Solidification/Stabilization Process of Fly Ash
Abstract
It is crucial to solidify/stabilize fly ash before its utilization or incorporating in landfill, owing to leaching toxicity. To date, solidification methods such as cement solidification and chemical solidification are widely used in China for their high efficiency and easy application. The chemical solidification method involves the transformation of hazardous waste into materials with low solubility, migration, and toxicity, by using the chemical reagents. Different chemicals, including gypsum, bleaching powder, sodium thiosulfate and sodium hydroxide, sodium sulfide, and organic polymer stabilizers can be utilized based on the kinds of heavy metals. Four types of chemicals were used, i.e., NaOH, ethylenediaminetetraacetic acid disodium, sodium sulfide, and thiourea, to explore the possibility of chemical stabilization for fly ash treatment. At last, a high-pressure formation method is performed to reduce landfill volume. Under a certain degree of pressure, fly ash can be shaped using a hydraulic press and specific mold, which can greatly reduce the amount of chemical agents required, as well as running costs.
Keywords
Fly ash; solidification; stabilization; organic chelating polymer; high-pressure formation; leaching toxicity
8.1 Solidification/Stabilization Process of Fly Ash
Fly ash can be treated in different ways. The traditional method, such as direct dumping, has been prohibited. The most frequent means used include
1. placement in a security landfill,
2. immobilization through solidification (cement) and then landfill or reuse and vitrification, and
3. separation of heavy metals by volatilization at high temperature or hydrometallurgical extraction by dissolution in an acidic or alkaline medium.
The objectives of solidification/stabilization are to achieve and maintain the desired physical properties of solidified waste with good antipermeability, leaching resistance, resistance to freezing and thawing, and adequate mechanical strength. Even better, the solidified waste could be recycled as resources, such as building foundation and subgrade materials. Moreover, the solidification/stabilization process should be simple and convenient, with a lower material and energy consumption as well as enlargement ratio. Meanwhile, the solidifying agent, which is abundant in source and cheap in price, should decrease the processing costs of the solidification/stabilization method.
The solidification/stabilization method utilizes chemically reactive agents to form stable solids that are nonhazardous, or less hazardous than the original materials. Solidification refers to the physical changes in the contaminated material when a certain binding agent is added. These changes include an increase in compressive strength, a decrease in permeability, and condensation of hazardous materials. Stabilization refers to the chemical changes that take place between the stabilizing agent (binding agent) and the hazardous constituents. The results of these changes should include a less soluble, less toxic constituent with hindered mobility. Although there are some differences in the concept between solidification and stabilization, the in situ technologies usually include the two methods, with different emphasis in any given project.
Most of the requirements above are principal, since one method or product can achieve all the objectives. When there is an outstanding result in a comprehensive comparison, it can be accepted and developed in practical application. There are plenty of pretreatment methods for fly ash. Table 8.1 shows the currently typical solidifying agents.
Table 8.1
Common Solidifying Agents for Fly Ash
| No. | Category | Solidifying Agents |
| 1 | Cements | Cements |
| 2 | Iron oxides, ferrite | |
| 3 | Phosphates | |
| 4 | Silicates | |
| 5 | Sulfide | Na2S, FeSO4, NaHS, polysulfide |
| 6 | Organic solidifying agents or chelators | EDTA |
| 7 | Organic acids (thiodiglycolic acid, sixthio guanidine acid) | |
| 8 | Thiourea | |
| 9 | Sodium diethyldithiocarbamate trihydrate | |
| 10 | Pyrrolidines | |
| 11 | Amines | |
| 12 | Carbamates | |
| 13 | Thiols |

The placement of fly ash in a security landfill will have an irreversible effect on the environment, as the heavy metals in the ash would never disappear in the landfill. Moreover, the cost of such a disposal method is unacceptably high in many cases, as the investment for a security landfill is always high. Immobilization using cement and other solidification agents often shows relatively low stabilization efficiency, according to long-term leachability tests. The capability of a cement matrix to capture and stabilize heavy metals is limited and it may deteriorate after long-term storage in a security landfill or in recycling uses such as road paving and surfacing material. Moreover, the volume of the solidified products usually increases considerably, leading to an increase in transportation cost. Modifications have been implemented to increase the long-term stabilization effects for the cement method. Three principally different cement solidification technologies exist:
1. solidification of unwashed (original) fly ash;
The first method produces a residue with high chlorine and heavy metal content levels. Consequently, a large amount of expensive high-quality cement with good hydraulic properties is used. In the second method, the soluble heavy metal chlorides are transformed into heavy metal hydroxides, which precipitate. After filtration and solidification with a small amount of cement, this process yields a residue with low chlorine content but high heavy metal content. However, the heavy metals will continuously release, though slowly, in a wet environment. Most heavy metals may be removed when the fly ash is washed in an acidic medium, which is actually a hydrometallurgical process, as most of the metals may dissolve in the acidic solution. Post-solidification treatment for the resultant residue seems unnecessary.
Evaporation or vitrification of heavy metals in the fly ash at high temperature have obvious shortcomings owing to their extremely high energy consumption and need for heavy investment in the treatment equipment. This is not a suitable and cost-effective technology for a small- or medium-scale incinerator.
8.2 Characterization and Leaching Toxicity of Fly Ash Used
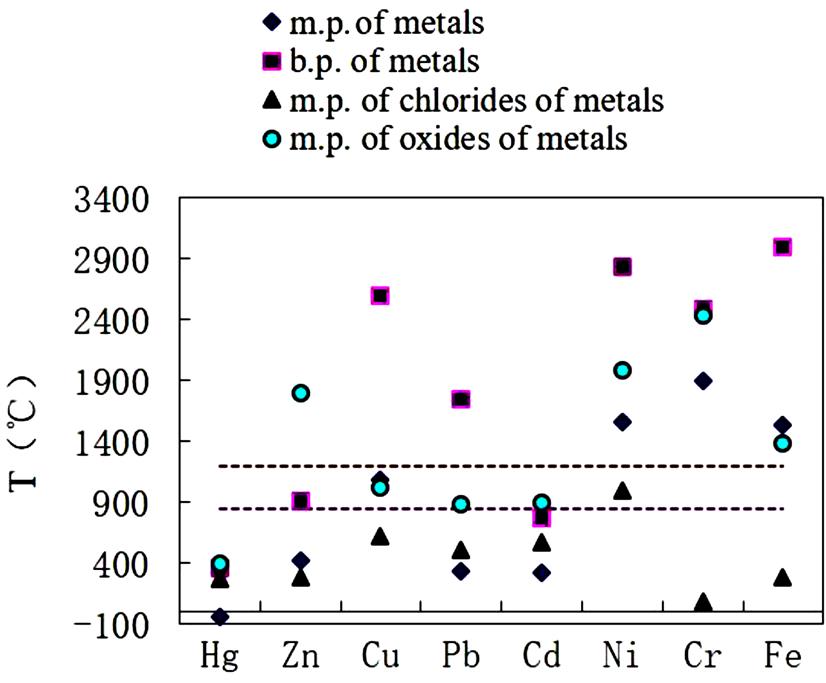
The elemental content of the fly ash is closely related to the melting and boiling points of the elements (metals or compounds) concerned (Table 8.2). In general, the lower the melting and boiling points for either elements or compounds (oxides, sulfates, and chlorides), the higher the content levels of the corresponding elements in the fly ash.
Table 8.2
Composition of the Fly Ashes Used (mg/kg)
| Hg | Zn | Cu | Pb | Cd | Ni | Cr | Fe | |
| Sample I | 49 | 4382 | 296 | 1480 | 24.6 | 60.1 | 115 | 25,742 |
| Sample II | 55 | 4389 | 330 | 1512 | 26.4 | 61.5 | 121 | 25,812 |
| Average | 52 | 4386 | 313 | 1496 | 25.5 | 60.8 | 118 | 25,777 |

Fig. 8.1 shows the boiling and melting points of the metals and their compounds of interest, as well as the temperature ranges of incineration in the Changzhou Municipal Solid Waste (MSW) Incinerator, as indicated between the two dotted lines. The metals of Ni, Cr, and Fe, and the oxides of Zn, Ni, Cr, and Fe would be less volatized, as the melting points of these compounds and metals fall above the incineration temperature range. However, the melting points of all the chlorides and most sulfates, and metallic Hg, Zn, Cu, Pb, and Cd, fall in or below this range, which implies that these substances would be more volatile under incineration conditions. Hence, it may be proposed that the heavy metals in the fly ash possibly originate from the vitalization of chlorides and sulfates of Zn, Ni, Cr, and Fe and chlorides, sulfate, and oxides of the other metals, and metallic elements for Hg, Zn, Cu, Pb, and Cd. Moreover, the metallic forms may be oxidized into oxides in the incineration process and then volatized as flue gas, as confirmed in the leachability tests.
Table 8.3 shows the leaching toxicity of the fly ash, determined using the China Toxicity Characteristic Leaching Procedure (TCLP) Standard, as described in the experimental section. It can be seen from Table 8.4 that 49% Cd, 16% Pb, 13% Zn, 6% Ni, and a few other metals can be leached out from the ash. Considering the great solubility of chlorides and sulfates of Zn, Cu, and Ni, these elements should be present predominantly as insoluble oxides in the fly ash. Nevertheless, as described above, the volatilization of oxides of these metals is poor. This implies that the oxides in the fly ash should originate from the chemical reactions that have taken place during the incineration process, including decomposition of sulfates and chlorides. However, it is somewhat difficult to reach such a conclusion for Hg, Pb, and Cr, as the solubility of their salts is much lower and the leachability would be also lower. Hence, the leachability toxicity of the fly ash may be reduced if the heavy metals can be transformed into oxides.
Table 8.3
The Physical Properties of Metals Concerned and Their Compounds
| Element | Melting Point (°C) | Boiling Point (°C) | Oxides | Chlorides | Sulfates |
| Hg | −39 | 357 | Decomposable above 400°C | M.P. 275°C, B.P. 301°C | Decomposable at the M.P. |
| Zn | 419 | 907 | Volatilization at 1800°C | M.P. 283°C, sublimation under calcination | Decomposable under calcination |
| Cu | 1083 | 2595 | M.P. 1026°C | M.P. 620°C, decomposable at 993°C | Decomposable at 560°C |
| Pb | 327 | 1744 | M.P. 886°C, B.P. 1516°C | M.P. 501°C, B.P. 950°C | M.P. 1170°C |
| Cd | 321 | 767 | Sublimation at 900°C | M.P. 570°C, B.P. 960°C | M.P. 1000°C |
| Ni | 1555 | 2837 | M.P. 1980°C | M.P. 1001°C | M.P. 31.5°C |
| Cr | 1900 | 2480 | M.P. 2435°C, B.P. 3000°C | M.P. 83°C | Decomposable at high temperature |
| Fe | 1535 | 3000 | M.P. 1377°C, decomposable at 3410°C | M.P. 282°C, B.P. 316°C | Decomposable at high temperature |

Table 8.4
Leachability Toxicity of Fly Ash (mg/L)
| Elements | Concentrations in the Leach Solution | Leachability (%) | Toxicity Standards Set in China | ||
| First Analysis | Second Analysis | Average | |||
| Hg | 0.0346 | 0.0309 | 0.03275 | 0.6 | 0.05 |
| Zn | 56.66 | 57.80 | 57.23 | 13.05 | 50 |
| Cu | 0.71771 | 0.70567 | 0.71169 | 2.27 | 50 |
| Pb | 23.96 | 25.15 | 24.56 | 16.42 | 3.0 |
| Ni | 0.30101 | 0.38794 | 0.34448 | 5.67 | 25 |
| Cd | 1.2057 | 1.3145 | 1.2601 | 49.42 | 0.3 |
| Cr | 0.13881 | 0.13575 | 0.13683 | 1.16 | 1.5 |
8.3 Solidification Methods for Fly Ash
8.3.1 Cement Solidification Process
The cement solidification method involves the mixture of fly ash, cement, and water in a certain proportion. By using the hydration reaction of active materials including SiO2, Al2O3, and CaO, etc., heavy metals can be bound in the cement in the form of hydroxides or complexes formed by different modes of hydration including adsorption, chemical absorption, sedimentation, ion exchange, and passivation. Meanwhile, cement can provide an alkaline environment which can inhibit the infiltration of heavy metals.
There are many kinds of cement used as solidification reagents, including ordinary Portland cement, Portland blast furnace slag cement, portland-pozzolana cement, alumina cement, and zeolite cement. The selection of solidification reagents should focus on the types and characteristics of waste, as well as the requirements of the solidification reagents. As a well-understood method for hazardous waste disposal, cement solidification has products including different shapes of granular, soil, or solid block, which depend on the combination of the cement content and the moisture content of waste. Also, it has the advantages of simple operation and technique, abundance and low cost, etc. However, it has the disadvantages of a higher volume enlargement ratio (1.5–2), and lower leaching character resistance. Moreover, toxic substances could gradually leach, which incurs a potential risk to the environment.
For a comparison, both traditional solidification methods using cement and asphalt and the latest methods using chemicals were studied in this project. For cement and asphalt solidification methods, ordinary Portland cement with No. 425 and No. 325 was used. A given weight of cement or asphalt was mixed with a similar weight of fly ash and added water, stirred and then injected into the mold with an effective volume of 70.7 × 70.7 × 70.7 mm. It was then dried naturally at room temperature. After 4 weeks of drying, the solidified products were broken into fine particles and then screened using a screener with a porosity of 5 mm. The screened powder was used for toxicity analysis. The results are presented in Tables 8.4–8.6. Obviously, higher cement or asphalt content levels in the solidified products will result in lower leachability. The selection of the ratios depends on the leaching toxicity limit. According to the China TCLP standard, the ratios of fly ash to cement should be lower than 2:1 for both types of cement (Tables 8.4 and 8.5) and the asphalt (Table 8.6) used, so that the leachability of all heavy metals can reach the standard (Table 8.7).
Table 8.5
Leachability of the Solidified Products Using No. 325# Cement (mg/L in the Leaching Solution)
| Sample No. | 1 | 2 | 3 | 4 | 5 |
| Ratios of fly ash to cement (g:g) | 3:1 1200:400 |
2:1 1000:500 |
1:1 800:800 |
1:2 500:1000 |
1:3 400:1200 |
| Zn | 12.937 | 3.3359 | 2.4326 | 2.4780 | 1.5321 |
| Cu | 0.67589 | 0.38451 | 0.22357 | 0.24510 | 0.17645 |
| Pb | 4.8976 | 1.8462 | 1.0024 | 1.0243 | 0.86542 |
| Cd | 0.10234 | 0.031274 | 0.020135 | 0.021347 | 0.021084 |
| Ni | 0.28025 | 0.31279 | 0.62590 | 0.72395 | 0.69637 |
| Cr | 0.28579 | 0.20965 | 0.19435 | 0.17463 | 0.17652 |

Table 8.6
Leachability of the Solidified Products Using No. 425# Cement (mg/L in the Leaching Solution)
| Sample No. | 1 | 2 | 3 | 4 | 5 |
| Ratios of fly ash to cement (g:g) | 3:1 1200:400 |
2:1 1000:500 |
1:1 800:800 |
1:2 500:1000 |
1:3 400:1200 |
| Zn | 15.024 | 4.2924 | 2.8618 | 2.5493 | 1.7405 |
| Cu | 0.70040 | 0.43212 | 0.25989 | 0.21370 | 0.18839 |
| Pb | 5.5777 | 1.9180 | 1.0596 | 1.0286 | 0.81516 |
| Cd | 0.10560 | 0.032977 | 0.019462 | 0.022526 | 0.021084 |
| Ni | 0.26025 | 0.14633 | 0.18070 | 0.38694 | 0.42217 |
| Cr | 0.37570 | 0.20271 | 0.18290 | 0.21820 | 0.23739 |
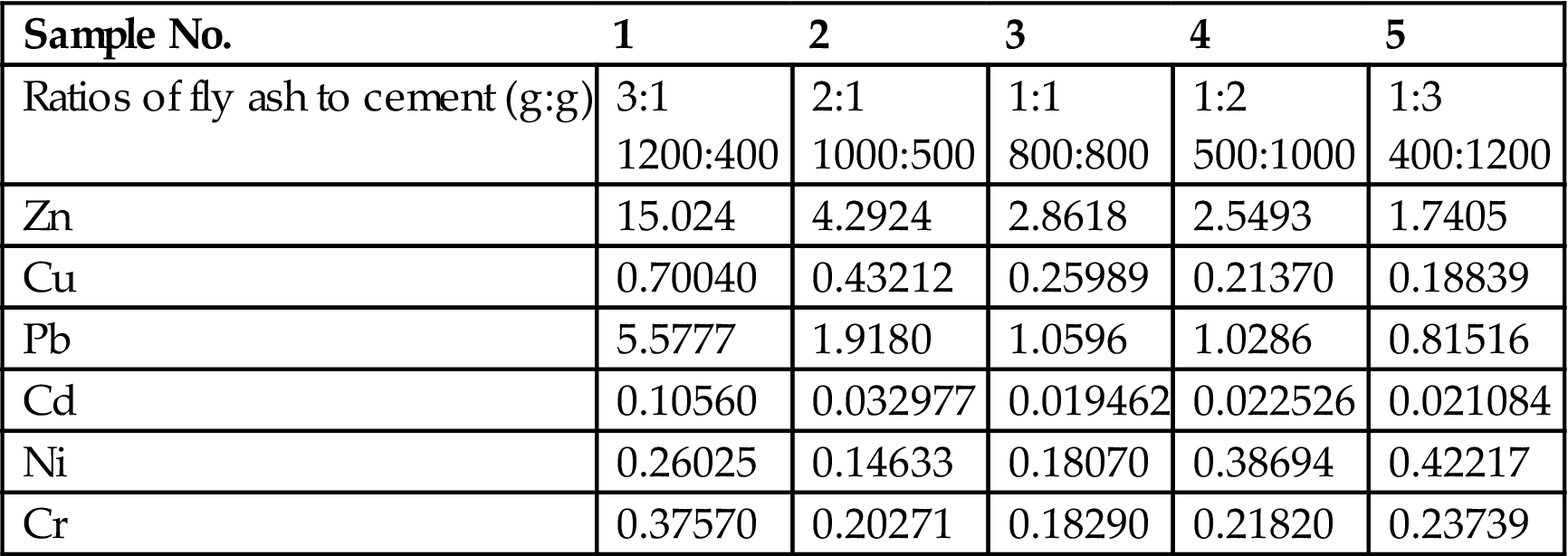
Table 8.7
Leachability of the Solidified Products Using Asphalt (mg/L in the Leaching Solution)
| Sample No. | 1 | 2 | 3 | 4 | 5 |
| Ratios of fly ash to asphalt (g:g) | 3:1 1200:400 |
2:1 1000:500 |
1:1 800:800 |
1:2 500:1000 |
1:3 400:1200 |
| Pb | 4.2377 | 1.2180 | 0.87822 | 0.45861 | 0.31516 |
| Cd | 0.014867 | 0.012342 | 0.0086957 | 0.0078541 | 0.0061711 |

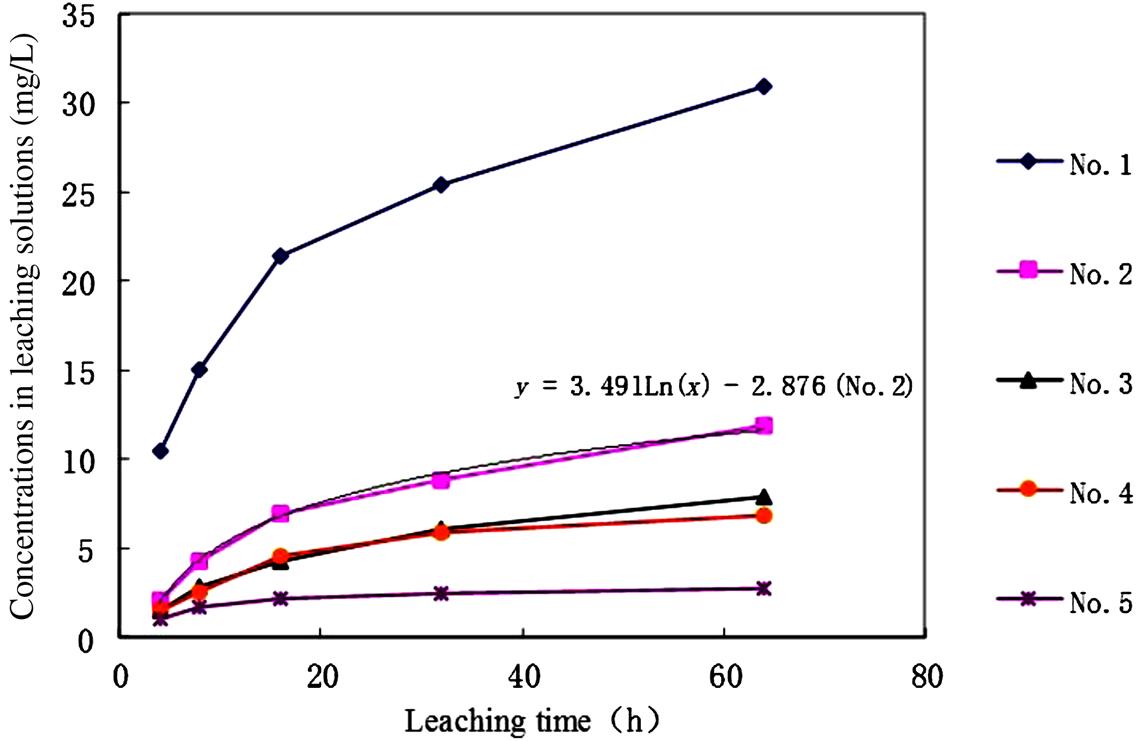
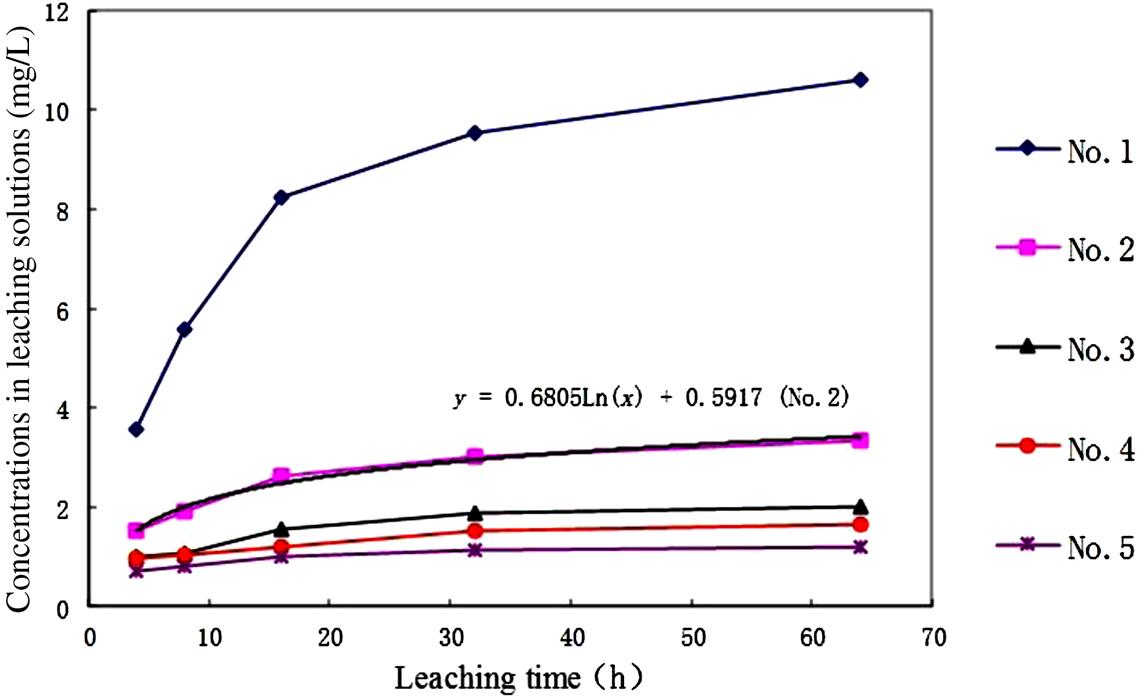
However, the solidification seems unable to fix the heavy metals firmly in the matrix of the solidified products when they are exposed to the aqueous solution for a long time, as shown in Figs. 8.2 and 8.3. Only Zn and Pb are observed, as the other metals have lower leachability.
Obviously, the leachability of Zn and Pb increases as the leaching time extends. It is easy to get an equation for No. 2 sample, as shown in the figures. It can be calculated from these two equations that it would take many years for Zn and 34 h for Pb to be leached to the level of the toxicity standard set by China, under the leaching conditions used (pH 5.8–6.8, while stirring). Hence, Pb would be more easily leached and should be especially controlled. Certainly, the solidified products would not be put in such an environmental medium, and the leaching rate would be much lower in the practical situation. However, it can be concluded that the cement-solidified product is not persistent as required by the increasingly stringent environmental constraints. The encapsulated heavy metals in the solidified products will be released gradually in the final disposal sites.
8.3.2 Pozzolanic Solidification Process
By using the pozzolanic characteristics of waste, in materials such as coal ash, fluidized bed incineration fly ash, cement kiln ash, and blast furnace slag after processing, heavy metals can be immobilized, and the products can be shaped into soft, fine particles or hard lumps. However, quantity added is higher than in the cement method, and so the weight of waste increased after treatment.
8.3.3 Thermoplastic Solidification Process
Thermoplastic material is one kind of special material with superior heat and resistance, for example, asphalt, paraffin, and polyethylene. The asphalt solidification method has the advantages of delivering small gap and high-density products. Thermoplastic material is characteristically leakproof for most solutions. Therefore, the pollutant release rate the of product is lower than those of others. The leaching ratio is 2–3 magnitudes smaller than that occurring with the cement solidification method, which is 10−4–10−6 g/cm2 d. However, this method requires expensive equipment and skilled workers. Owing to its flammability, most thermoplastic material carries the risk of volatility in operation. The asphalt solidification method is not suitable for waste with high salt content (nitrate or chloride), and thereby it is inappropriate for the treatment of fly ash.
8.3.4 Chemical Solidification Process
The chemical solidification method involves the transformation of hazardous waste into materials with low solubility, migration, and toxicity, by using chemical reagents. Compared with other solidification/stabilization methods, the chemical solidification method for fly ash has the advantages of simple technique and good solidification and stabilization results, as well as being cheap.
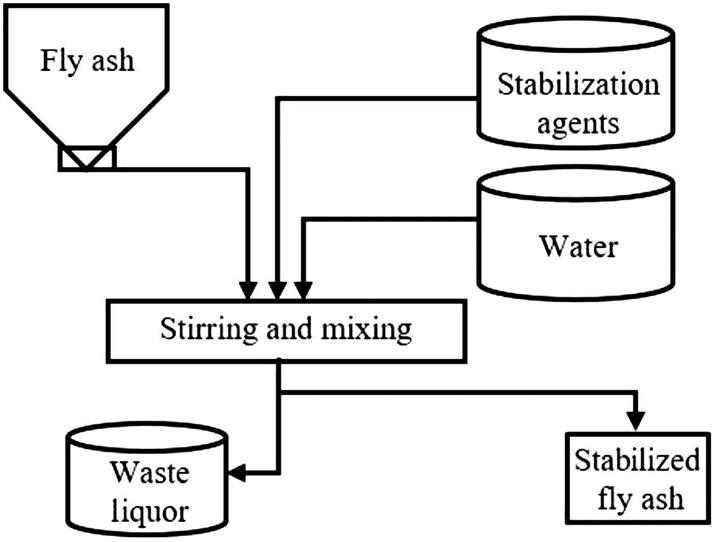
When dealing with hazardous waste, different chemicals, including gypsum, bleaching powder, sodium thiosulfate and sodium hydroxide, sodium sulfide, and organic polymer stabilizers such as heavy metal rock mixtures, phosphate, etc. can be utilized, based on the kinds of heavy metals involved. The most obvious characteristic of the chemical solidification method is that the volume enlargement ratio is far lower than those of other methods. Fig. 8.4 shows the technological process of the chemical solidification method for fly ash.
1. Phosphate stabilization
A phosphate radical (PO43−) can combine with more than thirty elements in fly ash and bottom ash, and there are more than three hundred kinds of natural mineral substances formed that have good stability for pH, and oxidation-reduction potential. It has been confirmed that the dissolvability of metal in fly ash, bottom ash, or a mixture can be controlled efficiently by adding phosphoric acid or orthophosphate. Moreover, the phosphate substances formed are of long-term geochemical stability. The phosphate radical (PO43−) has an excellent capacity of stabilizing bivalent heavy metal ions (Pb2+, Zn2+, Cd2+, etc.), especially for Pb2+. The cost of phosphate stabilization could be higher than that of cement stabilization, while the less weight gain can be obtained for the former. Furthermore, phosphate stabilization is an important method for fly ash treatment in America.
2. Ferrous sulfate stabilization
Alkaline fly ash was mixed into a saturated FeSO4 solution, Fe(OH)2 was formed, then settled immediately. The suspending liquid was aerated, and Fe(OH)2 was oxidated to ferric oxide. Meanwhile, the trace metal was combined with ferric oxide, so that the trace metal content decreased, and the soluble salts were removed from fly ash, for dissolving into the solution. The principle of ferrous sulfate stabilization is that Fe (II) is transformed into Fe (III) with oxygen and water, and the ion reaction is as follows:
The FeOOH thus formed is of large specific surface area, and stabilizes fly ash in a way lying between chemical bond attraction and physical adsorption.
3. Sulfide stabilization
Sulfides, including inorganic sulfides (such as Na2S) and organic sulfide, are used as mercury adsorbents for flue gas purification systems and precipitants for waste water treatment in a wet scrubbing system. The sulfide stabilization is effectual under normal operating parameters, while the stability of organic sulfide is disputed with reference to biodegradation.
4. Polymeric chelant stabilization
Polymeric chelant stabilization has a good effect on fly ash stabilization. For the same metal, the product from polymeric chelant stabilization was more stable than hydroxide, carbonate, and sulfide, while the cost is also the highest among the chemical agent stabilization systems. Though there are many kinds of chelants, some chelants could result in secondary pollution. Caution is required in evaluating chelant when applying.
5. Mixed chemicals stabilization
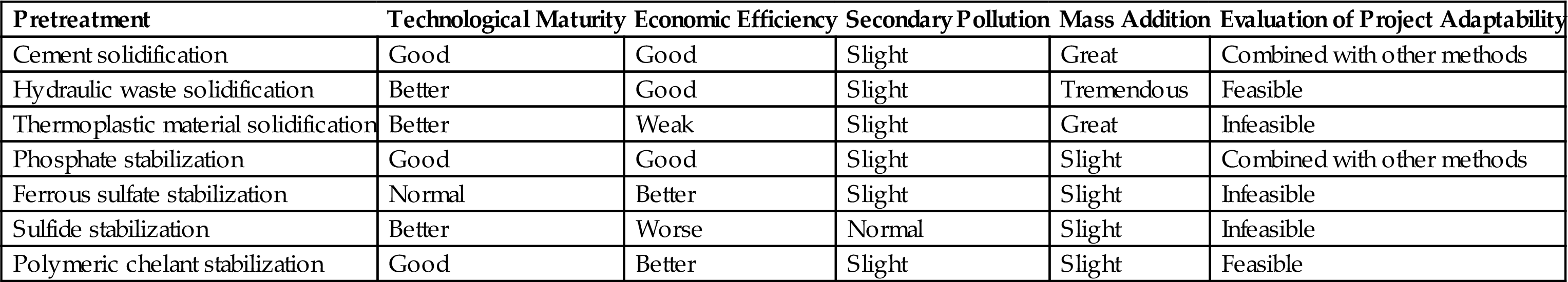
For the best cost performance, two or more methods are often combined in practical engineering, such as chemical stabilization+cement solidification, chemical stabilization+thermal treatment, water scrubbing+thermal treatment, water scrubbing+chemical stabilization+low-temperature sintering, water (acid) scrubbing+chemical stabilization, polymeric chelant stabilization+cement solidification, and so on, as shown in Table 8.8.
Table 8.8
Comparison of Solidification/Stabilization Processes
| Pretreatment | Technological Maturity | Economic Efficiency | Secondary Pollution | Mass Addition | Evaluation of Project Adaptability |
| Cement solidification | Good | Good | Slight | Great | Combined with other methods |
| Hydraulic waste solidification | Better | Good | Slight | Tremendous | Feasible |
| Thermoplastic material solidification | Better | Weak | Slight | Great | Infeasible |
| Phosphate stabilization | Good | Good | Slight | Slight | Combined with other methods |
| Ferrous sulfate stabilization | Normal | Better | Slight | Slight | Infeasible |
| Sulfide stabilization | Better | Worse | Normal | Slight | Infeasible |
| Polymeric chelant stabilization | Good | Better | Slight | Slight | Feasible |
8.4 Treatment of Fly Ash with Four Stabilizers
There are considered to be many naturally stabilized chemical forms of heavy metals in nature. Hence it is completely possible to convert the heavy metals in fly ash to their naturally stabilized chemical forms, such as sulfides. An alternative is to extract the heavy metals from the fly ash by acidic or alkaline treatment, so that their contents can be reduced to an environmentally acceptable level. In this task, four types of chemicals were used, i.e., NaOH, ethylenediaminetetraacetic acid disodium (EDTA), sodium sulfide, and thiourea, to explore the possibility of chemical stabilization for fly ash treatment.
8.4.1 Treatment of Fly Ash with NaOH Solution
To 10 g of the fly ash, add 100 mL of 0.1 mol/L, 0.5 mol/L, 1 mol/L, 2 mol/L, and 5 mol/L NaOH solution, stirred on the oscillator for 24 h, and then left to stand for 16 h. It was then filtered, and the aqueous solution analyzed. The leaching residue was also dissolved in a nitric acid solution, and the heavy metal contents determined. Zn, Pb, and Cd appeared in the samples.
Table 8.9 shows the Pb and Cd content levels in the leaching solutions and leaching residues. The quantities of lead extracted increased with the increases of initial NaOH concentrations. This phenomenon can be found also in the alkaline treatment of Electric Arc Furnace dusts and oxidized zinc ores. Inorganic compounds of lead, especially oxides, carbonates, phosphates, arsenates, etc., can dissolve in both strong acidic and strong alkaline solutions. However, as predicted, the extraction of Cd remains unchanged as the initial NaOH concentrations increase.
Table 8.9
| No. | 1 | 2 | 3 | 4 | 5 | |
| NaOH (mol/L) | 0.1 | 0.5 | 1 | 2 | 5 | |
| Pb | Concentration in the leaching solutions (mg/L) | 29.83 | 36.21 | 60.98 | 72.18 | 85.02 |
| Pb leached (%) | 19.94 | 24.20 | 40.76 | 48.25 | 56.83 | |
| Content in the leaching residues (mg/kg) | 1196 | 1122 | 868 | 763 | 628 | |
| Cd | Concentration in the leaching solutions (mg/L) | 0.53290 | 0.53316 | 0.52143 | 0.50499 | 0.52917 |
| Cd leached (%) | 20.90 | 20.91 | 20.45 | 19.80 | 20.75 | |
| Contents in the leaching residues (mg/kg) | 20.40 | 20.15 | 20.27 | 20.45 | 20.19 | |
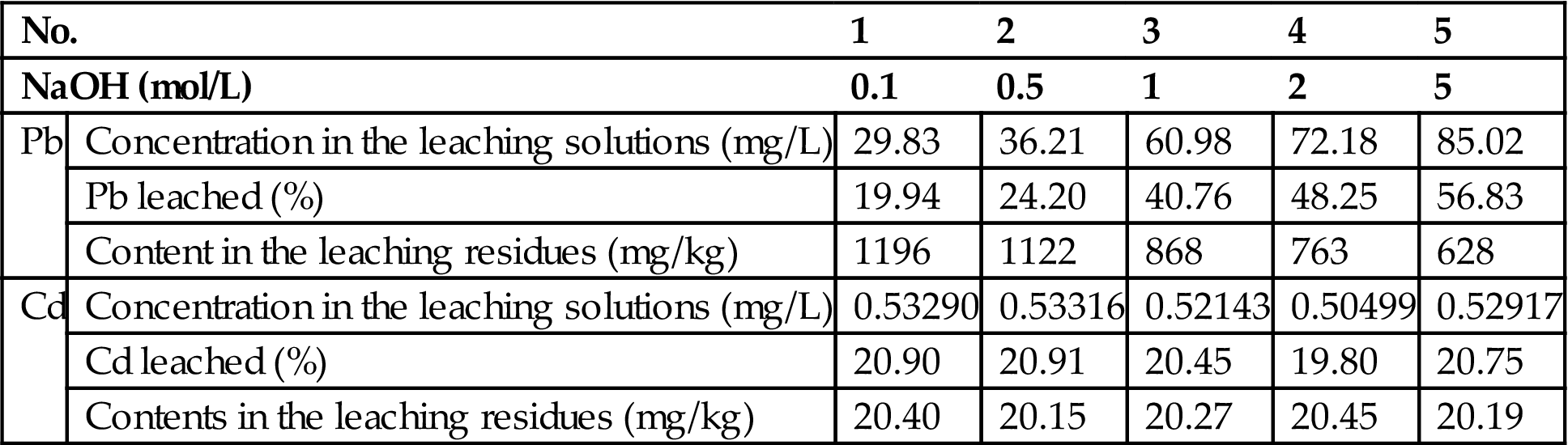
The dependence of lead and cadmium leaching on pH and NaOH concentrations clearly shows that extraction of lead increases significantly, while that of cadmium decreases as pH values or NaOH concentrations increase (Fig. 8.5). The chemical reactions can be expressed as follows.
At lower pH values, the oxides of lead and cadmium dissolve in acidic leaching solutions:
As pH values increase, insoluble lead and cadmium hydroxides form, so that the leaching rate decreases until reaching pH 9:
Lead hydroxides dissolve when pH (owing to NaOH concentrations) increases so that leaching increases, while the leaching of cadmium remains at a lower value, contributed by chemical equilibrium:
In strong NaOH solutions, PbSO4 can also dissolve to form soluble Na2Pb(OH)2SO4:
Moreover, the Pb and Cd in the leaching residues are at 628 mg/kg and 20.19 mg/kg respectively, and even the fly ash is treated at 5 M NaOH solutions. According to Table 8.9, around 20% and 56% respectively of Pb and Cd can be leached at 5 M NaOH solution, and then the possible concentrations in the leaching solutions of the residues would be 10.3 mg/L for Pb and 0.998 mg/L for Cd, which are beyond the scope of the leaching toxicity standard. Therefore, NaOH solutions can be used for the part extraction and recovery of lead and zinc from, but not for ultimate stabilization of, the fly ash.
8.4.2 Treatment of Fly Ash with EDTA Solution
It is considered that EDTA salt is an excellent complex agent and may be used for the stabilization of heavy metals in fly ash by dissolving the soluble salts so that the leaching toxicity can be reduced.
To 10 g of the fly ash, add 100 mL of EDTA solution with 0.01 mol/L, 0.02 mol/L, 0.05 mol/L, 0.1 mol/L, and 0.2 mol/L, leached at ambient temperature for 24 h in an oscillator with a frequency of 110 ± 10/min. Leave to stand for 16 h and then filter. Analyze the aqueous solution using ICP. The leaching residues were dissolved in a nitric acid solution after thoroughly washing with water, and the contents of Pb and Cd determined. The results are shown in Table 8.10.
Table 8.10
Extraction and Stabilization of Fly Ash by Treatment with EDTA Solutions
| No. | 1 | 2 | 3 | 4 | 5 | |
| EDTA(mol/L) | 0.01 | 0.02 | 0.05 | 0.1 | 0.2 | |
| Pb | Concentration in leaching solutions (mg/L) | 27.91 | 35.74 | 90.63 | 108.6 | 118.2 |
| Pb leached (%) | 18.66 | 23.89 | 60.58 | 72.59 | 79.01 | |
| Content in leaching residues (mg/kg) | 1226 | 1137 | 568 | 434 | 314 | |
| Cd | Concentration in leaching solutions (mg/L) | 1.2875 | 1.3950 | 1.8020 | 1.8673 | 1.9128 |
| Cd leached (%) | 50.49 | 54.70 | 70.67 | 73.23 | 75.01 | |
| Content in leaching residues (mg/kg) | 12.75 | 11.47 | 7.651 | 6.630 | 6.375 | |
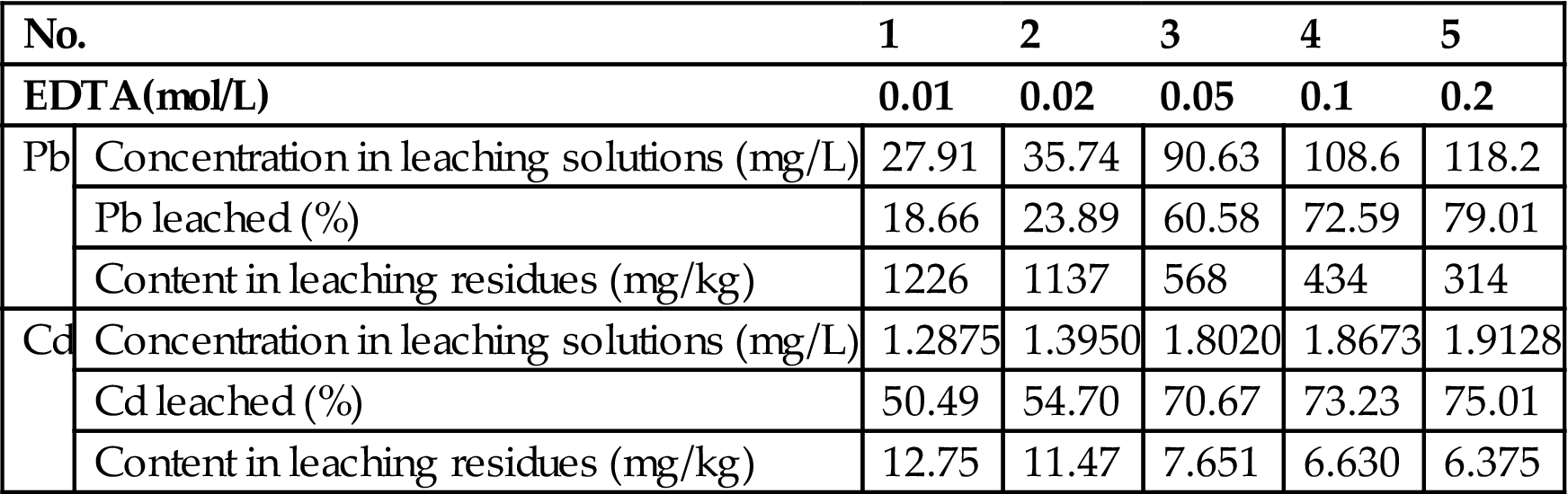
It is clear that extraction of both lead and cadmium from fly ash increases with the increase of EDTA concentrations. Over 70% of both Pb and Cd can be leached at an EDTA concentration of 0.1 M. Hence, the leachability toxicity would be reduced after the fly ash is treated with EDTA solutions.
When EDTA concentration was increased from 0.05 to 0.1 mol/L, the leaching of Pb and Cd increased from 60.58% and 70.67% to 72.59% and 73.23%, respectively. Hence, an EDTA solution with a concentration of 0.05 mol/L may be used to assess the leaching in practical applications. In this case, the content levels of Pb and Cd in the leaching residues were 568 and 7.6 mg/kg, reaching the leachability toxicity standard.
8.4.3 Stabilization with Sodium Sulfide (Na2S)
The sulfides of lead, zinc, cadmium, etc., have been present in nature for a long time. An effective approach to stabilize the heavy metals therefore is by transferring the soluble forms of sulfides to their insoluble forms. The solubility products of the heavy metals decrease in a sequence as
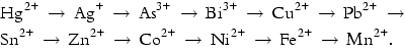
The former metal will be precipitated in preference to the latter.
To 10 g of the fly ash, add 0.1795 g, 0.5 g, 1 g, 2 g, 4 g, and 6 g Na2S·9H2O, and 100 mL water. Allow leaching for 24 h in an oscillator with a frequency of 110 ± 10/min. Leave the resulting mixture to stand for 16 h and then filter. Analyze Pb and Cd content in the aqueous solutions using ICP. The results are shown in Table 8.11.
Table 8.11
Stabilization of Fly Ash with Na2S
| No. | 1 | 2 | 3 | 4 | 5 | 6 | |
| Na2S·9H2O added (g) | 0.1795 | 0.5 | 1 | 2 | 4 | 6 | |
| S2+ (mol) | 0.00075 | 0.00208 | 0.00416 | 0.00833 | 0.01665 | 0.02498 | |
| Fly ash/sodium sulfide (wt%) | 1.8 | 5 | 10 | 20 | 40 | 60 | |
| C = [Zn2++Pb2++…] (mol) | 1.0301×10−4 | ||||||
| S2+/C (molar ratios) | 7.3 | 20 | 40 | 81 | 161 | 243 | |
| Contents in the leaching solutions (mg/L) | Pb | 7.265 | 2.737 | 1.265 | 0.73712 | 0.12579 | 0.10112 |
| Cd | 0.12342 | 0.10659 | 0.095372 | 0.089752 | 0.053296 | 0.044881 | |

It can be seen from Table 8.11 that the leaching decreased with the increase of sodium sulfide added. When the sodium sulfide (Na2S·9H2O) reached 0.5 g (5% of the fly ash weight) and 0.18 g (1.8% of the fly ash weight), respectively, Pb and Cd could be stabilized to a level of the leachability toxicity standard. It has been found that the increase in volume of the sulfide-stabilized ash is completely negligible. Hence, sodium sulfide should be an excellent stabilizer for fly ash.
8.4.4 Stabilization of Fly Ash with Thiourea
It has been proved that heavy metals can take insoluble forms with the addition of thiourea, an organic precipitant. For comparison, in this test, thiourea is also used as one of the stabilizers.
To 10 g of fly ash, add 0.1795 g, 0.5 g, 1 g, 2 g, 4 g, and 6 g thiourea, and 100 mL water, respectively. Allow leaching to proceed for 24 h in an oscillator with a frequency of 110±10/min. Leave it to stand for 16 h and then filter. Analyze Pb and Cd contents in the aqueous solutions by ICP. The results are shown in Table 8.12.
Table 8.12
Stabilization of Fly Ash with Thiourea
| No. | 1 | 2 | 3 | 4 | 5 | 6 | |
| Thiourea added in grams | 0.0460 | 0.0760 | 0.1649 | 0.3928 | 0.7950 | 1.5345 | |
| Thiourea in mol | 0.00060 | 0.00100 | 0.00217 | 0.00516 | 0.01044 | 0.02016 | |
| Thiourea/Fly ash (wt%) | 0.46 | 0.76 | 1.65 | 3.93 | 7.95 | 15.34 | |
| C = [Zn2++Pb2++…] (mol) | 1.0301×10−4 | ||||||
| Thiourea/C (molar ratios) | 5.8 | 9.7 | 21 | 50 | 101 | 196 | |
| Contents in the leaching solutions (mg/L) | Pb | 3.572 | 1.256 | 0.9798 | 0.5589 | 0.09182 | 0.08782 |
| Cd | 0.11220 | 0.10220 | 0.084152 | 0.067321 | 0.039271 | 0.025245 | |
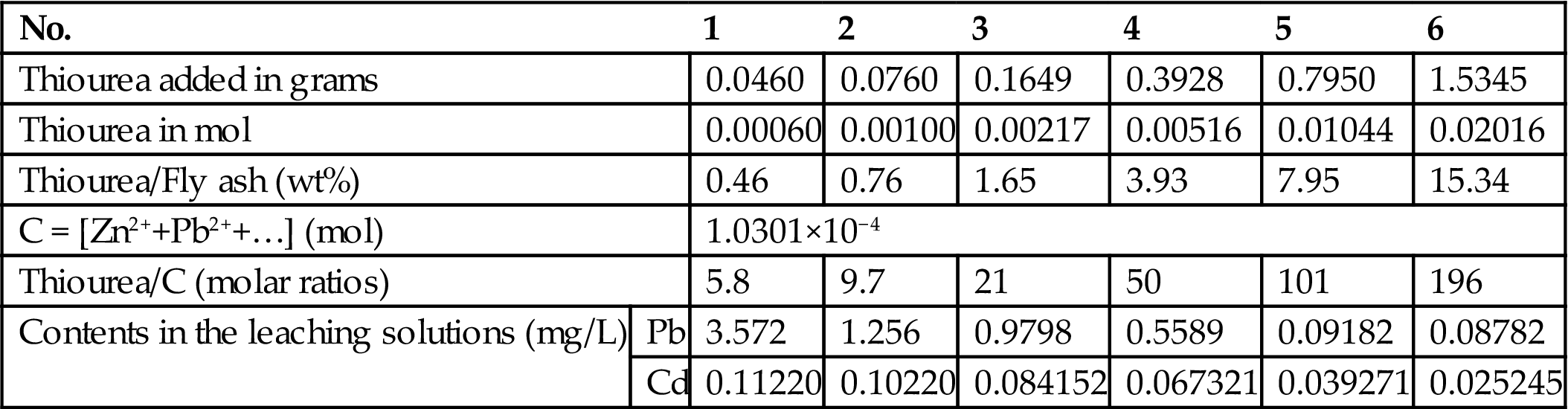
When the level of added thiourea reaches 0.076 g (0.76% of the fly ash) and 0.046 g (0.46% of the fly ash), the leachability toxicity of the stabilized products will be reduced to the standard. The quantity needed is much lower than that of sodium sulfide. From Tables 8.11 and 8.12, it can be seen that the sodium sulfide and thiourea added far exceed the results of the chemical stoichiometry, implying that some of the stabilizer content would be consumed in the formation of other soluble metals such as Fe, Ca, Mg, etc. For comparison, it can be easily calculated that 5% of Na2S·9H2O (0.00208 mol for 10 g of the fly ash) is equivalent to 0.76% of thiourea (0.001mol H2NCSNH2 for 10 g of the fly ash), in terms of the leachability level of Cd and Pb from the fly ash. Hence, the stabilizing capability of thiourea is greater than that of sodium sulfide.
8.4.5 Comparison of Four Stabilizers
The results are shown in Table 8.13. Obviously, thiourea was preferable to sodium sulfide, and sulfide to EDTA. NaOH alone cannot be used for stabilization of fly ash. The main purposes of stabilization or solidification with regard to the heavy metals in fly ash are in fact to reduce the dissolution rate of the metals into the environment in landfill or other disposal sites, using the current leachability toxicity standards as the assessment tool. The function of the immobilization of heavy metals may depend on the stabilization and solidification methods used. Traditional methods include cement and asphalt solidification. However, the life of cement and asphalt products is also limited, depending on the quality of the cement and asphalt used. These solidified products may be disposed of by being used as the materials for road surfaces, substitutes for bricks for construction sites, or just placement in a landfill. It is understandable that these products would age and the heavy metals encapsulated would be released gradually.
Table 8.13
Comparison of Stabilization Methods by the Chemicals Used
| Items | NaOH | EDTA | Sodium Sulfide | Thiourea |
| Mechanism | Dissolution of zinc, lead from the ash to reduce the leachability toxicity of these two metals | Dissolution of the metals to reduce the leachability toxicity | Transferring the soluble forms into insoluble forms | Transferring the soluble forms into insoluble forms |
| Advantages | Possible recovery of the dissolved metals | Possible of reaching the leaching toxicity standard. No volume expansion | Excellent stabilization for heavy metals. No or little volume expansion | Excellent stabilization for heavy metals. No or little volume expansion. Very little amount of agent needed |
| Disadvantages | Impossible to make leaching residues reach leaching toxicity | Difficult to regenerate the complex agent owing to the stability of the complexes formed | Possible dissolution of sulfides when exposed to acidic rain or environment, for example, in a landfill | Possible dissolution of sulfides when exposed to acidic rain or environment, for example, in a landfill |

One of the main purposes of MSW incineration is to reduce the volume of the MSW. Nevertheless, such a volume reduction will be disturbed when the fly ash and bottom ash are solidified with cement or asphalt. Hence, such a solidification method is not a good choice.
The most obvious advantage of chemical stabilization of fly ash is the negligible volume expansion. The addition of sulfides to the fly ash transfers the soluble forms of heavy metals into the insoluble forms, which have existed in nature as mineral ores for a long time. This should become a reliable method for fly ash treatment. Certainly, chemically stabilized products would not allow exposure to the acidic environment, thus preventing the dissolution of heavy metals, as in the case of cement and asphalt solidified products.
The long-term effects of chemically stabilized products should be explored to justify the effectiveness of the stabilization of these products. Nevertheless, from the viewpoint of economy, the cost using the chemical stabilization method should be lower than that using high-temperature volatilization and evaporation of heavy metals or vitrification as a whole for fly ash.
From the viewpoint of the long-term stabilization effect for stabilized or solidified products, combinations of cement and asphalt solidification and chemical stabilization may be more effective for the safety of the fly ash. Before cement or asphalt solidification, the heavy metals in the fly ash are first chemically stabilized by chemical agents such as sodium sulfide. As a result, the leaching toxicity may be much lower than that of fly ash processed using the single means of solidification. On the other hand, if the heavy metals in the fly ash could be partially extracted by chemical agents such as sodium hydroxide and EDTA, and then solidified by cement, the leaching toxicity would also be reduced as a consequence. It can be expected that such a combination would increase the safety of the fly ash discharged into the environment.
The quantity of the cement or asphalt or chemical agent needed for safe disposal of the fly ash depends on the contents and chemical forms of heavy metals in the fly ash, as well as the leaching toxicity standards set in the nations concerned. Stricter standards may be set in the future owing to environmental constraints. In this case, disposal methods should be selected correspondingly so that the resulting stabilized products can meet legislative requirements.
The heavy metals in the fly ash generated in MSW incinerators can be stabilized effectively using sodium sulfide and thiourea as stabilizers. In this case, the volume expansion due to the addition of chemicals is negligible. Another effective treatment method for fly ash is to leach the heavy metals by using complex agents such as EDTA. NaOH can be used for the leaching of zinc, but the resultant leaching residues should be treated further, and therefore it is not a suitable chemical agent. A comparison between the cement and asphalt solidification and chemical stabilization is made. The results of the latter have little volume expansion.
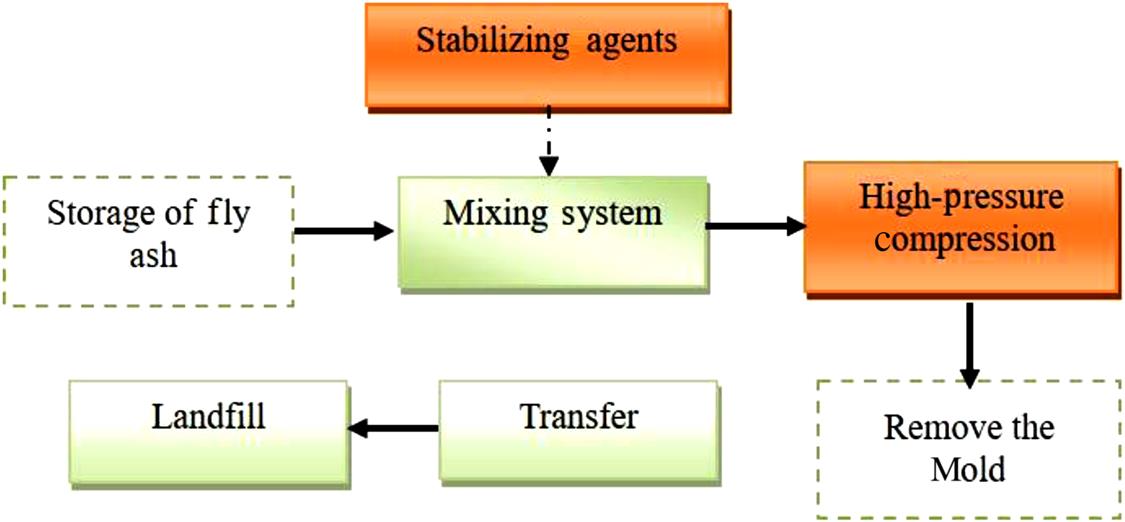
8.5 High-pressure Compression for a Volume Reduction of Fly Ash
Dedicated landfill is the most important type of site for hazardous waste disposal after the waste is stabilized chemically and physically. Conventional solidification and stabilization for fly ash, using cement and chemicals, leads to a significant increase in volume. Organic polyphosphoric acid and organic polyphosphate can be selected as the stabilizers, with around 3% of the fly ash treated.
High pressure is used to compress powder materials in order to reduce volume and enhance strength. In this work, hydraulic equipment is applied for the compression of fly ash into blocks with high density. The main technical parameters of several machines for high-pressure compression are shown in Table 8.14.
Table 8.14
Main Technical Parameters of Several Machines for High-Pressure Compression
| Type | Unit | 40T | 63T | 100T | 160T | 200T | 315T | 400T | 500T | 630T | 1000T | 1250T | 1600T |
| Nominal pressure | kN | 400 | 630 | 1000 | 1600 | 2000 | 3150 | 4000 | 5000 | 6300 | 10,000 | 12,500 | 16,000 |
| Ejector force | kN | 63 | 100 | 200 | 250 | 400 | 630 | 630 | 1000 | 1250 | 1000 | 1000 | 1200 |
| Maximum hydraulic working pressure | MPa | 25 | 25 | 26.2 | 26 | 25 | 25 | 25 | 26 | 26 | 26 | 26 | 26 |
| Slide stroke | mm | 400 | 500 | 500 | 500 | 700 | 800 | 800 | 900 | 900 | 900 | 900 | 1000 |
| Maximum opening height | mm | 600 | 700 | 900 | 900 | 1120 | 1250 | 1400 | 1500 | 1500 | 1500 | 1500 | 1900 |
| Size: | |||||||||||||
| Width | mm | 400 | 500 | 580 | 800 | 900 | 1200 | 1200 | 1400 | 1600 | 1500 | 1600 | 1600 |
| Length | mm | 400 | 500 | 710 | 800 | 900 | 1200 | 1260 | 1400 | 1600 | 1500 | 1800 | 1800 |
| Ejector stroke | mm | 120 | 160 | 200 | 200 | 250 | 300 | 300 | 400 | 400 | 400 | 400 | 450 |
| Slide speed: | |||||||||||||
| Downward | mm/s | 40 | 76 | 100 | 100 | 100 | 100 | 100 | 100 | >100 | 100 | >100 | 200–150 |
| Working | mm/s | 10 | 10 | 8–15 | 8–15 | 8–15 | 8–15 | 8–15 | 10 | 12 | 8–15 | 10 | 10 |
| Return | mm/s | 60 | 60 | 60 | 60 | 70 | 70 | 70 | 80 | 65 | 80 | 80 | >75 |
| Machine shape size: | |||||||||||||
| Length | mm | 1484 | 1900 | 2076 | 2650 | 2780 | 3440 | 3440 | 4100 | 4390 | 2340 | 6000 | 6500 |
| Width | mm | 1000 | 1100 | 1200 | 1600 | 2120 | 2220 | 2220 | 2420 | 2520 | 3000 | 3800 | 3900 |
| Height | mm | 2406 | 2430 | 3300 | 3350 | 3690 | 3900 | 4050 | 5348 | 4860 | 6000 | 6500 | |
| Motor power | kW | 5.5 | 5.5 | 7.5 | 15 | 18.5 | 22 | 22 | 37 | 68 | 75 | 90 | |
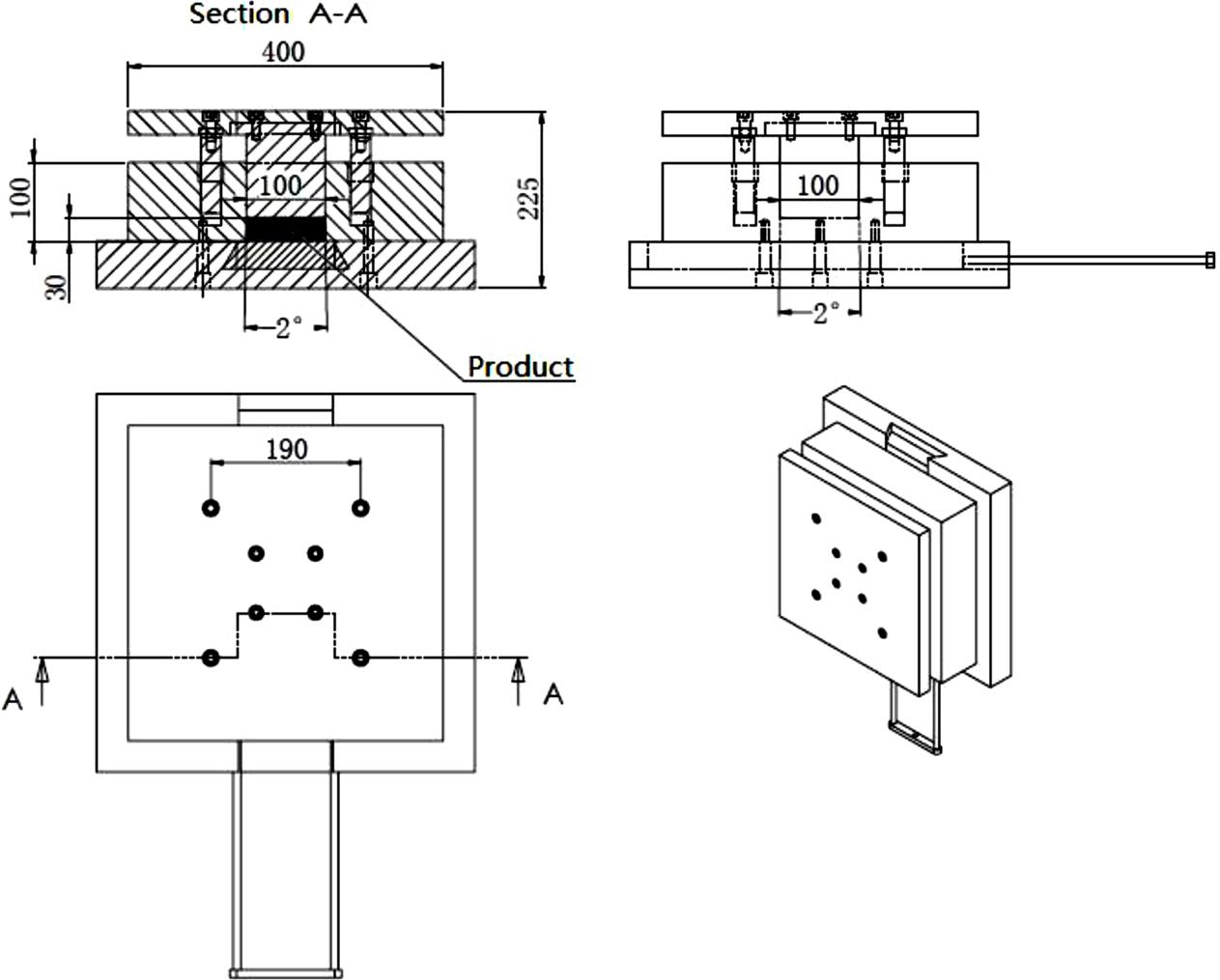
The mold used for compressing fly ash is presented in Figs. 8.6A and 8.6B; it has five parts. From top to bottom, the three components in the upper part play an important role in compression, and the top of the base is used to withstand the downward-pressing force, ensuring that the mold is pressed uniformly. The male mold is the key component for compressing fly ash directly and finally making fly ash into block form, under force. The female mold surrounds the male mold to prevent fly ash from leaking. The main purpose of the ejector pin is to press the fly ash block sout of the mold. The function of the base is to protect the four upper parts under huge pressure.
8.5.1 Operation of High Hydraulic Pressure Compression of Fly Ash
The aim of high hydraulic pressure is to compress fly ash so as to reduce the pollution emitted into the atmosphere, increasing the density of the fly ash and facilitating the landfill. Compared to conventional systems, this technology differs in its high hydraulic pressure compressing system and demolding system.
As shown in Fig. 8.7, the density of fly ash increases obviously with increasing pressure. When the pressure was below 100 MPa, the density was increasing sharply. When the pressure was above 100 MPa, the rate of increase of density was falling dramatically, which indicated that it was effective and economic to compress fly ash at a pressure of around 100 MPa. The initial density of the fly ash was 0.79 g/cm3, and it increased to 1.9 g/cm3 at 100 MPa, meaning approximately 60% of volume reduction.
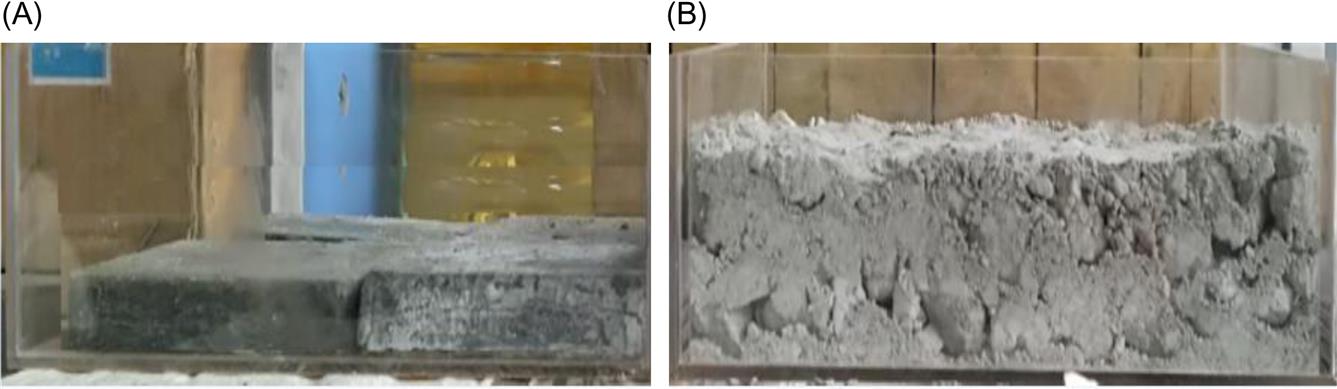
The fly ash looks very loose. Therefore, when fly ash is compressed, the space among the particles will be reduced. Furthermore, the fly ash particles were anomalous and porous, and the structure of fly ash particles would be destroyed when a huge external force was applied.
In order to study the trends of heavy metal leaching toxicity in the compressed fly ash blocks, eight kinds of heavy metals (As, Cd, Cr, Cu, Hg, Ni, Pb, and Zn) were monitored. The detailed data of the pressure and the heavy metal leaching toxicity are shown in Table 8.15. It can be seen that the leaching toxicity had been greatly reduced after being compressed.
Table 8.15
The Leaching Concentrations of Heavy Metals in Differently Compressed Fly Ash
| Pressure/MPa | Chinese National Standard | As | Cd | Cr | Cu | Hg | Ni | Pb | Zn |
| mg/L | 1.5 | 0.3 | 10 | 50 | 0.05 | 10 | 3 | 50 | |
| 60 | mg/L | ND | ND | 0.277 | 0.039 | 0.044 | ND | 26.1 | 1.91 |
| 100 | mg/L | ND | ND | 0.279 | 0.051 | 0.058 | ND | 28.9 | 2.09 |
| 200 | mg/L | ND | ND | 0.258 | 0.030 | 0.052 | ND | 25.8 | 2.98 |
| 280 | mg/L | ND | ND | 0.256 | 0.055 | 0.050 | ND | 33.6 | 2.43 |
| 360 | mg/L | ND | ND | 0.270 | 0.038 | 0.053 | ND | 27.1 | 2.14 |
| 380 | mg/L | ND | ND | 0.275 | 0.036 | 0.056 | ND | 26.3 | 2.09 |
| 0 | mg/L | ND | ND | 0.93 | 0.068 | 0.512 | ND | 38.09 | 7.42 |
| 60 (stabilized) | mg/L | ND | ND | ND | ND | ND | ND | ND | ND |

Note: The fly ash of the last group was chemically stabilized on site by the company using organic chelating agents, and those in the others were the original fly ash.
In the stabilizing process of fly ash, the stabilizer is generally added in excess, and prone to the phenomenon of uneven mixing, resulting in substantial waste of the stabilizer. As shown in Table 8.15, high pressure can reduce the heavy metal leaching toxicity of original fly ash to some extent, and it is feasible to mix the original fly ash with the stabilized fly ash by means of high-pressure compression, and the resulting substance can meet heavy metal leaching toxicity standards (Table 8.16).
Table 8.16
Leaching Concentrations of Heavy Metals from the Mixture of Stabilized and Original Fly Ash
| The Mass Ratio of Original and Stabilized Fly Ash | Elements(mg/L) | Cr | Cu | Hg | Pb | Zn |
| Limit Standard | 10 | 50 | 0.05 | 3 | 50 | |
| 10:0 | 0.315 | ND | ND | 3.42 | 0.377 | |
| 9:1 | 0.298 | ND | ND | 3.28 | 0.338 | |
| 8:2 | 0.255 | ND | ND | 3.16 | 0.334 | |
| 7:3 | 0.239 | ND | ND | 3.09 | 0.305 | |
| 6:4 | 0.237 | ND | ND | 3.08 | 0.297 | |
| 5:5 | 0.184 | ND | ND | 2.67 | 0.29 | |
| 4:6 | 0.179 | ND | ND | 2.48 | 0.282 | |
| 3:7 | 0.155 | ND | ND | 0.734 | 0.28 | |
| 2:8 | 0.15 | ND | ND | 0.671 | 0.267 | |
| 1:9 | 0.148 | ND | ND | 0.242 | 0.264 | |
| 0:10 | 0.144 | ND | ND | 0.064 | 0.167 |
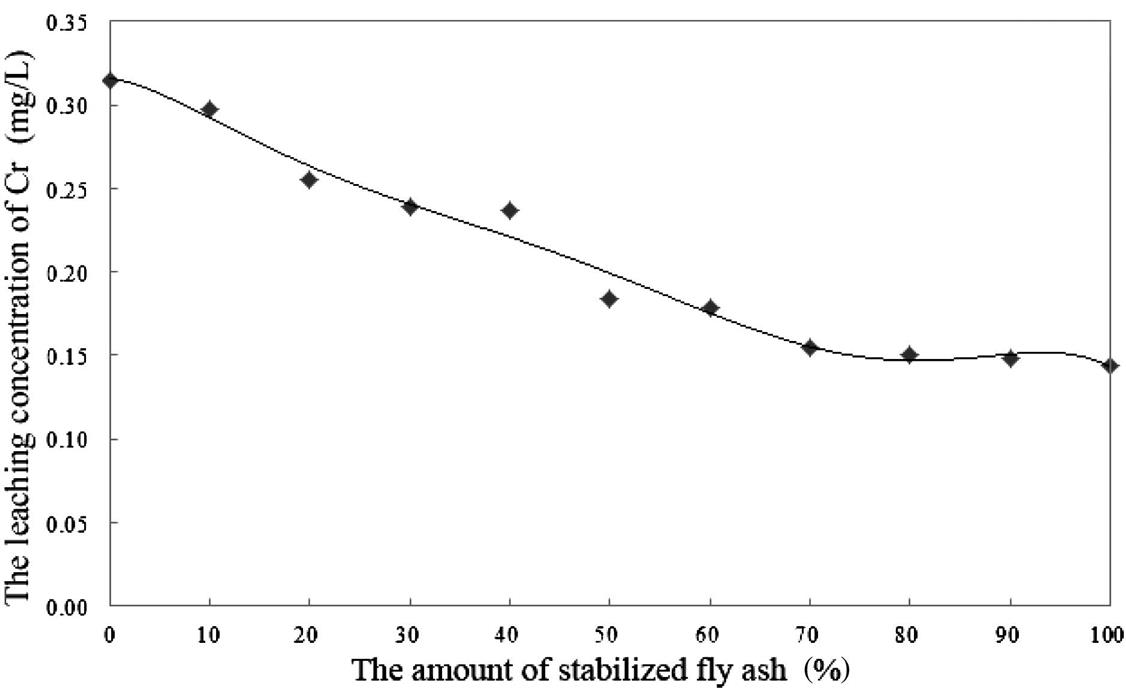
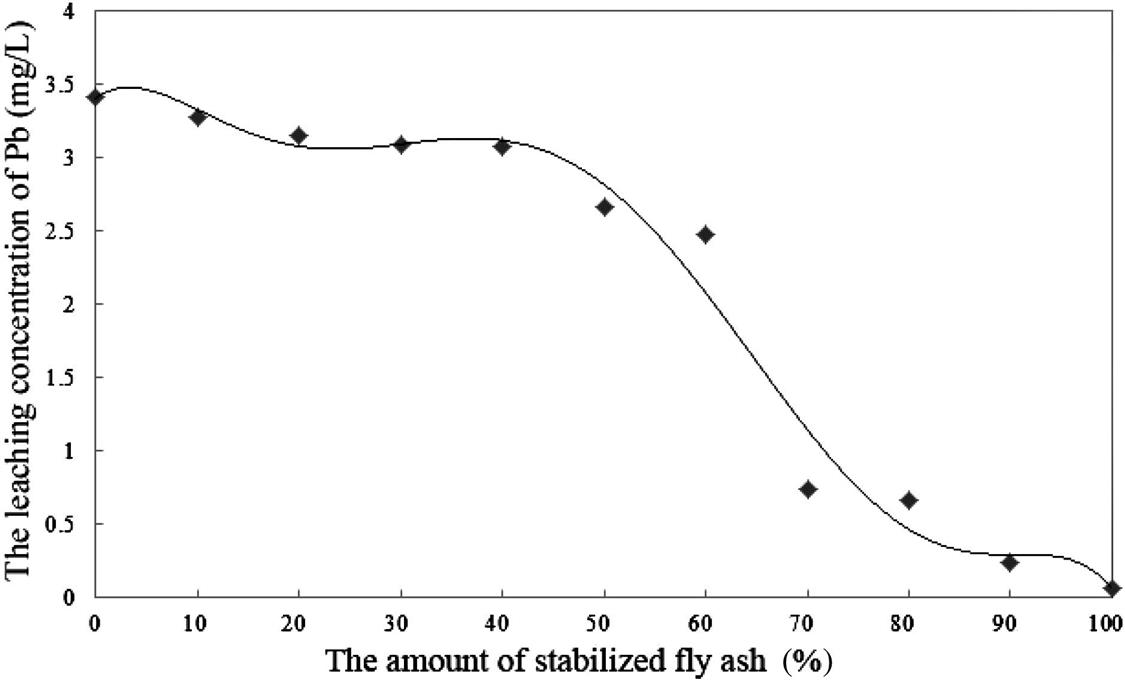
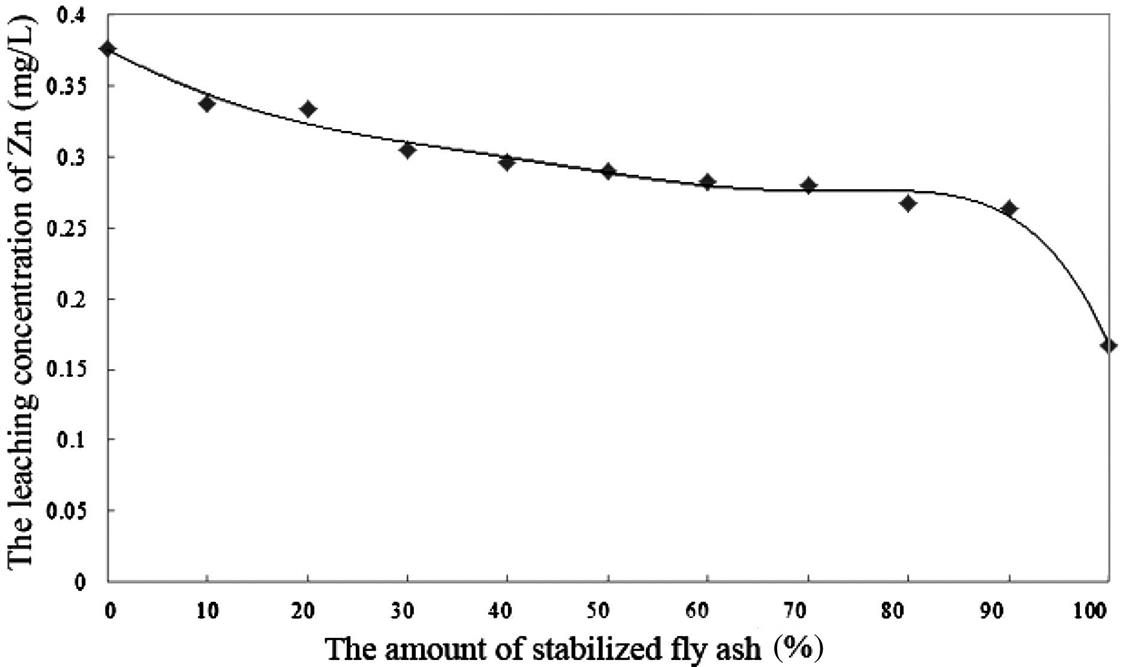
The leaching extent of fly ash will be reduced after compression. As a result, Cu and Hg were both below the detection limits. When the proportion of the original fly ash was less than 50%, the leaching toxicity of the other heavy metals could also meet the standard, as shown in Figs. 8.8–8.10.
From Figs. 8.8–8.10, it is easy to establish that the leaching toxicity of heavy metals decreased at almost every step. In the fly ash stabilizing process, the stabilizer sprays unevenly, and is usually excessive in quantity. During the compressing process, not only must the density of fly ash increase, but also the original and the stabilized fly ash must mix sufficiently. This makes for full contact between the original fly ash and the excessive stabilizer in the stabilized fly ash, and this improves the utilization efficiency of the stabilizer. Hence, mixing the original fly ash with stabilized fly ash actually means reusing the excess stabilizer that has not been utilized during the previous stabilizing process.
As shown in Fig. 8.9, the decrease in Pb concentration can be basically divided into three stages. When the dosage of stabilized fly ash was less than 55%, the leaching concentration decreased slowly. When the dosage of stabilized fly ash was 55–75%, it presented a sudden drop in this decrease in leaching concentration. When the dosage was more than 75%, the leaching concentration decreased slowly again. The reason for the big drop in the second stage may be that when the proportion of stabilized fly ash rose up to a certain amount, the stabilizer was mixed up with heavy metals in fly ash under high pressure. Since the concentration of Pb was the highest heavy metal concentration in fly ash, it would take priority in reacting with the stabilizer. When the dosage exceeded the optimum level, it was useless to increase the dosage of the stabilized fly ash.
8.5.2 Process Design of High Hydraulic Pressure Compression of Fly Ash
1. Project scale
The disposal capacity of the project is designed to 15–200 t/d.
2. Project purpose
The purpose of this project is to pretreat the fly ash by compressing it to a block, which can not only solve the atmospheric pollution caused by transporting fly ash in the landfill process, but also reduce the volume of fly ash and increase landfill capacity.
3. Project plan
According to the scale and targets, this project includes the plan of applying a four-pillar hydraulic machine as the treatment equipment for the fly ash, which will reduce the amount of fly ash dust emitted into the atmosphere by more than 99% and increase the fly ash density by more than 200%, significantly reducing the cost of final disposal.
This project intends to use two sets of four-pillar hydraulic machines of 500–2000 t, with capacity of 3–20 t/h (working 8 h) per set, which can meet the demands of fly ash treatment. A flowchart of the high-pressure compressing process for fly ash is given in Fig. 8.11.
1. High hydraulic pressure compressing system
Four-pillar hydraulic machine and mold.
The block needs to be demolded after compressing, and since stabilizers are of a certain adhesivity, there is a little difficulty involved in getting the block out of the mold, and so the demolding system is required. The system contains the demolding plate and ejector pin.
3. The scheme design of high-pressure compression for fly ash
The key step of the high-pressure compression of fly ash is to load the fly ash into the block and to compress the fly ash by exerting 20–100 MPa pressure via hydraulic equipment, with the purpose of reducing volume, and increasing density and the capacity of landfill. Simultaneously, the large bulk of fly ash in the landfill also reduces air pollution in the transporting, unloading, and landfill processes.
The main structures involved in the project of the high-pressure compression of fly ash include one compressing room, one fly ash block shed, and one fly ash storage silo. The compressing room is made up mainly of a high-pressure compressing system and a demolding system. The fly ash storage silo has a link to the computer room, so that the fly ash can be transported to the high-pressure compressing system continuously. Fly ash blocks which have been compressed are stacked in the fly ash block shed.
Main technical parameters
1. The properties of the fly ash (inside the fly ash storage silo)
Treatment capacity: 15–200 t/d
2. The high-pressure compressing parameters
Compressing pressure: 20–100 MPa
The main machinery and equipment is set out in Table 8.17.
Table 8.17
Main Machines for High-Pressure Compression of Fly Ash
| No. | Machines | Types | Amount |
| 1 | Kiloton class four-pillar hydraulic machine | 68 kW | 2 |
| 2 | Pressing mold | volume = 0.4 m3 | 3 |
| 3 | Fly ash storage silo | 25 m3 R × H = 3.2 m × 3 m | 1 |
| 4 | Demolding system | 3 | |
| 5 | Transfer machine | Capacity = 1 t, power = 5 kW | 1 |
| 6 | Meter | 1 | |
| 7 | Electric cable bridge | 1 | |
| 8 | Guide funnel | 1 |

High pressure normally changes the physical or chemical forms in the environment. Under a certain amount of pressure, fly ash is shaped using a hydraulic press and a specific mold. In this process, the self-assembly of some crystals occurs and the consequent enhanced density of the fly ash makes leaching and weathering more difficult in a landfill. Clumping in fly ash dramatically reduces its leaching toxicity, owing to the high density and low permeability.
When a lighter powder turns into a block, the volume of fly ash can be reduced by 50 to 80%, which decreases the risk of dust blowing, and at the same time, extends the operational life of landfill. In addition, this process can greatly reduce the amount of solidifying agents required, as well as running cost. High-pressure compression of fly ash is a good prospect as a final disposal system.