Weathering Process and Biological Dechlorination of Bottom Ash
Abstract
Copper leaching of bottom ash (BA) codisposed with refuse subject to short-term accelerated weathering, preparation of fresh simulated municipal solid waste (MSW) landfill leachate, evolution of mineralogical and chemical properties, changes of thermal analysis patterns are fully provided. Influences of weathering on Cu fractionation, Cu leaching in synthetic precipitation leaching procedure (SPLP) and toxicity characteristic leaching procedure (TCLP), Cu leaching in codisposal leaching procedure are presented. The higher contents of plastics and kitchen waste lead to the higher chlorine level (0.6–0.7 wt.%) of the BA. Biological dechlorination of incineration BA and geoenvironmental weathering/deterioration of landfilled BA glass are explored. The codisposal of refuse and BA has been widely used in the form of either multilayered landfill or mixed landfill, and the BA is commonly utilized as landfill cover. Weathering process transforms some Cu that is bound to organic matter and carbonates in BA into the residual fractions. The results of SPLP and TCLP leaching study shows that Cu leachability increases. Weathering could be an effective method for decreasing Cu leaching when BA is codisposed with refuse. The high content of chlorine causes the corrosion of reinforce steel in concrete, so it is necessary to remove the chlorine from BA before it can be reused as the raw material for cement production. A correlative analysis is conducted to evaluate the effect of the MSW components and collection mode on the heavy metal and chlorine characteristics in BA. The insoluble chlorine in BA exists primarily as aluminum oxide chloride, which is produced under the high temperature (1250°C) in incinerators. Biological dechlorination process of BA with aged refuse in landfill can decrease the Cl to less than the target level (0.18 wt.%) within 30 weeks. It is still hard to give a definite conclusion regarding whether glass evolution in BA may decrease or increase the leaching amount of toxic elements from ash deposit, but can provide information in terms of assessment of the long time-scale behavior of pollutants in ashes and thus helps choose environmentally sound management strategy for ash deposits.
Keywords
Bottom ash; codisposal; weathering process; copper leaching; biological dechlorination; landfill
4.1 Weathering Process and Copper Leaching from Bottom Ash Codisposed with Refuse on Weathering
Physical weathering and chemical weathering are usually be distinguished as the two categories of weathering processes happening in nature, although these two processes are separated in description, actually they work together to break down big particles to smaller fragments or to generate minerals more adopted to the environment.
Dissolution, carbonation, hydration, oxidation, and chelating are usually thought to be the main processes that control the chemical weathering processes. Development of joints, crystal growth, and heat are thought to be the main mechanisms of physical weathering. These processes are considered to be the main weathering processes happened in a landfill.
pH, electrical conductivity (EC), temperature, etc., of bottom ash (BA) will be influenced by weathering in nature. Weathering can greatly influence some movable elements such as Cl, more than 50% of which flow out from the piles due to natural weathering. However, according to the 18-month monitoring data of a BA pile under natural weathering, the concentration of Pb and Zn were less than 25 μg/L.
As the result of weathering, not only the total concentration, but also the chemical form will change. Chemical form is as important as the total concentration, which can determine the behavior, the toxicity and the mobility of an element. Mineral phases change during weathering, an illite-like basal spacing in a weathered rim region is observed in a weathered glassy phase of BA. Gympsum, ettringite, and Na2Si2O5 appear in a 4-month aging BA. Some researches indicated the influence of carbonation on the weathering of BA, a precipitation consisted mainly Al-rich amorphous minerals, calcite, and possibly gibbsite were formed during the carbonation of BA in the laboratory.
Compared with fresh BA, humic substances increase during landfill, the heavy metals transform among different phases. With respect to the heavy metals present in BA, sequential extraction procedures (SEPs) have been applied to fractionation of BA into different fractions under different conditions. Leaching tests are widely used as tools to estimate the release potential of constituents from waste materials over a range of possible waste management activities, including recycling or reuse, to assess the efficiency of waste treatment and disposal processes.
Weathering of BA indicated that, well-ordered primary clay (illite) is found to be generated from glasses in a 12-year old municipal solid waste (MSW) incineration BA. Minerals that form under weathering, such as Fe oxyhysroxides and carbonates always have a high trapping capacity of heavy metals and will act as a stable trapping medium under atmosphere condition. Leaching tests are supposed to describe the weathering phenomenon of heavy metals, and by combining leaching tests with some comprehensive instrument analyses, the major chemical forms which control the leaching behaviors of heavy metals could be discovered.
Geochemical weathering process will change the compositions of BA at landfill. For example, due to washing out of Ca, Na, K, Cl, Ti, and S, their concentrations increase with landfill depth and landfill time. Other elements including Si, Al, Fe, Mg, and P have a higher total contents with the development of weathering. The change of total contents might indicate the dissolving speed. According to the results of leaching, the contents of heavy metals of the soluble fraction in water such as Pb, Cr, Zn, and Cu decrease after landfill treatment, which might be related to the washing out or immobilization process of the soluble fractions. Results of availability test indicate that Cu, Zn, and Cd have a relatively higher leachability than Pb and Cr. When compared with fresh residues, the availability of Cu, Zn, and Cd of weathered residues is higher than in fresh residues. Results of the European Community Bureau of Reference (BCR) SEP indicate that the differences in leaching properties of heavy metals are mainly due to the differences of chemical forms. As the weathering of MSW residues progress, heavy metals are adsorbed on iron/manganese oxyhydroxides or combined with organic carbon and sulphur, since the stability of these chemical forms is different under the same leaching conditions.
The codisposal of refuse and BA has been widely used in the form of either multilayered landfill or mixed landfill, and the BA is commonly utilized as landfill cover. It has been proposed to use BA as landfill cover construction materials for liner, protection layer, and drainage layer as replacement of traditional construction materials such as natural minerals (e.g., gravel, sand, till, and clay) or synthetic products (e.g., geomembranes). Mixed landfill of BA and refuse can also assist organic fraction anaerobic digestion, upgrade landfill gas, and enhance landfill stabilization. Therefore, mixed landfill of BA and refuse in sanitary landfill is also encouraged in China according to the Ministry of Environment Protection of China (GB 16889-2008).
Generally, the potential of heavy metals leaching from BA is limited due to its strong acid-neutralizing capacity (ANC) by hydroxides, silicates, and carbonates in BA when it is deposited in landfill. However, when BA is codisposed with refuse, the decrease of pH in leaching environment due to hydrolyzes/acidification process during anaerobic digestion could eventually dissolve and mobilize heavy metals in BA. The codisposal of BA and refuse can pose a risk to the environment due to heavy metal leaching.
Among various technologies such as size separation, washing, weathering, chemical extraction, and immobilization, because of the simplicity and high cost-effectiveness, the weathering apparently is the most commonly used method. Weathering can significantly change chemical and mineralogical characteristics of the material by hydrolysis of Ca, Al, Na, and K, dissolution/precipitation of hydroxides and salts of these main cations, carbonation, neutralization of pH, and neo-formation of clay-like minerals from glasses. The mineralogical changes caused by weathering processes result in changes in chemical properties of BA, including ANC, pH, redox potential, and sorption, and ion-exchange capacity. Moreover, the neoformed reactive and sorptive solid substrates can affect the leaching, solubility, and complexation of heavy metals in BA. The formation of secondary phases, such as aluminosilicates, and gypsum, can lead to the formation of cement-like materials, which may act as binder materials.
Among the heavy metals in BA, copper (Cu) is of particular concern. Firstly, the content of Cu is relatively high in BA. Secondly, it has a high potential toxicity especially to aquatic biota, and can be leached in considerable amounts to ecosystem. The neoformed aluminum (hydro)-oxides in MSW incinerator (MSWI) BA may act as reactive binder materials to keep Cu from leaching. Reduction of Cu leaching by weathering may result from formation of insoluble Cu oxides. The leaching of Cu with the process of weathering treatment is attenuated when situated in inorganic acid solution, but is enhanced in acetic acid solution. However, the effect of weathering process on Cu leaching is seldom studied and far from clarified in fresh landfill leachate, which is characterized by a relative low pH value and high organic acid and dissolved organic matter (DOM) concentrations.
In this work, the effect of short-term accelerated weathering on the leaching behavior of Cu when BA was codisposed with refuse was investigated. Changes of chemical and mineralogical properties of BA at different stages of short-term accelerated weathering were examined. The extractability of Cu of the weathered BA was then evaluated using codisposal leaching procedure and standard leaching protocols [i.e. synthetic precipitation leaching procedure (SPLP) and toxicity characteristic leaching procedure (TCLP)]. The data shown in this work could be of significance in evaluating the short-term accelerated weathering as an effective pretreatment method to decrease Cu leaching prior to its codisposal with refuse.
Fresh BA sample was collected from Pudong Yuqiao Waste to Energy Plant, Shanghai, China. The plant consisted of three furnaces (made by Alstom, Levallois-Perret, France) together with two sets of 8500 kW power generation steam turbines with an average MSW treatment capacity of 1000 t/d. The BA was water quenched and magnetic separated before sampling, and the samples were sieved through 4 mm before use. Portion of the sample was digested using microwave total digestion method and analyzed using an Optima 2100 DV inductively coupled plasma optical emission spectrometry (ICP-OES) (PerkinElmer, USA). The chemical characteristics of the BA sample indicated that Cu (642 mg kg−1) is one of the major heavy elements.
Short-term accelerated weathering was carried out in a lab-scale air-tight column reactor (10 cm in diameter ×50 cm) where a slow air flow (30 L/min) was blown by a pump (HAILEA V-30, China), and this process lasted for 408 h. Different BA samples, i.e., fresh BA (S0, pH =12.4), weathered BA for 48 h (S1, pH =10.8), weathered BA for 144 h (S2, pH =9.9) and weathered BA for 408 h (S3, pH =9.4), were taken from the column reactor after the different weathering times (thoroughly homogenized before sampling). The pH of BA sample dropped sharply from 12.4 to 10.2 at the first 72 h, further decreased to 9.5 at 216 h, and then kept relatively steady afterwards. The moisture content of BA gradually decreased from 23.4 to 3.0% during weathering process, which is different from ageing process in a natural environment with rainy periods.
4.1.1 Preparation of Fresh Simulated MSW Landfill Leachate
In order to obtain fresh simulated MSW landfill leachate in the acidification stage, a simulated landfill was constructed. The waste composition consisted of 15% (w/w) inorganic matters and 85% (w/w) organic matters, dominated by kitchen wastes (ca. 50%) according to the local waste characteristics in Shanghai. The simulated MSW landfill had a height length 160 cm and inside diameter 80 cm was operated under anaerobic condition at an atmosphere temperature of 20–35°C. After 50 days anaerobic digestion, landfill leachate with a pH of 4.84, COD of 41,700 mg/L and Cu of 0.03 mg/L were collected from the simulated landfill.
Two standard leaching protocols, i.e. SPLP and TCLP, were carried out on the fresh and weathered BA. In the SPLP, 2 g samples were added into 40 mL acid solution at pH of 4.2 (made by adding the 60/40 weight percent mixture of sulfuric and nitric acids to water). In the TCLP, 2 g samples were, respectively, added into 40 mL extraction fluid 1# (pH =2.88) and 2# (pH =4.93) (according to US EPA Test Method 1311). After shaking for 24 h at 25°C, the pH values in solution were measured by a digital pH meter (PHS-3C), the concentrations of Cu and Al were determined by the Optima 2100 DV ICP-OES, dissolved organic carbon (DOC) was determined by total organic carbon (TOC)-V CPH/CPN (Shimadzu Corporation, Japan). All the leaching experiments were conducted in triplicate.
Fresh simulated landfill leachate was adopted as extraction fluid to simulate the Cu leaching potential when BA was codisposed with refuse. The codisposal leaching procedure was carried out for 24 h or 15 days. In the 24 h test, simulated landfill leachate was passed through 0.45 μm membrane before use. Then BA was added into the filtered landfill leachate with a liquid–solid ratio of 20:1. In view of the pH variation of acid-phase leachate from MSW landfill, the initial pH of the suspension were adjusted to 4.50, 6.00 and 7.50 using organic acids (acetic: butyric =4:1) or 0.1 M NaOH solution. The suspension was shaken for 24 h by a rotary oscillator at 25°C. In the 15-day test, BA sample was added into raw simulated landfill leachate with a liquid–solid ratio of 20:1, and the suspension was shaken on a rotary shaker at 90 rpm at 25°C. After 15 days, the size charge fractionation (SCF) procedure was also introduced to classify Cu in leachate. The fractionation products of Cu were divided into three kinds, namely particulate and colloidal matter >0.45 μm, free anions/nonlabile complexes <0.45 μm and free cations/labile complexes <0.45 μm. To measure Cu concentration in the codisposal leaching procedure, 5 mL simulated landfill leachate was firstly digested using 8 mL mixed solution (65% HNO3:30% H2O2 = 7:1) using Microwave Digestion System ETHOS (Milestone, s.r.l, Italy), and the Cu concentrations were determined using ICP-OES.
SEP was adopted to analyze Cu speciation transformation in BA. Fractionation of Cu in fresh and weathered BA samples was conducted using SEP, where Cu was classified into five operationally defined fractions including exchangeable phase, carbonates phase, Fe/Mn oxides, organic matter phase, and residual (silicates) phase. Triplicate was used in SEP and Cu concentrations in each solution of each step were analyzed using ICP-OES.
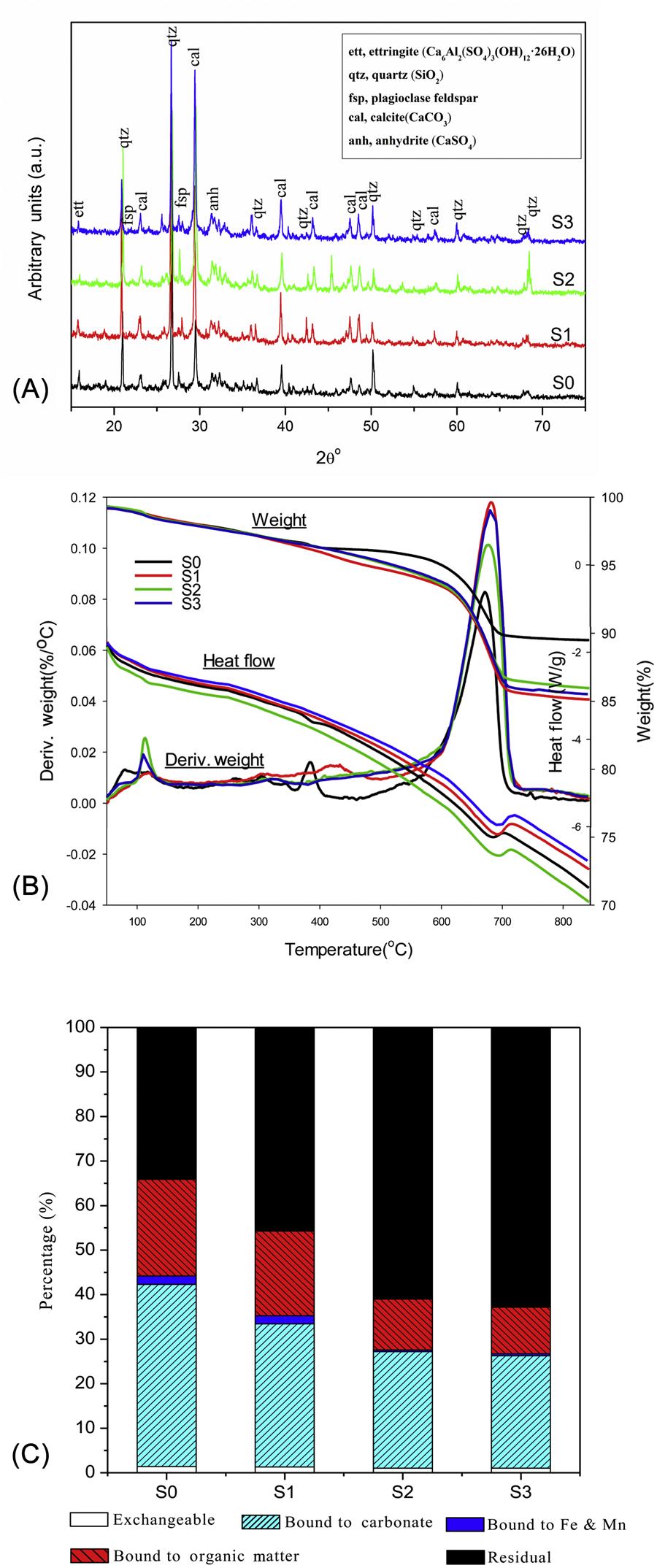
X-ray diffraction (XRD) and thermal analysis were used to characterize surface mineral composition and thermo-reaction properties of fresh and weathered BA samples. In the XRD analysis, AD 8 Advance powder diffractometer (Bru-ker AXS Inc., Germany) was employed with accelerating voltage at 40 kV and current at 40 mA. The samples (<5 μm) were examined at room temperature over the 2θ range 10–90° using graphite monochromatic sCu Kα radiation. The step scan was 0.02° and the measuring time 0.1 s step−1. The diffractograms were obtained with Diff-plus and analyzed using MDI Jade 5.0 software. Thermal gravimetric analysis and differential thermal gravimetric analysis (TGA/DTG) were performed using SDT Q600 simultaneous thermal analyzer (TA, USA). The samples were heated from 50 to 850°C at a heating rate 10°C min−1 under nitrogen flow (100 mL min−1).
4.1.2 Evolution of Mineralogical and Chemical Properties
From the X-ray diffractograms of the fresh and weathered BA samples (Fig. 4.1A), the principal minerals were identified to quartz (SiO2), calcite (CaCO3), anhydrite (CaSO4), plagioclase feldspar and ettringite (Ca6Al2(SO4)3(OH)12·26H2O) in both fresh and weathered ash samples. However, it was obvious that the content of CaCO3 increased over weathering period, which was attributed to the reaction between CO2 and portlandite-like minerals in BA. Short-term accelerated weathering changed the mineralogical characteristic of BA. Additionally, the diffractogram of the weathered samples showed a relatively low intensity in peaks for ettringite compared to the fresh sample, and this might result from the carbonation of ettringite. The result is different from natural weathering, in which ettringite is usually formed, hinting that short-term accelerated weathering cannot be compared directly to natural weathering possibly.
4.1.3 Changes of Thermal Analysis Patterns
Fig. 4.1B showed TG/DTG/DSC data of fresh and weathered BA samples. The data of DSC were calculated from DTA results. Suggested by a weak peak in the DTG curves, the weight loss occurred at about 120°C, and this might be attributed to the evaporation of free water in BA. At temperature of 360–410°C, a weight loss was detected only in fresh sample (S0), impling that part of organic matters (hydrophilic fraction mainly) in BA were gradually oxidated. The largest difference in peaks of BA samples appeared at around 600–750°C, which was mainly because of CO2-loss of calcium carbonate and pyrolysis of some other inorganic matters. The significant increase in intenties of peaks at around 600–750°C for the weathered BA suggested the formation of carbonate minerals during the weathering. The phenomenon was also confirmed by the results of XRD test.
4.1.4 Influence of Weathering on Cu Fractionation
Fig. 4.1C showed the fractionational variation of Cu in BA samples over the weathering process. Organic matter bound fraction, carbonate bound, and residual fraction were the main fractions of Cu in BA, and Cu in exchangeable (<1.5%) or bound to Fe–Mn oxides (<2.0%) was minimal. The weathering process reduced Cu in the organic matter bound fraction from 21.7% (139.4 mg kg−1, dry wt, similarly hereinafter) to 10.4% (67.0 mg kg−1), probably due to the degradation of organic matter in BA. The phenomenon was consistent with previous researches. Meanwhile, the carbonate bound fraction of Cu in the weathered BA decreased from 40.9% (262.5 mg/kg) to 25.2% (162.0 mg/kg), the exchangeable fraction decreased from 1.4% (9.1 mg/kg) to 1.0% (6.6 mg/kg), and the residual fraction increased significantly from 34.1% (219.1 mg/kg) to 62.8% (403.2 mg/kg). In general, weathering process transformed some Cu that was bound to organic matter and carbonates in BA into the residual fraction.
4.1.5 Influence of Weathering on Cu Leaching in SPLP and TCLP
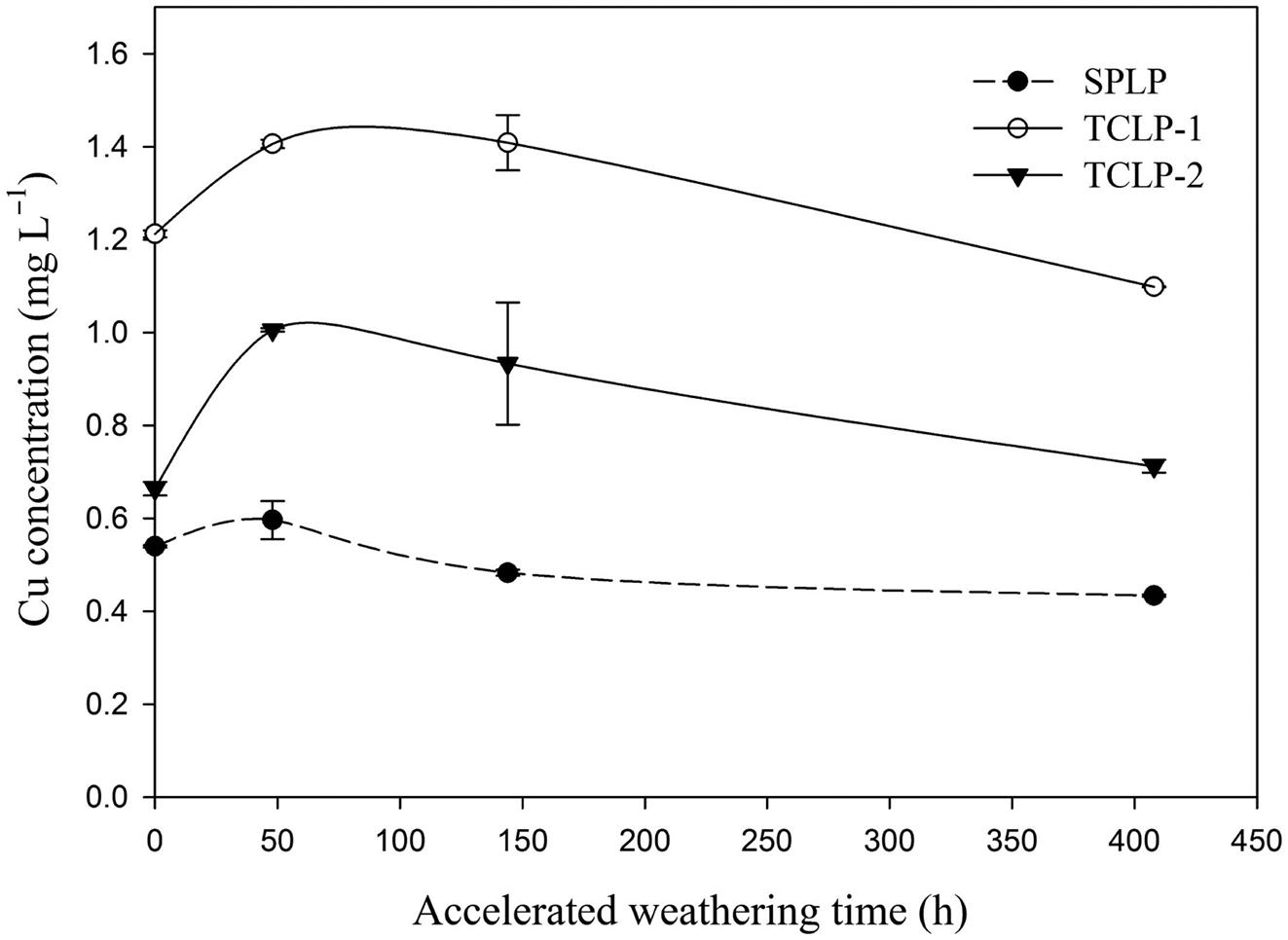
The results of SPLP leaching study showed that Cu leachability increased from 0.54 to 0.60 mg/L within the first 48 h, followed by a subsequent decrease to 0.43 mg/L (Fig. 4.2). This singular extraction profile might be accounted for the variation of DOC and Al concentrations in the leachates over the weathering time. The concentration of DOC in leachate continuously decreased from 63.3 to 56.7 mg/L in the course of weathering, most likely due to the stronger adsorption of fulvic acid (FA) onto the BA matrix. The aluminum concentration in leachate peaked initially and then leveled off in the SPLP test (Fig. 4.2), which was similar to the trend of Cu leaching in weathering period. The trend of Al leaching could be explained by the increase of amorphous Al oxyhydroxides (possibly carbonation of ettringite) and immobilization of Al as hydroxocomplexes or other water insoluble phases. As a result, the extraction profile of Cu in SPLP might be contributed by the adsorption of Cu on natural humic substances and complexation of Cu onto newly formed aluminum hydroxides, as suggested by recent findings.
The results of TCLP leaching indicated that 144-h accelerated weathering might have facilitated Cu leaching, and no beneficial effect was observed even after BA was weathered for 408 h (Fig. 4.3A). The maximum Cu leaching was found for 48 h weathered BA, in which the concentration of Cu increased from 1.21 to 1.41 mg/L for TCLP-1 and from 0.66 to 1.00 mg/L for TCLP-2. Meantime, the final pH of leachate decreased from 10.5 to 6.9 for TCLP-1 and from 12.2 to 9.6 for TCLP-2 after 48-h weathering. The dramatic decreases of ANC in weathered BA must have enhanced extraction of Cu from weathered BA. The leached Cu concentration of BA after 408-h accelerated weathering was slightly decreased from 1.21 to 1.10 mg/L for TCLP-1, while the concentration was slightly increased from 0.66 to 0.71 mg/L for TCLP-2. These results revealed that weathering had no beneficial effects on Cu leaching in TCLP. As expected, the concentration of DOC soared from ca. 60 mg/L to 2700 mg/L when acetic acid was introduced as leaching liquid in TCLP (Fig. 4.3B). Meanwhile, the Al concentration in the leachate decreased consistently during the weathering period in TCLP.
4.1.6 Influence of Weathering on Cu Leaching in Codisposal Leaching Procedure
The Cu leaching in codisposal leaching procedure for 24 h to 15 days was shown in Figs. 4.4 and 4.5. In the 24-h leaching study, the amount of leached Cu decreased consistently over the weathering period at pH 4.50. On pH 6.00 and 7.50, the leached Cu increased initially and followed by a subsequent decrease. For 408-h accelerated weathered BA, the Cu leaching concentration changed from 5.43, 2.03 and 1.86 mg/L at the beginning of weathering to 2.49, 0.83 and 1.10 mg/L at pH 4.50, 6.00 and 7.50, respectively. Although the leaching pH in codisposal leaching procedure evidently decreased with weathering process from 9.18–11.57 to 6.30–8.18, the Cu leaching concentration of the 408-h weathered BA was effectively reduced by 41–59% when the BA was situated in simulated landfill leachate with different pH conditions.
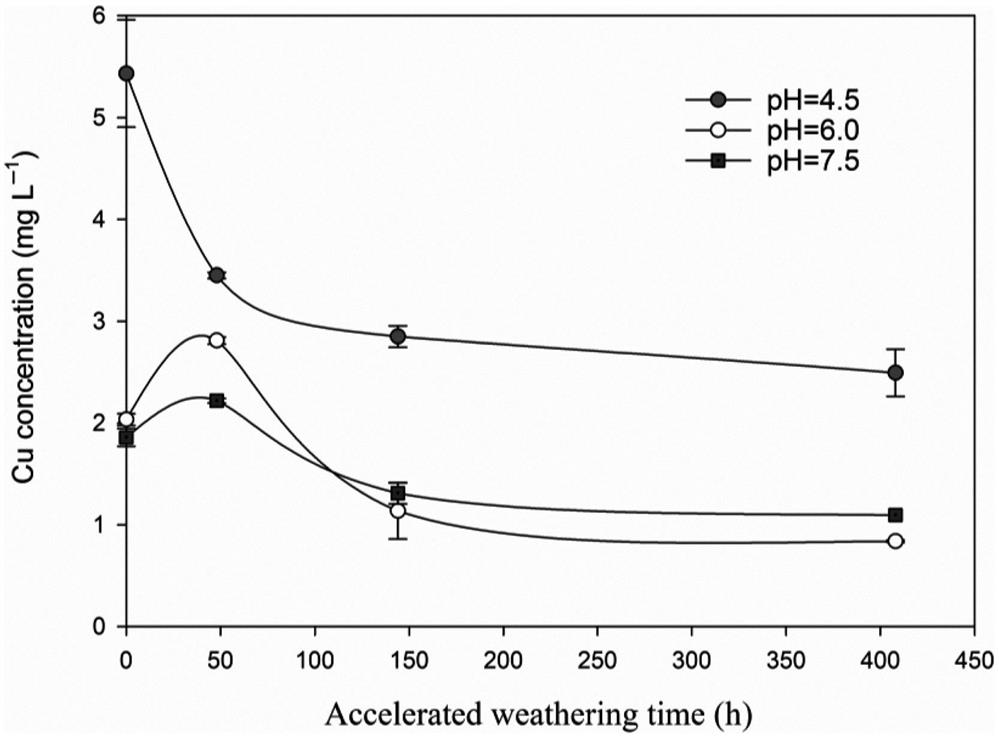
In the 15-day leaching experiment, the leached Cu concentrations of weathered BA decreased significantly (Fig. 4.5). The amount of total leached Cu decreased from 5.71 to 4.73, 0.44 and 0.65 mg/L after 48, 144, and 408 h accelerated weathering, respectively. Moreover, the concentration of Cu of free or labile complex fraction (the fraction with the highest mobility and bio-toxicity) decreased by 40.0, 95.8 and 97.6% after 48, 144, and 408 h accelerated weathering, respectively (Fig. 4.6). These results suggested that accelerated weathering could be an effective method for decreasing Cu leaching when BA was codisposed with refuse.
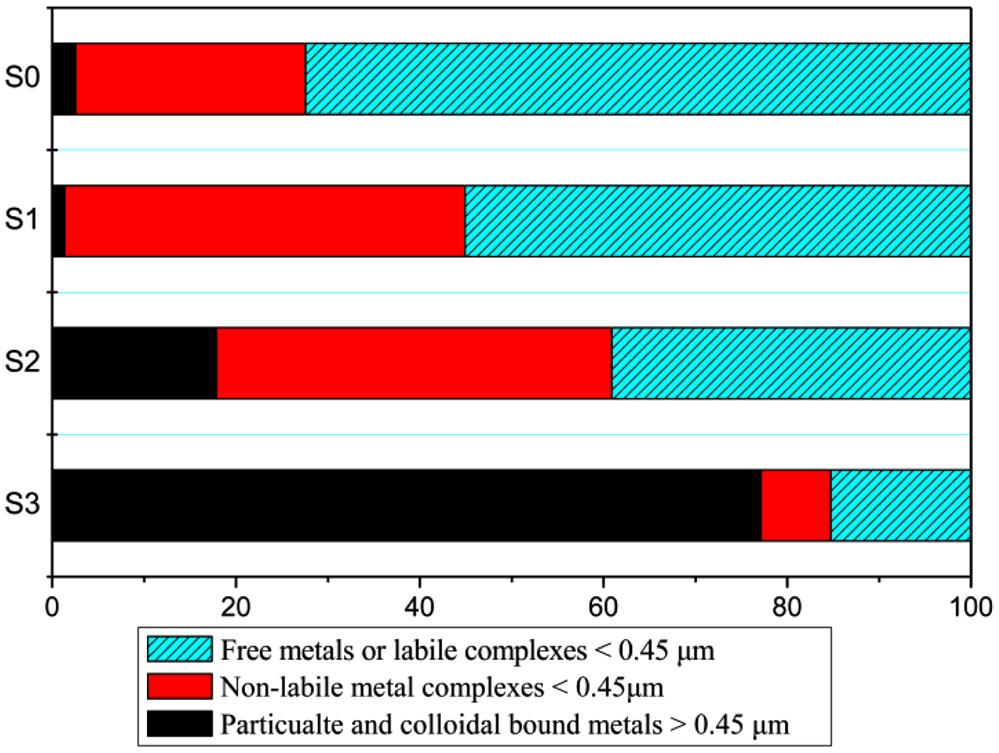
SCF procedure also revealed that the portion of free and labile fraction was consistently decreased from 72.4 to 55.0, 39.1 and 15.2%, and the portion of particulate and colloidal bound fraction was changed from 2.6 to 1.4, 17.9 and 77.1% after 48, 144, and 408 h accelerated weathering, respectively (Fig. 4.6). The Cu distribution analysis showed that a great part of leached Cu were bound to particulate or colloidal, and only a small portion of free and labile fraction Cu were leached from 408-h weathered BA.
The leaching of Cu was determined by the physical and chemical properties of BA and the leaching environment. The basic parameters of BA such as ANC and weight loss on ignition were changed during the weathering process. Meanwhile, mineralogical characteristics including the changes of Cu fractionation and the newly formed minerals were also observed using XRD and SEP. According to the leaching results, weathering had little or no beneficial effect on Cu leaching in SPLP and TCLP. However, the weathering process had significantly reduced Cu leaching concentrations in codisposal leaching procedure.
The Cu fractionation analysis showed that large amount of Cu in BA existed in carbonates, organic matters and silicates fractions, and the mobilization of Cu was most likely driven by exchangeable Cu, Cu-carbonates and Cu-organic matters dissolution from BA. The final concentration of leached Cu was probably also determined by secondary reactions such as adsorption and Cu–DOM complexation.
When BA was quenched in leaching liquid, part of organic carbon in BA would also be leached. The leaching of organic carbon might include humic acid (HA), fumic acid, and hydrophilic organic carbon (Hy) such as aliphatic acids or carbohydrates. Thus, the leachate DOC of BA in SPLP, which was in the concentration of ca. 60 mg/L, would mainly be attributed by fumic acid and Hy.
The leached Cu from BA probably had a very high affinity for organic ligands in leaching environment. When inorganic acid solution (HNO3 and H2SO4) was selected as leaching liquid in SPLP, fumic acid was believed to be the main complexing agent. The decrease of Cu availability, the absorption of fumic acid and Cu complexes onto neoformed Al hydroxides could explain the reduction of Cu leaching to a large extent. When hydrophilic organic acid–CH3COOH was introduced in TCLP, the acetic acid might be of a more important complexing agent for Cu than FA, and thus the adsorption of Cu and fumic acid complexes to hydro (oxide) minerals might have played a comparably minor role in this process. Moreover, the distinct decrease of ANC of weathered BA would facilitate Cu leaching significantly. In brief, the enhanced Cu leaching by Hy complexation and the decline of ANC would had resulted in little overall benefit of Cu leaching in TCLP with weathering.
When situated in fresh simulated landfill leachate in codisposal leaching procedure, the DOM species and concentration in simulated landfill leachate would affect Cu leaching process of BA, resulting in difference in Cu leaching with SPLP and TCLP. As DOM in fresh leachate from different landfills in China contains approximately 10% HA, 50% fumic acid and 40% Hy, it can be concluded that both fumic acid and Hy in landfill leachate play an important role in Cu leaching process in the codisposal leaching procedure. Two possible explanations can be provided for the reduction of Cu leaching of weathered BA when codisposed with refuse: (1) the transformation of mineralogical characteristics of weathered BA, such as redistribution of Cu from labile fractions to less mobile fractions, reduces the amount of Cu available for complexation with fumic acid or Hy; (2) as new hydro(oxide) minerals are formed during the weathering process, those minerals not only compete for Cu in adsorption due to more available binding sites, they are also extremely active in adsorption because of reactive surfaces. Studies are needed to further distinguish these potential mechanisms.
The results showed that simulated landfill leachate would facilitate Cu leaching from BA significantly. Compared with the artificial leaching solution (inorganic acid and acetic acid), the leaching procedures such as SPLP and TCLP may have underestimated Cu leaching when BA was codisposed with refuse. Because of the significant difference of Cu leaching, we believe that SPLP and TCLP may be useful or effective enough to assess heavy metal leaching risk for reuse BA As SCM, but they are not suitable for assessing Cu leaching when BA is codisposed with refuse.
Short-term accelerated weathering was applied on BA to investigate its effect on Cu leaching when BA was codisposed with refuse. Both standard leaching protocols, i.e. SPLP and TCLP, and codisposal leaching procedure of Cu were conducted. Fractionation study showed that short-term weathering transformed some Cu-organic matter and Cu-carbonates to Cu-silicates. The standard leaching experiment showed that weathering had little or no beneficial effect on Cu leaching in SPLP and TCLP, which could be explained by the adsorption and complexation of Cu with DOM. However, the Cu leaching of weathered BA was reduced significantly when situated in fresh simulated landfill leachate, probably due to the weakening effect of Cu complexation with FA or hydrophilic fractions and/or intensifying Cu absorption to neoformed of hydro (oxide) minerals in weathered BA. The 24-h leaching test showed that the amount of leached Cu was decreased effectively from 5.43, 2.03 and 1.86 mg L−1 at the beginning of weathering to, 2.49, 0.83 and 1.10 mg L−1 after 408 h weathered treatment at pH 4.50, 6.00 and 7.50, respectively, while the 15-day codisposal leaching test indicated that 408-h weathering reduced total Cu leaching by 86.3% and Cu in free or labile complex fraction by 97.6%. Accelerated weathering can be an effective pretreatment method to decrease Cu leaching when BA is codisposed with refuse.
4.2 Characterization of Chlorine and Heavy Metals for the Potential Recycling of Bottom Ash as Cement Additives
The manufacture of Portland cement clinker consumes a large amount of raw materials (limestone, clay, etc.) as well as plenty of energy (850 kcal/kg clinker) along with massive emissions of CO2 (around 0.85 kg of CO2/kg clinker) attributing to approximately 5% of global carbon emissions. MSWI BA composing of amorphous silica (usually more than 50 wt.%), alumina, iron oxide, and calcium oxide is quite similar in composition with commonly used cement additives such as ground-granulated blast-furnace slag and pulverized coal fly ash (FA), suggesting that MSWI BA has a significant potential to be reused as cost–effective raw materials in cement production. More than 60% of the world’s total cement are produced and consumed by emerging markets, most notably, China. Thus, for these rapidly developing countries, reusing incineration residues as replacements for raw materials in cement manufacturing has become an important option, which enables MSW to be completely recycled while reducing the consumption of natural resources.
Many studies have been conducted on the feasibility of recycling MSWI residues as secondary raw resources for cement products. Some researchers have described the potential use of BA for concrete production. However, the high content of heavy metals increases the pollution risk of recycled materials made from incineration residues. Moreover, the high levels of alkali chloride in MSWI BA can lead to blockages in cement production devices such as cyclone pipes, resulting in kiln shutdown. Besides, the migration of chloride into concrete can accelerate the corrosion of embedded steel.
With consideration of serious problems caused by chlorine and heavy metals in the process of reusing MSWI BA, the systemic correlative analysis between the level of chlorine and heavy metals and MSW composition is valuable and significant, especially in mixed collection system of undeveloped countries. But the study focusing on the incinerators with mixed collection mode of waste in developing countries is still lacked. In addition, for the chemical characteristic of MSWI BA, previous study only focuses on the solubility of chlorine in BA. There is few report investigating the distribution and chemical form of insoluble chloride.
4.2.1 Chlorine and Heavy Metals as Factor for Potential Recycling of Bottom Ash as Cement Additives
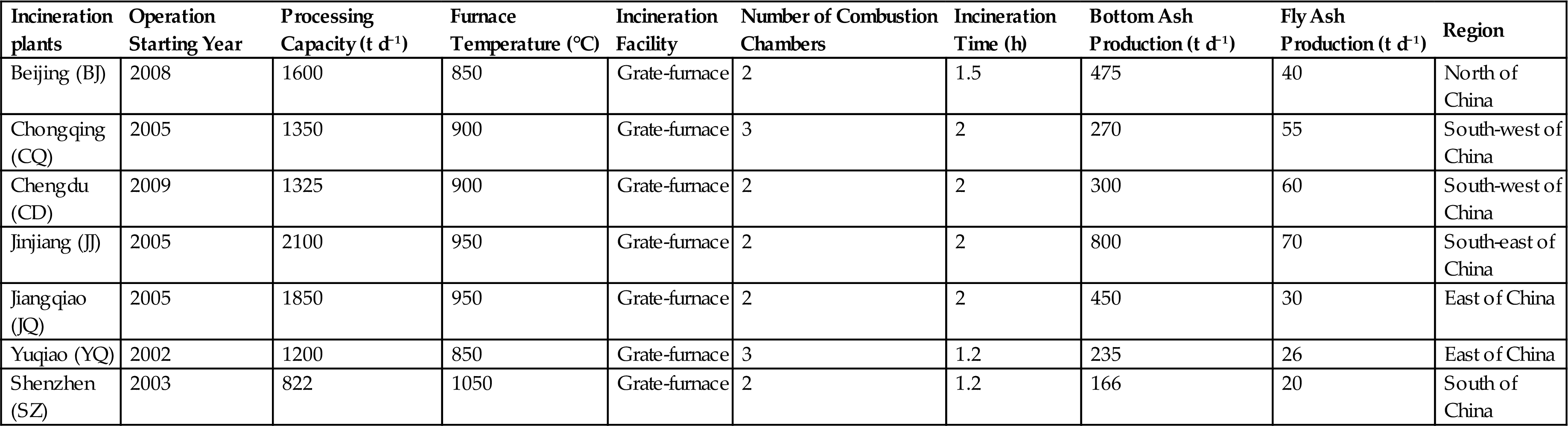
The BA samples used in this study were obtained from seven MSW incineration plants in six representative cities in China. The sampling seasons does influence the sample composition. But this article mainly focuses on the regional differences of FA. The effects of different waste input in different regions on the characteristics of BA were mainly concerned. Therefore, in order to avoid the seasonal effects, all the sampling was conducted in July, 2013. The systematic sampling method was applied to collect the FA samples from the BA conveyors. BA is the solid waste discharged at predetermined time intervals, therefore the samples were taken at the same time interval of BA discharge. Generally, the sampling time interval was 0.24–0.3 h. Forty to fifty samples were collected during the sampling period of 12 h in each MSW incineration plant. Each sample weighed 1–1.25 kg and the total sample amount was 50 kg. Samples of different sampling time points were mixed by quartering method to make it representative and then to be analyzed. Details of the seven sampling incineration plants are shown in Table 4.1.
Table 4.1
Operating Conditions in Incineration Plants
| Incineration plants | Operation Starting Year | Processing Capacity (t d−1) | Furnace Temperature (°C) | Incineration Facility | Number of Combustion Chambers | Incineration Time (h) | Bottom Ash Production (t d−1) | Fly Ash Production (t d−1) | Region |
| Beijing (BJ) | 2008 | 1600 | 850 | Grate-furnace | 2 | 1.5 | 475 | 40 | North of China |
| Chongqing (CQ) | 2005 | 1350 | 900 | Grate-furnace | 3 | 2 | 270 | 55 | South-west of China |
| Chengdu (CD) | 2009 | 1325 | 900 | Grate-furnace | 2 | 2 | 300 | 60 | South-west of China |
| Jinjiang (JJ) | 2005 | 2100 | 950 | Grate-furnace | 2 | 2 | 800 | 70 | South-east of China |
| Jiangqiao (JQ) | 2005 | 1850 | 950 | Grate-furnace | 2 | 2 | 450 | 30 | East of China |
| Yuqiao (YQ) | 2002 | 1200 | 850 | Grate-furnace | 3 | 1.2 | 235 | 26 | East of China |
| Shenzhen (SZ) | 2003 | 822 | 1050 | Grate-furnace | 2 | 1.2 | 166 | 20 | South of China |
The chemical composition of the BA was determined using X-ray fluorescence (XRF) (EDX-720, Shimadzu, Japan). The heavy metal (except mercury) content was determined by acid digestion of the samples in a microwave oven, followed by inductively coupled plasma atomic emission spectroscopy (ICP-OES, 720ES, Agilent, USA). The acid digestion procedure was briefly as follows: 6 mL of HNO3 (65%), 2 mL of HF (40%) and 2 mL of H2O2 (30%) were added to 0.1 g dried and exactly weighed sample, and then the samples were digested in a microwave oven. All of the sample digestions were carried out in triplicate. The mercury content was measured by a direct mercury analyzer (DMA-80, Milestone Srl, Italy).
The MSWI BA was abraded to the particle size below 150 μm and freeze-dried. One gram BA samples were added to 50 mL of 14.2 v/v% nitric acid solution. After shaken for 30 min, the samples were heated to 100°C for 5 min and then cooled to room temperature. After the supernatant was filtered, the total chlorine content of the samples was measured by ion chromatography (ICS-5000, ThermoFisher, USA). The soluble chlorine content was measured using 50 mL of hot water at 50°C as an extraction medium instead of 14.2 v/v% nitric acid solution. After shaken for 30 min, the sample solution was filtered and analyzed by ion chromatography. The insoluble chlorine content was calculated as the difference between the total chlorine content and the soluble chlorine content.
The chemical speciation of chlorine in samples was characterized by synchrotron radiation X-ray diffraction (XRD), using the BL14B1 beamline at the Shanghai Synchrotron Radiation Facility, China and X-rays with a wavelength of λ = 1.24 Å. The BL14B1 is a bending magnet beamline, and the storage ring energy of SSRF is 3.5 GeV.
These seven facilities are described in Table 4.1. The seven MSW incineration facilities are in six cities, which are located in the east, south, south-west, south-east, and north of China, respectively. The FA and the BA that were generated from the incinerators comprised 1.7–4.5 and 20–29 wt.% of the total solid waste, respectively. However, the BA in the Jinjiang incineration plant accounted for almost 40% of the input waste, which may have resulted from the large proportion of dust that primarily consisted of inorganic matter in the raw refuse.
Table 4.2 shows our survey results of the refuse compositions for the six cities. The food refuse from Jinjiang city accounted for less than 20 wt.% of the total refuse, whereas the kitchen waste proportion of other cities were nearly 50 wt.% or even more. In addition, the rubber–plastic content of Jinjiang city refuse was also higher than that of the other cities. Jinjiang is a county-level city that is located in the south-eastern part of Fujian province, China. It is the largest production base of travel/sport shoes in China and has more than 3000 footwear production factories. Byproducts from footwear production inevitably mix with MSW, resulting in a high proportion of rubber and plastic in the refuse. Plastic are commonly thought to be the main source of chlorine in MSW. This opinion was also further verified by the chlorine content measurement in Section 3.4. United Nations Educational, Scientific, and Cultural Organization named Chengdu a “City of Gastronomy” in 2010, and food culture is an important part of the leisure culture in Chengdu. Statistical data for Chengdu shows that approximately 9000 restaurants and canteens are located in the central urban area of the city and the per capita consumption in catering industry is 3428 yuan per year, which is much higher than the average status all around China, 1990 yuan · (person · a)−1. Chongqing cuisine is a branch of Sichuan cuisine that is noted for its distinctive spicy and pungent flavors. Chongqing is also the birthplace of the hot pot in China, and hot pot restaurants can be found everywhere. Thus, kitchen waste, which is usually regarded as the source of soluble chlorine, accounts for a higher proportion of the total refuse in Chengdu and Chongqing than the other investigated cities.
Table 4.2
Refuse Compositions for Six Cities in 2012 (wt.%)
| Cities | Food | Paper | Textile | Wood | Glass | Rubber–plastic | Dust | Metal |
| Shanghai | 51.9 | 9.4 | 2.8 | 1.5 | 4.3 | 13.5 | 1.1 | 0.9 |
| Beijing | 55.2 | 14.3 | 4.3 | 0.5 | 4.3 | 14.8 | 6.4 | 0.2 |
| Shenzhen | 47.8 | 13.7 | 10.3 | 2.9 | 5.1 | 13.9 | 5.8 | 0.7 |
| Chongqing | 55.8 | 15.1 | 3.0 | 2.6 | 1.5 | 15.1 | 13.2 | 0.2 |
| Chengdu | 69.0 | 9.8 | 1.8 | 2.7 | 2.6 | 9.1 | 1.8 | 0.7 |
| Jinjiang | 18.7 | 6.3 | 4.6 | 3.4 | 2.1 | 26.2 | 37.8 | 0.9 |

4.2.2 Chemical Composition of MSWI Bottom Ash from Different Citys
The chemical composition is the foundation of feasibility of reusing of MSWI BA as cement additives. Table 4.3 lists the XRF analysis results for the chemical composition of the BA samples, which are expressed in terms of oxides. The following elements were present in proportions above 1 wt.%: silica (SiO2), aluminum oxide (Al2O3), calcium oxide (CaO), iron oxide (Fe2O3), potassium oxide (K2O), phosphorus pentoxide (P2O5), magnesium oxide (MgO), sulfur trioxide (SO3), and sodium oxide (Na2O), which accounted for approximately 56.2–79.6 wt.% of the BA samples in total. The construction and demolition wastes could be a main source of aluminum, calcium, and silicon in BA. For the seven incineration plants, the SiO2, Al2O3, and CaO contents, which are usually the three primary components of the BA, ranged from 19.9 to 39.3, 5.91–11.4 and 9.3–25.4 wt.%, respectively, and in accordance with the previous reports about elemental composition of MSWI BA. The results also indicate that Chinese MSWI BA has the similar compositions of the raw materials used in cement production. The Jinjiang BA had the highest SiO2 and Al2O3 contents in the BA samples among the different cities. Silicon is a primary component of street or road dust. Many research studies have shown that Si accounts for over 20% of the total mass of dust. Thus, the higher Si content in the Jinjiang BA compared with that of the other cities may have resulted from a large proportion of dust in the Jinjiang MSW.
Table 4.3
Chemical Composition (wt.%) of Bottom Ash Determined by XRF
| Elemental Composition | JQ | YQ | BJ | SZ | CQ | CD | JJ |
| Na2O | 2.02 | 2.48 | 1.61 | 1.43 | 2.35 | 2.62 | 1.67 |
| MgO | 2.28 | 1.92 | 2.16 | 1.01 | 2.05 | 2.10 | 1.34 |
| Al2O3 | 5.96 | 5.91 | 7.97 | 6.97 | 7.59 | 11.4 | 8.34 |
| SiO2 | 21.3 | 27.4 | 19.9 | 23.2 | 24.4 | 33.1 | 39.3 |
| P2O5 | 3.26 | 3.22 | 1.94 | 1.60 | 4.75 | 2.79 | 1.05 |
| SO3 | 3.29 | 2.13 | 1.84 | 1.37 | 2.50 | 3.36 | 0.95 |
| K2O | 1.34 | 1.40 | 1.67 | 1.45 | 1.34 | 1.64 | 1.61 |
| CaO | 23.4 | 20.0 | 16.2 | 13.3 | 20.8 | 15.5 | 9.38 |
| TiO2 | 0.61 | 0.56 | 0.65 | 0.90 | 0.71 | 0.90 | 0.57 |
| MnO | 0.13 | 0.10 | 0.07 | 0.07 | 0.13 | 0.10 | 0.08 |
| Fe2O3 | 5.77 | 5.77 | 4.52 | 5.92 | 7.94 | 7.05 | 5.78 |
| CuO | 0.10 | 0.11 | 0.04 | 0.24 | 0.12 | 0.05 | 0.05 |
| ZnO | 0.29 | 0.35 | 0.23 | 0.68 | 0.45 | 0.32 | 0.20 |
| BaO | 0.15 | 0.14 | 0.20 | 0.27 | 0.20 | – | 0.15 |

The SO3 contents of the BA from the different cities ranged from 0.95 to 3.36 wt.% and the average was 2.26 wt.%. The addition of industrial waste and refuse from drop-off centers, which consisted primarily of building materials, increased the sulfur content of the MSWI BA. The presence of sulfate in incineration residues makes recycling these residues as cement raw materials undesirable, because of the potential corrosion caused by the resultant of reaction between sulfur oxides and water. Thus, it is very necessary to develop an effective desulphurization technology for the reuse of MSWI BA in cement production.
4.2.3 Heavy Metal Content of MSWI Bottom Ash
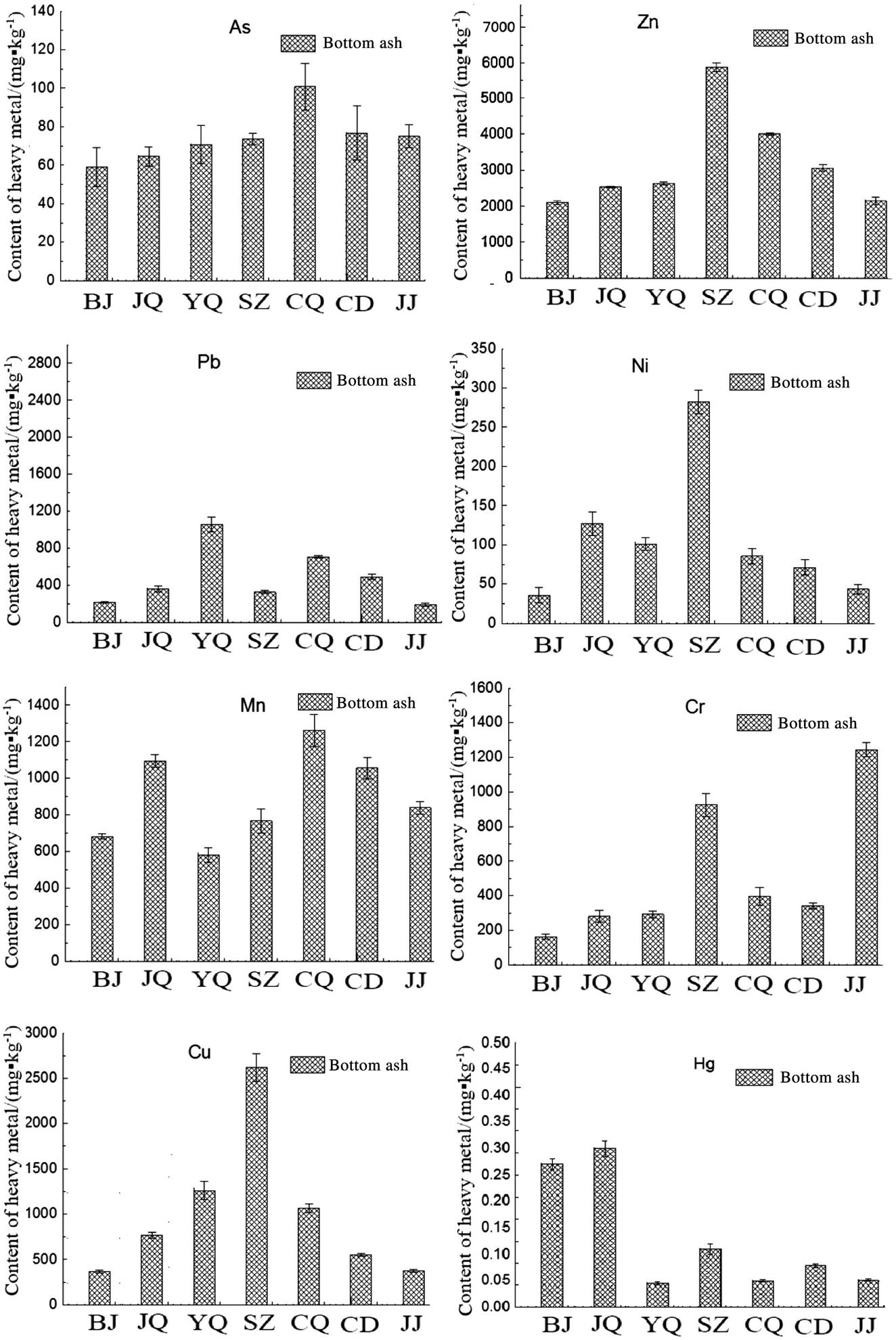
Heavy metal content is another influencing factor on reusing BA in cement production concerning the potential environmental risk. Fig. 4.7 clearly shows that kinds of heavy metals classified as hazardous were present in the BA. Zinc, cadmium, lead, and arsenic are all volatile metal elements. Cadmium and cadmium oxide are preferentially converted into CdCl2 during incineration. Furthermore, cadmium, cadmium chloride and, to a limited extent, cadmium oxide can all be vaporized. Thus, cadmium was either not present in the BA samples or its concentration was below the detection limit. Zinc is not as volatile as cadmium, but ZnCl2 and traces of metallic zinc were volatilized. However, there was limited conversion into ZnCl2 because a substantial amount of zinc was already present in the waste in oxide form. Therefore, the bulk of the zinc eventually remained in the BA. Cr, Mn, and Cu are less volatile than zinc and cadmium and thus tend to be transferred to the BA during incineration. In summary, the BA showed high concentrations of the following heavy metals (in decreasing order of abundance): Zn, Cu, Pb, and Cr, which was in accordance with the previous reports about MSW incineration residuals. Besides, the Zn and Pb content in each sample was both nearly 1 g kg−1.
There were significant differences among the heavy metal contents of the different MSWI BA samples. The Shenzhen BA had the highest concentrations of Zn, Cu, and Ni in the BA samples from all of the cities. This high concentration could be attributed to the widespread presence of these metals in raw refuse. Shenzhen is a fairly developed city in China, and the electronics industry is an important segment of the city’s economic structure. Moreover, waste classification and recovery has not been widely adopted in Shenzhen; thus, industrial waste inevitably mixes with domestic refuse and is transferred to the incineration plant.
The heavy metal content in BA was determined by the input waste and incinerator operating conditions, such as the combustion temperature, furnace type, feed rate, residence time and so on. Table 4.4 summarizes the results of a series of experiments carried out on the composition and metal content of the bulk waste. In the aforementioned study, rubber, textiles, and plastics were the primary sources of chromium. As discussed above, Jinjiang city is the largest travel/sport shoes production base of China, and scraps from shoe production inevitably mix with MSW, resulting in a high Cr level in the MSW. So the Jinjiang BA exhibited the highest chromium level (i.e., 1244 mg kg−1) compared with the BA samples from the other cities, which contained less than 400 mg kg−1 of chromium.
Table 4.4
Metal Content of Shredded Bulky Waste
| Waste | As | Cd | Cr | Cu | Pb | Zn |
| Plastics | 0.18 | 3.6 | 2.3 | 1 | 7 | 16 |
| Textiles | – | 0.02 | 4.3 | 2 | 5 | 27 |
| Plastic film | – | – | 0.2 | 3 | 2 | 1.2 |
| Paper | – | – | – | 0.1 | 0.2 | 1.0 |
| Power cords | 0.03 | – | 1.3 | 9399 | 37 | 3.0 |
| Sponge | 0.02 | – | 0.7 | 22 | 12 | 21 |
| Electric circuit boards | 0.06 | 2.5 | 1.5 | 1193 | 37 | 35 |
| Cans | – | – | 0.1 | 6 | 0.1 | 0.5 |
| Rubber | 0.14 | 0.3 | 4.7 | 12 | 10 | 98 |
| Wood | – | 0.6 | – | 11 | 31 | 155 |
| Residuals | – | – | 19.4 | 10,787 | 75 | 1809.5 |
| Total | 0.43 | 7.02 | 34.5 | 21,436.1 | 216.3 | 2167.2 |

Notes: unit: (mg kg−1-shredded bulky waste), ‘–’: not detected.
Lead is extremely toxic and can damage the nervous system, kidneys, and reproductive system, particularly in children. Many industries, such as the coatings, automotive, storage battery, aeronautical, and steel industries generate large quantities of wastewater that contain varying lead concentrations. The Shanghai Yuqiao MSW incineration plant is located in the Kangqiao industrial region of the New Pudong District in Shanghai. The storage battery and automotive factories in Shanghai Yuqiao once caused lead poisoning in children, which may indirectly explain the high lead content of the Shanghai Yuqiao BA.
Almost all of the heavy metal (except for Hg) levels in the Beijing BA were lower than those in the other samples, which can be attributed to the relatively sound waste classification and recovery in Beijing; thus, waste separation and recovery may be the most effective and direct way of reducing heavy metal pollution.
In summary, the heavy metal contents of BA samples from few cities exhibited exceeded the standard (GB 5085-2007). MSWI BA was even considered as general solid waste. BA is more widely used as a raw material for aggregate and cement products than FA; thus, the environmental impact of using BA should be seriously considered. This study demonstrates that mixing industrial waste into MSW increases the levels of some heavy metals, such as Cr and Pb. Therefore, inappropriate waste handling, storage, collection, and disposal practices pose environmental and public health risks. Appropriate and safe MSW management, for example, the separate collection of recyclable solid waste, is of utmost importance in creating a healthy environment.
4.2.4 Chlorine Content in Bottom Ash from Different Citys
Polyvinylchloride plastic is the dominant contributor of chlorine to MSW at 63–72 wt.%, and the other major sources of chlorine are paper, kitchen waste, and leather. Kitchen waste has a low chlorine content of 1 wt.%, but its high proportion in Chinese MSW results in a 45 wt.% contribution to the chlorine load. In addition, kitchen waste primarily consists of water-soluble alkali chloride salts and thus leads significantly to high temperature corrosion. According to the mass balance of chlorine during the incineration process, almost all of the organic chlorides are converted to HCl, Cl2, and polycyclic aromatic hydrocarbon (PAHs) in the flue gas at a temperature of 700°C, whereas the inorganic chlorides tend to primarily occur in the bottom of the incinerators at the same temperature. The BA is the target material in the present study; thus, it is important for us to consider the inorganic chlorides in kitchen waste.
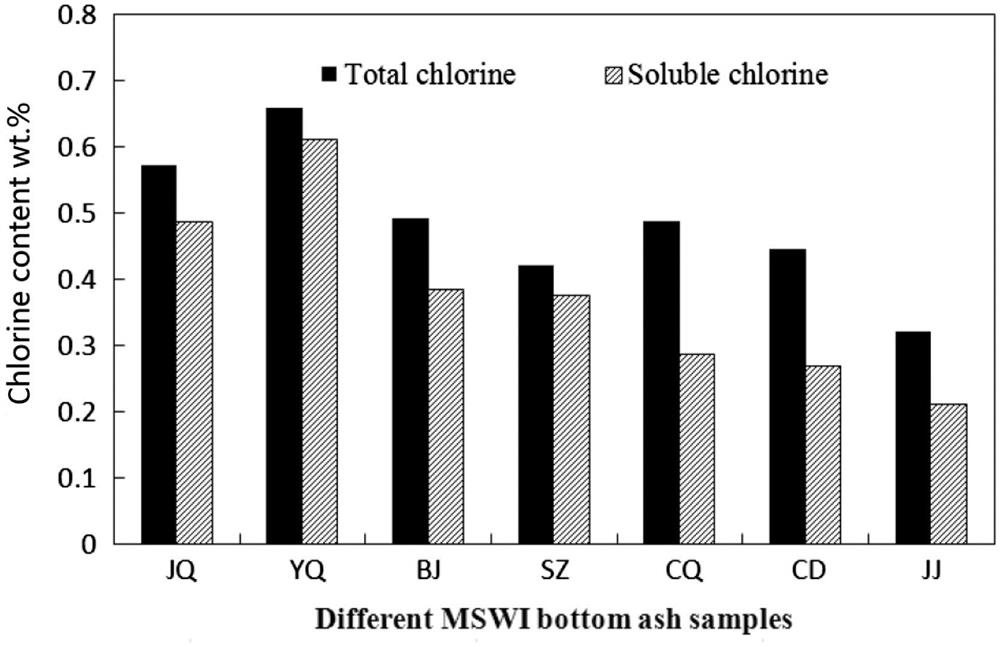
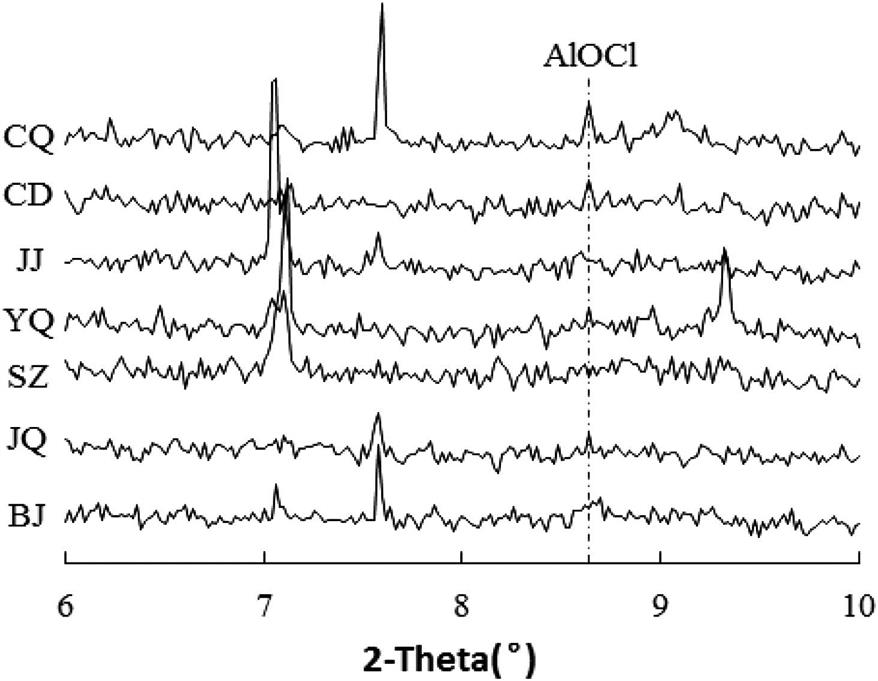
The total chlorine content of the seven BA samples varied from 0.32 to 0.66 wt.%, and the soluble chlorine varied from 59 to 93 wt.%, as shown in Fig. 4.8. The Jinjiang BA had the lowest chlorine level of all of the cities, most likely because of the fairly low proportion of the kitchen waste, which accounted for only 18.7 wt.% of the total refuse. Chengdu had the largest proportion of kitchen waste; however, Chengdu unexpectedly exhibited the lowest proportion of plastic. The chlorine content in the BA from Chengdu is relative to the other cities. Thus, plastic and other refuse containing chlorine are also important contributors to the chlorine content of the BA, which supports the argument that chlorine levels in MSW incineration residues could be reduced significantly through plastics removal. The Shanghai Jiangqiao, Shanghai Yuqiao, and Beijing BA was produced by cities with comparatively developed economic levels and tended to have high chlorine contents with low proportions of insoluble chlorine. This result may be attributed to the inappropriate waste handling, storage, and collection practices in less developed regions, which may lead to the mixing of industrial waste into MSW. A washing process could remove most of the soluble chlorine from the BA. Washing with water rendered the Shanghai Yuqiao BA more suitable for reuse than that of the other cities, even though this ash contained the highest level of chlorine of all of the cities. The Chongqing and Chengdu BA may be unsuitable for cement production because these ash samples had high insoluble chlorine levels that could not be eliminated by water washing. In conclusion, separating plastic waste and kitchen waste from other refuse is an effective way of reducing the chlorine content of the BA.
4.2.5 Chemical Speciation of Chlorine in Bottom Ash from Different Citys
The synchrotron XRD analysis showed that aluminum oxide chloride (AlOCl) was one of the major mineral phases in the insoluble chlorine in Chinese MSWI BA (see Fig. 4.9), which is quite different from the previous report that the primary insoluble chlorine of BA is Friedel’s salt (3CaO·Al2O3·CaCl2·10H2O). Besides, from the small-angle diffraction pattern, it can be also noticed that there are peaks located in the 2θ range of 7–7.5°, which may be due to the presence of soluble chlorine salt, Ca6(CO3)2(OH)7Cl. Comparing Figs. 4.8 and 4.9 clearly shows that the peak intensity of AlOCl and the insoluble chlorine concentration exhibited the same trends. The Chongqing and Chengdu BA, which contained higher levels of insoluble chlorine comparing with the BA from the other cities, also had higher peak intensities for AlOCl, suggesting that the insoluble chlorine in Chinese MSWI BA existed primarily as AlOCl. The AlOCl is mainly produced at local high temperature (about 1250°C) in the incinerator, the reaction equation as follows:
So prohibiting the material with high content of chloride mixing with MSW by separated collection system and accelerating waste mixing in incinerators to avoid local overheating are the efficient methods to inhibit the formation of insoluble chlorine.
4.3 Biological Dechlorination of Incineration Bottom Ash
The unseparated solid waste collection system, especially the high content of food residue, make the chlorine content of BA reach up to 1–30%, which is quite higher than chlorine content standard of cement (600 mg/kg). The high content of chlorine causes the corrosion of reinforce steel in concrete, so it is necessary to remove the chlorine from BA before it can be reused as the raw material for cement production. Although soluble chlorine of BA can be easily removed by washing method, insoluble chlorine, such as friedel salt (3CaO·Al2O3·CaCl2·10H2O) and hydrocalumite, are difficult to be removed. Hence, it is urgent to develop a dechlorination technology to remove insoluble chlorine with low environmental impacts and low operational cost.
Biological dechlorination technology enables the decomposition of refractory chlorine minerals to soluble chlorine compounds. Organic acid, carbonate, and sulfate ions produced biologically from carbon sources play an important role for the decomposition of refractory chlorine minerals. In addition, organic acids are also effective for the removal of toxic heavy metals like copper and lead owing to the complexation with heavy metals.
Three lysimeters with the size of 5 ×5 ×2.5 m were built in Shanghai Refuse Landfill. About 235 t of BA were set in a lysimeter. The mixture of BA and aged refuse was added in the other lysimeters. Weight-base ratio of aged refuse to the mixture was 20%. BA was sampled at five different points on the surface of lysimeters periodically. Contents of soluble and insoluble chlorine in BA were measured based on Japanese Industry Standard A1154 method (JIS A1154). At first, BA was abraded less than 150 μm and freeze-dried. 10 g of BA samples were added into 50 mL of 14.2 v/v% nitric acid solution. After 30 min shaking, heated to 100°C for 5 min and then cooled to room temperature. After supernatant was filtrated, chlorine concentration in sample solution was measured by ion-chromatography (DIONEX Co., DX-120). Content of total chlorine in BA was calculated using this analysis results. To measure content of soluble chlorine, 50 mL of hot water at 50°C was used as extraction medium instead of 14.2 v/v% nitric acid solution. After 30 min shaking, sample solution was filtrated and analyzed by ion-chromatography. Content of insoluble chlorine was calculated the difference between total chlorine content and soluble chlorine content.
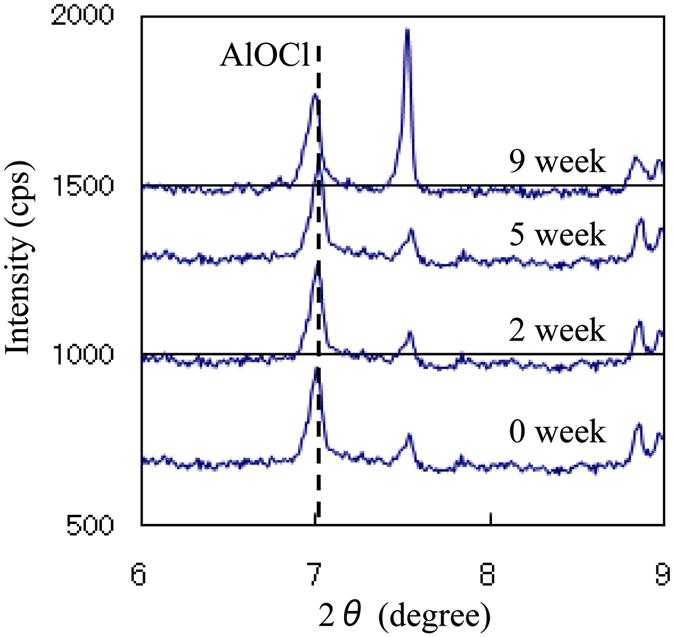
Chlorine contents of BA from 0 to 30 weeks of dechlorination experiments were shown in Fig. 4.10. Because concentration of chlorine in BA should be less than 0.18 wt% to meet Chinese standard of chlorine content for cement clinker, 0.18 wt% is the target level to evaluate chlorine removal efficiency. In addition, dechlorination period is preferred to be 1 year (55 weeks) or less for lower operational cost of the dechlorination. The results of all lysimeters indicated that chlorine was removed to less than the target level within 30 weeks, which means a successful efficiency of chlorine removal. No significant difference of dechlorination efficiency among all lysimeters indicated that aged refuse had negligible effect on chlorine removal.

Because organic acid, carbonate, and sulfate anions can promote the decomposition of insoluble chlorine, in particular Friedel’s salt (3CaO·Al2O3·CaCl2·10H2O), aged refuse was used as organic compounds source to produce them biologically. However, experimental results were contrast to the expectation. Negligible effect of aged refuse for the dechlorination implies poor production of organic acid, carbonate, and sulfate anions. However, the ratio of carbon to nitrogen (C/N ratio) of aged refuse was 15.6, which was almost the same with garbage compost and within optimum range for soil microorganism activity (C/N ratio: 10–20). Therefore, it might be difficult to explain less chlorine in Chinese BA was not Friedel’s salt, organic acid, and anions might be noneffective on the decomposition of insoluble chlorine minerals.
The experiment result implied the different chemical/mineral form of insoluble chlorine in Chinese BA. Fig. 4.10 shows that the concentration of total chlorine decreases gradually with time. However, concentration of insoluble chlorine showed large fluctuation. This indicated that the generation and decomposition of insoluble chlorine occurred in parallel at the same time. This was also monitored in dechlorination experiments using Japanese BA.
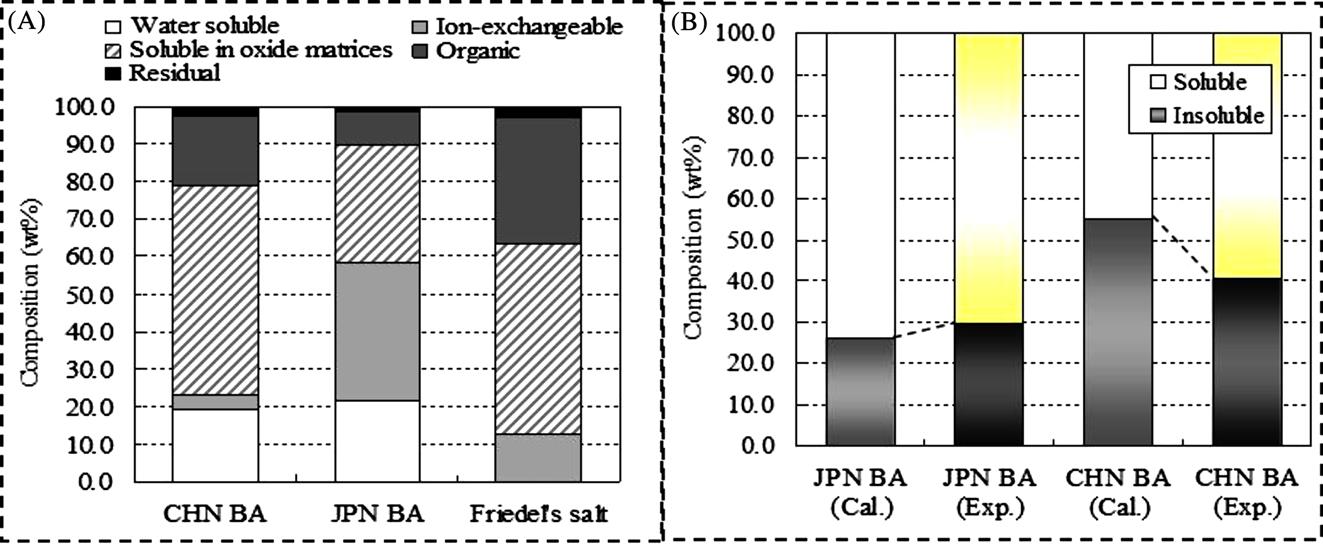
The results of sequential extraction for Chinese and Japanese MSWI BA are illustrated in Fig. 4.11. The composition of categorized chlorine for Chinese MSWI BA was 19% for “water-soluble”, 4% for “ion-exchangeable”, 56% for “soluble in oxide matrices”, 19% for “organic”, and 2% for “residual”. Because water-soluble, ion-exchangeable, and “soluble in oxide matrices” form of chlorine were more soluble than organic and residual form, sequential extraction results indicated that chlorine in Chinese MSWI BA was relatively more soluble than that in Japanese MSWI BA. This result had good agreement with the results of JIS A1154. Based on the composition of categorized chlorine for purely synthesized Friedel’s salt, the compositions of “soluble” and “insoluble” chlorine for Chinese and Japanese MSWI BA were calculated assuming that all insoluble chlorine was Friedel’s salt, as shown in Fig. 4.11. Calculation results were compared with experimental results by JIS A1154. This comparison indicated that 88.4% of insoluble chlorine in Japanese MSWI BA was Friedel’s salt. On the other hand, calculated insoluble chlorine in Chinese MSWI BA was quite larger than experimental result, which meant that the assumption used in this calculation was wrong and major mineral phase of insoluble mineral phase in Chinese MSWI BA was not Friedel’s salt.
Synchrotron XRD analysis clearly suggested that AlOCl was one of major mineral phase of insoluble chlorine in Chinese MSWI BA. XRD analysis result of fresh MSWI BA was shown in Fig. 4.12. If AlOCl was major mineral of insoluble chlorine, peak intensity of AlOCl and experimental measurement result of insoluble chlorine (JIS A1152) should have the same variation trend. They were compared and illustrated in Fig. 4.13. In this comparison, relative peak intensity of AlOCl based on quartz peak intensity was used to enable direct comparison among different MSWI BA samples. Peak intensity of AlOCl and insoluble chlorine concentration had good agreement, which suggested that insoluble chlorine in Chinese MSWI BA existed mainly as AlOCl.

Biological dechlorination of Chinese MSWI BA using three semi-plant scale lysimeters was investigated. This study could yield two successful results; successful chlorine removal efficiency and original identification of insoluble chlorine mineral in Chinese MSWI BA. Dechlorination experiment series showed that chlorine concentration in MSWI BA decreased less than target level (0.18 wt%) within 30 weeks. The combination of sequential extraction method with synchrotron XRD analysis suggested that AlOCl was major mineral phase of insoluble chlorine in Chinese MSWI BA. Although biological dechlorination experiments showed no significant effect of aged refuse on chlorine removal, it could be explained by different decomposition mechanism of AlOCl. The identification of insoluble chlorine will help understand the mechanism of biological dechlorination.
4.4 Geoenvironmental Weathering/Deterioration of Landfilled Bottom Ash Glass
Glass is generally considered as a strong and stable material, however deterioration of glass (either natural or man-made, e.g. volcanic glass, window glass, vitrified waste matrices, coal ash glass) has long been discovered and reported by geochemists, mineralogist, geologist as well as material scientists. The fundamental decay mechanisms are the same for all types of alkali-silicate glass. However, the specific deterioration/weathering behavior of the BA glass phase may be influenced by factors of, e.g. interior characteristic of the glass phase and exterior environmental conditions.
The BA glass phase normally acts as a host material for certain toxic elements. For example, many heavy metals are incorporated into the glass matrix in the form of metallic compounds (Cu–Fe, Cu–Pb–S etc.). However, up to now limited understanding of the alteration behavior of the glass matrix and its releasing or up-taking of toxic elements has been revealed. The glass phases are generally regarded as the principal incineration products of solid waste. XRD-based bulk analysis was conducted for calculation the glass content in MSW-BA. The results revealed that the glass content normally account for approximately 45–55 wt% of MSWI-BA with slight deviations among the ash samples of different sources.
Triangular diagrams, plotted on basis of glass network former (Si as the essential network former), intermediate former (Al normally acts as a representative intermediate former) and modifier (primarily Na, K, Ca, and Mg as network modifiers), are often used to relate glass composition to its durability. Glass with higher percentage of network former and lower percentage of modifier would point to a higher durability.
Glass deterioration normally initiates from the rim of the glass entity or along the wall of fissures or vesicles in the glass matrix. For the BA glass with little or no embedded compounds, glass deterioration proceeds by preferential dissolution of certain elements (predominantly alkalis, and occasionally alkaline-earth elements), leaving a depleted alteration layer which could subsequently be disrupted into minute fragments by OH− attacking.
Alteration of the glass phase to the gel phase takes place as the first stage of weathering. In geology, the process is termed as “palagonitization” which usually happens to igneous rocks. The gel, as the first stable product of glass alteration, will undergo an advanced aging process in which the gel reacts with the surrounding fluid and crystallizes to new phases (e.g. to smectite for igneous rock). To make the whole weathering process easily understandable, all the reactions involved in this weathering pattern could be abstracted as: (1) precipitation of gel-palagonite. The dissolved glass constituents precipitated in an open space, forming a gel phase principally composed of Ca and Al; (2) zoning crystallization. Interior reorganization took place in the gel phase, forming a new crystallized phase.
The compositional properties of the primary glass are essential parameters controlling the weathering process of glass. Durability of glass decreases when glass contains lower content of network formers and higher content of modifiers. In addition to the compositional feature of the secondary BA glass phase, its durability is also intimately related with glass texture or structure. Given that the external environment is the same, the BA glass phases with more homogeneous nature and less embedded compounds (spinels and metallic inclusions) appears more resistant to alteration, basically keeping its original structure and composition at least within the first few decades. Only the glass rim with a thickness of about 10 μm undergoes weathering by losing certain soluble ions (especially Na and Ca). Whereas, the BA glass phases that contain more embedded compounds tends to be more sensitive in the course of weathering, resulting in a deterioration thickness of a few hundred microns. Moreover, the cracks and vesicles that formed in the incineration or cooling process increase the specific area that contacts with the percolated fluid, thus leading to a more fragile structure to weathering.
Dissolution of glass normally abbeys the procedures of, firstly selective extraction of certain elements, followed by a progressive partial dissolution of the glass network, contemporaneously accompanied by formation of various gel phases. If these two procedures proceed successively, then evolution of glass happens incongruently.
Coexistence of both congruent and incongruent dissolution of the BA glass phases in landfills could be attributed to the specific pH conditions, ranging from highly alkaline to nearly neutral. High pH favors dissolution of silica. Effective dissolve of Si takes place at pH ≥9, resulting in a more vulnerable Si-network for destruction. In an oxidizing environment and at pH >3, Fe and Al will precipitate in the pores of the gel-palagonite as oxides/hydroxides. It is pertinent to mention that the fluid pH in the studied landfill site normally ranges from 7.5 to 12. This concurs with observations that the newly formed gel-palagonite in BA is highly variable but generally enriched nature of Al and Fe. The yellow to brown color of the gel-palagonite is indicative of the presence of iron in the +3 oxidation state.
Evolution of the BA glass phases produces various gel phases. In petrology, the term “gel” had been applied to describe the intermediate phases that derived from the volcanic glass. The formation of gel is generally viewed as an incipient stage in the evolution of glass to clay-like minerals. An advanced stage usually follows the gel formation, namely “gel evolution”, in which the gel will be crystallized to new phases via interior reorganization or reaction with surrounding fluid, such evidence has been illustrated in the present study.
Time is definitely an essential factor influencing petrogenesis of disposed incineration residues. However, time-dependent evolution features could not be easily deduced by using ash samples of 0–20 years old, which may partially be ascribed to the complex glass characteristics and environmental factors. Moreover, the highly variable thickness of the newly formed gel phases, even within samples of the same disposal year, disables a reliable estimation of the alteration rate of the BA glass. However, generally it can be concluded that deterioration of the BA glass phases proceeds in a relatively faster manner than that of natural glass owing to the complex textural and compositional features and alkaline weathering conditions. In spite of the relatively faster evolution speed of glass phases in BA deposit, there is still one more point worth mentioning, completion of the whole process still need a long time.
Natural weathering of the BA glass phases is quite significant from the standpoint of environmental safety, as might be expected from the fact that all the reactions involved in glass evolutions may locally change the properties of the leaching solution by releasing or entrapping certain toxic constituents. However, it is still hard to give a definite conclusion regarding whether glass evolution may decrease or increase the leaching amount of toxic elements from ash deposit. Nevertheless, knowledge of the characteristics of the deterioration/weathering products of the BA glass phases provides us valuable information in terms of assessment of the long time-scale behavior of pollutants in ashes and thus helps to choose environmentally sound management strategy for ash deposits.
4.5 Leaching Behavior and Alterations of Heavy Metals in Bottom Ash under Geochemical Weathering at Landfill
Leaching tests are widely used as tools to estimate the release potential of constituents from waste materials over a range of possible waste management activities, including recycling or reuse, to assess the efficiency of waste treatment processes. Therefore, in order to test the weathering influences to MSWI residues in a landfill, leaching tests were conducted in this research.
Weathering was used for indicating total reactions that possibly occurred in the landfill. It is a general term for the alteration by chemical, mechanical, and biological processes at or near the Earth’s surface, in response to environmental conditions. Geochemical weathering was used to indicate the weathering of MSW incineration residues in a landfill. Geochemical weathering of MSWI residues was different from the weathering of rocks, due to a high pH, high salinity, loose structures, high porocity, and high water content. These differences induced a high weathering speed.
Leaching tests were supposed to be able to describe the weathering phenomenon of heavy metals, and by combining leaching tests with some comprehensive instrument analysis, the major chemical forms which controlled the leaching behaviors of heavy metals can be discovered.
4.5.1 Field Survey and Sampling of a MSWI Residues Landfill
Bore sampling was implemented at a landfill in Dec 2004. The landfill site started since the year 1988, with a planned total landfill area of 29,058 m2, landfill capacity of 209,020 m3. Only MSWI residues from the incineration plant located beside the landfill and some incombustible waste have been landfilled. The percentage (based on wet weight) of fresh BA, after chemical treated FA and incombustible waste was about 65, 20, and 15%, respectively. According to the TEM survey and landfill records, one drilling point as shown in Fig. 4.14 was selected to conduct the sampling. Total depth of landfill waste was about 6 m; sampling depth was from surface (0 m) to near bottom (5 m). Samples from 0–1, 1–2, 2–3, 3–4 and 4–5 m were well mixed and represented the corresponded landfill depth MSWI residues. Every bore sample was divided into four sub samples in situ using quartation methods. First sub sample’s temperature and leaching test was conducted in situ right after sampling; physical properties were analyzed by using the second sub sample; chemical properties were analyzed using the third sub sample; and the remaining samples were stored below 4°C in refrigerators for a future use.

*Fresh residues mean a mixture of 25% fly ash and 75% bottom ash (weight percent)
**All data is means of three samples.
The analysis included chemical composition analysis, leaching test, and sequential extraction, as shown in Table 4.5. After removing the incombustible solid waste from the bore samples, the samples were mixed, quartered, 2 kg (<13 mm) were picked out and crushed to around 2 mm particle size which is used for the JLT46 test, and then 500 g of them were crushed to around 100 μm for total composition analysis, availability test, and sequential extraction test.
Table 4.5
Summary of the Leaching Tests Applied
| Leaching conditions | Japan | Holland | Proposed by SMT Program of European Commission | ||
| Test name | JLT46 | Availability test | BCR-step 1 | BCR-step 2 | BCR-step 3 |
| Partical size | <2 mm | <125 μm | <125 μm | <125 μm | <125 μm |
| Solution | Pure water | Pure water +HNO3 | Acetic acid (0.11 mol/L) | HydroxylammoniumChloride (0.1 mol/L) | Hydrogen peroxide (8.8 mol/L)Ammonium acetate (1 mol/L) |
| pH | – | 7.4 (fixed) | – | 2 (initial) | 2 (initial) |
| Weight (g) | >50 | 16 | 1 | Residue of step 1 | Residue of step 2 |
| L/S (mL/g) | 10 | 100 | 40 | 40 | 50 |
| Shaking | Horizontal shaking | Stirring | End over end shaking | ||
| Exraction time (h) | 6 | 3h×2 | 16 | 16 | 16 |
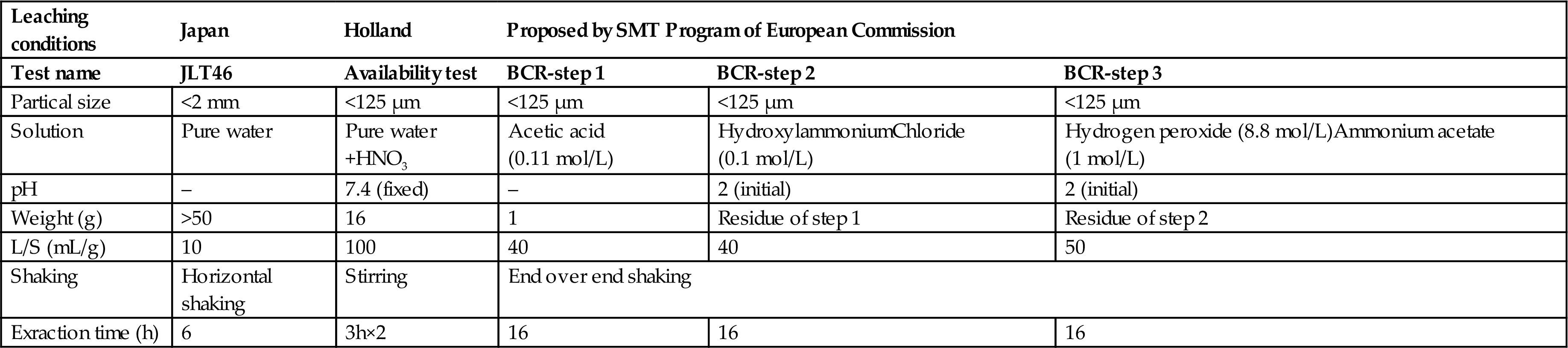
Total composition was analyzed by XRF. JLT46 is a leaching test in the Environmental Agency Notification No. 46 of Japan to test the reused materials and soil, it is a Japanese national leaching test standard, particle size <2 mm, deionized water and test samples, L/S =10, 6 h shaking, filtrate with a 0.45 µm filter are regulated in this standard. The long-term leaching test applied for assessing environmental risk (leaching potential) of the use of BA named availability test, and regulated as Dutch availability test NEN7341 was also applied to the samples.
The availability test was thought can represent the leaching behavior of oxyanionic species under the first leaching step, which is at pH 7 and at pH 4 is considered to be representative of more severe natural situations. The small particle size ensures a good water contact with the sample and minimizes the diffusion barrier of the particles. The high L/S ratio minimizes solubility limits of the constituents to be analyzed. SEP, the three-step SEP proposed by the Standards, Measurements, and Testing Program of the European Union was performed to the samples. Table 4.5 shows the experimental conditions for leaching tests and sequential extraction test. Chemical compositions were analyzed by Rigaku zsx-101e; concentrations of Pb, Cr, Zn, Cd, Cu were analyzed by Shimadzu ICPS-7000; TOC, inorganic carbon (IC), and TN were analyzed by Shimadzu TOC-Z. All the tests and experiments were conducted as three parallel tests.
4.5.2 Characterization of the Fresh and Weathered Residues in the Landfill
Fig. 4.14 shows the total contents of major compositions and heavy metals analyzed by XRF. Fresh and landfilled residues were analyzed, landfill ages for the drilling core residues were recognized as following: 0.5–2 years (0–1 m), 2–3 years (1–2 m), 3–4 years (2–3 m), 6–8 years (3–4 m), 8–10 years (4–5 m). When comparing fresh residues with landfilled residues, concentrations of the fresh residues were similar to the surface 0–2 m landfilled residues. This might due to a short landfill time and less change occurred.
All ten major compositions were divided into two groups according to the change of concentrations with depth: Group 1, which included CaO, Na2O, K2O, TiO2, and S; Group 2, which included SiO2, Al2O3, Fe2O3, MgO, and P2O5. Concentrations of compositions in Group 1 decreased with landfill depth and landfill time, concentrations of compositions in Group 2 were on the opposite side. Contents in Group 1 were thought to have a high solubility in fresh residues or change to soluble forms due to weathering and these contents were easy to be washed away by rainfall. Contents in Group 2 were thought to have a low solubility in fresh residues or change to insoluble forms due to weathering, therefore the concentrations increased with landfill depth and time.
For the fresh residues, concentrations of Pb, Zn, and Cu (3–5 m depth) were higher than in landfilled residues, concentrations of Cd and Cr were lower than in landfilled residues. Pb and Zn were thought to have a low leachability, therefore they were retained in the landfill. For Cd and Cr, it was suggested that soluble fractions were dissolved and went down the landfill with leachate. For Cu, it was suggested that soluble fractions were dissolved and went through landfill from upper layers to lower layers. However, due to the high trapping capacities of bottom layers after weathering, Cu was withheld in the lower layers. The other reason might be the influence of mass balance, since the leachability of heavy metals was lower than the major soluble major contents, therefore due to the mass decrease, the contents of heavy metals became relatively high. In order to know the leaching potential of the landfilled residues, the following leaching tests were conducted.
4.5.3 Influences of Weathering to the Leaching Behavior of MSW Incineration Residues
Concentrations of heavy metals, IC, TOC, and pH of the leachate obtained from the leaching test (JLT46) were analyzed as shown in Table 4.6. Results indicated that pH decreased with depth, this might be explained by the effect of washing out of alkaline compositions. Other possible reasons of the low pH were supposed to be the oxidization of sulfide or generation of carbonic acid from carbon dioxide.
Table 4.6
Results Obtained after Performing the Japanese Regulated Leaching Test* (JLT46) (unit: mg/L (%), i.e. Leachate Concentration (leaching rate))a
| Depth (m) | pH | IC (mg/L) | TOC (mg/L) | Leached Heavy Metals, mg/L (%) | |||
| Pb | Cr | Cd | Cu | ||||
| Fresh residue | 12.1 | 1.2 | 22.1 | 1.43 (0.09) | 0.11 (0.30) | <0.005 | 0.55 (0.34) |
| 0–1 | 11.4 | 1.4 | 21.7 | 0.01 (0.006) | 0.04 (0.09) | <0.005 | 0.75 (0.38) |
| 1–2 | 11.7 | 0.7 | 17.9 | 0.03 (0.02) | 0.01 (0.04) | <0.005 | 0.52 (0.24) |
| 2–3 | 10.2 | 4.2 | 19.8 | 0.05 (0.03) | 0.01 (0.02) | <0.005 | 0.32 (0.14) |
| 3–4 | 9.9 | 3.8 | 7.9 | 0.03 (0.01) | 0.01 (0.02) | <0.005 | 0.17 (0.04) |
| 4–5 | 9.8 | 3.2 | 5.1 | 0.01 (0.004) | <0.005 | <0.005 | 0.09 (0.02) |
| Thresholds | – | – | – | <0.01 | <0.05 | <0.01 | – |

aRegulated threshold of JLT46-a Japanese leaching test applied to soils and reused materials, deionized water, L/S=10, 6 h shaking.
Leaching concentrations of Pb, Cr, and Cu were lower in the landfilled residues than in the fresh residues. When comparing different depth landfilled residues, the leachate concentrations were lower in deeper layers. Low leaching concentrations in the landfilled residues might be explained by decrease of water-soluble fractions after landfill, or it might be due to the low pH of the leachate. The leachate concentrations of Cd and Cr were lower than the regulated value.
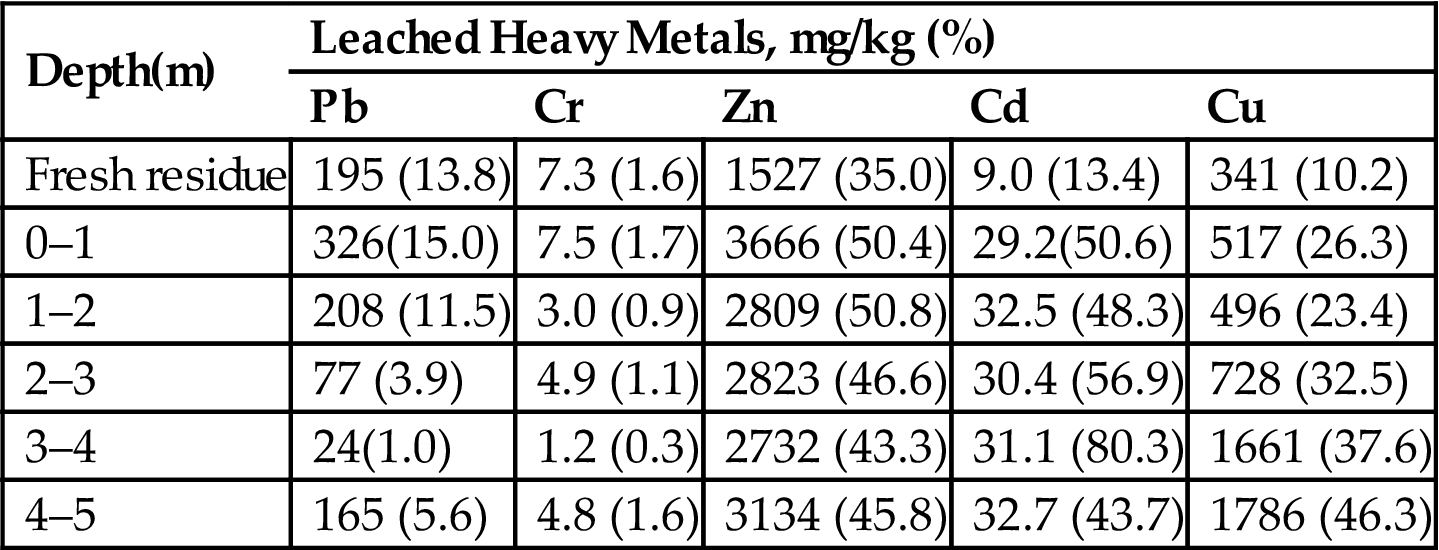
In order to evaluate the long-term leaching behavior of the landfilled residues, Availability test was also conducted. Leachate concentrations and leaching ratio are shown in Table 4.7. Results indicated that for the landfilled residues, the availability was: Cr (0.3–1.7%)<Pb (1.0–15.0%)<Cu (23.4–46.3%)<Zn (43.3–50.8%)<Cd (43.7–80.3%). With landfill depth, the availability of Cu increased, the availability of Pb and Zn decreased. When they were compared with fresh residues, Pb, and Cr had a lower availability, Zn, Cd, and Cu had a higher availability in the landfilled residues.
Table 4.7
Results Obtained After Performing the Availability Test (NEN7341) Leaching Test (unit: mg/kg (%), i.e. Leachate Contents (Leaching Ratio))
| Depth(m) | Leached Heavy Metals, mg/kg (%) | ||||
| Pb | Cr | Zn | Cd | Cu | |
| Fresh residue | 195 (13.8) | 7.3 (1.6) | 1527 (35.0) | 9.0 (13.4) | 341 (10.2) |
| 0–1 | 326(15.0) | 7.5 (1.7) | 3666 (50.4) | 29.2(50.6) | 517 (26.3) |
| 1–2 | 208 (11.5) | 3.0 (0.9) | 2809 (50.8) | 32.5 (48.3) | 496 (23.4) |
| 2–3 | 77 (3.9) | 4.9 (1.1) | 2823 (46.6) | 30.4 (56.9) | 728 (32.5) |
| 3–4 | 24(1.0) | 1.2 (0.3) | 2732 (43.3) | 31.1 (80.3) | 1661 (37.6) |
| 4–5 | 165 (5.6) | 4.8 (1.6) | 3134 (45.8) | 32.7 (43.7) | 1786 (46.3) |
4.5.4 Influences of Weathering to Chemical Forms of Heavy Metals
Fig. 4.15 shows the extracted ratio of Pb, Cd, Cr, and Zn from the landfilled residues under the three-step sequential extraction (BCR) and availability test (NEN7341). Results indicated that the total extracted ratio from BCR was: Cr (11–32%)<Pb (31–51%)<Cu (50–99%)<Zn (56–71%)<Cd (52–94%). If we compare leaching concentrations from the three steps, the contents extracted from step1 were Cr <Pb<Cu <Cd <Zn, the contents extracted from step 2 were Cr <Cd<Cu <Zn <Pb, and the contents extracted from step 3 were Zn <Cr<Pb <Cu <Cd. When the extracted concentrations from BCR were compared with the availability test, the available contents were recognized to have a good correlation with the BCR three steps extractions. For Pb, Cr, and Zn, the available contents from NEN7341 were less than total the steps 1 and 2. For Cd and Cu (3–5 m landfill depth) the available contents from the availability test were higher than total of the steps 1 and 2. For Pb, Cr, and Zn, the chemical forms that adsorbed by iron, aluminum, and manganese oxyhydroxides were supposed to be more stable than Cd and Cu.

Total contents of Fe2O3, Al2O3 were higher in the lower layers of landfilled residues (Fig. 4.14), however, total contents of heavy metals which absorbed on the iron/manganese oxyhydroxides were not always higher in the lower layers. This means that not only the total compositions of these major compositions but also their chemical forms or reactivities are very important to understand the chemical behaviors of heavy metals during weathering.
Chemical composition, water leaching test (JLT46) and acid leaching test (NEN7341 availability test) were conducted to explain the short-term and long-term leaching behavior of the fresh and landfilled MSWI residues. BCR SEP was conducted to clarify the chemical forms of change in heavy metals due to weathering in the landfill. By the combination of the leaching tests and SEP test, transformations of heavy metals during landfill were suggested. At the same time, the influences of major compositions to heavy metals were also suggested. Results of this study indicated the following.
Major geochemical weathering phenomenon included change in major compositions: due to washing out of Ca, Na, K, Cl, Ti, and S, concentrations increased with landfill depth and landfill time. Other elements including Si, Al, Fe, Mg, and P had higher total contents with development of weathering. Change of total contents might indicate the dissolving speed. From the results of water leaching (JLT46), water-soluble fraction of heavy metals decreased for all the Pb, Cr, Zn, and Cu after landfill. This might be due to washing out or immobilization of the soluble fractions.
Results of availability tests indicated that Cu, Zn, and Cd had a relatively higher leachability than Pb and Cr. When compared with fresh residues, the availability of Cu, Zn, and Cd of weathered residues was higher than in fresh residues. Differences in leaching properties of heavy metals were mainly due to the differences in chemical forms. As weathering of MSW residues, heavy metals were adsorbed on iron/manganese oxyhydroxides or were combined with organic carbon and sulphur, since the stability of these chemical forms was different under leaching conditions, the leaching test showed different leaching behavior of the heavy metals.