Tailings Disposal
The disposal of mill tailings is a major environmental problem, which is becoming more serious with the increasing exploration for metals and the working of lower-grade deposits. Apart from the visual effect on the landscape of tailings disposal, the major ecological effect is usually water pollution, arising from the discharge of water contaminated with solids, heavy metals, mill reagents, and sulfur compounds. Waste must therefore be disposed of in both an environmentally acceptable and, if possible, economically viable manner. Disposal is governed by legislation and may involve long-term rehabilitation of the site.
Keywords
Tailings dam types; backfill; submarine disposal; environmental issues; acid rock drainage
16.1 Introduction
The disposal of mill tailings is a major environmental problem, which is becoming more serious with the increasing exploration for metals and the working of lower-grade deposits. Apart from the visual effect on the landscape of tailings disposal, the major ecological effect is usually water pollution, arising from the discharge of water contaminated with solids, heavy metals, mill reagents, and sulfur compounds (Chalkley et al., 1989). Waste must therefore be disposed of in both an environmentally acceptable and, if possible, economically viable manner (Ritcey, 1989). Disposal is governed by legislation and may involve long-term rehabilitation of the site.
The nature of tailings varies widely; they are usually transported and disposed of as a slurry of high water content, but they may be composed of very coarse dry material, such as the float fraction from dense medium plants. Due to the lower costs of mining from open pits, ore from such locations is often of very low grade, resulting in the production of large amounts of fine tailings.
16.2 Methods of Disposal of Tailings
The methods used to dispose of tailings have developed due to environmental pressures, changing milling practice, and realization of profitable applications. Early methods included discharge of tailings into rivers and streams, which is still practiced at some mines, and the dumping of coarse dewatered tailings on to land. The many nineteenth-century tips seen in Cornwall and other parts of Britain are evidence of this latter method. Due to the damage caused by such methods, and the much finer grinding necessary on most modern ores, other techniques have been developed.
16.2.1 Tailings Dams
The design, construction, and operation of tailings dams is a major consideration for most new mining projects, as well as for many existing operations (Vick, 1984).
It is economically advantageous to site the impoundment close to the mine, but this imposes limits on site selection. The type of tailings embankment is generally determined by the local seismic activity, water clarification requirements, tailings properties and stability, tailings size distribution, foundation and hydrological conditions, and environmental factors (Azizli et al., 1995). The ground underlying the dam must be structurally sound and able to bear the weight of the impoundment. If such a site cannot be found close to the mine, it may be necessary to pump the tailings, at a high slurry density, to a suitable location.
Tailings dams may be built across river valleys, or as curved or multi-sided dam walls on valley sides, this latter design facilitating drainage. On flat, or gently sloping ground, lagoons are built with walls on all sides of the impoundment.
The disposal of tailings adds to the production costs, so it is essential to make disposal as cheap as possible. This requirement led initially to the development of the once commonly used upstream method of tailings dam construction, so named because the centerline of the dam moves upstream into the pond.
In this method, a small starter dam is placed at the extreme downstream point (Figure 16.1) and the dam wall is progressively raised on the upstream side. The tailings are discharged by spigoting off the top of the starter dike and, when the initial pond is nearly filled, the dike is raised and the cycle repeated. Various methods are used to raise the dam; material may be taken from the dried surface of the previously deposited tailings and the cycle repeated, or more commonly, the wall may be built from the coarse fraction of the tailings, separated out by cyclones, or spigots, the fines being directed into the pond (Figure 16.2 and 16.3).

The main advantages of the upstream construction are the low cost and the speed with which the dam can be raised by each successive dike increment.
The method suffers from the disadvantage that the dam wall is built on the top of previously deposited unconsolidated slimes retained behind the wall. There is a limiting height to which this type of dam can be built before failure occurs and the tailings flow out and, because of this, the upstream method of construction is now less commonly used. Several major failures have involved tailings dams constructed with the upstream method (van Zyl, 2014).
The downstream method has evolved as a result of efforts to devise methods for constructing larger and safer tailings dams. This method produces safer dams both in terms of static and seismic loading (Azizli et al., 1995). It is essentially the reverse of the upstream method, in that as the dam wall is raised, the centerline shifts downstream, and the dam remains founded on coarse tailings (Figure 16.4). Most procedures involve the use of cyclones to produce sand for the dam construction.
Downstream dam building is the only method that permits design and construction of tailings dams to acceptable engineering standards. All tailings dams in seismic areas, and all major dams, regardless of their location, should be constructed using some form of the downstream method. The major disadvantage of the technique is the large amount of sand required to raise the dam wall. It may not be possible, especially in the early stages of operation, to produce sufficient sand volumes to maintain the crest of the tailings dam above the rising pond levels. In such cases, either a higher starter dam is required or the sand supply must be augmented with borrowed fill, such procedures increasing the cost of tailings disposal.
The centerline method (Figure 16.5) is a variation of that used to construct the downstream dam and the crest remains in the same horizontal position as the dam wall is raised. It has the advantage of requiring smaller volumes of sand-fill to raise the crest to any given height. The dam can thus be raised more quickly and there is less trouble keeping it ahead of the tailings pond during the early stages of construction. Care, however, must be exercised in raising the upstream face of the dam to ensure that unstable slopes do not develop temporarily.
Very stable tailings dams can be constructed from open-pit over-burden, or waste rock, according to the local circumstances. An example is shown in Figure 16.6. Waste rock is considered an interesting construction material when available in sufficient quantities because of its ideal mechanical and geotechnical properties, such as well-graded particle size distribution, high internal friction angle, and high pore-water dissipation capacity (Julien and Kissiova, 2011). Since the tailings are not required for the dam construction, they may be fed into the pool without separation of the sands from the slimes. In some cases, the output of overburden may not be sufficient to keep the dam crest above the tailings pond, and it may be necessary to combine waste rock and tailings sand-fills to produce a safe economical dam.
Erosion of dams due to wind and rain can affect the stability and produce environmental problems. Many methods are used to combat this, such as vegetation of the dam banks (Hill and Nothard, 1973) and chemical stabilization to form an air- and water-resistant crust.
There is little doubt that tailings dams have visual impact. Perhaps the most conspicuous is the downstream type, whose outer wall is continually being extended, and cannot be re-vegetated until closure. There are, however, few reasons why dam walls should not be landscaped at some stage in their life, and many dams have been designed to permit early visual integration with the environment (Down and Stocks, 1977a). An example is the impoundment at Flambeau, North Wisconsin, USA (Shilling and May, 1977), where a rock-fill dam wall 18 m high, 24 m wide at the crest, and 111 m wide at the base was designed to minimize both visual and pollution effects (Figure 16.7). The wall consists of a clay core, with the downstream side faced with non-pyrite rock and covered with top-soil, permitting re-vegetation, and consequently, reduced visual impact.
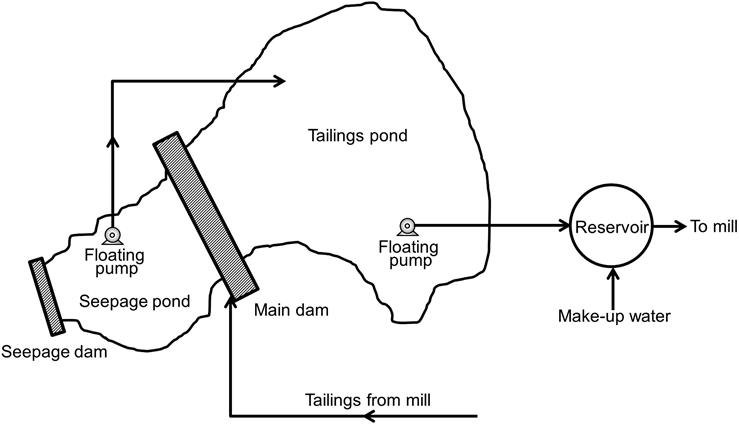
Figure 16.8 shows a generalized representation of water gain and loss at a tailings impoundment (Down and Stocks, 1977b). With the exception of precipitation and evaporation, the rates and volumes of the water can be controlled to a large extent. It is more satisfactory to attempt to prevent the contamination of natural waters rather than to treat them afterwards, and if surface run-off to the dam is substantial, then interception ditches should be installed. It is difficult to quantify the amount of water lost to groundwater, but this can be minimized by selecting a site with impervious foundations, or by sealing with an artificial layer of clay. Seepage through the dam wall is often minimized by an impervious slimes layer on the upstream face of the dam, but this is expensive, and many mines prefer to encourage free-drainage of the dam through pervious, chemically barren material. In the case of upstream dams, this can be a barren starter dike, while with downstream and centerline constructions, a free-draining gravel blanket can be used. A small seepage pond with impervious walls and floors situated below the main dam can collect this water, from where it can be pumped back into the tailings pond. If the dam wall is composed of metal-bearing rock, or sulfide tailings, the seepage is often highly contaminated due to its contact with the solid tailings, and may have to be treated separately.
The tailings are often treated with lime to neutralize acids and precipitate heavy metals as insoluble hydroxides before pumping to the dam. Such treated tailings may be thickened, and the overflow, free of heavy metals, returned to the mill (Figure 16.9), thus reducing the water and pollutant input to the tailings dam.
Assuming good control of the above inputs and outputs of dam water, the most important factor in achieving pollution control is the method used to remove surplus water from the dam. Decant facilities are required on all dams to allow excess free water to be removed. Inadequate decant design has caused major dam failures. Many older dams used decant towers with discharge lines running through the base of the dam to a downstream pump-house. Failures of such structures were common due to the high pressures exerted on the pipelines, leading to uncontrolled losses of fluids and tailings downstream. Floating, or movable, pump-houses situated in the tailings pond are now in common use.
Recycling of decant water is becoming more important. As much water as possible must be reclaimed from the tailings pond for reuse in the mill and the volume of fresh make-up water used must be kept to a minimum. The difference between the total volume of water entering the tailings pond and the volume of water reclaimed plus evaporation losses must be stored with the tailings in the dam. If that difference exceeds the volume of the voids in the stored tailings, there becomes a surplus of free water that can build up to tremendous quantities over the life of a mine. A typical dam-reclaim system is shown in Figure 16.10.
The main disadvantage of water reclamation is the recirculation of contaminants to the mill, which can interfere with processes such as flotation. Water treatment may overcome this, at little or no extra cost, as similar treatment would be required for the effluent discharge in any case.
16.2.2 Backfill
It is common practice in underground mines, in which the method of working requires the filling of mined-out areas, to return the mill tailings underground. This method has been used since the beginning of the 20th century in South Africa’s gold mines (Stradling, 1988), and is now used in many modern underground mines. Backfilling involves the return of tailings or waste rock mixed with water and with or without hydraulic binders into empty stopes. After a curing period of a few months, the backfill can act as effective ground support and allow recovering ore from an adjacent stope, which otherwise would have been left as ground support. Backfilling worked-out stopes also reduces the volume of tailings that must be impounded on the surface; typically 40–60% of all tailings produced by the mine can be returned underground as backfill, therefore the footprint of the required tailings surface impoundment is significantly reduced.
Three main types of backfill are in use worldwide: hydraulic fill, rockfill, and cemented paste backfill. Hydraulic fill is produced with de-slimed tailings and implies the use of binders to provide mechanical strength to the backfill mass once cured. Hydraulic fill is transported underground by gravity or pumping as a 60–70% (by weight) solids slurry. Significant amounts of water and binder are recovered after deposition in the stope and must be pumped to the surface. Rockfill is produced with waste rock distributed underground by truck or conveyor belts. In some cases, a binder slurry can be added to improve mechanical strength.
Cemented paste backfill has been applied since the 1990s and has rapidly gained popularity because of its advantages over hydraulic backfill, notably: use of the total tailings (no de-sliming required), improved mechanical strength, and possibility to return underground sulfidic tailings prone to acid rock drainage (ARD) (Belem and Benzaazoua, 2008). Cemented paste backfill consists of a mixture of tailings, water, and binder (e.g., Portland cement) that is usually pumped into underground stopes as a 70–80% solids paste, as shown in Figure 16.11. The choice and dosage of binder depends on the composition of tailings and water, and on the required mechanical strength, and ultimately on economics. Generally, the amount of binder used is between 3% and 7% of the dry tailings mass. Binder is the major expense in a paste backfill operation, therefore there is incentive to substitute part of the cement content with by-products from other industries with adequate properties, such as fly ash and blast-furnace slag (Peyronnard and Benzaazoua, 2011). Paste backfill recipes must be optimized for each site through laboratory testing and regular follow-up, since the tailings and water properties have an influence on strength acquisition.
16.2.3 Densified Tailings
Advances in the disposal of tailings using semidry or dry techniques offer a number of advantages over the wet disposal techniques. Collectively, these techniques are known as “densified tailings,” and require that tailings be thickened or dewatered prior to disposal. The densified tailings techniques comprise dry stacking (or filtered tailings), thickened tailings, and paste tailings. These are all schemes that improve water and reagent recovery and decrease tailings volumes and footprint, which greatly assists site rehabilitation. Although disposal of densified tailings has benefits, these techniques require a detailed understanding of the rheology and transport of the densified tailings (Nguyen and Boger, 1998).
The thickened tailings technique involves the use of thickeners to bring the tailings to a solid content between 50% and 70%, to make the tailings mass more homogeneous and self-supporting, while reducing the size of the dams (Robinsky, 1999). Furthermore, thickened tailings disposal areas do not require settling ponds because of the improved water recovery at the thickening stage. This method of disposal was used at the Ecstall (Kidd Creek) operation at Texasgulf Canada Ltd. (Amsden, 1974). The tailings disposal area consists of 3,000 acres enclosed by a gravel dike. Mill tailings are thickened and pumped to a central spigoting location inside the dam. The system is designed to build a mountain of tailings in the central area and thus keep the height of the perimeter dike to a minimum.
Paste tailings are produced by thickening and filtering the tailings slurry up to 70–85% solids. The benefits of a high solids content are the improved hydrogeotechnical properties of the tailings (Bussière, 2007). Binders may be added to paste tailings, but in lower proportion compared to underground paste backfill, and may provide stabilization of contaminants (such as arsenic) in the cemented tailings matrix (Benzaazoua et al., 2004). This method of disposal is in use at the Bulyanhulu Mine (Tanzania). The paste tailings have a solids content of approximately 73% and are transported by pipeline and deposited by a series of deposition towers placed at the center of each cell forming the tailings storage facility. The use of paste tailings allows for better water recovery, reduced tailings storage facility footprint, minimization of contaminant transport, lower wind erosion, and possible progressive site rehabilitation (Theriault et al., 2003).
Dry stacking refers to the disposal of tailings having a solid content above 85%, usually obtained by thickening and filtering. At such low moisture content, the tailings have to be transported to the tailings storage facility by truck or conveyor belt. This method is particularly useful in arid climates, or when water handling issues are significant (Davies and Rice, 2001). For example, the Raglan Mine in Quebec is located in an arctic environment and uses dry stacking to recycle most of its process water and transports the tailings by truck to the impoundment area, where they are integrated into the permafrost (Bussière, 2007).
Densified tailings is expected to become more common in operating mines, particularly those with low ore grade and high throughput, which produce large volumes of tailings and require large impoundment areas. For those high tonnage operations, a reduction in tailings volume has a significant impact on dam size and the area of disturbed land.
16.2.4 In-pit Disposal
The disposal of tailings in mined-out open pits can be an alternative to constructing a new tailings storage facility, provided that the open pit and the tailings have the appropriate characteristics. The main risk associated with in-pit disposal is the contamination of the groundwater network by leachates from the tailings. This risk is minimized for open pits that act as water sinks, that is, groundwater flows toward the pit, and/or when the bedrock is mostly impervious (not fractured). Hydrogeological studies of the pit area must be undertaken to confirm the suitability of the pit to safely contain the tailings while maintaining the quality of the groundwater (Eary, 2011).
16.2.5 Submarine Disposal
For operations that are close to the sea, submarine tailings disposal is an alternative to conventional tailings disposal, provided the governmental regulations permit disposal in such a manner. The basic submarine tailings disposal design comprises a tailings line to a de-aeration/mixing chamber, with a seawater intake line, and discharge to location and depth allowing gravity flow of a coherent density to the final sedimentation area. Such systems can place mine tailings at locations and depths constraining environmental impact to restricted areas of the seabed and deep water turbidity (Ellis et al., 1995). This form of tailings disposal attracts considerable attention from environmental groups, as the final disposal of the tailings is not in a controlled impoundment, but is released directly into the lower levels of the ocean and can therefore affect the deep sea ecosystem. The process is increasingly used in the Asia-Pacific region where on-land disposal options are problematic. In comparison to tailing retention on land, the mining industry has argued that submarine tailings disposal in the Asia-Pacific region is safer for the local people and the environment as the land is unsuited to the construction of tailings dams due to the natural topography, regular seismic activity, and high rainfall (McKinnon, 2002). Due to the complexity of the decision-making process for the viability of submarine tailings disposal, tools such as an expert system have been developed to assist mining project planners explore the feasibility of this method of tailings disposal (Ganguli et al., 2002).
16.2.6 Reprocessing and Reuse of Tailings
The most satisfactory way of dealing with tailings is to make positive use of them, such as reprocessing in order to recover additional values, or to use them as a useful product in their own right, for example, the use of coarse (20–30 mm) DMS float as railway ballast and aggregate. Tailings can be used as a component of mortar for concrete or as fined-grained construction material. Depending on the jurisdiction, the reuse of tailings may be encouraged either on the mine site as construction material or for other usage outside the mine site. The incentive to strive for sustainable development should lead to new alternatives for tailings reuse; phytomining where plants, natural or genetically modified, are used to uptake metals is one emerging possibility (Moskvitch, 2014).
Reprocessing the tailings may in some cases become economically attractive, especially for older tailings produced when the processing methods were not as efficient as they are today. With the global drop in ore grades, yesterday’s wastes may become tomorrow’s resources. Mining and comminution costs are also significantly lower when reprocessing tailings than with typical ore (Muir et al., 2005).
16.3 Environmental Issues
Apart from geotechnical stability issues, the most serious problem associated with the disposal of tailings is the release of polluted water, and this has been extensively investigated. The main effects of pollution are due to the effluent pH, which may cause ecological changes; dissolved heavy metals, such as copper, lead, zinc, etc., which can be lethal to fish-life if allowed to enter local water-courses; mill reagents, which are usually present in only very small quantities, but, nevertheless, may be harmful; and suspended solids, which should be minimal if the tailings have sufficient residence time in the dam, thus allowing the solids to settle and produce a clear decant.
16.3.1 Cyanide and Ammonia Management
Complexes of metals with cyanide and ammonia are especially prone to stabilization and solubilization in alkali solutions and may require special treatment other than straightforward neutralization by lime. Although natural degradation occurs to some extent, this is of little value in many cases during the winter months in some locales, when the tailings ponds may be ice-covered. Several processes have been developed to treat cyanide-bearing effluent, these occasionally being derived from cyanide used as a flotation reagent (see Section 12.17.3) but especially derived from cyanide use in gold extraction (Scott and Ingles, 1981). Alkaline chlorination, whereby cyanide is oxidized to cyanate, is one route (Eccles, 1977), but cyanides can also be effectively destroyed by oxidation with ozone (Jeffries and Tczap, 1978) or hydrogen peroxide, and by reactions with sulfur dioxide and air (Lewis, 1984), processes that are used industrially. Other methods include electrochemical treatment, ion exchange, and volatilization of hydrogen cyanide. In the latter method, which has been proven at full scale, the tailings are acidified to produce hydrogen cyanide which is volatilized by intensive air-sparging, while simultaneously recovering the evolved gas in a lime solution for recycling. The aerated, acidified barren solution is then re-neutralized to precipitate the metal ions.
Cyanide destruction may also cause other issues, such as production of thiocyanates and ammonia during SO2-air treatment (Mudder et al., 2001; Gould et al., 2012), so it is imperative to test and identify potential problems before selecting a treatment option.
Ammonium ions enter the systems from the use of the blasting agent ammonium nitrate fuel oil (ANFO), from amines used as flotation reagents, and from certain hydrometallurgical operations. There is the possibility of biological control, using bacteria (nitrifiers) that gain their energy for growth from oxidation of ammonia (Kapoor et al., 2003).
16.3.2 Acid Rock Drainage
Acid rock drainage (ARD) (or acid mine drainage, AMD) is considered the most significant environmental issue related to mine waste management. It is produced through the natural oxidation of sulfidic minerals by air and water, accelerated by bacterial action (thiobacillus); thus exposed sulfide-bearing tailings (and waste rock) are prone to ARD generation. Pyrite and pyrrhotite are the main ARD generating sulfide minerals and are found in many deposits associated with base metals, gold and coal. The resulting acid leaches other heavy and toxic metals into the ARD (Ritcey, 1989). (Under other conditions, primarily moisture levels 3–8%, the same oxidation reactions can cause sulfide self-heating, a topic introduced in Chapter 2.)
The mineralogical nature of the tailings can provide some natural pollution control. For instance, the presence of alkaline gangue minerals such as limestone can render metals less soluble and neutralize oxidation products. Such ores thus present fewer problems than sulfide ores associated with neutral-acid gangues. Several testing methods have been developed to identify potentially acid-generating tailings, either based on the balance between acid-producing and neutralizing minerals (Sobek et al., 1978; Miller et al., 1991; Lawrence and Scheske, 1997) or on the reaction rates of acid generation and neutralization processes (Bussière et al., 1997; Morin and Hutt, 1997; Lapakko and White, 2000).
Once ARD generation has begun, it is difficult to control and stop. Therefore, several jurisdictions impose regulations on ARD prevention, usually by obligating mine operators to reclaim their tailings ponds at the end of mine-life, or preferably, during operation. To prevent ARD, the main approaches involve the removal of one of the components for sulfide oxidation, either air (oxygen), water, or the sulfide minerals themselves. The selection of the ARD prevention approach is based on tailings characterization, climatic conditions at the site, social and economic considerations, and desired performance. In humid climates, covers to limit oxygen transport are generally favored. Water covers, where tailings are deposited under a water layer in an impoundment built with impervious dikes, are efficient at limiting oxygen transport because oxygen solubility and diffusion in water are several orders of magnitude lower than in air (Davé et al., 1997). However, the long-term geotechnical stability risk of maintaining impervious dikes led to the development of alternative dry cover systems that induce lower pressures on the confining structures. Dry covers of various types can offer similar performance as water covers to limit oxygen transport when at least one cover layer maintains a high water content (Nicholson et al., 1989). Different types of materials can be used as dry covers: natural geomaterials (clay, silt, sand), industrial materials (neutral tailings, neutral waste rock, sludge), geomembranes and geocomposites (Aubertin et al., 1997; Yanful et al., 1999; Demers et al., 2008). Dry cover systems consist of single to multilayered covers; the selection of the appropriate cover system for a given site depends on material availability, site characteristics and required performance. In arid climates, water exclusion, by covering with different soils layers or with impervious geomembranes, is usually the most appropriate option (Williams et al., 1997; Zhan et al., 2001). In permafrost conditions, tailings may be integrated into the frozen soil, as the cold temperatures significantly reduce sulfide mineral oxidation rates (Elberling, 2001, 2005). However, global climate changes may affect the permafrost conditions and more robust ARD strategies must be developed for arctic tailings disposal and reclamation.
A novel approach to preventing ARD is the argument that since oxidative processes cause ARD, then reductive processes will counteract the oxidation. There are examples where reductive reactions have occurred naturally and eliminated acid drainage. They appear to be related to the formation of biofilms hosting oxygen-consuming bacteria (acidophilic heterotrophs), which under the right conditions can outcompete the bacteria promoting oxidation (Kalin et al., 2012). This is an example of the application ecological engineering principles to solving the ARD problem (Kalin, 2005).
When ARD has begun, chemical treatment of acid effluents is essential, neutralization by lime usually being performed, which precipitates the heavy metals, and promotes flocculation as well as reducing acidity. Furthermore, a number of wastewater treatment techniques are available, such as physical adsorption methods using active carbon, bentonite clay or mineral slimes, biological oxidation of organics, biological sulfate reduction for metal precipitation as sulfides, removal of ionic species by ion exchange resins, and reverse osmosis (Rao and Finch, 1989; Younger et al., 2002; Lawrence et al., 2003). Passive treatment methods, such as wetlands and limestone drains, are proposed as alternatives to chemical treatment and are considered a more suitable solution for long-term treatment of ARD (Bernier et al., 2002; Neculita et al., 2007).
Rather than just treat, there may be options for recovery of heavy metals (and other resources) (Rao, 2006). For instance, it has been established that various microorganisms that may be recovered by flotation are able to abstract heavy metal ions from aqueous solutions, thus making it possible not only to solve some industrial environmental problems, but also to recover a currently wasted product (Veneu et al., 2014).
As a specific case, a major concern is control of selenium in discharge waters, especially from the mining of coal (Harrison et al., 2013). Several techniques are employed: ion exchange, zero-valent iron (ZVI), and active biological treatment. Ion exchange removes selenium by exchanging selenate for ions on a resin backbone. ZVI refers to using elemental iron to reduce the oxidized forms of selenium (selenite, selenate) to elemental selenium. The ZVI can be in powder, granular, or fiber forms. The elemental selenium is insoluble and exists as nanoparticles embedded in the iron and is recovered. In active biological treatment, certain bacteria gain their energy from reduction of selenites and selenates to selenium. One engineered system is to use a fluidized bed reactor (FBR). Solid-liquid separation is required to recover the biomass with the selenium. Two full-scale FBR-based treatment plants are scheduled for operation in 2014.
It is evident that there is much research potential in these areas. Particular attention is being given to the modification of mineral processing operations to mitigate environmental impact. The ultimate way of avoiding water-based environmental impact is to operate dry mineral processes, and consideration is being given to such options, particularly in arid areas.