Chapter 19
Diagnostics
Mitsuhiro Ebara
International Research Center for Materials Nanoarchitectonics (WPI-MANA), National Institute for Materials Science (NIMS), 1-1 Namiki, Tsukuba, 305-0044, Japan
19.1 Introduction
Early detection of infectious diseases plays a crucial role in all treatment and prevention strategies because rapid and accurate identification of the underlying agent using diagnostic testing is essential to select the correct control measure, such as containment, confinement, antimicrobials, and vaccines. However, many infections remain undetected worldwide due to poor diagnostic tools. In those areas, infection is often undiagnosed and therefore untreated, or diagnosed at the late stage when treatment becomes less effective. Infectious diseases are leading causes of mortality and morbidity globally [1, 2]. Pathogen detection can utilize tests such as enzyme-linked immunosorbent assay (ELISA). ELISA can detect disease-specific protein biomarkers in human bodily fluids to provide both diagnostic and prognostic value [3]. Nucleic acid testing (NAT) is also utilized for infectious diseases. However, these technologies are usually performed in centralized laboratories using high-end instrumentation and skilled personnel. Therefore, the World Health Organization (WHO) has established a set of criteria to guide the development of point-of-care (POC) diagnostic tools in resource-limited settings, which are (i) Affordable, (ii) Sensitive, (iii) Specific, (iv) User friendly, (v) Rapid and robust, (vi) Equipment-free, and (vii) Deliverable to end users, abbreviated as “ASSURED” [4] (Table 19.1).
Table 19.1 The ideal rapid test: ASSURED criteria
| A = Affordable |
| S = Sensitive |
| S = Specific |
| U = User-friendly (simple to perform in a few steps with minimal training) |
| R = Robust and rapid (results available in less than 30 min) |
| E = Equipment-free |
| D = Deliverable to those who need them |
One of the major limitations in POC tests is the accuracy at low levels compared to laboratory assays. Protein biomarkers are usually used as diagnostic targets (analytes), but are dilute in the complex milieu of human blood. Separating biomarkers from the background and enriching them to easily detectable concentrations will overcome this issue. Microscale diagnostic technologies have been employed, especially in distributed diagnostic and home healthcare technologies, with great success to address this need [5, 6]. Microscale technologies reduce sample volumes and ease fluid handling constraints, while providing modularity. The vast majority of the molecular components employed in these technologies have remained constant and an opportunity exists to develop new molecular tools to address inherent issues. Current technologies rely on traditional chromatographic techniques that suffer from poor diffusion of large biomarkers and limited activity of surface-bound capture moieties. While microfluidic systems primarily operate at the microscale (i.e., millionths of a meter), nanotechnology has contributed new concepts and will likely play an increasing role in the diagnostic area.
In the following sections of this chapter, some of the recently developed technologies associated with two major clinical diagnostic tests – immunoassays and nucleic acid amplification tests – are introduced. Certain applications of the stimuli-responsive polymer-based diagnostic tests are also discussed. The chapter ends with an overview of some of the future trends in stimuli-responsive polymer applications in diagnostics.
19.2 Immunoassays
An immunoassay is a test that relies on biochemistry to measure the presence and/or concentration of an analyte. The analyte can be large proteins, antibodies that a person has produced as a result of an infection or small molecules. Immunoassays have been utilized for various clinical applications since the mid-1960s and have revolutionized the care of patients [7]. Immunoassays detect biomarkers in the sample fluid via the recognition using the immobilized antibodies at solid surfaces. The signal from an immunoassay resulted from an enzyme acting on a substrate to yield a colored solution with the amount of color in the solution being equivalent to the amount of antigen in the test solution (Figure 19.1). The performance of immunoassays is intrinsically associated with specimen quality and quantity because the biomarker concentration and the specimen volume define the absolute number of available target molecules for detection. Because the antibody-biomarker binding occurs at a solid–liquid interface, immunoassays can utilize a significantly higher amount (≥10-fold) of antibodies to overcome the slow reaction kinetics [8, 9]. Another challenge for immunoassays is nonspecific binding, which raises the assay background noise and compromises the limit of detection [10]. The rarity of a disease biomarker in a broad sample background can lead to crippling signal-to-noise problems because the rare antigens themselves can be lost at various places when they are adsorbed nonspecifically to the device surfaces.
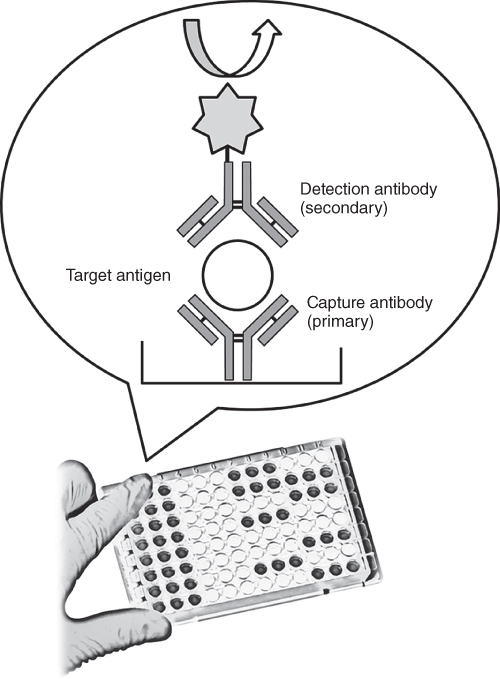
Figure 19.1 A sandwich ELISA. (1) Plate is coated with a capture antibody; (2) sample is added, and any antigen present binds to capture antibody; (3) detecting antibody is added, and binds to antigen; (4) enzyme-linked secondary antibody is added, and binds to detecting antibody; (5) substrate is added, and is converted by enzyme to detectable form.
More recently, the many complex steps of an immunoassay have been compressed into a simplified format for the end user. The format is dependent on the flowing of the specimen together with the assay components through a nitrocellulose test strip. In this format, binding of the antibody to the target antigen is directly observable by the user due to the accumulation of dyed microbeads that will bind to a specific location on the nitrocellulose yielding a colored line that is easily observable by the user. Lateral flow test (lateral flow assays (LFA)) is one such technology, and has been used as a rapid assay to diagnose various infectious diseases [11, 12]. Compared to common laboratory techniques such as ELISA, however, LFA exhibits significantly lower sensitivity, which results in numerous false negatives [13]. Therefore, LFA cannot be widely utilized for early disease diagnosis. One of the major reasons for the low sensitivity is the extremely low concentration of the biomarkers in a sample fluid. In order to regain clinical utility for LFA, approaches for concentrating biomarkers prior to detection have been employed to enhance the test's sensitivity [14, 15].
19.3 Nucleic Acid Tests
NAT for infectious diseases is almost exclusively performed in centralized laboratories using high-end instrumentation and skilled personnel. However, NAT at POC is beginning to enter clinical practice in developed and developing countries [16–18]. Key advantages of nucleic acid targeting technologies over protein targeting are that often they can provide earlier diagnosis as well as identification of disease-associated alleles. These allow healthcare providers to select effective treatment for an individual (e.g., personalized medicine) in a timely manner. As a result, there has been a strong push to expand the accessibility of nucleic acid targeting technologies worldwide. Unfortunately, nucleic acid targeting tests are much more complicated than protein targeting tests, and often they require trained personnel. To illustrate, the main steps for the assays targeting nucleic acid biomarkers include sample preparation (cell lysis to expose nucleic acid, purification and concentration of nucleic acid), amplification of targeted nucleic acid, and detection of the amplified products. Current systems for clinical diagnostic applications are mainly polymerase chain reaction (PCR)-based ones and are still relatively complex and expensive.
NAT involves three main steps: sample preparation, amplification, and detection. Integration of all three steps into a POC-compatible format has been demonstrated in some real-time PCR-based benchtop systems. In most cases, lysis is an initial process required to break open intact structures of targeted species and to release nucleic acids [19, 20]. Additional purification or extraction of the released nucleic acids from the lysate may be conducted to minimize inhibitory effects on downstream amplification. Following lysis, nucleic acids are typically purified through solid-phase extraction, which involves capturing nucleic acids onto a solid support, followed by wash steps and elution for downstream amplification. Therefore, purification and concentration of target nucleic acid prior to detection is also important for NAT to enhance the sensitivity.
19.4 Stimuli-Responsive Biomarker Separations
Since there has been a renewal of interest in purifying and concentrating dilute biomarkers as mentioned, a considerable number of studies have been conducted on designing of novel nanomaterials to meet these applications [21]. Some special types of polymers, for example, have emerged as a very useful class of polymers and have their own special chemical properties and applications in various areas [22–24]. These polymers are coined with different names, based on their physical or chemical properties like, “stimuli-responsive” or “environmental-sensitive” or “smart” or “intelligent” polymers. We shall use further on the name “stimuli-responsive” polymers for such polymer systems in this chapter. The characteristic feature is their ability to respond to very slight changes in the surrounding environment. The environmental trigger behind these transitions can be either change in temperature or pH shift, increase in ionic strength, presence of certain chemicals, and so on. More recently, changes in electric and magnetic fields and light or radiation forces have also been reported as stimuli for these polymers. Since the conjugation of a stimuli-responsive polymer to a single biomolecule can generate a nanoscale switch, there has been a renewal of interest in applying such polymers in diagnostic systems. Stayton and Hoffman, for example, have developed a series of nanosized thermos-responsive poly(N-isopropylacylamide) (PNIPAAm)-protein conjugates that separate and enrich analytes from solution and enable detection (Figure 19.2) [25, 26]. Because smart conjugates bind analytes in solution prior to separation, conjugate–analyte binding avoids steric and mass transport limitations associated with surface-based techniques [27, 28]. The author and coworkers have further developed a reversible microchannel surface capture system for stimuli-responsive grafted bioanalytical beads [29]. PNIPAAm was grafted onto polydimethylsiloxane (PDMS) surfaces by a UV-mediated graft polymerization from a photoinitiator that was preadsorbed in the channel wall. The switchable hydrophilic/hydrophobic properties were controlled by varying the photo-illumination times and/or the initiator concentration [30]. The surface traps captured PNIPAAm-grafted nanobeads uniformly above the lower critical solution temperature (LCST) and facilitated their rapid release as the temperature was reversed to below the LCST (Figure 19.3). A pH-responsive surface trap has been also constructed in the channel wall using the same methods. The proposed microfluidic systems promises “smart” protein biomarker separation and enrichment by combining with advanced microfluidic flow and mixing technologies.

Figure 19.2 Thermo-induced immunoseparation system using a stimuli-responsive polymer.

Figure 19.3 Design concept for smart diagnostic system that utilizes biomolecular-polymer-nanoparticle hybrids for enhancing analyte capture rates and assay sensitivity.
19.5 Stimuli-Responsive Diagnostics in the Developing World
The stimuli-responsive diagnostic systems may be also adopted for civilian healthcare in the developing world since these systems must be inexpensive, but also accurate, reliable, rugged, and well suited to the medical and social contexts of the developing world [31, 32]. The developing world does not have access to many of the best medical diagnostic technologies; for example, of the world's population of 6.1 billion people, 3 billion people lack basic sanitation, 2 billion do not have access to electricity, and more than 1 billion lack basic healthcare services and clean drinking water [33]. During the past decades, many efforts have been made for developing near-patient/home-testing approach in the developing world [34]. Simple and rapid immunoassays such as lateral flow strip tests, for example, have been one of the successfully used diagnostic technologies in the developing world. The use of saliva as a sample is also a suitably challenging issue because blood sampling is not easy in some locations where there are few highly trained healthcare workers [35, 36]. While a saliva test has many advantages over a serum test (i.e., less expensive and less invasive vial), many of the indicator molecules in saliva are dilute and their concentrations are very low. Taking these problems into account, the “stimuli-responsive” switchable surface trap systems mentioned will overcome this issue because the proposed systems can separate and enrich indicators with simple “on–off” switch control of the external stimuli such as friction heat, light, and magnet, without using electrical power. A reversible microchannel surface capture system has been further developed for bioanalytical samples [29, 30, 37]. The capture/release efficiency and enrichment of PNIPAAm–antibody conjugates in PNIPAAm-grafted PDMS microchannels have been investigated using a helical flow, circular microreactor. Mixing and recirculation substantially increase the conjugate release rate and sharpness once the temperature has dropped below the phase transition temperature. The concentration of protein–polymer conjugates could be achieved by a continuous conjugate flow into the heated recirculator, allowing nearly linear enrichment of the conjugate reagent from larger volumes. This capability was shown with anti-p24 HIV monoclonal antibody reagents that were enriched over fivefold by using this protocol. pH-responsive surface traps have also been constructed in the channel wall by the same methods.
Magnetic nanoparticles have also been developed for diagnostic target isolation, because smaller particles display better association and binding properties to the target analytes [38, 39]. A reversible surface capture system for smart bioconjugates has also been demonstrated on porous membrane filters for the detection of the malaria antigen Plasmodium falciparum histidine-rich protein 2 (PfHRP2) [40]. The carboxyl end groups of semi-telechelic PNIPAAm were modified with tetrafluorophenol to yield amine-reactive ester groups for conjugation to amine groups of anti-streptavidin and anti-PfHRP2 antibodies. Stimuli-responsive membranes were constructed from 1.2-µm-pore-size, hydroxylated, nylon-6,6 filters (Figure 19.4). The PNIPAAm-grafted membranes showed greater than 80% anti-streptavidin capture efficiency. The PfHRP2 antigen could be processed and detected at clinically relevant concentrations of this malaria biomarker. These studies provide insight into the mechanism of smart polymer–protein conjugate capture and release in grafted channels and show the potential of this purification and enrichment module for processing diagnostic samples. Thus, stimuli-responsive polymer-based systems are useful to regulate flow, concentrating biomarkers, and eliminating the need for external power.
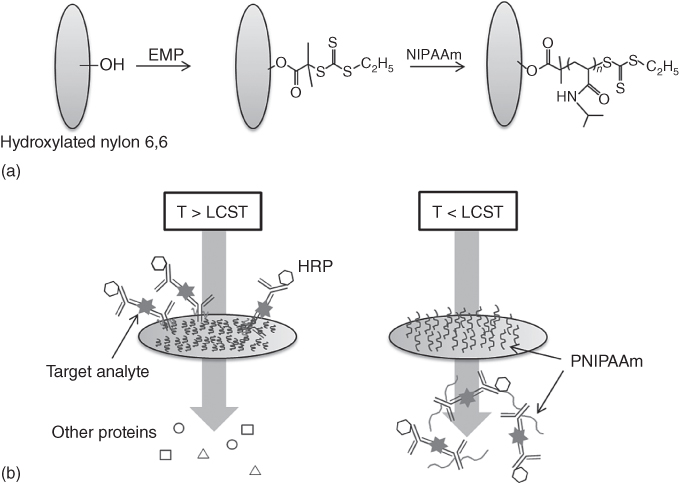
Figure 19.4 Stimuli-responsive fluidic system for purifying and concentrating diagnostic biomarkers using temperature-responsive antibody conjugates and membranes.
19.6 Conclusions
Although this chapter focuses solely on the roles of smart polymer-based nanotechnologies within the fields of diagnostics, they have an extensive range of applications. They are expected to help address many problems facing today's society. These technologies, for example, may make it possible to manufacture programmable materials that require less energy to produce less waste than conventional materials. Recent progress in polymeric synthesis has led to intriguing new “stimuli-responsive” polymer systems, resulting from novel approaches for synthesizing random, block, or graft copolymers as well efficient control of molecular weight and its distributions. These materials will enable us to develop better, faster, cheaper, and more powerful technology that has the potential for improvements in health, safety, and quality of life, particularly in resource-limited environments such as aerospace or low-infrastructure sites such as the developing world.
References
- 1 Centers for Disease Control. Cdc.gov (2015) Estimating Seasonal Influenza-Associated Deaths in the United States: CDC Study Confirms Variability of Flu| Seasonal Influenza (Flu) | CDC http://www.cdc.gov/flu/about/disease/us_flu-related_deaths.htm (accessed 30 October 2015).
- 2 World Health Organization. Who.int (2015) WHO | HIV/AIDS http://www.who.int/gho/hiv/en/ (accessed 30 October 2015).
- 3 Anderson, N.L. (2010) Clin. Chem., 56, 177.
- 4 Peeling, R.W., Holmes, K.K., Mabey, D., and Ronald, A. (2006) Sex. Transm. Infect., 82, 5.
- 5 Dupuy, A., Lehmann, S., and Cristol, J. (2005) Clin. Chem. Lab. Med., 43, 1291.
- 6 Toner, M. and Irimia, D. (2005) Annu. Rev. Biomed. Eng., 7, 77.
- 7 Yalow, B.R.S. and Berson, S.A. (1960) J. Clin. Invest., 39, 1157.
- 8 Klenin, K.V., Kusnezow, W., Langowski, J., and Klenin, K.V. (2005) J. Chem. Phys., 214, 715.
- 9 Glaser, R.W. (1993) Anal. Biochem., 213, 152.
- 10 Soukka, T., Härmä, H., Paukkunen, J., and Lövgren, T. (2001) Anal. Biochem., 73, 2254.
- 11 Zhou, G., Mao, X., and Juncker, D. (2012) Anal. Chem., 84, 7736.
- 12 Quach, C., Newby, D., Daoust, G., Rubin, E., and Mcdonald, J. (2002) Clin. Diagn. Lab. Immunol., 9, 925.
- 13 Cêtre-Sossaha, C., Billecocqb, A., Lancelota, R., Defernezc, C., Favrec, J., Bouloyb, M., Martineza, D., and Albinaa, E. (2009) Prev. Vet. Med., 90, 146.
- 14 Kehrel, M.K.M., Dassinger, N., Merkl, S., and Vornicescu, D. (2012) Phys. Status Solidi A, 209, 917.
- 15 Nash, M.A., Waitumbi, J.N., Hoffman, A.S., Yager, P., and Stayton, P.S. (2012) ACS Nano, 6, 6776.
- 16 Raja, S., Ching, J., Xi, L., Hughes, S.J., Chang, R., Wong, W., McMillan, W., Gooding, W.E., McCarty, K.S., Chestney, M., Luketich, J.D., and Godfrey, T.E. (2005) Clin. Chem., 51, 882.
- 17 Tanriverdi, S., Chen, L., and Chen, S. (2010) J. Infect. Dis., 201, S52.
- 18 Goldmeyer, J., Haijing, L., McCormac, M., Cook, S., Stratton, C., Lemieux, B., Kong, H., Tang, W., and Tang, Y.-W. (2008) J. Clin. Microbiol., 46, 1534.
- 19 Belgrader, P., Hansford, D., Kovacs, G.T.A., Venkateswaran, K., Mariella, R. Jr., Milanovich, F., Nasarabadi, S., Okuzumi, M., Pourahmadi, F., and Northrup, M.A. (1999) Anal. Chem., 71, 4232.
- 20 Doebler, R.W., Erwin, B., Hickerson, A., Irvine, B., Woyski, D., Nadim, A., and Sterling, J.D. (2009) J. Assoc. Lab Autom., 14, 119.
- 21 Ebara, M. (ed.) (2016) Biomaterials Nanoarchitectonics, Elsevier.
- 22 Ratner, B.D., Hoffman, A.S., Schoen, F.J., and Lemons, J.E. (eds) (2004) Biomaterials Science: An introduction to materials in medicine, 2nd edn, Elsevier.
- 23 Malgras, V., Ji, Q., Kamachi, Y., Mori, T., Shieh, F.-K., Wu, K.C.-W., Ariga, K., and Yamauchi, Y. (2015) Bull. Chem. Soc. Jpn., 88, 1171.
- 24 Lowe, A.B. and McCormick, C.L. (2001) in Stimuli-Responsive Water Soluble and Amphiphilic Polymers, ACS Symposium Series, vol. 780 (ed. C.L. McCormick), American Chemical Society, p. 1.
- 25 Kulkarni, S., Schilli, C., Grin, B., Müller, A.H., Hoffman, A.S., and Stayton, P.S. (2006) Biomacromolecules, 7, 2736.
- 26 Kulkarni, S., Schilli, C., Müller, A.H., Hoffman, A.S., and Stayton, P.S. (2004) Bioconjugate Chem., 15, 747.
- 27 Malmstadt, N., Hoffman, A.S., and Stayton, P.S. (2004) Lab Chip, 4, 412.
- 28 Malmstadt, N., Yager, P., Hoffman, A.S., and Stayton, P.S. (2003) Anal. Chem., 75, 2943.
- 29 Ebara, M., Hoffman, J.M., Hoffman, A.S., and Stayton, P.S. (2006) Lab Chip, 6, 843.
- 30 Ebara, M., Hoffman, J.M., Stayton, P.S., and Hoffman, A.S. (2007) Radiat. Phys. Chem., 76, 1409.
- 31 Yager, P., Edwards, T., Fu, E., Helton, K., Nelson, K., Tam, M.R., and Weigl, B.H. (2006) Nature, 442, 412.
- 32 Urdea, M., Penny, L.A., Olmsted, S.S., Giovanni, M.Y., Kaspar, P., Shepherd, A., Wilson, P., Dahl, C.A., Buchsbaum, S., Moeller, G., and Hay Burgess, D.C. (2006) Nature, 73–79.
- 33 Black, R.E., Morris, S.S., and Bryce, J. (2003) Lancet, 361, 2226.
- 34 Price, C.P. (2001) Br. Med. J., 332, 1285.
- 35 Fu, E., Chinowsky, T., Foley, J., Weinstein, J., and Yager, P. (2004) Rev. Sci. Instrum., 75, 2300.
- 36 Fu, E., Foley, J., and Yager, P. (2003) Rev. Sci. Instrum., 74, 3182.
- 37 Ebara, M., Hoffman, A.S., Stayton, P.S., and Lai, J.J. (2013) Langmuir, 29, 5388.
- 38 Lai, J.J., Hoffman, J.M., Ebara, M., Hoffman, A.S., Estournès, C., Wattiaux, A., and Stayton, P.S. (2007) Langmuir, 23, 7385.
- 39 Lai, J.J., Nelson, K.E., Nash, M.A., Hoffman, A.S., Yager, P., and Stayton, P.S. (2009) Lab Chip, 9, 1997.
- 40 Golden, A.L., Battrell, C.F., Pennell, S., Hoffman, A.S., Lai, J.J., and Stayton, P.S. (2010) Bioconjugate Chem., 21, 1820.
