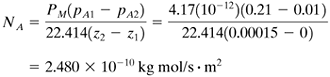6.5. MOLECULAR DIFFUSION IN SOLIDS
6.5A. Introduction and Types of Diffusion in Solids
Even though rates of diffusion of gases, liquids, and solids in solids are generally slower than rates in liquids and gases, mass transfer in solids is quite important in chemical and biological processing. Some examples are leaching of foods, such as soybeans, and of metal ores; drying of timber, salts, and foods; diffusion and catalytic reaction in solid catalysts; separation of fluids by membranes; diffusion of gases through polymer films used in packaging; and treating of metals at high temperatures by gases.
We can broadly classify transport in solids into two types of diffusion: diffusion that can be considered to follow Fick's law and does not depend primarily on the actual structure of the solid, and diffusion in porous solids where the actual structure and void channels are important. These two broad types of diffusion will be considered.
6.5B. Diffusion in Solids Following Fick's Law
1. Derivation of equations
This type of diffusion in solids does not depend on the actual structure of the solid. The diffusion occurs when the fluid or solute diffusing is actually dissolved in the solid to form a more or less homogeneous solution—for example, in leaching, where the solid contains a large amount of water and a solute is diffusing through this solution, or in the diffusion of zinc through copper, where solid solutions are present. Also, the diffusion of nitrogen or hydrogen through rubber, or in some cases diffusion of water in foodstuffs, can be classified here, since equations of similar type can be used.
Generally, simplified equations are used. Using the general Eq. (6.2-14) for binary diffusion,
Equation 6.2-14
![]()
the bulk-flow term, (cA/c)(NA + NB), even if present, is usually small, since cA/c or xA is quite small. Hence, it is neglected. Also, c is assumed constant, giving for diffusion in solids
Equation 6.5-1
![]()
where DAB is diffusivity in m2/s of A through B and is usually assumed constant, independent of pressure for solids. Note that DAB ≠ DBA in solids.
Integration of Eq. (6.5-1) for a solid slab at steady state gives
Equation 6.5-2
![]()
For the case of diffusion radially through a cylinder wall of inner radius r1 and outer r2 and length L,
Equation 6.5-3
![]()
Equation 6.5-4
![]()
This case is similar to conduction heat transfer radially through a hollow cylinder, as shown in Fig. 4.3-2.
The diffusion coefficient DAB in the solid as stated above is not dependent upon the pressure of the gas or liquid on the outside of the solid. For example, if CO2 gas is outside a slab of rubber and is diffusing through the rubber, DAB would be independent of pA, the partial pressure of CO2 at the surface. The solubility of CO2 in the solid, however, is directly proportional to pA. This is similar to the case of the solubility of O2 in water being directly proportional to the partial pressure of O2 in the air by Henry's law.
The solubility of a solute gas (A) in a solid is usually expressed as S in m3 solute (at STP of 0°C and 1 atm) per m3 solid per atm partial pressure of (A). Also, S = cm3(STP)/atm · cm3 solid in the cgs system. To convert this to cA concentration in the solid in kg mol A/m3 using SI units,
Equation 6.5-5
![]()
Equation 6.5-6
![]()
EXAMPLE 6.5-1. Diffusion of H2 Through Neoprene MembraneThe gas hydrogen at 17°C and 0.010 atm partial pressure is diffusing through a membrane of vulcanized neoprene rubber 0.5 mm thick. The pressure of H2 on the other side of the neoprene is zero. Calculate the steady-state flux, assuming that the only resistance to diffusion is in the membrane. The solubility S of H2 gas in neoprene at 17°C is 0.051 m3 (at STP of 0°C and 1 atm)/m3 solid · atm and the diffusivity DAB is 1.03 × 10−10 m2/s at 17°C. Solution: A sketch showing the concentration is shown in Fig. 6.5-1. The equilibrium concentration cA1 at the inside surface of the rubber is, from Eq. (6.5-5),
Figure 6.5-1. Concentrations for Example 6.5-1.
Since pA2 at the other side is 0, cA2 = 0. Substituting into Eq. (6.5-2) and solving,
|
2. Permeability equations for diffusion in solids
In many cases the experimental data for diffusion of gases in solids are not given as diffusivities and solubilities but as permeabilities, PM, in m3 of solute gas A at STP (0°C and 1 atm press) diffusing per second per m2 cross-sectional area through a solid 1 m thick under a pressure difference of 1 atm pressure. This can be related to Fick's equation (6.5-2) as follows:
Equation 6.5-2
![]()
From Eq. (6.5-5),
Equation 6.5-7
![]()
Substituting Eq. (6.5-7) into (6.5-2),
Equation 6.5-8
![]()
Equation 6.5-9
![]()
Permeability (PM) is also given in the literature in several other sets of units. For the cgs system, the permeability is given as cm3(STP)/(s · cm2C.S. · atm/cm). In some cases in the literature the permeability is given as cm3(STP)/(s · cm2C.S. · cm Hg/cm thickness). These are related as follows:
Equation 6.5-10
![]()
Equation 6.5-11
![]()
When there are several solids 1, 2, 3, . . . , in series and L1, L2, . . . , represent the thickness of each, then Eq. (6.5-8) becomes
Equation 6.5-12
![]()
where pA1 − pA2 is the overall partial pressure difference.
3. Experimental diffusivities, solubilities, and permeabilities
Accurate prediction of diffusivities in solids is generally not possible because of the lack of knowledge of the theory of the solid state. Hence, experimental values are needed. Some experimental data for diffusivities, solubilities, and permeabilities are given in Table 6.5-1 for gases diffusing in solids and solids diffusing in solids.
| Solute (A) | Solid (B) | T (K) | DAB, Diffusion Coefficient [m2/s] | Solubility, S
| Permeability, PM
| Ref. |
|---|---|---|---|---|---|---|
| H2 | Vulcanized rubber | 298 | 0.85(10−9) | 0.040 | 0.342(10−10) | (B5) |
| O2 | 298 | 0.21(10−9) | 0.070 | 0.152(10−10) | (B5) | |
| N2 | 298 | 0.15(10−9) | 0.035 | 0.054(10−10) | (B5) | |
| CO2 | 298 | 0.11(10−9) | 0.90 | 1.01(10−10) | (B5) | |
| H2 | Vulcanized neoprene | 290 | 0.103(10−9) | 0.051 | (B5) | |
| 300 | 0.180(10−9) | 0.053 | (B5) | |||
| H2 | Polyethylene | 298 | 6.53(10−12) | (R3) | ||
| O2 | 303 | 4.17(10−12) | (R3) | |||
| N2 | 303 | 1.52(10−12) | (R3) | |||
| O2 | Nylon | 303 | 0.029(10−12) | (R3) | ||
| N2 | 303 | 0.0152(10−12) | (R3) | |||
| Air | English | |||||
| leather | 298 | 0.15−0.68 × 10−4 | (B5) | |||
| H2O | Wax | 306 | 0.16(10−10) | (B5) | ||
| H2O | Cellophane | 311 | 0.91−1.82(10−10) | (B5) | ||
| He | Pyrex glass | 293 | 4.86(10−15) | (B5) | ||
| 373 | 20.1(10−15) | (B5) | ||||
| He | SiO2 | 293 | 2.4−5.5(10−14) | 0.01 | (B5) | |
| H2 | Fe | 293 | 2.59(10−13) | (B5) | ||
| Al | Cu | 293 | 1.3(10−34) | (B5) |
For the simple gases such as He, H2, O2, N2, and CO2, with gas pressures up to 1 or 2 atm, the solubility in solids such as polymers and glasses generally follows Henry's law and Eq. (6.5-5) holds. Also, for these gases the diffusivity and permeability are independent of concentration, and hence pressure. For the effect of temperature T in K, the ln PM is approximately a linear function of 1/T. Also, the diffusion of one gas, say H2, is approximately independent of the other gases present, such as O2 and N2.
For metals such as Ni, Cd, and Pt, where gases such as H2 and O2 are diffusing, it has been found experimentally that the flux is approximately proportional to (![]() ) so Eq. (6.5-8) does not hold (B5). When water is diffusing through polymers, unlike the simple gases, PM may depend somewhat on the relative pressure difference (C9, B5). Further data are available in monographs by Crank and Park (C9) and Barrer (B5).
) so Eq. (6.5-8) does not hold (B5). When water is diffusing through polymers, unlike the simple gases, PM may depend somewhat on the relative pressure difference (C9, B5). Further data are available in monographs by Crank and Park (C9) and Barrer (B5).
EXAMPLE 6.5-2. Diffusion Through a Packaging Film Using PermeabilityA polyethylene film 0.00015 m (0.15 mm) thick is being considered for use in packaging a pharmaceutical product at 30°C. If the partial pressure of O2 outside the package is 0.21 atm and inside it is 0.01 atm, calculate the diffusion flux of O2 at steady state. Use permeability data from Table 6.5-1. Assume that the resistances to diffusion outside the film and inside are negligible compared to the resistance of the film. Solution: From Table 6.5-1, PM = 4.17(10−12) m3 solute(STP)/(s · m2 · atm/m). Substituting into Eq. (6.5-8),
Note that a film made of nylon has a much smaller value of permeability PM for O2 and would make a more suitable barrier. |
4. Membrane separation processes
In Chapter 13 a detailed discussion is given of the various membrane separation processes for gas separation by membranes, dialysis, reverse osmosis, ultrafiltration, and microfiltration.
6.5C. Diffusion in Porous Solids That Depends on Structure
1. Diffusion of liquids in porous solids
In Section 6.5B we used Fick's law and treated the solid as a uniform homogeneous-like material with an experimental diffusivity DAB. In this section we are concerned with porous solids that have pores or interconnected voids in the solid which affect the diffusion. A cross section of a typical porous solid is shown in Fig. 6.5-2.
Figure 6.5-2. Sketch of a typical porous solid.

For the situation where the voids are filled completely with liquid water, the concentration of salt in water at boundary 1 is cA1 and at point 2 is cA2. The salt, in diffusing through the water in the void volume, takes a tortuous path which is unknown and greater than (z2 − z1) by a factor τ, called tortuosity. Diffusion does not occur in the inert solid. For a dilute solution using Eq. (6.3-5) for diffusion of salt in water at steady state,
Equation 6.5-13
![]()
where ε is the open void fraction, DAB is the diffusivity of salt in water, and τ is a factor which corrects for the path longer than (z2 − z1). For inert-type solids τ can vary from about 1.5 to 5. Often the terms are combined into an effective diffusivity:
Equation 6.5-14
![]()
EXAMPLE 6.5-3. Diffusion of KCl in Porous SilicaA sintered solid of silica 2.0 mm thick is porous, with a void fraction ε of 0.30 and a tortuosity τ of 4.0. The pores are filled with water at 298 K. At one face the concentration of KCl is held at 0.10 g mol/liter, and fresh water flows rapidly past the other face. Neglecting any other resistance but that in the porous solid, calculate the diffusion of KCl at steady state. Solution: The diffusivity of KCl in water from Table 6.3-1 is DAB = 1.87 × 10−9 m2/s. Also, cA1 = 0.10/1000 = 1.0 × 10−4 g mol/cm3 = 0.10 kg mol/m3, and cA2 = 0. Substituting into Eq. (6.5-13),
|
2. Diffusion of gases in porous solids
If the voids shown in Fig. 6.5-2 are filled with gases, then a somewhat similar situation exists. If the pores are very large so that diffusion occurs only by Fickian-type diffusion, then Eq. (6.5-13) becomes, for gases,
Equation 6.5-15
![]()
Again the value of the tortuosity must be determined experimentally. Diffusion is assumed to occur only through the voids or pores and not through the actual solid particles.
A correlation of tortuosity versus the void fraction of various unconsolidated porous media of beds of glass spheres, sand, salt, talc, and so on (S8) gives the following approximate values of τ for different values of ε: ε = 0.2, τ = 2.0; ε = 0.4, τ = 1.75; ε = 0.6, τ = 1.65.
When the pores are quite small in size and on the order of magnitude of the mean free path of the gas, other types of diffusion occur, which are discussed in Section 7.6.