Chapter 15
Nanoarchitectonics Approach for Sensing
Katsuhiko Ariga
World Premier International (WPI) Research Center for Materials Nanoarchitectonics (MANA), National Institute for Materials Science (NIMS), 1-1 Namiki, Tsukuba, 305-0044, Japan
15.1 Introduction
Sensing is one of the most important applications for life activities. Bio-systems cannot avoid influences from external stimuli and interactions with external chemicals. Therefore, development of sensing materials and systems (or devices) would contribute to improvements in life through detection of dangerous stuff and finding necessary materials. In addition to research efforts for sophisticated sensing device apparatus, exploration of materials systems with high sensitivity and selectivity is a crucial factor for preparation of good sensors. Good sensing materials systems can be obtained both by synthesis of guest molecules and materials with recognizing target guests and fabrication of material structures to facilitate sensing capabilities. These efforts are based heavily on nanomaterials and nanostructures. It apparently relies on nanoarchitectonics [1–10].
Of course, plenty of research efforts on exploration of sensing materials have been extensively done so far. However, architecting sensing units into hierarchic construction has not been fully explored. In fact, regulation of biological activities is based on molecular interactions within complexes of functional molecules/materials in highly hierarchic structures. Development of bio-like hierarchic structures would be an important key in the preparation of advanced sensors. Therefore, approaches based on fabrication of hierarchic nanostructures using the nanoarchitectonics concept would be attractive for sensor technology.
However, fabrication of hierarchic structures cannot be easily achieved as seen in biological systems. One possible methodology for fabrication of hierarchic functional structures would be a multistep process in which nanostructured materials can be first synthesized and the resulting materials are then assembled into further advanced structures. For example, layer-by-layer (LbL) assembly of nanostructured materials is one of the easily accomplishable ways [11–13]. Based on this strategy, various sensors have been architected through LbL assemblies of nanocarbons and mesoporous materials. In this chapter, several examples of sensing systems with LbL nanomaterials are introduced.
15.2 Layered Mesoporous Carbon Sensor
One of the most advantageous points of the LbL assembly in fabrication of hierarchic nanostructures is its high versatility. The LbL technique is available for layer assembly of huge kinds of materials including various polymers, molecular assemblies, biomaterials, and inorganic nanomaterials. In addition, the LbL processes can be done with totally inexpensive apparatuses such as beakers and tweezers and by very simple procedures such as dipping and spraying. This technique can be used in the fabrication of functional hierarchic structures using nanostructured building units that can be synthesized separately.
The first example of LbL nanomaterials sensors is shown in Figure 15.1 in which a sensor based on LbL structures prepared from mesoporous carbon materials (CMK-3) with the aid of polyelectrolytes is illustrated [14]. Because mesoporous carbon materials CMK-3 do not have surface charges necessary for electrostatic LbL procedure, the surfaces of CMK-3 materials were partially oxidized through treatment with ammonium persulfate enabled to introduce negatively charged carboxylate groups. The modified CMK-3 materials were assembled alternately with cationic polyelectrolyte, poly(diallyldimethylammonium chloride) (PDDA), onto a quartz crystal microbalance (QCM) plate.

Figure 15.1 A sensor based on LbL structure of mesoporous carbon materials (CMK-3) and polyelectrolyte on a QCM plate.
(Ariga et al. 2008 [14]. Reproduced with permission of John Wiley and Sons.)
Sensing capabilities of the architected sensors toward bioactive components were investigated in aqueous phase. After injection of guests into the aqueous phase, detection of the guest substances was evaluated by frequency shifts upon adsorption of the guests. For example, the prepared sensors exhibited high sensitivity to tannic acid. Sensing signals, frequency shifts toward adsorption of tannic acid are much greater that those for adsorption of caffeine and catechin. This selectivity to tannic acid is due to the molecular structure of tannic acid. Tannic acid has a multiple phenyl ring structure that is advantageous for interaction with the carbon surface upon π–π interactions and hydrophobic effects. In addition, selective sensing is also resulted from the size matching between tannic acid and carbon mesopore channel. Tannic acid has a roughly circular shape with an approximate diameter of 3 nm that fits well with the CMK-3 nanochannel.
Findings of this sensing research are not limited to high selectivity to a particular guest, tannic acid. Highly cooperative adsorption behaviors upon adsorption of tannic acid into carbon mesopore channel were suggested from the sigmoidal profile of adsorption quantities of tannic acid to the CMK-3 at low concentrations. The observed cooperative nature of guest adsorption can be interpreted with enhanced guest–guest interaction including effective π–π and/or hydrophobic interactions within a confined environment. This finding is important for interpretation of molecular interaction, especially nonspecific interaction, within a confined space, the so-called nanospace. It is also related to the understanding of various processes of biological systems.
15.3 Layered Graphene Sensor
Another important detection target in environmental issues is sensing of toxic aromatic hydrocarbons in gas phase. For facilitated detection of aromatic hydrocarbons, architecting π-electron-rich nanostructures onto sensor devices was investigated through the LbL assembly of reduced graphene oxide nanosheets and aromatic ionic liquid (Figure 15.2) [15].
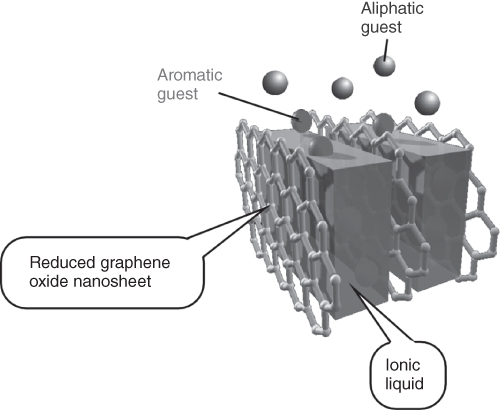
Figure 15.2 An LbL assembly of reduced graphene oxide nanosheets and ionic liquid with selective adsorption of aromatic guest molecules.
(Ji et al. 2010 [15]. Reproduced with permission of John Wiley and Sons.)
In the first process, graphene oxide nanosheets were disassembled upon oxidation of graphite materials under acidic conditions. The prepared graphene oxide nanosheets were reduced in the presence of aromatic ionic liquid, immidazolium derivatives, in the second step. This second process resulted in the formation of cationic complexes between reduced graphene oxide nanosheets and ionic liquid. The resulting cationic complexes were then assembled into layered nanostructures alternately with anionic polyelectrolytes poly(sodium styrene sulfonate) (PSS) on a QCM electrode. Detailed observation of the formed LbL nanoarchitectonics by transmission electron microscopy (TEM) revealed aligned organization of reduced graphene oxide nanosheets with enhanced layer spacing by filling interlayer spaces with ionic liquid.
Detection behaviors for toxic aromatic molecules by the prepared LbL structures on QCM sensor plates were roughly investigated by very simple procedures, exposure of the sensors to solvent vapors under an ambient atmosphere. This in situ sensing revealed enhanced sensitivity to aromatic gas substances over the other substances. For example, much greater selectivity for benzene vapor over cyclohexane (more than 10 times) was observed (Figure 15.3), while these two guest molecules possess similar molecular sizes, molecular weights, and vapor pressures. Higher sensitivity was similarly confirmed for the other aromatic targets such as pyridine and toluene. As can be expected, a nanoarchitected π-electron-rich two-dimensional nanospace within layered structures of reduced graphene oxides and aromatic ionic liquid would be the ideal medium for selective adsorption and enhanced detection.
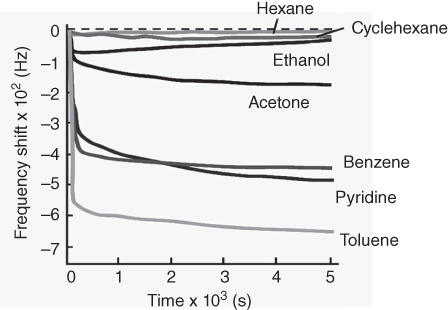
Figure 15.3 Sensing profiles (frequency shifts) of various gaseous guests by the QCM sensor with the LbL film of reduced graphene oxide nanosheets and ionic liquid.
15.4 Hierarchic Carbon Capsule Sensor
As more advanced nanoarchitectonic fabrication for sensing application, hierarchic nanocarbon structures were demonstrated through LbL assembly of mesoporous carbon capsules. Using zeolite crystals as templates, mesoporous carbon capsules were synthesized. The resulting capsule has a homogeneous rectangular shape with sizes of 1000 × 700 × 300 nm3 and 35-nm-thick mesoporous walls. The latter mesoporous wall has a uniform pore-size distribution centered at 4.3 nm in diameter, and a specific surface area of 918 m2 g−1. Because the mesoporous carbon capsules do not have surface charge, the capsules were coated with a charged surfactant prior to the LbL process. Surfactant-coated mesoporous carbon capsules were assembled with counterionic polyelectrolyte into LbL nanoarchitectures on a QCM plate (Figure 15.4) [16].

Figure 15.4 An LbL film of mesoporous carbon capsules with polyelectrolyte on a QCM plate.
(Adapted from Yoon et al. 2009 [16].)
Sensing of organic gas molecules was investigated simply by exposing the QCM sensor devices with the carbon capsule LbL films to saturated vapors over neat solvents. The in situ frequency shifts of the QCM sensor exhibited a general tendency of higher sensitivity to aromatic hydrocarbons such as benzene and toluene, as similarly seen in the other carbon-based sensing systems. If we compare guest molecules with various functional groups (acetic acid, ammonia, butylamine, aniline, and pyridine), the sensing films with the carbon capsules have a great affinity for aromatic guests, that is, aniline and pyridine (Figure 15.5).
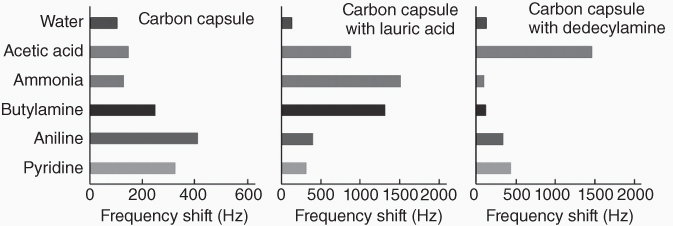
Figure 15.5 Gas sensing selectivity of QCM sensors with three kinds of mesoporous carbon capsules.
Because of the hierarchic nature of the sensing film structure, selectivity of these sensors can be easily modulated by impregnation or doping of the second sensing components in the capsule inside. Impregnation of the carbon capsule with an acidic second component, lauric acid, resulted in the greatest affinities for nonaromatic amines and the second highest affinity for acetic acid. In this case, the affinity between nonaromatic amines and aromatic amines was drastically reversed only through the presence of the acidic additive. Increased affinity would be originated from acid–base interaction, and, therefore, a stronger base aliphatic amine guest probably exhibits greater sensitivity. In turn, impregnation with a basic second component, in the carbon capsule LbL systems, changes the highest affinity to acetic acid. As demonstrated in these examples, the prepared hierarchic sensing structures are capable of easy modulation of sensing selectivity that would be highly beneficial for adjusting and optimizing sensor capabilities to target guests.
15.5 Cage-in-Fiber Sensor
As seen in the former examples, designs of carbon nanostructures would be important keys to control sensor capabilities. In many cases, such nanostructures can be synthesized using a template. Therefore, careful designing and selection of the template structure for nanostructure synthesis would be crucial in nanoarchitectonics for advanced sensors. A novel cage-type nanocarbon, carbon nanocage, was invented by Vinu et al. using large three-dimensional cage-type face-centered cubic mesoporous silica materials (KIT-5) as inorganic templates [17]. This well-engineered nanocarbon material has a huge surface area (1600 m2 g−1) and specific pore volume (2.10 cm3 g−1) with facilitated material diffusion within pores. Therefore, carbon nanocage materials have excellent capability to remove toxic hydrophobic materials from aqueous solution [18]. Figure 15.6 illustrates much better performances of carbon nanocages in the removal of the model dye (Alizarin Yellow) as compared with activated carbons and conventional mesoporous carbon, CMK-3.

Figure 15.6 Comparisons of dye-removal capability among (a) activated carbon, (b) carbon nanocage, and (c) conventional mesoporous carbon CMK-3: the structure of the carbon nanocage is only a simplified illustration.
Using carbon nanocage materials as sensing components, sensor systems with a novel type of hierarchic structure, cage-in-fiber structural motif, were developed for selective sensing for aniline (Figure 15.7) [19].The carbon nanocage materials were first dispersed in host polymer, poly(methyl methacrylate). The resulting mixture was used for fabrication of electrospun nanofibrous films. This hierarchic structure can enhance sensitivity with rapid response to a selected guest gas. The most interesting finding of this sensing system would be enhanced sensitivity to aniline (11.9 Hz ppm−1) as compared with the volatile aromatic organic compounds such as benzene (0.2 Hz ppm−1) and toluene (0.4 Hz ppm−1). While the binding constant for benzene is estimated to be 36.4 M−1, that for aniline becomes 1852.1 M−1. Even when flows between benzene and aniline were alternately applied, large sensing responses appeared only with the flow of aniline. At the surface of the carbon nanocage, small amounts of carbonyl group exist due to partial oxidation, resulting in selective interaction with basic aniline in addition to π–π interaction of carbon frameworks. The developed sensing system would be useful to detect lung cancer at its early stage, and this detection can be done through a simple breath check.
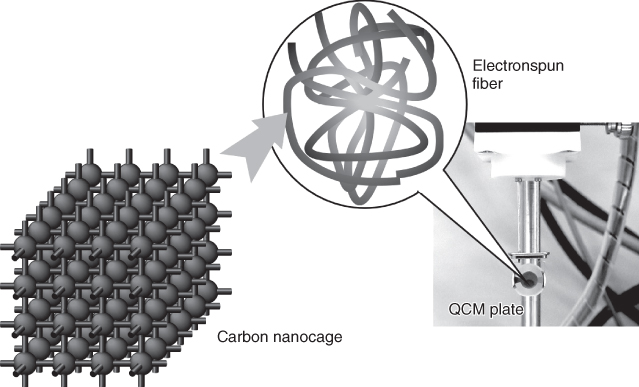
Figure 15.7 Nanostructure carbon with cage-in-fiber structural motif on a QCM sensor plate: the structure of the carbon nanocage is only a simplified illustration.
(Adapted from Izawa et al. 2013 [19].)
15.6 Summary
Sensing is a very important application because it reveals the presence of chemicals, concentration of chemicals, and interactive nature of chemicals. These factors are unavoidable for life activities. Both finding useful drugs and detecting toxic substances rely on sensing functions. In addition to improvements in sensing device apparatuses, exploration of sensing materials is crucial for development of sensing technologies. Sensing materials are not only good functional materials but also good structured materials. Both materials nature and structures have to work well for improvements in sensing performance. As shown in various sensors in this chapter, nanoarchitectonics approach to construct hierarchic structures is really useful for advanced high performance. Because nanoarchitectonics concept was just recently initiated, further developments of sensor designing and fabrications based on the nanoarchitectonics concepts are highly anticipated.
References
- 1 Aono, M. (2011) Sci. Technol. Adv. Mater., 12, 040301.
- 2 Aono, M., Bando, Y., and Ariga, K. (2012) Adv. Mater., 24, 150.
- 3 Ariga, K., Ji, Q., Hill, J.P., Bando, Y., and Aono, M. (2012) NPG Asia Mater., 4, e17.
- 4 Aono, M. and Ariga, K. (2016) Adv. Mater., 28, 989.
- 5 Ariga, K., Li, M., Richards, G.J., and Hill, J.P. (2011) J. Nanosci. Nanotechnol., 11, 1.
- 6 Ramanathan, M., Hong, K., Ji, Q., Yonamine, Y., Hill, J.P., and Ariga, K. (2014) J. Nanosci. Nanotechnol., 14, 390.
- 7 Ariga, K. and Aono, M. (2016) Jpn. J. Appl. Phys., 55, 1102A6.
- 8 Ariga, K., Yamauchi, Y., Ji, Q., Yonamine, Y., and Hill, J.P. (2014) APL Mater., 2, 030701.
- 9 Ishihara, S., Labuta, J., Van Rossom, W., Ishikawa, D., Minami, K., Hill, J.P., and Ariga, K. (2014) Phys. Chem. Chem. Phys., 16, 9713.
- 10 Ariga, K., Minami, K., and Shrestha, L.K. (2016) Analyst, 141, 2629.
- 11 Ariga, K., Hill, J.P., and Ji, Q. (2007) Phys. Chem. Chem. Phys., 9, 2319.
- 12 Ariga, K., Yamauchi, Y., Rydzek, G., Ji, Q., Yonamine, Y., Wu, K.C.-W., and Hill, J.P. (2014) Chem. Lett., 43, 36.
- 13 Rydzek, G., Ji, Q., Li, M., Schaaf, P., Hill, J.P., Boulmedais, F., and Ariga, K. (2015) Nano Today, 10, 138.
- 14 Ariga, K., Vinu, A., Ji, Q., Ohmori, O., Hill, J.P., Acharya, S., Koike, J., and Shiratori, S. (2008) Angew. Chem. Int. Ed., 47, 7254.
- 15 Ji, Q., Honma, I., Paek, S.-M., Akada, M., Hill, J.P., Vinu, A., and Ariga, K. (2010) Angew. Chem. Int. Ed., 49, 9737.
- 16 Ji, Q., Yoon, S.B., Hill, J.P., Vinu, A., Yu, J.-S., and Ariga, K. (2009) J. Am. Chem. Soc., 131, 4220.
- 17 Vinu, A., Miyahara, M., Sivamurugan, V., Mori, T., and Ariga, K. (2005) J. Mater. Chem., 15, 5122.
- 18 Ariga, K., Vinu, A., Miyahara, M., Hill, J.P., and Mori, T. (2007) J. Am. Chem. Soc., 129, 11022.
- 19 Kosaki, Y., Izawa, H., Ishihara, S., Kawakami, K., Sumita, M., Tateyama, Y., Ji, Q., Krishnan, V., Hishita, S., Yamauchi, Y., Hill, J.P., Vinu, A., Shiratori, S., and Ariga, K. (2013) ACS Appl. Mater. Interfaces, 5, 2930.
