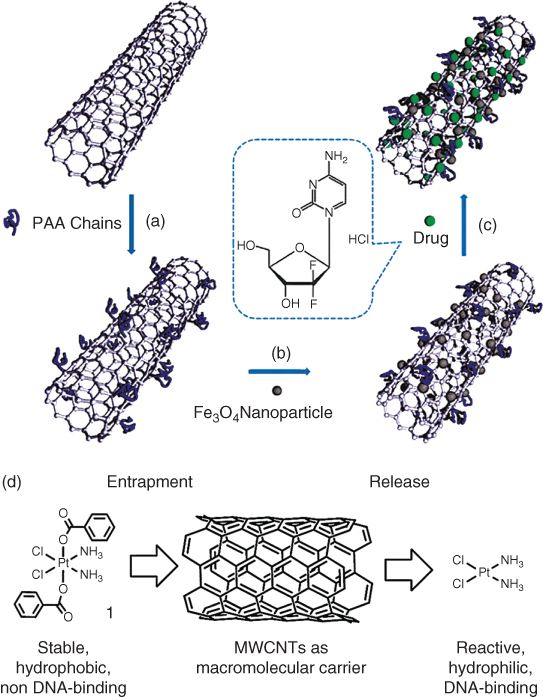
Figure 4.2 Synthesis of hydrophilic multiwalled carbon nanotubes externally loaded with magnetite nanoparticles. (a) Grafting of PAA onto the carbon nanotubes via in-situ free radical polymerization; (b) Deposition of Fe3O4 nanoparticles on the surface via chemical co-precipitation method; (c) External loading of gemcitabine by physical adsorption.
((a–c) Yang et al. 2009 [16]. Reproduced with permission of Royal Society of Chemistry.)
(d) Hydrophobic trapping of platinum(IV) prodrug within MWNTs and controlled release.(Li et al. 2012 [17]. Reproduced with permission of Royal Society of Chemistry.)
Pristine CNTs are hydrophobic, so surface modification to achieve hydrophilic surfaces is necessary for medical applications. The functionalization techniques can involve covalent as well as non-covalent strategies. The surface of CNTs can be covalently modified; then further functionalization with hydrophilic organic molecules can be employed for increased solubility in aqueous environments [18]. Poly(ethylene glycol) is perhaps the most common species for functionalization in medical applications, which can increase the dispersity of CNTs in aqueous solution as well as enhance biocompatibility of CNTs [19].
4.2.3 Uptake of Carbon Nanotubes
In general, CNTs must be able to enter cells in order to deliver their cargo to cells. It has been proved that various types of functionalized CNTs have the ability to be taken up by many different types of cells and can migrate intracellularly through different cellular barriers [20]. Figure 4.3 shows the uptake of CNTs by human promyelocytic leukemia (HL60) cells and human T-cells (Jurkat). It has been stipulated that the pathway of cellular uptake is endocytosis. Fluorescently labeled functionalized nanotubes were incubated with HL60 cells for 1 h in order to determine if the CNTs can enter the cells. The cells were washed twice, collected by centrifugation, and resuspended in growth medium. Confocal microscopy showed CNTs aggregating around the cell membranes as well as in the interior of the cells Figure 4.3 [21].
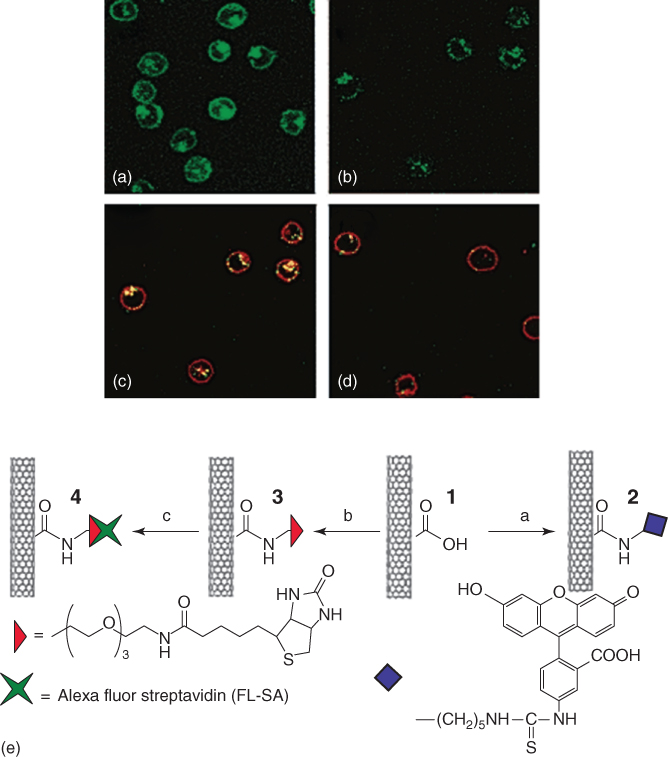
Figure 4.3 Confocal images of cells after incubation in solutions of functionalized single-wall carbon nanotubes (SWCNTs) (a) after incubation in 2, (b) after incubation in a mixture of 4 (fluorescence due to SA), and the endocytosis marker FM 4-64 at 37 °C (image shows fluorescence in certain areas only), (c) same as (b) except with added fluorescence shown due to FM 4-64 stained endosomes, (d) same as (b) after incubation at 4 °C.
(Kam et al. 2004 [21]. Reproduced with permission of American Chemical Society.)
Functionalized MWNTs have also been proved to be able to enter cells. Surface functionalization generally increases the uptake of CNTs [22]. There are different theories as to how CNTs enter cells. An interesting theory is that MWNTs enter cells in different ways depending on aggregation. The theory suggests that bundles of MWNTs enter cells via endocytosis, while single tubes enter by directly penetrating the cell membrane [23].
4.2.4 Hybrid Materials
In addition to functionalization, CNTs can be used to synthesize hybrid materials. One example is a hydrogel–CNT hybrid. CNTs can be covalently and non-covalently bonded to hydrogels in a matrix. Hydrogels are biocompatible and have a high water content, which makes them useful in drug delivery [13]. Drugs can be loaded onto the hydrogels via swelling and eventually released. However, they have poor mechanical properties. This problem is solved if hydrogel–CNT hybrids are synthesized. The mechanical strength, drug release profile, remote actuation capabilities, and biological interactions of these materials are better than that of pure hydrogels [13].
4.2.5 Vaccine Treatment
CNTs can also be used as scaffolds in vaccine compositions. It is essential to facilitate the presentation of the antigen to the immune system; this can be achieved using scaffolds. Characteristics that make CNTs ideal scaffolds in vaccine compositions include high stability on shelves as well as in vivo. They are also capable of binding to many different types of antigens. In addition, they can bind to many antigens at once, either of the same kind or different kinds [24]. A study was performed in which a B-cell epitope of the foot and mouth disease virus (FMDV) attached to CNT amine groups with a biofunctional linker [12]. The bioconjugate could then be administered to the patient and used as a vaccine. It has been shown that there was a larger response in terms of antibody recognition and attachment with the bioconjugates when compared to free antigens [12, 25].
4.2.6 Cancer Treatment
SWCNTs can covalently bond with small drug molecules at the ends of tubes or at defect sites on the side wall. Anticancer drugs were successfully delivered by SWNTs to cancer cells through a phospholipid-mediated process. Platinum(IV) complex units were conjugated to SWCNTs and released via reduction inside the low pH environment within the cancer cells [14]. A safer, more efficient process involves conjugating SWCNTs with tumor-targeting ligand units that are capable of recognizing and binding to cancer-specific receptors and inducing receptor-mediated endocytosis. This would minimize unnecessary healthy cell death as in the case of chemotherapy, reducing or eliminating associated side effects as a result [14]. Another type of therapy to treat cancer is immunotherapy, which is a type of treatment in which an immune response is either induced, enhanced, or suppressed [26]. It can be used to treat cancer by enhancing an immune response to cancerous tumors. It has been reported that CNTs are capable of activating innate as well as adaptive immune responses [27].
4.2.7 Gene Therapy
RNA interference is a unique way of curing disease by silencing genes. This is achieved by utilizing short interfering RNA molecules (siRNA) and delivering them to cells. Efficient delivery of siRNA molecules is crucial to effective gene silencing. It has been proved that SWCNTs can be used to transport and deliver siRNA molecules, and they are more efficient than other transfection agents when it comes to lamin A/C gene silencing [28]. CNTs not only can be used for gene silencing but they can also be used for enhancing gene expression. Ammonium-functionalized SWCNTs are able to facilitate the delivery of plasmid DNA, which enables expressions of marker genes [29]. It is essential that genetic material is protected from alteration in the delivery process. Transporting genetic material within CNTs achieves this goal. Genetic material transported in CNTs has been shown to maintain the ability to express proteins [30].
4.2.8 Toxicity
CNTs are inorganic, nonbiodegradable particles and, therefore, they may accumulate in the body and be potentially harmful. Exposure to CNTs can result in inflammation, epithelioid granulomas, and fibrosis [15]. Prolonged exposure to airborne CNTs could potentially lead to large lesions in the lungs. CNTs have been shown to be cytotoxic to several different cell types. However, the mechanism of CNT toxicity has not been studied in depth, and dosimetric analyses of CNT in the cell culture system are lacking [31]. However, peptide-functionalized SWCNTs are capable of moving across cell membranes and concentrating in the cytoplasm of fibroblasts and phagocytic cells without showing toxic effects. A similar study showed that CNTs were able to transport large functional groups into cells without inducing an immune response. The conflicting reports can be resolved as follows: carbon nanotube toxicity might depend on the type of functionalization [32].
4.3 Halloysite-Nanotube-Based Carriers for Drug Delivery
4.3.1 Halloysite Nanotubes: A Biocompatible Clay with Drug Delivery Capacity
Halloysite is a naturally available clay with a hollow tubular morphology generated by the rolling of its aluminosilicate sheets [33, 34]. As a consequence, the inner surface is composed of gibbsite octahedral sheet (Al−OH) groups with positive electrical potential, whereas the external surface consists of siloxane groups (Si−O−Si) with negative charge [35, 36]. Halloysite nanotubes (HNTs) are quite polydispersed in size depending on their geological origin [37]. The length is about 1000 nm, while the outer and internal diameters range between 50–80 and 10–15 nm, respectively [34]. Halloysite is a biocompatible and environmentally safe nanomaterial, as shown by several in vitro and in vivo studies [38–48]. The first biocompatibility experiments performed on fibroblast and human breast cells highlighted that HNTs are nontoxic to the cells and are even much less harmful than NaCl [41]. A recent review [49] evidenced that HNTs are proper and efficient nanocarriers for drug delivery systems of chemically and biologically active molecules because of their peculiar structural properties as well as their lack of cell toxicity, which represents the major prerequisite for their usage within pharmaceutical and biomedical applications. The HNT cavity was successfully employed for the encapsulation and controlled release of proteins [50, 51], DNA [52, 53], and functional compounds with antimicrobial [54–59], anticorrosion [55, 60–62] and antioxidant [63] activities. The loading of the HNT lumen can be easily achieved from saturated drug solutions accompanied by cyclic vacuum pumping in/out [64, 65]. According to the HNT geometrical characteristics, a successful drug loading ranges between 5 and 10 wt% of the nanotube weight, which corresponds to the complete loading of the internal cavity [47].
4.3.2 Modified Halloysite Nanotubes with a Time-Extended Effect on the Drug Release
Typically, pristine nanotubes exposed to aqueous media exhibit a complete release of water-soluble drugs within 4–12 h [47], which is not appropriate for some applications that needed a longer time efficiency of the entrapped active molecules. Both the formation of tube end stoppers and the HNT coating with polymeric layers were proposed for designing modified nanotubes with slower release kinetics [42, 63, 66, 67]. Figure 4.4a shows the benzotriazole release curves from pristine halloysite, HNTs coated with urea–formaldehyde (UF), and with copper tube end stoppers.

Figure 4.4 (a) Comparison of benzotriazole release curves from pristine halloysite, halloysite encapsulated with urea–formaldehyde encapsulation, and with copper end tube stoppers. (b) Formation of the tube end Cu-benzotriazole stoppers.
(Lvov et al. 2016 [47]. Reproduced with permission of John Wiley & Sons.)
A time-extended effect on the drug release was observed for both modified nanotubes. In particular, the slowest kinetics release was observed for the nanotubes capped with copper-inhibitor clogs because of the chelation of Cu(II) ions by released inhibitors, which are predominantly at the tube ends (Figure 4.4b). Similarly, the formation of dextrin (or glycogen) tube end stoppers (Figure 4.5a) was successfully employed to control the release of brilliant green (BG) inside human lung carcinoma cells (A549). Specifically, the BG release was triggered by the enzymatic dextrin decomposition (mediated trough the intercellular glycosyl hydrolases). Consequently, the destruction of the malignant cells was enhanced with respect to that observed for pure HNTs loaded with BG (Figure 4.5b).

Figure 4.5 (a) SEM image of a dextrin cap on the end of the functionalized nanotube. (b) Resazurin assay results demonstrating the LD50 value (50% death level) of BG-loaded HNTs for Hep3b cells.
(Reproduced with permission from [59].)
4.3.3 Covalently Functionalized Halloysite Nanotubes as Drug Delivery Systems Sensitive to Specific External Stimuli
Recently, covalent functionalization of halloysite surfaces was investigated with the aim of obtaining hybrid nanostructures with controlled drug release capability. These hybrid nanostructures are sensitive to external stimuli, such as temperature [35], pH [42], and intracellular enzymes [41]. The release kinetics of curcumin (an anticancer agent) from HNTs functionalized with chitosan is much faster at cell lysate than that at pH 7.4 [43]. Accordingly, the curcumin loaded in the modified nanotubes exhibited a specific toxicity toward cancer cells. The grafting of poly(N-isopropylacrylamide) (PNIPAAM) onto the HNT external surface allowed the generation of an efficient nanocarrier for thermoresponsive curcumin release [68]. Temperature-sensitive features were observed due to the coil-to-globule transition of the polymer at the LCST (lower critical solution temperature) occurring around 32 °C. In addition, the curcumin release was affected by the pH conditions. The in vitro kinetics studies conducted at 37 °C highlighted that the curcumin release was larger at pH = 6.8 (about 10 wt%) with respect to that estimated at pH = 1 (about 2.5 wt%). Interestingly, the thermoresponsive PNIPAAM/HNT carrier was complementary to the triazole/HNT hybrid [69], where a full release was observed only in acidic medium. Similarly, the release of cardanol (an anticancer agent) from the clay nanotubes functionalized with triazolium salt was favored by a decrease in pH [70]. Covalently functionalized HNTs were successfully employed as dual-responsive nanocarriers [71]. Figure 4.6 illustrates the scheme for HNT-Cur prodrug with a controlled curcumin release on dependence of both intracellular glutathione (GSH) and pH conditions.
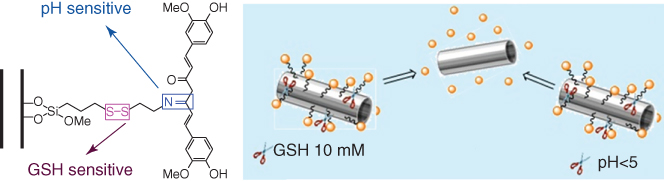
Figure 4.6 Schematic representation of HNTs-Cur prodrug with controlled curcumin release.
(Reproduced with permission from [71].)
4.3.4 Hybrids Based on Halloysite Nanotubes as Dual Drug Delivery Systems
Multicavity nanostructures for co-delivery of drugs can be successfully obtained by the selective modification of the HNT external surface. The grafting of the HNT shell with amphiphilic cyclodextrin (CD) generated hybrid nanomaterials with loading abilities toward silibinin (Sil) and quercetin (Que), which possess different affinities toward the CD cavity and the halloysite lumen [40]. The amounts of flavonoids encapsulated in the hybrid nanomaterial were 6.1 and 2.2 wt% for silibinin and quercitin, respectively [40]. Accordingly, the CD/HNT system is a proper nanocarrier for the loading/delivery of both drugs that exhibit synergic effects in anticancer therapy as demonstrated by in vitro cytotoxicity assays (Figure 4.7) [40]. Similar composite nanocarriers were obtained by the covalent modification of the HNT outer surface with CD glycocluster [72]. The preparation of the dual drug delivery system was based on a green protocol using absolvent-free microwave irradiation [72]. The CD glycocluster/HNTs showed efficient carrier ability toward silibinin and curcumin, which present a pH-dependent affinity toward the functionalized nanotubes. Slower kinetics release was observed for both natural drugs. Furthermore, supramolecular interactions were exploited to design composite nanostructures with tunable loading capacity. Composite materials based on HNT and cucurbit[6]uril (CB[6]) molecules were effective for the entrapment of essential oils because of the formation of hydrophobic cavities onto the halloysite surfaces [73]. The CB[6]/HNT hybrid was successfully employed as a nanofiller for pectin-based biofilms with antioxidant and antimicrobial properties.
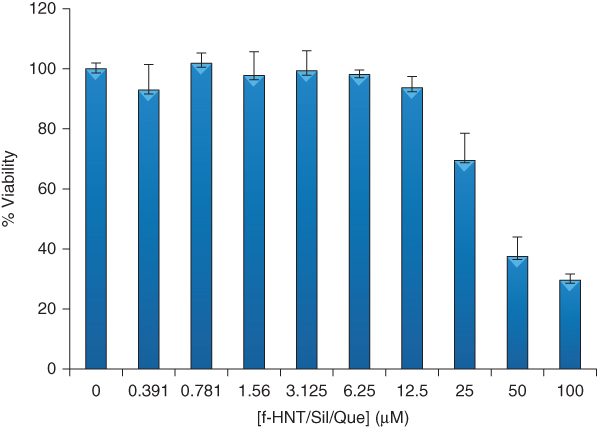
Figure 4.7 MTS test for the cell viability of 8505C cells cultured for 72 h in the presence of f-HNT/Sil/Que.
(Massaro et al. 2015 [40]. Reproduced with permission of Royal Society of Chemistry.)
4.4 Tubular Nanosized Drug Carriers: Uptake Mechanisms
Both CNTs and HNTs are considered as transporters for various cargos including medical formulations for target drug delivery. Mechanisms of anisotropic particle uptake (including CNTs, HNTs, and nanorods) in mammalian and non-mammalian cells were studied by numerous authors over the past few decades, but still this task remains not fully understood. In general, there are four main pathways of nanoparticle internalization (Figure 4.8): energy-independent membrane piercing by passive diffusion (a), calveolae-mediated endocytosis (b), phagocytosis (c), and clathrin-mediated endocytosis (d).

Figure 4.8 The biological mechanisms of internalization of nanotubes: (a) membrane piercing, (b) calveolae-mediated endocytosis, (c) phagocytosis, and (d) clatrin-mediated endocytosis.
Most of the studies based on the use of endocytosis inhibitors have demonstrated that the mechanism of CNT uptake is the energy-dependent endocytosis. For example, Kam et al. [21] have demonstrated that SWCNTs) functionalized with small molecules and proteins can enter both nonadherent and adherent cell lines (CHO and 3T3). Based on early publications [74, 75] they have proposed that the nanotubes nonspecifically associate with hydrophobic regions of the cell surface and are internalized via endocytosis. Fluorescence microscopy has been employed for detection of nanotubes inside the cell cytoplasm using green fluorescent conjugate for visualization of SWCNTs. Incubation at 4 °C completely inhibited the endocytosis [74, 75]. Red FM 4-64 marker was used to stain endosomes which formed around nanotubes during endocytosis; yellow color was observed due to overlapping of green fluorescence from nanotubes and red-stained endosomes. This observation is the direct evidence for endocytosis of nanotubes which accumulate in the cytoplasm of the cells after internalization, and the uptake pathway is consistent with endocytosis.
Haniu et al. have reported that CNT internalization was suppressed by cytochalasin D, an endocytosis inhibitor, in different types of cells [76]. In the other study, this group has used two types of endocytosis inhibitors with different targets: chlorpromazine as a clathrin-mediated endocytosis inhibitor and indomethacin as a caveolae-mediated endocytosis inhibitor [77, 78]. It was proved that internalization of MWCNTs was suppressed by both types of endocytosis inhibitors. In contrast, Kostarelos et al. [20] reported that the cellular uptake of functionalized CNT is not affected by sodium azide, which is also known as an endocytosis inhibitor. They also have revealed that the process of CNT internalization is independent of cell type including bacterial and non-mammalian eukaryotic cells such as Saccharomyces cerevisiae and Cryptococcus neoformans. However, the studies performed by Haniu et al. demonstrated that the cellular uptake changes in response to cell differentiation and is inhibited by endocytosis inhibitors [77, 78]. However, the comparison of these studies is not correct enough because the MWCNTs used were not functionalized or labeled with fluorescein isothiocyanate. Moreover, Tabet et al. demonstrated that the mechanism of MWCNT uptake may depend on their modification or its absence [79] whereas Kostarelos et al. postulated that the internalization process is independent of the nature of functional groups [20].
Maruyama et al. [80] have demonstrated the MWCNT uptake into nonphagocytic cell lines (human bronchial epithelial cells (HBECs) and human mesothelial cells (HMCs)) as well as their accumulation in the lysosomes of the cells. They used a standard technique with corresponding endocytosis inhibitors and determined that clathrin-mediated endocytosis inhibitors significantly suppressed MWCNT uptake, whereas caveolae-mediated endocytosis and macropinocytosis were also found to be involved in MWCNT uptake. Apparently, the mechanism of CNT endocytosis represents the combination of three pathways: clathrin-mediated endocytosis, caveolae-mediated endocytosis, and macropinocytosis; wherein clathrin-mediated endocytosis plays a key role and other pathways may be also involved in varying degrees.
As was demonstrated recently, the uptake of CNTs into cells is a size-dependent process [81]. Kang et al. [82] showed that the large MWCNTs were not taken up by cells through endocytosis due to size limitations, whereas the single-walled CNTs can enter human HepG2 hepatocellular carcinoma cells via endocytosis. Surface characteristics are also critical in CNT uptake: oxidized nanotubes are more likely to enter cells via endocytosis with the help of specific ligands [83–85] because the oxidation of nanotubes introduces carboxyl groups, which provides a negative charge to their surface. This in turn results in repulsion from the plasma membrane unless the CNT has ligands that are recognized with a high affinity by the cell. Some ligands (folic acid [83], albumin [84], and epidermal growth factor [85]) have been reported to facilitate uptake by specific cell types.
The size and possibility of nanotubes to form clusters and aggregates must be taken into account when it comes to the rational design of CNT-based carriers for cell therapy. A recent review by Raffa et al. [86] summarized the works that described the mechanism of cellular uptake of nanotubes and demonstrated that the degree of dispersion, the formation of supramolecular complexes, and the nanotube length play a crucial role in the process of uptake. Relatively big aggregates – bundles, clusters, or single dispersed nanotubes 1 µm or more in length – enter the cells by phagocytosis. Endocytosis appears to be the internalization pathway for CNT-forming supramolecular structures; wherein passive diffusion is the internalization mechanism for submicron CNTs that do not form supramolecular complexes.
In addition, Yaron et al. [87] have demonstrated that relatively small single-wall CNTs enter the mammalian cells by energy-dependent endocytosis, which was determined as suppression of nanoparticle transport at 4 °C compared with 37 °C. The authors also examined the possibility for physical penetration of SWCNTs through the plasma membrane. Using electrochemical impedance spectroscopy and Langmuir monolayer film balance measurements, they demonstrated that Pluronic-stabilized SWCNTs associated with membranes but did not penetrate through the membrane. They described the stages of SWCNT uptake by cells:
- 1. Adsorption of SWCNTs onto the cell surface and penetration into the outer leaflet of the bilayer;
- 2. Increasing of membrane tension and induction of imbalance between the outer leaflet and the inner leaflet;
- 3. Local disturbances in membrane tension, which formed from the previous stage, stimulating endocytosis as the membrane attempts to regulate tension;
- 4. Formation of endosomes, which shrink during processing;
- 5. Distribution of SWCNTs inside the cytoplasm by disruption of the endosomes or lack of lysosomal processing.
Earlier, Jin et al. [3] have observed that SWCNTs incorporated into and expelled from NIH-3T3 cells using a perfusion microscope cage. This study represents the first conclusive evidence of SWCNT exocytosis. The rate of excluded nanotubes closely matches the endocytosis rate with negligible temporal offset. Therefore, in this study, the authors identify the exocytosis pathway that leads to the previously observed aggregation and accumulation of SWCNT within the cells.
Hong et al. also demonstrated the reverse process of endocytosis, exocytosis of single nanotubes or molecules on plasmonic substrates [88]. In this work, cellular uptake and transmembrane displacements show clear correlation with temperature and clathrin assembly on cell membranes. This strongly suggests that the cellular entry mechanism for a nanotube molecule is clathrin-dependent endocytosis through the formation of clathrin-coated pits on the cell membrane (Figure 4.9).
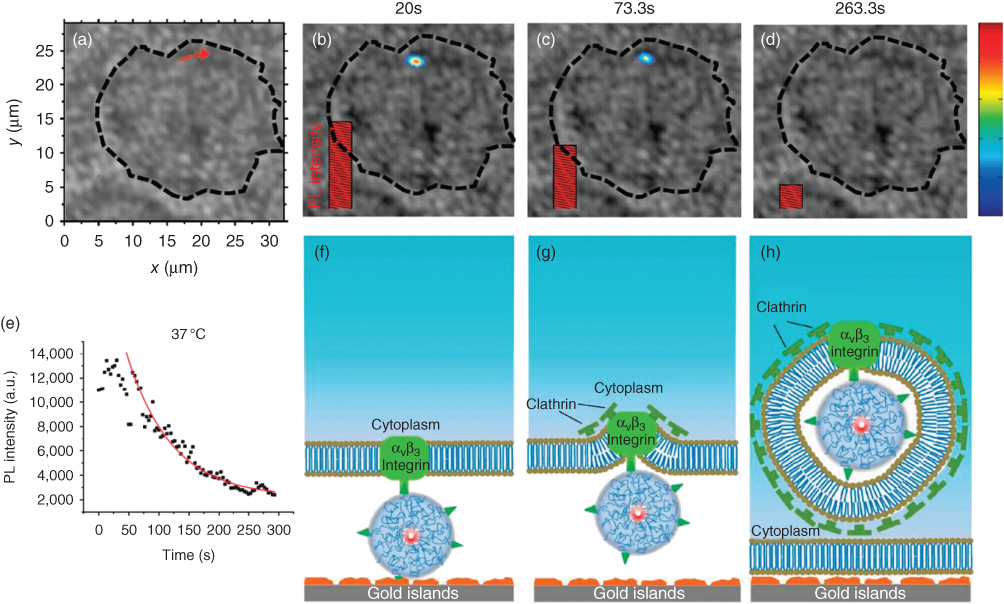
Figure 4.9 Carbon nanotubes imaging and tracking on a plasmonic gold substrate at 37 °C during endocytosis.
(Zhang et al. 2009 [89]. Reproduced with permission of John Wiley & Sons.)
Halloysite is a nontoxic material which is a promising candidate for the formation of effective and safe drug delivery systems. The mechanism of HNT internalization has been studied in some works, and most of them proposed that clay nanotubes enter the mammalian cells via energy-dependent endocytosis. For example, Massaro et al. and Zhang et al. postulated in their works that halloysite-drug complexes' internalization mechanism presumably involves an endocytosis process and nanotubular containers could be taken up into the cytosol by endosomes (Figure 4.10) [72, 89].

Figure 4.10 Schematic illustration of HNT endocytosis process. (Adapted from Massaro et al. 2016 [72] and Liu et al. 2015 [90].)
Liu et al. have observed under confocal microscope the internalization and localization of HNTs inside A549 cells [42]. The results of this work show that HNTs can readily internalize into live cells and aggregate together around the nucleus region (Figure 4.11). This phenomenon has also been demonstrated in our previous work [57] using a combination of enhanced dark-field and fluorescent microscopy techniques.
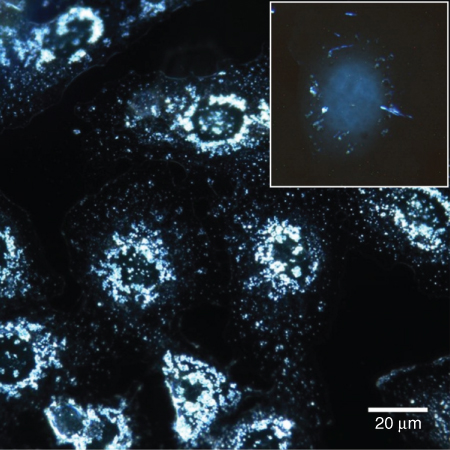
Figure 4.11 Enhanced dark-field microscopy of HNTs taken up byA549 human cells in monolayer. Inset indicates the penetration of HNTs into cell in suspension culture. Note the perinuclear distribution of nanotubes.
Liu et al. [90], using the autofluorescence properties of curcumin, demonstrated the intracellular uptake of curcumin-loaded HNT-g-CS nanoparticles by fluorescence microscopy. They showed that HNTs began to enter the cell membrane at 2 h, entered into the cells at 4 h, and the cytoplasm was filled at 8 h. It was also observed that the cells treated with curcumin-functionalized HNTs showed profound fluorescence intensity in the cytoplasm at 12 h. Therefore, the authors speculated that the observed higher uptake of HNTs might be due to lysosome-mediated endocytosis, which has already been found in other systems.
Yang et al. [91] proposed that the drug-loaded nanotubes could penetrate the plasma membrane directly and/or via endocytosis mechanism due to their unique needle-like morphology similar to CNTs [87]. They observed the doxorubicin-loaded HNT internalization, but the authors suggest that the exact mechanism is yet to be decyphered, and they revealed that nanotubes can remain in both the cell cytoplasm and nuclei with a prolonged residue time. This provides the possibility of inducing cell apoptosis via the mitochondrial injury pathway. The authors hypothesized that HNT-g-COS would promote the anticancer efficiency of DOX through synergistic mechanism of both the mitochondria and nuclei injury (Figure 4.12).

Figure 4.12 Illustration of the HNT-g-COS synthesis and doxorubicine loading process and uptake process of nanotubes by cells and cell apoptosis mechanism.
(Yang et al. 2016 [91]. Reproduced with permission of American Chemical Society.)
4.5 Conclusions
Nanotubes are excellent candidates for fabrication of nanoarchitectonic drug delivery vehicles due to their structural and chemical properties. CNTs are theoretically useful in a variety of medical applications; however, there are many concerns on their possible toxicity. Loaded CNTs can be used for vaccine treatment, gene therapy, and cancer treatment due to their efficient penetration into cells. CNT composites are prospective materials that can be used as tissue scaffolds and artificial implants. They have been used as electro-reactive components in model biosensors, but no real medical applications were reported. There may be exceptional cases where CNTs themselves did not show toxic effects; however, CNTs are still not safe to use indiscriminately in vivo. CNTs are not biodegradable and exact mechanisms of their removal from organisms have not been determined [12–15, 32, 92]. HCTs are a natural nanosized material currently used in fabrication of drug delivery vehicles. The exceptional biocompatibility of halloysite facilitates its use in a number of biomedical applications. The mechanisms of cellular internalization of nanotubes have been briefly overviewed, although there is still need for additional studies. The future research in fabrication of drug delivery vehicles using nanotubes is focused on designing novel functional (bio)macromolecules to render the nanotubular drug containers with the ability for targeted delivery and triggered slow release inside the target cells.
References
- 1 Elsabahy, M. and Wooley, K.L. (2012) Design of polymeric nanoparticles for biomedical delivery applications. Chem. Soc. Rev., 41, 2545–2561.
- 2 Karimi, M., Ghasemi, A., Zangabad, P.S., Rahighi, R., Basri, S.M.M., Mirshekari, H., Amiri, M., Pishabad, Z.S., Aslani, A., Bozorgomid, M., Ghosh, D., Beyzavi, A., Vaseghi, A., Aref, A.R., Haghani, L., Bahrami, S., and Michael, R. (2016) Hamblin smart micro/nanoparticles in stimulus-responsive drug/gene delivery systems. Chem. Soc. Rev., 45, 1457–1501.
- 3 Jin, H., Heller, D.A., and Strano, M.S. (2008) Single-particle tracking of endocytosis and exocytosis of single-walled carbon nanotubes in NIH-3T3 cells. Nano Lett., 8, 1577–1585.
- 4 Kumar, S., Rani, R., Dilbaghi, N., Tankeshwar, K., and Kim, K.-H. (2017) Carbon nanotubes: a novel material for multifaceted applications in human healthcare. Chem. Soc. Rev., 46, 158–196.
- 5 Ariga, K., Ji, Q., Nakanishi, W., Hill, J.P., and Aono, M. (2015) Nanoarchitectonics: a new materials horizon for nanotechnology. Mater. Horiz., 2, 406–413.
- 6 Tang, F., Li, L., and Chen, D. (2012) Mesoporous silica nanoparticles: synthesis, biocompatibility and drug delivery. Adv. Mater., 24, 1504–1534.
- 7 Nurunnabi, M., Parvez, K., Nafiujjaman, M., Revuri, V., Khan, H.A., Feng, X., and Lee, Y.-K. (2015) Bioapplication of graphene oxide derivatives: drug/gene delivery, imaging, polymeric modification, toxicology, therapeutics and challenges. RSC Adv., 5, 42141–42161.
- 8 Hilder, T.A. and Hill, J.M. (2009) Modeling the loading and unloading of drugs into nanotubes. Small, 5, 300–308.
- 9 Yendluri, R., Otto, D.P., De Villiers, M.M., Vinokurov, V., and Lvov, Y.M. (2017) Application of halloysite clay nanotubes as a pharmaceutical excipient. Int. J. Pharm., 521, 267–273.
- 10 Bertolino, V., Cavallaro, G., Lazzara, G., Milioto, S., and Parisi, F. (2017) Biopolymer-targeted adsorption onto halloysite nanotubes in aqueous media. Langmuir, 33, 3317–3323. doi: 10.1021/acs.langmuir.7b00600
- 11 Leporatti, S. (2017) Halloysite clay nanotubes as nano-bazookas for drug delivery. Polym. Int., 66, 1111–1118. doi: 10.1002/pi.5347
- 12 Bianco, A., Kostarelos, K., and Prato, M. (2005) Applications of carbon nanotubes in drug delivery. Curr. Opin. Chem. Biol., 9, 674–679.
- 13 Cirillo, G., Hampel, S., Spizziri, U., Parisi, O., Picci, N., and Lemma, F. (2014) Carbon nanotubes hybrid hydrogels in drug delivery: a perspective review. BioMed Res. Int., 2014, 1–17.
- 14 Chen, J., Chen, S., Zhao, X., Kuznetsova, L., Wong, S., and Ojima, I. (2008) Functionalized single-walled carbon nanotubes as rationally designed vehicles for tumor-targeted drug delivery. J. Am. Chem. Soc., 130, 16778–16785.
- 15 Alshehri, R., Ilyas, A., Hasan, A., Arnaout, A., Ahmed, F., and Memic, A. (2016) Carbon nanotubes in biomedical applications: factors, mechanisms, and remedies of toxicity. J. Med. Chem., 59, 8149–8167.
- 16 Yang, D., Yang, F., Hu, J., Long, J., Wang, C., Fu, D., and Ni, Q. (2009) Hydrophilic multi-walled carbon nanotubes decorated with magnetite nanoparticles as lymphatic targeted drug delivery vehicles. Chem. Commun., 29, 4447.
- 17 Li, J., Yap, S.Q., Chin, C.F., Tian, Q., Yoong, S.L., Pastorin, G., and Ang, W.H. (2012) Platinum(IV) prodrugs entrapped within multiwalled carbon nanotubes: selective release by chemical reduction and hydrophobicity reversal. Chem. Sci., 3 (6), 2083.
- 18 Sadegh, H. and Shahryari-ghoshekandi, R. (2015) Functionalization of carbon nanotubes and its application in nanomedicine: a review. Nanomed. J., 2 (4), 231–248.
- 19 Lay, C.L., Liu, J., and Liu, Y. (2011) Functionalized carbon nanotubes for anticancer drug delivery. Expert Rev. Med. Dev., 8 (5), 561–566.
- 20 Kostarelos, K., Lacerda, L., Pastorin, G., Wu, W., Wieckowski, S., Luangsivilay, J., Godefroy, S., Pantarotto, D., Briand, J.-P., Muller, S., Prato, M., and Bianco, A. (2007) Cellular uptake of functionalized carbon nanotubes is independent of functional group and cell type. Nat. Nanotechnol., 2, 108–113.
- 21 Kam, N.W., Jessop, T.C., Wender, P.A., and Dai, H. (2004) Nanotube molecular transporters: internalization of carbon nanotube − protein conjugates into mammalian cells. J. Am. Chem. Soc., 126 (22), 6850–6851.
- 22 Al-Jamal, K.T., Nerl, H., Müller, K.H., Ali-Boucetta, H., Li, S., Haynes, P.D., Jinschek, J.R., Prato, M., Bianco, A., Kostarelos, K., and Porter, A.E. (2011) Cellular uptake mechanisms of functionalised multi-walled carbon nanotubes by 3D electron tomography imaging. Nanoscale, 3 (6), 2627.
- 23 Mu, Q., Broughton, D.L., and Yan, B. (2009) Endosomal leakage and nuclear translocation of multiwalled carbon nanotubes: developing a model for cell uptake. Nano Lett., 9 (12), 4370–4375.
- 24 Scheinberg, D.A., Mcdevitt, M.R., Dao, T., Mulvey, J.J., Feinberg, E., and Alidori, S. (2013) Carbon nanotubes as vaccine scaffolds. Adv. Drug Delivery Rev., 65 (15), 2016–2022.
- 25 Salvadormorales, C., Flahaut, E., Sim, E., Sloan, J., Hgreen, M., and Sim, R. (2006) Complement activation and protein adsorption by carbon nanotubes. Mol. Immunol., 43 (3), 193–201.
- 26 Battigelli, A., Ménard-Moyon, C., and Bianco, A. (2014) Carbon nanomaterials as new tools for immunotherapeutic applications. J. Mater. Chem. B, 2 (37), 6144–6156.
- 27 Wang, C., Xu, L., Liang, C., Xiang, J., Peng, R., and Liu, Z. (2014) Immunological responses triggered by photothermal therapy with carbon nanotubes in combination with anti-CTLA-4 therapy to inhibit cancer metastasis. Adv. Mater., 26 (48), 8154–8162.
- 28 Kam, N.W., Liu, Z., and Dai, H. (2005) Functionalization of carbon nanotubes via cleavable disulfide bonds for efficient intracellular delivery of siRNA and potent gene silencing. J. Am. Chem. Soc., 127 (36), 12492–12493.
- 29 Singh, R., Pantarotto, D., Mccarthy, D., Chaloin, O., Hoebeke, J., Partidos, C.D., Briand, J.-P., Prato, M., Bianco, A., and Kostarelos, K. (2005) Binding and condensation of plasmid DNA onto functionalized carbon nanotubes: toward the construction of nanotube-based gene delivery vectors. J. Am. Chem. Soc., 127 (12), 4388–4396.
- 30 Lacerda, L., Bianco, A., Prato, M., and Kostarelos, K. (2006) Carbon nanotubes as nanomedicines: from toxicology to pharmacology. Adv. Drug Delivery Rev., 58 (14), 1460–1470.
- 31 Hirano, S., Fujitani, Y., Furuyama, A., and Kanno, S. (2010) Uptake and cytotoxic effects of multi-walled carbon nanotubes in human bronchial epithelial cells. Toxicol. Appl. Pharmacol., 249 (1), 8–15.
- 32 Yinghuai, Z., Peng, A., Carpenter, K., Maguire, J., Hosmane, N., and Takagaki, M. (2005) Substituted carborane-appended water-soluble single-wall carbon nanotubes: new approach to boron neutron capture therapy drug delivery. J. Am. Chem. Soc., 127, 9875–9880.
- 33 Joussein, E., Petit, S., Churchman, J., Theng, B., Righi, D., and Del Vaux, B. (2005) Halloysite clay minerals — a review. Clay Miner., 40, 383–426.
- 34 Lvov, Y. and Abdullayev, E. (2013) Functional polymer–clay nanotube composites with sustained release of chemical agents. Prog. Polym. Sci., 38, 1690–1719.
- 35 Du, M., Guo, B., and Jia, D. (2010) Newly emerging applications of halloysite nanotubes: a review. Polym. Int., 59, 574–582.
- 36 Yuan, P., Southon, D., Liu, Z., Green, M.E.R., Hook, J.M., Antill, S.J., and Kepert, C.J. (2008) Functionalization of halloysite clay nanotubes by grafting with γ-aminopropyltriethoxysilane. J. Phys. Chem. C, 112, 15742–15751.
- 37 Pasabakhsh, P., Churchman, G.J., and Keeling, J.L. (2013) Characterisation of properties of various halloysites relevant to their use as nanotubes and microfibre fillers. Appl. Clay Sci., 74, 47–57.
- 38 Fakhrullin, G.I., Akhatova, F.S., Lvov, Y.M., and Fakhrullin, R.F. (2015) Toxicity of halloysite clay nanotubes in vivo: a caenorhabditis elegans study. Environ. Sci. Nano, 2 (1), 54–59.
- 39 Kryuchkova, M., Danilushkina, A., Lvov, Y., and Fakhrullin, R. (2016) Evaluation of toxicity of nanoclays and graphene oxide in vivo: a paramecium caudatum study. Environ. Sci. Nano, 3 (2), 442–452.
- 40 Massaro, M., Piana, S., Colletti, C.G., Noto, R., Riela, S., Baiamonte, C., Giordano, C., Pizzolanti, G., Cavallaro, G., Milioto, S. et al (2015) Multicavity halloysite-amphiphilic cyclodextrin hybrids for co-delivery of natural drugs into thyroid cancer cells. J. Mater. Chem. B, 3 (19), 4074–4081.
- 41 Vergaro, V., Abdullayev, E., Lvov, Y.M., Zeitoun, A., Cingolani, R., Rinaldi, R., and Leporatti, S. (2010) Cytocompatibility and uptake of halloysite clay nanotubes. Biomacromolecules, 11 (3), 820–826.
- 42 Liu, M., Chang, Y., Yang, J., You, Y., He, R., Chen, T., and Zhou, C. (2016) Functionalized halloysite nanotube by chitosan grafting for drug delivery of curcumin to achieve enhanced anticancer efficacy. J. Mater. Chem. B, 4 (13), 2253–2263.
- 43 Shutava, T.G., Fakhrullin, R.F., and Lvov, Y.M. (2014) Spherical and tubule nanocarriers for sustained drug release. Curr. Opin. Pharmacol., 18, 141–148.
- 44 Joo, Y., Sim, J.H., Jeon, Y., Lee, S.U., and Sohn, D. (2013) Opening and blocking the inner-pores of halloysite. Chem. Commun., 49 (40), 4519–4521.
- 45 Fakhrullin, R.F. and Lvov, Y.M. (2016) Halloysite clay nanotubes for tissue engineering. Nanomedicine, 11 (17), 2243–2246.
- 46 von Klitzing, R., Stehl, D., Pogrzeba, T., Schomäcker, R., Minullina, R., Panchal, A., Konnova, S., Fakhrullin, R., Koetz, J., Möhwald, H., and Lvov, Y.M. (2017) Halloysites stabilized emulsions for hydroformylation of long chain olefins. Adv. Mater. Interfaces, 4 (1), 1600435.
- 47 Lvov, Y., Wang, W., Zhang, L., and Fakhrullin, R. (2016) Halloysite clay nanotubes for loading and sustained release of functional compounds. Adv. Mater., 28 (6), 1227–1250.
- 48 Konnova, S.A., Lvov, Y.M., and Fakhrullin, R.F. (2016) Magnetic halloysite nanotubes for yeast cell surface engineering. Clay Miner., 51 (3), 429–433.
- 49 Shchukin, D.G., Sukhorukov, G.B., Price, R.R., and Lvov, Y.M. (2005) Halloysite nanotubes as biomimetic nanoreactors. Small, 1 (5), 510–513.
- 50 Chao, C., Liu, J., Wang, J., Zhang, Y., Zhang, B., Zhang, Y., Xiang, X., and Chen, R. (2013) Surface modification of halloysite nanotubes with dopamine for enzyme immobilization. ACS Appl. Mater. Interfaces, 5 (21), 10559–10564.
- 51 Tully, J., Yendluri, R., and Lvov, Y. (2016) Halloysite clay nanotubes for enzyme immobilization. Biomacromolecules, 17 (2), 615–621.
- 52 Shamsi, M.H. and Geckeler, D.V. (2008) The first biopolymer-wrapped non-carbon nanotubes. Nanotechnology, 19 (7), 075604.
- 53 Lee, Y., Jung, G.-E., Cho, S.J., Geckeler, K.E., and Fuchs, H. (2013) Cellular interactions of doxorubicin-loaded DNA-modified halloysite nanotubes. Nanoscale, 5, 8577–8585.
- 54 Aguzzi, C., Viseras, C., Cerezo, P., Salcedo, I., Sánchez-Espejo, R., and Valenzuela, C. (2013) Release kinetics of 5-aminosalicylic acid from halloysite. Colloids Surf., B, 105, 75–80.
- 55 Abdullayev, E., Sakakibara, K., Okamoto, K., Wei, W., Ariga, K., and Lvov, Y. (2011) Natural tubule clay template synthesis of silver nanorods for antibacterial composite coating. ACS Appl. Mater. Interfaces, 3 (10), 4040–4046.
- 56 Tan, D., Yuan, P., Annabi-Bergaya, F., Liu, D., Wang, L., Liu, H., and He, H. (2014) Loading and in vitro release of ibuprofen in tubular halloysite. Appl. Clay Sci., 96, 50–55.
- 57 Dzamukova, M.R., Naumenko, E.A., Lvov, Y.M., and Fakhrullin, R.F. (2015) Enzyme-activated intracellular drug delivery with tubule clay nanoformulation. Sci. Rep., 5, 10560.
- 58 Viseras, M.T., Aguzzi, C., Cerezo, P., Viseras, C., and Valenzuela, C. (2008) Equilibrium and kinetics of 5-aminosalicylic acid adsorption by halloysite. Microporous Mesoporous Mater., 108 (1–3), 112–116.
- 59 Lvov, Y.M., DeVilliers, M.M., and Fakhrullin, R.F. (2016) The application of halloysite tubule nanoclay in drug delivery. Expert Opin. Drug Delivery, 13 (7), 977–986.
- 60 Abdullayev, E. and Lvov, Y. (2010) Clay nanotubes for corrosion inhibitor encapsulation: release control with end stoppers. J. Mater. Chem., 20 (32), 6681–6687.
- 61 Luo, Z., Song, H., Feng, X., Run, M., Cui, H., Wu, L., Gao, J., and Wang, Z. (2013) Liquid crystalline phase behavior and sol–gel transition in aqueous halloysite nanotube dispersions. Langmuir, 29 (40), 12358–12366.
- 62 Fakhrullin, R.F., Tursunbayeva, A., Portnov, V.S., and L'vov, Y.M. (2014) Ceramic nanotubes for polymer composites with stable anticorrosion properties. Crystallogr. Rep., 59 (7), 1107–1113.
- 63 Massaro, M., Riela, S., Guernelli, S., Parisi, F., Lazzara, G., Baschieri, A., Valgimigli, L., and Amorati, R. (2016) A synergic nanoantioxidant based on covalently modified halloysite-trolox nanotubes with intra-lumen loaded quercetin. J. Mater. Chem. B, 4 (13), 2229–2241.
- 64 Price, R.R., Gaber, B.P., and Lvov, Y. (2001) In-vitro release characteristics of tetracycline HCl, khellin and nicotinamide adenine dineculeotide from halloysite; a cylindrical mineral. J. Microencapsul., 18 (6), 713–722.
- 65 Suh, Y.J., Kil, D.S., Chung, K.S., Abdullayev, E., Lvov, Y.M., and Mongayt, D. (2011) Natural nanocontainer for the controlled delivery of glycerol as a moisturizing agent. J. Nanosci. Nanotechnol., 11 (1), 661–665.
- 66 Lvov, Y.M., Shchukin, D.G., Mohwald, H., and Price, R.R. (2008) Halloysite clay nanotubes for controlled release of protective agents. ACS Nano, 2 (5), 814–820.
- 67 Wei, W., Minullina, R., Abdullayev, E., Fakhrullin, R., Mills, D., and Lvov, Y. (2014) Enhanced efficiency of antiseptics with sustained release from clay nanotubes. RSC Adv., 4 (1), 488–494.
- 68 Cavallaro, G., Lazzara, G., Massaro, M., Milioto, S., Noto, R., Parisi, F., and Riela, S. (2015) Biocompatible poly(N-isopropylacrylamide)-halloysite nanotubes for thermoresponsive curcumin release. J. Phys. Chem. C, 119 (16), 8944–8951.
- 69 Riela, S., Massaro, M., Colletti, C.G., Bommarito, A., Giordano, C., Milioto, S., Noto, R., Poma, P., and Lazzara, G. (2014) Development and characterization of co-loaded curcumin/triazole-halloysite systems and evaluation of their potential anticancer activity. Int. J. Pharm., 475 (1–2), 613–623.
- 70 Massaro, M., Colletti, C.G., Noto, R., Riela, S., Poma, P., Guernelli, S., Parisi, F., Milioto, S., and Lazzara, G. (2015) Pharmaceutical properties of supramolecular assembly of co-loaded cardanol/triazole-halloysite systems. Int. J. Pharm., 478 (2), 476–485.
- 71 Massaro, M., Amorati, R., Cavallaro, G., Guernelli, S., Lazzara, G., Milioto, S., Noto, R., Poma, P., and Riela, S. (2016) Direct chemical grafted curcumin on halloysite nanotubes as dual-responsive prodrug for pharmacological applications. Colloids Surf., B, 140, 505–513.
- 72 Massaro, M., Riela, S., Baiamonte, C., Blanco, J.L.J., Giordano, C., Lo Meo, P., Milioto, S., Noto, R., Parisi, F., Pizzolanti, G. et al (2016) Dual drug-loaded halloysite hybrid-based glycocluster for sustained release of hydrophobic molecules. RSC Adv., 6 (91), 87935–87944.
- 73 Biddeci, G., Cavallaro, G., Di Blasi, F., Lazzara, G., Massaro, M., Milioto, S., Parisi, F., Riela, S., and Spinelli, G. (2016) Halloysite nanotubes loaded with peppermint essential oil as filler for functional biopolymer film. Carbohydr. Polym., 152, 548–557.
- 74 Silverstein, S.C., Steinman, R.M., and Cohn, Z.A. (1977) Endocytosis. Annu. Rev. Biochem., 46, 669–722.
- 75 Vida, T.A. and Emr, S.D. (1995) A new vital stain for visualizing vacuolar membrane dynamics and endocytosis in yeast. J. Cell Biol., 128, 779–792.
- 76 Haniu, H., Saito, N., Matsuda, Y., Kim, Y.A., Park, K.C., Tsukahara, T., Usui, Y., Aoki, K., Shimizu, M., Ogihara, N., Hara, K., Takanashi, S., Okamoto, M., Ishigaki, N., Nakamura, K., and Kato, H. (2011) Elucidation mechanism of different biological responses to multi-walled carbon nanotubes using four cell lines. Int. J. Nanomed., 6, 3487–3497.
- 77 Yumoto, R., Suzuka, S., Oda, K., Nagai, J., and Takano, M. (2012) Endocytic uptake of fitcalbumin by human alveolar epithelial cell line A549. Drug Metab. Pharmacokinet., 27, 336–343.
- 78 Haniu, H., Saito, N., Matsuda, Y., Tsukahara, T., Maruyam, K., Usuie, Y., Aoki, K., Takanashi, S., Kobayashi, S., and Nomura, H. (2013) Culture medium type affects endocytosis of multi-walled carbon nanotubes in BEAS-2B cells and subsequent biological response. Toxicol. In Vitro, 27, 1679–1685.
- 79 Tabet, L., Bussy, C., Setyan, A., Simon-Deckers, A., Rossi, M.J., Boczkowski, J., and Lanone, S. (2011) Coating carbon nanotubes with a polystyrene-based polymer protects against pulmonary toxicity. Part. Fibre Toxicol., 2011 (8), 3.
- 80 Maruyama, K., Haniu, H., Saito, N., Matsuda, Y., Tsukahara, T., Kobayashi, S., Tanaka, M., Aoki, K., Takanashi, S., Okamoto, M., and Kato, H. (2015) Endocytosis of multiwalled carbon nanotubes in bronchial epithelial and mesothelial cells. BioMed Res. Int., 2015, Article ID 793186, 9 pp.
- 81 Nagai, H. and Toyokuni, S. (2012) Differences and similarities between carbon nanotubes and asbestos fibers during mesothelial carcinogenesis: shedding light on fiber entry mechanism REVIEW. Cancer Sci., 103, 1378–1390.
- 82 Kang, B., Chang, S., Dai, Y., Yu, D., and Chen, D. (2010) Cell response to carbon nanotubes: size-dependent intracellular uptake mechanism and subcellular fate. Small, 6, 2362–2366.
- 83 Kang, B., Yu, D.C., Chang, S.Q., Chen, D., Dai, Y.D., and Ding, Y. (2008) Intracellular uptake, trafficking and subcellular distribution of folate conjugated single walled carbon nanotubes within living cells. Nanotechnology, 19, 375103.
- 84 Iancu, C., Mocan, L., Bele, C. et al (2011) Enhanced laser thermal ablation for the in vitro treatment of liver cancer by specific delivery of multiwalled carbon nanotubes functionalized with human serum albumin. Int. J. Nanomed., 6, 129–141.
- 85 Bhirde, A.A., Patel, V., Gavard, J. et al (2009) Targeted killing of cancer cells in vivo and in vitro with EGF-directed carbon nanotube-based drug delivery. ACS Nano, 3, 307–316.
- 86 Raffa, V., Ciofani, G., Vittorio, O., Riggio, C., and Cuschieri, A. (2010) Physicochemical properties affecting cellular uptake of carbon nanotubes REVIEW. Nanomedicine, 5, 89–97.
- 87 Yaron, P.N., Holt, B.D., Short, P.A., Lösche, M., Islam, M.F., and Dahl, K.N. (2011) Single wall carbon nanotubes enter cells by endocytosis and not membrane penetration. J. Nanobiotechnol., 9, 45.
- 88 Hong, G., Wu, J.Z., Robinson, J.T., Wang, H., Zhang, B., and Dai, H. (2012) Three-dimensional imaging of single nanotube molecule endocytosis on plasmonic substrates. Nat. Commun., 3, 700.
- 89 Zhang, S., Li, J., Lykotrafitis, G., Bao, G., and Suresh, S. (2009) Size-dependent endocytosis of nanoparticles. Adv. Mater., 21, 419–424.
- 90 Liu, H.-Y., Du, L., Zhao, Y.-T., and Tian, W.-Q. (2015) In vitro hemocompatibility and cytotoxicity evaluation ofhalloysite nanotubes for biomedical application. J. Nanomat., 2015, Article ID 685323, 9 pp.
- 91 Yang, J., Wu, Y., Shen, Y., Zhou, C., Li, Y.F., He, R.R., and Liu, M. (2016) Enhanced therapeutic efficacy of doxorubicin for breast cancer using chitosan oligosaccharide modified halloysite nanotubes. ACS Appl. Mater. Interfaces, 8, 26578–26590.
- 92 He, H., Pham-Huy, L., Dramou, P., Xiao, D., Zuo, P., and Pham-Huy, C. (2013) Carbon nanotubes: applications in pharmacy and medicine. BioMed Res. Int., 12, Article ID 578290.
