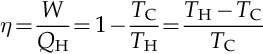Energy Science and Thermodynamics
Abstract
Energy utilization is greatly affected by the laws of thermodynamics. The first law of thermodynamics states that energy cannot be created or destroyed; it can merely be transformed from one type to another. So, if energy cannot be destroyed, why do we need new energy supplies continuously and what do we mean when we say we use energy? The answer lies in the second law of thermodynamics, which can be stated in many forms. One form of this law says that it is impossible to convert heat into work without rejecting some of that energy to a heat sink. This means that there is a thermodynamic limit to the amount of energy that be captured from energy sources. Eventually, the energy sources are degraded into less usable forms of energy. Understanding these laws is vital for understanding many of the mistakes that people make about energy. It is also vital to understand the heat engines that have powered our economies for the past 200 years.
Energy utilization is greatly affected by the laws of thermodynamics. The first law states that energy cannot be created or destroyed, merely transformed from one type to another. If energy cannot be destroyed, why do we need new energy supplies continuously? What do we mean when we say we use energy? The answer lies in the second law of thermodynamics, which can be stated in many forms. One form of this law says that it is impossible to convert heat into work without rejecting some of that energy to a heat sink. Moreover, the heat that is transferred to and from a heat engine depends on the temperature of the heat source and the heat sink. This means that there is a thermodynamic limit on the amount of energy that can be captured from energy sources. Eventually, these energy sources are degraded into less usable forms of energy—particularly heat that is dissipated into the atmosphere and eventually out to the rest of the universe.
The First Law of Thermodynamics
The first law of thermodynamics states that energy cannot be created or destroyed; it can only be transformed from one type of energy to another. This seems like an obvious statement, one with which most students of science and engineering are eminently familiar. It leads to a relatively simple equation: during any process, the energy in equals the energy out. This law was not always obvious to scientists. In fact, up until the nineteenth century, scientists were under the mistaken belief that heat was not a form of energy at all, but that heat had mass. This was part of the caloric theory of heat, which had its origins in the medieval notion that there were only four elements that made up the universe: earth, air, fire and water. There was considerable evidence to support this theory. For example, if you burned a piece of wood you would see smoke and other vapors emanating from it that would disappear into and became part of the air. During the burning, you would see heat and flames, which clearly indicated that fire was one of the elements making up the wood. The end result would be ash which would become part of the earth. This ash could not be burned and weighed less than the original piece of wood because of the air and fire that it had lost. There are also many substances out of which you can squeeze water, including fruit, vegetables and the earth itself. These observations led to the mistaken belief that heat or fire was an element and as such had mass. There were many observations that disputed this belief, but scientists had explained away these observations.
The big breakthrough in the development of the first law of thermodynamics was made by an American engineer called Benjamin Thompson. Thompson was born in rural Woburn, Massachusetts, on March 26, 1753; his birthplace is preserved there as a museum. He was educated mainly at the Woburn schools, although he sometimes managed to get to Cambridge to attend lectures by Professor John Winthrop of Harvard College. It was there he learned about the caloric theory of heat. In 1769 when Thompson was 16, he began to conduct his own experiments to try to understand the nature of heat, and he corresponded with friends about these experiments (these records of his thoughts are still in existence today). In 1785 at the age of 32, he moved to Bavaria in Germany where he spent about 12 years. Some of his duties there were to reorganize the army and to establish workhouses for the poor. He made studies on the fuels used for lighting, heating and cooking, including their relative costs and efficiencies. He also constructed the English Garden in Munich in 1789; it remains there today and is known as one of the largest urban public parks in the world. Thompson was greatly admired for this work, and in 1791 he was named a Count of the Holy Roman Empire and granted the formal title of Count Rumford.
In 1797 while Thompson was working in Munich (the capital city of Bavaria), he was made responsible for the boring of cannon barrels using sharp cutting tools in a lathelike machine. He observed that when the blade became blunt, the lathe had to work harder using more energy. At the same time, the barrel became hotter. This suggested to him that there might be a link between work and heat and that heat could be a form of energy, not mass. He made a series of measurements on the cannons, linking the amount of work to the rise in temperature of the cannon. He began to realize that the link between work and heat was that heat was a form of energy, not the caloric matter of the current scientific thinking. In a paper published in 1798 entitled, An Experimental Enquiry Concerning the Source of the Heat Which Is Excited by Friction, Thompson argued that heat was not caloric matter; instead, heat, work and motion are all forms of energy. Thompson (now Count Rumford) described how he immersed a cannon barrel in water and arranged for a blunted cutting tool. He showed that while he was cutting the barrel the water could be boiled and that the supply of frictional heat continued as long as the machine kept boring. He pointed out that the weight of the cannon and the material removed remained the same no matter how much work was done or how hot the barrel became. He also noted that the only thing communicated to the barrel was the energy of motion. This was a fundamental breakthrough that rejected the caloric theory of heat, the accepted theory of the day. While his work did cause some hostility, it was subsequently important in establishing the law of conservation of energy, or the first law of thermodynamics.
While the term “energy” had not been coined yet, it appears that it was first used by Thomas Young in 1807—a few years after Benjamin Thompson’s observations on the cannon. A further step in the development of the first law of thermodynamics on the conservation of energy was formulated in 1837 by Karl Mohr, who stated:
…besides the 54 known chemical elements there is in the physical world one agent only, and this is called energy. It may appear, according to circumstances, as motion, chemical affinity, cohesion, electricity, light and magnetism; and from any one of these forms it can be transformed into any of the others.
Mohr had not included heat in his conservation principle because, despite Thompson’s breakthrough, many scientists still clung to the caloric theory that maintained that heat could neither be created nor destroyed. Thompson had shown that mechanical energy can be converted into heat. Savery, Newcomen, Watt, and other purveyors of the steam engine had shown that heat can be converted into mechanical energy, which contradicts and disproves the caloric theory. Thus, the principle of conservation of energy assumes that heat is a form of energy and that heat and mechanical work can be made to be interchangeable.
The first explicit statement of the first law of thermodynamics was given by Rudolf Clausius in 1850, 53 years after Thompson’s breakthrough. Clausius stated:
There is a state function E, called “energy,” whose differential equals the work exchanged with the surroundings during an adiabatic process.
This is a rather precise but tricky statement, requiring some explanation. The word “adiabatic” means that there is no heat transferred in the process. Clausius recognized that heat is a form of energy that could be transferred into other forms of energy. If the process is adiabatic, any change in the energy of the system is equal to the work done by the system. If the process is not adiabatic, the heat transfer must also enter the equation.
A more modern explanation of the first law of thermodynamics is that there are many forms of energy—kinetic, mechanical, potential, chemical, electrical, internal, nuclear, heat, and work. All of these can be converted from one form into another, but the total amount of energy is always conserved. The implications of this are very important. When fuel is burned, the chemical energy of the chemical bonds is released as heat. If the main purpose is to heat up the living space, energy must be directed into heating up air or water. The heated medium is pumped around the living space to distribute the energy to where it is wanted and in the correct form. If the goal is to create mechanical energy or work, heat has to be converted into work. This can be easily done in heat engines such as the internal combustion engine or the steam engine, which is an external combustion engine. If the goal is to create electrical energy, heat must first be transformed into mechanical energy and then into electrical energy.
The sun generates energy through nuclear reactions. In this process, hydrogen atoms are fused together under intense heat and pressure to form helium atoms. The helium atoms contain slightly less mass than the hydrogen atoms used to create them. This extra mass is converted into energy according to Einstein’s famous equation, E = mc2. This energy is then stored in the sun as internal energy and nuclear energy and can be transferred to the earth as heat and radiation.
One of the most efficient and clean forms of energy on the earth is hydroelectric power. In this process, the sun vaporizes water from the ocean, and it falls on high mountains as rain or snow and flows into rivers. At high altitudes, the water has potential energy that originally was driven by the nuclear energy of the sun. The potential energy of this water is captured by building a dam and then letting the water flow through a mechanical turbine, converting the potential energy into mechanical energy and ultimately into electrical energy. At each step of this process, energy is being conserved. It should be noted that most of the energy utilized on the earth originated on the sun. The main exception to this rule is the geothermal energy deep in the earth, which is discussed in Chapter 11. This energy, which is due to nuclear processes under the earth, is captured via heat transfer and utilized.
Wind energy can also be converted into electrical energy. Wind energy is actually kinetic energy due to the movement of the air molecules, which are moved by the sun’s energy. This energy is used to turn the blades of wind turbines, effectively converting the kinetic energy of the air molecules into the kinetic energy of the blades, which in turn is converted into the electrical energy used to power our homes and industries.
None of these conversions are 100% efficient. Some energy is always lost due to friction and other such inefficiencies; however, that energy is not destroyed. It usually ends up as low-grade heat energy that serves to heat up the world or the universe slightly. There are other inefficiencies in the energy conversion process, and these are dealt with in the second law of thermodynamics.
If energy cannot be created or destroyed, merely transformed from one type to another, why do we need new energy supplies continuously and what do we mean when we say we use energy? This answer lies in the second law of thermodynamics.
The Second Law of Thermodynamics
The second law of thermodynamics puts a limit on how efficient the energy conversion processes can be. Even though energy transferred as heat is indeed energy transfer, there is something different about heat energy. The way scientists define heat is somewhat different than the way most people understand heat. If two bodies of different temperatures are placed in contact with one another, there will be a transfer of energy from the hot body to the cold body and that transfer will continue until the two bodies are the same temperature. This transfer of energy is called heat. The temperature of each body is due to its “internal energy.” This energy is stored as the kinetic energy of vibrating and moving molecules and is a function of the temperature and pressure of the material. The hotter the material, the faster the molecules move. When energy is transferred as heat, the hotter body loses some of its internal energy and the colder body gains some internal energy. It is therefore incorrect to say a hot body contains heat; it contains internal energy and it can transfer some of that energy to a colder body as heat. The colder body then converts that energy to internal energy. The transfer of that internal energy is what is called heat. The reader can now go back to the previous section on the first law of thermodynamics and see all my deliberate errors written about the word heat. For instance, I said that in the sun “hydrogen atoms are fused together under intense heat and pressure to form helium atoms.” This is not correct; I should have said that “hydrogen atoms are fused together at very high temperatures and pressures to form helium atoms.”
There are three different heat transfer methods: conduction, convection and radiation.
How much heat can be transferred between two bodies depends on their temperatures and the method of heat transfer. Heat energy cannot be converted entirely into work or mechanical energy, electricity or any other form of energy. This has a big impact on the efficiency of heat engines. When fuel is burned in a heat engine, the chemical energy that is stored in the chemical bonds of the fuel molecules is released. This energy is transferred to the engine as heat; however, not all of that heat can be converted into mechanical energy. When people were developing and trying to perfect heat engines, such as the internal combustion engine, they discovered this limitation and had to understand it. The result was the formulation of the second law of thermodynamics.
The seminal work in this area was due to a French engineer called Sadi Carnot. In 1824, he published a paper entitled, Reflections on the Motive Power of Fire and the Machines Needed to Develop This Power. This paper presented the idea that the amount of work done by a heat engine is due to the flow of heat from a hot to a cold body. Carnot’s understanding of heat was still mired in the incorrect caloric theory of heat, but his conclusions were still valid. His analysis determined that the theoretical heat that could be transferred to the heat engine was proportional to the temperature difference between the heat source (the hot body) and the heat sink (the cold body). This analysis allowed him to calculate the theoretical efficiency of a heat engine, which turned out to be much lower than the efficiency of other energy conversion processes.
Using Carnot’s analysis, several people were able to deduce different statements of the second law of thermodynamics. Some of these are:
1. It is impossible to produce work in the surroundings using a cyclic process connected to a single heat reservoir (Thomson, 1851).
2. It is impossible to carry out a cyclic process using an engine connected to two heat reservoirs that will have as its only effect the transfer of a quantity of heat from the low-temperature reservoir to the high-temperature reservoir (Clausius, 1854).
3. In any process, the entropy of the universe increases, causing it to tend towards a maximum (Clausius, 1865).
This third statement introduces the concept of entropy and puts the law on a more mathematical basis. Since the amount of heat transferred in any process depends on the temperature of the body transferring the heat, entropy is defined as the heat transferred divided by the temperature, T, at which it is transferred. Giving entropy the symbol S and the heat transferred the symbol Q, by definition:
![]()
A certain understanding of entropy is required to fully understand the limitations of energy usage. This is illustrated in Appendix A and discussed in the next section.
One of the consequences of the second law of thermodynamics is that, when you burn fuel to drive a heat engine, only some of the heat from the fuel can be converted to work in the engine. The rest must be rejected to a heat sink, which is usually the atmosphere surrounding the engine. Consequently, heat engines are inherently inefficient. Another consequence is that heating your house with an electric heating element is going to be much more expensive than using most other fuels. This is because the electricity has been created using an inefficient heat engine where some of the heat had to be rejected to the surroundings. If your goal is to increase the temperature of some space (such as your house), it is better to burn a fuel directly and capture as much of that heat in your house as possible. The efficiency of direct heating by burning a fuel is much higher than creating electricity where some of the heat must be lost. All of this was analyzed by Sadi Carnot using his Carnot cycle and published in 1824.
Sadi Carnot was the eldest son of a French Revolutionary named Lazare Carnot and was born on June 1, 1796, during the height of the French Revolution. Sadi studied at the École Polytechnique beginning in 1812. By the time Sadi graduated in 1814, Napoleon’s empire was on the run and European armies were invading France. During Napoleon’s return to power in 1815, Sadi’s father, Lazare Carnot, was Minister of the Interior for a few months. Following Napoleon’s final defeat later that year, Lazare fled to Germany, never to return to France.
Sadi Carnot was an army officer for most of his life, but in 1819 he semiretired from the army and began to devote his attention to designing steam engines. These engines were the main workhorses of Europe, particularly Britain, and were used for pumping water from mines, dredging ports and rivers, grinding wheat, and spinning and weaving cloth; however, they were somewhat inefficient. The import of the more advanced British steam engines into France after the war showed Carnot how far the French had fallen behind in their technology. He was particularly dismayed that the British had progressed so far through the genius of a few engineers who lacked any real scientific education. British engineers had also accumulated and published reliable data about the efficiency of many types of engines under actual running conditions; they argued about the merits of low and high pressure engines and of single-cylinder and multi-cylinder engines.
Carnot understood implicitly that great civilizations need to harness energy to advance their technology. Convinced that France’s inadequate utilization of steam was a factor in its downfall, he began to write a nontechnical work on the efficiency of steam engines. Other workers before him had examined the question of improving the efficiency of steam engines by comparing the expansion and compression of steam with the production of work and consumption of fuel. In his essay, Réflexions sur la puissance motrice du feu et sur les machines propres à développer cette puissance (Reflections on the Motive Power of Fire and the Machines Needed to Develop This Power), published in 1824, Carnot gave a lot of attention to the theory of the process not concerning himself, as others had done, with its mechanical details.
Carnot stated that, in a steam engine, motive power is produced when heat “drops” from the higher temperature of the boiler to the lower temperature of the condenser, just as water, when falling, provides power in a waterwheel. He worked within the theoretical framework of the caloric theory of heat, assuming that heat was a gas that could be neither created nor destroyed. Though this assumption was incorrect and Carnot himself had doubts about it even while he was writing his essay, many of his results were nevertheless true. One of these was his prediction that the efficiency of an idealized engine depends only on the temperature of its hottest and coldest parts and not on the substance (steam or any other fluid) that drives the mechanism.
Carnot understood that every thermodynamic system exists in a particular thermodynamic state. When a system is taken through a series of different states and finally returned to its initial state, a thermodynamic cycle is said to have occurred. In the process of going through this cycle, the system may perform work on its surroundings, thereby acting as a heat engine. The cycle that he proposed and used in his analysis is now known as the Carnot cycle. A system undergoing a Carnot cycle is called a Carnot heat engine, although such a “perfect” engine is only theoretical and cannot be built in practice.
The mathematical details of the Carnot cycle are shown in Appendix A, but it is not necessary to fully understand those details to appreciate its usefulness. The Carnot cycle when acting as a heat engine, consists of the following four steps:
1. Reversible and isothermal expansion of the working fluid at the “hot” temperature, TH (isothermal heat addition). During this step, the fuel is burned creating the hot temperature and causing the working fluid or gas to expand. The expanding gas makes the engine’s piston do work on the surroundings. As the piston is forced to move, it drives a shaft which converts the work into kinetic energy. The gas expansion is propelled by the absorption of heat from the high temperature reservoir created by the burning fuel.
2. A reversible and adiabatic (isentropic) expansion of the working fluid (isentropic work output). Remember that adiabatic means that there is no heat transferred. Isentropic means that the entropy of the system remains constant. For this step, the piston and cylinder are assumed to be thermally insulated (adiabatic), thus they neither gain nor lose heat. The gas continues to expand, working on the surroundings. When gas expands it also cools, losing energy. Since the process is insulated, however, it cannot lose that energy as heat. This forces the gas to continue to do work by driving the piston. This expansion of the gas causes it to cool to the “cold” temperature, TC.
3. Reversible isothermal compression of the gas at the “cold” temperature, TC (isothermal heat rejection). In this step, the surroundings do work on the gas, which causes a quantity of heat to flow out of the gas to the low temperature reservoir.
4. Isentropic compression of the gas (isentropic work input). Once again, the piston and cylinder are assumed to be thermally insulated (or adiabatic). During this step, the surroundings, through the piston, do work on the gas, compressing it and causing the temperature to rise to TH. At this point, the gas is in the same state as at the start of step one.
The antithesis of a heat engine is a refrigerator. A heat engine burns fuel as part of a thermodynamic cycle to create heat that is converted into mechanical energy. A refrigerator sends the cycle in the opposite direction and uses electrical energy to create mechanical energy that then pumps heat from the cold body to the hotter body.
The efficiency of the heat engine, η, is defined as the work produced divided by the heat input from the hot reservoir. In Appendix A the efficiency is calculated as follows:
Where,
W is the work done by the system (energy exiting the system as work).
QH is the heat put into the system (heat energy entering the system).
TC is the absolute temperature of the cold reservoir.
TH is the absolute temperature of the hot reservoir.
This efficiency describes the fraction of the heat energy extracted from the hot reservoir and converted to mechanical work. A Rankine cycle is usually the practical approximation of a Carnot cycle for a steam engine. It is shown, in Appendix A, that for any cycle operating between temperatures TH and TC, none can exceed the efficiency of a Carnot cycle.
Carnot’s theorem is a formal statement of this fact: No engine operating between two heat reservoirs can be more efficient than a Carnot engine operating between those same reservoirs. Equation 3.1 gives the maximum efficiency possible for any engine using the corresponding temperatures. A corollary to Carnot’s theorem states that: All reversible engines operating between the same heat reservoirs are equally efficient. The right-hand side of Equation 3.1 gives what may be a more easily understood form of the equation: the theoretical maximum efficiency of a heat engine equals the difference in temperature between the hot and cold reservoir divided by the absolute temperature of the hot reservoir. To find the absolute temperature in degrees Kelvin, add 273.15° to the Celsius temperature. To find the absolute temperature in degrees Rankine, add 459.6° to the Fahrenheit temperature. Looking at the formula in Equation 3.1, an interesting fact becomes apparent. Lowering the temperature of the cold reservoir will have more effect on the ceiling efficiency of a heat engine than raising the temperature of the hot reservoir by the same amount. In the real world, this may be difficult to achieve since the cold reservoir is often an existing ambient temperature, such as the atmosphere.
In other words, maximum efficiency is achieved if no new entropy is created in the cycle. In practice, the required dumping of heat into the environment to dispose of excess entropy leads to a reduction in efficiency. Equation 3.1 gives the efficiency of any theoretically reversible heat engine.
Carnot realized that in reality it is not possible to build a thermodynamically reversible engine. Real heat engines are less efficient than indicated by Equation 3.1. Nevertheless, Equation 3.1 is extremely useful for determining the maximum efficiency that could ever be expected for a given set of thermal reservoirs.
There are four practical heat engine cycles in wide use today, each trying to approximate the Carnot thermodynamic cycle. They are
1. The Otto cycle, which is the basis of the gasoline engine.
2. The Diesel cycle, commercialized in the Diesel engine.
3. The Rankine cycle, the basis for steam engines widely used today in power plants to generate electricity.
4. The Brayton cycle used in gas turbines that are used to generate electricity or provide thrust.
There is also the Stirling cycle that can be used to make a practical external combustion heat engine, but this engine has never been commercialized. Despite this there is a lot of interest in developing Stirling engines because a large variety of fuels can be used to drive such engines, including solar energy. The Stirling engine is an alternative to the Rankine cycle engine.
The entropy statement of the second law also allows scientists to analyze chemical reactions, the phase behavior of fluids, and many other seemingly unconnected processes. It also explains why people say they use energy when they are actually converting energy from one form into another. When fuel is burned to generate energy, chemical energy is converted into heat and then some of that heat energy is converted into electricity. Some of it is also rejected to the atmosphere where it is no longer usable. This electricity creates light in a lightbulb, which is also lost as heat to the atmosphere.
If the fuel is used to power an internal combustion engine to drive an automobile after some of the heat is rejected to the atmosphere, the rest of the fuel’s energy creates useful and usable kinetic energy. All of that kinetic energy is eventually lost as frictional heat, which is also lost to the atmosphere. All the energy we “use” becomes lost as heat that has been mostly transferred to the atmosphere, some of which is then radiated through space to other parts of the universe.
Another consequence of the first and second laws of thermodynamics is that perpetual motion machines are not possible. The first law simply states that if you set a machine in motion by supplying it with energy it could keep running forever in a frictionless environment. You could not extract more energy back out of it than you put in because that would violate the first law. The second law says that you cannot even get as much out as you put in because some of the energy is lost as heat via friction. Perpetual motion machines fall into two categories: those that violate the first law of thermodynamics and those that violate the second law of thermodynamics.
The entropy parameter is also a measure of the randomness of the universe, and the second law states that the randomness of the universe is increasing. In other words, as processes unfold, the elements of the universe tend to a more disordered state.
The answer to the question “What do we mean when we say we use energy?” is that the available energy is used and then converted into unavailable energy. Electrical energy, potential energy, kinetic energy and chemical energy in fuels are all available forms of energy. Energy lost to the atmosphere as heat becomes mostly unavailable energy. It is hard to extract energy from the atmosphere because the temperature is not high enough. The cumulative effect of energy lost to the atmosphere or the ocean is that it is also continuously radiated to the rest of the universe where it becomes completely unavailable. The second law of thermodynamics governs this process.
The laws of thermodynamics have many more applications than have been shown here, but that is beyond the scope of this book. Here it is simply necessary to have a little understanding of the laws of thermodynamics so that the energy processes can be understood a little better.