Chapter 2
Architectonics in Nanoparticles
Qingmin Ji1,2, Xinbang Liu2 and Ke Yin3
1Nanjing University of Science and Technology, Herbert Gleiter Institute of Nanoscience, 200 Xiaolingwei, Nanjing, 210094, China
2Nanjing University of Science and Technology, School of Materials Science and Engineering, 200 Xiaolingwei, Nanjing, 210094, China
3Electric Power Simulation and Control Engineering Center, Nanjing Institute of Technology, No. 1 Hongjing Avenue, Jiangning Science Park, Nanjing, 211167, China
2.1 Introduction
Within the field of nanotechnology, zero-dimensional nanoparticles are one of the most prominent and promising candidates for technological applications [1]. Taking a close look at their impact in the research areas, we can get impressive lists of studies or applications of nanoparticles in fields including biomedical, energy, electronics, sensors, catalysis, filtrates, and so on. With the intensive developments in nanoscience and nanotechnology, the creation of novel nanostructures requires not only high regularity but also high complexity, which poses great challenges to the fabrication of nanoparticles. Architectonics of nanoparticles, which means the manipulation at micro and nano dimensions, will impart multifunctions of nanostructures and explore a range of new properties or applications (Figure 2.1) [2]. The concept of architectonics of nanoparticles is involved in the harmonization of various techniques and phenomena including chemical fabrications and structural control induced by physical stimuli and self-assembly/organization [3]. In this section, we focus on the recent research efforts in the creation of architectured nanoparticles through synthesis strategies.
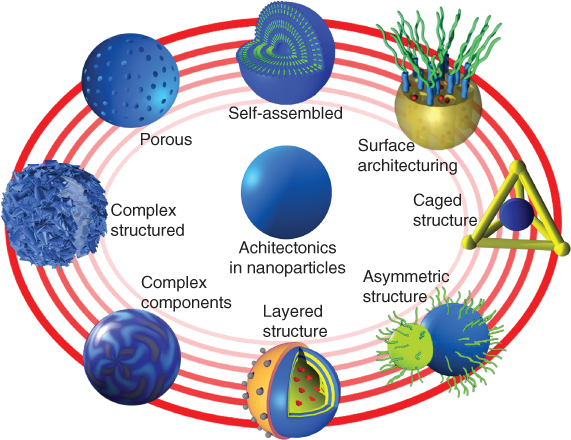
Figure 2.1 The scheme for nanoparticles with various architectures.
According to the material nature of nanoparticles, they can be “soft” nanoparticles, which are based on polymers or molecular assemblies, or “hard” nanoparticles, which are made up of metals, inorganic materials, or their hybrids. Herein, we briefly introduce the design and fabrication of novel nanostructured nanoparticles, highlighting the principles and mechanisms of architectonics. We also summarize their related superior properties from the architectured nanoparticles.
2.2 Soft Nanoparticles
The design of “soft” nanoparticles can be traced back to the emergence of nanotechnology in the past decades. It became an important part of research in nanoscience and has met intensive investigations due to their high application potentials in electronic, photonic, or biotechnology [4]. Especially for the applications in biomedicine, soft nanoparticles with well-defined architectures and functionalities may satisfy the need to combine bulk loading, target delivery, and stimuli-responsive release. The improvement in properties and the discovery of new functionalities by structural architecturing are key goals for the design of soft nanoparticles.
2.2.1 Smart Polymer Nanoparticles
Polymer nanoparticles have constituted by far the most studied “soft” particles in the literature. In general, they can be distinguished into two major families according to their applications. One is related to drug delivery or biomedical applications, and the other is for electronic or optoelectronic properties.
2.2.1.1 Multi-Responsive Polymer Nanoparticles for Biological Therapy
A number of synthetic or natural polymers have been studied and used for biomedical applications [5]. However, only limited molecules could be used as constituents of drug delivery vesicles, due to the drastic concerns of toxicity and biocompatibility. The backbone chemistry of polymer nanoparticles may affect their stability, biodegradability, biocompatibility, biodistribution, and cellular fate. Therefore, the precise control over the nanostructures from both the chemical and physical traits is critically important in the design of polymer nanoparticles for biomedical delivery applications. Attractive “smart” properties of polymer nanoparticles can be modulated to be responsive to pH, enzymes, temperature, and even external stimuli like near-infrared (IR) or UV–vis irradiation, magnetic fields, or ultrasound vibrations [6].
The pH- and temperature-responsive nanoparticles are among the most studied dual-sensitive nanosystems. On the basis of the pH- or temperature-sensitive components, the further incorporation with functional additives in the structures may accomplish more visualized responses according to the external stimuli. Lin and coworkers constructed pH- and temperature-sensitive hydrogel nanoparticles with dual photoluminescence (PL) for bioprobes [7]. The hydrogel nanoparticles consisted of the thermo- and pH-responsive copolymers of poly(N-isopropylacrylamide) (PNIPAM) and poly(acrylic acid) (PAA). A red-emission rare-earth complex and a blue-emission quaternary ammonium tetraphenylethylene derivative (d-TPE) with similar excitation wavelengths are inserted into the hydrogel nanoparticles. The PL intensities of the nanoparticles show a linear temperature response in the range from 10 to 80 °C (red emission) and a linear pH response between pH 6.5 and 7.6 (blue emission). These dual-emission nanoparticles provide highly sensitive detection in the case of cancer cells. They can act as a quite promising sensing platform, which has potential applications in biology and chemistry, including bio- or chemosensors, biological imaging, cancer diagnosis, and externally activated release of anticancer drugs.
Smartness of the novel responsive systems relies on the structural design according to the requirements of the specific delivery. For example, oxidative stress and a reduced pH are important stimuli targets for intracellular delivery and for delivery to diseased tissue. Ideal materials were thus required to be able to deliver bioactive agents selectively under those conditions. Almutairi and coworkers designed a pH- and oxidation-responsive nanoparticle based on polythioether ketal, which can undergo programmed degradation in response to reactive oxygen species (ROS) and acidic pH (Figure 2.2) [8]. This dual-sensitive nanoparticles functioned akin to an “AND” logic gate in circuits. The polymeric backbone transforms from hydrophobic to hydrophilic following exposure to ROS, which then allows rapid acid-catalyzed degradation of ketal groups under mildly acidic environments. These responses finally accelerate the release of ovalbumin inside the nanoparticles. Cellular uptake studies proved that these dual-sensitive nanoparticles delivered and released fluorescence-labeled ovalbumin into macrophage cells much more efficiently than poly(lactic-co-glycolic acid) (PLGA) nanoparticles.

Figure 2.2 Logic gate nanoparticles that show a dual response to reactive oxygen species (ROS) and low pH.
(Adapted from Mahmoud et al. 2011 [8].)
The past several years have witnessed a rapid progress in the development of multi-stimuli-responsive polymeric nanoparticles for programmed site-specific drug delivery [9]. These multifunctional nanoparticles are able to elegantly address the challenging issues of current nanoparticulate drug delivery systems including aspects of preparation, drug loading, in vivo stability, tumor targetability, tumor cell uptake, and intracellular drug release. These architecturing features for smart functionalities have offered unprecedented control of drug delivery and release profiles leading to superior development in vitro and/or in vivo biological therapy.
2.2.1.2 Optoelectrical Polymer Nanoparticles
The polymer nanoparticles for electrical and optical properties or photoluminescence are mainly composed of conjugated polymers, such as polyaniline, polypyrrole, polythiophenes, and poly(p-phenylenevinylene)s [10]. Because of the difficulty in satisfying these rigorous structural requirements for photoluminescence and charge transport, a challenge has remained in the development of novel high-performance π-conjugated systems for nano-optoelectronics. The desirable optical and electrical properties of π-conjugated molecules for these applications depend on their primary molecular structure and their intermolecular interactions such as molecular packing or ordering in the condensed states [11]. Liu and coworkers reported the preparation of self-assembled fluorescent organic nanoparticles in water by means of calixarene-induced aggregation of a tetraphenylethene derivative (QA-TPE) mediated by p-sulfonatocalix[4]arenes [12]. The self-assembled nanoparticles showed interesting photo-switching behaviors. Free QA-TPE is nonfluorescent, owing to intramolecular rotations of the phenyl rings. In contrast, the self-assembled nanoparticles that formed upon complexation of QA-TPE with p-sulfonatocalix[4]arene exhibited aggregation-induced emission fluorescence (λem = 480 nm), as a result of the inhibition of rotations. Upon UV light irradiation, free QA-TPE was cyclized to the corresponding diphenylphenanthrene, which showed typical fluorescence of a π-conjugated system at λem = 385 nm, whereas the nanoparticles were nonfluorescent upon irradiation due to the aggregation-caused quenching. This system allows programmed modulation of TPE fluorescence at two different emission wavelengths by means of host–guest complexation and irradiation. The fluorescent nanoparticles present highly integrated modes into a single molecular unit and can exhibit modulation of fluorescence by multiple stimuli, which is expected to be more adaptable to practical applications.
Although basically π-conjugated polymers are inherited with a hydrophobic nature, water-soluble conjugated polymers have been receiving increasingly greater attention owing to their potential applications in the areas of biosensing, bioimaging, and optoelectronics [13]. The formation into spherical morphology provides an alternative state to achieve good water solubility or dispersibility of the conjugated polymers. The water-dispersible conjugated polymer nanoparticles (CPNs) were mainly prepared by mini-emulsion and reprecipitation. However, only limited highly hydrophobic conjugated polymers which do not carry functional groups to be further modified can be successfully used to obtain nanoparticles by those processes. Physical-stimuli-intrigued molecular changes in the polymer structures open a pathway to obtain both water-dispersible and functional nanoparticles. Demir, Tuncel, and coworkers synthesized mechanically stable, water-dispersible CPNs in shelled architecture via cross-linking strategy [14]. Polyfluorene derivatives containing azide groups were designed to facilitate the inter- and intra-cross-linking of polymer chains. As azide groups can be decomposed under UV light to form very reactive species (nitrene species), it therefore allows the further functionalization of the nanoparticles. The cross-linking by UV light on the polymer nanoparticles strengthened the mechanical stability of nanoparticles and also accomplished emission color tuning of the core–shell structures. Due to an energy transfer from the blue-emitting core to the green-yellow-emitting shell, a broad band covering the blue to yellow region of the visible spectrum generates white emission. The light sources designed from these nanoparticles have high correlated color temperature and high S/P value, which exhibit high efficiency under low optical power levels. The CPNs can also be integrated as color-conversion light-emitting diodes (LEDs) to show their use as luminophores. These water-dispersible and mechanically stable CPNs may show great potential both in optoelectronic applications including in LEDs, photovoltaics, inkjet printing, and in the construction of organic light-emitting diodes (OLEDs).
Optoelectrical polymers are an attractive class of materials because they combine the optical and electronic properties of semiconductors with the processing advantages and mechanical properties of polymers. These materials hold enormous promise for the next generation of optoelectrical devices, such as LEDs, transistors, molecular switches, photovoltaic cells, chemical and biological sensors, and large-area flexible displays. They also become one of the most rapidly expanding research areas for polymers.
2.2.2 Nanoparticles from Biomimetic Assembly
Numerous highly functional assemblies which perform complicated tasks in nature have always inspired researchers' attempts to emulate them. The investigation of nature's principles to produce fascinating and functional complex assemblies has developed into the period of requiring highly interdisciplinary efforts based on chemistry, biotechnology, and materials science. The strategies based on biomimetic-assembly processes usually utilize the environmentally benign and “green” experimental conditions, unlike the harsh conditions used in the traditional chemical synthesis [15]. The morphology and size of the formed nanoparticles may also be controlled in nanoscale dimensions. The rational design on those assembled structures can generate modular, multifunctional nanocontainers for smart delivery [16].
Considering the biomedical application of nanoparticles, we must mention the biomimetic carrier: liposomes. Liposomes are the most clinically established nanometer-scale systems that are used to deliver therapeutic drugs, genes, vaccines, and imaging agents. Liposomes consist of single or multiple concentric lipid bilayers, which offer biocompatibility, biodegradability, reduced toxicity, and capacity for various sized cargo. With tremendous advances in design and engineering of lipids, a new generation of lipid nanoparticles has been developed in response to external stimuli (e.g., pH, temperature, enzymes, etc.). Functional metal nanoparticles, proteins, viruses, and genes can be incorporated into the lipid layer of the nanoparticles, which not only strengthen the capability to trigger drug release at specific sites but also surface properties, stability, and overall biocompatibility [17].
Viruses traditionally have a major role in the study of infection and disease, and also have been explored as gene vectors for biotherapy. Despite their high gene transfer and expression efficacy, virus vectors may bring on a variety of adverse side effects like tissue toxicity or severe immune responses. Much effort has been devoted to alleviate their drawbacks. Kostarelos and coworkers successfully engineered adenovirus (Ad)-loaded lipid nanoparticles by enveloping Ad with a pH-sensitive lipid layer of dioleoylphosphatidylethanolamine and cholesteryl hemisuccinate (DOPE:CHEMS) [18]. As these lipid nanoparticles could successfully escape the endosomes and traffic to the perinuclear region, they showed levels of gene expression similar to those of naked Ad both in vitro and in vivo. This system has also the engineering flexibility in the combination of virion therapeutic component with imaging probes, which thus can allow optical tracking of viruses.
The size, shape, and surface chemistry of nanoparticles can affect circulation half-life, cell-specific internalization, excretion, toxicity, and efficacy. Although great efforts have been exerted to design polymeric and liposome systems for effective drug delivery, most of those nanoparticles are still heterogeneous in size, composition, and surface chemistry, which may lead to suboptimal performance and potential toxicity. Nanoparticles, which developed directly from large macromolecules such as proteins and DNA, and so on, are thus found to be more effective and safe in the delivery system [19]. Due to the easily programmable design on the molecular structures, these kinds of systems exhibit advantages of the nontoxic biodegradability and homogeneous biodistribution of the targeting ligands for more efficient and safe delivery. DNA/siRNA can be fabricated into novel tetrahedral nanoparticles with a well-defined size, deliver siRNAs into cells, and silence target genes in tumors (Figure 2.3) [20]. The overhang design of the DNA strands allows for specific hybridization of complementary siRNA sequences. Therefore, the full control over the spatial orientation of the siRNA and the location and the density of cancer-targeting ligands can be achieved. These nanoparticles showed more than four times longer blood circulation time than the parent siRNA in vivo. Robust gene silencing with both intra-tumor and systemic injection into tumors can also be accomplished without any detectable immune response. The macromolecules may also directly self-assemble as a container and serve as a versatile delivery nanoplatform. Chung, Kang, and coworkers developed protein-cage-based nanoparticles by the usage of ferritin protein cage isolated from the hyperthermophilic archaeon (Pyrococcus furiosus) [21]. The spherical cage possesses inner and outer diameters of 8 and 12 nm, respectively. Antibodies can be loaded into the nanocage without chemical modification. As antibodies offer an almost unlimited range of specific targeting moieties, their incorporation into the protein nanocages brings attractive targeting functions for effective targeted drug delivery and diagnosis [22].
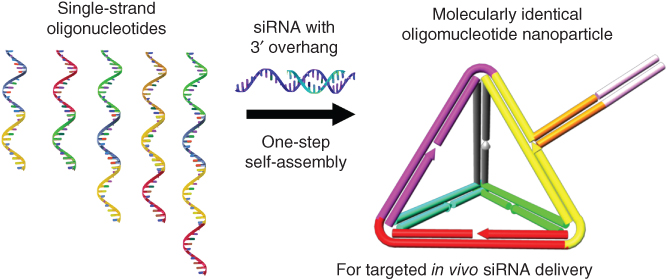
Figure 2.3 Programmable self-assembly of nucleic acid nanoparticles for targeted in vivo siRNA delivery.
(Adapted from Lee et al. 2012 [20].)
2.3 Hierarchical Architecturing of Solid Nanoparticles
The design of hierarchically structured nano- and microparticles of different sizes, porosities, surface areas, compositions, and internal structures is important for new or enhanced application properties of their applications in a variety of fields. Major progress has been made in recent years concerning the synthesis of hierarchical structured materials with tailored nanostructure, in particular due to the discovery in the 1990s of mesoporous silica. A large amount of work has been dedicated to the structural designs on the hierarchical architectures at nanometer dimension. Relying on the bottom-up designs, higher order architectures of inorganic or hybrid materials can also be constructed, which can be through the structural transcription, directed-assembly, templated-assembly, or layer-by-layer (LbL) assembly techniques. In this part, we mainly focus on the hierarchical structures of porous nanoparticles and layered nanoparticles.
2.3.1 Porous Nanoparticles
Porous materials play a very important role in the processes of molecular transport, adsorption, catalysis, and separation. The most popular and widely applicable method is template-directed assembly, which affords ordered materials in a range of length scales. According to the states of the templates being involved in the formation process of porous nanoparticles, they can be soft templates, in the process of which inorganic precursors co-assemble with the template molecules, and hard templates, in which inorganic precursors are covered on the templates with well-defined morphologies. With the precise control on the reaction conditions and the templates, a variety of mesostructures have been explored, including hexagonal, cage-like cubic, cubic bicontinuous, and platelet-ordered structures, and even quasicrystalline structures [23]. Besides the control on morphologies, the production process for industrial scale is also explored, which may greatly develop the applications of mesoporous nanoparticles in industrial applications [24]. Through adjusting the amount of small organic amines (SOAs) in the cetyltrimethylammonium (CTA+)-templated formation process, Zhang et al. successfully synthesized MSNs with diameters <130 nm and small pore sizes of 2–3 nm for the first time in kilogram scale and under mild reaction conditions. Various morphologies of stellate (ST), raspberry (RB), or wormlike (WO) could also be controlled. Owing to the soft particle binder of SOAs, the process allows the easy recovery, redispersion, and a high-yield large-scale production of MSNs.
Quasicrystals is a new class of ordered state with both noncrystallographic rotational symmetry and quasiperiodic translational symmetry. Their sharp Bragg peaks are distinct from nonequilibrium states such as glassy or amorphous materials. Recent advances in the fabrication of quasicrystals have increased the length scales into the mesoscale range (2–50 nm). The formation of templated mesoporous silicas by surfactant micelles can be tuned to produce mesoporous materials with quasicrystalline ordering. Xiao and Fujita et al. first reported the formation of hard-matter quasicrystals in mesoporous silica, which exhibited 12-fold (dodecagonal) symmetry in both electron diffraction and morphology [25]. Using an anionic surfactant system of N-myristoyl-l-glutamic acid (C14GluA), they obtained three crystal structures (space groups Pm3n, P42/mnm, and Cmmm) merely by varying the alkalinity in the synthesis [26]. The nonequilibrium nature of the growth process caused quasicrystalline tiling in mesoporous silicas and resulted in the formation of dodecagonal quasicrystals. The mechanism is expected to serve as a conceptual guide for synthesizing quasicrystals in a broader class of soft-matter systems as well.
Besides the design of the geometrical variations in the silica pore mesostructure, the synthesis of analogous multicompartment porous nanoparticles was also succeeded by the precise control on the growth process. Suteewong et al. reported a one-pot synthesis method for a class of MSNs containing both cubic and hexagonally structured compartments within one nanoparticle (Figure 2.4) [27]. Through adjusting the concentrations of ethyl acetate (EtOAc) in the hexadecyltrimethylammonium bromide (CTAB)-directed sol–gel synthesis, the charge state of silicate precursors can be varied, and may induce a structural change in the CTAB templates. A core with cage-like cubic mesoporous morphology and up to four branches with hexagonally packed cylindrical mesopores can be formed. The results suggest a pathway toward high levels of architectural complexity in locally amorphous, mesostructured nanoparticles, and could enable tuning of different pore environments of nanoparticles for specific applications.

Figure 2.4 Template-directed formation of multicompartment mesoporous silica nanoparticles with branched shapes.
Not only porous silica but the formation of other porous metal nanoparticles by templates has also attracted great interest, as metal nanoparticles may bring superior optical/electrical properties or excellent performance on the catalysis and sensing [28]. The porous features of metal nanoparticles are also essential to affect their application properties. It should be noted that in comparison with silica, metal precursors may react much faster, and have relatively worse compatibility with the structural- directing molecules. Therefore, to stabilize the complex structures of metal precursors and template molecules, polymers are likely chosen as the templates. Yamauchi and coworkers used a core–shell–corona-type triblock copolymer [poly(styrene-b-2-vinylpyridine-b-ethylene oxide), PS-b-P2VP-b-PEO] as the pore-directing agent for porous metal nanoparticles (Figure 2.5) [29]. Negatively charged PtCl42− ions can interact with the protonated P2VP+ blocks, while the free PEO chains prevent the aggregation of the Pt nanospheres. The size of the mesopores can be finely tuned by varying the length of the PS chain. This system can effectively control reactive domains in the polymer backbone and avoid aggregation of micelles during the synthesis. Homogeneous mesoporous Pt nanospheres with large pore size (35 nm) were successfully obtained and can be used as active electrocatalysts for methanol oxidation, which showed higher catalytic activity when compared with nonporous nanospheres or commercial Pt black. Mesoporous metal spheres with multimetallic compositions (Pt–Pd, Pt–Pd–Ru, etc.) may also successfully be synthesized by the core–shell–corona-type triblock copolymers. The selective binding effects from the polymer templates present a promising route for the design of superior porous metal materials.

Figure 2.5 Polymeric micelles for the synthesis of mesoporous platinum nanospheres.
Besides relying on the templates for directing the formation of pores in the structures, a self-templated strategy is also developed, where inorganic template particles themselves can occur as a dissolution-regrowth process under certain reaction conditions. The nanofeatures of structures may change after the regrowth and lead to more complex structures when compared with the morphology of the solid template. Ji et al. prepared cell-like flake-shell capsules from simple silica nanoparticles [30]. Spontaneous formation of flake-shell capsules occurred during a hydrothermal process (Figure 2.6). Gradual dissolution of silica from the surface of the nanoparticles and precipitation as silica nanosheets in the vicinity of the parent particle finally formed into hollow spherical capsules consisting of assembled silica nanosheets. The porous capsules showed advantages in the loading of various molecules (DNA, proteins, nanoparticles, small drug molecules). The networked structures also possess dynamically structural flexibility, which can respond to external stimuli and exhibit controlled release of drug molecules without needing surface modification. Although inorganic structures, the silica-nanosheet-assembled capsules also present flexibility somehow reminiscent of cells or other soft lipid assemblies.
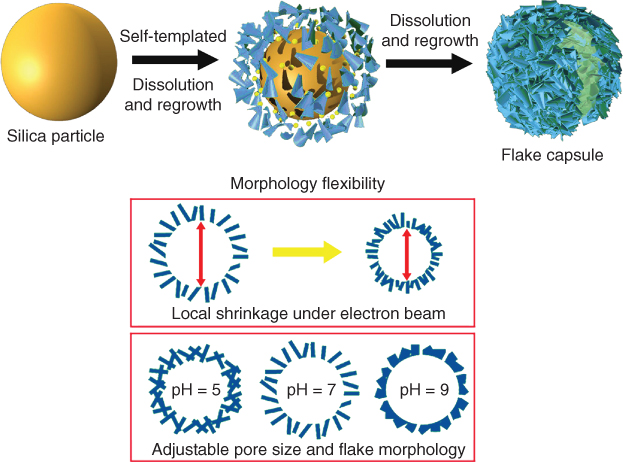
Figure 2.6 Self-templated formation of flake-shelled silica capsule with morphology flexibility to stimuli.
2.3.2 Layered Nanoparticles
In most of the nanoparticles mentioned, the formation of the morphologies is basically based on a one-pot synthetic process. However, the nanostructures of nanoparticles can be manipulated through alternatively more programmable pathways. LbL assembly is a versatile technology which has been widely employed for the layered nanoarchitectonics of various materials including organic polymers, biomaterials, inorganic substances, and supramolecular assemblies. The assembly structures that result may exhibit functions complementary or synergistic to those of the component layers. Most popular modes of LbL assembly are based on an electrostatic mechanism. Continuous assembly between positively and negatively charged materials affords layered architectures with a great freedom in the number of layers and their sequence. Similar processes can be extended to other interactions such as metal coordination, hydrogen bonding, covalent bonding, supramolecular inclusion, bio-specific recognition, charge-transfer complex formation, and stereo-complex formation [31].
In addition to the formation of LbL structures by solution dipping, various techniques like spin coating and spray drying can also be exerted to the facile LbL process. Decher, Voegel, and coworkers first developed the spray LbL technique for the successive ionic layer adsorption and reaction [32]. The spray drying strategy was used mainly for the formation of planar LbL films in the beginning, which attracted attention due to the dramatically speedy assembly process. In the process for multilayer films, polyelectrolytes and components were separately sprayed, either sequentially or simultaneously, on the supporting substrate. DeSimone, Hammond, and coworkers further optimized this method for imparting function on nanoparticles via a rapid, scalable, and high-throughput way (Figure 2.7) [33]. By using the particle replication in non-wetting templates (PRINT) process, PRINT particles were first fabricated and stored as particle arrays on the harvesting layer. After the arrays were cross-linked under vapor-phase glutaraldehyde/concentrated acid treatment, sequential sprays were applied for the LbL cycles of polycation-wash–polyanion-wash on the arrays. The functionalized particles were finally collected by sonication of the arrays in water. The PRINT particles coated with hyaluronic acid were demonstrated to achieve targeted interactions with CD44 receptors and were found in high levels on aggressive breast cancer cells. This spray-assisted LbL process has provided an exciting platform for large-scale production of built-to-order functional nanoparticle systems. It can achieve exquisite control over particle geometry (size, shape) and composition (cargo, carrier system), which may optimize the important parameters of nanoparticles for biomedicine applications.

Figure 2.7 Scalable and rapid fabrication of functionalized particles by spray-assisted layer-by-layer PRINT process.
(Adapted from Morton et al. 2013 [33].)
There is no doubt that LbL technology allows an extreme control over capsule properties and to introduce multifunctionality into drug delivery devices at a scale unmet in a much easier way. Besides the polymer-based nanoparticles, the versatile LbL technique may be also exerted for pure inorganic nanoparticles. Upconversion nanoparticles (UCNPs) are a new generation of fluorophores which can convert near-IR radiations into visible radiations via a nonlinear optical process [34]. They can overcome some of the limitations of conventional bio-labels including organic dyes and quantum dots. With the properties of low toxicity and high signal-to-noise ratio, they show great potential for highly complex bioimaging applications both in vitro and in vivo. The one-pot heating-up method is one of the most commonly used ways to synthesize the UCNPs (H-UCNPs), which may easily cause unhomogeneous local relative distribution of the rare earth dopants in the structures. Zhao, Zhang, and coworkers developed the homogeneous doping approach based on the successive layer-by-layer method (SLbL) (Figure 2.8) [35]. At the surface of the NaGdF4:Yb,Er initial seeds, Gd, Yb, Er ions were introduced into the shells by a heterogeneous radial growth process. This SLbL protocol resulted in the formation of uniform UCNPs with homogeneous distribution of dopant ions and controllable doping concentration, which may reduce the cross-relaxation and facilitate the energy transfer between dopant ions. Therefore, the overall upconversion luminescence emission intensity and lifetime of the SLbL–UCNPs were remarkably enhanced compared with that of the H-UCNPs.
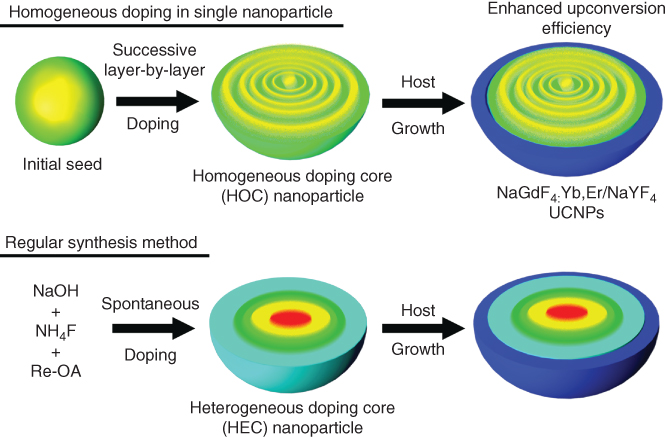
Figure 2.8 Engineering homogeneous doping in upconversion nanoparticles by successive layer-by-layer process.
(Adapted from Wang et al. 2014 [35].)
The LbL technique provides a high level of control over individual particle variables and presents an exciting paradigm for the multilayered particles with functionalities. Through increasing interdisciplinary researches, it will undoubtedly accelerate the development of the next generation of nanoparticles for various applications.
2.4 Janus (Asymmetric) Nanoparticles
Colloidal particles are fundamental to nature and technology. In terms of length scale, they bridge small molecules (Å), which can only be approached by modern tools of electron microscopy, and big objects (100 µm), which can be easily observed by the human eye. Janus particles are colloid-sized particles which possess two regions of different surface chemical composition. De Gennes first named “Janus particles” in his Nobel Prize address. He borrowed the name “Janus” from the Roman God who has two faces looking in opposite directions to describe this special class of nanoparticles [36]. These Janus particles could be very useful in controlling molecular recognition and self-assembly processes, which are some of the more intriguing and challenging aspects of current materials science [37]. The development of chemical synthetic methods now provides access to Janus particles with a wide range of sizes, shapes, and materials.
Many strategies have been developed for the fabrication of Janus particles with diverse functionalities and a scale-up production quantity [38]. The approaches include surface sputtering or coating, Pickering emulsions, biphasic electrified jetting, or techniques exploiting phase separation in confined spaces. The key synthesis procedure is partial masking of the surfaces of the particles prior to selective engineering and functionalization of the exposed particle surfaces. The most challenging is the precision engineering on the smaller side of the Janus particle.
Despite the direct fabrication purely via self-assembly, various techniques such as consecutive transformation, microcontact printing, and so on are developed for the manipulation of the morphologies of Janus particles [2d, 39]. Gröschel et al. conceived a versatile and simple pathway toward large amounts of Janus micelles with tunable Janus balance based on selective cross-linking of multicompartment micelles (MCMs) from linear ABC triblock terpolymers (Figure 2.9) [40]. Depending on the volume ratios of A and B (VA/VB) and the ratio of the corona-forming block (NC/(NA + NB)), different kinds of spherical core-compartmentalized multicompartment micelles (core-MCM) could be formed in a selective solvent for the C block. This concept was successfully applied to a wide range of triblock terpolymers and for different MCM architectures as well. It has emerged as a straightforward, robust, and versatile procedure, even directly suitable for further transfer into technologies.
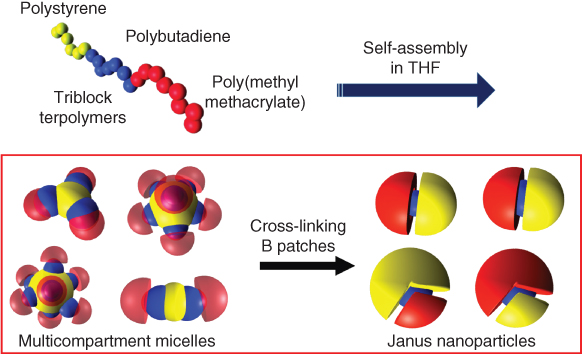
Figure 2.9 The formation of soft, nanoscale Janus particles with tunable Janus balance by triblock terpolymers.
(Adapted from Gröschel et al. 2012 [40].)
Asymmetric particles from metals have also attracted great interest, since the combinations of two or more different metal phases may bring collective effects on magnetic, electrical, semiconductive, optical, optoelectronic, or catalytic properties. Like that of polymeric Janus particles, the fabrication of inorganic asymmetric particles also requires partial coverage of the surfaces of metal particles. Acharya and coworkers designed ultrasmall asymmetric nanostructures through selective deposit Au at the ends of PbS nanorods of 12 nm [41]. The preferential adsorption of Au that occurs at the rod tips is due to their higher reactivity with the sulfur terminals at the mixed {100} planes of PbS nanorods. The deposition of Au probes at the tips of nanorods modifies the nature and energies of PbS rod orbitals, which forms efficient electron conduction channels for electronic transport along the rods. Such a modified metal interface should have paramount importance on different materials combinations and promote technological advancement of transport-based devices in sub-nanometer scale [42].
Despite extensive progress in asymmetric particles of polymers, metals, or hybrids, there is still an urgent need for the development of more effective synthetic protocols and creation of more complicated nanoparticles. The precise manipulation of the asymmetry architectures will have a significant implication for nanoparticle-controlled assembly as well as spatially selective functionalizations for various applications.
2.5 Functional Architectures on the Surface of Nanoparticles
Controlling surface functionalities at the nanometer scale is a critical issue for improving the performance of nanoparticles in applications. The surface functionalization may not only alter the surface characteristics (wetting or adhesion properties) but also bring about new properties of nanoparticles in sensing, imaging, catalysis, and targeting [2c, 3c, 43]. The creation of specific surface architectures on nanoparticles for selective response to external stimuli has been considered as a promising approach for novel drug carriers, nanosensors, bioprobes, photocatalysts, optoelectronics devices, and so on.
Different nanoparticles, including metals, metal oxides, carbon, polymers, and mesoporous silica, can adapt surface architectures by covalent or non-covalent bonding with different nanoscaled organic and inorganic materials on the surface. The unlimited combinations of the distinct properties of inorganic, organic, or even bioactive components have attracted considerable attention. So far, many novel advanced functionalized nanoparticles with well-controlled surface architectures and multiple functions have been created and applied in various fields.
The non-covalent construction of surface architectures on nanoparticles is mainly based on the LbL technique, as mentioned. Polymers, nanostructures, and biologically relevant materials can be deposited on the surface of solid spheres with designable layer orders. Hammond and coworkers ultilized a trilayer architecture of poly-l-lysine (PLL) modified with iminobiotin, the linker protein neutravidin and biotin-end-functionalized poly(ethylene glycol) (PEG) on the polystyrene (PS) nanoparticle [44]. This surface design made the nanoparticles gain tumor cell selectivity via the erosion of LbL layers. The PLL layer improves cellular uptake of nanoparticles. The neutravidin layer can be easily decomposed at pH 4–6 (tumor environment). The PEG layer acts as an antifouling polymer to avoid rapid reticuloendothelial system (RES) clearance and thus allows the accumulation of nanoparticles in tumor interstitials.
A more stable and wider choice of surface architectures can be introduced on the surface of nanoparticles by covalent interactions. The marriage of organic and materials chemistry can lead to the birth of more novel hybrid nanostructures and combine the beneficial characteristics and properties of both core materials and surface functional components. The supermolecular architectures on nanoparticles can be used as switches to modulate reversibly the range of properties like optical, fluorescent, electrical, magnetic, and also control the release of drug molecules [45]. Mattoussi and coworkers reported the control on the architecture, coordination, and reactivity of luminescent quantum dots (QDs) by an amino acid central scaffold (Figure 2.10) [46]. Utilizing l-aspartic acid, they designed a versatile platform that allowed the controllable coupling of one or more lipoic acid (LA) groups, one or more PEG moieties, along with terminal reactive groups. These ligands with various architectures and selective reactivity were then capped on the surface of gold nanoparticles and QDs. The nanoparticles ligated with bis(LA)-PEG exhibit remarkable colloidal stability over a broad range of biological conditions. As reactive groups including azide, amine, and carboxylic acid can be easily introduced into the ligands, the functionalized QDs conjugated with biomolecules (transferrin protein, peptide, etc.) could also be achieved. These surface cappings on QDs or AuNPs were shown to promote energy transfer interactions and cellular uptake; therefore, the ligand-covered nanoparticles have great promise in biosensing and imaging applications.
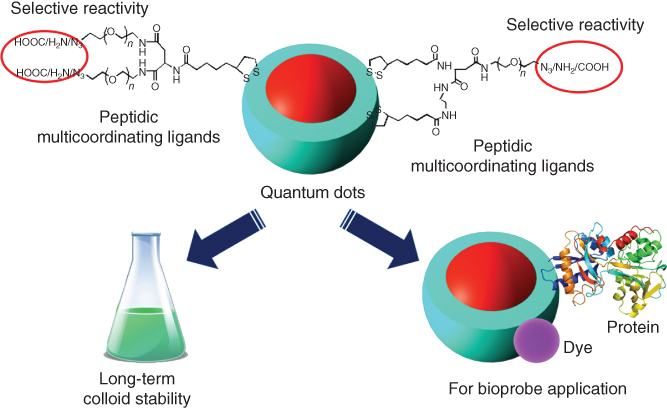
Figure 2.10 Control of the surface architecture and reactivity of nanoparticles by peptidic multicoordinating ligands on surface.
(Adapted from Zhan et al. 2015 [46].)
2.6 Summary
The creation of complex nano- and microparticles attracts not only fundamental interest but also has practical significance in fields such as biomedicine, optics, catalysis, and electronics. The concepts of architecting for the design of novel complex nanostructures are developing rapidly. The breakthrough applications lie in the exploitation of unique or organized structures. There are certainly many possibilities in architectonics, which could be further discovered in the near future. Here, we intend to present a brief but comprehensive introduction on the relevant advances in synthesis, the combined collective properties and applications from architectured nanoparticles. We focus on certain relevant materials and architectured structures. It should provide a knowledge base on the architectonics in nanoparticles. It may also promote the scientists to open new directions for future cross-disciplinary developments. Although fantastic and fast progress has been achieved in the past years, many questions remain unsolved and, therefore, many challenges still exist. We expect more progress in the development of new synthesis concepts for more highly defined architectures of nanoparticles, facile synthetic strategies for future nanotechnologies, and new application areas.
References
- 1 (a) Nie, Z., Petukhova, A., and Kumacheva, E. (2010) Properties and emerging applications of self-assembled structures made from inorganic nanoparticles. Nat. Nanotechnol., 5, 15–25;(b) Giljohann, D.A., Seferos, D.S., Daniel, W.L., Massich, M.D., Patel, P.C., and Mirkin, C.A. (2010) Gold nanoparticles for biology and medicine. Angew. Chem. Int. Ed., 49, 3280–3294.
- 2 (a) Grzelczak, M., Vermant, J., Furst, E.M., and Liz-Marzan, L.M. (2010) Directed self-assembly of nanoparticles. ACS Nano, 4, 3591–3605;(b) Schärtl, W. (2010) Current directions in core–shell nanoparticle design. Nanoscale, 2, 829–843;(c) Klajn, R., Stoddart, J.F., and Grzybowski, B.A. (2010) Nanoparticles functionalised with reversible molecular and supramolecular switches. Chem. Soc. Rev., 39, 2203–2237;(d) Gröschel, A.H., Walther, A., Löbling, T.I., Schacher, F.H., Schmalz, H., and Müller, A.H. (2013) Guided hierarchical co-assembly of soft patchy nanoparticles. Nature, 503, 247–251.
- 3 (a) Ariga, K., Ji, Q., McShane, M.J., Lvov, Y.M., Vinu, A., and Hill, J.P. (2012) Inorganic nanoarchitectonics for biological applications. Chem. Mater., 24, 728–737;(b) Lu, Z.D. and Yin, Y.D. (2012) Colloidal nanoparticle clusters: functional materials by design. Chem. Soc. Rev., 41, 6874–6887;(c) Kao, J., Thorkelsson, K., Bai, P., Rancatore, B.J., and Xu, T. (2013) Toward functional nanocomposites: taking the best of nanoparticles, polymers, and small molecules. Chem. Soc. Rev., 42, 2654–2678.
- 4 (a) Grzybowski, B.A. and Huck, W.T.S. (2016) The nanotechnology of life-inspired systems. Nat. Nanotechnol., 11, 585–592;(b) Maggini, L. and Bonifazi, D. (2012) Hierarchised luminescent organic architectures: design, synthesis, self-assembly, self-organisation and functions. Chem. Soc. Rev., 41, 211–241;(c) Yan, W., Xu, L., Xu, C., Ma, W., Kuang, H., Wang, L., and Kotov, N.A. (2012) Self-assembly of chiral nanoparticle pyramids with strong R/S optical activity. J. Am. Chem. Soc., 134, 15114–15121.
- 5 (a) Tanner, P., Baumann, P., Enea, R., Onaca, O., Palivan, C., and Meier, W. (2011) Polymeric vesicles: from drug carriers to nanoreactors and artificial organelles. Acc. Chem. Res., 44, 1039–1049;(b) Elsabahy, M. and Wooley, K.L. (2012) Design of polymeric nanoparticles for biomedical delivery applications. Chem. Soc. Rev., 41, 2545–2561.
- 6 (a) Cheng, R., Meng, F., Deng, C., Klok, H.-A., and Zhong, Z. (2013) Dual and multi-stimuli responsive polymeric nanoparticles for programmed site-specific drug delivery. Biomaterials, 34, 3647–3657;(b) Crucho, C.I.C. (2015) Stimuli-responsive polymeric nanoparticles for nanomedicine. ChemMedChem, 10, 24–38;(c) Stuart, M.A., Huck, W.T., Genzer, J., Müller, M., Ober, C., Stamm, M., Sukhorukov, G.B., Szleifer, I., Tsukruk, V.V., Urban, M., Winnik, F., Zauscher, S., Luzinov, I., and Minko, S. (2010) Emerging applications of stimuli-responsive polymer materials. Nat. Mater., 9, 101–113;(d) Hu, J., Zhang, G., and Liu, S. (2012) Enzyme-responsive polymeric assemblies, nanoparticles and hydrogels. Chem. Soc. Rev., 41, 5933–5949;(e) An, X., Zhu, A., Luo, H., Ke, H., Chen, H., and Zhao, Y. (2016) Rational design of multi-stimuli-responsive nanoparticles for precise cancer therapy. ACS Nano, 10, 5947–5958.
- 7 Zhao, Y., Shi, C., Yang, X., Shen, B., Sun, Y., Chen, Y., Xu, X., Sun, H., Yu, K., Yang, B., and Lin, Q. (2016) pH- and temperature-sensitive hydrogel nanoparticles with dual photoluminescence for bioprobes. ACS Nano, 10, 5856–5863.
- 8 Mahmoud, E.A., Sankaranarayanan, J., Morachis, J.M., Kim, G., and Almutairi, A. (2011) Inflammation responsive logic gate nanoparticles for the delivery of proteins. Bioconjugate Chem., 22, 1416–1421.
- 9 (a) Cheng, L., Wang, C., Feng, L.Z., Yang, K., and Liu, Z. (2014) Functional nanomaterials for phototherapies of cancer. Chem. Rev., 114, 10869–10939;(b) Gohy, J.F. and Zhao, Y. (2013) Photo-responsive block copolymer micelles: design and behavior. Chem. Soc. Rev., 42, 7117–7129;(c) Jochum, F.D. and Theato, P. (2013) Temperature- and light-responsive smart polymer materials. Chem. Soc. Rev., 42, 7468–7483.
- 10 (a) Vincent, B. (1995) Electrically conducting polymer colloids and composites. Polym. Adv. Technol., 6, 356–361;(b) McQuade, D.T., Pullen, A.E., and Swager, T.M. (2000) Conjugated polymer-based chemical sensors. Chem. Rev., 100, 2537–2574;(c) Thomas, S.W. III, Joly, G.D., and Swager, T.M. (2007) Chemical sensors based on amplifying fluorescent conjugated polymers. Chem. Rev., 107, 1339–1386.
- 11 (a) Pecher, J. and Mecking, S. (2010) Nanoparticles of conjugated polymers. Chem. Rev., 110, 6260–6279;(b) Tuncel, D. and Demir, H.V. (2010) Conjugated polymer nanoparticles. Nanoscale, 2, 484–494.
- 12 Jiang, B.-P., Guo, D.-S., Liu, Y.-C., Wang, K.-P., and Liu, Y. (2014) Photomodulated fluorescence of supramolecular assemblies of sulfonatocalixarenes and tetraphenylethene. ACS Nano, 8, 1609–1618.
- 13 (a) Howes, P., Green, M., Bowers, A., Levitt, J., Suhling, K., and Hughes, M. (2010) Phospholipid encapsulated semiconducting polymer nanoparticles: their use in cell imaging and protein attachment. J. Am. Chem. Soc., 132, 3989–3996;(b) Fisslthaler, E., Sax, S., Scherf, U., Mauthner, G., Moderegger, E., Landfester, K., and List, E.J.W. (2008) Inkjet printed polymer light emitting devices fabricated by thermal embedding of semiconducting polymer nanospheres in an inert matrix. Appl. Phys. Lett., 92, 183305;(c) Huebner, C.F., Roeder, R.D., and Foulger, S.H. (2009) Nanoparticle electroluminescence: controlling emission color through Forster resonance energy transfer in hybrid particles. Adv. Funct. Mater., 19, 3604–3609;(d) Landfester, K. (2001) The generation of nanoparticles in miniemulsions. Adv. Mater., 13, 765–768.
- 14 Park, E.-J., Erdem, T., Ibrahimova, V., Nizamoglu, S., Demir, H.V., and Tuncel, D. (2011) White-emitting conjugated polymer nanoparticles with cross-linked shell for mechanical stability and controllable photometric properties in color-conversion LED applications. ACS Nano, 5, 2483–2492.
- 15 (a) Whitesides, G.M. and Grzybowski, B. (2002) Self-assembly at all scales. Science, 295, 2418–2421;(b) Zhang, S. (2003) Building from the bottom up. Mater. Today, 6, 20–27.
- 16 (a) Zhang, C., Wang, W., Liu, T., Wu, Y., Guo, H., Wang, P., Tian, Q., Wang, Y., and Yuan, Z. (2012) Doxorubicin-loaded glycyrrhetinic acid-modified alginate nanoparticles for liver tumor chemotherapy. Biomaterials, 33, 2187–2196;(b) Guo, P. (2010) The emerging field of RNA nanotechnology. Nat. Nanotechnol., 5, 833–842;(c) Khaled, A., Guo, S., Li, F., and Guo, P. (2005) Controllable self-assembly of nanoparticles for specific delivery of multiple therapeutic molecules to cancer cells using RNA nanotechnology. Nano Lett., 5, 1797–1808;(d) Uchida, M., Kosuge, H., Terashima, M., Willits, D.A., Liepold, L.O., Young, M.J., McConnell, M.V., and Douglas, T. (2011) Protein cage nanoparticles bearing the LyP-1 peptide for enhanced imaging of macrophage-rich vascular lesions. ACS Nano, 5, 2493–2502.
- 17 (a) Janib, S.M., Moses, A.S., and MacKay, J.A. (2010) Imaging and drug delivery using theranostic nanoparticles. Adv. Drug Delivery Rev., 62, 1052–1063;(b) Al-Jamal, W. and Kostarelos, K. (2011) Liposomes: from a clinically established drug delivery system to a nanoparticle platform for theranostic nanomedicine. Acc. Chem. Res., 44, 1094–1104.
- 18 Van den Bossche, J., Al-Jamal, W.T., Yilmazer, A., Bizzarri, E., Tian, B., and Kostarelos, K. (2011) Intracellular trafficking and gene expression of pH-sensitive, artificially enveloped adenoviruses in vitro and in vivo. Biomaterials, 32, 3085–3093.
- 19 (a) Petros, R.A. and DeSimone, J.M. (2010) Strategies in the design of nanoparticles for therapeutic applications. Nat. Rev. Drug Discovery, 9, 615–627;(b) Choi, H.S., Liu, W., Liu, F., Nasr, K., Misra, P., Bawendi, M.G., and Frangioni, J.V. (2010) Design consideration for tumour-targeted nanoparticles. Nat. Nanotechnol., 5, 42–47;(c) Weissleder, R., Kelly, K., Sun, E.Y., Shtatland, T., and Josephson, L. (2005) Cell-specific targeting of nanoparticles by multivalent attachment of small molecules. Nat. Biotechnol., 23, 1418–1423;(d) Lv, H., Zhang, S., Wang, B., Cui, S., and Yan, J. (2006) Toxicity of cationic lipids and cationic polymers in gene delivery. J. Controlled Release, 114, 100–109.
- 20 Lee, H., Lytton-Jean, A.K.R., Chen, Y., Love, K.T., Park, A.I., Karagiannis, E.D., Sehgal, A., Querbes, W., Zurenko, C.S., Jayaraman, M., Peng, C.G., Charisse, K., Borodovsky, A., Manoharan, M., Donahoe, J.S., Truelove, J., Nahrendorf, M., Langer, R., and Anderson, D.G. (2012) Molecular self-assembly nucleic acid nanoparticles for targeted in vivo siRNA delivery. Nat. Nanotechnol., 7, 389–393.
- 21 Kang, H.J., Kang, Y.J., Lee, Y.-M., Shin, H.-H., Chung, S.J., and Kang, S. (2012) Developing an antibody-binding protein cage as a molecular recognition drug modular nanoplatform. Biomaterials, 33, 5423–5430.
- 22 (a) Carter, P. (2001) Improving the efficacy of antibody-based cancer therapies. Nat. Rev. Cancer, 1, 118–129;(b) Allen, T.M. (2002) Ligand-targeted therapeutics in anticancer therapy. Nat. Rev. Cancer, 2, 750–763.
- 23 (a) Malgras, V., Ji, Q., Kamachi, Y., Mori, T., Shieh, F.-K., Wu, K.C.-W., Ariga, K., and Yamauchi, Y. (2015) Templated synthesis for nanoarchitectured porous materials. Bull. Chem. Soc. Jpn., 88, 1171–1200;(b) Liu, Y., Goebl, J., and Yin, Y. (2013) Templated synthesis of nanostructured materials. Chem. Soc. Rev., 42, 2610–2653.
- 24 Zhang, K., Xu, L.-L., Jiang, J.-G., Calin, N., Lam, K.-F., Zhang, S.-J., Wu, H.-H., Wu, G.-D., Albela, B., Bonneviot, L., and Wu, P. (2013) Facile large-scale synthesis of monodisperse mesoporous silica nanospheres with tunable pore structure. J. Am. Chem. Soc., 135, 2427–2430.
- 25 Xiao, C., Fujita, N., Miyasaka, K., Sakamoto, Y., and Terasaki, O. (2012) Dodecagonal tiling in mesoporous silica. Nature, 487, 349–353.
- 26 Frank, F.C. and Kasper, J.S. (1959) Complex alloy structures regarded as sphere packings. II. Analysis and classification of representative structures. Acta Crystallogr., 12, 483–499.
- 27 (a) Suteewong, T., Sai, H., Hovden, R., Muller, D., Bradbury, M.S., Gruner, S.M., and Wiesner, U. (2013) Multicompartment mesoporous silica nanoparticles with branched shapes: an epitaxial growth mechanism. Science, 340, 337–341;(b) Suteewong, T., Sai, H., Cohen, R., Wang, S., Bradbury, M., Baird, B., Gruner, S.M., and Wiesner, U. (2011) Highly aminated mesoporous silica nanoparticles with cubic pore structure. J. Am. Chem. Soc., 133, 172–175;(c) Suteewong, T., Sai, H., Bradbury, M., Estroff, L.A., Gruner, S.M., and Wiesner, U. (2012) Synthesis and formation mechanism of aminated mesoporous silica nanoparticles. Chem. Mater., 24, 3895–3905.
- 28 (a) Zhang, J. and Li, C.M. (2012) Nanoporous metals: fabrication strategies and advanced electrochemical applications in catalysis, sensing and energy systems. Chem. Soc. Rev., 41, 7016–7031;(b) Valtchev, V. and Tosheva, L. (2013) Porous nanosized particles: preparation, properties, and applications. Chem. Rev., 113, 6734–6760;(c) Yang, S. and Luo, X. (2014) Mesoporous nano/micro noble metal particles: synthesis and application. Nanoscale, 6, 4438–4457;(d) Song, H. (2015) Metal hybrid nanoparticles for catalytic organic and photochemical transformations. Acc. Chem. Res., 48, 491–499.
- 29 (a) Li, Y., Bastakoti, B.P., Malgras, V., Li, C., Tang, J., Kim, J.H., and Yamauchi, Y. (2015) Polymeric micelle assembly for the smart synthesis of mesoporous platinum nanospheres with tunable pore sizes. Angew. Chem. Int. Ed., 54, 11073–11077;(b) Jiang, B., Ataee-Esfahani, H., Li, C.L., Alshehri, S.M., Ahamad, T., Henzie, J., and Yamauchi, Y. (2016) Mesoporous trimetallic PtPdRu spheres as superior electrocatalysts. Chem. Eur. J., 22, 7174–7178;(c) Li, Y.Q., Li, C.L., Bastakoti, B.P., Tang, J., Jiang, B., Kim, J., Shahabuddin, M., Bando, Y., Kim, J.H., and Yamauchi, Y. (2016) Strategic synthesis of mesoporous Pt-on-Pd bimetallic spheres templated from a polymeric micelle assembly. J. Mater. Chem. A, 4, 9169–9176;(d) Jiang, B., Li, C.L., Imura, M., Tang, J., and Yamauchi, Y. (2015) Multimetallic mesoporous spheres through surfactant-directed synthesis. Adv. Sci., 2, 1500112.
- 30 (a) Ji, Q., Guo, C., Yu, X., Ochs, C., Hill, J.P., Caruso, F., Nakazawa, H., and Ariga, K. (2012) Flake-shell capsules: adjustable inorganic structures. Small, 8, 2345–2349;(b) Manoharan, Y., Ji, Q., Yamazaki, T., Chinnathambi, S., Chen, S., Singaravelu, G., Hill, J.P., Ariga, K., and Hanagata, N. (2012) Effect of molecular weight of polyethyleneimine on loading of CpGO ligodeoxynucleotides onto flake-shell silica nanoparticles for enhanced TLR9-mediated induction of interferon-alpha. Int. J. Nanomed., 7, 3625–3635;(c) Ji, Q., Hill, J.P., and Ariga, K. (2013) Shell-adjustable hollow “soft” silica spheres as a support for gold nanoparticles. J. Mater. Chem. A, 1, 3600–3606;(d) Ji, Q., Ishihara, S., Terentyeva, T.G., Deguchi, K., Ohki, S., Tansho, M., Shimizu, T., Hill, J.P., and Ariga, K. (2015) Manipulation of shell morphology of silicate spheres from structural evolution in a purely inorganic system. Chem. Asian J., 10, 1379–1386.
- 31 (a) Ariga, K., Yamauchi, Y., Rydzek, G., Ji, Q., Yonamine, Y., Wu, K.C.-W., and Hill, J.P. (2014) Layer-by-layer nanoarchitectonics: invention, innovation, and evolution. Chem. Lett., 43, 36–68;(b) Becker, A.L., Johnston, A.P.R., and Caruso, F. (2010) Layer-by-layer-assembled capsules and films for therapeutic delivery. Small, 6, 1836–1852.
- 32 Popa, G., Boulmedais, F., Zhao, P., Hemmerlé, J., Vidal, L., Mathieu, E., Félix, O., Schaaf, P., Decher, G., and Voegel, J.-C. (2010) Nanoscale precipitation coating: the deposition of inorganic films through step-by-step spray-assembly. ACS Nano, 4, 4792–4798.
- 33 Morton, S.W., Herlihy, K.P., Shopsowitz, K.E., Deng, J., Chu, K.S., Bowerman, C.J., DeSimone, J.M., and Hammond, P.T. (2013) Scalable manufacture of built-to-order nanomedicine: spray-assisted layer-by-layer functionalization of PRINT® nanoparticles. Adv. Mater., 25, 4707–4713.
- 34 (a) Haase, M. and Schaefer, H. (2011) Upconverting nanoparticles. Angew. Chem. Int. Ed., 50, 5808–5829;(b) Chen, G., Qiu, H., and Prasad, P.N. (2014) Upconversion nanoparticles: design, nanochemistry, and applications in theranostics. Chem. Rev., 114, 5156–5214;(c) Zhou, J., Liu, Q., Feng, W., Sun, Y., and Li, F. (2015) Upconversion luminescent materials: advances and applications. Chem. Rev., 115, 395–465;(d) Park, Y.I., Lee, K.T., Suh, Y.D., and Hyeon, T. (2015) Upconverting nanoparticles: a versatile platform for wide-field two-photon microscopy and multi-modal in vivo imaging. Chem. Soc. Rev., 44, 1302–1317.
- 35 Li, X., Wang, R., Zhang, F., and Zhao, D. (2014) Engineering homogeneous doping in single nanoparticle to enhance upconversion efficiency. Nano Lett., 14, 3634–3639.
- 36 de Gennes, P.G. (1992) Soft matter. Science, 256, 495–497.
- 37 (a) Jiang, S., Chen, Q., Tripathy, M., Luijten, E., Schweizer, K.S., and Granick, S. (2010) Janus particle synthesis and assembly. Adv. Mater., 22, 1060–1071;(b) Du, J. and O'Reilly, R.K. (2011) Anisotropic particles with patchy, multicompartment and Janus architectures: preparation and application. Chem. Soc. Rev., 40, 2402–2416;(c) Lattuada, M. and Hatton, T.A. (2011) Synthesis, properties and applications of Janus nanoparticles. Nano Today, 6, 286–308;(d) Kim, S.-H., Lim, J.-M., Lee, S.-K., Heo, C.-J., and Yang, S.-M. (2010) Biofunctional colloids and their assemblies. Soft Matter, 6, 1092–1110;(e) Li, F., Josephson, D.P., and Stein, A. (2011) Colloidal assembly: the road from particles to colloidal molecules and crystals. Angew. Chem. Int. Ed., 50, 360–388.
- 38 (a) Hu, J., Zhou, S., Sun, Y., Fang, X., and Fabrication, L.W. (2012) Properties and applications of Janus particles. Chem. Soc. Rev., 41, 4356–4378;(b) Walther, A. and Müller, A.H.E. (2013) Janus particles: synthesis, self-assembly, physical properties, and applications. Chem. Rev., 113, 5194–5261;(c) Higuchi, T., Tajima, A., Motoyoshi, K., Yabu, H., and Shimomura, M. (2008) Frustrated phases of block copolymers in nanoparticles. Angew. Chem. Int. Ed., 47, 8044–8046.
- 39 (a) Gröschel, A.H., Schacher, F.H., Schmalz, H., Borisov, O.V., Zhulina, E.B., Walther, A., and Müller, A.H.E. (2012) Precise hierarchical self-assembly of multi compartment micelles. Nat. Commun., 3, 710;(b) Jiang, S. and Granick, S.A. (2009) Simple method to produce trivalent colloidal particles. Langmuir, 25, 8915–8918.
- 40 Gröschel, A.H., Walther, A., Löbling, T.I., Schmelz, J., Hanisch, A., Schmalz, H., and Müller, A.H.E. (2012) Facile, solution-based synthesis of soft, nanoscale Janus particles with tunable Janus balance. J. Am. Chem. Soc., 134, 13850–13860.
- 41 Khan, A.H., Ji, Q., Ariga, K., Das, B., Sarma, D.D., and Acharya, S. (2011) Synthesis and metallic probe induced conductance of Au tipped ultranarrow PbS rods. Chem. Commun., 47, 8421–8423.
- 42 (a) Liang, S., Liu, X.-L., Yang, Y.-Z., Wang, Y.-L., Wang, J.-H., Yang, Z.-J., Wang, L.-B., Jia, S.-F., Yu, X.-F., Zhou, L., Wang, J.-B., Zeng, J., Wang, Q.-Q., and Zhang, Z. (2012) Symmetric and asymmetric Au-AgCdSe hybrid nanorods. Nano Lett., 12, 5281–5286;(b) Seh, Z.W., Liu, S., Zhang, S.-Y., Bharathi, M.S., Ramanarayan, H., Low, M., Shah, K.W., Zhang, Y.-W., and Han, M.-Y. (2011) Anisotropic growth of titania onto various gold nanostructures: synthesis, theoretical understanding, and optimization for catalysis. Angew. Chem. Int. Ed., 50, 10140–10143.
- 43 (a) Oh, J.-H., Park, D.H., Joo, J.H., and Lee, J.-S. (2015) Recent advances in chemical functionalization of nanoparticles with biomolecules for analytical applications. Anal. Bioanal. Chem., 407, 8627–8645;(b) Sedlmeier, A. and Gorris, H.H. (2015) Surface modification and characterization of photon-upconverting nanoparticles for bioanalytical applications. Chem. Soc. Rev., 44, 1526–1560.
- 44 (a) Poon, Z., Chang, D., Zhao, X., and Hammond, P.T. (2011) Layer-by-layer nanoparticles with a pH-sheddable layer for in vivo targeting of tumor hypoxia. ACS Nano, 4, 4284–4294;(b) Dreaden, E.C., Morton, S.W., Shopsowitz, K.E., Choi, J.-H., Deng, Z.J., Cho, N.-J., and Hammond, P.T. (2014) Bimodal tumor-targeting from microenvironment responsive hyaluronan layer-by-layer (LbL) nanoparticles. ACS Nano, 8, 8374–8382;(c) Wen, C.-Y., Wu, L.-L., Zhang, Z.-L., Liu, Y.-L., Wei, S.-Z., Hu, J., Tang, M., Sun, E.-Z., Gong, Y.-P., Yu, J., and Pang, D.-W. (2014) Quick-response magnetic nanospheres for rapid, efficient capture and sensitive detection of circulating tumor cells. ACS Nano, 8, 941–949.
- 45 (a) Lai, C.Y., Trewyn, B.G., Jeftinija, D.M., Jeftinija, K., Xu, S., Jeftinija, S., and Lin, V.S.Y. (2003) A mesoporous silica nanosphere-based carrier system with chemically removable CdS nanoparticle caps for stimuli-responsive controlled release of neurotransmitters and drug molecules. J. Am. Chem. Soc., 125, 4451–4459;(b) Lin, L.-S., Cong, Z.-X., Cao, J.-B., Ke, K.-M., Peng, Q.-L., Gao, J., Yang, H.-H., Liu, G., and Chen, X. (2014) Multifunctional Fe3O4@polydopamine core–shell nanocomposites for intracellular mRNA detection and imaging-guided photothermal therapy. ACS Nano, 8, 3876–3883.
- 46 Zhan, N., Palui, G., Kapur, A., Palomo, V., Dawson, P.E., and Mattoussi, H. (2015) Controlling the architecture, coordination, and reactivity of nanoparticle coating utilizing an amino acid central scaffold. J. Am. Chem. Soc., 137, 16084–16097.
