How Is Energy Measured
Abstract
The units used to measure energy are varied, but it is useful to understand the equivalencies between those units. Where do we get our energy from today? Are energy sources different in different countries according to the local resources? Many units of energy have a historical basis. Understanding this basis helps us to understand the reason for the many and varied units of energy. Each unit can be related to the others using unit conversions.
The units for energy are surprisingly varied, but it is useful to understand the equivalencies between the different units and the reason for their existence. To derive the units for energy, it is necessary to review the equations from which energy is calculated. While it is not important to fully understand these equations, some equivalencies between the various units used for energy will be discussed later in this chapter. It is also useful to understand that there are various unit systems; however, in any one equation, it is necessary that a consistent unit system be used. The easiest and most widely used unit system is Le Système Internationale d'Unités, the SI unit system. Some people have also referred to this system as the metric system.
Table 2.1 shows the basic units for the common parameters in three different unit systems, the SI system, the British or American customary system, and the centimeter-gram-second (CGS) system.
Table 2.1
Units for Energy Parameters in Three Unit Systems
| Parameter | SI | British | CGS |
| Mass | kilograms (kg) | slugs | grams (g) |
| Distance | meters (m) | feet (ft) | centimeters (cm) |
| Time | seconds (s) | seconds (s) | seconds (s) |
| Velocity | m/s | ft/s | cm/s |
| Acceleration | m/s2 | ft/s2 | cm/s2 |
| Force | newtons (N) | pounds (lbf) | dynes |
| Energy | joules (J) | ft-lbf | erg |
| Power | watt (W) | ft-lbf/s | erg/s |

The three basic units in any unit system are mass, distance, and time. In the SI system, the basic units are kilograms (kg), meters (m), and seconds (s). Velocity is the change in distance with time and is represented as meters per second (m/s). Acceleration is the rate of change of velocity and is represented as meters per second squared (m/s2). According to Newton’s second law of motion, force is equal to the mass times the acceleration. In the SI system, force is measured in newtons (N).
![]()
![]()
Energy is derived from the equation for work (work and heat are forms of energy). Work is equal to force times the distance moved and, in the SI system, is measured in joules (J).
![]()
![]()
Power is the rate of doing work or the rate of energy conversion. In the SI system, it is measured in watts (W).
![]()
![]()
When units are written out in full, capital letters are not used. This includes those units that are named after a person such as newtons, joules, and watts. That is the convention. When units named after a person are abbreviated, they are done so with capital letters. For example, newtons are abbreviated as N, joules are abbreviated as J, and watts are abbreviated as W.
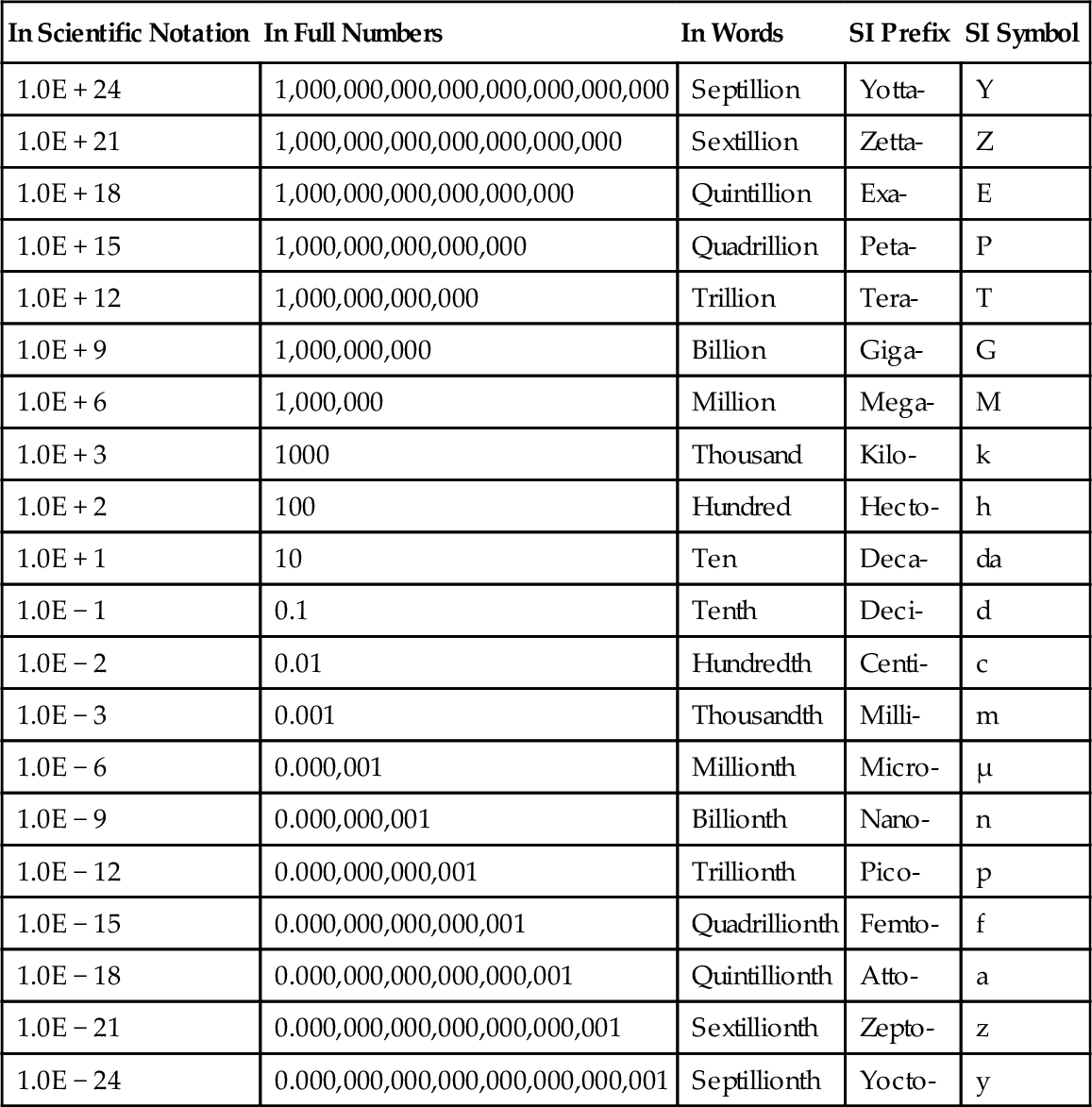
When calculating the amount of energy used by a large country or by the whole world in a year, the numbers can be quite large. When you look at the energy required to accelerate a single subatomic particle in a nuclear reaction, the numbers are very small. That is why in the SI system a variety of prefixes are used to denote a unit being multiplied by a power of 10; for example, the prefix kilo means 1000, 103, or in scientific notation 1E + 3. The prefixes for the powers of 10 are shown in Table 2.2. The large number prefixes are usually abbreviated with a capital letter and the small power prefixes are abbreviated with a small letter; for example, 1E + 15 joules = 1015 joules = 1 petajoule = 1 PJ. Similarly, 1E − 15 joules = 10− 15 joules = 1 femtojoule = 1 fJ. In Figure 1.3, you can see the energy reserves for various countries in exajoules (EJ); 1 exajoule = 1018 joules = 1 EJ. Later in the chapter you will see that 1 BTU is equal to 1055.056 J; 1 EJ is roughly equal to 1 quadrillion BTU, which is sometimes abbreviated as quads. The energy reserves for countries are often given in quads or exajoules, which are roughly equivalent to each other.
Table 2.2
Prefixes Used in SI Units
| In Scientific Notation | In Full Numbers | In Words | SI Prefix | SI Symbol |
| 1.0E + 24 | 1,000,000,000,000,000,000,000,000 | Septillion | Yotta- | Y |
| 1.0E + 21 | 1,000,000,000,000,000,000,000 | Sextillion | Zetta- | Z |
| 1.0E + 18 | 1,000,000,000,000,000,000 | Quintillion | Exa- | E |
| 1.0E + 15 | 1,000,000,000,000,000 | Quadrillion | Peta- | P |
| 1.0E + 12 | 1,000,000,000,000 | Trillion | Tera- | T |
| 1.0E + 9 | 1,000,000,000 | Billion | Giga- | G |
| 1.0E + 6 | 1,000,000 | Million | Mega- | M |
| 1.0E + 3 | 1000 | Thousand | Kilo- | k |
| 1.0E + 2 | 100 | Hundred | Hecto- | h |
| 1.0E + 1 | 10 | Ten | Deca- | da |
| 1.0E − 1 | 0.1 | Tenth | Deci- | d |
| 1.0E − 2 | 0.01 | Hundredth | Centi- | c |
| 1.0E − 3 | 0.001 | Thousandth | Milli- | m |
| 1.0E − 6 | 0.000,001 | Millionth | Micro- | μ |
| 1.0E − 9 | 0.000,000,001 | Billionth | Nano- | n |
| 1.0E − 12 | 0.000,000,000,001 | Trillionth | Pico- | p |
| 1.0E − 15 | 0.000,000,000,000,001 | Quadrillionth | Femto- | f |
| 1.0E − 18 | 0.000,000,000,000,000,001 | Quintillionth | Atto- | a |
| 1.0E − 21 | 0.000,000,000,000,000,000,001 | Sextillionth | Zepto- | z |
| 1.0E − 24 | 0.000,000,000,000,000,000,000,001 | Septillionth | Yocto- | y |
When you receive your electricity bill, the units for the energy used are not joules but kilowatt-hours (kWh). Given that a watt is a joule per second and there are 3600 s in an hour and there are 1000 watts in a kilowatt, 1 kWh = 3,600,000 joules. A typical monthly electric bill might show that you have used 500 kWh charged out at about 10c/kWh. If they had shown the energy usage in joules, it would have been 1.8E + 9 joules, or 1.8 gigajoules (GJ). The electric company uses kWh instead of GJ because people understand kilowatt-hours better than they understand gigajoules. As the electricity bill always uses kWh, electricity consumption and generation tends to be reported in various multiples of watt-hours instead of joules or multiples of joules.
A typical large coal-fired or nuclear-powered electric plant can produce about 1 billion joules of electricity per second, or 1 GJ/s, or 1 gigawatt (GW). Sometimes this is shown as 1000 megawatts (MW) but it really is just a different way of saying the same thing. In 1 day, the 1 GW plant can nominally produce 24 GWh of electricity. In 1 year, the same plant operating 24 h/day and 365 days/year can nominally produce 8760 GWh of electricity. You could also write this as 8.76 terawatt-hours (TWh), which is equivalent to 8.76E + 12 × 3600 joules of energy, or 31.536 PJ.
There are various other units for energy that are also used for various historical reasons. The British Thermal Unit (BTU or Btu) is a unit of energy that, as originally defined in Great Britain, is the amount of energy needed to heat one pound of water by one degree Fahrenheit (°F). Its value can vary depending on the temperature so the International Standards Organization (ISO) has recently set the value to be fixed at 1055.056 joules. A more widely used value, based on the International Steam Table (IT) calorie and defined by the Fifth International Conference on the Properties of Steam (London, July 1956), sets the value of the calorie at exactly 4.1868 joules. This makes the BTU equal to 1055.05585 joules; however, the value set by ISO (1 BTU = 1055.056 joules) is not uncommon.
The calorie is a unit of energy of French origin that, as originally defined, was the amount of energy needed to heat 1 g of water by 1 °C. Its value can also vary depending on the temperature. ISO has recently set the value to be 4.184 joules; however, the value based on the IT calorie was defined by the Fifth International Conference on the Properties of Steam (London, July 1956) to be exactly 4.1868 joules. This conversion factor is also called thermal calories, abbreviated as calth.
The calorie is most widely used today to specify the energy gained by human beings when food is digested. Interestingly, one calorie is such a small unit of energy that the food industry uses the kilocalorie to determine the quantity of energy in food; however, they call a kilocalorie a Calorie (with a capital C) and use Calories and kilocalories interchangeably. Understandably, this can create some confusion. I once knew an engineer who looked up the latent heat of melting for ice cream and found it to be 350 J/g. He then looked up the nutritional data on ice cream and found that it contained three Calories per gram of ice cream. He converted the 350 J/g to 84 Calories/g and came to the incorrect conclusion that it took more energy for the body to melt the ice cream than it gained from digesting it. He reasoned that he could eat as much ice cream as he wanted without gaining weight. After eating several gallons of ice cream and gaining about 15 pounds, he realized that something was wrong. He then discovered the confusion between Calories (which are really kilocalories) and Calories. While it does take 84 Calories/g to melt the ice cream, the energy gained from digesting the ice cream is 3 kilocalories/g, or 3000 Calories/g. I am sure that others have been similarly confused by this difference.
An electron-volt (eV) is another specialized unit of energy, particularly common in nuclear reactions. It is defined as the amount of kinetic energy gained by a single unbound electron when it accelerates through an electric potential difference of one volt. Its use in nuclear reactions will be seen later in Chapter 7.
![]()
The erg is the unit of energy in the CGS unit system. While this unit system is not very commonly used today and was replaced by the SI unit system, you will occasionally see the erg unit used. It is easy to show that one erg is exactly equal to 10− 7 J, or 100 nanojoules (nJ). These other (non-SI) units do not use the same prefixes as the SI units, but instead use scientific notation or words for powers of 10, shown in Table 2.2; for example, 7 × 1015 BTU can be written as 7E + 15 BTU or written out as 7 quadrillion BTU, which can be abbreviated as 7 quads.
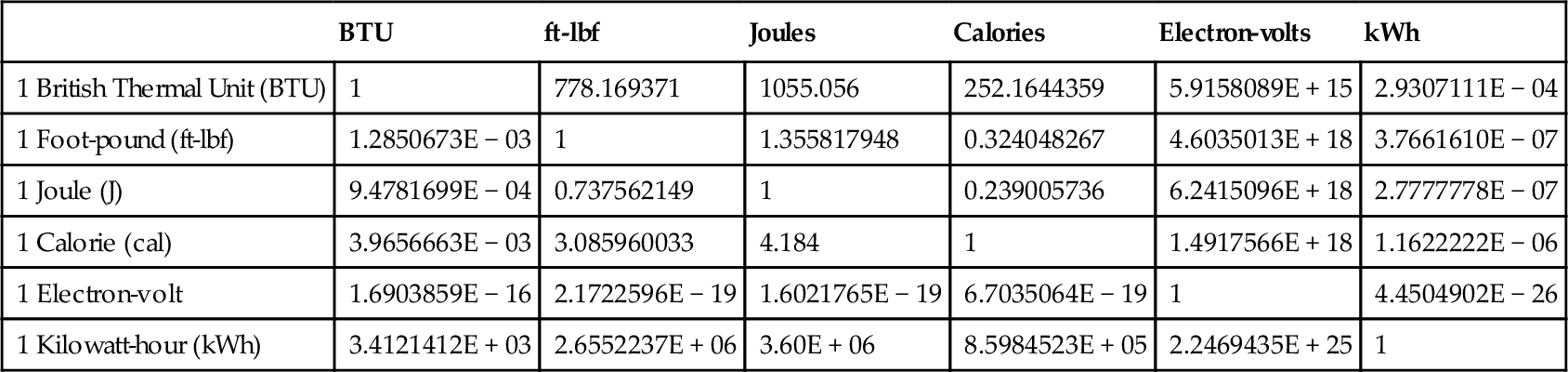
Table 2.3 shows the conversions between the important energy unit values. The numbers in the table show how many of the units at the top of the column are equal to one of the units on the left-hand side; for example, 1 Calorie = 4.184 joules.
Table 2.3
Energy Conversions
| BTU | ft-lbf | Joules | Calories | Electron-volts | kWh | |
| 1 British Thermal Unit (BTU) | 1 | 778.169371 | 1055.056 | 252.1644359 | 5.9158089E + 15 | 2.9307111E − 04 |
| 1 Foot-pound (ft-lbf) | 1.2850673E − 03 | 1 | 1.355817948 | 0.324048267 | 4.6035013E + 18 | 3.7661610E − 07 |
| 1 Joule (J) | 9.4781699E − 04 | 0.737562149 | 1 | 0.239005736 | 6.2415096E + 18 | 2.7777778E − 07 |
| 1 Calorie (cal) | 3.9656663E − 03 | 3.085960033 | 4.184 | 1 | 1.4917566E + 18 | 1.1622222E − 06 |
| 1 Electron-volt | 1.6903859E − 16 | 2.1722596E − 19 | 1.6021765E − 19 | 6.7035064E − 19 | 1 | 4.4504902E − 26 |
| 1 Kilowatt-hour (kWh) | 3.4121412E + 03 | 2.6552237E + 06 | 3.60E + 06 | 8.5984523E + 05 | 2.2469435E + 25 | 1 |
Sometimes when energy values of different sources are compared, people convert the energy from different fuels into tons of oil equivalent or tons of coal equivalent. To do this, they have to define the energy content of one ton of standard oil. The energy content of crude oil varies from oil to oil but, for the purpose of this conversion, the equivalent oil has been defined as 10 Gcalth/ton, or 41.868 GJ = 1 ton of oil equivalent (toe). Similarly, one ton of coal equivalent (tce) is defined as 7 Gcalth/ton of coal; therefore, 29.3076 GJ = 1 tce.
The average energy content of various fuels in BTUs is shown in Table 2.4.
Table 2.4
Average Energy Content of Various Fuels
1 Kilowatt-hour of electricity ≈ 3412 Btu
1 Cubic foot of natural gas ≈ 1000 Btu
1 Therm of natural gas = 100,000 Btu
1 Gallon of crude oil ≈ 138,095 Btu
1 Barrel of crude oil ≈ 5,800,000 Btu
1 Gallon of fuel oil ≈ 149,690 Btu
1 Gallon of gasoline ≈ 125,000 Btu
1 Gallon of ethanol ≈ 84,400 Btu
1 Gallon methanol ≈ 62,800 Btu
1 Gallon of gasohol (10% ethanol, 90% gasoline) ≈ 120,900 Btu
1 Gallon of e-85 (85% ethanol, 15% gasoline) ≈ 90,500 Btu
1 Gallon of kerosene ≈ 135,000 Btu
1 Gallon of diesel fuel ≈ 138,690 Btu
1 Gallon of (lpg) ≈ 95,475 Btu
1 Pound of coal ≈ 8100-13,000 Btu
1 Ton coal ≈ 16,000,000-26,000,000 Btu
1 Ton wood (dry) ≈ 16,000,000-18,000,000 Btu
1 Standard cord of wood ≈ 17,000,000-30,000,000 Btu
1 Face cord of wood ≈ 6,000,000-8,000,000 Btu
Many homes in the United States and throughout the world are heated with natural gas. One cubic foot of natural gas (measured at standard atmospheric conditions) contains, in approximate round numbers, 1000 BTUs or 1 million joules (1 MJ) of energy when burned. When natural gas is bought and sold, it is usually measured in volumes of one thousand cubic feet (1 MCF for short). The M comes from the Latin word mille, which means thousand, so 1 MCF of natural gas contains ~ 1 million BTUs, or 1 billion joules (1 GJ) of energy. In the United States, invoices sent to homeowners by natural gas utilities showed the gas usage in CCF, which stood for one hundred cubic feet. The Latin word for hundred is centum; hence, one hundred cubic feet was abbreviated CCF. It makes more sense to charge the user for the energy content of the gas used rather than the volume. By consensus, the utility companies switched to charging for the BTUs used. They adopted the therm as the basic unit of energy, and this is what is now displayed in customer invoices. As shown in Table 2.4, 1 therm = 100,000 BTUs. One CCF of gas contains about 100,000 BTU. So, the CCF value previously used and the therm unit currently used are approximately the same.
Hydrocarbon Fuels
Table 2.5 shows the energy content of the widely used hydrocarbon fuels. The gallon used here is the U.S. gallon, which is equal to 0.96894 imperial gallons. One barrel, a unit widely used in the oil business, is equal to 42 U.S. gallons, which is 5.61458 cubic feet and 158.98 liters.
Table 2.5
Average Energy Content of Hydrocarbon Fuels
| Fuel | BTU/Barrel | BTU/Gallon |
| Crude oil | 5,855,795 | 139,424 |
| Motor gasoline | 5,250,000 | 125,000 |
| Aviation gasoline | 5,005,224 | 119,172 |
| Jet fuel | 5,434,926 | 129,403 |
| L.P.G. | 4,054,470 | 96,535 |
| Propane | 3,836,000 | 91,333 |
| Ethane | 3,082,000 | 73,381 |
| Butane | 4,326,000 | 103,000 |
| Kerosene | 5,670,000 | 135,000 |
| #1 Distillate | 5,706,000 | 135,857 |
| #2 Distillate | 5,825,000 | 138,690 |
| #4 Distillate | 6,062,000 | 144,333 |
| Residual oil | 6,287,000 | 149,690 |
Wood Fuels
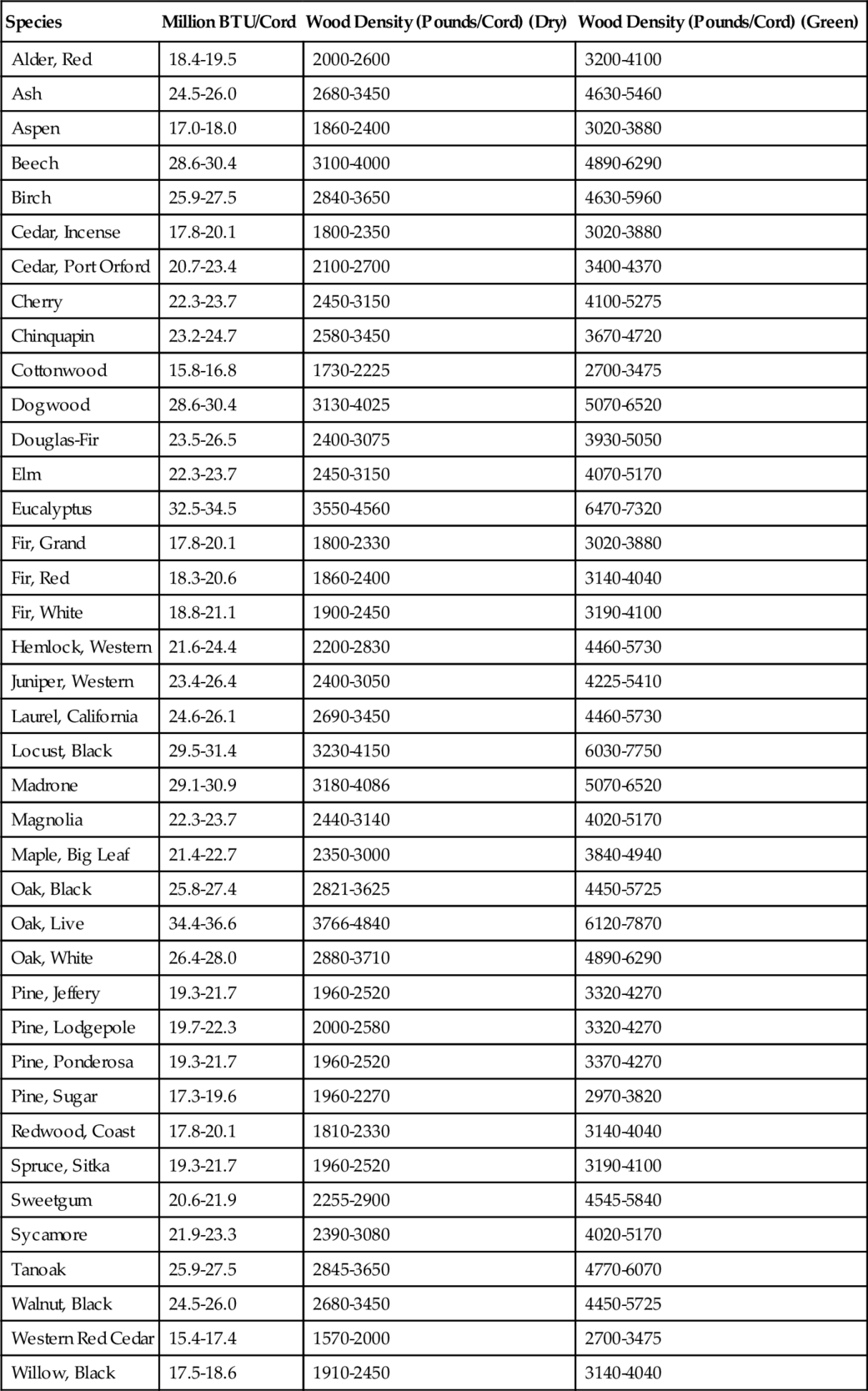
Table 2.6 shows the energy content of various types of woods when burned. Soft woods such as pine, fir, willow, and aspen have less energy per unit volume. In terms of weight, most woods have similar energy content per unit weight: 8250 BTU/pound for dried nonresinous woods (such as aspen, oak, and maple) and 9150 BTU/pound for dried resinous woods (such as pine and western red cedar). The cord is the common unit for wood and one cord is defined as a volume of 128 cubic feet (4′ × 4′ × 8′ is a typical arrangement to give a volume of one cord). In Table 2.6, the lower value of the range assumes 70 cubic feet of wood per cord (i.e., 58 ft3 of the cord is air). The higher value of the range assumes 90 cubic feet of wood per cord (i.e., 38 ft3 of the cord is air). The green weight assumes the wood has not been dried and the moisture content is ~ 50%. The dry weight assumes the wood has been dried to a moisture content of 12%. Green wood is harder to burn because of the high moisture content.
Table 2.6
Wood Energy Content and Density Values
| Species | Million BTU/Cord | Wood Density (Pounds/Cord) (Dry) | Wood Density (Pounds/Cord) (Green) |
| Alder, Red | 18.4-19.5 | 2000-2600 | 3200-4100 |
| Ash | 24.5-26.0 | 2680-3450 | 4630-5460 |
| Aspen | 17.0-18.0 | 1860-2400 | 3020-3880 |
| Beech | 28.6-30.4 | 3100-4000 | 4890-6290 |
| Birch | 25.9-27.5 | 2840-3650 | 4630-5960 |
| Cedar, Incense | 17.8-20.1 | 1800-2350 | 3020-3880 |
| Cedar, Port Orford | 20.7-23.4 | 2100-2700 | 3400-4370 |
| Cherry | 22.3-23.7 | 2450-3150 | 4100-5275 |
| Chinquapin | 23.2-24.7 | 2580-3450 | 3670-4720 |
| Cottonwood | 15.8-16.8 | 1730-2225 | 2700-3475 |
| Dogwood | 28.6-30.4 | 3130-4025 | 5070-6520 |
| Douglas-Fir | 23.5-26.5 | 2400-3075 | 3930-5050 |
| Elm | 22.3-23.7 | 2450-3150 | 4070-5170 |
| Eucalyptus | 32.5-34.5 | 3550-4560 | 6470-7320 |
| Fir, Grand | 17.8-20.1 | 1800-2330 | 3020-3880 |
| Fir, Red | 18.3-20.6 | 1860-2400 | 3140-4040 |
| Fir, White | 18.8-21.1 | 1900-2450 | 3190-4100 |
| Hemlock, Western | 21.6-24.4 | 2200-2830 | 4460-5730 |
| Juniper, Western | 23.4-26.4 | 2400-3050 | 4225-5410 |
| Laurel, California | 24.6-26.1 | 2690-3450 | 4460-5730 |
| Locust, Black | 29.5-31.4 | 3230-4150 | 6030-7750 |
| Madrone | 29.1-30.9 | 3180-4086 | 5070-6520 |
| Magnolia | 22.3-23.7 | 2440-3140 | 4020-5170 |
| Maple, Big Leaf | 21.4-22.7 | 2350-3000 | 3840-4940 |
| Oak, Black | 25.8-27.4 | 2821-3625 | 4450-5725 |
| Oak, Live | 34.4-36.6 | 3766-4840 | 6120-7870 |
| Oak, White | 26.4-28.0 | 2880-3710 | 4890-6290 |
| Pine, Jeffery | 19.3-21.7 | 1960-2520 | 3320-4270 |
| Pine, Lodgepole | 19.7-22.3 | 2000-2580 | 3320-4270 |
| Pine, Ponderosa | 19.3-21.7 | 1960-2520 | 3370-4270 |
| Pine, Sugar | 17.3-19.6 | 1960-2270 | 2970-3820 |
| Redwood, Coast | 17.8-20.1 | 1810-2330 | 3140-4040 |
| Spruce, Sitka | 19.3-21.7 | 1960-2520 | 3190-4100 |
| Sweetgum | 20.6-21.9 | 2255-2900 | 4545-5840 |
| Sycamore | 21.9-23.3 | 2390-3080 | 4020-5170 |
| Tanoak | 25.9-27.5 | 2845-3650 | 4770-6070 |
| Walnut, Black | 24.5-26.0 | 2680-3450 | 4450-5725 |
| Western Red Cedar | 15.4-17.4 | 1570-2000 | 2700-3475 |
| Willow, Black | 17.5-18.6 | 1910-2450 | 3140-4040 |
Units of Power
Power is simply the rate of doing work, or the rate of energy conversion. In the SI units system, this is measured in watts (which is equal to joules per second). In the British units system (also known as American customary units) it is given in ft-lbf/s. Another common power unit is horsepower (hp), which is defined as 550 ft-lbf/s. As its name implies, this unit was meant to estimate the approximate peak rate at which a horse can work. Other than horsepower, the other power conversion factors are the same as energy conversions if time is measured in the same units (seconds, hours, days, or years).
![]()
A little calculation exercise is useful here. A human being can work at the maximum rate of about ¼ hp, so in 1 h a human can do:
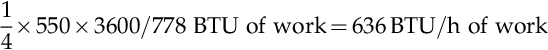
If that person is paid $10/h for their labor, you will be getting 63.6 BTU of work for each dollar spent. In Table 2.5, you will see that one gallon of gasoline contains about 125,000 BTU. At $3.50/gallon, energy in the form of gasoline can be purchased for about 35,700 BTU for each dollar spent. At this rate, gasoline is 560 times cheaper than human labor. Put another way, compared to human labor at $10/h, gasoline is worth $1965/gallon. This illustrates the tremendous value that the discovery and refining of crude oil has had on the well-being of humans. Energy was made much cheaper and society has advanced because of this discovery.
In his 1943 paper in the American Anthropologist discussed in Chapter 1, Leslie White makes this point even more dramatically. He says, “In the US the energy expended is now about 13.5 horsepower hours per day per capita, which is the equivalent of 100 human slaves for each person.”
