Ethanol, Biodiesel, and Biomass
Abstract
In the United States, ethanol is generated from corn and has been strongly promoted as a renewable and clean energy source. However, careful analysis shows that it is not carbon neutral because significant pollution is generated from the farming machinery used to grow the corn and from the fuel used for the distillation of the alcohol. Moreover, diverting corn for energy use has driven up the price of corn-based foods. Ethanol is rapidly falling out of favor again, and the same issues apply to biodiesel. The only feedstock that makes sense for biodiesel is waste vegetable oil. But the supply of such oil is limited.
In many respects, biomass has had a bright past. Prior to 1900, it was the chief energy source used in the world, and it continues to maintain a strong presence today. In Chapter 1, Figure 1.1 shows that biomass was overtaken by coal as the number one energy source in the world around 1910 (depending on the data source). The main biomass energy source represented in Figure 1.1 is wood. People have cut down trees or gathered fallen wood and burned them for fuel since prehistoric times. Wood and other biomass was burned to heat houses and other buildings and to provide fuel for cooking food. In fact, it continues to be used that way today. When I was a boy growing up in the small town of Chinchilla (in Queensland, Australia), our house had a wood-burning stove in the kitchen. Not only was it used for cooking, but also it heated the hot water for the house and was the only source of space heating. We regularly bought a load of eucalyptus wood from a supplier, and one of the chores required of my brothers and me was to split the wood with an ax into pieces small enough to be burned in the stove. We kept that stove burning almost continuously. Apart from this wood, the only other energy source used in our house was electricity that powered the lights, a washing machine (no dryer) and a single radio. We even solar dried our clothes on a clothesline. Today, homes are typically heated with natural gas, propane or heating oil. Clothes are dried in a dryer powered by gas or electricity. While wood is a less common energy source today, particularly in the Western world, many people would still like to see its use expanded in the future.
Biomass is viewed as a clean and renewable energy source because it can be renewed and replaced by growing new plants and trees. When new trees are grown, they capture carbon dioxide from the atmosphere that is created when they are burned. If every tree that is burned is replaced by a new tree, biomass is truly renewable and carbon neutral. This, however, has not always been true. In the middle ages in Europe, most of the European forests were cut down and burned, but were not replaced. This was done not only to provide an energy source, but also to provide potash for glass and soap manufacturing. This practice was not carbon neutral or renewable because new trees were not grown to replace the ones chopped and burned.
Various forms of biomass, including wood, can be also converted into ethanol and other fuels to provide transportation fuels. In the United States, ethanol is generated from corn and has been strongly promoted as a renewable and clean energy source. Careful analysis, however, shows that it is not carbon neutral because significant pollution is generated from the farming machinery used to grow the corn and from the fuel used for distillation of the alcohol. Moreover, diverting the corn to energy use has driven up the price of corn-based foods. The idea that a vital food resource is being used to make fuel to drive our automobiles is distasteful to some people. Due to this, ethanol is rapidly falling out of favor again in the United States. In Brazil, a large ethanol fuel program is based on sugarcane which has many advantages over corn-based fuel. It is cheaper and produces bagasse (the fibrous residue that remains after sugarcane is crushed to extract the juice)—a by-product that can be used as an energy source to distill the ethanol.
Biomass has three relevant components: cellulose, starches, and sugars. All of these can be burned directly to release energy. Starches and sugars can also be readily converted into ethanol via the biological fermentation process; however, this process is only capable of making ethanol up to about 12% purity. After that, the microorganisms begin to die because the ethanol they are producing as a waste product becomes poisonous to them. Consequently, after fermentation, the ethanol produced must be concentrated by a distillation process. This takes energy to achieve. Cellulose can also be converted into liquid fuels (such as ethanol, gasoline, or diesel) or burned to provide heat.
Ethanol Production
The largest producers of fuel ethanol in the world are shown in Table 12.1. The top two are the United States and Brazil that dominate world production. The largest producer, the United States, makes all of its ethanol from corn stocks. The second largest producer, Brazil, uses sugarcane.
Table 12.1
Fuel Ethanol Production in the World (Thousands of Barrels of Ethanol per Day)
| Rank | Country | 2005 | 2006 | 2007 | 2008 | 2009 |
| 1 | USA | 254.7 | 318.6 | 425.4 | 605.6 | 713.5 |
| 2 | Brazil | 276.4 | 306.1 | 388.7 | 466.3 | 449.8 |
| 3 | China | 20.7 | 24.1 | 28.7 | 34.3 | 37.0 |
| 4 | France | 2.5 | 5.0 | 9.3 | 17.0 | 21.5 |
| 5 | Canada | 4.4 | 4.4 | 13.8 | 16.3 | 18.7 |
| 6 | Germany | 2.8 | 7.4 | 6.8 | 10.0 | 13.0 |
| 7 | Spain | 5.2 | 6.9 | 6.2 | 5.9 | 8.0 |
| 8 | Jamaica | 2.2 | 5.2 | 4.9 | 6.4 | 6.9 |
| 9 | Thailand | 1.2 | 2.2 | 3.0 | 5.7 | 6.9 |
| 10 | India | 3.7 | 4.1 | 4.5 | 4.6 | 5.8 |
| 11 | Colombia | 0.5 | 4.6 | 4.7 | 4.4 | 5.2 |
| 12 | Australia | 0.4 | 1.3 | 1.4 | 2.5 | 3.5 |
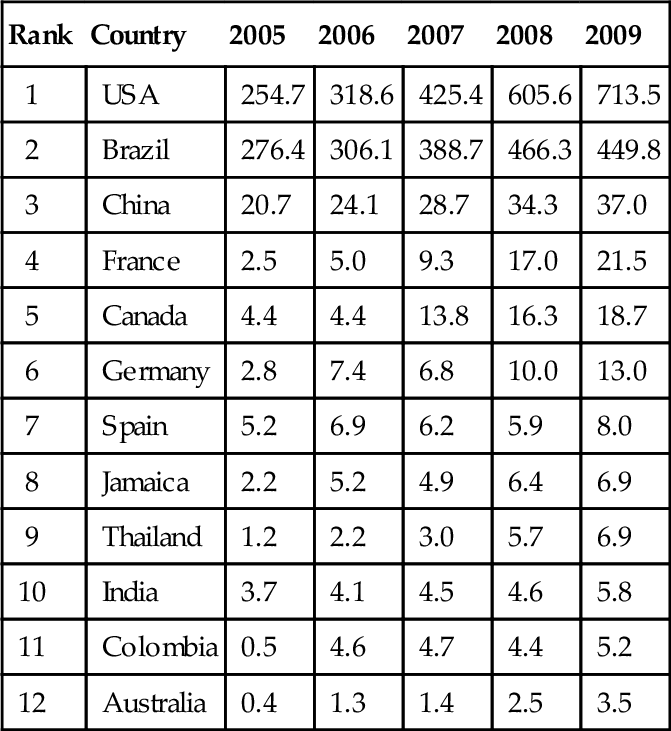
Data Source: EIA.
The monthly fuel ethanol and biodiesel production (in barrels per day) in the United States, for the past 30 years, is shown in Figure 12.1. The United States is now producing over 900,000 barrels per day of fuel ethanol, around 5% of the country’s liquid fuel supply. Beginning in 2002, the fuel ethanol production was ramped up in response to subsidies instituted by the federal government. The main argument for these subsidies was that ethanol is a clean-burning renewable fuel with a high octane number. Its octane value using the (R + M)/2 formula is 99, which is significantly higher than regular gasoline (which is around 85-87). As explained in the next section, however, ethanol also has a low-energy density that results in a lower fuel economy than gasoline.
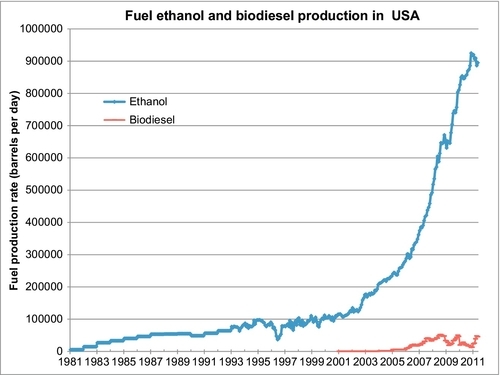
The second supporting argument for ethanol subsidies is that it is produced from indigenous sources in the United States and displaces imported oil from the fuel supply. The arguments against this are that ethanol is primarily produced from corn. This leads to an increase in the price of corn which is an important element of the U.S. food supply. Moreover, if imported oil is used to plow the fields and harvest the corn and to distill the fermented ethanol, the amount of displaced imported oil is not nearly as high as the ethanol production numbers indicate. This factor is a function of the fuel production efficiency measured by the energy balance in producing ethanol.
Most of the ethanol produced in the world is generated from corn or sugarcane. Whether corn or sugarcane is used, it needs to be grown, collected, crushed, processed into pure sugar, fermented and distilled into pure ethanol. All of these steps require energy use. The total amount of energy input into the process compared to the energy released by burning the resulting ethanol fuel is known as the energy balance. Energy balance estimates from different sources can be contradictory and can inspire passionate intellectual debates. Several credible researchers (e.g., Pimentel, Keeney and DeLuca, Ho) have concluded that the energy balance is actually negative. In other words, it takes more energy to produce a gallon of ethanol than is recovered when it burns as fuel. A comprehensive study by Shapouri, Duffield and Wang of the USDA, completed in 2002, concluded that the ratio of energy out to energy in (the energy balance’s defining number) is 1.34. This means that 34% more energy is obtained from the ethanol fuel than is required to produce it. To get to that number, the authors include energy credits for co-products produced along with the ethanol. These include distiller’s dried grains, corn oil, corn gluten meal, and corn gluten feed from wet milling.
Opponents often overstate this number, assuming that the one unit of energy used to produce the 1.34 energy units of ethanol is all imported oil. In actual fact, a lot of the energy used to distill the ethanol is domestic coal or natural gas. In 2007, National Geographic Magazine estimated that the energy balance number is 1.3 (i.e., one barrel equivalent of fossil-fuel energy is required to create 1.3 barrels of oil equivalent energy from the resulting corn ethanol). The energy balance for sugarcane ethanol produced in Brazil is more favorable: one unit of fossil-fuel energy is required to create eight energy units from the sugarcane-derived ethanol. Sugarcane is easier to grow than corn and the sugarcane bagasse (the dried cane cellulosic residue) is usually the fuel burned to distill the fermented ethanol.
Most of the corn ethanol produced in the United States is blended at a ratio of 10% ethanol to 90% gasoline and thus is known as E10. Pure ethanol (E100) and an 85% ethanol blend (E85) are also available. E85 is the most common blend available in Brazil.
Fuel Economy of Ethanol
Pure ethanol has a high octane number (99), but an energy density that is 32.5% lower than gasoline (see Table 2.4). This means that adding ethanol in any proportion to regular gasoline will increase the octane number, but will lower the fuel economy. This might seem contradictory because most people believe that high octane fuels will give them a higher fuel economy (measured as miles per gallon). Octane rating, however, is not a measure of the energy content of the fuel, but instead is a measure of its ability to burn at high compression ratios without auto-igniting.
In theory, all vehicles have a fuel economy that is directly proportional to the fuel’s energy density; however, there are many variables that affect the performance of a particular fuel in a particular engine. The compression ratio of the engine has a significant effect. Using the data in Table 2.4, ethanol contains approximately 32.5% less energy per unit volume than gasoline. In theory, burning pure ethanol in a vehicle will result in a 32.5% reduction in miles per gallon compared to burning pure gasoline. Given that ethanol has a higher octane rating, the engine can be made more efficient by raising its compression ratio. This can make the fuel economy of ethanol almost constant for any blend. This is not easy to do. So, ethanol blends in practice give a lower fuel economy. For E10 (10% ethanol and 90% gasoline), the effect is small (3.3%) when compared to conventional gasoline and even smaller when compared to boutique and reformulated gasoline blends. For E85 (85% ethanol), the effect becomes more significant. E85 will produce about 27.5% lower mileage than gasoline and hence will require more frequent refueling. Flex fuel vehicles are more optimized towards ethanol blends. Based on EPA tests for 2006 E85 models, the average fuel economy for E85 vehicles resulted in 25.6% lower mileage than regular unleaded gasoline.
Biodiesel Production
Figure 12.1 also shows the production of biodiesel in the United States. Biodiesel production now has a capacity of about 45,000 barrels a day, significantly less than ethanol production. It is also somewhat cyclical in nature, although Figure 12.1 only shows the commercial production of biodiesel. There is also a lot of biodiesel produced in small batch systems for personal use. The EIA data plotted in Figure 12.1 does not reflect this noncommercial production.
The name biodiesel implies that the same diesel fuel made from crude oil can be manufactured from biological materials, but this is not true. Diesel fuel is a mixture of long-chain alkane molecules, such as decane (the C10 alkane), dodecane (the C12 alkane), and eicosane (the C20 alkane). What has come to be known as biodiesel is actually a mixture of methyl esters that are manufactured from oils and fats. All oils and fats, whether from animal or vegetable sources, are made up of fatty acids called triglycerides. There are 14 principal triglycerides or fatty acids found in oils and fats. These are shown in Table 12.2. The percentage of the 14 triglycerides found in common fat and oil sources is shown in Table 12.3. All of these triglycerides can be reacted with methanol to make methyl esters. Methyl esters can be burned in diesel engines as diesel fuel. These esters have a high cetane number. This makes them a good blending stock to combine with regular crude oil-based diesel fuel, but they can also be burned directly in diesel engines. They do, however, have one major disadvantage: they have a relatively high pour point, which means they tend to gel up (solidify) in cold weather. This disadvantage can be overcome when they are blended with regular crude oil-based diesel fuel at low proportions. They can only be used directly as diesel fuel (known as B100) in the summer or in warm climates.
Table 12.2
The Fourteen Principal Fatty Acids Found in Fats and Oils
| Fatty Acid | No. of Carbons and Double Bonds | Chemical Structure | Melting Point (°C) | Boiling Point (°C) |
| Caprylic | C8 | CH3(CH2)6COOH | 16.5 | 239 |
| Capric | C10 | CH3(CH2)8COOH | 31.3 | 269 |
| Lauric | C12 | CH3(CH2)10COOH | 43.6 | 304 |
| Myristic | C14 | CH3(CH2)12COOH | 58 | 332 |
| Palmitic | C16:0 | CH3(CH2)14COOH | 62.9 | 349 |
| Palmitoleic | C16:1 | CH3(CH2)5CH | 33 | – |
| Stearic | C18:0 | CH3(CH2)16COOH | 69.9 | 371 |
| Oleic | C18:1 | CH3(CH2)7CH | 16.3 | – |
| Linoleic | C18:2 | CH3(CH2)4CH | − 5 | – |
| Linolenic | C18:3 | CH3(CH2)2CH | − 11 | – |
| Arachidic | C20:0 | CH3(CH2)18COOH | 75.2 | – |
| Eicosenoic | C20:1 | CH3(CH2)7CH | 23 | – |
| Behenic | C22:0 | CH3(CH2)20COOH | 80 | – |
| Eurcic | C22:1 | CH3(CH2)7CH | 34 | – |
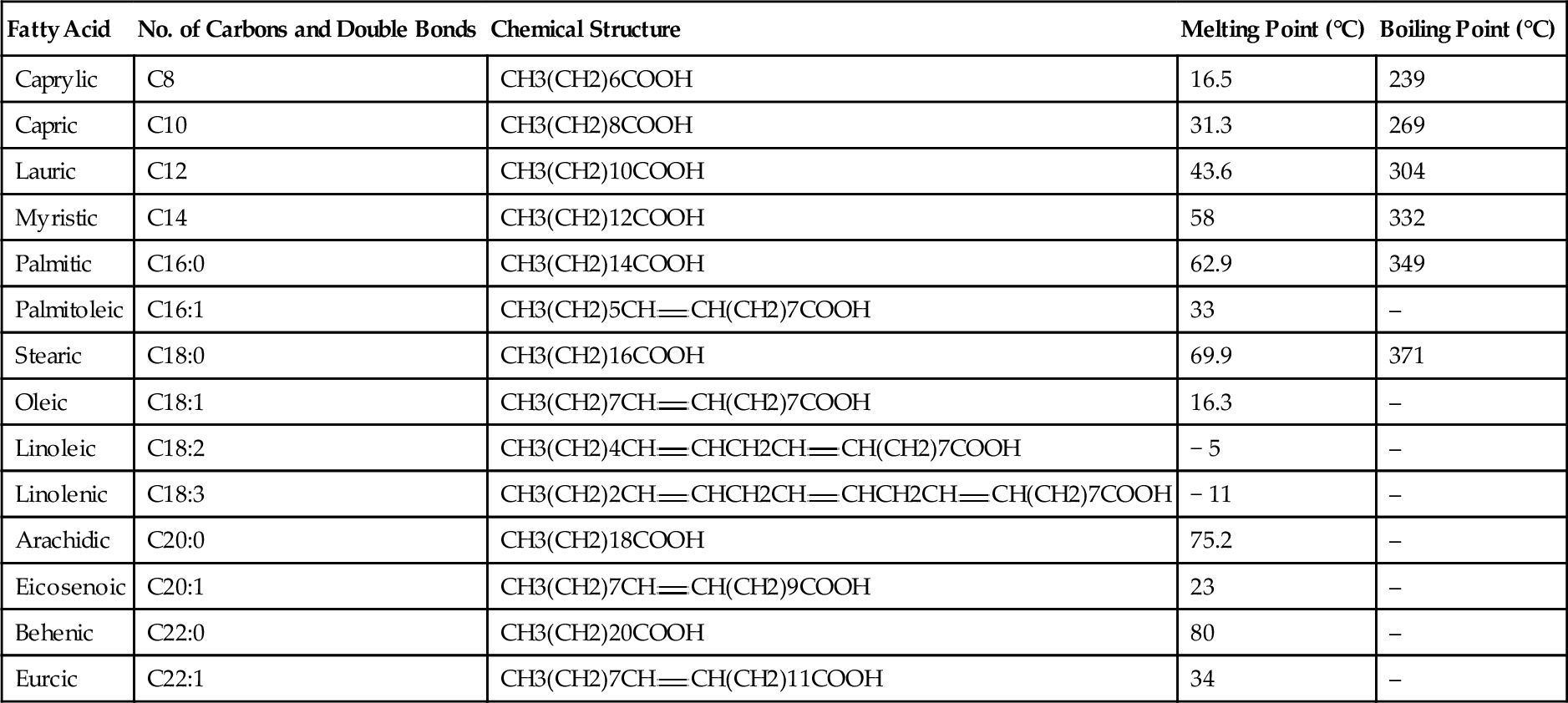
Table 12.3
Percentage of Fatty Acid Types in Various Oils and Fats
| Fatty Acid Fat or Oil | C8:0 | C10:0 | C12:0 | C14:0 | C16:0 | C16:1 | C18:0 | C18:1 | C18:2 | C18:3 | C20:0 C22:0 | C20:1 C22:1 | Other |
| Yellow Grease | 1 | 23 | 1 | 10 | 50 | 15 | |||||||
| Tallow | – | – | 0.2 | 2-3 | 25-30 | 2-3 | 21-26 | 39-42 | 2 | – | 0.4-1 | 0.3 | 0.5 |
| Lard | – | – | – | 1 | 25-30 | 2-5 | 12-16 | 41-51 | 4-22 | – | , | 2-3 | 0.2 |
| Butter | 1-2 | 2-3 | 1-4 | 8-13 | 25-32 | 2-5 | 25-32 | 22-29 | 3 | – | 0.4-2 | 2-1.5 | 1-2 |
| Coconut | 5-9 | 4-10 | 44-51 | 13-18 | 7-10 | – | 1-4 | 5-8 | 1-3 | – | – | – | – |
| Palm Kernal | 2-4 | 3-7 | 45-52 | 14-19 | 6-9 | 0-1 | 1-3 | 10-18 | 1-2 | – | 1-2 | – | – |
| Palm | – | – | – | 1-6 | 32-47 | – | 1-6 | 40-52 | 2-11 | – | – | – | – |
| Safflower | – | – | – | – | 5.2 | – | 2.2 | 76.3 | 16.2 | – | – | – | – |
| Peanut | – | – | – | 0.5 | 6-11 | 1-2 | 3-6 | 39-66 | 17-38 | – | 5-10 | – | – |
| Cottonseed | – | – | – | 0-3 | 17-23 | – | 1-3 | 23-41 | 34-55 | – | – | 2-3 | – |
| Corn | – | – | – | 0-2 | 8-10 | 1-2 | 1-4 | 30-50 | 34-56 | – | – | 0-2 | – |
| Sunflower | – | – | – | – | 6 | – | 4.2 | 18.7 | 69.3 | 0.3 | 1.4 | – | – |
| Soybean | – | – | – | 0.3 | 7-11 | 0-1 | 3-6 | 22-34 | 50-60 | 2-10 | 5-10 | – | – |
| Rapeseed | – | – | – | – | 2-5 | 0.2 | 1-2 | 10-15 | 10-20 | 5-10 | 0.9 | 50-60 | – |
| Linseed | – | – | – | 0.2 | 5-9 | – | 0-1 | 9-29 | 8-29 | 45-67 | – | – | – |
| Tung | – | – | – | – | – | – | – | 4-13 | 8-15 | 72-88 | – | – | – |
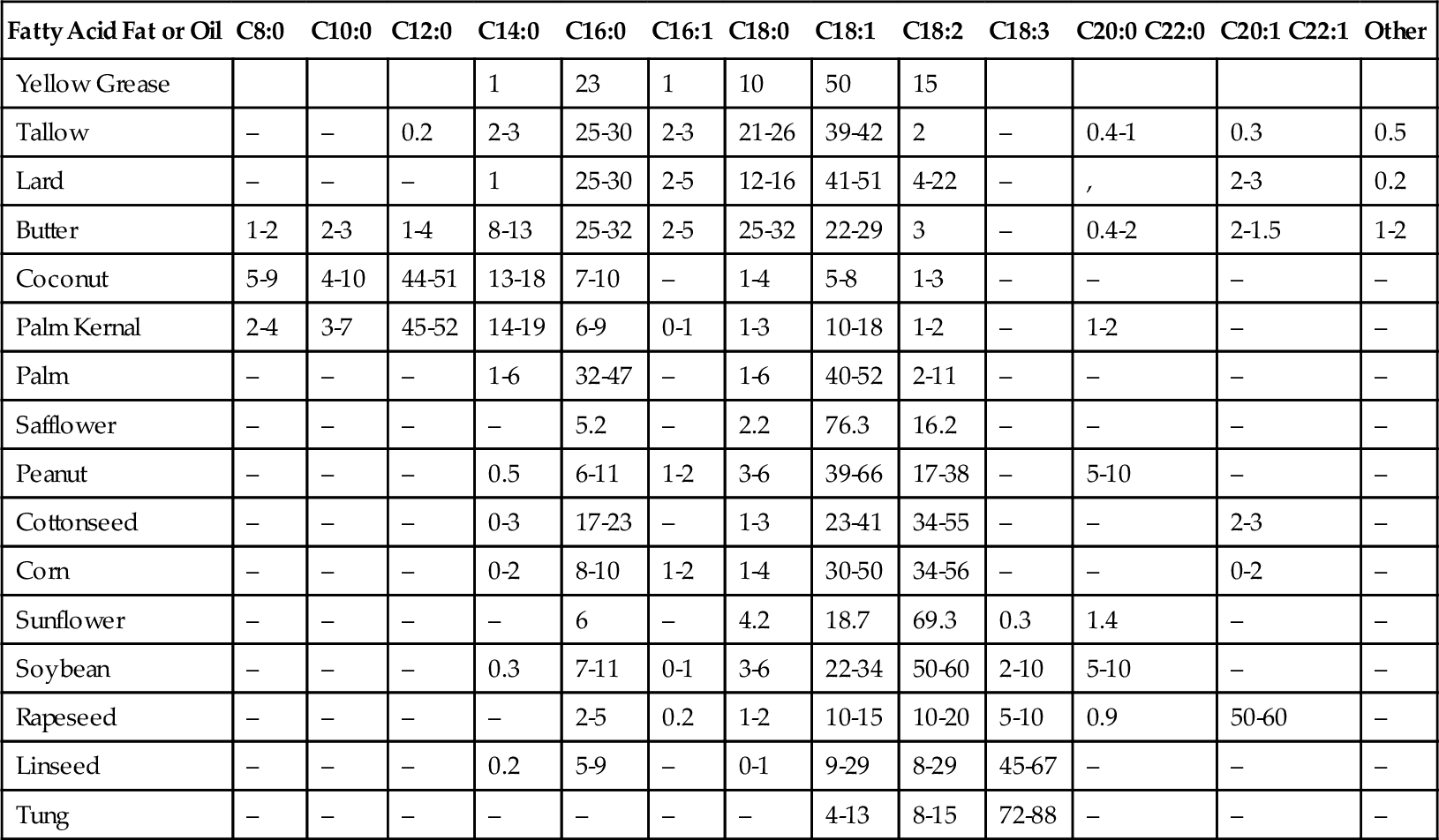
Biodiesel is typically made by reacting Straight Vegetable Oil (SVO), Waste Vegetable Oil (WVO), or animal tallow with methanol, which is known as the trans-esterification reaction. WVO, also known as yellow grease, is mostly composed of C18 fatty acids with one double bond and C16s with no double bonds. The volume of biodiesel produced is about equal to 90% of the volume of WVO that is supplied to the reaction process; the other 10% is primarily glycerol. In general, the reaction is
![]()
The catalyst used is either sodium hydroxide (NaOH) or potassium hydroxide (KOH). The glycerol is a by-product that has many uses. While most uses require small volumes, it can also be made into methanol in a process described later.
A lot of biodiesel is made from WVO in small home-processing kits similar to the one shown in Figure 12.2 from Reliance Energy Resources.
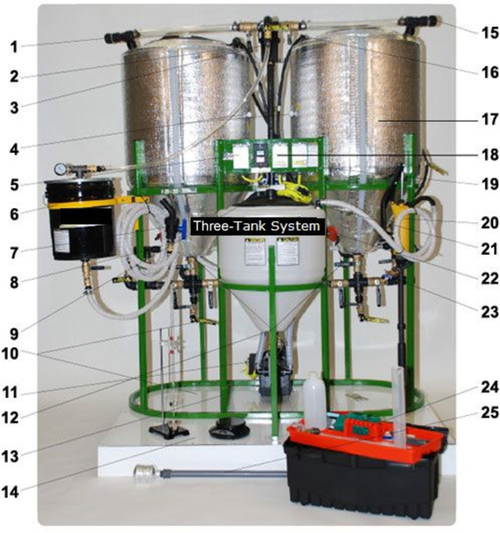
The WVO is fed from a transportation container to one of the large tanks where the biodiesel process starts. The small central tank holds a mixture of methanol and catalyst (sodium or potassium hydroxide) to react with the vegetable oil. These chemicals are mixed in the correct proportions and fed into one or both of the large tanks containing the WVO and then the components react for a set period of time. After the reaction has finished and the glycerol has separated from the biodiesel by gravity, the glycerol is drained off and a water wash occurs. Mist washing is a critical part of making high-quality biodiesel. The mist heads are metered at a precise flow rate to achieve thorough washing. The water wash occurs in the same tank where the reaction occurred.
After the biodiesel is water washed, it is allowed to settle so that all of the water comes out of the biodiesel. The settling of the glycerol and water from the biodiesel is assisted by using a sonic agitator that reduces the duration of the settling period from about a day to a few hours. Once the settling is complete, the biodiesel can be separated from the water and pure biodiesel remains. The purpose of washing the methyl ester layer is to get rid of impurities such as dissolving glycerols and the remaining traces of catalyst. The methyl ester is usually tested to make sure that the quality meets the minimum standards for diesel fuel. The main waste by-product from this process, glycerol, is currently not very valuable due to the high production that has occurred in the United States by home and commercial biodiesel producers. There is, however, a solution to this problem as discussed below.
The trans-esterification of oil involves a reaction between the triglycerides and methanol in the presence of the catalyst, yielding a mixture of fatty acid methyl esters, which is a combination of the biodiesel and glycerol. One problem associated with the use of WVO is the high content of free fatty acids (FFAs), which can neutralize the caustic (KOH or NaOH) catalyst and form fatty acid salts as an unwanted product. Free fatty acids are components of the triglyceride molecule that break off during the cooking process. When there are too many FFAs in the cooking oil mixture, the oil is no longer useful for cooking and must be discarded or converted into biodiesel. The triglyceride-methanol reaction is catalyzed (sped up) by the hydroxides (NaOH or KOH). These hydroxides are not consumed by the triglyceride-methanol reaction, but the FFAs do react with the hydroxides resulting in loss of catalyst. Elimination of FFAs can be done through esterification using methanol and a sulfuric acid catalyst before proceeding to the trans-esterification step. After the esterification pretreatment, WVO is heated to about 120 °F in order to speed up the trans-esterification reaction. The trans-esterification product obtained is allowed to separate, and the aqueous layer containing some traces of glycerol and catalyst is drained out.
Another important part of the system shown in Figure 12.2 is an Ultra-Sonic Frequency Generator (USFG). The USFG improves the performance of the system in two ways: it causes the reaction to proceed at a faster rate and in higher yields, and it aids in the settling process between the organic and aqueous layers. It is thought that the USFG does this by improving agitation and mixing throughout the system. The improvement in mixing is due to induced cavitation of the liquid. This is effected by creating alternating low and high pressure bubbles, which cause certain areas to collapse rapidly and increases the shear force gradients. The ultra-sonication also aids in the separation process. Typically, a biodiesel batch will separate in 10-24 h. With the addition of an USFG, this time can be reduced to less than one hour. An USFG can also improve catalyst efficiency. The amount of catalyst needed for the average batch can be reduced by 50%. Other benefits from ultra-sonication include higher purity in the glycerol by-product and less necessity for excess methanol in the reaction.
Glycerol is the only major by-product in this process. Its main characteristics include
• It is a neutral, sweet tasting, colorless, thick liquid that can be frozen to a thick paste and has a high boiling point
• It is not poisonous or toxic
• It can easily dissolve in water or alcohol, but not in oils. It is also a good solvent. There are many substances which will dissolve in glycerol that will not dissolve in water or alcohol
• It is highly hygroscopic, which means that it absorbs water from the air. For example, if you leave a beaker of glycerol exposed to the air, it would quickly become a miscible mixture that is 80% glycerol and 20% water
• It is widely used to manufacture capsules, suppositories, ear infection remedies, anesthetics, cough remedies, lozenges, and gargles
• It is also used to moisten, sweeten or preserve many foods and drinks such as soft drinks, candies, cakes, meat, and cheese
• It is used in textiles to soften the yarn and to lubricate fibers of different kinds
• It is used as a lubricant in food processing to manufacture resin coatings, to add flexibility to rubber and plastic and as a building block to manufacture flexible foams
• It is used to manufacture dynamite and to create components that are used in radios and neon lights.
Despite all of these uses, the biodiesel industry has created an oversupply of glycerol, which has caused its price to plummet.
It is possible to transform glycerol back into methanol, which is the preferred method of disposal. In fact, there are catalysts available on the market which can perform this conversion at low temperatures and pressures. Edman Tsang, a researcher in the Department of Chemistry at the University of Oxford (England), has patented a technology that uses a metal catalyst to convert glycerol into methanol by reacting it with hydrogen. This process works at a temperature of 100 °C (212 °F) and at 20 bars of pressure. The process produces only methanol and no by-products according to the reaction
![]()
![]()
Once produced, the methanol can then be reused in the next triglyceride batch. The net overall biodiesel reaction then becomes
![]()
Adoption of this solution will solve the glycerol oversupply problem.
Direct Conversion of Woody Biomass into Gasoline, Diesel and Other Liquid Fuels
The cellulose in woody lignin-based biomass (such as trees) and the bagasse from sugarcane and switch-grass cannot be converted into liquid fuels as easily as sugars and starches. This is the subject of much research. Throughout history, the energy available in woody biomass has been traditionally released by direct burning. The resulting energy can be captured as heat to maintain the temperature of a living space, which has been its primary use. It can also be converted into electricity by using the heat energy to generate steam to drive a steam turbine. Because so much energy is consumed as liquid fuels, a process for converting the woody biomass into liquid fuels is very desirable. One way to do this is via the syngas pathway. To do this, wood lignins are first converted into syngas (hydrogen and carbon monoxide) in a gasifier by reacting it with steam and oxygen. The syngas is then used to make diesel or gasoline typically using the Fischer-Tropsch process or the methanol pathway. The same methods are used to make liquid fuels from natural gas or coal. These processes are covered in Chapter 6 for natural gas and in Chapter 13 for coal.
One of the more innovative biomass-to-liquid fuel conversion processes belongs to a newly formed company called KiOR, which was founded in 2007 by Khosla Ventures (KV) and a group of catalyst scientists who had an idea for making renewable fuels from cellulosic biomass through a one-step catalytic process. The KiOR process for catalytic conversion of biomass to a hydrocarbon fuel has significant implications because it is a simple and inexpensive process. It uses a relatively abundant low-cost feedstock, trees and, in KiOR’s particular case, the Southern Yellow Pine.
KV made a $10 million investment in KiOR to fund the Company’s R&D program and pilot plant. The pilot plant was an opportunity for KiOR’s proof of concept: that cellulosic feedstock could be converted into renewable fuel through a scalable, relatively inexpensive catalytic process. Encouraged by technical success at the pilot scale, KiOR accelerated the time scale after just a few months of pilot testing and began work on a demonstration unit, with a capacity to process 10 dry tons of wood and produce up to 15 barrels of product per day. This is a small production volume, but a commercial unit would be 1000 barrels per day. KiOR then arranged meetings with three southeastern state governors to discuss the facility's location. Within one week, KiOR had agreed to build a small commercial scale plant in Mississippi due to Governor Haley Barbour’s enthusiasm for the project and the benefits it could offer his state. The MS legislature approved a $75 million interest-free loan for the plant in September 2010. By the first quarter of 2011, construction was under way for an initial-scale commercial production facility in Columbus, Mississippi, which is shown in Figure 12.3.

The company’s technology platform combines its new catalyst systems with a process based on existing FCC technology, a standard process used in oil refining. The efficiency of KiOR’s process, called biomass fluid catalytic cracking, and the proven nature of catalytic cracking technologies allow for significant cost advantages including lower capital and operating costs versus traditional biofuel producers. KiOR processes its renewable oil product in a conventional hydrotreater, which is a standard process unit used in oil refineries, into gasoline and diesel blend-stocks that can be combined with existing fossil-based fuels and used in vehicles on the road today.
KiOR began construction of its first commercial scale facility, located in Columbus, Mississippi, in early 2011. The facility will produce gasoline and diesel from Southern Yellow Pine. The gasoline and diesel product is intended as a blending-stock with regular fossil fuels. KiOR has already established purchase agreements for the site’s entire fuel output. The company chose the Columbus location due to the site’s proximity to abundant, locally grown tress and its access to a shipping infrastructure. The facility is designed as an initial scale commercial facility, processing 500 dry tons of wood per day. Once fully operational, it is expected to produce over 11 million gallons of gasoline, diesel and fuel oil blend-stocks annually. This is equivalent to 720 barrels of oil per day, a very small commercial size production plant, but economically significant.
KiOR currently has sales agreements in place with Hunt Refining, Catchlight Energy (a joint venture between Chevron Corporation and Weyerhaeuser Company) and FedEx Corporate Services. Catchlight Energy also has an agreement with KiOR to supply the biomass feedstock for the facility. The facility will cost approximately $190 million to build, and production is scheduled to commence in the second half of 2012.
In the third quarter of 2012, KiOR planned to begin construction of a larger production facility in Newton, Mississippi. KiOR is designing this plant as its standard commercial production plant, which will process approximately 1500 tons of biomass per day, three times the size of its Columbus facility at 2150 barrels per day. By expanding its standard commercial facilities in discrete, independent projects that are each viable on their own, KiOR believes it will have the flexibility to coordinate its growth in response to capital availability and market conditions.
Environmental Issues
The biomass energy source is not without environmental impact. The burning of wood may be renewable, but the environmental impacts can be so severe that the burning of wood in open fireplaces is highly regulated in most communities. These effects, however, can be mitigated. In general, the more efficient the stove, the less pollution it produces. In most places, there are regulations covering how efficient a wood-burning stove must be. According to the U.S. Environmental Protection Agency, the use of wood for residential heating contributes up to 50% of the organic air pollutants being released into the atmosphere, some of which are carcinogenic. During winter months, in areas where wood is the principal heating fuel, wood stoves produce as much as 80% of these types of pollutants. Robert McCrillis of the EPA says, “… In the field, it is the installation and how the stove is operated that has the largest effect on how it performs. Public education on the correct use of a word-burning stove is part of the current regulations.…” To meet federal clean air standards, some areas are regulating the use of wood stoves and banning open fireplaces in new constructions. In order to curb pollution, some communities only allow the installation of EPA-approved Phase II stoves, which combust the wood more completely and are more energy efficient.
In the United States, the manufacturing of ethanol from corn generates significant amounts of pollutants in the planting and harvesting of the corn and the distillation of the fermented alcohol. Moreover, the low energy balance ratio (1.34) for corn-based ethanol means that it generates more pollution than just carbon dioxide. If a policy was adopted in the United States which stated that the corn used for ethanol production had to be cultivated and harvested with equipment powered by pure ethanol and that distillation and processing had to be fueled by ethanol or waste corn stalks and husks, the exact net production of ethanol would be demonstrated. This may result in a lot less ethanol being produced in the country.
The Future of Biomass
There is really only a small and limited future for biomass in the energy supply. It does not make sense to be converting part of the nation’s food supply into fuel. The production of ethanol from corn in the United States should be reduced and the corn redirected back into the food supply. Some additional trees could be grown and burned for fuel or processed into liquid fuels via the gasification and Fisher-Tropsch routes or using the KiOR catalysis process. The arable land available to cultivate trees for this purpose is, however, limited. Most of the available land is already being devoted to growing crops and wood for paper. While it is possible to grow some trees, this will not have a substantial impact on future energy supply. Similarly, growing food crops such as rapeseed, soy and corn to be processed directly from SVO into biodiesel also does not make sense. Again, these crops are needed for the food supply. It does make sense to process waste material, such as WVO and tallow into biodiesel; however, the supply of these is so limited that it will always be a small niche market. Generally, this means that energy from biomass cannot and should not increase much.
