Metallic Ore Minerals
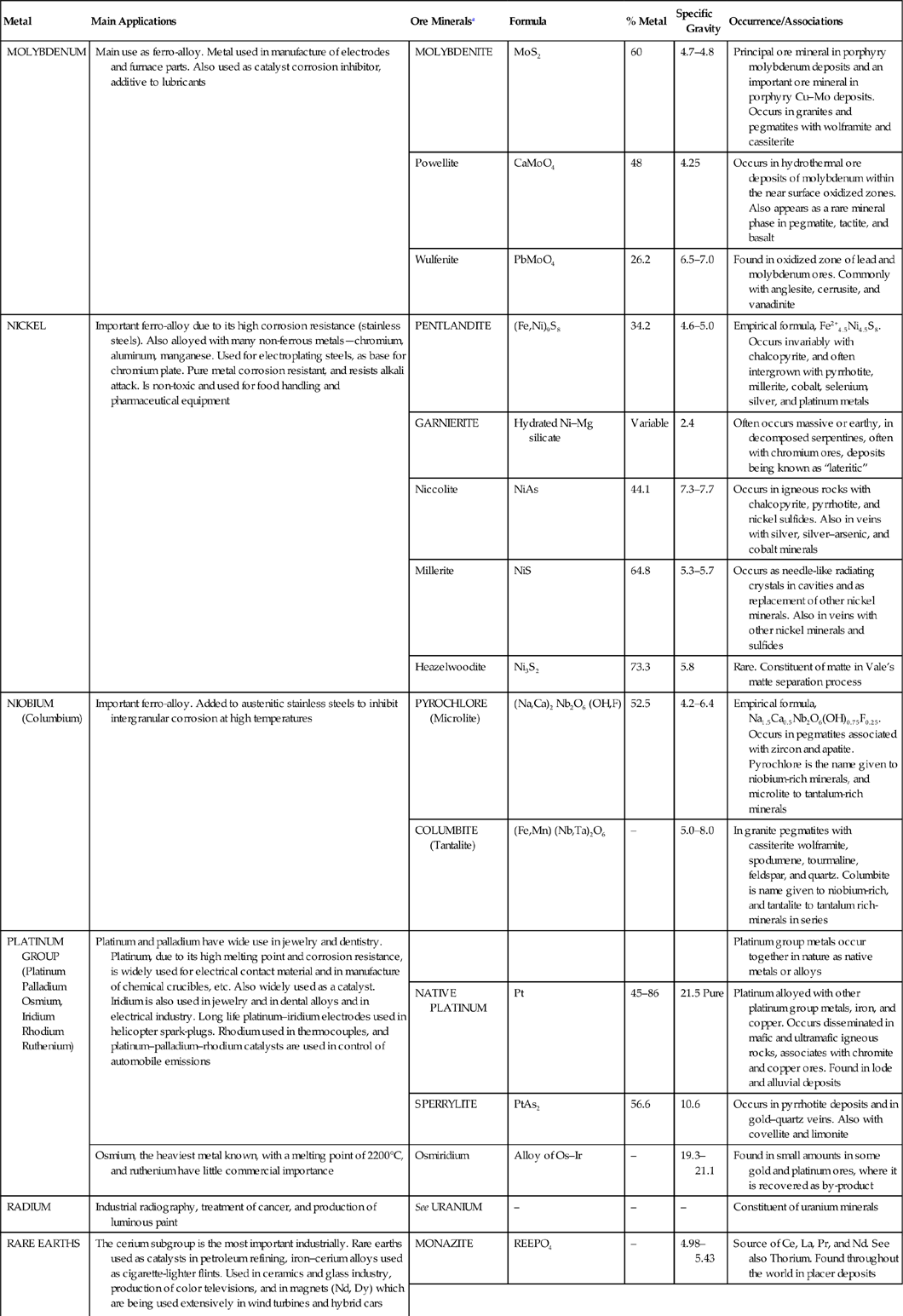
| Metal | Main Applications | Ore Mineralsa | Formula | % Metal | Specific Gravity | Occurrence/Associations |
| ALUMINUM | Where requirements are lightness, high electrical and thermal conductivity, corrosion resistance, ease of fabrication. Forms high tensile strength alloys | BAUXITE | – | – | 3.2–3.5 | Bauxite, which occurs massive, is a mixture of minerals such as diaspore, gibbsite, and boehmite with iron oxides and silica. Occurs as residual earth from weathering and leaching of rocks in tropical climates |
| Diaspore | AlO(OH) | 45.0 | 3.2–3.5 | |||
| Gibbsite | Al(OH)3 | 34.6 | 2.38–2.42 | |||
| Boehmite | AlO(OH) | 45.0 | 3.2–3.5 | |||
| ANTIMONY | Flame-resistant properties of oxide used in textiles, fibers, and other materials. Alloyed with lead to increase strength for accumulator plates, sheet, and pipe. Important alloying element for bearing and type metals | STIBNITE | Sb2S3 | 71.8 | 4.5–4.6 | Main ore mineral. Commonly in quartz veins and in limestone replacements. Associates with galena, pyrite, realgar, orpiment, and cinnabar |
| ARSENIC | Limited use in industry. Small amounts alloyed with copper and lead to toughen the metals. In oxide form, used as insecticide | Arsenopyrite | FeAsS | 46.0 | 5.9–6.2 | Widely distributed in mineral veins, with tin ores, tungsten, gold and silver, sphalerite, and pyrite. Since production of metal is in excess of demand, it is commonly regarded as gangue |
| Realgar | AsS | 70.1 | 3.5 | Often associated in mineral veins in minor amounts | ||
| Orpiment | As2S3 | 61 | 3.4–3.5 | |||
| BERYLLIUM | Up to 4% Be alloyed with copper to produce high tensile alloys with high fatigue, wear, and corrosion resistance, which are used to make springs, bearings, and valves, and spark-proof tools. Used for neutron absorption in nuclear industry. Used in electronics for speakers and styluses | BERYL | Be3Al2Si6O18 | 5 | 2.6–2.9 | Only source of the metal. Often mined as gemstone—emerald, aquamarine. Commonly occurs as accessory mineral in coarse-grained granites (pegmatites) and other similar rocks. Also in calcite veins and mica schists. As similar density to gangue minerals difficult to separate other than by hand-sorting |
| BISMUTH | Pharmaceuticals; low-melting-point alloys for automatic safety devices, such as fire-sprinklers. Improves casting properties when alloyed with tin and lead | Native | Bi | 100 | 9.7–9.8 | Minor amounts in veins associated with silver, lead, zinc, and tin ores |
| Bismuthinite | Bi2S3 | 81.2 | 6.8 | Occurs in association with magnetite, pyrite, chalcopyrite, galena, and sphalerite, and with tin and tungsten ores. Majority of bismuth produced as by-product from smelting and refining of lead and copper | ||
| CADMIUM | Rust-proofing of steel, copper, and brass by electroplating and spraying; production of pigments; negative plate in alkali accumulators; plastic stabilizers | Greenockite | CdS | 77.7 | 4.9–5.0 | Found in association with lead and zinc ores, and in very small quantities with many other minerals. Due to volatility of the metal, mainly produced during smelting and refining of zinc, as a by-product |
| CESIUM | Low ionization potential utilized in photoelectric cells, photomultiplier tubes, spectro-photometers, infra-red detectors. Minor pharmaceutical use | POLLUCITE | Cs2Al2Si4O12.2H2O | 40.3 | 2.9 | Occurs in pegmatites of complex mineralogical character. Rare mineral |
| Lepidolite (Lithium mica) | K(Li, Al)3 (Si, Al)4O10 (OH,F)2 | – | 2.8–2.9 | Occurs in pegmatites, often in association with tourmaline and spodumene. Often carries traces of rubidium and cesium | ||
| CHROMIUM | Used mainly as alloying element in steels to give resistance to wear, corrosion, heat, and to increase hardness and toughness. Used for electroplating iron and steel. Chromite used as refractory with neutral characteristics. Used in production of bichromates and other salts in tanning, dyeing, and pigments | CHROMITE | FeCr2O4 | 46.2 | 4.1–5.1 | Occurs in olivine and serpentine rocks, often concentrated sufficiently into layers or lenses to be worked. Due to its durability, it is sometimes found in alluvial sands and gravels |
| COBALT | Used as alloying element for production of high-temperature steels and magnetic alloys. Used as catalyst in chemical industry. Cobalt powder used as cement in sintered carbide cutting tools | Smaltite | CoAs2 | 28.2 | 5.7–6.8 | Smaltite and cobaltite occur in veins, often together with arsenopyrite, silver, calcite, and nickel minerals |
| Cobaltite | CoAsS | 35.5 | 6.0–6.3 | |||
| Carrolite | CuCo2S4 | 38.0 | 4.8–5.0 | Carrolite and linnaeite sometimes occur in small amounts in copper ores. Cobalt is usually only a minor constituent in ores such as lead, copper, and nickel and extracted as by-product | ||
| Linnaeite | Co3S4 | 57.8 | 4.8–5.0 | |||
| COPPER | Used where high electrical or thermal conductivity is important. High corrosion resistance and easy to fabricate. Used in variety of alloys—brasses, bronzes, aluminum bronzes, etc. | CHALCOPYRITE | CuFeS2 | 34.6 | 4.1–4.3 | Main ore mineral. Most often in veins with other sulfides, such as galena, sphalerite, pyrrhotite, pyrite, and also cassiterite. Common gangue minerals quartz, calcite, dolomite. Disseminated with bornite and pyrite in porphyry copper deposits |
| CHALCOCITE | Cu2S | 79.8 | 5.5–5.8 | Often associated with cuprite and native copper. Constituent of matte in Vale’s matte separation process | ||
| BORNITE | Cu5FeS4 | 63.3 | 4.9–5.4 | Associates with chalcopyrite and chalcocite in veins | ||
| COVELLITE | CuS | 66.5 | 4.6 | Sometimes as primary sulfide in veins, but more commonly as secondary sulfide with chalcopyrite, chalcocite, and bornite | ||
| CUPRITE | Cu2O | 88.8 | 5.9–6.2 | Found in oxidized zone of deposits, with malachite, azurite, and chalcocite | ||
| MALACHITE | Cu2CO3(OH)2 | 57.5 | 3.6–4.0 | Frequently associated with azurite, native copper, and cuprite in oxidized zone | ||
| Native | Cu | 100 | 8.9 | Occurs in small amounts with other copper minerals | ||
| Tennantite | Cu12As4S13 | 51.5 | 4.4–4.6 | Tennantite and tetrahedrite found in veins with silver, copper, lead, and zinc minerals | ||
| Variable | Variable | |||||
| Tetrahedrite | Cu12Sb4S13 | 45.8 | 4.4–5.1 | Tetrahedrite more widespread and common in lead–silver veins | ||
| Variable | Variable | |||||
| Azurite | Cu3(CO3)2(OH)2 | 55.3 | 3.8–3.9 | Occurs in oxidized zone. Not as widespread as malachite | ||
| Enargite | Cu3AsS4 | 48.4 | 4.4 | Associates with chalcocite, bornite, covellite, pyrite, sphalerite, tetrahedrite, baryte, and quartz in near-surface deposits | ||
| GALLIUM | Electronics industry for production of light-emitting diodes. Used in electronic memories for computers | Occurs in some zinc ores, but no important ore minerals | – | – | – | About 90% of production is a direct by-product of alumina output. Also found in coal ash and flue dusts |
| GERMANIUM | Electronics industry | Argyrodite | Ag8GeS6 | 6.4 | 6.1 | Occurs with sphalerite, siderite, and marcasite. No important ore minerals. Chief source is cadmium fume from sintering zinc concentrates |
| GOLD | Jewelry, monetary use, electronics, dentistry, decorative plating | NATIVE | Au | >85 | 12–20 | Disseminated in quartz grains, often with pyrite, chalcopyrite, galena, stibnite, and arsenopyrite. Also found alluvially in stream or other sediments. South African “banket” is consolidated alluvial deposit |
| (invariably alloyed with Ag and Cu, and other metals) | ||||||
| Sylvanite | (Au,Ag)2Te4 | 34.4 | 7.9–8.3 | Sylvanite empirical formula Au0.75Ag0.25Te2. Tellurides occur in Kalgoorlie gold ores of Western Australia | ||
| Calaverite | AuTe2 | 43.6 | 9.0 | |||
| HAFNIUM | Naval nuclear reactors, flashbulbs, ceramics, refractory alloys, and enamels | No ore minerals | – | – | – | Produced as co-product of zirconium sponge |
| INDIUM | Electronics, component of low-melting-point alloys and solders, protective coating on silverware and jewelry | Occurs as trace element in many ores | – | – | – | Recovered from residues and flue dusts from some zinc smelters |
| IRON | Iron and steel industry | HEMATITE | Fe2O3 | 69.9 | 5.3 | Most important iron ore mineral. Occurs massive. Also in igneous rocks and veins, and as ooliths or cementing material in sedimentary rocks |
| MAGNETITE | Fe3O4 | 72.3 | 5.1–5.2 | The only ferromagnetic mineral. Widely distributed in several environments, including igneous and metamorphic rocks; and beach-sand deposits | ||
| Goethite | FeO(OH) | 62.9 | 4.0–4.4 | Widespread occurrence, associated with hematite and limonite | ||
| Limonite | FeO(OH)·nH2O | Variable | 2.9–4.3 | Natural rust, chief constituent being goethite. Often associates with hematite in weathered deposits | ||
| Siderite | FeCO3 | 48.3 | 3.7–3.9 | Occurs massive in sedimentary rocks and as gangue mineral in veins carrying pyrite, chalcopyrite, galena | ||
| Pyrrhotite | FeS | 63.6 | 4.6 | In monoclinic form the only magnetic sulfide mineral. Occurs disseminated in igneous rocks, commonly with pyrite, chalcopyrite, and pentlandite. Usually regarded as gangue | ||
| Variable | Variable | |||||
| Pyrite | FeS2 | 46.7 | 4.9–5.2 | One of most widely distributed sulfide minerals. Used for production of sulfuric acid, but often regarded as gangue | ||
| LEAD | Batteries, corrosion resistant pipes and linings, alloys, pigments, radiation shielding | GALENA | PbS | 86.6 | 7.4–7.6 | Very widely distributed, and most important lead ore mineral. Occurs in veins, often with sphalerite, pyrite, chalcopyrite, tetrahedrite, and gangue minerals such as quartz, calcite, dolomite, baryte, and fluorite. Also in pegmatites, and as replacement bodies in limestone and dolomite rocks, with garnets, feldspar, diopside, rhodonite, and biotite. Often contains up to 0.5% Ag and is important source of this metal |
| Cerussite | PbCO3 | 77.5 | 6.5–6.6 | In oxidized zone of lead veins, associated with galena, anglesite, smithsonite, and sphalerite | ||
| Anglesite | PbSO4 | 68.3 | 6.1–6.4 | Occurs in oxidation zone of lead veins | ||
| Jamesonite | Pb4FeSb6S14 | 40.1 | 5.5–6.0 | Rare mineral occurring in veins with galena, sphalerite, stibnite | ||
| LITHIUM | Lightest metal. Lithium carbide used in production of aluminum. Used as base in multipurpose greases; used in manufacture of lithium batteries. Large application in ceramics industry. Very little use in metallic form | SPODUMENE | LiAlSi2O6 | 3.7 | 3.0–3.2 | Occurs in pegmatites with lepidolite, tourmaline, and beryl |
| Amblygonite | LiAlPO4(OH,F) | Variable | 3.0–3.1 | Rare mineral occurring in pegmatites with other lithium minerals | ||
| Lepidolite | K(Li,Al)3 (Si,Al)4O10 (H,F)2 | Variable | 2.8–3.3 | Mica occurring in pegmatites with other lithium minerals | ||
| Tourmaline | Complex borosilicate of Al, Na, Mg, Fe, Li, Mn | – | 3.0–3.2 | Not a commercial source of metal. Some crystals used as gems. Occurs in granite pegmatites, schists, and gneisses | ||
| 50–70% world’s Li reserves are estimated to be in Solar de Uyuni salt flat in Bolivia, now in process of being extracted | ||||||
| MAGNESIUM | Small amounts used in aluminum alloys to increase strength and corrosion resistance. Used to desulfur blast-furnace iron. Added to cast-iron to produce nodular iron. Used in cathodic protection, as a reagent in petrol processing and as reducing agent in titanium, and zirconium production. Structural uses where lightness required—magnesium die castings | Most magnesium extracted from brine, rather than ore minerals | ||||
| Dolomite | MgCa(CO3)2 | 13 | 2.8–2.9 | Mineral used in manufacture of refractories. Occurs as gangue mineral in veins with galena and sphalerite. Also occurs widely as rock-forming mineral | ||
| Magnesite | MgCO3 | 28.6 | 3.0–3.2 | Used mainly for cement and refractory bricks. Often associates with serpentinite | ||
| Carnallite | KMgCl3·6H2O | 8.6 | 1.6 | Occurs with halite and sylvite | ||
| Brucite | Mg(OH)2 | 41.4 | 2.4 | Occurs in dolomitic limestones and veins with talc, calcite, and in serpentine | ||
| MANGANESE | Very important ferro-alloy. About 95% of output used in steel and foundry industry. Balance mainly in manufacture of dry cells and chemicals | PYROLUSITE | MnO2 | 63.2 | 4.5–5.0 | Often found in oxidized zone of ore deposits containing manganese. Also in quartz veins and manganese nodules |
| Manganite | MnO(OH) | 62.5 | 4.2–4.4 | Occurs in association with baryte, pyrolusite, and goethite and in veins in granite | ||
| Braunite | 3Mn2O3·MnSiO3 | 63.6 | 4.7–4.8 | Occurs in veins with other manganese minerals | ||
| Psilomelane | (Ba,H2O)2 Mn5O10 | 38.5 | 3.3–4.7 | Found with pyrolusite and limonite in sediments or quartz veins | ||
| MERCURY | Electrical apparatus, scientific instruments, manufacture of paint, electrolytic cells, solvent for gold, manufacture of drugs and chemicals | CINNABAR | HgS | 86.2 | 8.0–8.2 | Only important mercury mineral. Occurs in fractures in sedimentary rocks with pyrite, stibnite, and realgar. Common gangue minerals are quartz, calcite, baryte, and chalcedony |
| MOLYBDENUM | Main use as ferro-alloy. Metal used in manufacture of electrodes and furnace parts. Also used as catalyst corrosion inhibitor, additive to lubricants | MOLYBDENITE | MoS2 | 60 | 4.7–4.8 | Principal ore mineral in porphyry molybdenum deposits and an important ore mineral in porphyry Cu–Mo deposits. Occurs in granites and pegmatites with wolframite and cassiterite |
| Powellite | CaMoO4 | 48 | 4.25 | Occurs in hydrothermal ore deposits of molybdenum within the near surface oxidized zones. Also appears as a rare mineral phase in pegmatite, tactite, and basalt | ||
| Wulfenite | PbMoO4 | 26.2 | 6.5–7.0 | Found in oxidized zone of lead and molybdenum ores. Commonly with anglesite, cerrusite, and vanadinite | ||
| NICKEL | Important ferro-alloy due to its high corrosion resistance (stainless steels). Also alloyed with many non-ferrous metals—chromium, aluminum, manganese. Used for electroplating steels, as base for chromium plate. Pure metal corrosion resistant, and resists alkali attack. Is non-toxic and used for food handling and pharmaceutical equipment | PENTLANDITE | (Fe,Ni)9S8 | 34.2 | 4.6–5.0 | Empirical formula, Fe2+4.5Ni4.5S8. Occurs invariably with chalcopyrite, and often intergrown with pyrrhotite, millerite, cobalt, selenium, silver, and platinum metals |
| GARNIERITE | Hydrated Ni–Mg silicate | Variable | 2.4 | Often occurs massive or earthy, in decomposed serpentines, often with chromium ores, deposits being known as “lateritic” | ||
| Niccolite | NiAs | 44.1 | 7.3–7.7 | Occurs in igneous rocks with chalcopyrite, pyrrhotite, and nickel sulfides. Also in veins with silver, silver–arsenic, and cobalt minerals | ||
| Millerite | NiS | 64.8 | 5.3–5.7 | Occurs as needle-like radiating crystals in cavities and as replacement of other nickel minerals. Also in veins with other nickel minerals and sulfides | ||
| Heazelwoodite | Ni3S2 | 73.3 | 5.8 | Rare. Constituent of matte in Vale’s matte separation process | ||
| NIOBIUM (Columbium) | Important ferro-alloy. Added to austenitic stainless steels to inhibit intergranular corrosion at high temperatures | PYROCHLORE (Microlite) | (Na,Ca)2 Nb2O6 (OH,F) | 52.5 | 4.2–6.4 | Empirical formula, Na1.5Ca0.5Nb2O6(OH)0.75F0.25. Occurs in pegmatites associated with zircon and apatite. Pyrochlore is the name given to niobium-rich minerals, and microlite to tantalum-rich minerals |
| COLUMBITE (Tantalite) | (Fe,Mn) (Nb,Ta)2O6 | – | 5.0–8.0 | In granite pegmatites with cassiterite wolframite, spodumene, tourmaline, feldspar, and quartz. Columbite is name given to niobium-rich, and tantalite to tantalum rich-minerals in series | ||
| PLATINUM GROUP (Platinum Palladium Osmium, Iridium Rhodium Ruthenium) | Platinum and palladium have wide use in jewelry and dentistry. Platinum, due to its high melting point and corrosion resistance, is widely used for electrical contact material and in manufacture of chemical crucibles, etc. Also widely used as a catalyst. Iridium is also used in jewelry and in dental alloys and in electrical industry. Long life platinum–iridium electrodes used in helicopter spark-plugs. Rhodium used in thermocouples, and platinum–palladium–rhodium catalysts are used in control of automobile emissions | Platinum group metals occur together in nature as native metals or alloys | ||||
| NATIVE PLATINUM | Pt | 45–86 | 21.5 Pure | Platinum alloyed with other platinum group metals, iron, and copper. Occurs disseminated in mafic and ultramafic igneous rocks, associates with chromite and copper ores. Found in lode and alluvial deposits | ||
| SPERRYLITE | PtAs2 | 56.6 | 10.6 | Occurs in pyrrhotite deposits and in gold–quartz veins. Also with covellite and limonite | ||
| Osmium, the heaviest metal known, with a melting point of 2200°C, and ruthenium have little commercial importance | Osmiridium | Alloy of Os–Ir | – | 19.3–21.1 | Found in small amounts in some gold and platinum ores, where it is recovered as by-product | |
| RADIUM | Industrial radiography, treatment of cancer, and production of luminous paint | See URANIUM | – | – | – | Constituent of uranium minerals |
| RARE EARTHS | The cerium subgroup is the most important industrially. Rare earths used as catalysts in petroleum refining, iron–cerium alloys used as cigarette-lighter flints. Used in ceramics and glass industry, production of color televisions, and in magnets (Nd, Dy) which are being used extensively in wind turbines and hybrid cars | MONAZITE | REEPO4 | – | 4.98–5.43 | Source of Ce, La, Pr, and Nd. See also Thorium. Found throughout the world in placer deposits |
| BASTNAESITE | REE(CO3)F | – | 4.9–5.2 | Source of Ce, La, Pr, and Nd. Widespread, one of the more common rare-earth carbonates. Also found in carbonatite plutons, e.g., Bayan Obo, Mongolia, and Mountain Pass, California. Has replaced monazite as chief source of REEs | ||
| XENOTIME | YPO4 | 48.4 | 4.4–5.1 | Rare but important source of yttrium, along with ion-adsorbed RE-bearing clays. Found in pegmatites and other igneous rocks | ||
| Other potential RE minerals: parasite, synchysite, pyrochlore, fergusonite, allanite, eudialyte | ||||||
| RHENIUM | Used as catalyst in production of low-lead petrol. Used as catalyst with platinum. Used extensively in thermocouples, temperature controls, and heating elements. Also used as filaments in electronic apparatus | Molybdenite | MoS2 | – | 4.7–4.8 | Rhenium occurs associated with molybdenite in porphyry Cu–Mo deposits, and recovered as by-product |
| RUBIDIUM | Rubidium and cesium largely interchangeable in properties and uses, although latter usually preferred to meet present small industrial demand | See CESIUM | Rubidium widely dispersed as minor constituent in major cesium minerals | |||
| SILICON | Used in steel industry and as heavy medium alloy as ferro-silicon. Also used to de-oxidize steels. Metal used as semi-conductor | QUARTZ | SiO2 | 46.7 | 2.65 | Commonest mineral, forming 12% of earth’s crust. Essential constituent of many rocks, such as granite and sandstone, and virtually sole constituent of quartzite rock |
| SELENIUM | Used in manufacture of fade-resistant pigments, photo-electric apparatus, in glass production, and various chemical applications. Alloyed with copper and steel to improve machinability | Naumanite | Ag2Se | 26.8 | 8.0 | Selenides occur associated with sulfides, and bulk of selenium recovered as by-product from copper sulfide ores |
| Clausthalite | PbSe | 27.6 | 8.0 | |||
| Berzelianite | Cu2Se | 38.3 | 6.7 | |||
| SILVER | Sterling ware, jewelry, coinage, photographic and electronic products, mirrors, electroplate, and batteries. Silver nitrate solutions and other silver compounds used as disinfectants and microbiocides | ARGENTITE | Ag2S | 87.1 | 7.2–7.4 | Closely associated with lead, zinc, and copper ores, and bulk of silver produced as by-product from smelting such ores |
| ACANTHITE | Ag2S | Stable polymorph of argentite below 180°C. Similar associations as argentite | ||||
| Native | Ag | Up to 100 | 10.1–11.1 | Usually alloyed with copper, gold, etc., and occurs in upper part of silver sulfide deposits | ||
| Cerargyrite | AgCl | 75.3 | 5.8 | Occurs in upper parts of silver veins together with native silver and cerussite | ||
| TANTALUM | Used in certain chemical and electrical processes due to extremely high corrosion resistance. Used in production of special steels used for medical instruments. Used for electrodes, and tantalum carbide used for cutting tools. Used in manufacture of capacitors | PYROCHLORE TANTALITE | See NIOBIUM | As well as ore minerals, certain tin slags are becoming important source of tantalum | ||
| TELLURIUM | Used in production of free machining steels, in copper alloys, rubber production, and as catalyst in synthetic fiber production | Sylvanite Calaverite | See GOLD | Produced with selenium as by-product of copper refining | ||
| These metal tellurides, which are important gold ores, and other tellurides of bismuth and lead, are most important sources of tellurium | ||||||
| THALLIUM | Very poisonous, and finds limited outlet as fungicide and rat poison. Thallium salts used in Clerici solution, an important heavy liquid | Occurs in some zinc ores, but no ore minerals | – | – | – | By-product of zinc refining |
| THORIUM | Radioactive metal. Used in electrical apparatus, and in magnesium–thorium and other thorium alloys. Oxide of importance in manufacture of gas-mantles, and used in medicine | MONAZITE | REEPO4 | – | 4.9–5.4 | Although occurring in lode deposits in igneous rocks such as granites, the main deposits are alluvial, beach-sand deposits being the most important source. Occurs associated with ilmenite, rutile, zircon, garnets, etc. |
| Thorianite | ThO2 | 87.9 | 9.3 | Occurs in some beach-sand deposits | ||
| TIN | Main use in manufacture of tin-plate, for production of cans, etc. Important alloy in production of solders, bearing-metals, bronze, type-metal, pewter, etc. | CASSITERITE | SnO2 | 78.8 | 6.8–7.1 | Found in lode and alluvial deposits. Lode deposits in association with wolfram, arsenopyrite, copper, and iron minerals. Alluvially, often associated with ilmenite, monazite, zircon, etc. |
| TITANIUM | Due to its high strength and corrosion resistance, about 80% of titanium used in aircraft and aerospace industries. Also used in power-station heat-exchanger tubing and in chemical and desalination plants | ILMENITE | FeTiO3 | 31.6 | 4.5–5.0 | Accessory mineral in igneous rocks especially gabbros and norites. Economically concentrated into alluvial sands, together with rutile, monazite, and zircon |
| RUTILE | TiO2 | 60 | 4.2 | Accessory mineral in igneous rocks, but economic deposits found in alluvial beach-sand deposits | ||
| TUNGSTEN | Production of tungsten carbide for cutting, drilling, and wear-resistant applications. Used in lamp filaments, electronic parts, electrical contacts, etc. Important ferro-alloy, producing tool and high-speed steels | WOLFRAMITE | (Fe,Mn)WO4 | Variable | 7.1–7.9 | Occurs in veins in granite rocks, with minerals such as cassiterite, arsenopyrite, tourmaline, galena, sphalerite, scheelite, and quartz. Also found in some alluvial deposits |
| SCHEELITE | CaWO4 | 63.9 | 5.9–6.1 | Occurs under same conditions as wolframite. Also occurs in contact with metamorphic deposits | ||
| URANIUM | Nuclear power plant fuel. Depleted uranium used in ammunition and in shielding for radioactive materials | PITCHBLENDE (URANINITE) | UO2 (variable—partly oxidized to U3O8) | 80–90 | 8–10 | Most important uranium and radium ore. Much of the World’s uranium comes from unconformity type deposits in which the uraninite is massive and hosted by sandstone a few tens of meters above a highly reducing metamorphic basement |
| Carnotite | K2(UO2)2(VO4)2 ·3H2O | 52.8 | 3.7–4.7 | Secondary mineral found in sedimentary rocks, also in pitchblende deposits. Source of radium | ||
| Coffinite | U(SiO4)1-x (OH)4x Variable | 72.6 | 5.1 | Empirical formula, U(SiO4)0.9(OH)0.4. Common secondary uranium mineral | ||
| Torbernite | Cu(UO2)2(PO4)2·8-12H2O | 48.0 | 3.2 | Empirical formula, Cu(UO2)2(PO4)2·11(H2O). Secondary uranium mineral | ||
| Autunite | Ca(UO2)2(PO4)2·12H2O | 48.3 | 3.1–3.2 | Oxidation product of uranium minerals | ||
| VANADIUM | Important ferro-alloy. Used in manufacture of specialty steels, such as high-speed tool steels. Increases strength of structural steels—used for oil and gas pipelines. Vanadium–aluminum master alloys used in preparation of some titanium-based alloys. Vanadium compounds used in chemical and oil industries as catalysts. Also used as glass-coloring agent and in ceramics, and growing use in storage batteries | PATRONITE | VS4 | 28.4 | 2.8 | Occurs with nickel and molybdenum sulfides and asphaltic material |
| CARNOTITE | See URANIUM | |||||
| Roscoelite (Vanadium mica) | K(V,Al,Mg)2 AlSi3O10(OH)2 | 9.9 | 3.0 | Empirical formula, KV5+0.8Al0.6Mg0.4AlSi3O10(OH)2. Found in epithermal Au–Ag–Te deposits and oxidized portions of sedimentary U-V ores | ||
| Vanadinite | Pb5(VO4)3Cl | Variable | 6.6–7.1 | Occurs in oxidation zone of lead, and lead–zinc deposits. Also with other vanadium minerals in sediments | ||
| ZINC | Corrosion protective coatings on iron and steel (“galvanizing”). Important alloying metal in brasses and zinc die-castings. About 50% consumed in form of compounds, e.g., zinc oxide as catalyst in manufacture of rubber, and white paint pigment; zinc sulfide as luminescent pigment | SPHALERITE | (Zn,Fe)S | 67.1 Pure ZnS | 3.9–4.1 | Most common zinc ore mineral. Range in Fe content. Frequently associated with galena, and copper sulfides in vein deposits. Also occurs in limestone replacements, with pyrite, pyrrhotite, and magnetite |
| Wurtzite | (Zn,Fe)S | High temperature polymorph of sphalerite | ||||
| Smithsonite (Calamine) | ZnCO3 | 52 | 4.3–4.5 | Mainly occurs in oxidized zone of ore deposits carrying zinc minerals. Commonly associated with sphalerite, galena, and calcite | ||
| Hemimorphite (Calamine) | Zn4Si2O7(OH)2 ·H2O | 54.3 | 3.4–3.5 | Found associated with smithsonite accompanying the sulfides of zinc, iron, and lead | ||
| Marmatite | (Zn,Fe)S | 46.5–56.9 | 3.9–4.1 | High Fe content sphalerite | ||
| Franklinite | Oxide of Zn,Fe,Mn | – | 5.0–5.2 | First identified at Franklin Mine | ||
| Zincite | ZnO | 80.3 | 5.4–5.7 | Zincite often occurs with franklinite and willemite | ||
| Willemite | Zn2SiO4 | 58.5 | 4.0–4.1 | |||
| ZIRCONIUM | Used, alloyed with iron, silicon, and tungsten, in nuclear reactors, and for removing oxides and nitrides from steel. Used in corrosion-resistant equipment in chemical plants | ZIRCON | ZrSiO4 | 49.8 | 4.6–4.7 | Widely distributed in igneous rocks, such as granites. Common constituent of residues of various sedimentary rocks, and occurs in beach sands associated with ilmenite, rutile, and monazite |
| Baddeleyite | ZrO2 | 74 | 5.5–6.0 | Forms in igneous rocks low in silica. Found in rocks containing potassium feldspar and plagioclase. Associated minerals include ilmenite, apatite, fluorite, and pyrochlore | ||






aUppercase is main mineral exploited.
..................Content has been hidden....................
You can't read the all page of ebook, please click here login for view all page.
