Characterization and Recycling of Bottom Ash
Abstract
As one of the byproducts of municipal solid waste (MSW) incineration, abundant bottom ash is generated in MSW incinerators. Composition of bottom ash and distribution of components in terms of size, mass, material (ceramic, metal, glass, molten slag), physical and chemical properties, and mineral content are fully explored and presented. Generally, metal, glass, plastics, wood, and paper may be present. The constituents of bottom ash larger than 4 mm in approximate diameter made up 60.3% and 58.3%, respectively; they can be screened and washed out easily and may be used as secondary materials in the construction industry. The metal objects taken from bottom ash include ferrous metal items such as iron sheeting, wire and nails, and nonferrous metal items such as aluminum pans and copper wire. Metal is distributed mainly in a particle size of 16–25 mm. The main mineral content of ashes is found to be SiO2, CaCO3, CaSO4, 3Al2O3 2SiO2, and CaAl2Si2O8. Moreover, ratios of SiO2 in bottom ash are higher than those in fly ash, this possibly being ascribed to the glass in the waste. The evolution of iron-rich constituents in bottom ash and the mineralogical characterization of incineration bottom ash are fully discussed, with an emphasis on heavy metal-bearing phases.
Keywords
Bottom ash; ferrous metal; chemical composition; component distribution
2.1 Components of Bottom Ash and Their Distribution
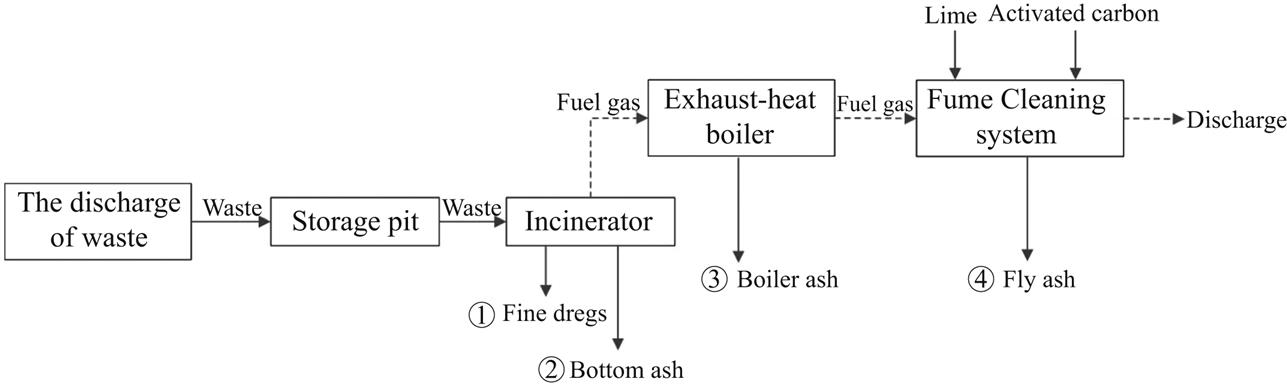
Bottom ash generated in the municipal solid waste (MSW) incineration process may consist of noncombustible inorganics, unburned carbon, and combustion ash, depending on the composition of the waste and the type of incinerators. The ash generation process is shown in Fig. 2.1. In general, the boiler ash is mixed with the slags and treated as bottom ash. The fly ash comes only from the baghouse.
Incineration ashes can be categorized into four types, according to the sites of production: fine dreg, bottom ash, boiler ash, and fly ash. Fine dreg always integrates with slag after dropping from the spaces among grates and then being gathered by the flue-dust retainer. It contains broken glass, molten aluminum ingots, and other metals. Bottom ash is generated from the furnace bottom after incineration, and should be water-cooled before outputting. It may contain fine ash and other residues such as iron wire, glass, and cement block. Boiler ash comes from the suspended particles in exhaust gas and then drops into the flue-dust retainer as blocked by boiler tube. It can be gathered alone or with bottom ash. Fly ash is the fine particles gathered by the fume cleaning system, predominantly the baghouse, and may contain CaCl2, CaSO4, Ca(OH)2, active carbon, and most importantly, dioxins and soluble heavy metals.
The bottom ash samples were taken from two different large-scale MSW incineration plants, labeled as A and B, respectively. Plant A was constructed and put into operation at the end of 2001, dealing with 1000 t of MSW per day, using tilted reverse reciprocating grates. Plant B was constructed and put into operation from 2003, with the same capacity as A and grates of the German hydraulic reciprocating type. Both of them use original wastes directly without any pretreatment. In the meantime, the fly ash from both plants was also taken for comparison. The bottom ash and fly ash from plant A and B were named to differentiate them (bottom ash A, fly ash A, bottom ash B and fly ash B).
Owing to the fact that the temperature of bottom ash is about 400°C when it is discharged from an incinerator, cooling equipment is needed. Water is usually used to cool down bottom ash, resulting in a moisture content of 11–18% in bottom ash.
Fresh bottom ash was black in a wet situation and changed to gray after air drying. It was found that the samples from different plants were very different both in color and composition. Bottom ash B had a larger amount of unburned organic than bottom ash A, and fly ash B was whiter than fly ash A.
The appearance of the bottom ash is shown in Fig. 2.2. It included metal, glass, plastics, wood, and paper. The glass was of different colors with silicate particles adhering to it. It comes mainly from all kinds of glass containers and utensils, such as bottles, glass windows and doors, and so on. The ceramics, including ceramic and porcelain chips, were mostly in molten form at a high temperature in the incinerator.

The molten slag (mineral substances) included inorganic compounds such as SiO2, CaCO3, CaO, CaO · Al2O3 · 2SiO2, MgCO3, barite, and gypsum, among others, which may also be present in fly ash. The metal articles were mainly everyday items such as aluminum pans, cans, copper wire, nails, and so on.
The organic materials in bottom ash included plastics, incompletely combusted organics, paper and paperboard, strips of cloth and artificial fiber, bone chips, and so on. In addition, the skin of some fruit, such as orange and banana, was also found in the bottom ash. For a grate furnace, the temperature at the center of the furnace may be 850–1100°C, but below 500°C on the grate, and some wastes cannot be burnt at this temperature. See Fig. 2.2 to view some of these.
2.2 Size Distribution in Bottom Ash
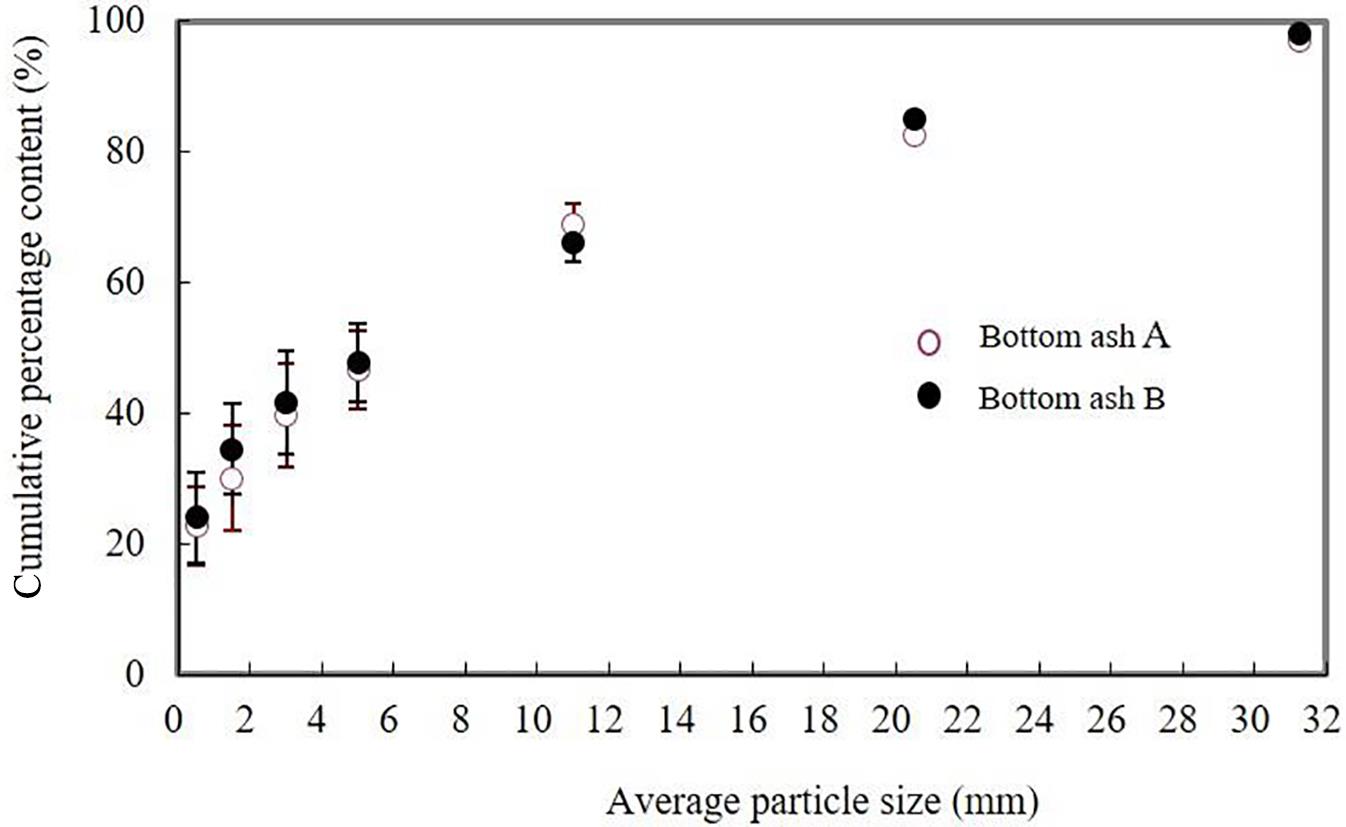
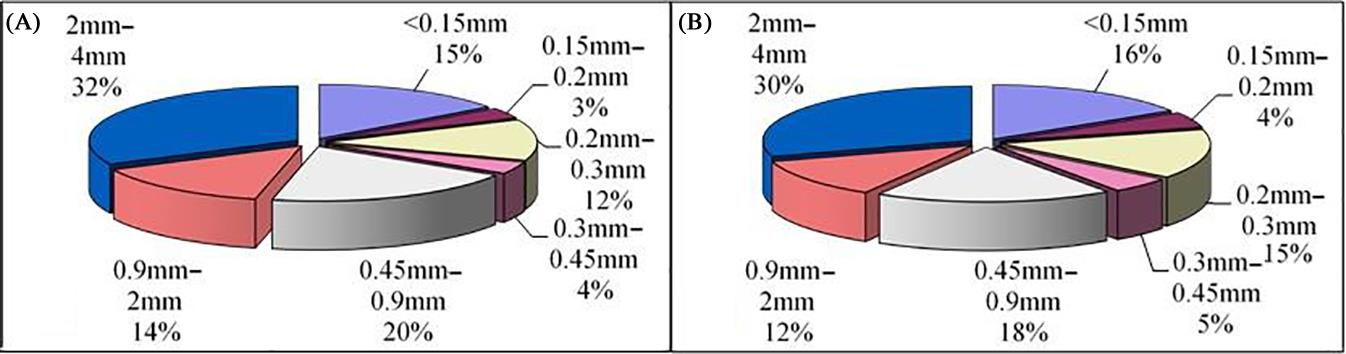
Fig. 2.3 shows the cumulative particle size distribution curve of bottom ash from plants A and B, which give fairly consistent results. The constituents of bottom ash larger than 4 mm in diameter made up 60.3% and 58.3%, respectively, can be screened and washed out easily; they may be used as secondary materials in the construction industry. The washing process helps to get rid of many soluble components and fine materials which adhere to the surface of. The constituents of bottom ash smaller than 1 mm in diameter made up about 20–25%, which included the ash from grates and boiler and mainly adhered to the surface of larger particles.
2.3 Mass Distribution in Bottom Ash
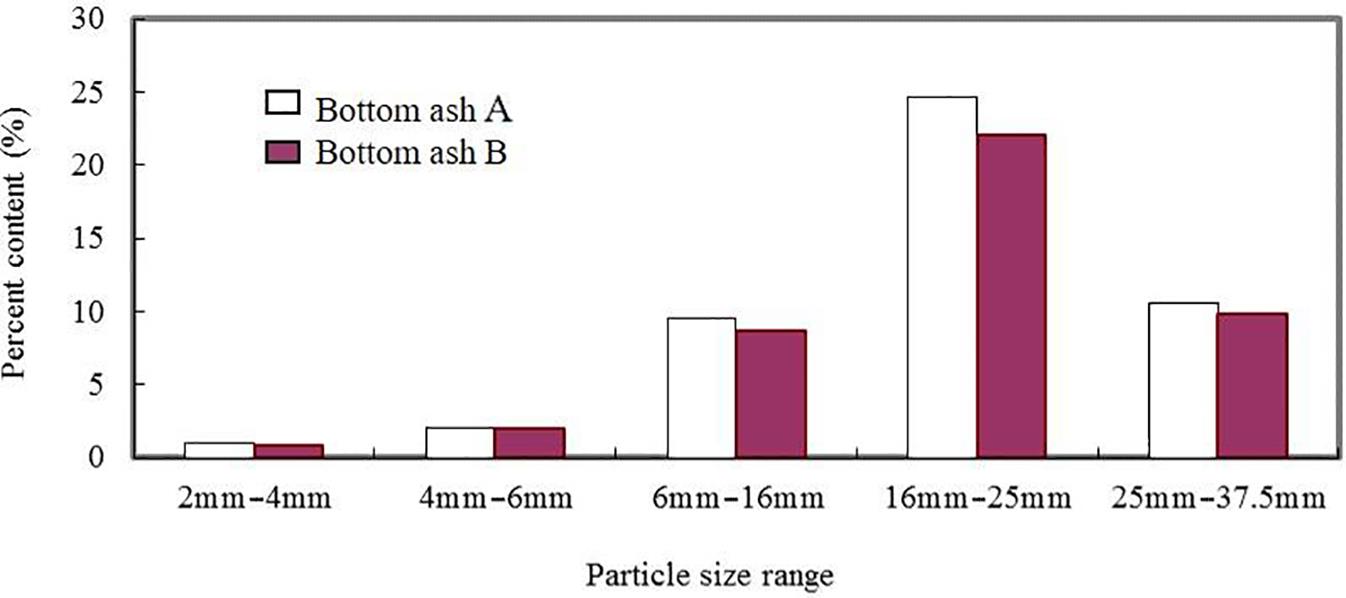
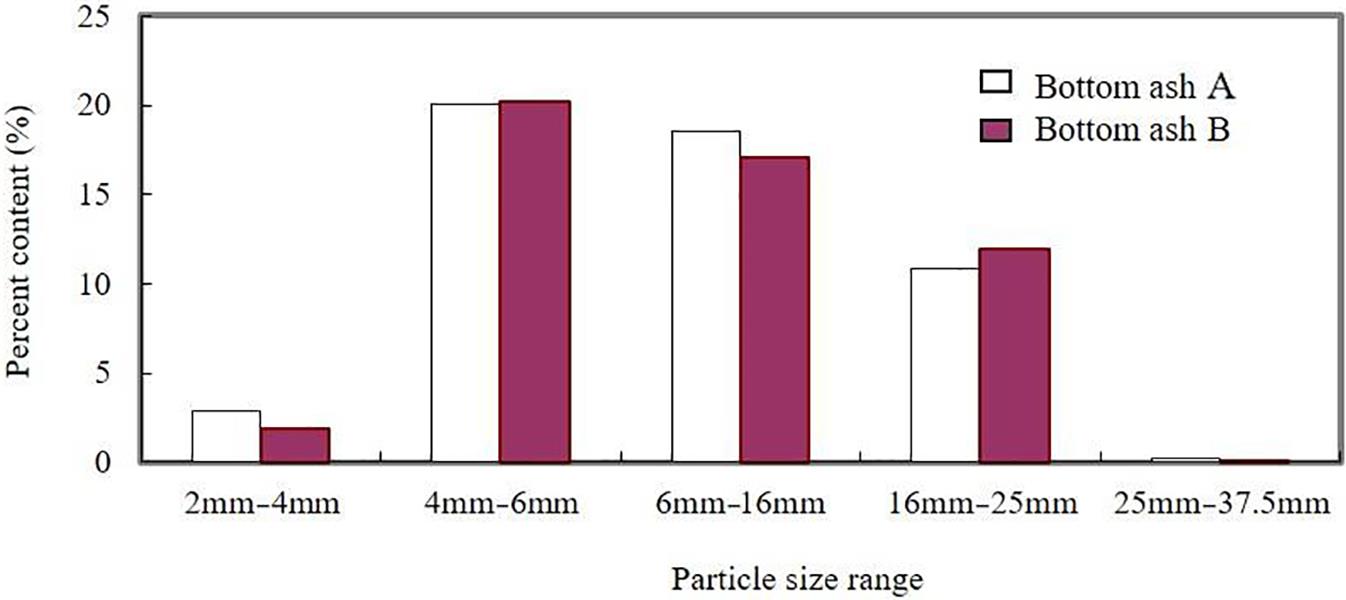
Mass distribution of bottom ash is given in Table 2.1, which shows figures for molten slag (64.09–66.42%), stone and brick (14.02–16.62%), ceramics (7.33–7.36%), glass (6.81–7.37%), metal products (3.67–4.10%), a small quantity of organics such as plastic, paper and wood, and so on. The size of stone and brick particles was always greater in approximate diameter than 25 mm, while that of slag and glass was smaller.
Table 2.1
Physical Fractionation of Bottom Ash from Municipal Solid Waste Incinerator
| Particle size (mm) | Component Content of Bottom Ash (%) | |||||||||||||
| Ceramic | Glass | Residues | Brick and Stone | Organics | Metal Products | Others | ||||||||
| A | B | A | B | A | B | A | B | A | B | A | B | A | B | |
| 2–4 | 0.99 | 0.83 | 2.91 | 1.86 | 93.38 | 94.77 | 1.04 | 1.31 | 0.58 | 0.72 | 1.10 | 0.51 | 0 | 0 |
| 4–6 | 2.08 | 1.98 | 20.04 | 20.18 | 61.05 | 63.98 | 12.83 | 9.29 | 1.53 | 2.11 | 2.06 | 2.06 | 0.41 | 0.40 |
| 6–16 | 9.50 | 8.71 | 18.54 | 17.09 | 47.56 | 49.40 | 20.14 | 20.98 | 1.00 | 1.23 | 2.95 | 2.37 | 0.31 | 0.22 |
| 16–25 | 24.64 | 22.09 | 10.89 | 11.98 | 39.46 | 40.50 | 10.50 | 7.63 | 0.38 | 1.75 | 14.03 | 15.97 | 0.10 | 0.08 |
| 25–37.5 | 10.62 | 9.81 | 0.21 | 0.08 | 32.15 | 33.14 | 50.12 | 50.7 | 1.93 | 3.18 | 4.97 | 3.09 | 0 | 0 |
| >37.5 | 4.97 | 5.66 | 1.50 | 1.51 | 5.00 | 5.20 | 81.26 | 77.49 | 1.99 | 5.22 | 5.28 | 4.92 | 0 | 0 |
| Total | 7.36 | 7.33 | 7.37 | 6.81 | 64.09 | 66.42 | 16.62 | 14.02 | 0.78 | 1.24 | 3.67 | 4.10 | 0.11 | 0.08 |
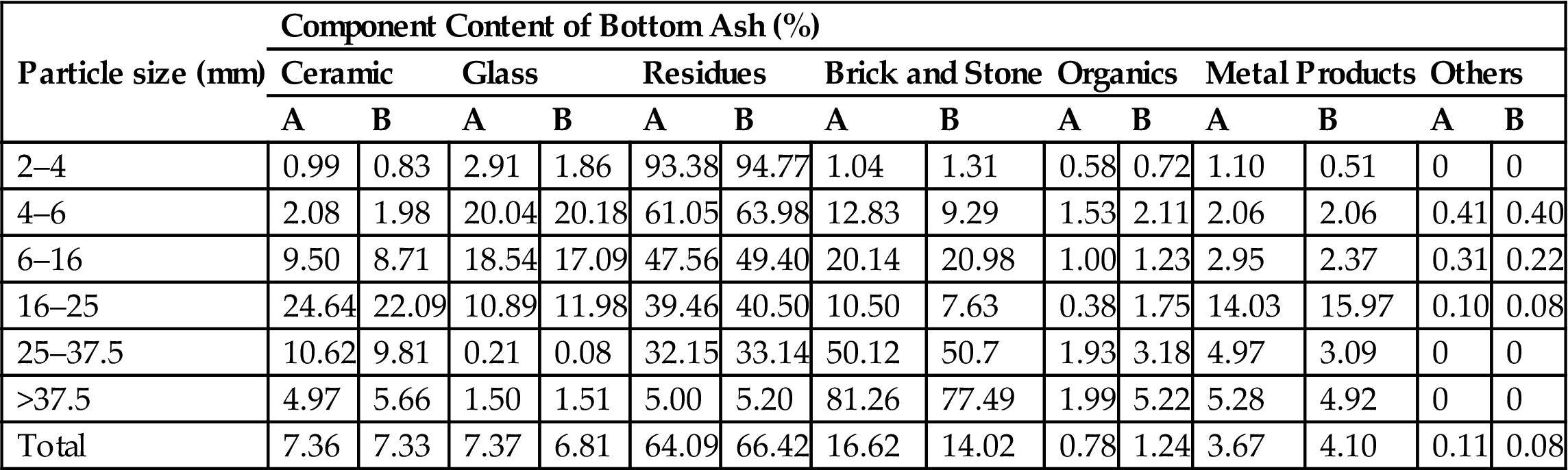
Note: Particles smaller than 2 mm in diameter are regarded as residues.
2.4 Ceramic Distribution in Bottom Ash
As seen in Fig. 2.4, the sizes of ceramic particles present in bottom ashes ranged from 16 to 25 mm in approximate diameter, which was bigger than those of glass.
2.5 Metal Items’ Distribution in Bottom Ash
Metal items from bottom ash included (Fig. 2.5) ferrous metal goods such as iron sheeting, wire and nails, and nonferrous metal goods such as aluminum pans and copper wire. They were distributed mainly at a particle size of 16 to 25 mm (diameter), as shown in Fig. 2.6. The metals should be removed before the bottom ashes are recycled for use as brickmaking material. For plant A, with a capacity of 1000 t/d, around 250–300 t/d bottom ash may be generated. In general, metal products may account for 3–5% of bottom ash, and 2700 t–4500 t/y may be collected thus.
2.6 Glass Distribution in Bottom Ash
Fig. 2.4 presents the distribution of glass in bottom ashes A and B. Particles from 4 to 16 mm in diameter made up the greater proportion of the ash, as the glass was shattered and crushed in the incinerators.
2.7 Molten Slag Distribution in Bottom Ash
Molten slag is the product of incineration at a high temperature. It contains mainly noncombustible inorganics, ash and residue from combustible material, additives, and chemical reaction products. Fig. 2.7 shows the distribution of molten slag in bottom ash, in which particles smaller than 2 mm in diameter were regarded as molten slag, and made up approximately 65% of total weight.
2.8 Physical and Chemical Properties of Bottom Ash
The physical and chemical properties of bottom ash particles with of 4 mm approximate diameter were analyzed, as shown in Fig. 2.8. Constituents of 2–4 mm diameter occurred the most frequently, followed by those of 0.45–0.9, 0–0.15, 0.9–0.2, and 0.2–0.3 mm. Items in the 0.3–0.45 mm and 0.15–0.2 mm ranges made up less than 5%.
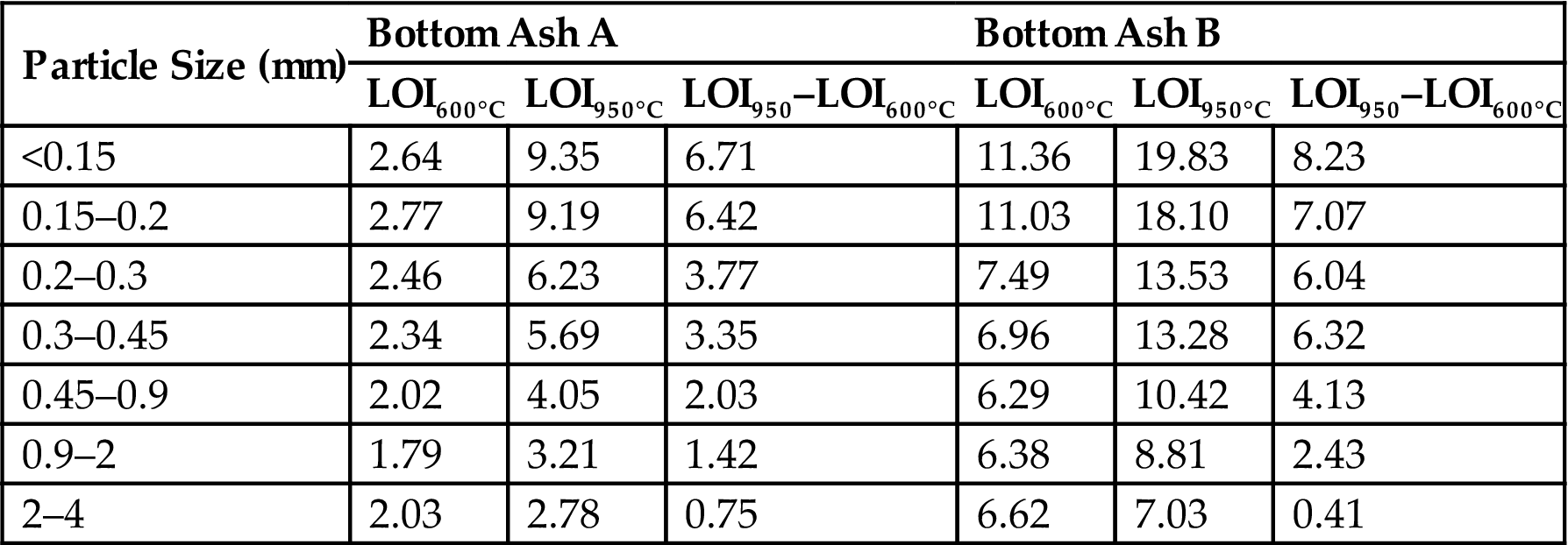
The loss on ignition (LOI) of 600°C reflects the volatile organic matter concentration in bottom ash, while the LOI of 950°C also includes the amount of carbon dioxide produced by the thermal decomposition of carbonate in the bottom ash.
The result are shown in Table 2.2. LOI represents the loss of ignition, and LOI 950–LOI 600°C can indirectly reflect the carbonate content of the ash. There was a big difference in loss of ignition between ashes A and B under the same conditions, with LOI for ash B higher than that for A, indicating that ash B had a higher volatile organic matter content. In addition, from the data of LOI950°C–LOI600°C it was possible to extrapolate that with the decrease in particle size of ashes, the carbonate content increased, especially for particles 0.15–0.2 mm or <0.15 mm in approximate diameter. Since the contents of organic matter in bottom ash could indirectly reflect the operating status of incinerators, it was possible also to discover that the operating status of incineration plant A was better than that of B.
Table 2.2
The Loss on Ignition of Bottom Ashes with Various Particle Sizes (%)
| Particle Size (mm) | Bottom Ash A | Bottom Ash B | ||||
| LOI600°C | LOI950°C | LOI950–LOI600°C | LOI600°C | LOI950°C | LOI950–LOI600°C | |
| <0.15 | 2.64 | 9.35 | 6.71 | 11.36 | 19.83 | 8.23 |
| 0.15–0.2 | 2.77 | 9.19 | 6.42 | 11.03 | 18.10 | 7.07 |
| 0.2–0.3 | 2.46 | 6.23 | 3.77 | 7.49 | 13.53 | 6.04 |
| 0.3–0.45 | 2.34 | 5.69 | 3.35 | 6.96 | 13.28 | 6.32 |
| 0.45–0.9 | 2.02 | 4.05 | 2.03 | 6.29 | 10.42 | 4.13 |
| 0.9–2 | 1.79 | 3.21 | 1.42 | 6.38 | 8.81 | 2.43 |
| 2–4 | 2.03 | 2.78 | 0.75 | 6.62 | 7.03 | 0.41 |
The bottom ash pH is listed in Table 2.3. The pH value of BA-A was 12.5–12.9, while the pH value of BA-B was 8.7–8.2. The pH value had a trend of rising slowly with the decrease of particle size.
2.9 Mineral Composition of Bottom Ash
The samples of bottom ash A were ground to a size of 0.075 mm (200 mesh) diameter, and the mineral composition analysis was carried out using an X-ray diffractometer (PW1710). The working voltage and working current were 40 kV and 20 A, respectively. The XRD map of incinerator ashes with different sizes is shown in Fig. 2.9. The main mineral composition of ashes was found to be SiO2, CaCO3, CaSO4, 3Al2O3 ∙ 2SiO2, and CaAl2Si2O8. The occurrences of SiO2 and 3Al2O3 · 2SiO2 in particles of 0.3–0.45 mm were the highest, while those of CaCO3 and CaAl2Si2O8 gradually increased with the decrease of particle size.
2.10 Chemical Composition of Bottom Ash
The essential constituents of BA-A and BA-B below 2 mm in diameter are listed in Table 2.4. SiO2 (35.3–42.3%), CaO (19–27.2%), Al2O3 (7.4–7.8%), Fe2O3 (3.9–5.1%) and a small amount of Na2O, K2O, MgO, and TiO2, were the main fractions, and belonged to the CaO–SiO2–Al2O3–Fe2O3 system.
Table 2.4
Essential Components of Ba-A and Ba-B Below 2 mm in Diameter (%)
| Components | BA-A | BA-B | Fly ash A | Coal Fly Ash |
| SiO2 | 35.3 | 42.3 | 20.5 | 54.9 |
| CaO | 27.2 | 19 | 35.8 | 8.7 |
| Al2O3 | 7.4 | 7.8 | 5.8 | 25.8 |
| Fe2O3 | 3.9 | 5.1 | 3.2 | 6.9 |
| P2O5 | 5.7 | 3.3 | 2.8 | |
| SO42− | 3.12 | 2.16 | 14.4 | 0.72 |
| MgO | 1.7 | 1.5 | 2.1 | 1.8 |
| Na2O | 2.1 | 3.1 | 3.7 | 0.6 |
| K2O | 1.6 | 1.7 | 4.0 | |
| Cl | 1 | 0.8 | 12.4 | |
| TiO2 | 0.7 | 0.6 | / | |
| ZnO | 0.4 | 0.3 | / | |
| BaO | 0.3 | 0.2 | / | |
| MnO | 0.1 | 0.1 | / | |
| CuO | 0.1 | 0.1 | / | |
| Loss on ignition | 8.7 | 12.0 | 22.0 |
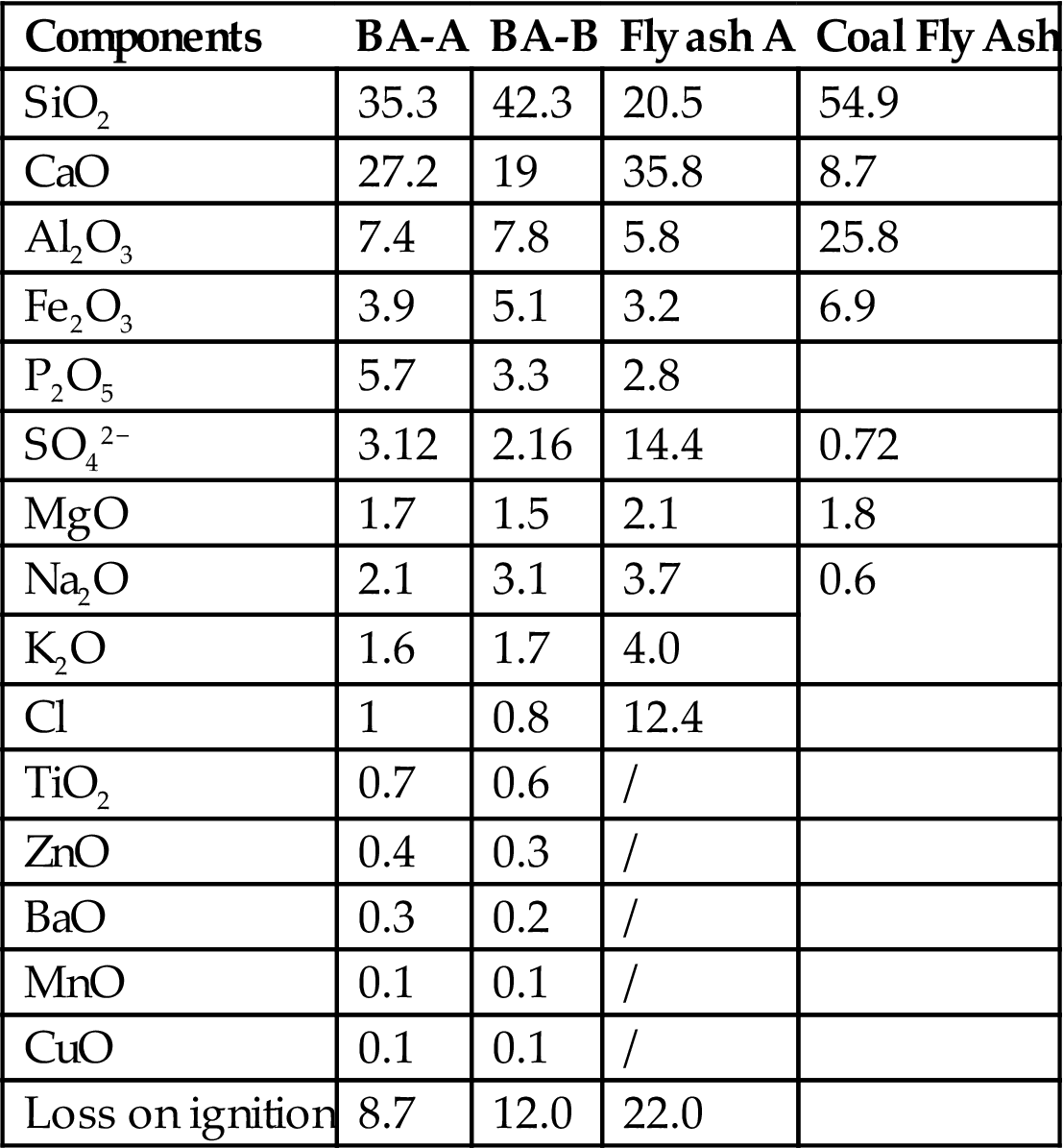
Ratios of SiO2 in bottom ash were higher than those in fly ash, which can possibly be ascribed to the presence of glass in the waste. The CaO content level in fly ash was obviously higher than those of BA, owing to the addition of an adsorbent, for example, Ca(OH)2 or CaO, used for adsorption of acid gas (HCl and SO2 et al.) produced in the waste incineration process. Oxides of Si, Al, and Fe, with a higher boiling point, may be present mainly in bottom ash, in contrast with oxides of Na, K, which are present mainly in fly ash, owing to their lower boiling point. The oxides with higher boiling points, especially Si, accounted for about 35% in BA-A, and up to 42.3% in BA-B, compared with 24.5% in the fly ash from the incinerator A. Similar trends for Fe, Al can also be observed. Thus, the oxides with higher boiling points and lower vapor pressure, i.e. Si, Al, Fe, etc., would remain in BA, while volatile oxides such as Na and K et al. were more likely to accumulate in fly ash.
Owing to the lower boiling points and higher volatility of sulphates and chlorides, S and Cl content levels in the fly ash were up to 12.0% and 12.4%, respectively, much higher than those in the bottom ash with S 1.8–2.6% and Cl 0.8–1.0%).
XRF analysis was applied to study the effect of particle sizes on the components of bottom ash B with below approximate diameters of 0.15 mm, 0.15–0.2 mm, 0.2–0.3 mm, 0.3–0.45 mm, 0.45–0.9 mm and 0.9–2 mm, and the results are listed in Table 2.5. Migration and transformation of heavy metals would occur in the incineration process, and they would finally be distributed in bottom ash, fly ash and flue gas in different forms and proportions.
Table 2.5
Chemical Components of Bottom Ash of Various Sizes (%)
| Components | <0.15 mm | 0.15–0.2 mm | 0.2–0.3 mm | 0.3–0.45 mm | 0.45–0.9 mm | 0.9–2 mm |
| SiO2 | 30.7 | 35.6 | 40.2 | 45.8 | 49.4 | 51.1 |
| CaO | 25.4 | 22.7 | 20.2 | 17.2 | 15.1 | 14.2 |
| Al2O3 | 7.2 | 7.4 | 7.7 | 7.8 | 8.2 | 8.4 |
| Fe2O3 | 4.5 | 4.7 | 5 | 5.1 | 5.5 | 5.8 |
| P2O5 | 3.6 | 3.5 | 3.4 | 3.7 | 3.1 | 2.8 |
| SO3 | 2.8 | 2.4 | 2 | 1.5 | 1.2 | 1 |
| Na2O | 1.8 | 2.1 | 2.6 | 2.6 | 4 | 4.5 |
| MgO | 1.6 | 1.5 | 1.4 | 1.3 | 1.4 | 1.5 |
| K2O | 1.5 | 1.6 | 1.7 | 1.8 | 1.7 | 1.7 |
| Cl | 1 | 0.9 | 0.8 | 0.7 | 0.6 | 0.6 |
| TiO2 | 0.7 | 0.6 | 0.5 | 0.5 | 0.5 | 0.5 |
| ZnO | 0.4 | 0.4 | 0.3 | 0.3 | 0.2 | 0.2 |
| BaO | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 |
| MnO | 0.2 | 0.2 | 0.1 | 0.1 | 0.1 | 0.1 |
| CuO | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
| PbO | 0.06 | 0.05 | 0.04 | 0.04 | 0.04 | 0.04 |
| Cr2O3 | 0.03 | 0.03 | 0.02 | 0.02 | 0.03 | 0.03 |

2.11 Mineralogical Characterization of Incineration Bottom Ash with an Emphasis on Heavy Metal-Bearing Phases
Approximately 77% of MSW is incinerated in Japan, and the generated residue accounts for nearly 64% of all waste disposed in landfill sites. The incineration process can reduce the weight of raw waste by up to about 80 wt%, and simultaneously results in high concentrations of heavy metals such as Pb, Cu, and Zn, which could be as much as five times that in raw waste. In recent years, strict attention has been paid to the potential impact of incineration residue on the surrounding environment. Various leaching tests (including upflow column leaching test CEN/TS 14405, pH dependence leaching test CEN/TS 14429, availability leaching test JLT19, etc.) are commonly used to estimate the mobilization behavior of pollutants in MSWI residue, followed by geochemical simulations based on mineral dissolution/precipitation, adsorption/precipitation or complexation with organic materials. However, the simulation is based on the pH variation only, without considering the evolutionary process of bottom ash constituents.
MSWI bottom ash has been reported as being a complex inorganic assemblage composed mainly of fine materials, melt components, small quantities of metallic components, synthetic ceramics, and stones, as well as unburned organic matter. Several complex silicates and oxides, the characterization of which remain undetermined, exist as primary melt components in bottom ash products. Owing to the multicomponent, partially amorphous characteristics of the bottom ash, combinatorial research methods including bulk analysis methods and microanalysis hold the promise of a more profound understanding of the primary and secondary phases in bottom ash. Heavy metals—the focal point of our research—are incorporated into these melt components (glass and minerals) that may undergo mineralogical changes when exposed to the environment. However, only a few studies have focused on the identification and characterization of heavy metal-bearing phases, as well as the weathering behavior of such phases.
2.11.1 Metallic Forms of Heavy Metals in Bottom Ash
The bulk chemical composition of bottom ash revealed that the incineration products were silicate-based materials, with various amounts of other elements. SiO2, CaO, Al2O3, FeO, MgO, Na2O, K2O, and TiO2 were the dominant components, while minor and trace components, such as P, Cl, S, Pb, Zn, Cu, Mn, Ni, Cr, etc., also exist in different proportions in the bottom ash.
The glass phases are generally regarded as the principal incineration products of MSWI bottom ash. Based on microanalysis by optical and electronic microscopy, the glass phases in bottom ash can generally be divided into: (1) melt glass formed during combustion in the furnace and (2) waste stream glass. The latter is usually categorized as refractory (residual) components that have survived incineration as remnants of waste glass input. The melt glass phases are the main melting products of ash under high temperature and complex incineration conditions, which serve as a matrix for bottom ash particles. Optical and electron microscopy analysis reveals the following characteristics of the melt glass phases: (1) isotrope, transparent or translucent locally shifting to brown, light blue, green, and red colors as observed by plane polarized light (PPL) with respect to its chemical composition, (2) having peculiar banding due to the existence of minute metallic inclusions, (3) highly vesicular owing to the entrapment of air and gas bubbles, and (4) coexistence of various well-formed mineral species, quench crystallites, and refractory components. Waste stream glass is typically isotropic, presenting a more uniform appearance with few or no vesicles, without a banding feature, and usually with only the surface partially melted during the combustion process.
Opaque metallic phases (in the center and the top right corner) and fractured quartz fragments exist as remnants of waste input, and a great portion of these waste remnants are set into the glass matrix during combustion. Vesicles of various sizes are easily visible using the PPL mode of optical microscopy. The main compositions of melt glass phases from different incineration facilities suggest a very strong resemblance in compositional properties. It is understood that silicon, aluminum, calcium, and also some alkaline and alkaline-earth elements are the main components of the melt glass.
Melilites, with a general chemical formula of (CaNa)2(AlMgFe2+)[(AlSi)SiO7], are typical high temperature products of metamorphosed impure limestone and basic alkaline volcanic minerals. Moreover, melilites were detected as common constituents of MSW slag at melting temperatures of 1150–1400°C. Comprehensive microscopy observations of thin sections indicate that melilites are among the most abundant silicate-based melt minerals in bottom ash, consisting of a series of solid solutions of end-members, of which the most important are gehlenite and akermanite. The melilites found in bottom ash usually exist as crystal aggregates embedded in a glass matrix, characterized by subhedral to euhedral microphenocrysts. Optical observation and compositional analysis by SEM/EDX indicate that the melilites usually have a similar chemical composition to that of the melt glass matrix, and that the entrapping of melilites makes the melt glass easily distinguishable from waste stream glass.
Pseudowollastonite (CaSiO3) is another silicate-based melt product of incineration, detected as radiating and fibrous aggregates. Quantitative analysis by electron microscope reveals that the pseudowollastonite in bottom ash contains a small amount of sodium, aluminum, and magnesium as additional substituents to calcium and silicon. A rough stoichiometric formula was estimated to be (Ca0.48–0.69Na0.06–0.21Mg0–0.18)(Si0.84–0.97Al0.03–0.18)O3, which implies undersaturation of cations, such as Ca, Na, and Mg. It should be noted that the pseudowollastonite shows a preference for embedding itself in high-silicon glass, and because the mandated temperature for MSWI furnaces in Japan is above 850°C, the pseudowollastonite might often not be detected in bottom ash.
The general chemical formula of spinel group minerals is A2+B23+O3, where A2+ represents a metal having a valence of plus two (commonly Fe2+, Mg2+, Zn2+, Mn2+, Ni2+, and in rare cases Cu2+, Pb2+, Co2+, Cd2+), and B represent metals having a valence of plus three (Al3+, Fe3+, Cr3+, and sometimes Ti4+). Optical microscopy and SEM/EDX analysis disclose that MSWI bottom ash contains a considerable amount of spinels group minerals, which show a preference for embedding themselves in melt glass as aggregates.
In addition to spinels, metallic inclusions are another group of minerals encapsulated in the melt glass matrix. Most of the metallic inclusions have sizes ranging from one micron (or even less) to hundreds of microns with spherical or subspherical shapes, existing as single inclusion or clustered aggregates. These metallic inclusions are the most common heavy metal-bearing phases, primarily including Fe–P, Fe–S, Fe–Cu, Cu–Sn, Cu–Zn, Cu–S, and Cu–Pb couple-dominated combinations, of which Fe–P alloys account for more than 80% of the existing metallic inclusions. Moreover, complex associations of the above couple-dominated compounds are regular products of the solid waste incineration process. Typical images of the metallic inclusions were collected and shown in the following content, in order to exhibit their characteristic properties.
Combinations of Cu and Sn, as well as affiliated metallic inclusions, were frequently detected in bottom ash. It is presumed that it was possibly a Cu-Sn combination piece that was melted in the incineration furnace, on account of its melting point of 700–900°C. The Cu element congregated forming metallic inclusions, while the Sn was oxidized to SnO2, owing to its low melting temperature. Occasionally, cuprous oxide (Cu2O) was observed as coating of Cu. No cupric oxide (CuO) was found in this study.
In addition to the melt glass phase and the various encapsulated compounds (silicate-mineral phase and nonsilicate-mineral phase), other phases, such as calcium-rich mineral phases and refractory phases, account for a great proportion of the bottom ash. It is well known that in a MSWI furnace, carbonates are calcined to form lime (CaO).
The refractory materials are products from the waste stream that survive the solid waste incineration process, consisting of waste glass, minerals, ceramics, and unburned materials. Although frequently found in bottom ash, they are of less significance in terms of their influence on heavy metals. The refractory minerals, including quartz, K-feldspar, plagioclase, biotite, and in rare cases mafic minerals (pyroxene and amphibole) and cordierite, usually remain intact, or only the rim is partially altered. The group of refractory phases, owing to their high resistance to extreme conditions (high temperatures), basically keep their original chemical compositions (compositions formed in natural conditions) during the combustion process, and the impurity components cannot easily be chemically incorporated into these phases.
As a thermodynamically unstable multicomponent material, MSWI bottom ash should undergo evolution immediately after its production. Of all the weathering reactions undergone by the bottom ash, alteration of the calcium-rich phases (e.g., carbonation) should govern the initial stage of weathering. In an effort to ratify this hypothesis, an accelerated weathering experiment was conducted at elevated experimental conditions. Subsamples of the bottom ash were collected periodically for characterization of the secondary mineralization phenomenon and corresponding mobilization behavior of the heavy metals.
In an advanced weathering stage, evolution of other phases (e.g., glass), which are comparatively resistant to external environmental factors, should take place. Silicate-based glass has been determined to be the dominant phase in bottom ash, accounting for approximately 50 wt% of the ash material. Additionally, the glass phase has long been reported as being a metastable material. The specific geochemical conditions of landfill sites (moderately to highly alkaline) should enhance the evolution process of the glass as well of as the encapsulated compounds, resulting in textural and chemical evolutions. However, it should be emphasized that the evolution of the glass phase, unlike carbonation, appears to proceed in a slow manner, and the evolution products are usually not well-crystallized phases. These characteristics necessitate the utilization of long-term weathered ash samples and effective analytical methods for studying this weathering phenomenon. In this pursuit, a ten-year naturally weathered bottom ash sample was utilized in an effort to illustrate the alteration phenomenon of glass with respect to the microanalysis results. The primary glass presents more or less uniform morphology regardless of the perfect vertical banding texture (alignments of minute metallic inclusions). The fissures in the glass matrix disconnected two pieces of glass from the parent material, which provides more accessible channels for the percolated fluid.
It is revealed that glass and nonsilicate minerals (spinels and metallic inclusions) are the most important constituents in bottom ash in respect of its safe reutilization or disposal. The amount of heavy metals in bottom ash was relatively high, and the heavy metals were principally concentrated in the nonsilicate minerals that preferentially embedded in the glass matrix.
It has been outlined that the glass phases are the main constituents of bottom ash (approximately 50 wt%), entrapping assorted mineral phases—either refractory fragments (quartz and plagioclase) or melt clusters (melilites, spinels, and metallic inclusions), and having numerous discrete vesicles and fractures that resulted from rapid quenching. In particular, the existence of these vesicles and fractures may increase accessibility to water and gas, in view of the weathering process of glass. The heterogeneous chemical composition of the melt glass with respect to its major components SiO2, CaO, Al2O3, Na2O, and K2O may suggest different weathering patterns (mechanism, weathering products, etc.).
The metals in bottom ash usually exist as various chemical combinations, e.g., several metals generally mixed together, or even with additional nonmetal elements. To gain a clear understanding of the behavior of bottom ash elements in the high temperature incineration process, particularly the behaviors of heavy metals, knowledge concerning the igneous process in geochemistry would be most helpful. According to Goldschmidt’s classification, the elements in the earth are categorized into four groups: lithophile (Na, K, Al, Si, Ti, P, O, Fe, Mn, Zn, etc.), siderophile (Fe, Ni, Cu, Zn, P, Mn etc.), chalcophile (Cu, Zn, Pb, S, Fe, Sn, etc.), and atmophile (H, N, and O). Only the elements detected in bottom ash are listed in the parentheses. Lithophile, siderophile, and chalcophile refer to the tendency of the element to partition into a silicate, metal, or sulfide liquid, respectively.
Magnetite, as well as a series of its Al-substituted and Ti-substituted varieties, is commonly identified in bottom ash. Heavy metals, such as Zn2+, Mn2+, and Cr3+, are frequently detected incorporated into these spinels in inconstant amounts. These findings are consistent with the fact that Zn2+, Mn2+, and Cr3+ have relatively high free energies (|ΔGf°|) of oxidation, correspondingly 237.9, 281.1, and 362.9 kJ per oxygen atom respectively at 827°C, indicating their easily oxidable properties.
Fe–P-dominant alloys are the most abundant metallic inclusions in bottom ash, comprising 60–95 wt% of Fe and P, and usually associated with other minor elements (Si, Ca, Al, Cu, Zn, and Pb). Apart from the Fe–P alloys, combinations of Fe and S were frequently detected in bottom ash, which provided sound evidence of the siderophile property of Fe. Cu and its combinations or compounds are used for kitchen utensils, electrical wire, pigments, catalysts, stabilizers, and many other items. These Cu-rich combinations were melted or partially melted, then recomposed, and finally formed as numerous metallic grains and scattered in the bottom ash. In this study, Cu was constantly found as metallic inclusions in thin sections of bottom ash, including element Cu (usually more than 83 wt% of Cu), alloys (5–60 wt% of Cu, mainly Cu–Sn, Cu–Fe, Cu–Zn, Cu–Pb), sulfide, and in rare cases, lower oxides of Cu (Cu2O). This is closely related to its strong resistance to oxidation, taking into account the low |ΔGf°| of oxidation at high temperature. When bottom ash is exposed to the atmosphere (as in landfilling or recycling), further oxidation of Cu metals and their combinations might take place.
The metal Pb and its compounds are used mainly for the production of Pb batteries, solder for electronic devices, paint materials, crystal glass, ink, and a variety of other useful things. Pb is of great interest not only because of its economic importance, but also because of its pollution of surrounding environments. From the viewpoint of geochemistry, Pb is chalcophile, and perhaps slightly siderophile. Pb cannot easily be incorporated into mineral species in natural systems, which might be ascribed to its large ionic radius (1.26 Å) and charge (plus two valence). However, MSWI bottom ash was identified as having certain specific behaviors. The long-term stability of these specific products should be noted, as it might be thermodynamically unstable in natural environmental systems.
A clear understanding of the distribution of the heavy metals is essential for analyzing their fate in view of disposal or recycling. Of the entire range of weathering phenomena in MSWI bottom ash, carbonation, and glass alteration are emphasized in that the corresponding weathering products play an essential role in influencing the behavior of the heavy metals. Pb, Zn, and Cu become more stable as the weathering process proceeds, which should be primarily attributed to the declined pH. Furthermore, carbonation is generally regarded as a dominant process controlling pH variation, especially in the first few decades after disposal.
Evolution of the glass phase into a relatively stable phase, although not as rapid as carbonation in the bottom ash, has been identified in 10-year naturally weathered samples. The degree of mobility of the glass elements largely depends upon the pH conditions of the percolated fluid.
Bottom ash is derived from the thermal processing of MSW. Part of the inorganic fraction of the waste turns into melt components (glass and minerals), and some of them survive the thermal processing to form heat-resistant refractory phases. The glass phases are the main constituents, accounting for approximately 50 wt% of bottom ash, with various chemical compositions. Therefore, it is not easy to suggest a uniform alteration manner upon their exposure to the environment. However, compositional values from different incineration plants show a comparative resemblance in their properties, which suggests that high temperature processing (above 850°C) of solid waste produces more or less uniform outcomes in respect of both chemical and mineralogical characteristics. All the melt mineral phases (silicate minerals and nonsilicate minerals), as well as some of the refractory phases embed in a melt glass matrix.
It is understood that the incineration process of MSW results in a significant increase in the quantity of heavy metals. Some of them are elements of environmental concern, such as Pb, Zn, Cu, Mn, and Cr. Over all the various phases in bottom ash, the nonsilicate minerals were detected to be the most significant concentrators of heavy metals. Cr, Zn, and Mn are ubiquitously incorporated into spinels, while Cu and Pb show evidence of opposite behaviors, which are substantially associated with Fe, Sn, and Zn, present as metallic inclusions bound in the silicate glass matrix. The behavior of heavy metals during the incineration process depends on their own physical and chemical properties. The geochemical classification by Goldschmidt presents a good explanation for elements of concern, especially for P, Cu, and Pb. It should be noted that, despite the heterogeneous chemical compositions of the numerous metallic inclusions, most of them were in the metallic state (not oxidized).
Generally, it was concluded that the long-term behavior of the elevated quantity of heavy metals depends to a great extent upon the behavior of the host phases, especially the weathering rate and alteration products of the calcium-rich phases and the silicate-based glass phases. Of all the factors related to the mobilization behavior of the heavy metals, the carbonation process is of particular importance with respect to their dominant role in pH variation, especially in the first few decades, in that pH is a key factor that controls the leaching behavior of the heavy metals. In addition to the newly formed carbonates, the glass-derived secondary products also contributed to the immobilization of the heavy metals. It appeared that glass-derived products should play a long-lasting role, since evolution of the glass phase proceeds in a relatively slow manner. A clear understanding of the various phases in MSWI bottom ash, as well as the possible alternation phenomenon, when subjected to weathering, is essential for prediction and explanation of the behavior of heavy metals of environmental concern.
2.11.2 Evolution of Iron-Rich Constituents in Bottom Ash
The iron content in MSWI bottom ash normally accounts for 100–200 g FeO per kg bottom ash. The mineralogical analysis of fresh MSWI bottom ash by synchrotron powder X-ray diffraction, optical microscopy, and SEM/EDX disclosed that iron principally exists as components of spinels (including magnetite (Fe3O4) and a series of Al or Ti-substituted varieties), Fe-rich metallic inclusions (Fe–P, Fe–S, Fe–Cu, Fe–Cu–Pb, etc.), hematite (Fe2O3), and unburned iron pieces. Spinels and Fe-rich metallic inclusions show a preference for embedding themselves in the glass matrix in the course of incineration, thus remaining isolated from the percolated fluid before breakdown of the glass matrix. Detailed information concerning spinels and metallic inclusions has been published in a previous paper. It should be noted that, despite the heterogeneous chemical compositions of the numerous metallic inclusions, most of them are in the metallic state (not oxidized), and should undergo chemical and mineralogical changes when exposed to the environment.
From comprehensive observation of thin section specimens of weathered bottom ash, it was found that several forms of secondary Fe-rich products developed. These secondary products occurred either as simple or as complex combinations that infilled the pores, cavities, and channels. In a comparison of the fresh and weathered bottom ash, it was noticed that hydrous iron oxides (mostly goethite and lepidocrocite) are the major secondary products from the parent Fe-rich constituents. In addition, iron may also go to other amorphous phases in association with other major and minor elements; and these amorphous phases are “gel”-like, nonstandard materials.
Goethite is one of the most stable hydrous iron oxides at ambient temperature and is, therefore, the end-member of many transformations. Fe-rich metallic inclusions were ubiquitously detected as encapsulated compounds in almost all silicate-based glass phases of bottom ash. Owing to the metallic properties of these compounds, when exposed to ambient environment, their evolution should take place.
It should be noted that dissolution of the metallic inclusions usually takes place in the course of glass alteration. The metallic inclusions primarily embed themselves in the glass matrix, which is protected from contacted with the percolated fluid. Additionally, these metallic inclusions exist mainly in their metallic forms (not oxidized). Breakdown of the glass entity directly triggers exposure to the percolated fluid, finally leading to the dissolution of the metallic inclusions. It is clear that the dissolution of the metallic inclusions depends to a large extent upon the degree of alteration of the glass matrix. In return, the dissolving of metallic inclusions may also affect the alteration behavior of the glass matrix.
Regardless of the year of disposal of the weathered bottom ash, transformation of the primary iron species (hematite, magnetite, ferrous metal fragments, etc.) was commonly detected, e.g., evolution of primary magnetite to hydrous iron oxide and corrosion of ferrous fragments. These reactions may dominate in an oxidic condition.
Hydrous iron oxides have been recognized as being solid phases which exert a significant effect on the behavior of a large range of environmentally relevant substances, particularly heavy metals. Hydrous iron oxides indirectly affect the environment by influencing the mobility of these heavy metals via functions of surface adsorption or incorporation. Since the last half of the 20th century, numerous research projects have been undertaken on the adsorption behavior of these iron oxides or iron oxide-rich materials toward pollutants. In the research field of MSWI bottom ash, hydrous iron oxides are also regarded as an important sorbent that inhibits heavy metals from leaching out of the bottom ash deposit. Generally, the growth of hydrous iron oxide and its sorption capacity of certain heavy metals in landfill depend considerably upon the properties of the prevailing geochemical conditions, particularly the property of the percolated fluid (e.g., pH, ion varieties and concentrations). Hence, the microstructure and chemical compositions of these secondary products may disclose the evolution history of the Fe-rich constituents as a function of time.
From the microanalysis and bulk analysis of MSWI bottom ash, it was realized that the primary and secondary phases, including magnetite, metallic inclusions, hematite, iron fragments, and hydrous iron oxide, are the major Fe-rich constituents, and transformations frequently occur among these constituents. Such transformation reactions occur under the influence of the prevailing geochemical conditions during the weathering process of bottom ash. The metallic inclusions in bottom ash are primarily iron-rich compounds, and usually associated with different proportions of nonhazardous elements (P, S, Si, Ca, Al, etc.) as well as hazardous elements (Cu, Zn, Pb, Ni, etc.). In addition, these metallic inclusions in the bottom ash exist largely in the metallic state. It was understood that such metallic inclusions are important concentrators for heavy metals, and so evolution of these specific products should be taken into consideration, as they might be thermodynamically unstable once exposed to an oxidized condition.
Regardless of the physical and chemical reactions involved in the weathering process of bottom ash, electrochemical corrosion may also be held responsible for dissolution of the metal fragments and metallic inclusions under appropriate geochemical conditions. For instance, the abundance of various ions in the percolated fluid (e.g., Cl−, SO42−, Ca2+, Na+, etc.) could enhance the corrosion process of iron. It has been reported that the reactivity of zero-valent iron surfaces was maintained at a high level when corrosion proceeded in high ionic strength water in the presence of oxygen. Besides, the presence of chloride ions was reported to be a major factor in promoting further corrosion of iron. Although the exact roles of these geochemical parameters in accelerating the corrosion process of iron is still not clear, there is no doubt that they are essential factors for the long-term behavior of the Fe-rich constituents (especially metal fragments and metallic inclusions) of bottom ash.
Based on the above discussions, it was perceived that the transformation of the various Fe-rich constituents is controlled primarily by the percolated fluid. Conversely, the properties of the percolated fluid could also be affected by the evolution process of the Fe-rich constituents.
Despite the chemical forms of iron in MSWI bottom ash, formation of secondary Fe-rich products (including goethite, lepidocrocite, hematite, magnetite, wustite, Fe–Si-rich gel phase) invariably occurred in the vicinity of the primary Fe-rich constituents (magnetite, hematite, Fe-rich metallic inclusions, etc.). Of all these secondary products, goethite is thermodynamically the most stable one under oxidic conditions and therefore constitutes the end-members of many transformation routes in the weathered bottom ash. The ubiquitous development of the goethite could be ascribed to the prevailing geochemical environment (alkaline and oxidic) in landfill site. The degree of the transformation between the various Fe-rich materials, to a large extent, depends upon the timescale of the weathering of the ash. Hematite and ferric metal are usually the most accessible materials and their evolution occurs in the early stages of weathering. Alterations of magnetite and Fe-rich metallic inclusions, in that they preferentially embed themselves in the melt glass matrix, take place together with breakdown of the glass matrix; thus, their transformation should predominate at a later stage of ash weathering.
It should be noted that natural weathering of bottom ash is quite significant from the standpoint of environmental safety, as might be expected from the fact that all the reactions involved in such evolutions may locally change the properties of the percolated fluid by entrapping the dissolved heavy metals (Pb, Zn, Ni, Cu, etc.). Consequently, the quantity of such heavy metals leaching from the ash deposit is expected to diminish. In this process it can be seen that transformation of the various Fe-rich materials in the naturally weathered bottom ash plays an indispensable role, as it could enhance the stability of the ash materials and minimize the potential risks to surroundings. Furthermore, immobilization of heavy metals by these secondary phases also enhances the feasibility of excavation of the landfilled (naturally weathered) MSWI bottom ash as construction materials, which enables the disposal of more fresh MSWI residues into the landfill site.