Nuclear Energy
Abstract
Nuclear energy grew rapidly during the 1960–1975 period in countries such as France, the United States, and Norway. But nuclear energy ran into problems in the 1970s because of public concern over the radioactive waste it generates, and this concern suppressed the further expansion of nuclear power. The public perception had begun to change in recent years, as concern about atmospheric carbon dioxide levels led to a renewed interest in energy sources not reliant on hydrocarbons. But, in 2010, a tsunami in Japan led to an accident at the Fukushima nuclear power plant, and the ensuing release of radioactive materials once again raised concerns about safety. At the same time, limited supplies of uranium have caused the price of that fuel material to go up. The solution to the shortage and resulting price increase is fast breeder reactors that use both uranium and thorium fuels. Unfortunately, this technology has not yet been perfected and commercialized.
Nuclear power had its genesis in Einstein’s theory of relativity which was first published as the special theory of relativity in 1905, although that is not actually what led scientists to generate nuclear power by splitting the atom. When heavy elements such as uranium (the heaviest naturally occurring element) are split into other elements using nuclear fission, the resulting elements are collectively slightly lighter than the original uranium atom. According to Einstein’s equation, the extra mass is converted into energy. Conversely, when light elements such as hydrogen (the lightest element) are fused into heavier elements (two hydrogen atoms fuse into helium), using nuclear fusion, they end up slightly lighter than the original hydrogen atoms used to create them. Again, according to Einstein’s equation, the extra mass is converted into energy.
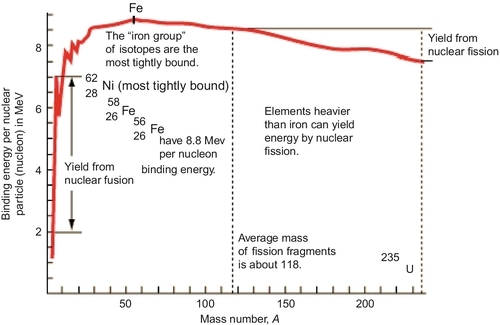
The energy released in each process (fission and fusion) is stored as the binding energy of the protons and neutrons in the nucleus of the atom. It may seem paradoxical that the fission of heavy elements and the fusion of lighter elements both release energy. This occurs because the energy required to bind neutrons and protons together to form atoms goes through a maximum near the middle elements of Iron and Nickel, as shown in the attached plot. Elements heavier than iron release energy when they are split by nuclear fission; elements lighter than iron release energy when they are fused together by nuclear fusion.
When scientists were probing the structure of the atom in the first half of the twentieth century they began using neutrons to bombard the nucleus. These experiments inevitably led to the splitting of uranium atoms into two roughly equal size pieces. The potential for splitting uranium atoms had been implied by work done with radium by Frederic and Irene Joliot-Curie (the daughter and son-in-law of Pierre and Marie Curie) in 1935. Enrico Fermi’s group in Italy was probably the first to split uranium atoms in 1937, but they struggled to understand the products of their uranium experiments. Fermi proved to himself and the world that the products were not any element in the range from element number 82 (lead) to element number 91 (protactinium). So he surmised that his experiments were creating trans-uranium elements, with an atomic number higher than 92. It was not until Otto Hahn and Fritz Strassman repeated the experiments in Germany in 1938 that they were able to prove that the uranium atoms were being split approximately in two and one of the products was barium. The Germans were so surprised and unsure of their results that they prevailed upon a former colleague, Lise Meitner, to examine their results and try to come up with an alternative explanation. Meitner had recently fled Nazi persecution in Germany. Instead of proposing an alternative explanation, she and her nephew (Otto Frisch) developed the theory for splitting the uranium atom into two using Einstein’s theory and equation and also predicted the energy that would be generated. Hungarian Leo Szilard, a former protégé and scientific collaborator with Albert Einstein, had previously patented the nuclear chain reaction process, surmising that any nuclear process that could generate neutrons could create a chain reaction that might be used to create a huge release of energy. He called the process “an atomic bomb.” When Szilard heard about the Hahn-Strassman experiment and saw it duplicated he realized that the splitting of Uranium-235 (U-235) with neutrons was exactly the process that could do this. Hahn called the process “nuclear fission.”
The first public demonstrations of the fission process were made in 1945 when a uranium fission bomb was dropped on Hiroshima in Japan and a plutonium fission bomb was dropped on Nagasaki, Japan. The results were horrifying and catastrophic, but this resulted in the surrender of Japan and the end of World War II. After World War II, the even more explosive hydrogen fusion bombs were developed by both the United States and Russia. These hydrogen fusion weapons were never deployed or used in any war; however, their explosive power and rapid release of energy have been demonstrated in test firings both above ground and underground. It can be seen from Figure 7.1 that a fusion process releases more energy than a fission process.
After World War II and the development of the hydrogen bomb, scientists turned their attention to harnessing this energy for the generation of electricity. The first plants that were developed utilized the easier to initiate and control uranium fission process. A typical nuclear fission power plant design is shown in Figure 7.2.
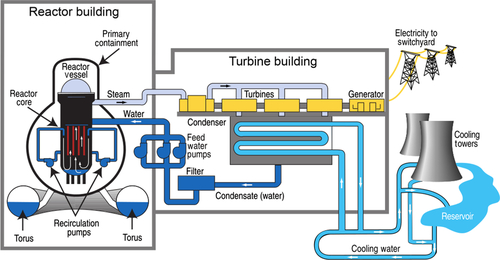
How does a nuclear power plant work? It is a relatively simple process. In the core of the reactor in the containment building, U-235 is bombarded with neutrons that split the uranium atom into two smaller atoms and also releases extra neutrons. These extra neutrons bombard other uranium atoms, splitting them and creating a chain reaction that sustains the reaction as long as the uranium fuel is present. The chain reaction can be controlled or stopped by the insertion of rods into the reaction chamber that absorbs some or all of the extra neutrons. These rods are usually made of boron or graphite. When the U-235 atoms are split, the extra binding energy is released and is transferred as heat to a water jacket surrounding the reaction core. The water absorbs the heat, raising its temperature, and vaporizes it into steam at high pressure. The steam is then fed to a steam turbine which generates the electricity.
When nuclear power plants were being designed in the 1950s, some nuclear engineers predicted that, with the deployment of nuclear power, electricity would become so cheap it would be too cheap to meter. The reason they came to this conclusion was that the amount of energy that could be extracted by splitting uranium atoms was enormous and the cost of the fuel relative to the energy generated was very small. Electricity, however, did not become too cheap to meter because the capital and operating costs of the nuclear power plants was so large that the electricity still had to be metered to pay back those large costs. The same arguments can be made about wind power and solar power. The sun and wind are free and cannot be metered, but in Chapter 9 you will see that wind power is still more expensive than fossil fuel power stations. The harnessing of solar energy discussed in Chapter 8 is so expensive due to high capital costs that it may never be more than a niche player.
Nuclear energy grew rapidly in the 1960-1975 period in countries such as the United States, France, Japan and Germany. It ran into problems in the 1970s due to the public concern raised by the radioactive waste it generated. These concerns were fueled by serious accidents at the Three Mile Island plant in Pennsylvania in 1979 and the Chernobyl disaster in the Ukraine in 1986. This suppressed its further expansion. In recent years, public perception had begun to change as concerns about atmospheric carbon dioxide levels lead to a renewed interest in nuclear power. The Fukushima nuclear power plant accident due to the earthquake and tsunami in northern Japan on March 11, 2011 has once again, however, focused attention on nuclear plant safety.
Most nuclear power is used to generate electricity. The United States and Russia do have some ships and submarines that are nuclear powered, but this represents a tiny fraction of the energy generated from nuclear reactions. The 29 countries with the largest nuclear power generation are shown in Table 7.1. The United States is far and away the largest user of nuclear energy with 841 TWh. France is the second largest with 411 TWh, and after that Japan, Russia, South Korea, and Germany round out the top six (only these six countries generate more than 130 TWh/year). According to the percentage of electricity generated by nuclear power shown in Table 7.2, France is number 1 with 76%, while the United States—which generates a healthy 20% of its electricity from nuclear power—is number 16 in that respect. For the entire world, the percentage of electricity generated by nuclear power is 13.42%.
Table 7.1
2009 Nuclear Energy Consumption (TWh)
| 1 | USA | 840.78 |
| 2 | France | 410.53 |
| 3 | Japan | 274.64 |
| 4 | Russian Federation | 163.58 |
| 5 | South Korea | 147.77 |
| 6 | Germany | 134.90 |
| 7 | Canada | 89.81 |
| 8 | Ukraine | 82.16 |
| 9 | China | 70.13 |
| 10 | United Kingdom | 69.19 |
| 11 | Spain | 52.89 |
| 12 | Sweden | 52.63 |
| 13 | Belgium & Luxembourg | 47.22 |
| 14 | Taiwan | 41.57 |
| 15 | Switzerland | 27.52 |
| 16 | Czech Republic | 27.11 |
| 17 | Finland | 23.65 |
| 18 | India | 16.82 |
| 19 | Bulgaria | 15.57 |
| 20 | Hungary | 15.42 |
| 21 | Slovakia | 14.08 |
| 22 | Brazil | 13.00 |
| 23 | South Africa | 12.12 |
| 24 | Romania | 11.76 |
| 25 | Lithuania | 10.85 |
| 26 | Mexico | 9.59 |
| 27 | Argentina | 7.99 |
| 28 | Netherlands | 4.23 |
| 29 | Pakistan | 2.85 |
Table 7.2
Percentage of Electricity Generated by Nuclear Power
| 1 | France | 75.69% |
| 2 | Lithuania | 70.68% |
| 3 | Belgium & Luxembourg | 55.88% |
| 4 | Slovakia | 53.72% |
| 5 | Ukraine | 47.52% |
| 6 | Hungary | 42.75% |
| 7 | Switzerland | 40.11% |
| 8 | Sweden | 38.95% |
| 9 | Bulgaria | 36.14% |
| 10 | Czech Republic | 33.59% |
| 11 | Finland | 33.04% |
| 12 | South Korea | 32.42% |
| 13 | Japan | 24.63% |
| 14 | Germany | 22.60% |
| 15 | Romania | 20.46% |
| 16 | USA | 20.26% |
| 17 | United Kingdom | 18.61% |
| 18 | Spain | 18.33% |
| 19 | Taiwan | 18.10% |
| 20 | Russian Federation | 16.47% |
| 21 | Canada | 14.16% |
| 22 | Argentina | 6.45% |
| 23 | South Africa | 4.67% |
| 24 | Netherlands | 3.77% |
| 25 | Mexico | 3.72% |
| 26 | Pakistan | 3.07% |
| 27 | Brazil | 2.78% |
| 28 | India | 1.93% |
| 29 | China | 1.88% |
The United States currently has 104 nuclear reactors. An aerial view of two of these reactors at the Diablo Nuclear Power Plant in Diablo Canyon, California, is shown in Figure 7.3.

The Uranium Fuel Cycle
All current nuclear power plants use U-235 as its fuel. There are a variety of uranium-based fuels that could be used, but U-235 is the simplest. The 235 refers to the atomic weight of the uranium atom that is used. Every uranium atom has 92 protons in the atomic nucleus and a varying number of neutrons. The atomic weight is collectively the total number of protons and neutrons in the atomic nucleus. There are three isotopes of uranium that exist in nature: U-234, U-235, and U-238. U-235 is easier to split than the other two isotopes because of the odd number of neutrons in the atom. When a neutron is fired at the atom with enough speed (kinetic energy), the U-235 atom absorbs the neutron and momentarily becomes U-236. The kinetic energy of the absorbed neutron causes the U-236 nucleus to begin vibrating and then it splits into two atoms of roughly equal size. Depending on the conditions, the products of the fission process are varied and range from Krypton, Strontium, Zirconium, Technetium to Cesium and Barium. When uranium is mined, it is recovered as uranium oxide (U3O8). Only about 0.7% of the uranium atoms in the U3O8 are U-235 atoms. Most of the other 99.3% of the uranium atoms are U-238. There are also a few U-234 atoms. The purified uranium oxide (U3O8) that is recovered from the mining operation is called yellowcake because of its color. It should be noted that some so-called yellowcake is actually brownish in color.
The next step in the cycle involves the conversion of the yellowcake into uranium hexafluoride (UF6) gas. This step is required to separate the uranium atoms into the three isotopes: U-234, U-235, and U-238. The U3O8 molecules are first chemically reacted into uranium hexafluoride (UF6) from which the individual U-235 and U-238 isotopes can be separated. This gas is sent to an enrichment plant where the isotope separation takes place. The United States currently has one operating enrichment plant, which uses a diffusion process to separate the isotopes. The slightly smaller and lighter U-235 atoms travel slightly faster than the U-238 atoms and are forced to diffuse through a porous membrane where they are collected and concentrated. The final product is still mostly U-238 but the concentration of U-235 has been raised from 0.7% to about 5%, and this mixture is now called enriched uranium fuel. Note that it is still molecular UF6.
There are other processes that are used for enrichment, and newer ones are being proposed and developed. Another enrichment technique that is used elsewhere is the gas centrifuge process, where the UF6 gas is spun at high speed in a series of cylinders which separates the lighter 235-UF6 and heavier 238-UF6 atoms based on their different atomic masses. The lighter U-235 travels faster than the U-238, causing it to separate. Other enrichment technologies that are currently being developed use laser technology to do the separation and concentration. One is called atomic vapor laser isotope separation and another is molecular laser isotope separation. These laser-based enrichment processes can achieve higher initial U-235 enrichment (isotope concentration) factors than the membrane or centrifuge processes and are capable of operating at higher throughput rates.
The next step in the production of nuclear fuel takes place at one of the five U.S. fuel fabrication facilities. Here, the enriched UF6 gas is reacted to form a black uranium dioxide powder (UO2). The powder is compressed into small pellets and sealed into metal fuel rods, which are long metal tubes about 1 cm in diameter. The fuel rods are then bundled together to make up a fuel assembly (see Figure 7.4). Depending on the reactor type, there are about 179-264 fuel rods in each fuel assembly and a typical reactor core holds 121-193 fuel assemblies.

These fuel rods can be handled safely without precautions because the uranium is only slightly radioactive and essentially all radiation is contained within the metal tubes. These fuel assemblies last up to 6 years and about one-third of the assemblies are changed out every 2 years.
The reactor core itself is a cylindrical arrangement of the fuel bundles, about 12 ft in diameter and 14 ft high. It is encased in a steel pressure vessel. The core has essentially no moving parts except for a small number of control rods that can be inserted to regulate the reaction. These control rods, typically made of boron or graphite, are designed to absorb some of the neutrons that are released by fission so that the reaction can be slowed or stopped as necessary.
Following their use in the reactor, the fuel assembly becomes highly radioactive and must be removed and stored under water for several years. Even though the fission reaction has stopped, the spent fuel continues to give off heat from the decay of radioactive elements that were created when the uranium atoms were split apart. The water cools the fuel and at the same time absorbs the radiation. As of 2002, there were over 165,000 spent fuel assemblies stored in about 70 interim storage pools throughout the United States. After cooling for several years, the spent fuel is moved to a concrete container for on-site storage.
About 80% of U-235 is consumed before the fuel rods are considered spent. This means that less than 4% of the uranium loaded into the reactor is consumed in the nuclear reactions. The rest of the uranium, which is mostly U-238, remains unchanged. Chemical processing of the spent fuel material to recover the remaining portion of fissionable products for use in fresh fuel assemblies is technically feasible. Some countries, such as France, reprocess spent nuclear fuel, but it is not permitted in the United States.
Eventually, it is intended that the spent nuclear fuel will be stored in a permanent underground repository. The United States designed and built the Yucca Mountain repository in Nevada for the purpose of storing this spent nuclear fuel but so far it has not been permitted for this purpose. The spent fuel continues to be stored on site at the nuclear reactors. This spent fuel, containing mostly U-238, could be reprocessed to enrich the U-235 or could be used as fuel in fast breeder reactors, which are discussed in the next section.
Fast Breeder Reactors
One of the problems with current nuclear reactor technology is that only some of the U-235 is consumed in the reactor and the U-235 represents less than 1% of the uranium atoms. The supply of uranium is limited, and nuclear power cannot be expanded much unless this limited uranium supply is increased. There are many other nuclear fission processes that rectify this situation. The fast breeder reactor is one such design that falls into the general class of breeder reactors. These reactors actually generate more fissile material than they use and can use a broader array of nuclear fuels and so they do not run into the supply limitation. Moreover, fast breeder reactors actually utilize the much more abundant U-238 atoms in its fuel process. Other breeder reactors can use thorium, which is three times more abundant than uranium.
Several fast breeders have been built and tested but, because their fission process is more complicated and their capital costs higher, they have not been as economically successful as standard U-235 nuclear reactors. If nuclear power is to be expanded, however, this technology has to be further developed in some form. In addition to the fast breeder reactor, other types of breeders are currently being developed. India, for example, is currently developing a thermal breeder reactor for commercial use. The Indian reactor uses thorium as its fuel. India has about one-third of the world’s reserves of thorium, so it makes economic sense for India to develop a breeder reactor that utilizes thorium.
Fast breeder reactors convert U-238 to Plutonium-239 (PU-239) under the action of fast moving neutrons. The PU-239 is fissile, whereas the U-238 is not. Due to the complexities in the handling of the fast moving neutrons, the standard nuclear reactors cannot be used.
One variation on the fast breeder reactor is the liquid metal fast breeder reactor shown in Figure 7.5. In this type of reactor, a liquid metal is used as the primary coolant. This liquid metal captures heat directly from the nuclear reactor core, then exchanges heat with water in the steam cycle. The steam is used to generate electricity in a steam turbine. The liquid metal is used as the primary coolant because water tends to slow down the fast neutrons needed for the nuclear reaction. Sodium, mercury, and lead have been used as the circulating liquid metal. In this reactor, even though the process “breeds” fissile material, the U-238 is actually consumed as fuel. Due to the fact that U-238 is much more abundant than U-235, the limited uranium supply is not a problem for nuclear expansion.
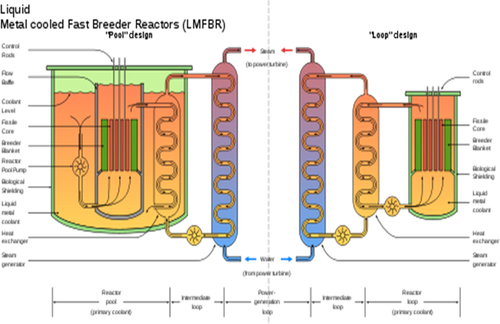
France built two fast breeders called the phenix and the superphenix and tested them extensively before shutting them down in 1996. The cleanup cost for the superphenix cost $1 billion. Germany spent €3.6 billion and 19 years to build a fast breeder reactor called the SNR-300, completing it in 1985. It sat idle for about 7 years before it was scrapped for political reasons without ever having run. The important point to note is that breeder technology is necessary if nuclear power is to be expanded.
Thorium-232 (Th-232) can be used in both fast breeder reactors and in thermal breeder reactors. It is first converted to U-233 according to the following breeder reaction:
![]()
In this reaction, the neutron bombards the nucleus of the Th-232, which then becomes Th-233. This then transmutes into 233-Protactinium (Pa-233) via beta decay. The Pa-233 undergoes a second beta decay to transmute into U-233, which is fissile in a similar manner to U-235. There are several advantages to using Th-232 as the fuel, particularly in thermal breeder reactors:
• It is more abundant than uranium
• Mined thorium consists of a single isotope (Th-232)
• It has a superior neutron usage
• It has favorable physical and chemical properties (higher melting point, higher thermal conductivity, lower coefficient of thermal expansion, and more stable oxides) which improve reactor performance, and
• It produces less toxic and shorter lived trans-uranium waste products that improve waste repository performance.
Uranium Reserves and Sources
Most of the uranium used in U.S. nuclear power reactors comes from foreign suppliers. Only about 14% comes from U.S. mined uranium. The operators of U.S. nuclear power reactors purchased and used a total of 50 million pounds of U3O8 during 2009, at a weighted-average price of $45.86 per pound. The 2009 total was a decrease of 7% compared with the 2008 total of 53 million pounds. 14% of the U3O8 delivered in 2009 was of U.S. origin at a weighted-average price of $48.92 per pound. Foreign-origin uranium accounted for the remaining 86% of deliveries at an average price of $45.35 per pound. Australian-origin and Canadian-origin uranium together accounted for 40% of the 50 million pounds. Uranium originating in the former Soviet Union (Kazakhstan, Russia, and Uzbekistan) accounted for 29% and the remaining 17% originated in Brazil, Czech Republic, Namibia, Niger, and South Africa.
The nuclear power plants purchased uranium of several different types: Uranium oxide (U3O8) concentrate was 69% of the deliveries in 2009, and natural UF6 and enriched uranium were 31%. During 2009, 17% of the uranium was purchased under spot contracts at a weighted-average price of $46.45 per pound. The remaining 83% was purchased under long-term contracts at an average price of $45.74 per pound.
The U.S. Energy Information Administration last updated its estimates of U.S. uranium reserves for year-end 2008. This represented the first revision of their estimates since 2004. The update is based mainly on analysis of company annual reports rather than geological analysis. At the end of 2008, their estimate of U.S. uranium reserves totaled 1227 million pounds of U3O8 at a maximum forward cost of up to $100 per pound of U3O8. At up to $50 per pound, U3O8, estimated reserves were 539 million pounds. Based on average 1999-2008 consumption levels, uranium reserves available at up to $100 per pound of U3O8 represented approximately 23 years’ worth of demand, while uranium reserves at up to $50 per pound of U3O8 represented about 10 years’ worth of demand; however, because domestic U.S. uranium production supplies only 14% on average of U.S. requirements for nuclear fuel, the effective years’ supply of domestic uranium reserves is actually seven times higher than these numbers.
In 2008, Wyoming led the nation in total uranium reserves in both the $50 and $100 per pound U3O8 categories, with New Mexico second. Taken together, these two states constituted about two-thirds of the estimated reserves in the country available at up to $100 per pound U3O8 and three-quarters of the reserves available at less than $50 per pound U3O8. By the mining method, uranium reserves in underground mines constituted 39% of the available product at up to $100 per pound of U3O8. This is followed by open-pit mining at 31% and in situ leaching (ISL) at 29%. At $50 per pound U3O8, however, uranium available through ISL was about 40% of the total reserves, somewhat higher than uranium in underground and open-pit mines in that cost category. ISL is the dominant mining method for U.S. production today.
Up until 2004, the average price being paid for uranium supplies was about $10 per pound. After that, the price increased steadily to $45 per pound due to increased demand and diminishing supplies. It briefly spiked at over $100 per pound in late 2007 and early 2008, but it then settled out at $45 per pound. As the price goes up, the supply increases because more uranium deposits become economic to mine. The low price discouraged the development of mines in the United States, but as the price has now increased, new U.S. supplies are being developed.
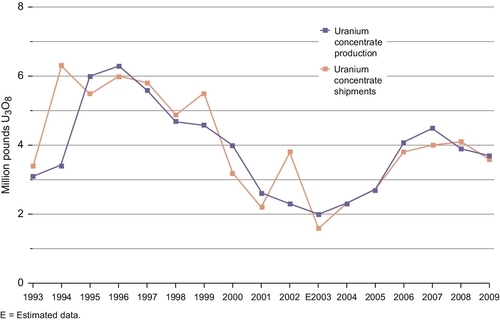
U.S. mines produced 4.1 million pounds of U3O8 in 2009, 7% more than in 2008. Fourteen underground mines produced ore-containing uranium during 2009, four more than 2008. Four ISL mining operations produced solutions containing uranium in 2009, two less than during 2008. Overall, during part or all of 2009, there were 18 U.S. mines that produced uranium to be processed into uranium concentrate. The total production of U.S. uranium concentrate (yellowcake) in 2009 was 3.7 million pounds, 5% below the 2008 level, from one U.S. mill (White Mesa Mill) and four ISL plants (Alta Mesa Project, Crow Butte Operation, Kingsville Dome, and Smith Ranch-Highland Operation). Shipments of uranium concentrate from these facilities were 3.6 million pounds in 2009, 12% below the 2008 level. The annual production of uranium from U.S. mines for the last 17 years is shown in Figure 7.6. Figure 7.7 shows the comparison between the annual production and annual shipments of uranium yellowcake from mines in the United States.

The United States has the capacity and reserves to increase its indigenous supply of uranium from U.S. sources but, at the moment, the nuclear power industry is highly dependent on foreign suppliers to fuel its nuclear power plants. This situation does not represent as large a balance of payments problem as does the large oil import bill because the 45 to 50 million pounds of yellowcake that it is currently importing only costs about $2.5 billion per year. It does, however, represent a significant security of supply issue, particularly if the United States wants to expand its nuclear power production. It is unlikely that the United States can increase its production of uranium enough to become self-sufficient in uranium. There is also some uncertainty about how long the foreign supplies will continue, particularly if nuclear power production was increased. This situation would be rectified if fast breeder reactors were commercialized.
The world supply of uranium is dominated by Kazakhstan, Australia, and Canada, with significant production coming from Russia, Uzbekistan, Namibia, and Niger. The world resources are sufficient for the current usage. If the price increases due to shortages, more dilute uranium deposits could be produced. The world price of uranium did spike in 2007-2008 due to strong demand, and it is likely to do so again if nuclear power demand picks up. The world reserves of uranium are shown in Table 7.3. These reserves are a function of the current world price, which is $45 per pound. At this price, Australia dominates in world reserves holding 1.673 million tons, which is 31% of the world’s known economic reserves of 5.404 million tons. The current usage rate of uranium is 53,663 tons/year, so the 5.404 million tons of reserves represent 100.7 years of reserve life at today’s usage rates. Based on the U.S. reserve estimates above, it could be projected that if the price of uranium doubled (which would not substantially affect the economics of today’s reactors), the amount of uranium reserves would also double. This would represent 200 years of supply at today’s usage rate. If fast breeder reactors were commercialized, the fuel supply from uranium would be increased by 150-fold. Clearly, the capacity to increase the supply of nuclear power exists, even with the current technology; however, substantial increases would require the commercialization of fast breeder technology.
Table 7.3
World Uranium Reserves 2009
| Tons U | % of world | EJ | |
| Australia | 1,673,000 | 31% | 292.9 |
| Kazakhstan | 651,000 | 12% | 114.0 |
| Canada | 485,000 | 9% | 84.9 |
| Russia | 480,000 | 9% | 84.0 |
| South Africa | 295,000 | 5% | 51.6 |
| Namibia | 284,000 | 5% | 49.7 |
| Brazil | 279,000 | 5% | 48.8 |
| Niger | 272,000 | 5% | 47.6 |
| USA | 207,000 | 4% | 36.2 |
| China | 171,000 | 3% | 29.9 |
| Jordan | 112,000 | 2% | 19.6 |
| Uzbekistan | 111,000 | 2% | 19.4 |
| Ukraine | 105,000 | 2% | 18.4 |
| India | 80,000 | 1.50% | 14.0 |
| Mongolia | 49,000 | 1% | 8.6 |
| Other | 150,000 | 3% | 26.3 |
| World total | 5,404,000 | 946.0 |
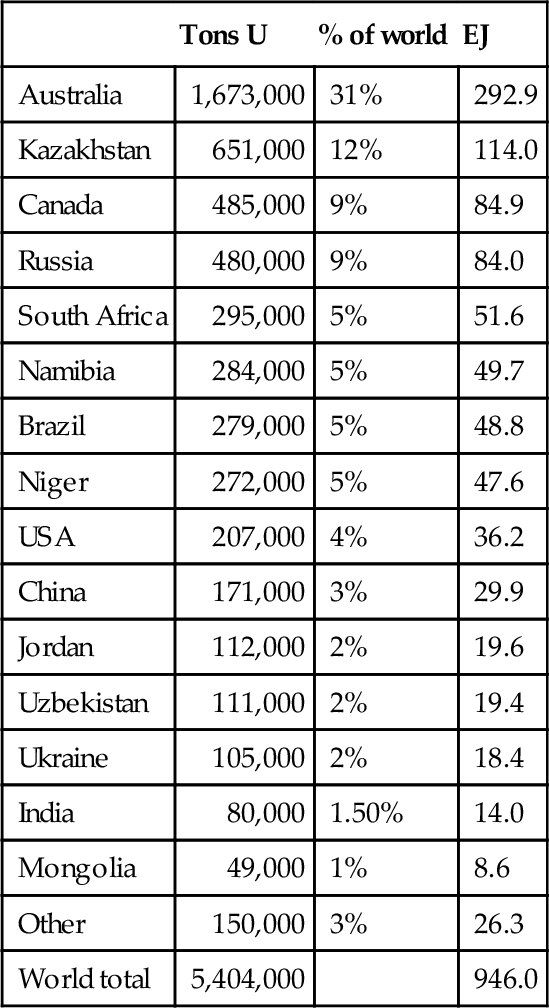
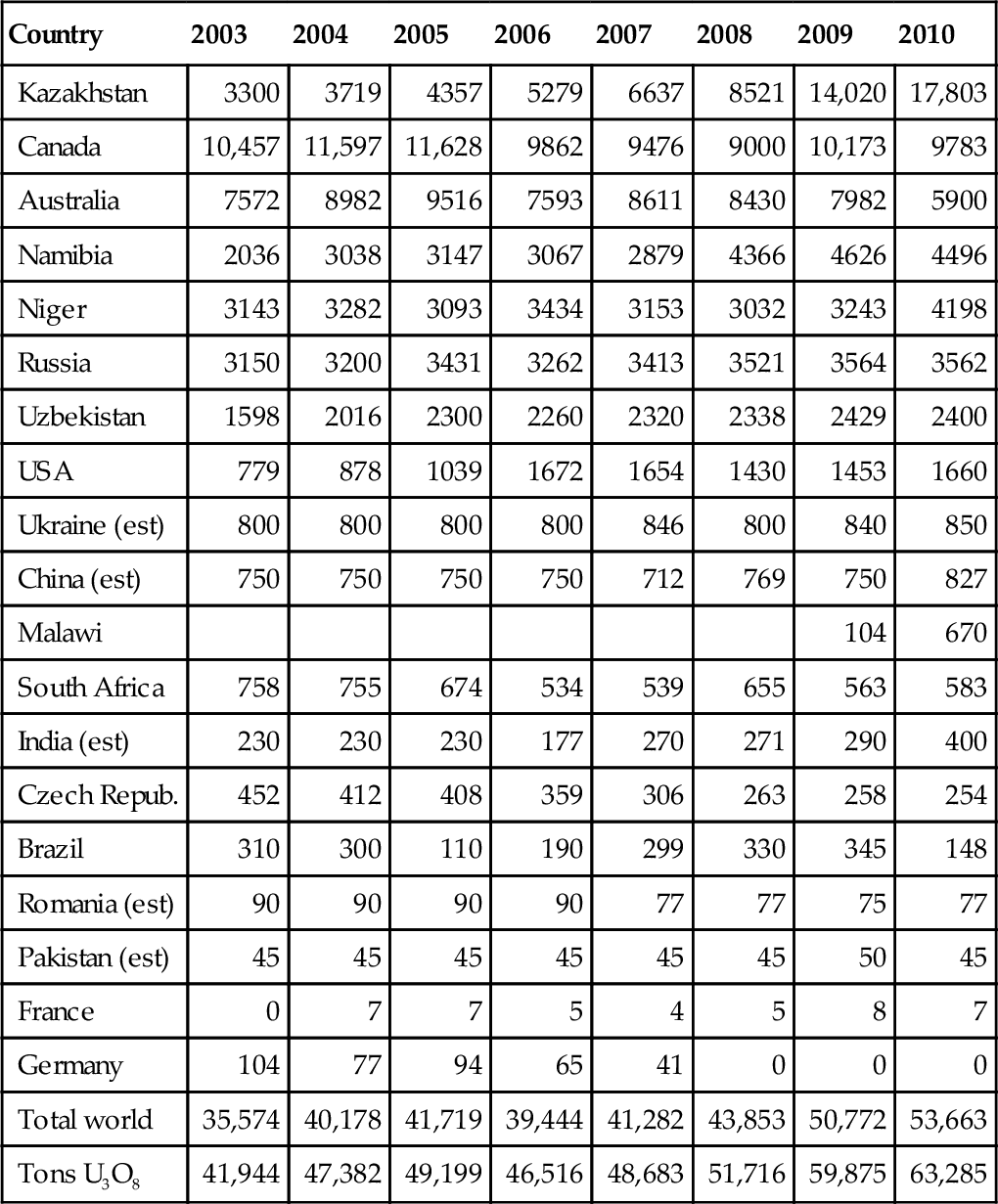
Table 7.3 conversions require some explanation. A 1-GW nuclear power plant requires approximately 200 short tons of uranium per year. In 1 year, the 1-GW plant can generate 8760 GWh of electricity. This equates to 43.8 GWh/ton, 48.63 GWh/ton, or 175.06 TJ/ton. This conversion factor has been used to convert the reserves of uranium into exajoules in Table 7.3. The current total world reserve of uranium is 946 EJ, of which 293 EJ are located in Australia, even though Australia produces substantially less than Kazakhstan and Canada. World uranium production for the past 8 years is shown in Table 7.4. In 2010, 53,663 tons of uranium was produced, representing 63,285 tons of U3O8 (yellowcake). In the past 4 years, production increases of 9% per year have been seen.
Table 7.4
World Uranium Production (Tons)
| Country | 2003 | 2004 | 2005 | 2006 | 2007 | 2008 | 2009 | 2010 |
| Kazakhstan | 3300 | 3719 | 4357 | 5279 | 6637 | 8521 | 14,020 | 17,803 |
| Canada | 10,457 | 11,597 | 11,628 | 9862 | 9476 | 9000 | 10,173 | 9783 |
| Australia | 7572 | 8982 | 9516 | 7593 | 8611 | 8430 | 7982 | 5900 |
| Namibia | 2036 | 3038 | 3147 | 3067 | 2879 | 4366 | 4626 | 4496 |
| Niger | 3143 | 3282 | 3093 | 3434 | 3153 | 3032 | 3243 | 4198 |
| Russia | 3150 | 3200 | 3431 | 3262 | 3413 | 3521 | 3564 | 3562 |
| Uzbekistan | 1598 | 2016 | 2300 | 2260 | 2320 | 2338 | 2429 | 2400 |
| USA | 779 | 878 | 1039 | 1672 | 1654 | 1430 | 1453 | 1660 |
| Ukraine (est) | 800 | 800 | 800 | 800 | 846 | 800 | 840 | 850 |
| China (est) | 750 | 750 | 750 | 750 | 712 | 769 | 750 | 827 |
| Malawi | 104 | 670 | ||||||
| South Africa | 758 | 755 | 674 | 534 | 539 | 655 | 563 | 583 |
| India (est) | 230 | 230 | 230 | 177 | 270 | 271 | 290 | 400 |
| Czech Repub. | 452 | 412 | 408 | 359 | 306 | 263 | 258 | 254 |
| Brazil | 310 | 300 | 110 | 190 | 299 | 330 | 345 | 148 |
| Romania (est) | 90 | 90 | 90 | 90 | 77 | 77 | 75 | 77 |
| Pakistan (est) | 45 | 45 | 45 | 45 | 45 | 45 | 50 | 45 |
| France | 0 | 7 | 7 | 5 | 4 | 5 | 8 | 7 |
| Germany | 104 | 77 | 94 | 65 | 41 | 0 | 0 | 0 |
| Total world | 35,574 | 40,178 | 41,719 | 39,444 | 41,282 | 43,853 | 50,772 | 53,663 |
| Tons U3O8 | 41,944 | 47,382 | 49,199 | 46,516 | 48,683 | 51,716 | 59,875 | 63,285 |
Nuclear Power Plant Safety
One of the battles that nuclear power has had to fight is the public perception that it is too risky to be used. Nuclear power generates radioactive waste. If this waste escapes into the environment, the consequences can be severe and include such things as radiation sickness, cancer, and death. The nuclear industry has a remarkably good safety record, but the consequences of a nuclear accident can be so catastrophic and the effects so long lasting that the standards of safety must be extremely high. Society cannot tolerate a mishap that would not only kill many people but would also render large areas of land uninhabitable. There have been a few incidents where public safety was put at risk but three accidents in particular stand out as fueling the fears of the public about expanded use of nuclear power.
The Three Mile Island Incident
The accident at the Three Mile Island Unit 2 (TMI-2) nuclear power plant near Middletown, Pennsylvania on March 28, 1979, caused no deaths or injuries to plant workers or members of the public. It was, however, the first serious nuclear accident and is still considered the most serious in U.S. nuclear power plant operating history. It also ushered in changes in nuclear reactor operations and accident response. Because the nuclear industry learned lessons from the event, the industry has been made much safer.
The accident began about 4:00 a.m. on March 28, 1979, when the main feed water pumps broke down and shut down the steam circulation to the turbines. The steam turbine and the main reactor shut down automatically, but reactor fuel was still generating heat and so the pressure in the primary cooling system began to increase. In order to prevent that pressure from becoming excessive, a pressure relief valve opened automatically. The valve should have closed again when the pressure decreased by a certain amount, but it did not. As a result, cooling water leaked out of the open valve and caused the core of the reactor to overheat. The plant did not have instruments that showed that the valve remained open or that showed the level of coolant in the core. Because adequate cooling was not available, the nuclear fuel overheated and the metal tubes which hold the nuclear fuel pellets ruptured and the fuel pellets began to melt. About one-half of the core melted during the early stages of the accident. Although the TMI-2 plant suffered a severe core meltdown, it did not lead to a breach of the walls of the containment building and it did not release large quantities of radiation to the environment.
Detailed studies of the radiological consequences of the accident have been conducted by many state and federal agencies. Estimates are that the average dose to about 2 million people in the area was only about 1 mrem. This is a very low radiation exposure, less than a single chest X-ray. There were no deaths or injuries and no evidence of cancer increases in the area.
The Chernobyl Disaster
On April 26, 1986, an accident occurred at Unit 4 of the nuclear power station at Chernobyl in the Ukraine in the former USSR. An aerial view of the plant taken in 1997, 11 years after the accident, is shown in Figure 7.8. The accident was apparently caused by a sudden power surge to the reactor controls. As a consequence, the reactor caught fire and exploded and released massive amounts of radioactive material into the environment. This radioactive material drifted downwind and was detected in Sweden within hours. Boron and sand were poured on the reactor from the air and then the damaged unit was encased in concrete. The three other units of the Chernobyl nuclear power station were subsequently restarted. The Soviet nuclear power authorities presented an initial report on the accident at an International Atomic Energy Agency meeting in Vienna, Austria, in August 1986.
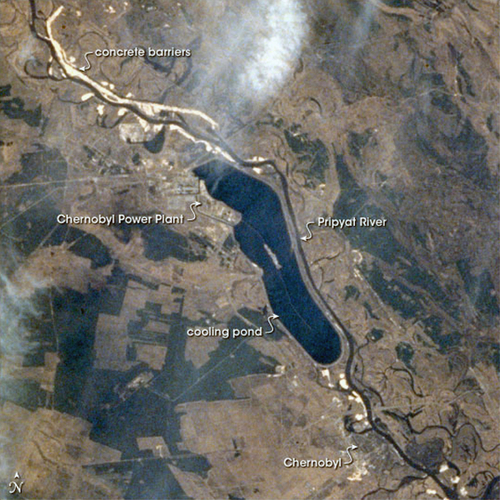
After the accident, access to the area in a 30-km (18-mile) radius around the plant was closed, except for persons requiring official access to the plant and to the immediate area for evaluating and dealing with the consequences of the accident and operation of the undamaged units. 116,000 people were evacuated from the most heavily contaminated areas in 1986 and another 230,000 people in subsequent years. Pripyat, the town near Chernobyl where most of the workers at the plant lived before the accident, was evacuated several days after the accident because of radiological contamination. It was included in the 30-km exclusion zone around the plant and is still closed to all but those with authorized access.
The Chernobyl accident caused many severe radiation effects almost immediately. Among the 600 workers present on the site at the time of the accident, two died within hours of the reactor explosion and 134 received high radiation doses and suffered from acute radiation sickness. Of these, 28 workers died in the first 4 months after the accident. Another 200,000 recovery workers involved in the initial cleanup work of 1986-1987 were exposed to high doses of radiation, between 0.01 and 0.50 grays. The gray is the SI unit for absorbed radiation, which is defined as one joule of deposited energy per kilogram of tissue. To assess the risk of radiation, the absorbed dose is multiplied by the relative biological effectiveness of the radiation to get the biological dose equivalent in rems or sieverts (1 Sv is equal to 100 rem or 100,000 mrem). As time went on, the number of workers involved in cleanup activities at Chernobyl rose to 600,000, although only a small fraction of these workers was exposed to dangerous levels of radiation. Both groups of cleanup and recovery workers may still become ill because of their radiation exposure so their health is still being monitored.
The Chernobyl accident also resulted in widespread contamination in the areas of Belarus, the Russian Federation, and the Ukraine—areas inhabited by millions of residents. Radiation exposure to residents evacuated from areas heavily contaminated by radioactive material from the Chernobyl accident also has been a concern. Average doses to Ukrainian and Belarusian evacuees were 17 and 31 mSv, respectively. Individual exposures ranged from a low of 0.1 to 380 mSv. The majority of the 5 million residents living in contaminated areas, however, received very small radiation doses, which are comparable to natural background levels, which are 1 mSv (or 100 mrem) per year.
The health of these residents has also been monitored since 1986 and to date there is no strong evidence for radiation-induced increases of leukemia or other cancers (other than thyroid cancer). An exception is a large number of children who in 1986 received substantial radiation doses to their thyroid after drinking milk contaminated with radioactive iodine. To date, about 4000 thyroid cancer cases have been detected among these children. Although 99% of these children were successfully treated, nine children in the three countries died from thyroid cancer. No other evidence of any effect on the number of adverse pregnancy outcomes, delivery complications, stillbirths or overall health of children has been observed among the families living in the most contaminated areas.
Apart from the increase in thyroid cancer after childhood exposure, no increase in overall cancer or noncancer diseases have been observed that can be attributed to the Chernobyl accident and exposure to radiation. Nevertheless, it is estimated that approximately 4000 radiation-related cancer deaths may eventually be attributed to the Chernobyl accident over the lifetime of the 200,000 emergency workers, 116,000 evacuees and 270,000 residents living in the most contaminated areas. This estimate is somewhat lower than initial speculations that radiation exposure would claim up to 100,000 lives.
The Fukushima-Daiichi Incident
The Fukushima-Daiichi nuclear incident was caused by a huge earthquake (9.0 on the Richter scale) off the northeast coast of the main Japanese Island of Honshu on March 11, 2011. The earthquake spawned a giant tsunami that created waves up to 128 ft tall in some areas adjacent to the earthquake. The waves were estimated at 45 ft tall when it swamped the Fukushima-Daiichi nuclear power plant, 120 miles away, about 1 h after the earthquake. The power plant is on the northeast coast of Honshu island about 120 miles southwest of the epicenter of the earthquake, which was located about 80 miles offshore from the city of Sendai.
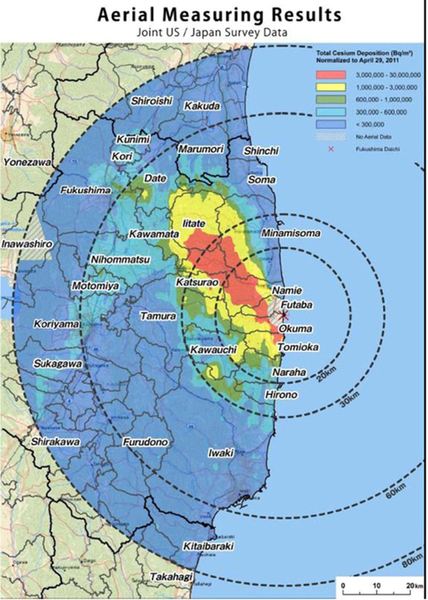
There are 6 reactors at the Fukushima-Daiichi nuclear power plant, but at the time of the quake, reactors 4, 5 and 6 had been shut down for maintenance. Reactors 1, 2 and 3 were operating, but they shut down automatically after the earthquake, with emergency electrical generators starting up to run the water pumps needed to cool the reactor cores. The plant was protected by a seawall designed to withstand a 19-ft tsunami, but when the 45-ft wave arrived the entire plant was flooded, knocking out the generators and the cooling water pumps. This caused a partial core meltdown in the operating reactors 1, 2 and 3. Explosions and severe damage occurred, and radiation leaked out into the surrounding countryside. The entire area, which had been badly damaged by the earthquake and tsunami, had to be evacuated to within 30 km of the plant. Figure 7.9 shows the cumulative radiation contamination as of April 29, 2011. No deaths or severe illnesses can be attributed to the accident, but the incident is ongoing at the time of publication.
Nuclear Fusion Reactors
It is clear from the discussion at the beginning of the chapter that nuclear fusion processes release much more energy than the fission processes; for example, the hydrogen bomb is far more potent than the uranium or plutonium bombs that were dropped on Hiroshima and Nagasaki in 1945. It would be expected, therefore, that a nuclear fusion reactor would be much more efficient and generate much more energy than a nuclear fission reactor. This was the expectation from nuclear scientists. Consequently, a lot of time and effort has been devoted to the development of a fusion reactor. Surprisingly, this effort has failed. In fact, so far no one has been able to build a fusion reactor that even produces more energy than it consumes. The hydrogen bomb clearly puts out more energy than it consumes, but for a nuclear power reactor, the reaction must be controlled so that the energy can be harnessed in an orderly fashion. To initiate the fusion reaction in a controlled environment, very high pressures and temperatures must be generated. This takes more energy than can be recovered from the resulting fusion process. In a hydrogen bomb, the nuclear fusion reaction is initiated by detonating a fission bomb. This technique cannot be used in a nuclear fusion reactor.
In the fusion process, two hydrogen atoms are fused into a helium atom; however, standard hydrogen (which is more than 99.8% of the hydrogen that exists on Earth) has just one proton and no neutrons in its nucleus, whereas a standard helium atom has two protons and two neutrons in its nucleus. The simplest way to create helium using nuclear fusion would suggest using an isotope of hydrogen called deuterium. Deuterium is a hydrogen atom that has one proton and one neutron in its nucleus. 0.0156% of the water molecules in seawater contain deuterium. Due to the large volume of seawater on Earth, there is also a large amount of deuterium that can be made available to fuel fusion reactors on a commercial scale.
This type of water containing deuterium is called heavy water, and it can be separated from seawater using the standard diffusion or centrifuge processes described earlier. The deuterium is liberated from the water using electrolysis to produce pure deuterium. When two deuterium atoms are fused with each other, however, one of the neutrons escapes and light helium (He-3) is created. A second possibility is one of the two deuterium atoms sheds a neutron that is captured by the other to create tritium (which is hydrogen with two neutrons and one proton in its nucleus). This is shown in the following two reactions:
![]()
![]()
It turns out that there is an easier fusion process to initiate and that is to fuse the two main isotopes of hydrogen, deuterium, and tritium, which produces standard helium plus one neutron.
![]()
Deuterium is twice as heavy as standard hydrogen (1H) which makes the separation from seawater rather easy compared to the uranium enrichment process. Tritium (3H or 3T) is also an isotope of hydrogen, but it does not occur naturally, so it is usually manufactured from lithium using the following nuclear reactions:
![]()
![]()
The reactant neutron can be supplied by the deuterium-tritium (D-T) fusion reaction shown above. The reaction with 6Li is exothermic, providing a small energy gain for the reactor. The reaction with 7Li is endothermic, but does not consume the neutron. At least some 7Li reactions are necessary because some neutrons are lost by reactions with other elements. Most reactor designs use the naturally occurring mix of lithium isotopes (7Li and 6Li).
The D-T nuclear reaction is the easiest fusion reaction to initiate, but it has some disadvantages.
• It produces neutrons that result in increased radioactivity within the reactor structure
• Only about 20% of the fusion energy yield appears in the form of charged particles, which limits the extent to which direct energy conversion techniques might be applied
• The tritium supply currently depends on lithium resources, which are less abundant than deuterium resources, and
• Tritium is radioactive and hard to handle.
The neutron flux in a commercial D-T fusion reactor has been estimated to be more than 100 times the neutron flux of current fission power reactors. In a commercial reactor, the neutrons produced could be used to react with lithium in order to create the tritium. This also channels the energy of the neutrons into the lithium. This reaction protects the reactor envelope from the neutron flux.
Though more difficult to initiate than the D-T reaction, the Deuterium-Deuterium (D-D) nuclear fusion reaction has certain advantages. The D-D reaction has two branches as shown above. The optimum energy for the initiation of the D-D reaction is 15 keV, which is slightly higher than the optimum for the D-T reaction. The first reaction does not produce neutrons, but it does produce tritium. Most of the tritium produced will be consumed in the D-T reaction before leaving the reactor, which reduces the tritium handling required, but also means that more neutrons are produced and that some of these are very energetic. The neutron produced from the second branch of the D-D reaction has an energy of only 2.45 MeV (0.393 pJ), whereas the neutron from the D-T reaction has an energy of 14.1 MeV (2.26 pJ), resulting in more isotope products and more material damage than occurs in the D-D reaction. Assuming complete tritium burn-up, the reduction in the fraction of fusion energy carried by neutrons is only about 18%, so the primary advantage of the D-D fuel cycle is that tritium production is not required. Other advantages are independence from limitations of lithium resources and less energetic neutron production.
Current research technology uses a magnetic torus to confine the deuterium fuel as plasma in a small enough space to create the reaction. Lasers have also been proposed to confine the plasma fuel.
The largest fusion reactor experiment so far has been the Joint European Torus (JET) in Culham, England. In 1997, JET produced a momentary peak of 16 MW of fusion power which was only 65% of the input power, with the output fusion power of 10 MW being sustained for just half a second. The next generation fusion reactor, ITER, is currently under construction in Cadarache, France. It is designed to produce 10 times more fusion power than the power put into the plasma, but it will only operate for a few minutes at a time. After 60 years of unsuccessful attempts to develop a commercial fusion reactor, research continues but there is considerable skepticism about its commercial viability.
There have been claims over the years of simpler cheaper methods of creating fusion reactions, but no commercial process has been demonstrated. The most famous of these experiments was the cold fusion claims of Martin Fleischman and Stanley Pons at the University of Utah in 1989. Pons and Fleishman announced results of an experiment where they electrolyzed heavy water using a palladium electrode. They measured more heat coming from the electrolysis chamber than was being input by the electrolysis electrodes and surmised that they had created cold fusion in the palladium electrode. Subsequent scrutiny discounted this as being caused by a fusion reaction. Nevertheless, some researchers still believe that cheap cold fusion processes are a possibility.
The Future of Nuclear Power
Because of the limited supply of uranium, it might seem that with current commercial technology, nuclear power might not be able to play an expanded role in the future energy resources of the world or of the United States. A doubling in the uranium price, however, is likely to lead to a 200-year supply, which would allow expansion of nuclear power to triple what it is now. Moreover, if public concerns about safety can be addressed, it is also feasible that breeder reactor technology does show promise for even more greatly increased nuclear power resources to be used. Breeder reactors can be based on both uranium (U-238) and thorium (Th-232) fuels, and there are sufficient quantities of these fuels to supply a lot of breeder reactor power for a very long time. Until the technology is further developed and commercial breeder reactors are built, however, nuclear power may remain at today’s levels.
