Thermal Characterization of Fly Ash
Abstract
Thermal characterization and use in ceramic tile making of fly ash is presented. Differential temperature analysis–thermogravimetric analysis analysis, elementary component variation, chemical component, radical component transformation, mineralogical component transformation, microstructure transfer, leaching toxicity and speciation transformation, as a function of temperature for fly ash, are provided. Use of fly ash in ceramic tile making is explored. 11 major elements in fly ash with contents over 1% and a sequence of O >Ca >Si >Cl >Al >Fe >Na >S >C >Mg are found in fly ash. Main elementary content and chemical component of the ash changes with increase of sintering temperature. It is inferred that heavy metals should be stabilized in the sintered fly ash due to generation of crystal and glass phases which constitute occluding body of these heavy metals. Microstructure of fly ash is strengthened with the increase of temperature, and porosity reduces. In addition, content of glass phases increases and particles are more closely cemented together as sintering temperature increases from 1000 to 1300°C, which is conducive to solidification of heavy metals. The leaching toxicity and speciation of heavy metals are also influenced by the increase of temperature.
Keywords
Fly ash; thermal analysis; sintering temperature; heavy metals; leaching toxicity
6.1 DTA–TGA Analysis for Fly Ash
This work performed differential temperature analysis (DTA)–thermogravimetric analysis (TGA) analysis as well as analysis of chemical, elementary, radical and mineralogical component transformation, microstructure transformation of sintered fly ash, and leaching toxicity, volatilization ratio and specification transformation of heavy metals as a function of temperature. Prior to analysis of characterization of heavy metals, fly ash was dried at 105°C for 24 h. The sampled fly ash was sintered at 400, 600, 800, 1000, 1200 and 1300°C, respectively, for 30 min in the muffle furnace (SK2–2.5–13D). After cooling down, the sintered fly ash went through thermal analysis.
To investigate major exothermic and endothermic reactions occurring in the heating process, thermal analyzer (SDT Q600) was used to perform differential DTA and TGA, following 10°C/min heating pattern.
Thermal analysis using thermal analyzer of fly ash is shown in Fig. 6.1, which involved TGA, DTA, and first derivative and second derivative analysis of TGA and DTA, of which the last analysis was used to determine temperature at which exothermic or endothermic reaction transferred. It was found that four endothermic peaks appeared: 225–350, 350–414, 446–481, 531–1241°C, and two exothermic peaks: 481–531, 1241–1400°C. The former three endothermic peaks formed due to the removal of physical adsorptive water, hydrate water, and constitutional water, respectively, and the last one between 531°C and 1241°C was caused by decomposition of Ca(OH)2, CaCO3, CaSO4 and chlorides. Exothermic peak between 481°C and 531°C formed due to burning of organic substances and it was very narrow, indicating that contents of organic component were negligible. While the exothermic peaks between 1241°C and 1400°C was caused by considerable chemical combinations and melting of fly ash, during which great glass phases and new minerals came into being. The fact that no exothermic peaks other than 481–531°C existed at temperature lower than 1241°C, indicated that the ash was relatively stable and showed little activity until 1241°C.
TGA analysis showed that its mass took on a ladder decrease, which was caused by the removal of water and decomposition of carbonates, silicates, aluminates, chlorides and other components. The first ladder, between 100°C and 350°C, formed due to the removal of physical adsorptive water, reflected by an endothermic peak in the DTA. 350–531°C, and the second ladder came into being due to the removal of hydration and institutional water as well as burning of organic substances. The decomposition process of Ca(OH)2 was demonstrated by a fast reduction of mass between 531°C and 660°C, and then there was a flat between 660°C and 781°C, indicating an end of the decomposition. Between 781°C and 956°C, there was a sharp decrease of mass by 15%, which was caused by decomposition of CaCO3, CaSO4, aluminum salts and partial chlorides. Mass loss between 956°C and 1241°C, registering 8%, was contributed to the continuative decomposition or volatilization of chlorides. The mass remained relatively constant when temperature exceeded 1241°C, which indicated an end of salt decomposition. Finally, considerable new silicates, aluminosilicates and calcium silicates came into being due to the combination of silicates with oxides such as Al2O3, CaO etc.
6.2 Elementary Component Variation as a Function of Temperature for Fly Ash
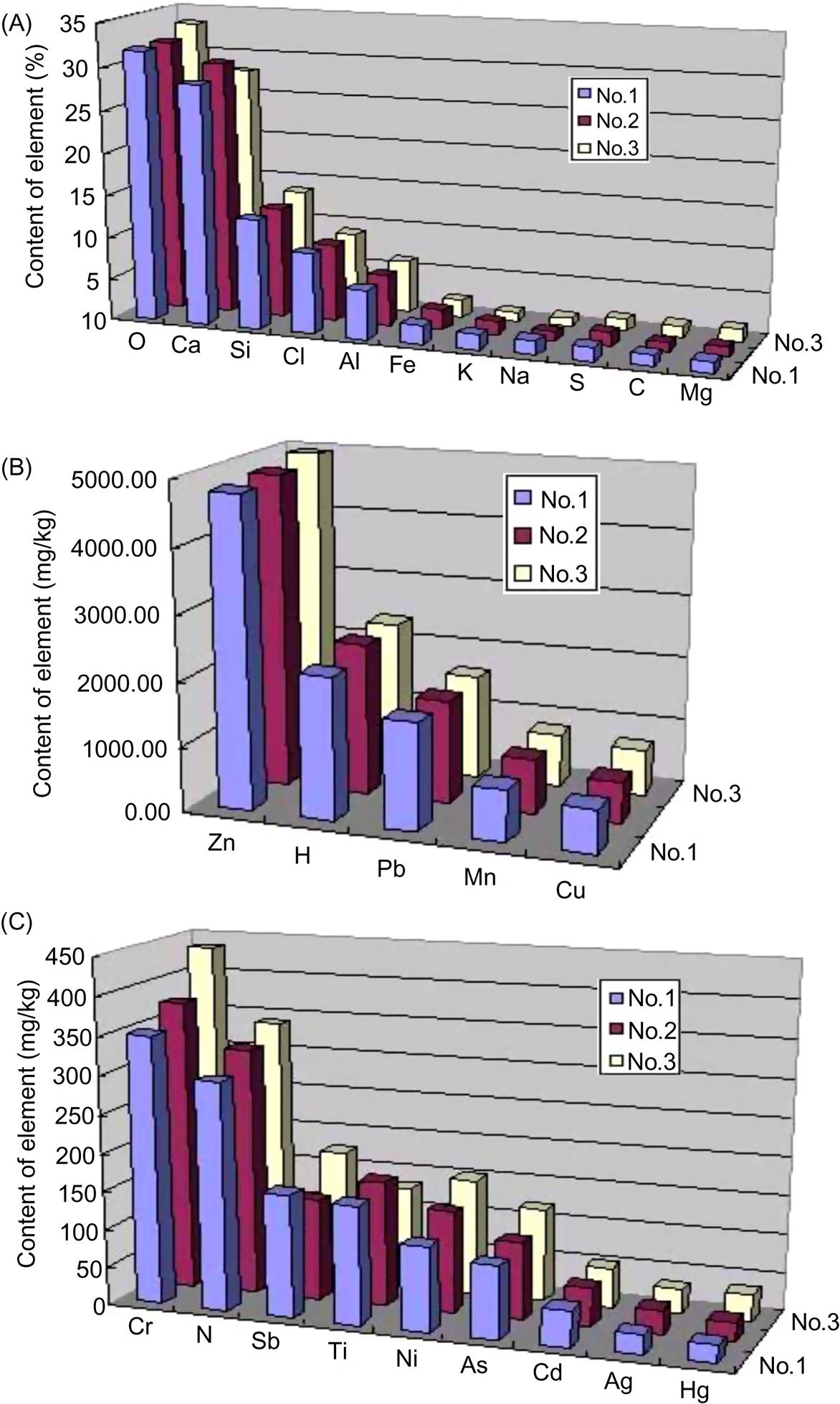
A total of 11 major elements in fly ash with contents over 1% with a sequence of O >Ca >Si >Cl >Al >Fe >Na >S >C >Mg were found in fly ash as given in Fig. 6.2. These elements accounted for around 97.5–98.7% of the ash. Content of Cl, around 9.23%, was relatively high, which should mainly exist in chlorides involving NaCl and KCl and other soluble salts as proved by sectioned scanning of X-ray diffraction (XRD) analysis. These soluble salts can increase leaching of heavy metals through ion exchange with them, and thus enhance toxicity of fly ash. In addition, Cl also exists in dioxins and other organic substances whose toxicity is very strong, and thus fly ash is a hazardous waste and should be treated safely.
Content of S, around 1.58%, was relatively high and existed mainly in sulfates and sulfides as proved though XRD analysis. These sulfates and sulfides, together with Ca, K, Mg and Na, inflict a negative influence on stabilization of fly ash in cement materials. However, Ca, K, Na, Mg and Fe, registering a high content in the ash, can help break Si-O, Al-O in quartz, silicates and aluminum salts to form new silicates and alumiosilicates as they are molecular network improver. Hence, they are conducive to reduction of energy consumption in making of fired construction materials, such as glazed tile, ceramic brick, ceramic glass etc. Si and Al, two important elements which form molecular network of salts, can shape backbone of silicates and aluminosilicates.
14 minor elements were detected and contents of them followed a sequence of Zn >Pb >Mn >Cu >Cr >N >Sb >Ti >Ni >As >H >Cd >Ag >Hg, as shown in Fig. 6.2. Contents of C and N, registering around 1.3 and 0.03%, respectively, were relatively low, demonstrating a low content of organic substances in the ash as proved by infrared radiation (IR) analysis. Among the minor elements of fly ash there are eight hazardous heavy metals which may harm environment through leaching out of the ash and thus should be stabilized.
With increase of sintering temperature, main elementary content of the ash changes, as shown in Fig. 6.3. Contents of Ca, Si, Al, Fe, K and Na stood almost constant, while that of O, C Cl and S reduced with increase of temperatures. Content of C reduced steadily especially when temperature exceeded 400°C, while that of S and Cl decreased considerably with increase of temperatures over 1000°C, indicating a fast decomposition or volatilization of chlorides and sulfates or sulfides over 1000°C. Content of C was close to zero at 600°C, indicating a through burning of organic substances at this temperature. Hence C, S, Cl and O are 4 main elements that cause mass loss of the ash at different temperatures, as shown in Fig. 6.4. The relationship of mass loss with temperature conforms to equation: y =9E −06x2 + 0.0036x − 0.339, among which y and x represent mass loss and temperature, respectively. R2 equals to 0.9955, indicating a relatively good simulation of the relationship.
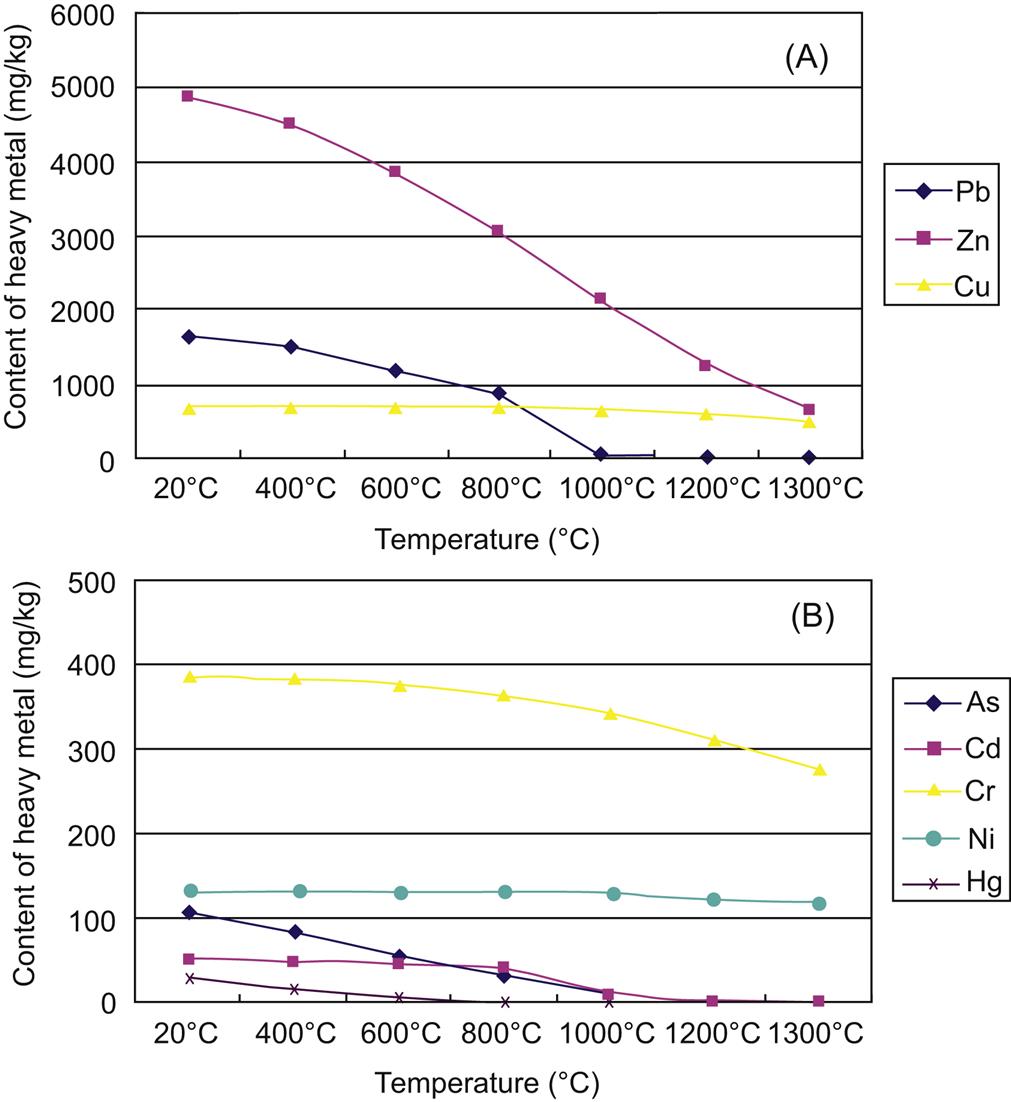
Content variations of heavy metals as a function of temperature is described in Fig. 6.5. Volatilization ratio of Hg increased considerably as sintering temperature rose from 400 to 1000°C, and was close to 100% after being sintered between 1000°C and 1200°C. Pb volatilized slowly as it increased from 400 to 800°C, flied away quickly from 800 to 1000°C, and was removed completely from fly ash Between 1000°C and 1200°C. Around 80% As and Cd flied away from fly ash sintered at 1000°C, and almost total As and Cd volatilized from fly ash sintered at 1200°C. Volatilization ratio of Zn was much smaller than that of the above four heavy metals after being sintered at the same temperature. Close to 65% Zn flied away from fly ash sintered at 1300°C and volatilization ratios of Cr, Ni and Cu were no more than 10% after being sintered at 1300°C.
Based on the above analysis of volatilization of heavy metals, volatilization ratio of heavy metals decreases as: Hg >Pb >Cd, As >Zn >Cr approximately Ni and Cu. It is easy for Hg, Cd, As and Cd to volatilize, and a little difficult for Zn and difficult for Ni, Cr and Cu to fly away from fly ash, with the increase of sintering temperature. Hence, exhaust gas from the thermal treatment of fly ash contains a lot of heavy metals, especially Hg, Pb, Cd and As, and thus requires further treatment.
6.3 Chemical Component Variation as a Function of Temperature for Fly Ash
Seven major chemical components were detected by XRF, and contents of them followed a sequence of CaO >SiO2 > Al2O3 > Fe2O3 > MgO >K2O >Na2O, as shown in Fig. 6.6. Total contents of these oxides exceeded 80%, and thus fly ash registered a SiO2–Al2O3–metal oxides system. CaO, a molecular network improver and with a content over 35% in the ash, can reduce energy consumption in making fired construction materials on the one hand and improve rupture strength and phase uniformity of the product on the other hand. Fe2O3, MgO, K2O and Na2O, constituting molecular network improvers like CaO, can reduce energy consumption by enhancing destroy of Si–O and Al–O, and finally they form new crystal phases or melt into glass phase. Total contents of these five molecular network improvers exceeded 40%, and thus fly ash is an active material as it can reduce energy consumption in production of fired construction materials. Content of chemical components, excluding K2O and Na2O whose contents decreased when temperature exceeded 1000°C, rose with increase of sintering temperature. Hence, fly ash at room temperature or sintered at high temperatures can be used as a slag for construction materials as its main contents are SiO2, Al2O3 and metal-oxides.
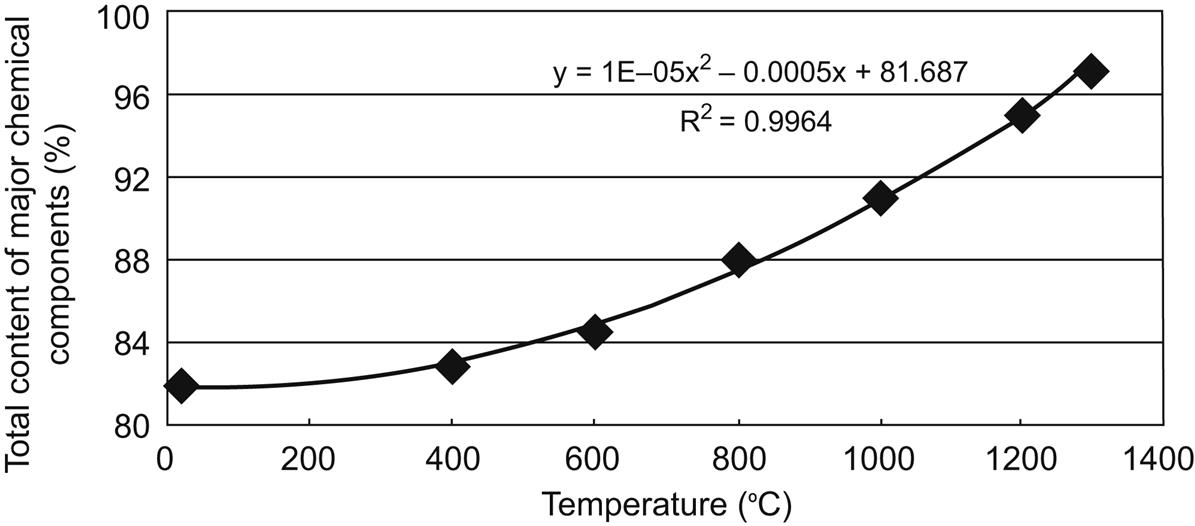
With increase of temperature, total content of chemical components of sintered fly ash increased, as shown in Fig. 6.7. Relationship of total contents with temperature conformed to equation: y = 1E−05x2 − 0.0005x + 81.687, among which x and y represent temperature and total content, respectively. R2 equals to 0.9964, indicating a good simulation of the equation for them.
6.4 Radical Component Transformation as a Function of Temperature for Fly Ash
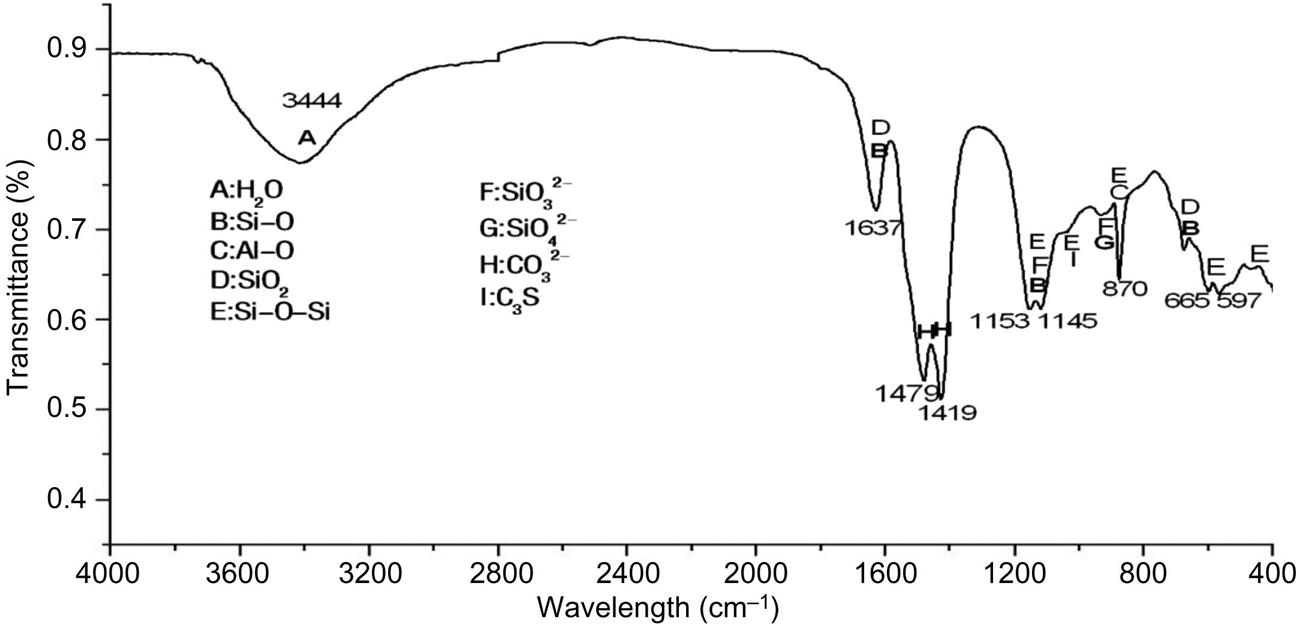
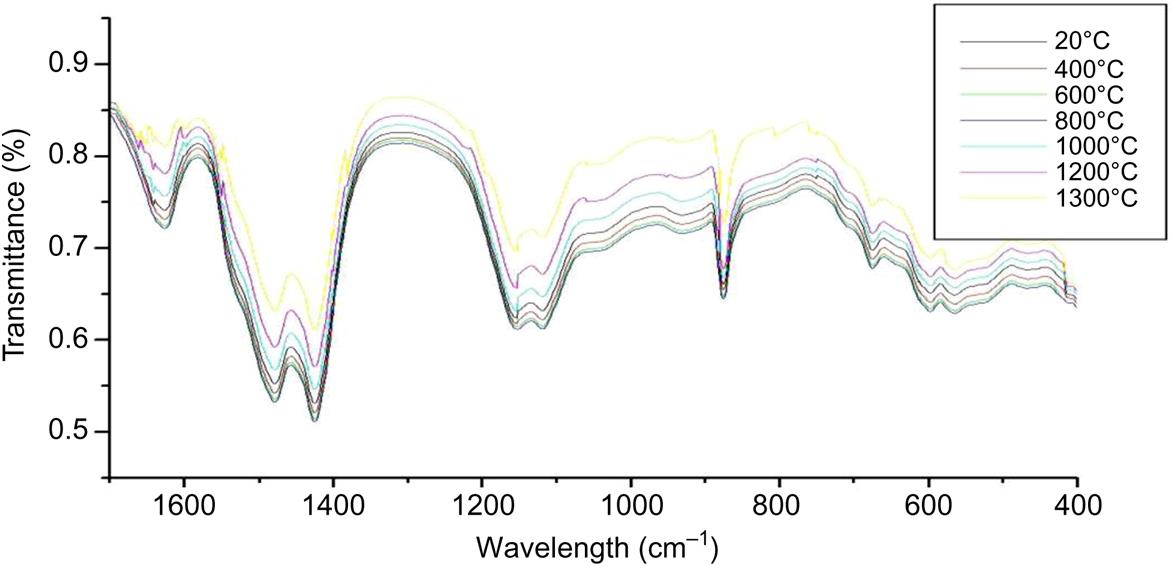
Six specific absorption bands at 3444, 1637, 1479–1419, 1153–1145, 870, 665–579cm−1 were detected by IR analysis, as shown in Fig. 6.8. Major radicals and minerals detected involved Si–O, Al–O, SiO2, Si–O–Si, SiO32−, SiO42−, CO32− and C3S, indicating that fly ash mainly contained silicates, aluminosilicates, calcium-silicates, quartz and carbonates. No radicals of organic substance were found in the IR spectrum, demonstrating that content of organic substance in fly ash was below detection limit of IR.
With rise of temperature, contents of crystal phases first increased from 20 to 800°C and then decreased from 800 to 1300°C, while content of glass phases registered a reverse trend, as shown in Fig. 6.9. This indicated that crystal phases formed as temperature increased from 20 to 800°C, which were transformed into glass phases when sintering temperature exceeded 800°C. When temperature was over 1200°C, a considerable increase in content of glass phases was seen in the IR spectrum, demonstrating occurrence of acute chemical combinations and formation of abundant glass phases. Hence, it is conferred that heavy metals should be stabilized in the sintered fly ash due to generation of crystal and glass phases which constitute occluding body of these heavy metals.
6.5 Mineralogical Component Transformation as a Function of Temperature for Fly Ash
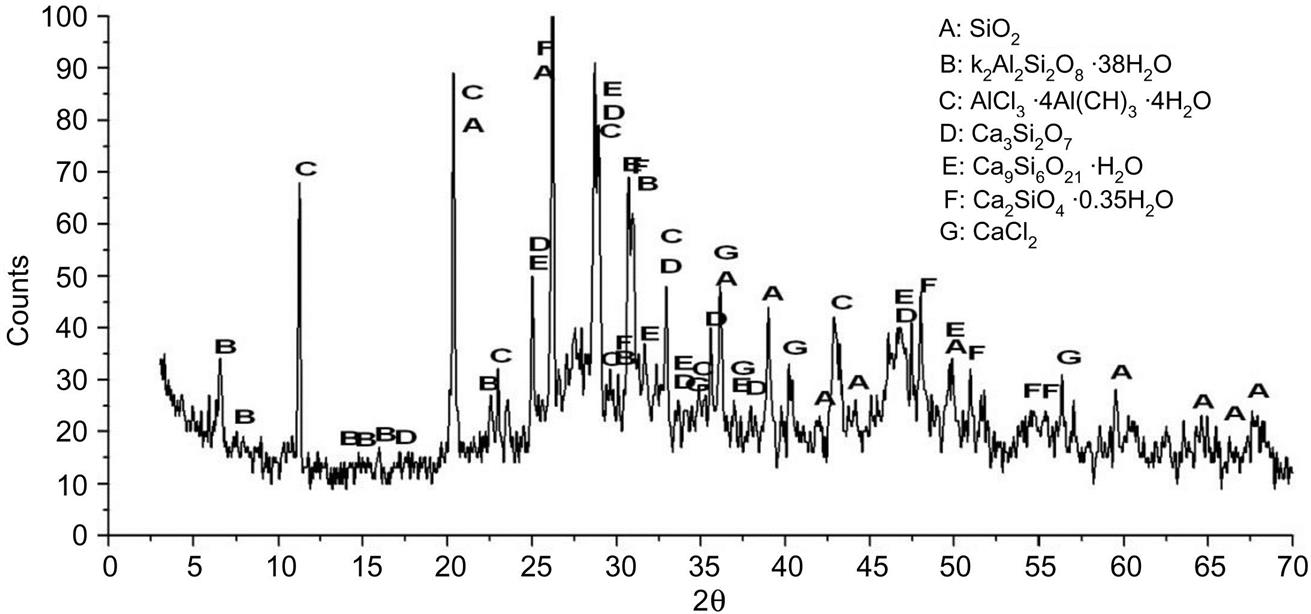
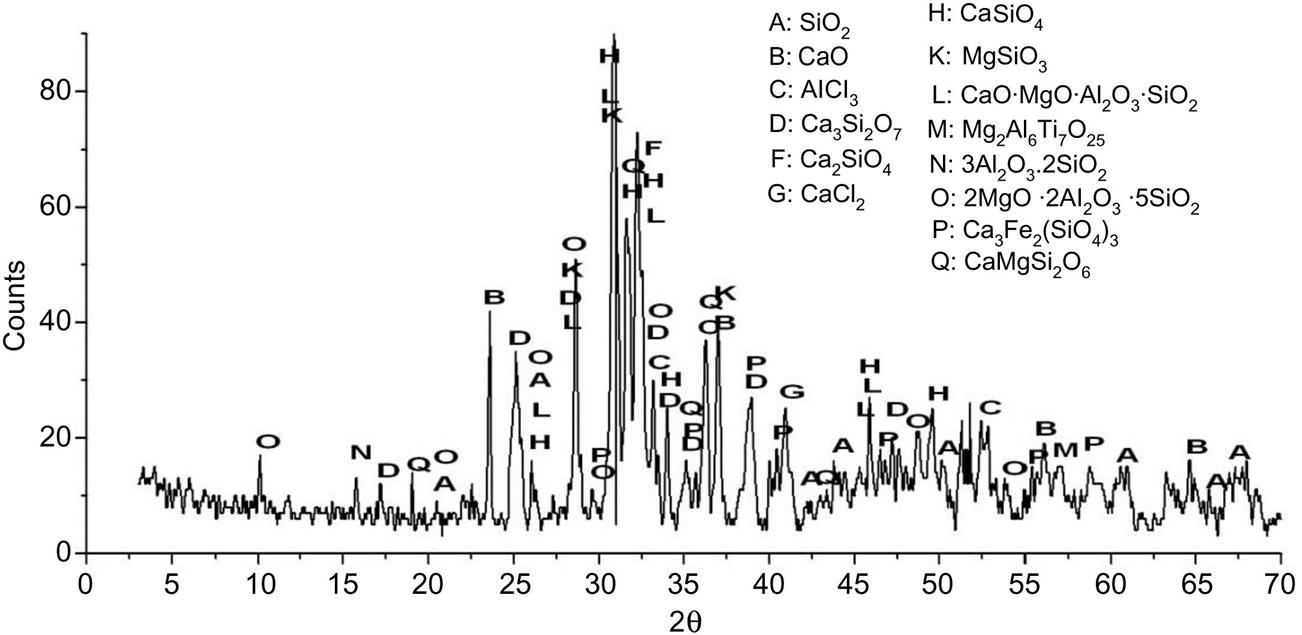
Major mineralogical components of fly ash involved SiO2, CaCl2, Ca3Si2O7, Ca2SiO4∙0.35H2O, Ca9Si6O21∙H2O, K2Al2Si2O8∙3.8H2O and AlCl3∙4Al(OH)3∙4H2O, as shown in Fig. 6.10. Contents of glass phases reached around 57%, which was conducive to reduction of energy in recycling of the ash.
After being sintered at 400°C, major components were SiO2, KAlSiO4, AlCl3, Ca3Si2O7, Al2O3, Ca2SiO4, CaCl2, CaCO3, Ca(OH)2 and CaSO4, as shown in Fig. 6.11. Diffraction peak of AlCl3∙4Al(OH)3∙4H2O at d = 3.93Å disappeared, which was decomposed into H2O, γ-Al2O3 and AlCl3 whose diffraction peaks appeared at d = 4.24, 8.04, 7.21, 7.8, 4.75 and 4.08 Å.
XRD analysis of fly ash sintered at 600°C is shown in Fig. 6.12. Diffraction peak of Ca(OH)2 at d = 5.4 Å disappeared and those of CaO appeared at d = 2.41, 1.7, 2.78 and 1.45 Å, demonstrating that Ca(OH)2 decomposed into CaO at this temperature.
As temperature risen to 800°C, diffraction peaks of CaCO3 and CaO at d = 3.86 and 2.41 Å decreased by 16.7% and around 50%, respectively, indicating that the former decomposed into CaO which reacted with SiO2 and formed Ca3Si2O7 whose diffraction peak at d = 5.17 Å increased by around 66.7%, as shown in Fig. 6.13. Although chemical combination as the above occurred at this temperature, major reactions were decomposition of salts and thus an endothermic peak emerged in the DTA analysis not an exothermic peak.
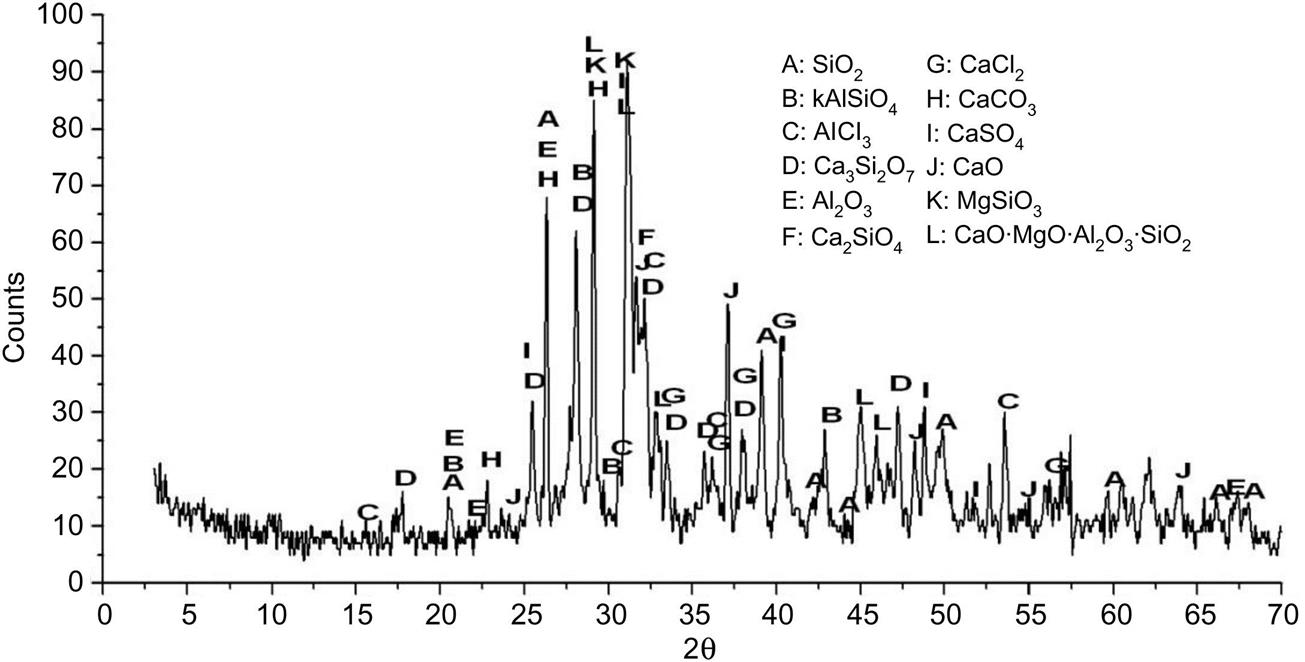
After being sintered at 1000°C, diffraction peak of mullite (3Al2O3 2SiO2) at d =5.39 Å appeared, which came into being due to combination of SiO2 with γ-Al2O3 (Fig. 6.14). Diffraction peaks of CaCO3 and CaSO4 at d = 3.86 and 1.87 Å, respectively disappeared, indicating a through decomposition of both salts. In addition, Mg2Al6Ti7O25 and CaSiO4 were detected, which came into being due to combination of oxides involving CaO, Al2O3, MgO, SiO2 etc.
XRD analysis of fly ash sintered at 1200°C showed that contents of crystal mullite decreased as its diffraction peak at d = 5.39 Å decreased considerably, which should be caused by transformation of crystal mullite to glass mullite (Fig. 6.15). In addition, contents of CaSiO4, MgSiO3 and CaO MgO Al2O3 SiO2 rose due to continuous combination of oxides and silicates.
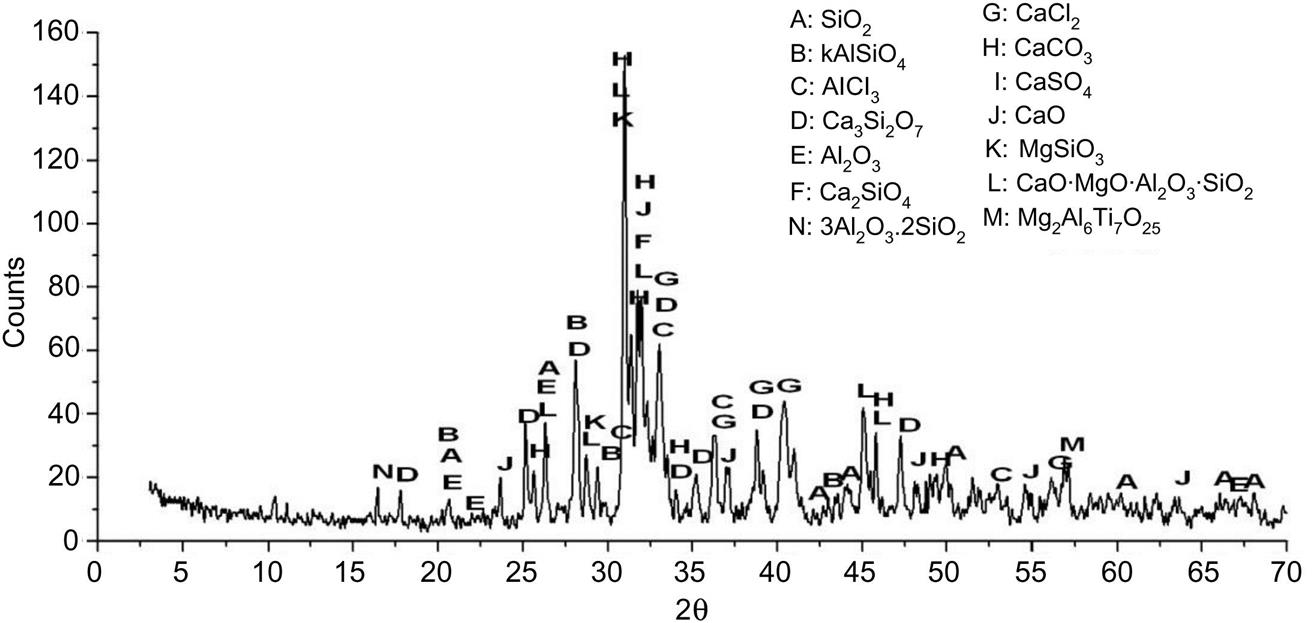
After being sintered at 1300°C, XRD analysis had a considerable increase of glass phase content, as shown in Fig. 6.16. In addition, cordierite (2MgO∙2Al2O3∙5SiO2), andradite (Ca3Fe2(SiO4))3, and enstatite (CaMgSi2O6) came into being due to considerable chemical combination which was proven by DTA analysis.
Based on the above analysis of mineralogical transformation as a function of temperature, it is concluded that salts, involving Ca(OH)2, CaCO3, CaSO4 etc., decompose firstly with increase of temperature. Then, oxides derived from the above decomposition process react with SiO2, forming silicates, calcium–silicates and aluminosilicates. Hence, pH value of sintered fly ash solution is reduced considerably, as shown in Fig. 6.17. In addition, contents of crystal phases first increased between room temperature and 800°C and then decreased between 800 and 1300°C, while that of glass phases registered a reverse trend.
From Fig. 6.17, it is clear that pH reduces relatively slowly with increase of temperature between 20°C and 600°C and decreases much more quickly from 600 to 1300°C, due to decomposition of salts and formation of new silicates, aluminosilicates and calcium–silicates, as described in XRD analysis.
6.6 Microstructure Transfer as a Function of Temperature for Fly Ash
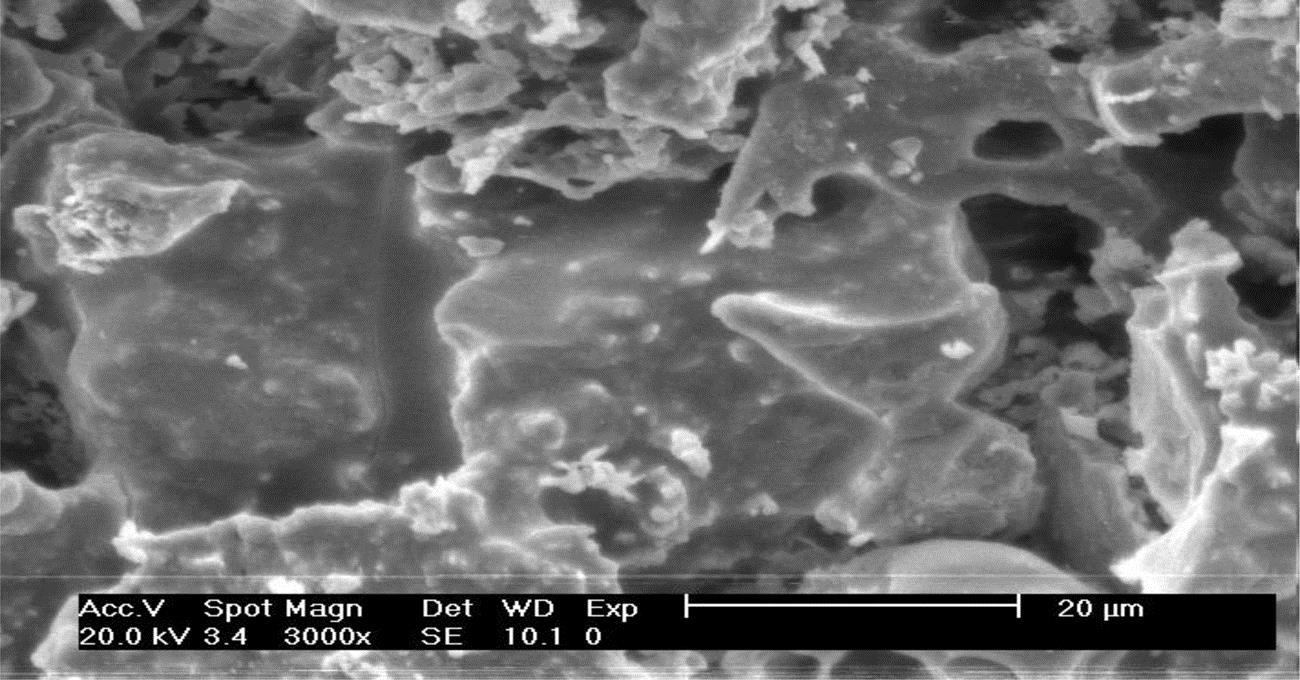
To investigate microstructure transfer as a function of temperature, fly ash At room temperature and that sintered at 1000 and 1300°C, respectively were chosen to perform scanning electron microscope (SEM) analysis. Particulates of municipal solid waste incinerator (MSWI) fly ash, even granular and less than 100 μm, had different shapes and sizes (Fig. 6.18), which was in conformity with its particulate size analysis. Among the particulates, glass phase existed which was conducive to increase of activity of the ash. The figure also indicated that the ash had a loose structure and a lot of holes among the particulates, which made it easy for heavy metals to be leached into the environment. Hence, leaching toxicity may be greatly reduced if its structure is cemented or hardened.
Mini-cracks disappeared according to SEM analysis of the ash sintered at 1000°C, as shown in Fig. 6.19. Obvious reaction can be seen at interfaces of particles and the interfaces were much more obscure in comparison with those at room temperature. Hence, porosity reduced considerably and density of the ash increased, which should be contributed to particle migration through liquid phases with the increase of sintering temperature.
From Fig. 6.20, it was clear that particles were more closely cemented together and their interfaces were invisible in the ash after being sintered at 1300°C, and a hard solid hole with high strength came into being. Apparently, heavy metals had been more tightly fixed in the solid and can hardly be leached out even at the acidic conditions. In addition, a lot of glass phases came into being at this temperature, which constituted one kind of cement material and were conducive to stabilization of fly ash.
From the above analysis, it is concluded that microstructure is strengthened with the increase of temperature, and porosity reduced. In addition, content of glass phases increases and particles are more closely cemented together as sintering temperature increases from 1000 to 13,000°C, which is conducive to solidification of heavy metals.
6.7 Leaching Toxicity as a Function of Temperature for Fly Ash
The results of leaching tests, in accordance with HVEP and ALT standards, are shown, respectively, in Tables 6.1 and 6.2. From Table 6.1, it can be seen that leaching toxicity, in accordance with HVEP standard, of heavy metals from fly ash sintered at 1000 and 1000°C-plus was lower than the standard value described in “Identification Standard of Hazardous Waste—Identification of Leaching Toxicity”.
Table 6.1
Leaching results of fly ash in accordance with HVEP standard (ppm)
| Tem (°C) | As | Cd | Cr | Cu | Hg | Ni | Pb | Zn |
| Room temperature | 0.057 | 0.159 | 1.631 | 1.034 | 0.693* | 0.019 | 48.13* | 9.98 |
| 400 | 0.049 | 0.147 | 1.013 | 0.954 | 0.401* | 0.011 | 31.04* | 8.01 |
| 600 | 0.037 | 0.114 | 0.897 | 0.632 | 0.162* | 0.008 | 15.04* | 7.25 |
| 800 | 0.023 | 0.092 | 0.572 | 0.421 | 0.039 | 0.006 | 3.19* | 6.19 |
| 1000 | 0.002 | 0.005 | 0.824 | 0.141 | ND | 0.001 | ND | 3.27 |
| 1200 | ND | ND | 1.582 | 0.012 | ND | ND | ND | 0.04 |
| 1300 | ND | ND | 1.018 | 0.008 | ND | ND | ND | ND |
| China standard | 1.5 | 0.3 | 10 | 50 | 0.05 | 10 | 3 | 50 |
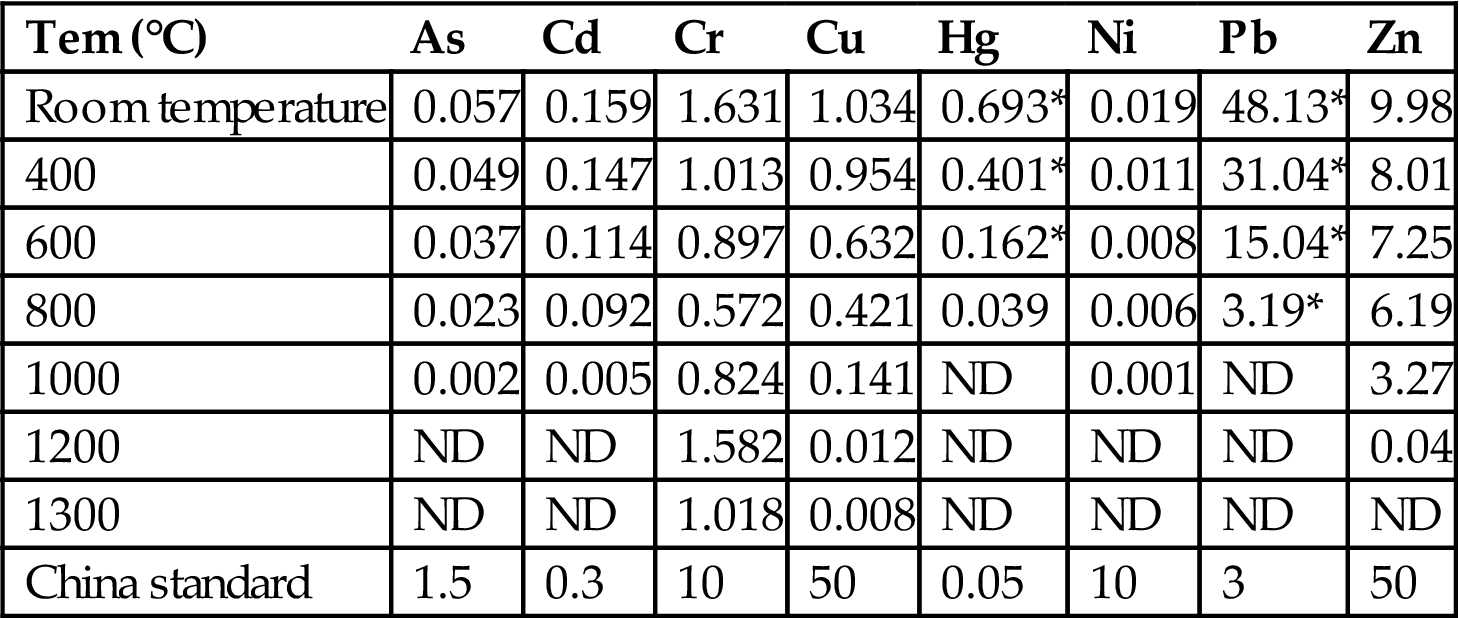
Note: ND and * represent, respectively, “not detected” and “leaching toxicity surpassing the limit”.
Table 6.2
Leaching results in accordance with ALT standard for fly ash (mg/kg)
| Tem (°C) | As | Cd | Cr | Cu | Hg | Ni | Pb | Zn |
| 25 | 7.25 | 29.65* | 39.76 | 69.04* | 1.38* | 21.56 | 748.1* | 1273* |
| 400 | 6.87 | 28.03* | 37.01 | 62.05* | 1.17* | 20.97 | 686.2* | 1101* |
| 600 | 4.98 | 17.65* | 31.36 | 55.36* | 0.69* | 19.02 | 332.8* | 978* |
| 800 | 2.69 | 9.05* | 25.34 | 48.32 | 0.28 | 17.78 | 102.8* | 634* |
| 1000 | 0.52 | 1.76 | 28.85 | 39.01 | 0.08 | 16.01 | 22.5 | 376 |
| 1200 | 0.02 | 0.05 | 36.98 | 20.65 | 0.01 | 13.25 | 2.7 | 101 |
| 1300 | ND | ND | 33.01 | 9.32 | ND | 8.53 | ND | 327 |
| China standard | 15 | 3 | 100 | 50 | 0.5* | 100 | 30 | 500 |

Note: ND and * represent, respectively, “not detected” and “leaching toxicity surpassing the limit”.
Leaching ratios, by ALT standard, of Cd, Cr, Cu, Pb and Zn exceeded the standard value until the sintering temperature reached 1000°C. Hence, leaching toxicity of heavy metals met the requirements described in “Identification Standard of Hazardous Waste—Identification of Leaching Toxicity”, only when the sintering temperature was no lower than 1000°C, and fly ash sintered at temperature 1000°C-min was potentially harmful to the environment in accordance with both standards. Besides, leaching toxicity, by both standards, of As, Cd, Cu, Hg, Ni, Pb and Zn decreased with increase of sintering temperatures, indicating that increase of temperature between 400°C and 1300°C was conducive to reduction of leaching toxicity of these heavy metals. Leaching toxicity of Cr decreased as temperatures increased from 400 to 800°C, and from 1200 to 1300°C, respectively, but increased when it rose from 800 to 1200°C. Consequently, increase of temperature between 800°C and 1200°C enhanced leaching of Cr into the environment. It is conferred that increase of leaching toxicity of Cr is caused by transformation of Cr3+ to Cr6+ whose solubility is much bigger, while its decrease between 1200°C and 1300°C should be caused by formation of glass phases which constitute cement materials of heavy metals and occlude Cr in them.
6.8 Speciation Transformation as a Function of Temperature for Fly Ash
Soluble Cd mainly existed in the acid solution, exchangeable and water-soluble phases, soluble Hg mainly in acid solution and water-soluble phases, while soluble Pb mainly appears in acid solution and soluble Zn mainly in organically bound and acid solution, in terms of fly ash at room temperature, as given in Fig. 6.21. Hence, it is relatively difficult for Pb and Zn to be leached out in environment without the existence of acids. However, in natural environment exposing to acid rain, acids always exist and the above four heavy metals tend to be extracted by acidic liquid after years. The aim of stabilization and sintering of the fly ash is to minimize leaching toxicity of these heavy metals by reducing their existence in water soluble, exchangeable, acid soluble and organically bound phases, as done in this work.
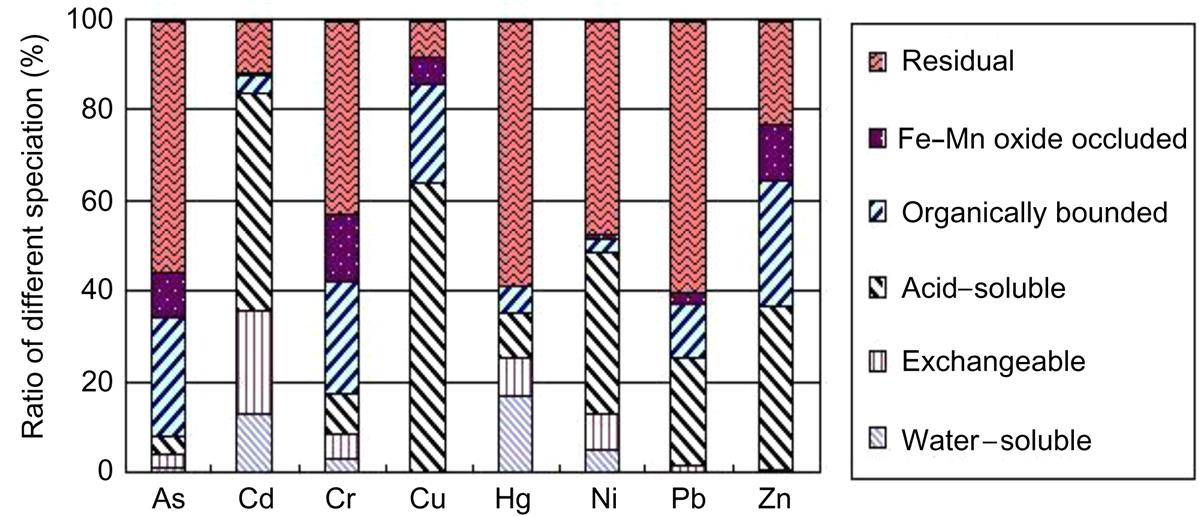
Sequential chemical results of fly ash sintered at 400, 600 and 800°C, respectively, are presented in Fig. 6.22(A)–(C). It was clear that ratios of soluble phase of each heavy metals decreased as sintering temperature increased from 400 to 800°C, indicating that increase of sintering temperature was conducive to stabilization of such heavy metals as As, Cd, Cr, Cu, Hg, Ni, Pb and Zn.
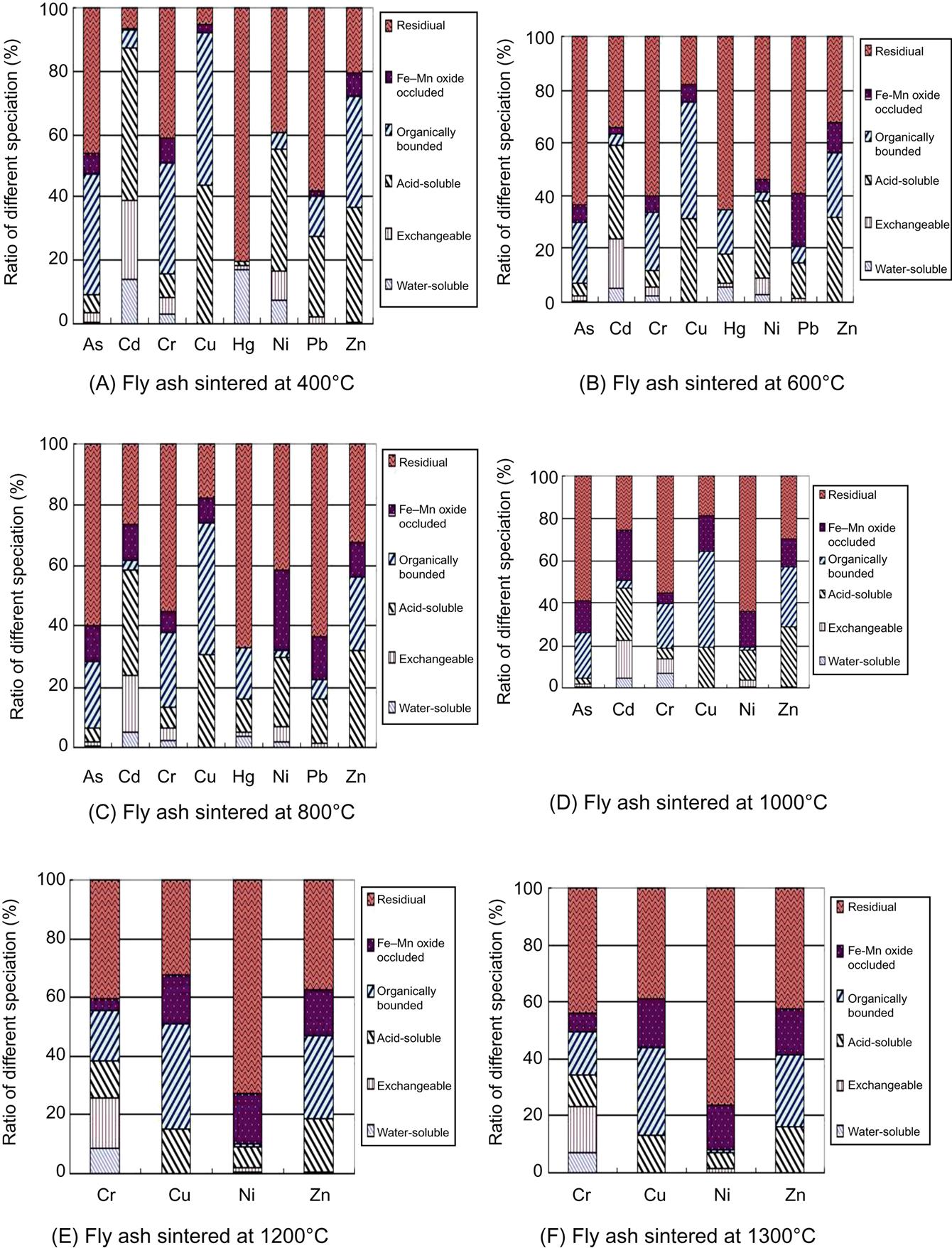
As temperature increased from 800 to 1000°C, most Hg and Pb flied away and their specification can’t be analyzed due to a trace content in the sintered fly ash. According to the sequential chemical extraction results of fly ash sintered at 1000°C in Fig. 6.22(D), ratios of soluble form of As, Cd, Cu, Ni, and Zn were reduced, respectively, showing that increase of sintering temperature from 800 to 1000°C enhanced stabilization of these heavy metals. While, ratios of soluble Cr increased as sintering temperature increased from 800 to 1000°C, indicating that increase of sintering temperature between 800°C and 1000°C was negative to stabilization of Cr. The reason is that increase of temperature enhances formation of silicates and alumino-silicates, which occlude most heavy metals except Cr in their lattice and thus they are hardly be leached out.
As and Cd almost flied away entirely at 1200°C, and their specification can’t be analyzed. Increase of sintering temperature from 1000 to 1300°C was conducive to stabilization of Cu, Ni, and Zn, as their soluble ratios were reduced, respectively, as shown in Fig. 6.22(D)–(F). While ratios of soluble Cr increased as temperature rose from 800 to 1200°C, but decreased again as temperature rose from 1200 to 1300°C with the same reason described in leaching toxicity analysis.
Based on the above specification analysis of heavy metals from fly ash sintered at different temperatures, it is found that increase of temperature is conducive to decrease of ratios of soluble As, Cd, Cu, Ni, Hg, Pb, Cu and Zn, and thus reduces leaching toxicity of these heavy metals. While, ratios of soluble Cr increase as sintering temperature rises from 800 to 1200°C, thus enhancing its leaching toxicity.
Four endothermic peaks: 225–350, 350–414, 446–481, 531–1241°C, and two exothermic peaks: 481–531, 1241–1400°C, were detected by DTA analysis. Mass of fly ash remained relatively constant when temperature exceeded 1241°C, which indicated an end of decomposition process of the salts and occurrence of considerable chemical combination. Hence, fly ash remains relatively stable until temperature exceeds 1241°C.
Contents of elementary components follow a sequence of O >Ca >Si >Cl >Al >Fe >Na >S >C >Mg >Zn >Pb >Mn >Cu >Cr >N >Sb >Ti >Ni >As >H >Cd >Ag >Hg. Contents of Ca, Si, Al, Fe, K and Na stand almost constant, while that of O, C, Cl, H, and S reduces with the increase of temperature. Volatilization ratio of heavy metals follows sequence of Hg >Pb >Cd, As >Zn >Cr approximately Ni and Cu.
Contents of chemical components follow a sequence of CaO >SiO2 > Al2O3 > Fe2O3 > MgO >K2O >Na2O, and fly ash registers a SiO2–Al2O3–metal oxides system as total contents of these oxides exceeds 80%. Content of chemical components, excluding K2O and Na2O whose contents decrease when temperature exceeds 1000°C, rises with increase of sintering temperature. Major radicals detected involve Si–O, Al–O, Si–O–Si, SiO32−, SiO42− CO32− and C3S. Content of crystal phases first increases between room temperature and 800°C and then decreases between 800°C and 1300°C, while that of glass phases registers a reverse trend.
Major mineralogical components of fly ash involve SiO2, CaCl2, Ca3Si2O7, Ca2SiO4∙0.35H2O, Ca9Si6O21∙H2O, K2Al2Si2O8∙3.8H2O and AlCl3∙4Al(OH)3∙4H2O. Glass phases account for around 57%, which is conducive to reduction of energy in recycling of the ash. Salts decompose firstly with increase of temperature and then oxides derived from the decomposition process react with SiO2, forming silicates, calcium-silicates and aluminosilicates.
Structure of fly ash is strengthened with the increase of temperature, and porosity reduced. In addition, content of glass phases increases and particles are more closely cemented together as sintering temperature increases from 1000 to 13,00°C, which is conducive to solidification of heavy metals.
Leaching toxicity analysis shows that leaching toxicity of As, Cd, Cu, Hg, Pb, Ni and Zn decreases with increase of sintering temperature from 400 to 1300°C. While that of Cr decreases with increase of temperature from 400 to 800°C, and from 1200 to 1300°C, respectively, but increases as it rises from 800 to 1200°C.
Sequential chemical extraction analysis indicates that increase of sintering temperature between 400°C and 1300°C is conducive to reduction of ratios of soluble As, Cd, Cu, Hg, Pb, Ni and Zn, respectively. Content of soluble Cr decreases with increase of temperature from 400 to 800°C, and from 1200 to 1300°C, respectively, but increases as it rises from 800 to 1200°C.