Hydrogen
Abstract
Many people have proposed hydrogen as a clean energy source because, when hydrogen is burned, its only byproduct is water. Rifkin has even gone as far as to say that we have reached the end of the hydrocarbon age and that hydrogen is the energy source of the future. However, this claim belies the fact that there are no indigenous hydrogen sources in the world. So, hydrogen has to be generated from other energy sources before it can be made available for use. The major methods for generating hydrogen rely on natural gas and coal as source materials. Consequently, hydrogen is not really an energy source but a method of energy storage. It also suffers from serious deficiencies in this regard. It is difficult to store and transport, and the technologies for doing this have not been developed. Moreover, once created, it has an energy density that is only one-sixth that of gasoline. As a result, it is not likely to play a serious role in the energy economy in the foreseeable future.
Many people have proposed hydrogen as a clean energy source because its only by-product is water when it is burned or reacted with oxygen. Rifkin (author of “The Hydrogen Economy”) has even gone as far as to say that the end of the hydrocarbon age has been reached and that hydrogen is the energy source of the future, the “forever fuel.” This claim, however, contradicts the fact that there are no indigenous hydrogen sources in the world. Hydrogen has to be generated from other energy sources before it can be made available for use. The major methods of generating hydrogen are from natural gas and coal. Consequently, hydrogen is not really an energy source but a method of energy storage. It also suffers from serious deficiencies in this regard. It is difficult to store and transport and the technologies for doing this have not yet been developed. Moreover, even when compressed to 10,000 psia, it has an energy density that is only 15% of that of gasoline. For any given fuel tank volume, a hydrogen-fueled vehicle will only travel 15% of the miles that a gasoline-fueled vehicle will travel with the same size fuel tank.
Hydrogen Technologies
Hydrogen can be burned in internal combustion engines (ICE) or converted to electricity in fuel cells. A fuel cell is a device that uses the chemical reaction between hydrogen and oxygen to produce electricity. The fuel cell has two electrodes sandwiched around an electrolyte. Hydrogen passes over one electrode and oxygen over the other, generating electricity, water and heat. Hydrogen is fed into the negative electrode, or “anode,” of the fuel cell. Oxygen (or air) enters the fuel cell through the positive electrode, or “cathode.” Under the action of a catalyst, the hydrogen atom splits into a proton and an electron, which take different paths to the cathode. The proton passes through the electrolyte. The electrons create a separate current that can be utilized before they return to the cathode to be reunited with the hydrogen and oxygen in a molecule of water. The electricity can be used directly to power an electric vehicle or to supply electric current to any other users such as houses or factories. There are several different fuel cell technologies, but the polymer electrolyte membrane (PEM) fuel cell, also called a proton exchange membrane fuel cell, is the technology proposed for use in automobiles. Figure 15.1 shows how a PEM fuel cell works. These cells have been widely used to provide electrical power in the space program.
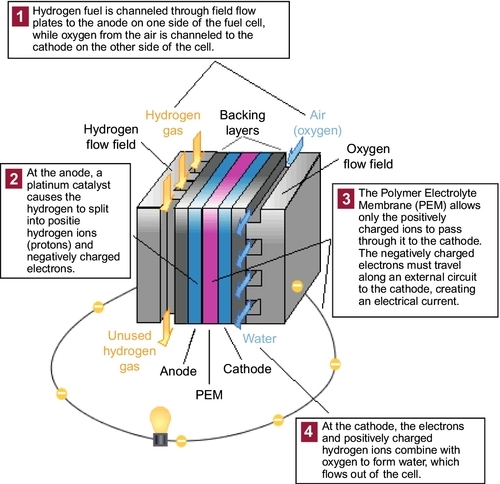
The principle of the fuel cell was first discovered by a German chemistry professor, Christian Schönbein, in 1838. Based on his work, the first fuel cell was demonstrated by Welshman William Grove in February 1839. The fuel cell he made used similar materials to today's phosphoric-acid fuel cell. It wasn’t until 1955 that Thomas Grubb, a chemist working for General Electric Company (GE), further modified the original fuel cell design by using a polystyrene ion-exchange membrane as the electrolyte. Three years later another GE chemist, Leonard Niedrach, devised a way of depositing platinum onto the membrane. This served as the catalyst for the hydrogen oxygen reaction and became known as the “Grubb-Niedrach” fuel cell. GE went on to develop this technology with NASA and McDonnell-Douglas, leading to its use in the first space program entitled “Project Gemini.” This was the first commercial use of a fuel cell.
In 1959, British engineer Francis Bacon developed a 5-kW stationary fuel cell and in the same year Harry Ihrig built a 15-kW fuel cell tractor for the Allis-Chalmers Company, which was widely demonstrated in the United States. This system used potassium hydroxide as the electrolyte and compressed hydrogen and oxygen as the reactants. Meanwhile, Bacon and his colleagues demonstrated that their five-kilowatt unit was capable of powering a welding machine.
In the 1960s, Pratt and Whitney adopted Bacon's technology for use in the U.S. space program to supply electricity and drinking water to the space modules. In 1991, the first hydrogen fuel cell automobile was developed by Roger Billings. He also developed the first hydrogen powered internal combustion engine. He did not attempt to commercialize them because he was more focused on his computer storage technology businesses, but to this day he retains a strong interest in hydrogen technologies.
United Technologies Corporation’s power subsidiary (UTC Power) was the first company to manufacture and commercialize a large, stationary fuel cell system for use as a co-generation power plant in hospitals, universities and large office buildings. UTC Power continues to market this fuel cell as the PureCell 200, a 200-kW system. In 2009, they added the PureCell 400 which was a 400-kW version. UTC Power is now the sole supplier of fuel cells to NASA for use in space vehicles, having supplied fuel cells for the Apollo missions and the Space Shuttle program. They are currently developing fuel cells for automobiles, buses and cell phone towers. Their PEM fuel cell has been shown to be capable of starting under freezing conditions.
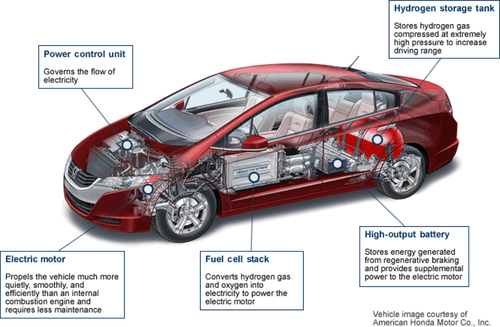
Most fuel cells designed for use in vehicles produce less than 1.16 volts of electricity, clearly not enough to power a vehicle. Multiple cells must be assembled into a fuel cell stack. The potential power generated by this stack depends on the number and size of the individual fuel cells that comprise the stack and the surface area of the PEM. Figure 15.2 shows the Honda fuel cell vehicle with the essential elements (the hydrogen storage tank, the fuel cell stack, the electric motor and the power control unit) cut away.
The Hydrogen Economy
The idea for utilizing hydrogen instead of fossil fuels to power houses and vehicles received a large boost in 2002 from a book by Jeremy Rifkin (2002) called The Hydrogen Economy. The jacket of the book claims that:
Rifkin takes us on an eye-opening journey into the next great commercial era in history. He envisions the dawn of a new economy powered by hydrogen that will fundamentally change the nature of our market, political and social institutions, just as coal and steam power did at the beginning of the industrial age. Rifkin observes that we are fast approaching a critical watershed for the fossil-fuel era, with potentially dire consequences for industrial civilization. While experts had been saying that we had another forty or so years of cheap available crude oil left, some of the world's leading petroleum geologists are now suggesting that global oil production could peak and begin a steep decline much sooner, as early as the end of this decade. Non-OPEC oil producing countries are already nearing their peak production, leaving most of the remaining reserves in the politically unstable Middle East. Increasing tensions between Islam and the West are likely to further threaten our access to affordable oil. In desperation, the U.S. and other nations could turn to dirtier fossil-fuels—coal, tar sand, and heavy oil—which will only worsen global warming and imperil the earth's already beleaguered ecosystems. Looming oil shortages make industrial life vulnerable to massive disruptions and possibly even collapse.
While the fossil-fuel era is entering its sunset years, a new energy regime is being born that has the potential to remake civilization along radical new lines, according to Rifkin. Hydrogen is the most basic and ubiquitous element in the universe. It is the stuff of the stars and sun and, when properly harnessed, it is the “forever fuel.” It never runs out and produces no harmful CO2 emissions. Commercial fuel-cells powered by hydrogen are just now being introduced into the market for home, office and industrial use. The major automakers have spent more than two billion dollars developing hydrogen cars, buses, and trucks, and the first mass-produced vehicles are expected to be on the road in just a few years.
The hydrogen economy makes possible a vast redistribution of power, with far-reaching consequences for society. Today's centralized, top-down flow of energy, controlled by global oil companies and utilities, will become obsolete. In the new era, says Rifkin, every human being could become the producer as well as the consumer of his or her own energy—a so called “distributed generation.” When millions of end-users connect their fuel-cells into local, regional, and national Hydrogen Energy Webs (HEWs), using the same design principles and smart technologies that made possible the World Wide Web, they can begin to share energy—peer-to-peer—creating a new decentralized form of energy use.
Hydrogen has the potential to end the world's reliance on imported oil and help diffuse the dangerous geopolitical game being played out between Muslim militants and Western nations. It will dramatically cut down on carbon dioxide emissions and mitigate the effects of global warming. Because hydrogen is so plentiful and exists everywhere on earth, every human being could be “empowered,” making it the first truly democratic energy regime in history.”
—Jeremy Rifkin (2002), The Hydrogen Economy.
These are heady claims, but do they stand up to any scientific scrutiny? While the potential of hydrogen will be examined in this chapter, one answer to that question is contained in a review of Rifkin's book by David Goodstein (Department of Physics, California Institute of Technology) that appeared in the March-April 2003 issue of American Scientist.
“The age of oil is about to end, which will come as a surprise to most people. Depletion of the rest of the fossil fuels may not be far behind. And if we do go on merrily burning up the planet's legacy, the result may be irreversible damage to our climate. This crucially important idea is the starting point of The Hydrogen Economy, a new book by Jeremy Rifkin, a former peace advocate who now crusades against biotechnology and various other perceived ills.
Rifkin believes that oil will be replaced by hydrogen fuel cells. Unlike the oil economy, which requires a top-down, capitalist-corporate order, the hydrogen economy will be something like the Internet, he says, with users who are also providers, generating their own hydrogen and sharing any surplus on the Hydrogen Energy Web. After all, unlike oil, hydrogen is everywhere: It's the most common element in the universe, the “forever fuel” that we can never run out of. The revolution brought about by the hydrogen economy will lead to a democratization of society and give a whole new meaning to the word globalization.
But wait a minute. Doesn't Rifkin understand that it takes energy to generate hydrogen from water, or from any other source? Well, yes, he even says so in a couple of places, but he seems to have trouble holding that thought. And when he does come to grips with it, he believes all the energy will come from “renewable resources—photovoltaic, wind, hydroelectric, geothermal, and biomass.” There will be no nuclear reactors in a world designed by Jeremy Rifkin. At present, all the renewables on Rifkin's list aside from hydroelectricity collectively generate less than 1 percent of our energy needs.
Is Rifkin's proposed solution physically possible? Well, yes, sort of, but it's extremely implausible that all the power generated today by fossil fuels, about 10 terawatts worldwide, could ever be replaced from those sources. Biomass is a terribly inefficient use of sunlight. There are only a few places on Earth where enough geothermal energy to generate electricity is within drilling distance of the surface. Hydroelectric capacity is already saturated, and wind is an intermittent, low-density (and often ugly) source of power. According to an article by Martin I. Hoffert and colleagues in the November 1, 2002, issue of Science (298:981-987), to replace the 10 terawatts with photovoltaics would require an array covering more than 200,000 square kilometers, whereas all the photovoltaic cells shipped between 1982 and 1998 would cover only 3 square kilometers.
Our best hope in the short run is that somebody will start building nuclear power plants in a hurry, before the oil starts to run out. In the longer run, when the fossil fuels are gone or sequestered and the uranium starts to run low, if we haven't yet brought thermonuclear energy under control, heroic measures like huge arrays of photovoltaics on Earth, or somewhat smaller ones in space (where the solar flux is about 8 times the average at the Earth's surface), may be in order. That is not to say we should not do our best to develop renewable energy sources. We certainly should. But they will not replace fossil fuels anytime soon.
Rifkin is certainly right to say that we will soon start running out of oil, that continued burning of fossil fuels is a grave threat to the Earth's climate, and that hydrogen, either in fuel cells or by combustion, is the best bet for the future of transportation. He has correctly identified the biggest problem we have. But this book is not part of the solution.”
—David Goodstein, Dept of Physics, California Institute of Technology.
Rifkin’s book certainly had a broad impact. In his State of the Union Address on January 28, 2003, President Bush embraced hydrogen as the fuel of the future, announcing the Freedom Car and Freedom Fuel Initiative. Then an article entitled How Hydrogen Can Save America appeared in the April 2004 issue of Wired magazine. The authors proposed a program equal in scope to the Apollo program that put men on the moon claiming:
“The cost of oil dependence has never been so clear. What had long been largely an environmental issue has suddenly become a deadly serious strategic concern. Oil is an indulgence we can no longer afford, not just because it will run out or turn the planet into a sauna, but because it inexorably leads to global conflict. Enough. What we need is a massive, Apollo-scale effort to unlock the potential of hydrogen, a virtually unlimited source of power. The technology is at a tipping point. Terrorism provides political urgency. Consumers are ready for an alternative. From Detroit to Dallas, even the oil establishment is primed for change. We put a man on the moon in a decade; we can achieve energy independence just as fast.”
—Wired magazine, April 2004
President Bush’s Hydrogen Initiative of 2003 provoked a response from the public radio show Car Talk with Tom and Ray Magliozzi, who are also syndicated nationally on many commercial radio stations. Here was their take on the Bush Administration’s program on hydrogen vehicles:
Dear Tom and Ray:
President Bush talked about a “hydrogen car” in his State of the Union address. Is this a realistic possibility during the Bush administration?
Ray: Maybe during the Jenna Bush administration, Jim. The technology itself works, but people “in the know” say it's going to be at least 20 years before hydrogen-powered cars are viable on a large scale—if then.
Tom: The main problems are: (1) the fuel cell “stacks” are still incredibly expensive to build, (2) the range of the cars is insufficient and (3) there's no national infrastructure (like gas stations) to support hydrogen. So it's not going to happen anytime soon.
Ray: So, why is the president talking about hydrogen-powered cars? Well, in my humble opinion, he's creating a distraction.
Tom: I think so, too. You probably know that we now import boatloads of foreign oil every day. And almost everybody agrees that this is not a good thing (except for the countries that sell us the oil). So what do you do about it?
Ray: Well, you can try to find more oil here at home, by drilling in Alaska's forests, for instance. Or you can force people to use less oil. The president knows that both of these options are pretty unpopular. So he's doing what any good politician would do: He's changing the subject.
Tom: Here's another reason why he might want to distract us from thoughts of fuel economy and foreign oil. With no pressure on American car companies to increase gas mileage, the Japanese have taken a significant lead in the most important new propulsion technology in decades: hybrid engines. Hybrid engines use battery power some of the time and gasoline power at other times, and they never have to be plugged in. They're a great way to increase mileage without sacrificing power or convenience. And you're going to see Americans adopting them in big numbers over the next five to 10 years.
Ray: Who makes the best-selling hybrid cars in America? Honda and Toyota. So, instead of urging America to make more fuel-efficient cars and cut down on foreign oil by raising the Corporate Average Fuel Economy (CAFE) standards, or urging U.S. manufacturers to catch up with the Japanese on hybrids—which would make a huge difference right away—the president's talk about hydrogen cars is, essentially, the old “Hey, everybody, look over there!”
—from Car Talk with Tom and Ray Magliozzi (2003)
Another very useful report written in response to Rifkin’s book is The Future of the Hydrogen Economy: Bright or Bleak? by Bossel et al. (2003). Their response was presented at the 2003 Fuel Cell Seminar, 3-7 November 2003 in Miami Beach, Florida. This report discusses hydrogen sources, hydrogen storage and hydrogen distribution. They state: “It seems that the recent surge of interest in a hydrogen economy is based more on visions than on hard facts. We are not at all against hydrogen, but we would like the discussion about the synthetic energy carrier to return to facts and physics. Hopefully, this study will help to identify chances and limitations of a hydrogen economy.”
Hydrogen has had important applications for a century, but it has never been an energy source or energy carrier for the reasons described by Bossel, Eliasson and Taylor. While hydrogen has important uses, it first has to be manufactured before it can be used. Hydrogen fuel does not exist on or in the Earth at all. In 1905, the Germans developed the Haber process to make ammonia from nitrogen and hydrogen. Ammonia is the starting point for making fertilizers and explosives. Hydrogen is also necessary for the refining of crude oil into high octane-low sulfur gasoline and high cetane-low sulfur diesel fuel. Consequently, one of the largest generators and users of hydrogen are petroleum refineries.
Hydrogen can be produced by several methods. To make hydrogen, you need an energy source and a hydrogen source. Today, the cheapest source of hydrogen is natural gas, which is both the energy source and the hydrogen source. The problem is that the resulting hydrogen has only 70% of the chemical energy of the original natural gas. Hydrogen can also be produced from oil, but the energy efficiency is a little worse. If hydrogen is derived from any fossil fuel, carbon dioxide will be created.
The primary ways in which natural gas, mostly methane (CH4), is converted to hydrogen involve reaction with either steam (steam reforming), oxygen (partial oxidation) or both in sequence (auto-thermal reforming). The overall reactions are:
![]()
![]()
In practice, gas mixtures containing carbon monoxide (CO) as well as carbon dioxide (CO2) and unconverted methane (CH4) are produced and require further processing. The reaction of CO with steam (the water-gas shift reaction) over a catalyst produces additional hydrogen and carbon dioxide (CO2). After separation, high-purity hydrogen (H2) is recovered.
The United States has lots of coal, and it can be used to make hydrogen. Coal is a cheap energy source, but not a good hydrogen source. Water must be used as a supplemental hydrogen source because coal has more energy than it has hydrogen. Syngas, a mixture of carbon monoxide and hydrogen, is made by gasifying the coal by reacting the water (as steam) with hot coal particles (see Chapter 13). This method has been used since the nineteenth century, but the energy efficiency is only 35%. The resulting hydrogen has 35% of the energy of the original coal.
Electricity can also be used to make hydrogen from water through electrolysis, an efficient but expensive process. Electrolysis of water has been known for centuries, and it is a common classroom demonstration. The reason why this method turns out to be expensive is once again due to the second law of thermodynamics. The electrical energy has been generated by burning fuels in a heat engine and converting the rotating energy to electrical energy, with an efficiency that is reduced by the second law of thermodynamics. In electrolysis, the electrical energy is then turned back into a hydrogen fuel.
Fossil fuel is the source of most of the energy used to make electricity in the United States. Hydrogen produced by electrolysis may be viewed as a convoluted way to convert fossil fuel to hydrogen. Fossil fuel (coal, natural gas and oil) can be converted to hydrogen directly without making electricity first. The direct methods use less fossil fuel for the same amount of produced hydrogen. Electricity from wind turbines or solar arrays can also make hydrogen through electrolysis, but this is inefficient and expensive.
Hydrogen is also expensive to store because it has a very low volumetric energy density. It is the simplest and lightest element; it is even lighter than helium. At atmospheric pressure, hydrogen is 2.93 times less energy dense than natural gas and 2933 times less energy dense than gasoline. Hydrogen, however, contains 3.1 times more energy than gasoline on a weight basis. This makes it useful for rocket fuel in the space program. To be useful for any transportation purposes, however, it must be made more energy dense. There are three ways to do this: hydrogen can be compressed, liquefied or chemically combined (Tables 15.1 and 15.2).
Table 15.1
Comparison of Properties of Hydrogen with Other Fuels
| Properties | Units | Hydrogen | Methane | Propane | Methanol | Ethanol | Gasoline |
| Chemical Formula | H2 | CH4 | C3H8 | CH3OH | C2H5OH | CxHy (x = 4-12) | |
| Molecular Weight | 2.02 | 16.04 | 44.1 | 32.04 | 46.07 | 100-105 | |
| Density (NTP) | kg/m3 | 0.0838 | 0.668 | 1.87 | 791 | 789 | 751 |
| lb/ft3 | 0.00523 | 0.0417 | 0.116 | 49.4 | 49.3 | 46.9 | |
| Normal Boiling Point | °C | − 253 | − 162 | − 42.1 | 64.5 | 78.5 | 27-225 |
| °F | − 423 | − 259 | − 43.8 | 148 | 173.3 | 80-437 | |
| Vapor Specific Gravity (NTP) | air = 1 | 0.0696 | 0.555 | 1.55 | N/A | N/A | 3.66 |
| Flash Point | °C | <−253 | − 188 | − 104 | 11 | 13 | − 43 |
| °F | <−423 | − 306 | − 155 | 52 | 55 | − 45 | |
| Flammability Range in Air | vol% | 4.0-75.0 | 5.0-15.0 | 2.1-10.1 | 6.7-36.0 | 4.3-19 | 1.4-7.6 |
| Auto Ignition Temperature in Air | °C | 585 | 540 | 490 | 385 | 423 | 230-480 |
| °F | 1085 | 1003 | 914 | 723 | 793 | 450-900 |
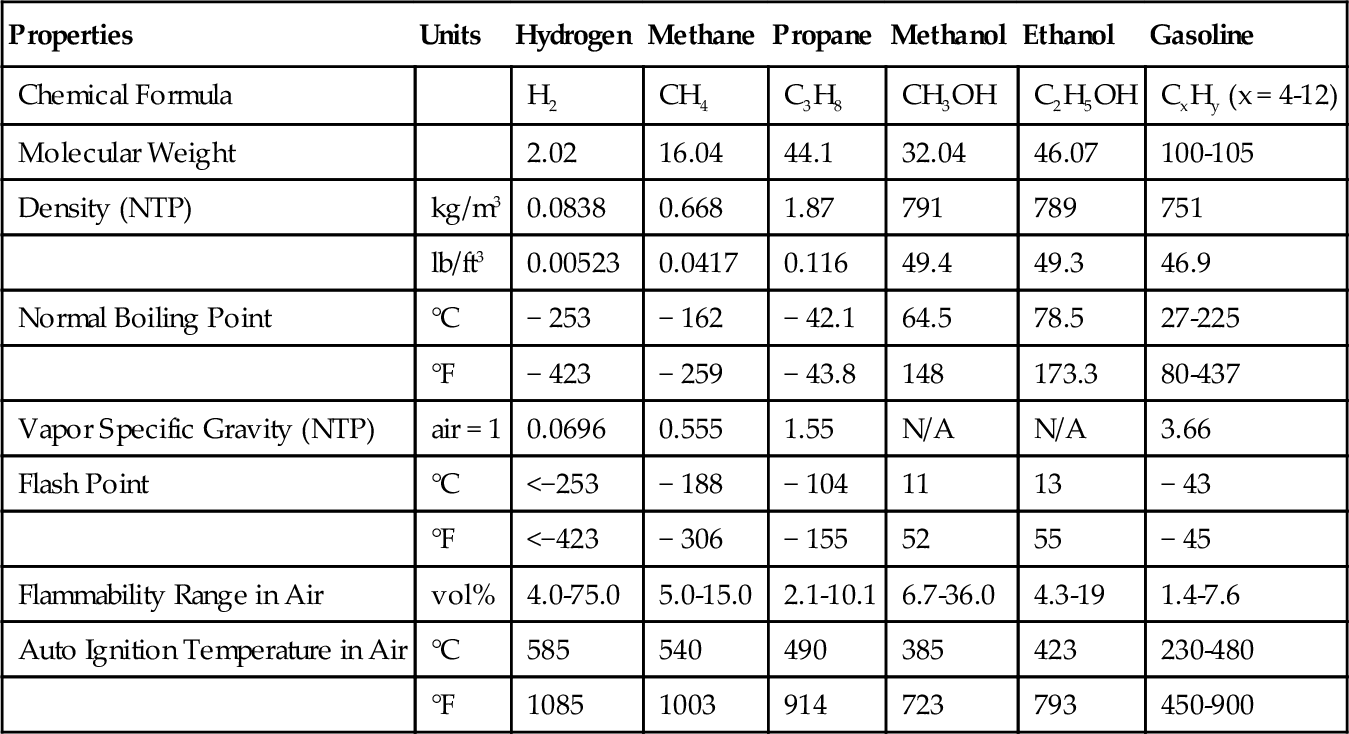
(Data Source: DOE)
Table 15.2
Energy Equivalencies of Fuels
| Hydrogen | Natural Gas | Crude Oil | Conventional Gasoline | Reformulated Gasoline (RFG) | California RFG | U.S. Conventional Diesel | Low-Sulfur Diesel | |
| (kg) | (million cubic feet) | (barrel) | (gallon) | (gallon) | (gallon) | (gallon) | (gallon) | |
| Hydrogen, 1 kg = | 1 | 0.000117 | 0.0211 | 0.992 | 1.014 | 1.011 | 0.896 | 0.889 |
| Natural Gas, 1 MMCF = | 8538 | 1 | 180.5 | 8468 | 8653 | 8628 | 7653 | 7591 |
| Crude Oil, 1 barrel = | 47.30 | 0.00554 | 1 | 46.91 | 47.94 | 47.80 | 42.40 | 42.06 |
| Gasoline, 1 gal = | 1.008 | 0.000118 | 0.0213 | 1 | 1.022 | 1.019 | 0.904 | 0.897 |
| RFG, 1 gal = | 0.987 | 0.000116 | 0.0209 | 0.979 | 1 | 0.997 | 0.884 | 0.877 |
| Calif RFG, 1 gal = | 0.989 | 0.000116 | 0.0209 | 0.981 | 1.003 | 1 | 0.887 | 0.880 |
| Diesel, 1 gallon = | 1.116 | 0.000131 | 0.0236 | 1.106 | 1.131 | 1.127 | 1 | 0.992 |
| LS Diesel, 1 gal = | 1.125 | 0.000132 | 0.0238 | 1.115 | 1.140 | 1.137 | 1.008 | 1 |
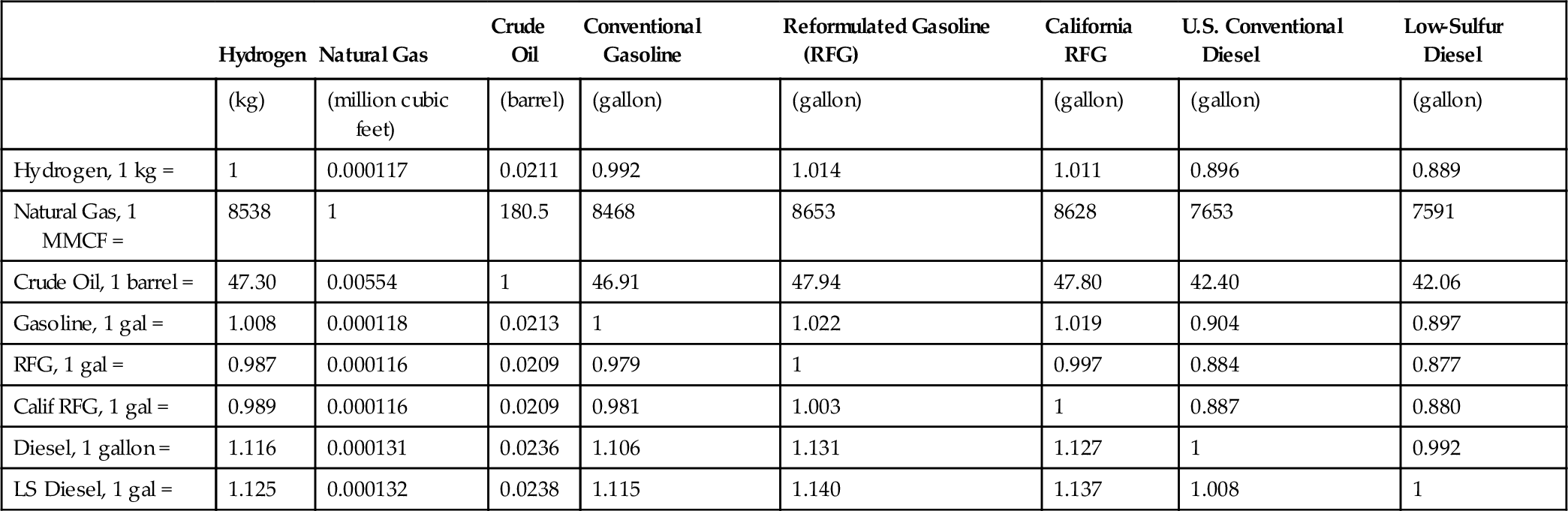
(Data Source: DOE)
Hydrogen compressed to a pressure of 10,000 psia occupies 6.05 times more volume than gasoline for the same energy, as shown in Table 15.3. This table also shows the energy density of liquid hydrogen, hydrogen at 10,000 psia, gasoline and ethanol on a volume basis as well as a mass basis. It is necessary to liquefy hydrogen or compress it to a pressure of 10,000 psia if a vehicle is to carry enough hydrogen fuel to be practical. It is very difficult to contain such high pressures safely in a lightweight tank. Catastrophic tank failures release as much energy as an equal weight of dynamite. A 10,000 psia tank made of high strength steel would weigh 100 times more than the hydrogen it contains. A truck or an automobile using a steel tank would be impractical as the tank would weigh nearly as much as the vehicle. High pressure hydrogen tanks made from carbon fiber have been considered as a solution. Carbon fiber is a material used in aircraft and sporting goods. At the present time, carbon fiber tanks are very expensive, too expensive to be practical. The DOE had proposed a performance goal as part of the Freedom Car initiative. Their goal is 4.5% as the ratio of hydrogen to tank weight at 10,000 psia.
Table 15.3
Energy Density of Hydrogen Compared to Other Fuels
| Fuels | BTU/lb | BTU/gal |
| Hydrogen at 10,000 psia | 61,127 | 20,534 |
| Liquid Hydrogen | 61,127 | 38,243 |
| Gasoline | 20,007 | 124,340 |
| Ethanol | 12,832 | 84,530 |
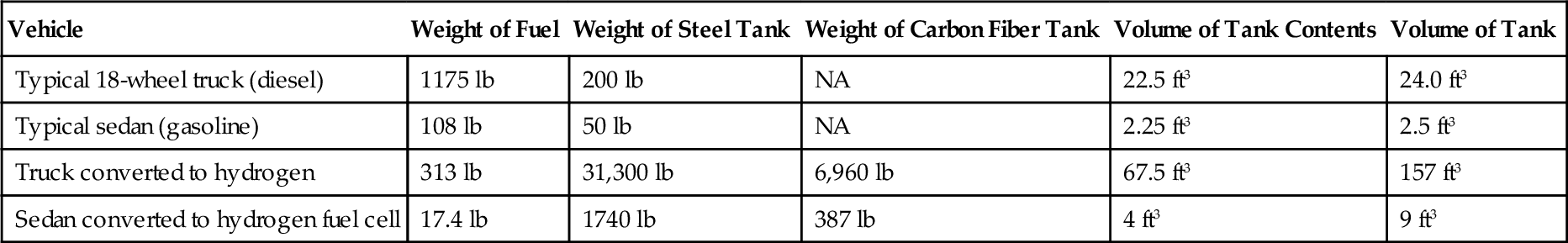
A typical 18-wheeled semitruck carries two 90-gallon tanks, providing a driving range up to 750 miles. A typical 4-cylinder sedan has an 18-gallon tank, providing a driving range up to 575 miles. The diesel engine achieves an efficiency of 35% at cruising speeds. The gasoline engine achieves an efficiency of 30% at cruising speed. Both vehicles could be converted to hydrogen operation. ICE could be used, resulting in an efficiency of 35%. Fuel cells could also be used, resulting in an efficiency of 45%. This information is summarized in Table 15.4. The space, weight and expense of steel tanks make them impractical. Any gains in energy efficiency would be offset by the losses incurred in hauling the very heavy tanks. Carbon fiber tanks of this size and performance do not exist; they are only goals. Gasoline, by contrast, requires only a small, low-tech tank.
Table 15.4
Hydrogen Fuel Tank Comparisons
| Vehicle | Weight of Fuel | Weight of Steel Tank | Weight of Carbon Fiber Tank | Volume of Tank Contents | Volume of Tank |
| Typical 18-wheel truck (diesel) | 1175 lb | 200 lb | NA | 22.5 ft3 | 24.0 ft3 |
| Typical sedan (gasoline) | 108 lb | 50 lb | NA | 2.25 ft3 | 2.5 ft3 |
| Truck converted to hydrogen | 313 lb | 31,300 lb | 6,960 lb | 67.5 ft3 | 157 ft3 |
| Sedan converted to hydrogen fuel cell | 17.4 lb | 1740 lb | 387 lb | 4 ft3 | 9 ft3 |
The laws of thermodynamics govern the amount of energy it takes to compress a gas. The physical properties of hydrogen make it the most difficult of all gases to compress. At 10,000 psia, a perfect, single-stage compressor consumes energy equal to 16% of the chemical energy in the hydrogen (this is the energy that gets instantly released in the event of a tank failure). It might be possible to use a series of multistage compressors with intercoolers to achieve 12%. This is an estimate extrapolated from an actual multistage compressor working at 3,000 psia.
It is technologically challenging to compress hydrogen to 10,000 psia. Higher pressure would not result in much volume reduction. At these pressures, hydrogen acts less like a gas and more like a liquid. The laws of thermodynamics also dictate that energy losses occur when hydrogen is transferred from a storage tank to a vehicle. The design of the transfer lines and the pressure fittings is critical in keeping energy losses low.
Liquid hydrogen also occupies 3.25 times more volume than gasoline for the same energy. Paradoxically, there is more hydrogen in a gallon of gasoline than there is in a gallon of liquid hydrogen. The advantage of hydrogen liquefaction is that a cryogenic hydrogen tank is much lighter. Hydrogen's physical properties mean hydrogen is harder to liquefy than any other gas except helium. There are significant and inevitable energy losses when hydrogen is liquefied.
Liquid hydrogen is colder than any other substance except liquid helium. The advantage of liquid hydrogen is that it can be stored in relatively lightweight tanks. A tank for cryogenic hydrogen is like a thermos bottle, but it must work much better. It consists of a tank within a tank with a vacuum between the two. The inner tank must be supported without conducting heat. This is very difficult to do in a tank designed for a vehicle. Bossel, Eliasson and Taylor estimate that a liquid hydrogen tank designed for automobile use would lose about 5% of its capacity every day through evaporation, in the same manner that LNG evaporates while it is being shipped in tankers. If you are not driving your liquid hydrogen fueled vehicle, its fuel would evaporate in about 20 days. Losses of this magnitude are acceptable for a taxicab fleet, for example, but unacceptable to most people. Hydrogen cannot be vented to the atmosphere because it is an explosion hazard and because it is a greenhouse gas. The vented hydrogen must be burned.
Hydrogen molecules exist in two forms, ortho (the electron spins are antiparallel) and para (the electron spins are parallel). Room temperature hydrogen is a mixture of the two. Liquid hydrogen, however, turns into pure para hydrogen over the course of a few days. The process releases enough heat to turn the liquid hydrogen to gas. Liquid hydrogen can be catalytically converted to all para during the liquefaction process. If this were not done, 30% of the hydrogen would escape in two days even in a perfect cryogenic tank. Conversion to the para form of hydrogen adds to the cost and complexity of liquefaction.
Certain alkali metal hydrides release hydrogen when exposed to water. These metal hydrides hold enough hydrogen to make them useful for transportation. 70% of the energy, however, is lost in the creation of the hydrides, making them unacceptable for widespread use. Certain metals (platinum, zirconium, lanthanum) can be formed into “sponges” for hydrogen. These sponges can only hold 1% of their weight in hydrogen and they are very expensive.
In both cases, the energy storage tank is heavy and expensive not to mention energy inefficient. These are not the qualities needed for vehicles designed for mass transportation. It is possible though for the technology to have a niche application. Long lasting, but expensive, batteries for laptop computers may use these hydrogen sources to power small fuel cells.
Another issue is the distribution of the hydrogen fuel. A 40-ton truck can deliver 26 tons of gasoline to a conventional gasoline filling station. One daily delivery is sufficient for a busy station. A 40-ton truck carrying compressed hydrogen can deliver only 400 kilograms. This is due to the weight of the tank, which is only capable of holding 3,000 psia of pressure. An empty truck will weigh almost as much as a full one. The compressed hydrogen tank must be robust. The energy used to compress the hydrogen to 3,000 psia would be released instantly if a tank ruptured. The fireball would cover a football field. Hydrogen is more energy dense than gasoline (by weight) and hydrogen-powered transportation is more energy efficient. Yet the hydrogen filling station will require 15 deliveries every day, everything else being equal. The energy cost of truck transport becomes unacceptable unless the source of hydrogen is very close to the point of use. A cryogenic truck could carry more hydrogen, but the energy cost to liquefy hydrogen makes this impractical in most cases.
Because hydrogen is a very small and reactive molecule, it is difficult to store and transport. It tends to diffuse through many materials and it makes most metals brittle and prone to failure. According to Bossel, Eliasson and Taylor, if it is transported by pipeline it takes about four times more energy to move hydrogen through the pipeline compared to natural gas. This leads them to make several significant conclusions, among them being:
Meanwhile, we find that the conversion of natural gas into hydrogen cannot be the solution of the future. Hydrogen produced by reforming natural gas may cost less (in both money and energy) than hydrogen obtained by electrolysis, but for most applications, natural gas is as good as, if not better than hydrogen. For use in road transport, if natural gas were converted to hydrogen, the well-to-wheel efficiency would be reduced and hence, for given final energy demand, the emission of CO2 would be increased. Moreover, for all stationary applications, the distribution of energy as electricity would be energetically superior to the use of hydrogen as an energy carrier.
After the release of Rifkin’s book and the announcement of the hydrogen initiatives by the Bush Administration, the National Research Council in conjunction with the National Academy of Engineering formed a Committee on Alternatives and Strategies for Future Hydrogen Production and Use to study the matter. The report on their findings entitled The Hydrogen Economy: Opportunities, Costs, Barriers, and R&D Needs was released in early 2004 (National Research Council and National Academy of Engineering, 2004). The report examined hydrogen end-use technologies, the distribution and storage of hydrogen, estimated costs of hydrogen supply, transition to hydrogen in vehicles, hydrogen production technologies and crosscutting issues (such as safety, research and international partnerships). Some of their conclusions were:
• There is a potential for eliminating almost all CO2 and criteria pollutants from vehicular emissions; however, there are currently many barriers to be overcome before that potential can be realized.
• There are other strategies for reducing oil imports and CO2 emissions. The DOE should keep a balanced portfolio of R&D efforts and continue to explore supply-and-demand alternatives that do not depend upon hydrogen. If battery technology improved dramatically, for example, all-electric vehicles might become the preferred alternative. Furthermore, hybrid electric vehicle technology is commercially available today, and benefits from this technology can therefore be realized immediately. Fossil-fuel-based or biomass-based synthetic fuels could also be used in place of gasoline.
They went on to identify the four principal research needs of a hydrogen economy:
1. To develop and introduce cost-effective, durable, safe, and environmentally desirable fuel cell systems and hydrogen storage systems. Current fuel cell lifetimes are much too short and fuel cell costs are at least an order of magnitude too high. An on-board vehicular hydrogen storage system that has an energy density approaching that of gasoline systems has not been developed; thus, the resulting range of vehicles with existing hydrogen storage systems is much too short.
2. To develop the infrastructure to provide hydrogen for the light-duty-vehicle user. Hydrogen is currently produced in large quantities at reasonable costs for industrial purposes. The committee’s analysis indicates that at a future, mature stage of development, hydrogen (H2) can be produced and used in fuel cell vehicles at reasonable costs. The challenge, with today’s industrial hydrogen as well as tomorrow’s hydrogen, is the high cost of distributing H2 to dispersed locations. This challenge is especially severe during the early years of a transition when demand is even more dispersed. The costs of a mature hydrogen pipeline system would be spread over many users as the cost of the natural gas system is today. The transition is difficult to imagine in detail. It requires many technological innovations related to the development of small-scale production units. Nontechnical factors such as financing, siting, security, environmental impact and the perceived safety of hydrogen pipelines and dispensing systems will also play a significant role. All of these hurdles must be overcome before there can be widespread hydrogen use. An initial stage during which hydrogen is produced at small scale near the small user seems likely. In this case, production costs for small production units must be sharply reduced, which may be possible with expanded research.
3. To reduce sharply the costs of hydrogen production from renewable energy sources over a time frame of decades. Tremendous progress has been made in reducing the cost of making electricity from renewable energy sources. Making hydrogen from renewable energy through the intermediate step of making electricity, a premium energy source, requires further breakthroughs in order to be competitive. Basically, these technology pathways for hydrogen production make electricity, which is converted to hydrogen, which is later converted by a fuel cell back to electricity. These steps add costs and energy losses that are particularly significant when the hydrogen competes as a commodity transportation fuel leading the committee to believe that most current approaches—except possibly that of wind energy—need to be redirected. The committee believes that the required cost reductions can be achieved only by targeted fundamental and exploratory research on hydrogen production by photo-biological, photochemical, and thin-film solar processes.
4. To capture and store (“sequester”) the carbon dioxide by-product of hydrogen production from coal. Coal is a massive domestic U.S. energy resource that has the potential for producing cost-competitive hydrogen. Coal processing, however, generates large amounts of CO2. In order to reduce CO2 emissions from coal processing, massive amounts of CO2 would have to be captured and safely and reliably sequestered for hundreds of years. Key to the commercialization of a large-scale, coal-based hydrogen production option (and also for natural-gas-based options) is achieving broad public acceptance, along with additional technical development, for CO2 sequestration.
For a viable hydrogen transportation system to emerge, all four of these challenges would have to be addressed. The committee, however, failed to identify the fundamental flaws of a hydrogen economy detailed by the Bossel, Eliasson and Taylor report. In the years since the report was released, none of their four challenges have been met. Consequently, a hydrogen-based economy has not become viable in terms of these criteria and nor is it likely to become viable in the future.
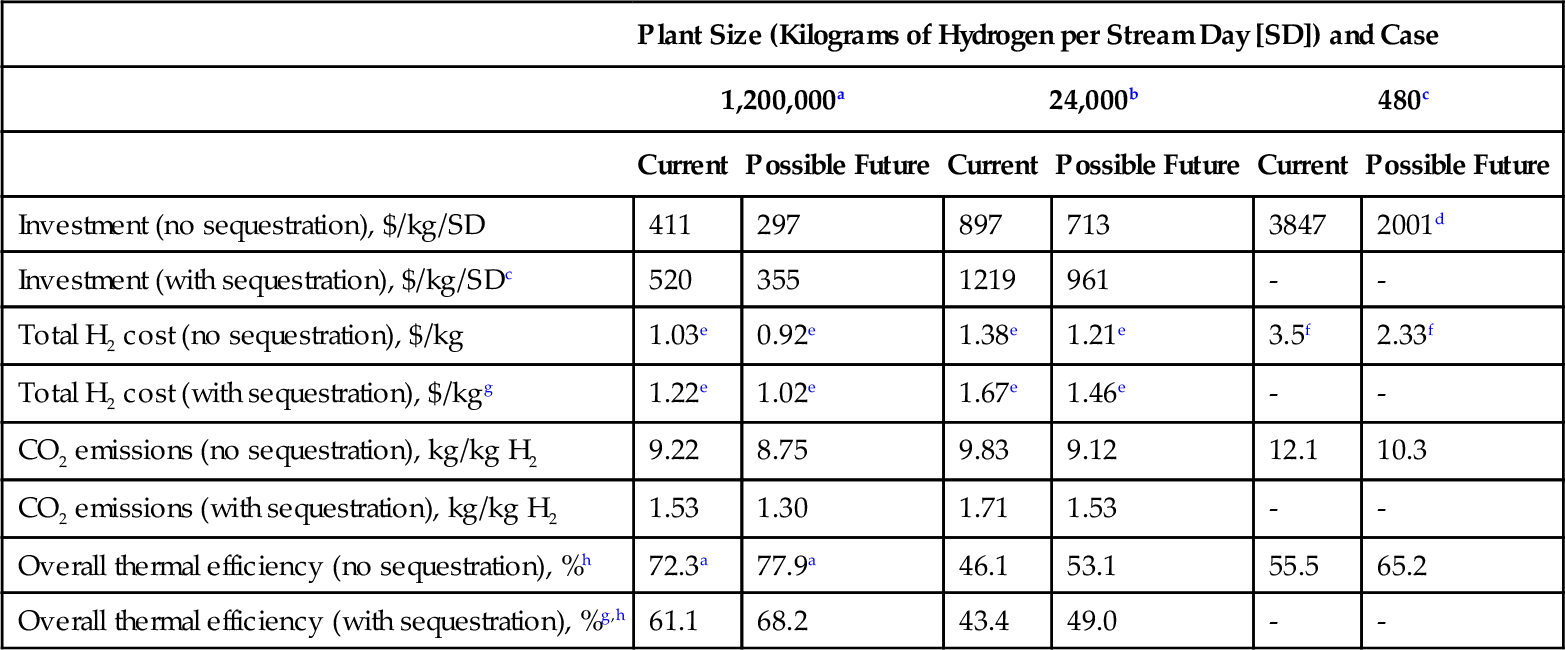
It is useful to document some of the data from the committee report. Table 15.5 shows the total investment required to manufacture hydrogen from natural gas, as well as the H2 production costs and overall thermal efficiency.
Table 15.5
Hydrogen Production Cost Data
| Plant Size (Kilograms of Hydrogen per Stream Day [SD]) and Case | ||||||
| 1,200,000a | 24,000b | 480c | ||||
| Current | Possible Future | Current | Possible Future | Current | Possible Future | |
| Investment (no sequestration), $/kg/SD | 411 | 297 | 897 | 713 | 3847 | 2001d |
| Investment (with sequestration), $/kg/SDc | 520 | 355 | 1219 | 961 | - | - |
| Total H2 cost (no sequestration), $/kg | 1.03e | 0.92e | 1.38e | 1.21e | 3.5f | 2.33f |
| Total H2 cost (with sequestration), $/kgg | 1.22e | 1.02e | 1.67e | 1.46e | - | - |
| CO2 emissions (no sequestration), kg/kg H2 | 9.22 | 8.75 | 9.83 | 9.12 | 12.1 | 10.3 |
| CO2 emissions (with sequestration), kg/kg H2 | 1.53 | 1.30 | 1.71 | 1.53 | - | - |
| Overall thermal efficiency (no sequestration), %h | 72.3a | 77.9a | 46.1 | 53.1 | 55.5 | 65.2 |
| Overall thermal efficiency (with sequestration), %g,h | 61.1 | 68.2 | 43.4 | 49.0 | - | - |
(Source: National Research Council and National Academy of Engineering Committee on Alternatives and Strategies for Future Hydrogen Production and Use, 2004)
a Includes compression of production hydrogen to pipeline of 75 atm.
b Includes compression of H2 prior to transport.
c Includes compression of H2 to 400 atm for storage/fueling vehicles.
d Includes estimated benefits of mass production.
e Based on natural gas at $4.50/million Btu.
f Based on natural gas at $6.50/million Btu.
g Includes capture and compression of CO2 to 135 atm for pipeline transport to sequestration site.
h Based on lower heating values for natural gas and hydrogen: includes hydrogen generation, purification, and compression, and energy imported from offsite as well as distribution and dispensing.
Figure 15.3 shows the unit investment cost (in 2004 $/kg/day) as a function of plant size. As expected, the economies of scale demand that plant sizes be large—greater than 1 million kg/day.
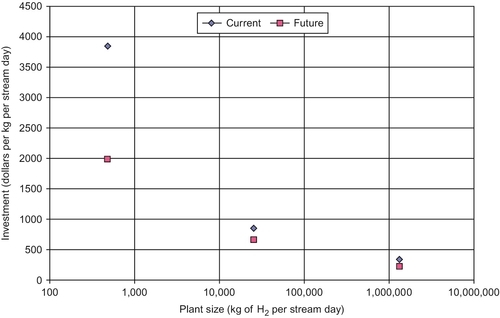
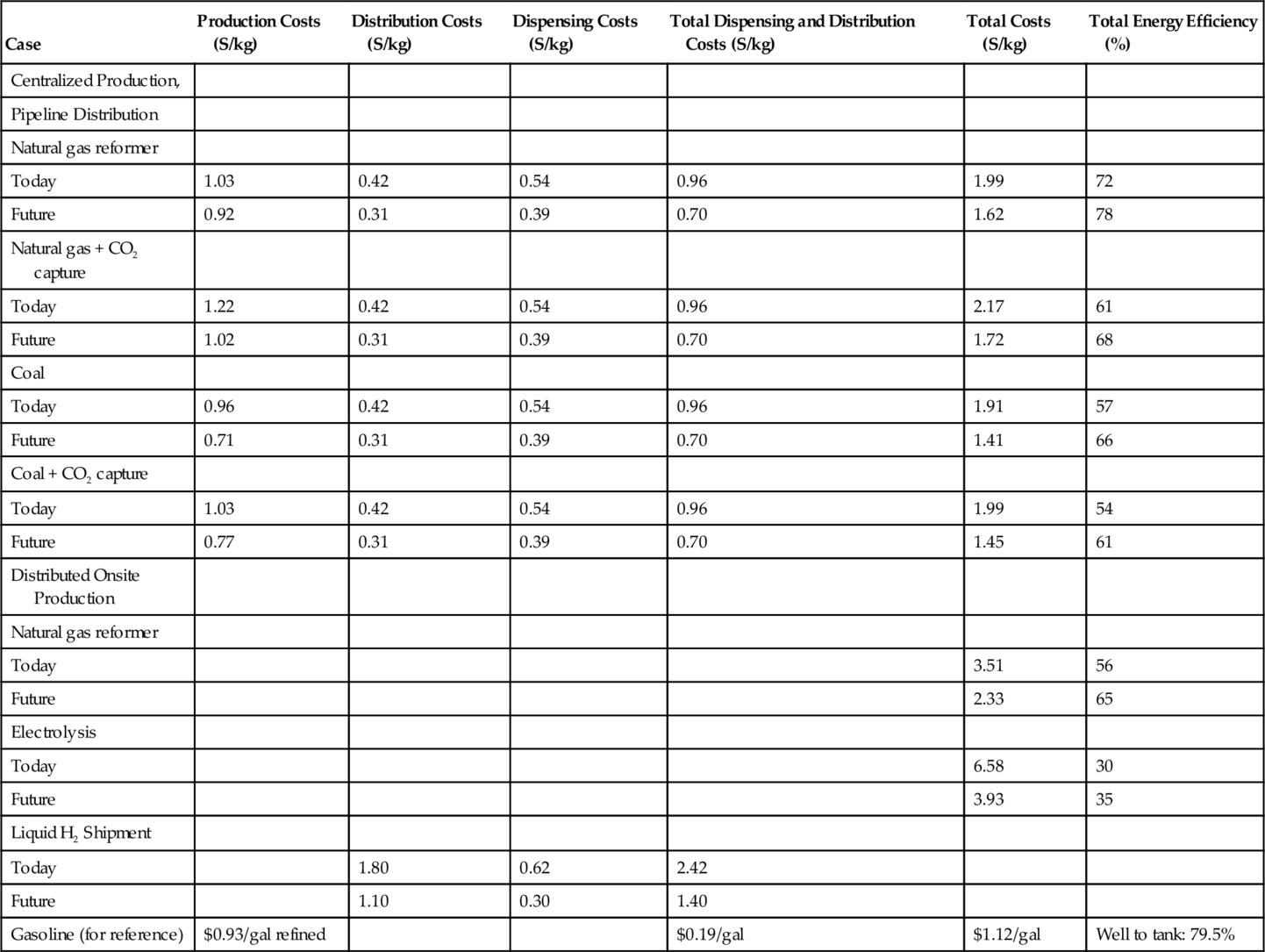
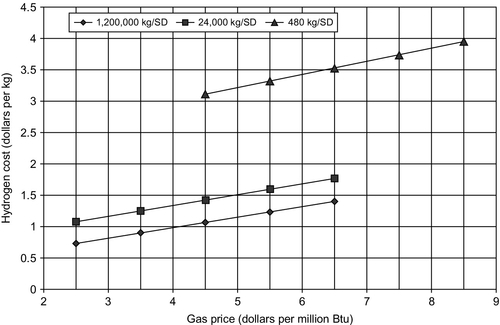
Table 15.6. shows the total costs of manufacturing hydrogen from methane and distributing it for sale to consumers in 2004. The current price is $1.99/kg (noting that 1 kg of H2 has about the same energy content as one gallon of gasoline). In 2004, the total cost of gasoline for reference was $1.12/gal. The distributed on-site cost of hydrogen using electrolysis was $6.58/kg, which is far too high to be useful. Figure 15.4 shows the hydrogen production cost as a function of plant size, and Figure 15.5 shows the hydrogen production cost as a function of both plant size and gas price.
Table 15.6
Total H2 Costs
| Case | Production Costs (S/kg) | Distribution Costs (S/kg) | Dispensing Costs (S/kg) | Total Dispensing and Distribution Costs (S/kg) | Total Costs (S/kg) | Total Energy Efficiency (%) |
| Centralized Production, | ||||||
| Pipeline Distribution | ||||||
| Natural gas reformer | ||||||
| Today | 1.03 | 0.42 | 0.54 | 0.96 | 1.99 | 72 |
| Future | 0.92 | 0.31 | 0.39 | 0.70 | 1.62 | 78 |
| Natural gas + CO2 capture | ||||||
| Today | 1.22 | 0.42 | 0.54 | 0.96 | 2.17 | 61 |
| Future | 1.02 | 0.31 | 0.39 | 0.70 | 1.72 | 68 |
| Coal | ||||||
| Today | 0.96 | 0.42 | 0.54 | 0.96 | 1.91 | 57 |
| Future | 0.71 | 0.31 | 0.39 | 0.70 | 1.41 | 66 |
| Coal + CO2 capture | ||||||
| Today | 1.03 | 0.42 | 0.54 | 0.96 | 1.99 | 54 |
| Future | 0.77 | 0.31 | 0.39 | 0.70 | 1.45 | 61 |
| Distributed Onsite Production | ||||||
| Natural gas reformer | ||||||
| Today | 3.51 | 56 | ||||
| Future | 2.33 | 65 | ||||
| Electrolysis | ||||||
| Today | 6.58 | 30 | ||||
| Future | 3.93 | 35 | ||||
| Liquid H2 Shipment | ||||||
| Today | 1.80 | 0.62 | 2.42 | |||
| Future | 1.10 | 0.30 | 1.40 | |||
| Gasoline (for reference) | $0.93/gal refined | $0.19/gal | $1.12/gal | Well to tank: 79.5% |
(Source: National Research Council and National Academy of Engineering Committee on Alternatives and Strategies for Future Hydrogen Production and Use, 2004)
Notes: The energy content of 1 kilogram of hydrogen (H2) approximately equals the energy content of 1 gallon of gasoline. Details of the analysis of the committee’s estimates in this table are presented in Chapter 5 and Appendix E of this report—see the discussion in this chapter.

The Future of Hydrogen and the Hydrogen Economy
Currently, hydrogen is being produced in chemical plants and petroleum refineries. It is being utilized to upgrade gasoline and diesel fuels and to make fertilizer and petro-chemicals. Those uses will continue to grow. It has future appeal to many people as a transportation fuel both in fuel cell-powered electric vehicles and for internal combustion engine-powered vehicles. The appeal emanates from the fact that the only product created from burning hydrogen is water. The hydrogen economy proposed by Rifkin, however, will never be realized for all the reasons documented by the Bossel, Eliasson and Taylor report. It is cheaper and better to transport and burn natural gas than convert the natural gas to hydrogen and burn hydrogen. Hydrogen is expensive to manufacture and distribute, and it is difficult to handle and store. If the goal is to make vehicles that have zero emissions, electric vehicles are probably a better alternative. Electric vehicles need better and cheaper batteries, and that seems to be a more controllable problem than all of the problems associated with a hydrogen economy. Hydrogen is also used to propel rockets into space, and it will continue to be used for that application. To eliminate carbon dioxide as a by-product of plane travel, hydrogen may find an additional application in plane travel, but at increased passenger costs.
