2
Nanodiamonds
Now the final allotrope of nanocarbon–nanodiamonds (NDs), and before that, there are diamonds. Famous or not, every diamond comes with a story, some publicly known and others remain in private talks. This is the story of the French Regent diamond.
2.1 Ah, Diamonds, Eternal Beautiful
Many famous diamonds today, including the French Regent diamond, originally came from India, each shadowed by a long trail of history [1]. Behind the flawless beauty of diamonds lies the dark side of human nature, reflecting personal selfishness and greediness, social and political power struggles, theft, robbery, murders, and even wars. The Regent diamond has it all. The original gem was said to have been discovered in 1701 by a slave working in a diamond mine at Kistna of Southern India. It presented as the opportunity of lifetime for the slave to gain his freedom. He hid the 410 carats stone, weighing 82 g, in a deep wound cut in his leg before fleeing to the south coast in search of a quick getaway to a new land and new life altogether. Shrouded by the agony of being caught and the risks of exposing his valuable treasure, he soon hooked up with an English skipper, entrusting the seaman with his valuable stone in exchange for a stealthy sail immediately. No one ever knew the fate of the slave, but the seaman came back alone with the stone in his pocket, which he sold quickly to a diamond merchant, Jamchund, for about £1000. Like a drunken sailor, he squandered the money as fast as it had come in and was last seen “hanging himself in remorse.”
Jamchund, well known for his dealings in the eastern region of India, and now holding the stone, proceeded to negotiate with the few potential buyers available to him, including the English governor of Fort St. George, Thomas Pitt, who eventually bought it at a price of £20 400. After Mr. Pitt’s purchase of the stone, suspicions lingered so strongly as to how the transaction actually took place, that upon returning to England in 1710 he felt obliged to send a public letter to the chief editor of the European Magazine clarifying the early history of the diamond and his negotiations with Jamchund. The story as told was never clear whether it was the fame of the Pitt diamond (as it was called at the time) or Mr. Pitt’s own reputation as a bold, temperamental, and sometimes lawless businessman that has fostered such sustained scandalous suspicions. Whatever it may have been, the criticism haunted Mr. Pitt for the remainder of his life.
By this time the diamond had been cut by a skillful jeweler in London named Harris, in a period over two years time (from 1704 to 1706), rendering a 136.8 carat diamond. It was a brilliant cut, which was a particular form of cut maximizing the reflecting facets in a cone shape, thereby channeling maximum light through the top of the diamond. In a broadly square shape, 1.2 inch by 1 inch, the diamond was cushioned in a 0.75‐inch depth and appeared to be perfectly white with a faintly pale blue “of the first water.” Its flawless cut presented brilliant speckles in a breathtaking beauty that no other diamond could match and, to this day, is still regarded as the finest diamond in the world.
In 1717, assisted by the powerful Scotch financier John Law, Mr. Pitt sold the diamond for a price of £135 000 to Philippe II, the Duke of Orleans “Regent of France,” ending Mr. Pitt’s enduring ordeal of seeking a Royal buyer. France, at that time under Louis XV, purchased the diamond upon the suggestion of Mr. Law that “France ought to possess a gem the finest ever seen in Europe.” From then on the diamond assumed its new title, the “Regent,” the name carried till this day.
The Regent made its public debut in 1721, when Louis XV wore it to attend a party at the Turkish embassy, where it immediately became the center of attention among the European royals and nobles. The next year at his coronation (25 October 1722), Louis XV had the Regent temporarily mounted on his new crown as the lead gem at the front center. Later, the King began to wear the Regent on his hat, a habit he kept for the rest of his life at the height of French art and culture in Europe. However, under the glamorous surface was the hardship wrought upon France by years of social unjustness and wars, in particular the Seven Years War, as so vividly characterized by historian Arthur Tilley [2]:
At this time the fable of the four cats became current: the thin cat was the people, the fat cat the financiers, the one‐eyed cat the ministry, and the blind cat the King who saw nothing and refused to see anything.
The King himself lived a good life and died in 1774, preceded by the death of his own son nine years earlier. The throne was therefore passed on to one of the grandsons, Louis XVI, who also inherited the Regent and used it again as the lead gem at his coronation, on 11 June 1775. Interestingly, Louis XVI also carried on his grandfather’s habit of wearing the gem on his hat. Was it a sign of glorification for his Grandpa or a final homage to the glory of the monarchy that was fading fast? History could never tell. Later, Queen Marie Antoinette was also seen to wear the Regent occasionally on her black velvet hat. In his reign, Louis XVI attempted some unsuccessful reforms bringing France into further financial hardship especially for those in the working class, fueling more social unrest. The dissatisfaction and anger of peasants finally erupted in the 1789 French Revolution. Louis XVI and Queen were both captured and later guillotined on 10 January 1792, which brought an end to the uninterrupted French monarchy of more than 1000 years.
One year earlier in 1791, a commission was convened by the National Assembly of the French First Republic including the most experienced jewelers in Paris at the time to inventory the French royal crown jewels, with the Regent leading a list of regalia including 9547 diamonds. The collection of the regalia was put on display for the first time to the general public at Garde‐Meuble (the Public Treasury). Early in September of 1792, as a precaution due to the growing violence in nearby Paris, the display was closed and all treasures safely stored away in the halls of Garde‐Meuble. On the morning of 17 September 1792, security guards discovered that the Regent and 11 cabinets full of the regalia had been stolen overnight – the robbery of the century had just been committed, which quickly sent shock waves throughout France and the rest of the world. As expected, an enormous effort was instituted to hunt for the thieves and every rock was turned in the entire country to look for the lost jewelry. In the end, the robbery was never solved.
One year later, in the midst of chaos with the Revolution, the Regent and some other regalia items were miraculously recovered from the attic of a house in the Allee des Veuves, Champs‐Elysees, at a tip from an anonymous letter addressed to the Commune. Rumors circulated wildly about this remarkable and highly unexpected reappearance of the royal jewelry, yet to this date, history could never reveal the true identity of the mysterious author(s) of the letter. The general consensus held by historians seemed to cite the great difficulties of reselling a diamond of such high profile as the main reason for the return of the Regent, or, is that because the fate of the Regent was already intertwined with the French politics? After all, this was not the only time the Regent was returned.
It was the Prussians who made the next return of the Regent after Napoleon’s defeat in the Battle of Waterloo, that lead to his exile to the island of Elba in 1814. Before that, following years of terrors and political power struggles of the France First Republic, Napoleon staged the Coup of 18 Brumaire in 1799 and seized the power from reign of the Directory, declaring essentially a dictatorship over France. Victories of his military campaigns in Europe (the Napoleonic wars) and the profits thereby brought to France had empowered Napoleon to become Emperor of the French by 1804. Napoleon appeared to have a special preference for the Regent among all other royal gems, and had placed the Regent in the pommel of his sword as depicted in the portraits by Jacques‐Louis David, First Painter to the Emperor. Later, it was removed to decorate his two‐edged sword: A wish of glorifying the Emperor’s undefeated might or a hope for a long‐lasting prosperous France? Unfortunately, neither came true. Shortly after losing the battle at Waterloo, Napoleon signed abdication of the throne and was exiled to Elba Island. His son and second wife, Marie Louis, went back to her home country, Austria, taking the Regent with her. Marie’s father, the Habsburg Emperor Francis II of Austria, who apparently was well acquainted with the value of the Regent and its history within the French royalty, returned the gem to France to avoid any unnecessary misunderstanding or, even worse, some regrettable disputes that could arise because of the gem. Obviously, the Habsburg Emperor valued peace for his own people, no matter how brief it might be at that time, above the possession of a gem.
Once back in the collection of French crown jewels, the Regent was mounted again on the crowns of Louis XVIII at his coronation (1814), as well as Charles X (1825) and Napoleon III (1852), during which time the people in France endured years of repaying the war debts caused by the Napoleonic military campaigns. Power struggles in the parliament and royal politics often delayed or derailed the much‐needed economic reforms that were necessary for reconstructing the nation. The parliament was filled with elites and wealthy nobles, suspicious of corruption and the abuse of power, which had widened the gaps between the rich and poor sending labor‐class people further into poverty and suffering. People’s resentment and frustrations continued to mount until finally they erupted into the Second Revolution in 1848. As the result, Charles X abdicated from the throne and the French Second Republic was established with Napoleon III, a nephew of Napoleon, the former Emperor of French, as the President who subsequently became the Emperor of France himself four years later.
Under Napoleon III’s government, France finally began to rebuild and grow in prosperity. The long‐awaited overhaul of the French banking systems and economic reforms significantly improved the country’s competiveness in export and trade. Industrialization greatly improved agricultural efficiency, resulting in surplus that finally eliminated the lasting cyclical famines. Urban infrastructure in Paris and other major cities in France were upgraded or reconstructed, attracting increasing number of foreign visitors from all over Europe. Napoleon III was skillful in international relations: Forging alliances with Britain, curbing Russia’s aggressiveness, signing favorable trading treaties, and expanding overseas business and colonies. France was emerging as a major player again in the Europe’s political landscape. Ironically, while the first Napoleon had left France enormous amounts of national debts after his short‐lived military glory, it was his nephew who actually revived France’s fortune.
In 1870, Napoleon III lost the Franco‐German War and was captured by Prussians in the Battle of Sedan. The French parliament immediately proclaimed its Third Republic forcing Napoleon III and his wife, Empress Eugenie, into exile in England. The former Emperor died three years later. On official records, the last royalty to wear the Regent was Empress Eugenie, who was reported to have the gem on her Grecian diadem probably before her exile. Since 1887, the Regent has been in the collection of the French Royal Treasury, still on display at the Louvre in Paris today [3].
The Regent has witnessed many changes in the world since its birth, but there are few things that never changed and one of them is that the Regent is still the world’s finest diamond and of eternal beauty.
2.2 Diamonds: From Structure to Classification
Traditions surrounding diamonds across so many cultures have imbued them with mystical aura. Their ability to endure down through the ages sets them apart from most other substances. But beneath all that history of glamour and status, diamonds are a natural material with a set of unique physical properties, capable of equally critical engineering and industrial utility, and now are making their contributions at a microscopic level to the wellbeing of humanity. We start this section with a discussion on the structure of diamond crystals from a scientific perspective.
2.2.1 Structure
The crystal structure of diamond is symmetric in all three dimensions, known as the diamond cubic lattice. It is the same structure shared by silicon, geranium, and some other alloys, in addition to carbon. Figure 2.1 shows a unit cell of the diamond cubic Bravais lattice, in which every carbon atom is represented by a sphere in a three‐dimensional matrix that can be repeated infinitely to fill the entire space [4]. A way to look at this crystal structure is by dividing the unit cell into eight smaller cubes, which can be further separated into two groups. The cube of the first group consists of five carbon atoms in a sp3 tetrahedral configuration with four carbons occupying four corners and the last one at the center of the cube. These carbon‐filled cubes are connected by two pairs of carbon atoms: One pair located in the lower half of the unit cell aligned diagonally, while the other pair in the upper half also aligned diagonally. These two pairs are staggered in perpendicular from each other. The cube of the other group, however, contains no carbon atoms at the center and, as a result, there are a total of eight carbon atoms [1 × 4+(⅛) × 4 × 8] in the unit cell.
Another way to view the diamond crystal structure is that it is composed of two interpenetrating face‐centered cubic lattices aligned in the direction of the body diagonal and displaced by ¼ length of the diagonal. All carbon atoms in the lattice are bonded tetrahedrally with a bond angle of 109.5° and a uniform carbon–carbon bond length of dcc = 0.154 nm throughout. Since the bond length corresponds to a translation between the two basis points, (0,0,0) and (1,1,1), it can be related to the width of the unit cell (called lattice constant, a) as dcc = a[(¼)2 + (¼)2 + (¼)2]1/2, which leads to a = 3.567 Å for diamond, a value confirmed by X‐ray diffraction measurement. The total number of carbon atoms in such a unit cell is also 8, calculated as 1 × 4+(½) × 6+(⅛) × 8. William Henry Bragg and William Lawrence Bragg were the first to determine the crystal structure of diamond in 1913, and were awarded the Nobel Prize in Physics 1915 “for their services in the analysis of crystal structure by means of X‐rays” [5].
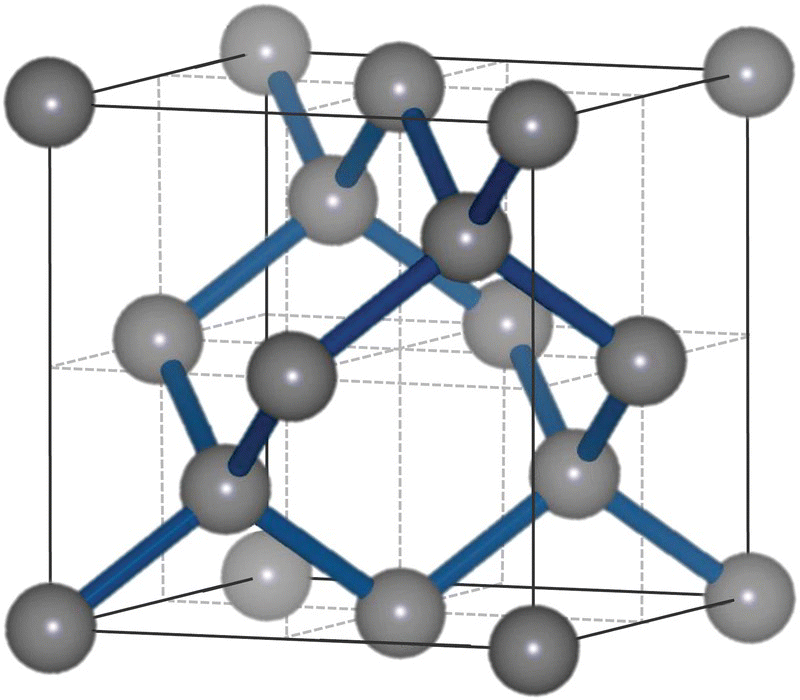
Figure 2.1 Unit cell of the diamond cubic crystal structure with a lattice constant of 3.57 Å. Carbon atoms are presented in gray spheres.
With eight carbon atoms squeezed in a 3.567‐Å cube, diamond has a mass density of 3.515 g cm−3 at room temperature and a number density of 1.76 × 1023 atoms cm−3, which is the highest of any material. Given this exceptionally compact structure, diamond shows several superlative physical properties not found in other carbon materials (Table 2.1) [6]. First, diamond is the hardest known material to date, about 40 times harder than stainless steel. Second, diamond has the highest thermal conductivity of any bulk material, 2000 W m−1 K−1 at 300 K, about 4 times more than copper. Third, diamond possesses the largest refractive index (n = 2.41 in the visible) of all dielectric materials, about 1.6 times as large as that of quartz. Because of these unique properties, together with their remarkable chemical inertness, diamond grits and powders have found a wide range of industrial applications over decades [7]. In 2015, the global rough diamond production from the world’s 54 largest diamond mines combined was estimated to be over 100 million carats, or 20 million grams [8], a high demand that comes at a steep price of harsh environmental hazards associated with the diamond mining [9].
Table 2.1 Typical physical properties of diamond.
Source: From Ref. [6].
| Property | Value | Unit |
| Hardness | 10 000 | kg mm−2 |
| Strength, tensile | >1.2 | GPa |
| Strength, compressive | >110 | GPa |
| Density | 3.52 | g cm−3 |
| Young’s modulus | 1220 | GPa |
| Thermal expansion coefficient | 0.0000011 | K−1 |
| Thermal conductivity | 20.0 | W cm−1 K−1 |
| Debye temperature | 2200 | K |
| Optical index of refraction (at 591 nm) | 2.41 | Dimensionless |
| Bandgap | 5.45 | eV |
2.2.2 Classification
A chemically pure and structurally perfect diamond is transparent and colorless, like a drop of pure water. This is because diamond is a wide bandgap semiconductor with an energy gap of Eg = 5.45 eV (Table 2.1) between valence and conduction bands (or 5.47 eV in [10]). Colors may arise from the presence of chemical impurities and/or structural defects in the diamond matrix. In gem and jewelry industry, color, clarity, cut, and carat weight (known as 4Cs) are four well‐accepted standards in describing the gem‐quality of diamond [11]. Scientists have been interested in the origin of diamond color not only to gain a better control of the color for market values, but also to learn the factors responsible for these interesting physical properties suitable for applications in electronics, optics, and even medicine.
Nitrogen is the most common impurity present in the crystal lattice of natural diamond [12]. In fact, the content of nitrogen both in quantity and structural form has been used to classify diamond materials (Table 2.2) [13]. Type I diamond contains up to 0.3% (3000 ppm) nitrogen and type II contains none or little nitrogen. Type I is further divided into two groups, Ia and Ib, depending on the nitrogen concentration and how these atoms are incorporated into the crystal lattice. The nitrogen atoms in type Ia diamond exist as aggregates, while in type Ib as atomically dispersed nitrogen entities. About 98% of natural diamonds belong to type Ia, containing nitrogen aggregates, with a strong optical absorption below 300 nm and thus colorless (Figure 2.2) [14], whereas the nitrogen atoms in type Ib diamond absorb strongly at a longer wavelength (i.e. 400 nm), imparting yellow color to diamond (Figure 2.3). There are also two groups of diamond in class II: Type IIa for pure diamond and type IIb for boron‐doped diamond. Type IIa diamond makes up 1–2% of all natural diamonds and is optically transparent with its transmittance spanning from 225 to 2000 nm (Figure 2.2).
Table 2.2 Classification of diamonds.
Source: From Ref. [13]. Reproduced with permission of Elsevier.
| Type | Feature | Color | Note |
| Ia | Nitrogen atom concentration up to 0.3%, aggregated nitrogen | Colorless | Most natural diamonds |
| Ib | Nitrogen atom concentration up to 500 ppm, singly substitutional nitrogen | Yellow, orange | Rare in nature, almost all HPHT diamonds |
| IIa | No or few nitrogen atoms | Colorless | Rare in nature, the “purest” diamonds |
| IIb | No or little nitrogen, boron‐doped | Blue, gray | Extremely rare in nature, p‐type semiconductor |
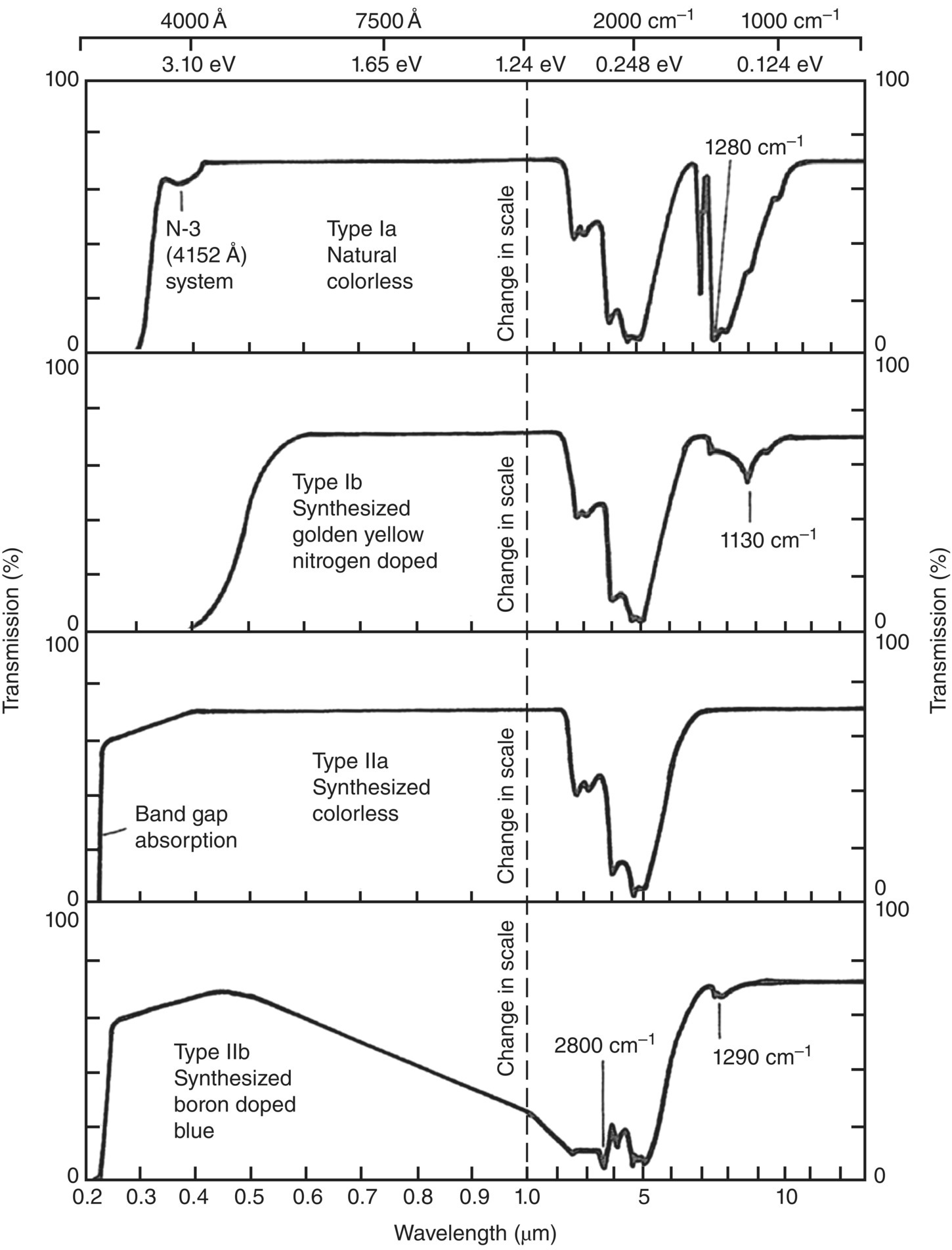
Figure 2.2 Optical transmission spectra of natural and synthetic diamonds of various types. Note the change in scale at 1 μm.
Source: Adapted with permission from Ref. [14].
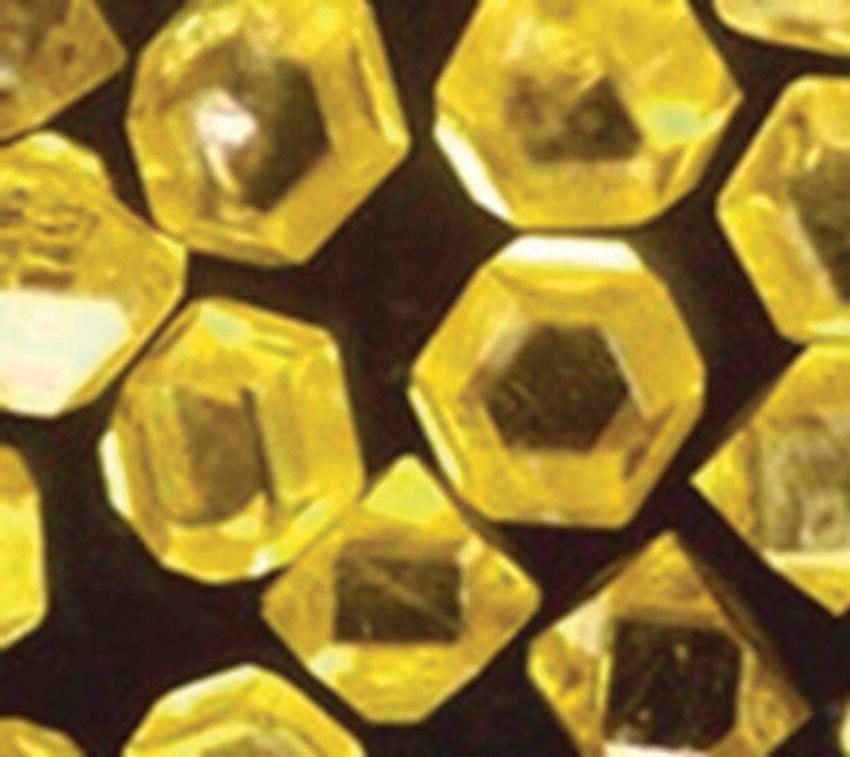
Figure 2.3 Diamond crystallites synthesized by the HPHT method. The particles, diameter approximately 400 μm, contain typically 100 ppm nitrogen.
To analyze the diamond composition in laboratories, the commonly used analytical instruments include thermal gravimetric analysis, mass spectrometry, infrared spectroscopy, Raman spectroscopy, ultraviolet‐visible spectroscopy, X‐ray diffraction, optical microscopy, electron microscopy, etc. Mid‐infrared spectroscopy (4000–400 cm−1 or 2.5–25 μm) is arguably the most useful tool to characterize the nitrogen concentration without causing damages to the crystals (cf., Section 3.1). As we will see throughout the later chapters, there are many examples and problems that cannot be solved without these instruments.
2.3 Diamond Synthesis
Being an allotrope of carbon in the sp3 configuration, diamond in fact is a metastable phase at normal temperature and pressure (Figure 2.4) [15]. It is less thermodynamically stable than graphite by 2.90 kJ mol−1 at 0 °C and 1 atm (i.e. the standard conditions for temperature and pressure). As a result, natural diamonds are typically formed in the lithospheric mantle at a depth of more than 140 km on Earth [16], discovered only after being forced to the earth surface via volcanic activities. At room temperature, diamond is converted to graphite at a very slow rate and notable structural changes only occur at temperatures above 1700 °C in a high vacuum [17]. For diamonds without defects inside the crystal lattice, graphitization starts at surface and proceeds gradually toward the core [18, 19].
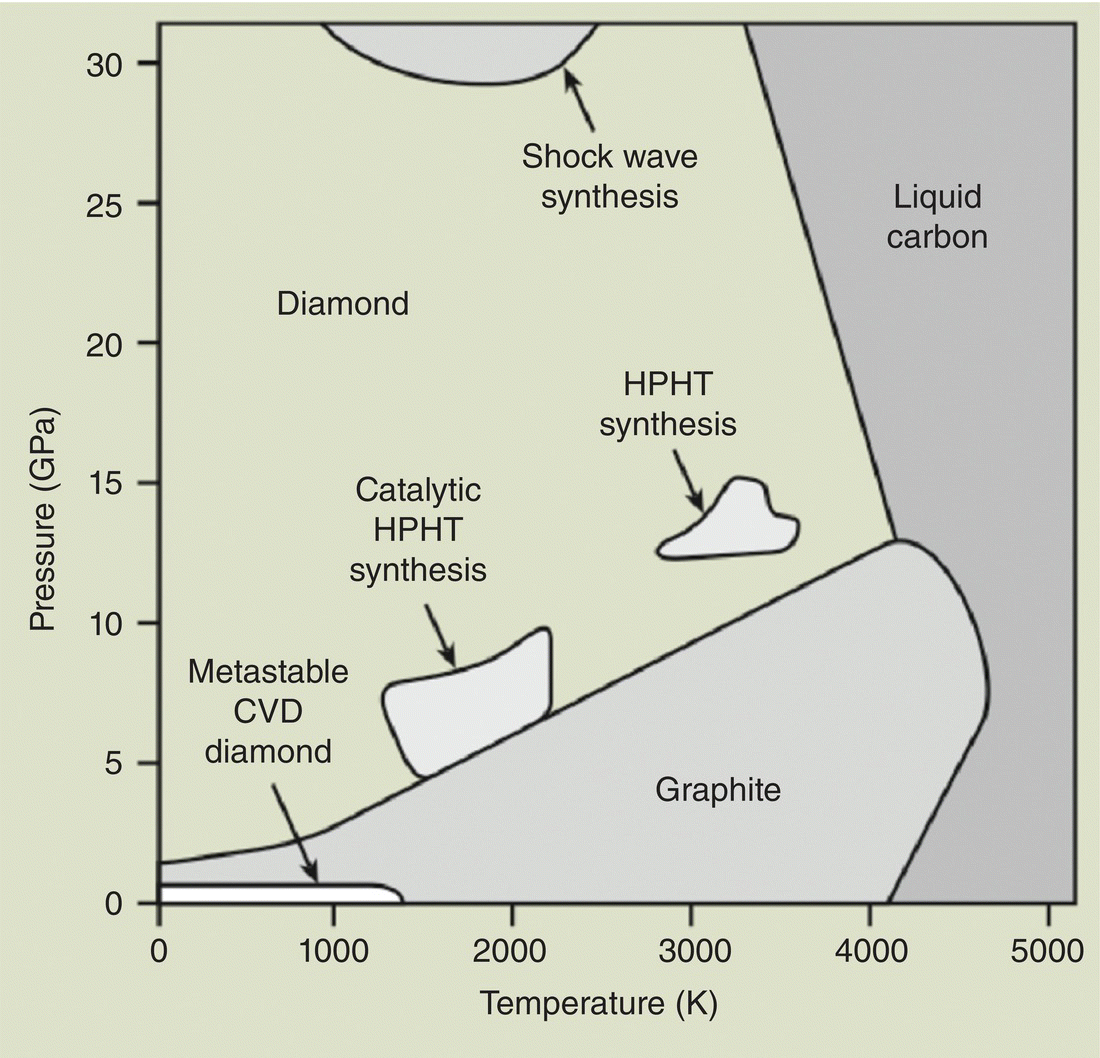
Figure 2.4 Phase and reaction diagram of carbon. The regions where the three synthesis methods, HPHT, CVD, and detonation (or shock wave), discussed in the text are indicated in the figure.
Source: Adapted with permission from Ref. [15]. Reproduced with permission of John Wiley & Sons.
Attempts to make diamonds artificially have a long history back to 1797, the year that diamond was first discovered to comprise only carbon atoms [20]. A famous example of the early attempts is the synthesis of diamonds by heating charcoal with iron inside a carbon crucible at temperatures above 3000 °C, followed by rapid cooling of the molten iron in water [21]. The reproducibility of this method, however, was poor. It was not until the 1950s that scientists were able to produce diamonds reliably in the laboratory, recreating the physical construction of what was once thought a singularity of nature. The first approach in this synthesis was to mimic nature by applying a high pressure (either static or transient) to graphite under a high temperature condition. Low‐pressure and low‐temperature methods were later developed to produce diamonds that is physically and chemically identical to those found in nature. However, the synthesis under such conditions often yielded a mixture of diamond, graphite, and amorphous carbon. Luckily, methods have been developed to overcome this problem by controlling the crystal formation kinetics such that the growth rate of diamond is higher than those of graphite and amorphous carbon [22]. Currently, the three commonly used methods for industrial production of synthetic diamonds are: High‐pressure high‐temperature (HPHT), chemical vapor deposition (CVD), and detonation.
2.3.1 HPHT
The first reproducible synthesis of “man‐made diamonds” with the HPHT method was demonstrated by a research group at General Electric in 1955 [23]. The method used a hydraulic press to generate a pressure in the range of 5–11 GPa while maintaining the temperature in the range of 1200–2200 °C. A special design of this ultra‐high pressure belt apparatus (Figure 2.5) was that both the chamber and pistons received lateral support from stressed binding rings, enabling the extreme conditions to be maintained for hours [24], which was a critical step in the synthesis. The addition of “solvent‐catalyst” consisting of nickel, cobalt, and iron also helped dissolve carbon, allowing the graphite‐to‐diamond conversion to proceed at lower temperatures and pressures as indicated in Figure 2.4.
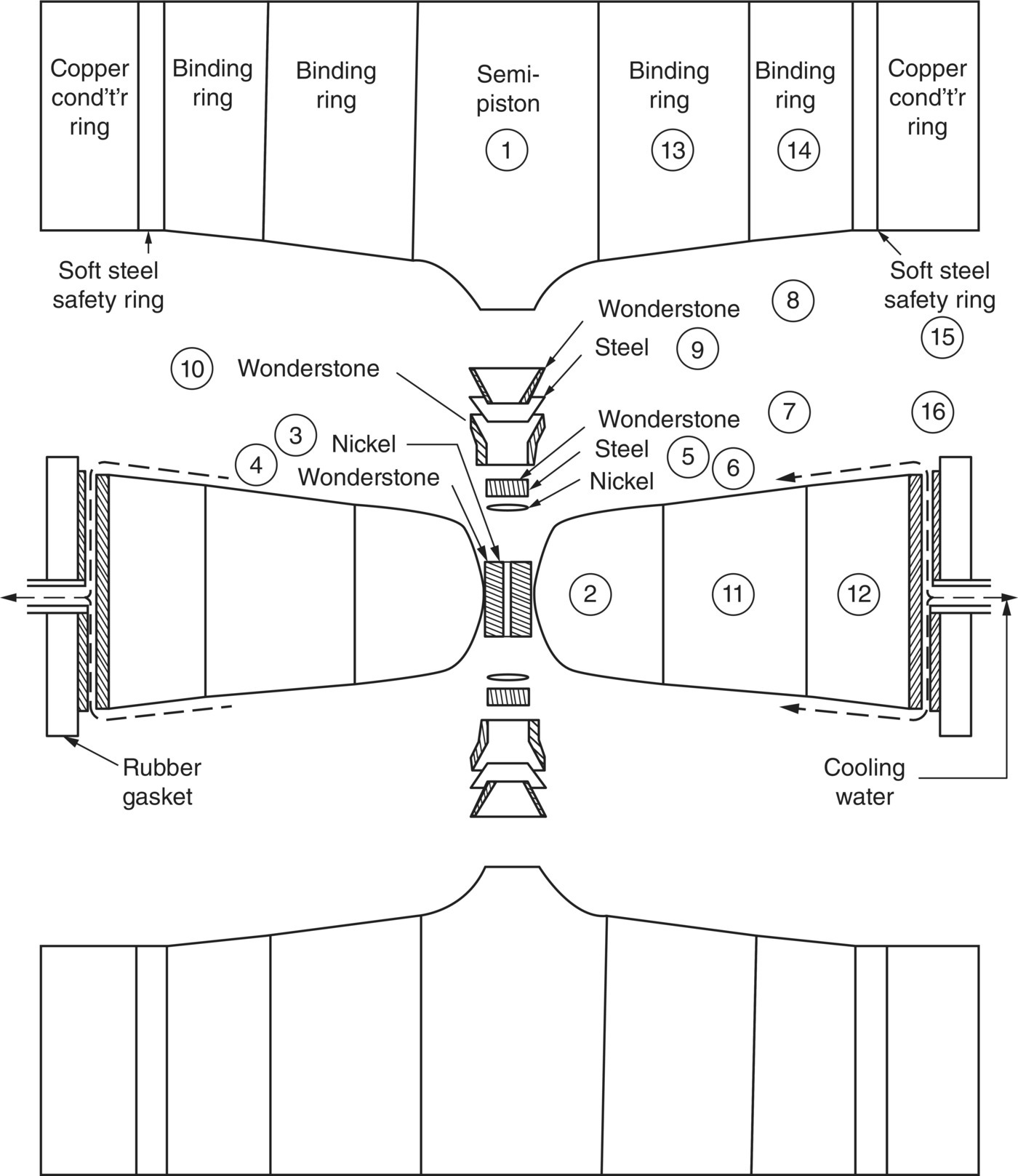
Figure 2.5 Ultrahigh pressure belt apparatus used by General Electric for the HPHT diamond synthesis.
Source: Reprinted with permission from Ref. [24]. Reproduced with permission of AIP Publishing LLC.
The diamond crystals grown by this sophisticate HPHT technique are of high quality and do not contain non‐diamond carbon structures (e.g. diamond‐like carbon, graphite, and amorphous carbon). Their sizes range from tens of nanometers to about 1 cm. A shortcoming of this method is that the diamonds grown are of type Ib with typical 100–200 ppm of single substitutional nitrogen atoms incorporated into the crystal lattice and thus appear yellowish (Figure 2.3). These man‐made diamonds can be easily distinguished from natural diamonds by measuring the concentrations of nitrogen contaminants with infrared spectroscopy (Table 2.2). Although the HPHT diamonds have not played a significant role in the mainstream diamond trade, they are monocrystalline and useful for a variety of industrial processes, including cutting and machining of mechanical components as well as polishing and grinding of optics.
2.3.2 CVD
Diamond growth through CVD was first reported in the 1960s [21]. The method grew diamonds by applying low‐pressure carbon‐containing gases on a chosen substrate. However, the rate of the growth in early experiments proved to be slow. This was mainly caused by the deposition of graphite, leading to the formation of mixed sp3/sp2 phases [25]. The breakthrough in the CVD diamond synthesis came from the discovery that the presence of a large amount of hydrogen atoms in the gas mixtures helped etch away sp and sp2 carbon atoms, enhancing the diamond growth [26]. The CVD‐based diamond growth has become an active and extensive area of research since the 1980s and stimulated industrial manufacture and use of diamond materials in many applications.
A typical source of carbon for CVD diamond growth is methane; however, other carbon‐containing gases also work. The growth requires a means of activating precursor molecules diluted in hydrogen, which is commonly performed through thermal methods (e.g. a hot filament) or in microwave plasma (Figure 2.6) [27]. The typical concentration of methane in the gas mixture is 0.5–2%. The growth pretreatments such as mechanical abrading, ultrasonic seeding, and ion bombardment are often used to prepare nucleation centers on the surface of the substrate, which is usually made of copper or silicon [25]. The CVD technology allows for the deposition of thin polycrystalline diamond films on areas up to 100 cm in diameter. Compared with HPHT, the method is less cost‐effective but is more versatile in producing diamond films of various kinds. For example, the addition of gases such as trimethyl boron to the gas mixtures facilitates the incorporation of boron defects into the diamond (i.e. doping of diamond), which in turn allows precise tuning of the physical properties (e.g. bandgap and electrical conductivity) of such diamond materials. The CVD films can later be milled to gain particles of various sizes and structures. Furthermore, diamond films with varying isotopic compositions of 12C and 13C are also synthesizable with this method [28].
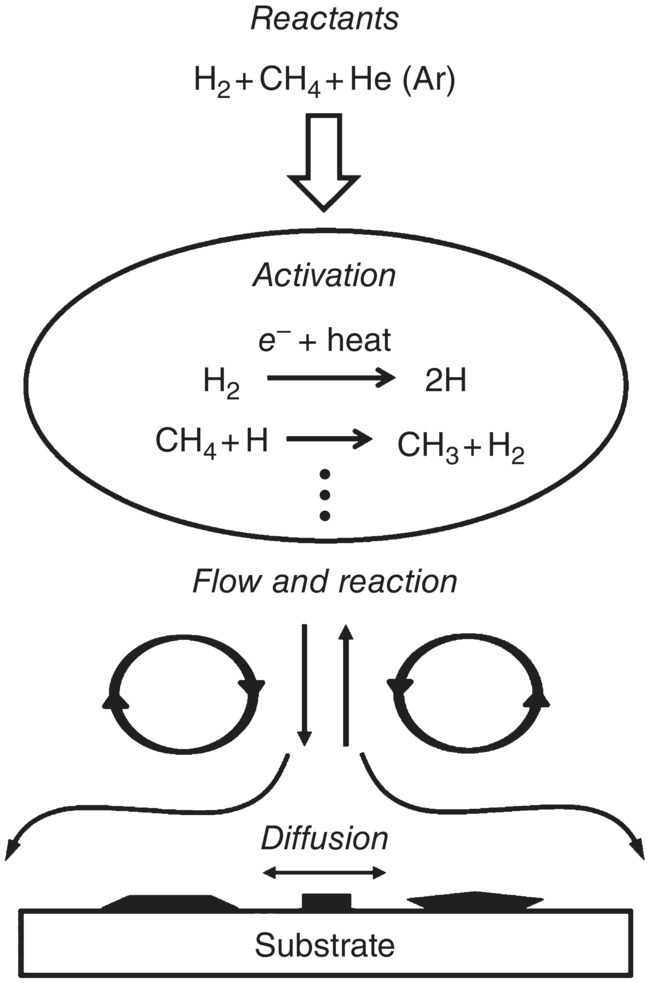
Figure 2.6 Schematic diagram of the CVD diamond growth mechanism. Methane is used as the carbon source in this example.
Source: Adapted with permission from Ref. [27].
2.3.3 Detonation
In 1963, a group of scientists in the Soviet Union discovered tiny crystallites of diamond particles in soot produced by detonating an oxygen‐deficient TNT/hexogen composition in an inert media [29]. The average size of the primary particles of these so‐called “detonation nanodiamonds (DNDs)” was only about 5 nm as a result that the shock waves, which compressed carbon into diamonds, generated by the explosion lasted only a fraction of a microsecond (Figure 2.4). For several reasons, including security measures in the USSR and a lack of interest in nanotechnology at that time, the application of this type of diamonds remained under‐exploited until recently [30].
DNDs have the smallest particle sizes among all synthetic diamonds. However, the transient explosion conditions in the reaction chamber inevitably cause severe agglomeration of the material, leading to an effective size of approximately 200 nm. Transmission electron microscopy (TEM) has revealed that the particles are linked by covalent bonds among surface functional groups and also by the soot structures surrounding every primary particle (Figure 2.7a). Therefore, thorough purification of the material is required in order to obtain graphite‐free NDs. Figure 2.7b shows a model explaining the unusually tight aggregation [31]. To isolate the primary particles, elaborate post‐processing deagglomeration and purification are often undertaken to break the covalent linkage between the disordered (sp and sp2) carbon atoms on the surfaces. A proven method is that of Osawa [32] using wet ball milling with ZrO2 microbeads. The resulting DND particles have a fairly uniform size distribution with a mean diameter of 4–5 nm. They are available in kilogram quantities in the form of colloidal sols, gels, and assemblies, suitable for various types of applications.
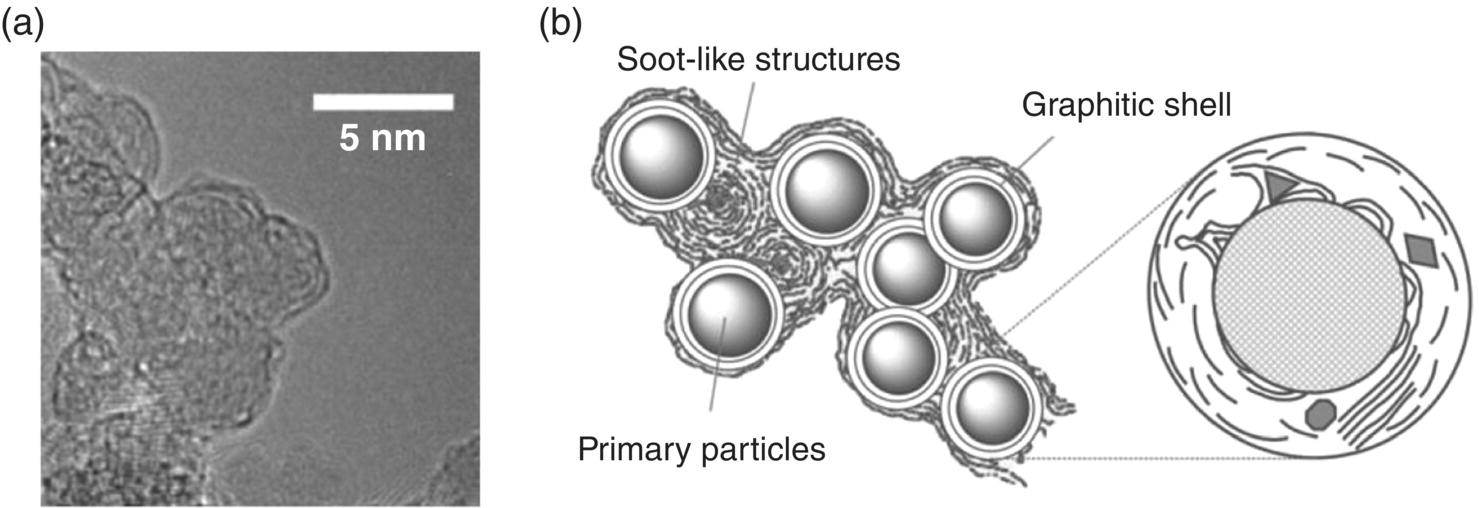
Figure 2.7 (a) High‐resolution TEM image of DNDs. The particles are surrounded by graphitic and soot‐like materials. (b) Structure model of the DND agglomerates.
Source: Reprinted with permission from Ref. [31]. Reproduced with permission of John Wiley & Sons.
2.4 Nanodiamonds: A Scientist’s Best Friend
While humans have valued the beauty of diamonds throughout history, as the story of the Regent vividly described earlier in this chapter, there are other virtues that diamonds have long been recognized equally valuable as a resourceful material. Rare in quantity, diamonds are the hardest material ever known to exist on the planet. What most people may not know, or has not fully recognized or utilized, is the other unique properties that make diamond distinctive from other materials. They include: (i) diamond is chemically inert, nontoxic, and biocompatible; (ii) the surface of diamond is readily derivatizable with a variety of functional groups; (iii) the structural defects in diamond are highly fluorescent and photostable. Leveraging these properties has opened doors to new developments and applications of the materials, particularly nanoscale diamonds, in biology and medicine [33].
As the diamond size decreases, the immediate question is: Do NDs share the same physical properties of diamonds given the same molecular structure in both? If not, to what extent ND begins to deviate its behaviors from diamonds? It is likely, as we have experienced with other nanomaterials, additional properties may occur and we will need to know the causes and how to control them effectively. We will discuss the size of NDs here and other size effects in the next chapters.
NDs belong to a broad family of nanocarbon including fullerenes, nanotubes, and graphenes, as discussed in Section 1.2. NDs can be loosely classified by size: Nanocrystalline (10–100 nm) or ultrananocrystalline (<10 nm) [34]. DNDs belong to the latter; they are polycrystalline and composed of a sp3‐hydridized diamond core coated with a sp2‐hybridized graphite shell or amorphous carbon (Figure 2.7a). The typical carbon content in these particles is in the range of 90%, in addition to 2% nitrogen atoms as impurities [35]. As a result, DNDs are not so suitable for optical applications but are more useful as drug‐delivery devices because of their small size (Chapter 13). HPHT‐NDs, in contrast, are monocrystalline with high optical transparency. These nanomaterials are produced by crushing and/or ball milling of micrometer‐ or millimeter‐sized diamond crystallites synthesized by HPHT methods and then separated by centrifugal force. Although their size is larger (typically 10–100 nm) and their size distribution is considerably broader than DNDs, they contain less sp2 carbon on surface and thus can be purified more easily in acids. Moreover, the HPHT‐ND particles can host a high‐density ensemble (>10 ppm) of fluorescent centers for applications as bioimaging agents (Chapter 6).
The smallest possible diamonds are the diamondoids, which are naturally present in crude oil [36]. With a formula of C10H16, adamantane is the simplest diamondoid molecule. It is a cycloalkane with a structural configuration similar to a segment of the diamond lattice (Figure 2.8). The molecule has a diameter of only approximately 0.5 nm, given a C─C bond length of 0.154 nm and a C─H bond length of 0.109 nm. A wide range of diamondoids consisting of 1–10 adamantane cages have been discovered and structurally identified in the laboratories with various spectroscopic techniques [37]. Larger diamondoids, such as cyclohexamantane (C26H30) with a size of more than 1 nm [38], are particularly interesting because they bridge the gap between hydrocarbon molecules and hydrogenated diamond nanocrystals. Compared to NDs with nonuniform size and irregular shape, these cage compounds are advantageous in having well‐defined structures and have attracted considerable attention of organic chemists to synthesize novel diamondoid‐based chemicals and materials. Recent developments of the techniques to derivatize diamondoids with various functional groups have expanded the research on these unique structures and the applications of these cage compounds in pharmaceutics, molecular electronics, and many other areas [39].
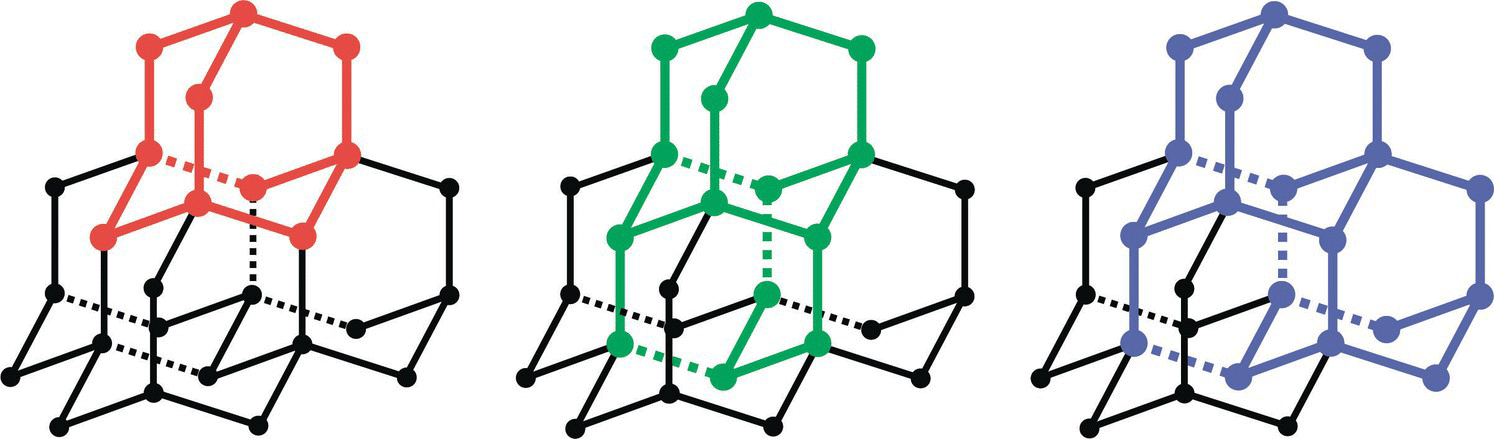
Figure 2.8 Structures of adamantane (red), diamantane (green), and triamantane (blue) as segments of the diamond lattice.
NDs that fill the size gap between hydrocarbon molecules and hydrogenated diamond nanocrystals, surprisingly, came from the outer space. In 1987, scientists discovered a new type of natural diamonds called meteoritic nanodiamonds, isolated from acid dissolution residues of primitive carbonaceous meteorites (Figure 2.9) [40]. The median diameter of these NDs was 2.6 nm, about 10–1000 times smaller than dust grains in interstellar space [41]. The concentration of the ND grains found in the Orgueil meteorite, a meteorite discovered in southwestern France in 1864, was up to 1400 ppm [42]. Further detailed analysis of the microstructures of the meteoritic NDs with high‐resolution TEM suggested that the predominant mechanism for the presolar diamond formation may be a vapor deposition process, rather than high‐pressure compression by shock waves [43]. Another notable finding of these studies is that ND is highest in abundance among all stardust particles (including SiC, graphite, and oxides) in meteorites [44]. This has led to a suggestion that NDs as small as 3 nm are thermodynamically more stable than graphite of similar size [45, 46]. It has also triggered an active search for interstellar NDs by astronomical observations in conjunction with laboratory studies. We will continue the discussion of this interesting topic in Chapter 14.

Figure 2.9 Diamond nanoparticles (white powders in vial) extracted from a piece of the carbonaceous Murchison meteorite.
Source: Reprinted with permission from Ref. [53].
The recent emergence of fluorescent nanodiamonds (FNDs) has sparked a new era of technological innovation with NDs for cell labeling, imaging, and tracking applications [47–50]. This technological advancement is made possible by high‐yield fabrication of these fluorescent carbon nanoparticles with excellent biocompatibility and unique optical properties (Chapter 6). A special attribute of FNDs is their bright and stable fluorescence originating from the nitrogen‐vacancy color centers (Chapter 3). When exposed to green‐yellow light, the FNDs emit non‐photobleaching tissue‐penetrating red photons, making them well suited for bioimaging applications. Contemporary research studies in the field have been focusing on applying the surface‐functionalized FNDs for nanoscale imaging and quantum sensing, as to be elaborated in Chapters 10 and 11, respectively. Moreover, the conjugation of FNDs with other nanoparticles to form hybrids has opened up exciting new horizons for this novel nanomaterial (Chapter 12).
Today, we are witnessing a rapid increase of interest in the use of ND particles in a broad range of science and technology areas. These include tribology, quantum information, catalysis, nanoscale sensing, biomedicine, and many more [51, 52]. We will provide comprehensive discussions of FNDs and their biomedical applications in the following chapters.
References
- 1 Streeter, E. (1882). The Great Diamonds of the World. G. Bell & Sons.
- 2 Tilley, A. (1922). Modern France. A Companion to French Studies. Cambridge University Press.
- 3 Mabille, G. (2001). Diamond, known as the “Regent”. http://www.louvre.fr/en/oeuvre‐notices/diamond‐known‐regent (accessed 16 April 2018).
- 4 Kittel, C. (2005). Introduction to Solid State Physics, 8e. Wiley.
- 5 The Nobel Prize in Physics 1915. Nobelprize.org. Nobel Media AB 2014. https://www.nobelprize.org/nobel_prizes/physics/laureates/1915 (accessed 16 April 2018).
- 6 Yoder, M.N. (1994). The vision of diamond as an engineered material. In: Synthetic Diamond: Emerging CVD Science and Technology (ed. K.E. Spear and J.P. Dismukes), 3–17. Wiley.
- 7 Tomlinson, P.N. (1992). Applications of diamond grits and composites. In: The Properties of Natural and Synthetic Diamond (ed. J.E. Field), 637–666. Academic Press.
- 8 Zimnisky, P. (2015). Global rough diamond production estimated to hit over 135M Carats in 2015. Kitco Commentary, Kitco.
- 9 The Environmental Literacy Council (2015). Diamond mining. https://enviroliteracy.org/land‐use/mineral‐resources/diamond‐mining (accessed 16 April 2018).
- 10 Clark, C.D., Dean, P.J., and Harris, P.V. (1964). Intrinsic edge absorption in diamond. Proc R Soc A 277: 312–329.
- 11 Dundek, M. (2009). Diamonds, 3e. Nobel Gems Publications.
- 12 Kaiser, W. and Bond, W.L. (1959). Nitrogen, a major impurity in common type I diamond. Phys Rev 115: 857–863.
- 13 Field, J.E. (1992). Applications of diamond grits and composites. In: The Properties of Natural and Synthetic Diamond (ed. J.E. Field), 669. Academic Press.
- 14 Pankove, J.I. and Qui, C.H. (1994). Optical properties and optoelectronic applications of diamond. In: Synthetic Diamond: Emerging CVD Science and Technology (ed. K.E. Spear and J.P. Dismukes), 401–418. Wiley.
- 15 Bundy, F.P. (1980). The P,T phase and reaction diagram for elemental carbon, 1979. J Geophys Res 85: 6930–6936.
- 16 Tappert, R. and Tappert, M.C. (2011). Diamonds in Nature: A Guide to Rough Diamonds. Springer.
- 17 Howes, V.R. (1962). The graphitization of diamond. Proc Phys Soc 80: 648–662.
- 18 De Vita, A., Galli, G., Canning, A., and Car, R. (1996). A microscopic model for surface‐induced diamond‐to‐graphite transitions. Nature 379: 523–526.
- 19 Adiga, S.P., Curtiss, L.A., and Gruen, D.M. (2010). Molecular dynamics simulations of nanodiamond graphitization. In: Nanodiamonds: Applications in Biology and Nanoscale Medicine (ed. D. Ho), 35–54. Springer.
- 20 Tennant, S. (1797). On the nature of the diamond. Philos Trans R Soc Lond 87: 123–127.
- 21 Hazen, R.M. (1999). The Diamond Makers. Cambridge University Press.
- 22 Spear, K.E. and Dismukes, J.P. (ed.) (1994). Synthetic Diamond: Emerging CVD Science and Technology. Wiley.
- 23 Bundy, F.P., Hall, H.T., Strong, H.M., and Wentorf, R.H. (1955). Man‐made diamonds. Nature 176: 51–55.
- 24 Hall, H.T. (1960). Ultra‐high‐pressure, high‐temperature apparatus: the “Belt”. Rev Sci Instrum 31: 125–131.
- 25 May, P.W. (2000). Diamond thin films: a 21st‐century material. Phil Trans R Soc A 358: 473–495.
- 26 Angus, J.C. and Hayman, C.C. (1991). Low‐pressure, metastable growth of diamond and ‘diamondlike’ phases. Annu Rev Mater Sci 241: 913–921.
- 27 Butler, J.E. and Woodin, R.L. (1993). Thin film diamond growth mechanisms. Phil Trans R Soc Lond A 342: 209–224.
- 28 Anthony, T.R. and Banholzer, W.F. (1992). Properties of diamond with varying isotopic composition. Diam Relat Mater 1: 717–726.
- 29 Danilenko, V.V. (2004). On the history of the discovery of nanodiamond synthesis. Phys Solid State 46: 595–599.
- 30 Vul’, A. and Shenderova, O. (2014). Detonation Nanodiamonds: Science and Applications. Pan Stanford.
- 31 Krueger, A., Ozawa, M., Jarre, G. et al. (2007). Deagglomeration and functionalisation of detonation diamond. Phys Stat Sol A 204: 2881–2887.
- 32 Osawa, E. (2008). Monodisperse single nanodiamond particles. Pure Appl Chem 80: 1365–1379.
- 33 Mochalin, V.N., Shenderova, O., Ho, D., and Gogotsi, Y. (2012). The properties and applications of nanodiamonds. Nat Nanotechnol 7: 11–23.
- 34 Williams, O.A. (2011). Nanocrystalline diamond. Diam Relat Mater 20: 621–640.
- 35 Shenderova, O.A., Vlasov, I.I., Turner, S. et al. (2011). Nitrogen control in nanodiamond produced by detonation shock‐wave‐assisted synthesis. J Phys Chem C 115: 14014–14024.
- 36 Marchand, A.P. (2003). Diamondoid hydrocarbons – delving into nature’s bounty. Science 299: 52–53.
- 37 Dahl, J.E., Liu, S.G., and Carlson, R.M. (2003). Isolation and structure of higher diamondoids, nanometersized diamond molecules. Science 299: 96–99.
- 38 Dahl, J.E.P., Moldowan, J.M., Peakman, T.M., and Carlson, R.M. (2003). Isolation and structural proof of the large diamond molecule, cyclohexamantane (C26H30). Angew Chem Int Ed 42: 2040–2044.
- 39 Schwertfeger, H., Fokin, A.A., and Schreiner, P.R. (2008). Diamonds are a chemist’s best friend: diamondoid chemistry beyond adamantine. Angew Chem Int Ed 47: 1022–1036.
- 40 Lewis, R.S., Ming, Y., Wacker, J.F. et al. (1987). Interstellar diamonds in meteorites. Nature 326: 160–162.
- 41 Lewis, R.S., Anders, E., and Draine, B.T. (1989). Properties, detectability and origin of interstellar diamonds in meteorites. Nature 339: 117–121.
- 42 Huss, G.R. and Lewis, R.S. (1995). Presolar diamond, SiC, and graphite in primitive chondrites: abundances as a function of meteorite class and petrologic type. Geochim Cosmochim Acta 59: 115–160.
- 43 Daulton, T.L., Eisenhour, D.D., Bernatowicz, T.J. et al. (1996). Genesis of presolar diamonds: comparative high‐resolution transmission electron microscopy study of meteoritic and terrestrial nano‐diamonds. Geochim Cosmochim Acta 60: 4853–4872.
- 44 Davis, A.M. (2011). Stardust in meteorites. Proc Natl Acad Sci U S A 108: 19142–19146.
- 45 Nuth, J.A. (1987). Small‐particle physics and interstellar diamonds. Nature 329: 589.
- 46 Badziag, P., Verwoerd, W.S., Ellis, W.P., and Greiner, N.R. (1990). Nanometre‐sized diamonds are more stable than graphite. Nature 343: 244–245.
- 47 Yu, S.J., Kang, M.W., Chang, H.C. et al. (2005). Bright fluorescent nanodiamonds: no photobleaching and low cytotoxicity. J Am Chem Soc 127: 17604–17605.
- 48 Chang, Y.R., Lee, H.Y., Chen, K. et al. (2008). Mass production and dynamic imaging of fluorescent nanodiamonds. Nat Nanotechnol 3: 284–288.
- 49 Wu, T.J., Tzeng, Y.K., Chang, W.W. et al. (2013). Tracking the engraftment and regenerative capabilities of transplanted lung stem cells using fluorescent nanodiamonds. Nat Nanotechnol 8: 682–689.
- 50 Hsiao, W.W.W., Hui, Y.Y., Tsai, P.C., and Chang, H.C. (2016). Fluorescent nanodiamond: a versatile tool for long‐term cell tracking, super‐resolution imaging, and nanoscale temperature sensing. Acc Chem Res 49: 400–407.
- 51 Greentree, A.D., Aharonovich, I., Castelletto, S. et al. (2010). Twenty‐first century applications of nanodiamonds. Opt Photonics News 21: 20–25.
- 52 Arnault, J.‐C. (ed.) (2017). Nanodiamonds: Advanced Material Analysis, Properties and Applications. Elsevier.
- 53 Beatty, K. (2003). A solar source for diamond dust? Sky & Telescope (23 July 2003). http://www.skyandtelescope.com/astronomy‐news/a‐solar‐source‐for‐diamond‐dust (accessed 16 April 2018).
