4
Surface Chemistry of Nanodiamonds
We discuss surface modification chemistry of nanodiamonds (NDs) in this chapter. The chemistry of a substance is ultimately determined by its molecular structure and physical conditions surrounding it. A quick look over the three allotropes of nanocarbon material reveals some subtle differences between the sp2 endcaps of fullerenes, the sp2 sidewalls of carbon nanotubes (CNTs), the sp2 sheets of graphenes, and their dangling carbon bonds along the edges (Figure 1.1–1.3). Both CNT and graphene have mixed chemical reactions as discussed in Section 1.2 and, depending where on a CNT or graphene, different chemical reactions may occur at different locations. Now with the ND’s sp3 saturated carbon bonds, it is the most stable bond configuration of carbon and therefore is expected to be chemically inert. Why would anyone care for the chemistry of such an inert material? Well, it is precisely because of the inertness (chemical stability) and other trademark properties (Table 2.1) that make ND an excellent nanomaterial for a diversified array of applications, ranging from laboratories to industries and from cosmetics to lubrications, including as a supporting medium for biomolecules. Similar to CNTs and graphenes, all chemical activities if/when occurs with NDs must happen on their surface. Fortunately, for a given amount, ND is equipped with a large surface area and a deep loading capacity perfectly for a nano‐sized carrier. But, the question is: How could it be possible for a tiny ND to carry a large load of cargo? We need to look into this matter of ND’s surface area a bit closer before any further discussion of chemistry.
Due to their small size, nanoparticles have a large specific surface area or a large surface‐area‐to‐volume ratio. The specific surface area is defined as the surface area of a material per unit mass (in m2 g−1). Consider a cubic ND particle with a length of l. Its specific surface area increases linearly as l is reduced, because the surface area scales with the length in the second power as 6l2, and the mass in the third power as ρl3, where ρ is the density. For ND cubes of l = 2.86 nm (or eight unit cells in one dimension) as an example (Figure 4.1), 1 g of these particles has a total surface area of 600 m2, which is about 1.5 times as large as that of a basketball court (15 m × 28 m = 420 m2). Out of the 4096 carbon atoms in each cube, nearly 20% of them are located on the surface. Clearly, the smaller the particle size, the more important role the surface atoms play in determining the chemistry. Therefore, prior to any practical use of NDs, one must understand their surface chemistry and their interactions with different target molecules in specific environments. In light of many potential applications of the carbon‐based nanoparticles in electronics, optics, and medicine, studies on the surface chemistry of NDs have been actively and extensively conducted over the past two decades [1]. This chapter provides a condensed but comprehensive overview of these research studies and developments of NDs for biological applications.

Figure 4.1 An idealized cubic ND with a perfect crystal structure and a length of 2.85 nm (or eight unit cells in one dimension).
4.1 Functionalization
As a carbon‐based nanoparticle, ND is inherently biocompatible. It is more suitable for applications in life science research than other nanoparticles such as gold, silver, and silica nanobeads. However, in order to make any use of them, NDs must be chemically modified and/or functionalized so that they are susceptible for ensuing interactions with the target molecules. Only atoms on the surface of NDs are subjected to such chemical modifications. This is because a bare ND’s surface always contains many dangling bonds, i.e. unsatisfied valence bonds associated with carbon atoms (Figure 4.1). These surface atoms, which are chemically unstable, can either bind to each other to form double bonds or with other atoms (such as H or O atoms) to fill their valence shells. The chemical reactions involved here essentially follow the basic principles of organic chemistry but are largely hindered by steric effects and thus less effective than small organic molecules in solution. The reactions are slow if the particles are large and have a monocrystalline structure.
NDs, either produced by the high‐pressure high‐temperature (HPHT), chemical vapor deposition (CVD), or detonation method, are always contaminated with residual chemical compounds from the manufacturing processes, leaving sp2 or graphitic carbon atoms on the surface. To remove these components, methods have been developed to treat the as‐formed ND materials in strong oxidative acids such as H2SO4/HNO3 mixtures [2] or ozone [3] at elevated temperatures. Consequently, these particles are derivatized with a variety of oxygen‐containing functional groups, including carboxylic (–COOH) and carbonyl (–C═O) groups as well as different alcohol (primary, secondary, and tertiary) and ether groups, etc. Fourier‐transform infrared (FTIR) spectroscopy, Raman spectroscopy, X‐ray photoelectron spectroscopy, thermal desorption mass spectrometry, and thermogravimetric analysis are common tools used to characterize the chemical compositions and surface terminations of these surface‐modified NDs [4]. Through FTIR, researchers have been able to distinguish different types of functional groups and adsorbates on the ND surface based on their signature vibrational frequencies. Furthermore, these analytical techniques can also detect subtle changes in the chemical composition before and after surface modification. For instance, the FTIR spectra of surface‐oxidized NDs often exhibit prominent absorption bands at 1700–1800 cm−1, ascribable to the ─C═O stretches of carbonyl, ketone, aldehyde, carboxylic acid, ester, and other oxygen‐containing groups (Figure 4.2). The spectra can be substantially simplified if a homogeneous layer of hydroxyl groups is formed on the surface by borane reduction [5].

Figure 4.2 IR transmission spectra of DNDs subjected to (a) LiAlH4 reduction, (b) borane reduction, (c) no treatment, (d) oxidation with HClO4, (e) oxidation with HNO3 and H2SO4 (1 : 1, v/v), and (f) reaction with ozone under ultraviolet irradiation.
Source: Reprinted with permission from Ref. [5].
High‐temperature gas treatments also serve as a means to modify the ND surface. Specifically, oxidation in air at temperatures higher than 500 °C has been applied to control the sp2/sp3 carbon ratios and the surface chemistry of detonation nanodiamond (DND) powders [6]. Treatment in hydrogen plasma at high temperatures can reduce ─C═O to ─C─OH and further to ─C─H groups under favorable conditions. Heating NDs in NH3 can lead to the production of a variety of nitrogen‐containing groups including ─NH2, ─C≡N, and moieties containing ─C═N. Similarly, heating NDs in Cl2 produces acylchlorides and in F2 produces the ─C─F groups. Annealing NDs in N2, Ar, or a vacuum at high temperatures completely eliminates all functional groups on the surface, converting sub‐10‐nm NDs such as DNDs to graphitic carbon nano‐onions. Krueger and coworkers [7] have presented a full description of all possible techniques for the chemical treatment and functionalization of ND surfaces. Figure 4.3 provides an overview of some common strategies for the surface modifications of NDs [8]. It illustrates how wet chemistry combined with high‐temperature gas treatments may attach various functional groups to the ND surface. An in‐depth review of the preparation of hydrogenated NDs and the characterization of their surface properties has been given by Arnault and Girard [9].

Figure 4.3 Overview of the commonly used methods for chemical modification and functionalization of ND surfaces.
Source: Reprinted with permission from Ref. [8]. Reproduced with permission of Nature Publishing Group.
Another useful method for the surface modification of NDs is by radical reactions. Benzoyl peroxide is one of the radical initiators to induce the reactions. Experiments of Tsubota et al. [10] demonstrated the feasibility of modifying hydrogenated diamond surface with various carboxylic acids using the peroxide radicals under mild conditions. The process could be greatly facilitated by ultrasonication and microwave irradiation, which initiated the radical copolymerization of surface‐graphitized NDs with molecules containing various functional groups, including –COOH, –NH2, or aliphatic moieties [11]. The development of these techniques, together with the chemical modification schemes as illustrated in Figure 4.3, gives researchers access to further conjugation of NDs with various bioactive ligands or biomolecules for diverse biotechnological and biomedical applications.
Now is a good time to ask a simple and yet critical question: How can we effectively control the surface affinity, namely, hydrophilic or hydrophobic surface? Conventional hydrogenation and fluorination are two obvious choices to prepare hydrophobic NDs. However, they both require high‐temperature gas treatments. Alternatively, wet chemistry offers a more versatile approach. A notable example in this direction is that of Hui et al. [12] who first treated acidified NDs with BH3 in tetrahydrofuran to homogenize their surfaces with hydroxyl groups through reductive reactions, and then terminated the hydroxylated ND surface with octadecyltrimethoxysilane through silanization (Figure 4.4a). As shown in the photograph of Figure 4.4b, the NDs were originally hydrophilic, staying preferably in water (the lower layer), but became highly hydrophobic and all suspended in the organic phase (toluene in this case) after surface modification. These hydrophobic particles can further be encapsulated in lipids to form liposomal NDs (cf., Section 4.3).

Figure 4.4 (a) Synthesis of hydrophobic HPHT‐NDs by reduction and silanization of acid‐treated NDs. (b) Photograph of 40‐nm HPHT‐NDs suspended in toluene/water before and after the reduction and silanization treatments.
Source: Adapted with permission from Ref. [12].
A most useful functional group derivatized on the ND surface is –COOH, which is capable of forming direct covalent conjugation with biomolecules as indicated in Figure 4.3. Zeta potential analysis, which measures the electrokinetic potential of a colloidal dispersion [13], serves as a convenient tool to identify the presence of –COOH groups on the surface. The potential describes the stability of a colloid and its tendency toward agglomeration. The colloid is considered stable when the zeta potential is lower than −30 mV or higher than +30 mV. It has been reported that NDs can form colloids with zeta potentials ranging from −40 to +40 mV, depending on their surface terminations [14–16]. For HPHT‐NDs treated with strong acid mixtures such as H2SO4/HNO3 (3 : 1, v/v) as an example, the colloids can have a zeta potential of −36 mV at pH ≥ 5 (Figure 4.5a) and exhibit high dispersibility in water [17]. The zero‐point potential occurs at pH 2.9, slightly smaller than pKa = 3.75 of HCOOH, meaning that their surface is negatively charged at neutral pH and in physiological medium (pH 7.4).
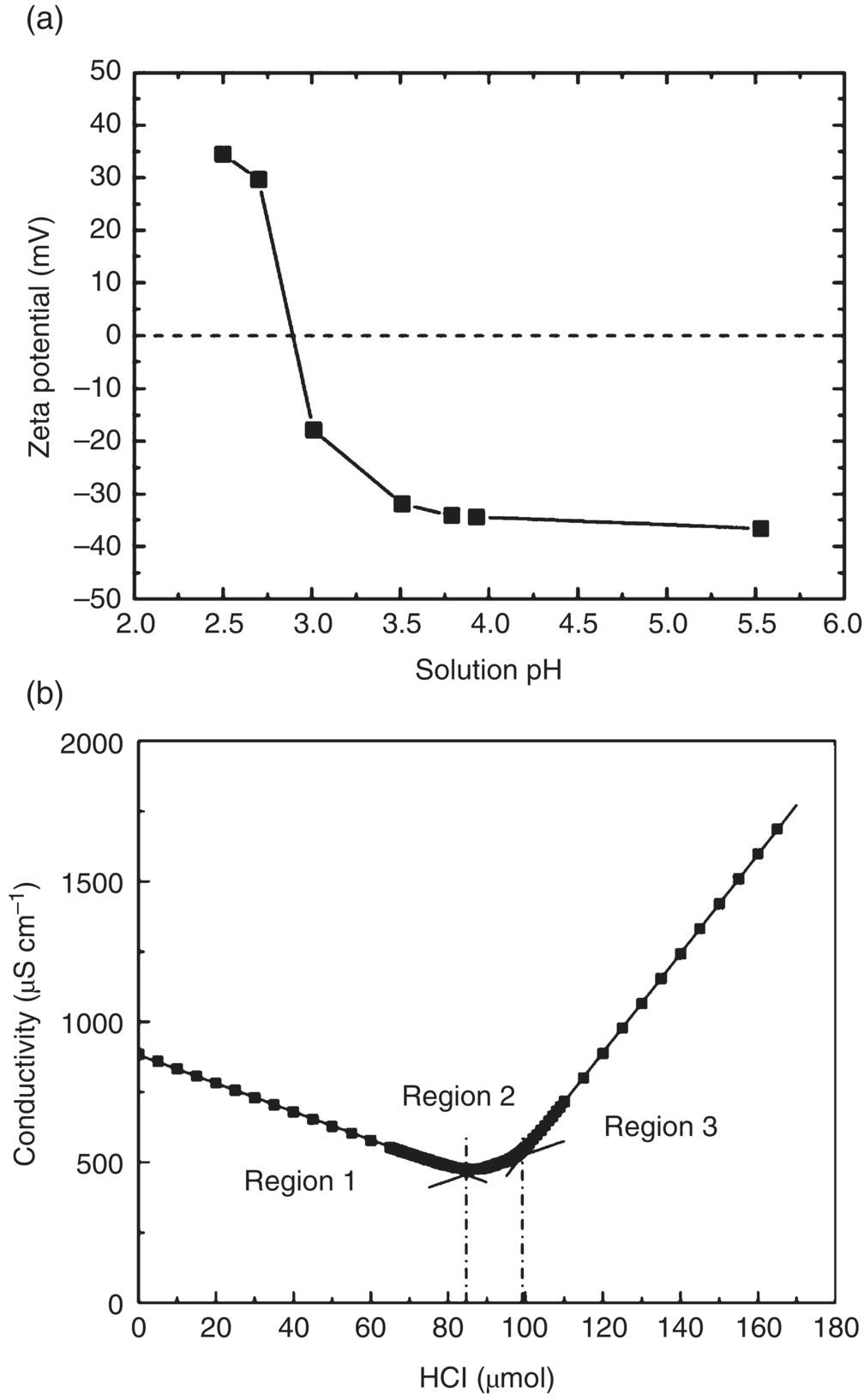
Figure 4.5 (a) Zeta potentials of oxidative‐acid‐treated HPHT‐NDs (nominal size ∼100 nm) as a function of solution pH. (b) Conductometric titration of oxidative‐acid‐treated 100‐nm HPHT‐NDs. In this titration, an excessive amount of NaOH was first added into the ND suspension and then neutralized with 0.1 N HCl.
Source: Adapted with permission from Ref. [17]. Reproduced with permission of Elsevier.
The zeta potential analysis described above provides only a qualitative measurement for the status of surface charge. What exactly is the amount of –COOH groups on the ND surface? To address this issue, Nguyen et al. [17] have adopted a conductometric back‐titration method that measures the electrolytic conductivity as a means to monitor the progress of a chemical reaction in solution [18]. Direct titration of the sample solution with NaOH is not suitable here because the surface carboxylic acid groups on the NDs are low in both acidity and density. Shown in Figure 4.5b is a typical backward titration curve obtained by first reacting the 100‐nm HPHT‐NDs with an excessive amount of 0.1 N NaOH, then titrating back with standardized 0.1 N HCl. The curve consists of three regions, of which the first region corresponds to the neutralization of excess OH−, the second one corresponds to the titration of the surface –COOH, and the third corresponds to the increase of solution H3O+ due to the addition of excess titrant, 0.1 N HCl. The conductivity is lowest when the solution OH− ions are completely neutralized. With this method, the researchers determined a quantity of 100 μmol g−1 for the carboxylate groups on the surface of the 100‐nm HPHT‐ND. However, due to the high polydispersity and irregular shape of the ND particles, only a rough estimate of approximately 7% could be obtained for the fraction of surface carbon atoms in carboxyl form. A higher percentage is anticipated for DNDs which are polycrystalline and contain more defects (and thus more sites to form –COOH groups) on their surface.
4.2 Bioconjugation
4.2.1 Noncovalent Conjugation
A ND's surface may contain a rich variety of functional groups, depending on how the nanomaterial is synthesized and subsequently chemically treated. The chemical treatment can change the properties of the particles from inert to highly reactive or even highly toxic in cells and living organisms. Conjugation of NDs with biomolecules is one of such chemical treatments and can be achieved by either physical adsorption through noncovalent interactions or covalent linkage with surface functional groups. A convenient way to characterize the outcome of the conjugation and the stability of the nanoparticle bioconjugates in solution is dynamic light scattering (DLS).
DLS is a technique measuring the temporal fluctuation of the intensities of scattered light from molecules or small particles undergoing Brownian motion in solution by using lasers as the light sources [19]. It provides information about the hydrodynamic sizes and size distribution profiles of the particles before and after surface modification. The first‐order result of the DLS measurement is the size‐dependent intensity distribution. This distribution, however, does not reflect directly the hydrodynamic diameters (dh) of the particles, because the intensity is proportional to ![]() , instead of dh. Thus, particles of larger size dominate the distribution in observation. It is possible to convert the intensity distributions to volume and number distributions according to the Mie scattering theory [20] if the particles are spherical, sizes are homogeneous, and their optical properties are known. For NDs, due to their irregular shape and large variation in size, the volume and number distributions derived from their intensity distributions are best used only for comparative purposes. Nonetheless, the comparison still sheds significant insight into the result of surface modification.
, instead of dh. Thus, particles of larger size dominate the distribution in observation. It is possible to convert the intensity distributions to volume and number distributions according to the Mie scattering theory [20] if the particles are spherical, sizes are homogeneous, and their optical properties are known. For NDs, due to their irregular shape and large variation in size, the volume and number distributions derived from their intensity distributions are best used only for comparative purposes. Nonetheless, the comparison still sheds significant insight into the result of surface modification.
An array of organic and biological molecules have been conjugated onto NDs either covalently or noncovalently. These include chemotherapeutic drugs [21–24], carbohydrates [25, 26], peptides [14, 27], proteins [17, 28], small interfering RNA [29, 30], and DNA [31, 32] (cf., Chapter 13 for further details). When attaching biomolecules such as proteins to NDs, it is crucial to confirm that their functionalities are conserved. Nguyen et al. [17] addressed this concern in a study of lysozyme adsorption and immobilization on the surface of 100‐nm HPHT‐NDs. They detected the hydrolytic activity of the enzyme after physical attachment and found that their activity was significantly lower than that of free lysozymes in aqueous solution. The relative activity was only about 15% at the surface coverage of 10%. The activity, however, could be boosted as the ND surface was blocked with supplementary proteins such as cytochrome c to create a more “crowded” environment. The tactic effectively increased the activity of ND‐bound lysozymes from 60 to 70%. Such a surface effect is expected to be found in other enzymes as well.
A question often asked is: What is the maximum loading capacity of proteins on a ND particle? The answer can be derived from a measurement of the adsorption isotherms of the protein of interest on NDs. Specifically, Kong et al. [33] investigated the high affinity capture of proteins (including cytochrome c, myoglobin, and serum albumin) by the acid‐treated HPHT‐NDs. They determined the loading capacity based on the change of protein concentration before and after adding diamond powders into the solution by ultraviolet‐visible (UV‐Vis) spectroscopy (Figure 4.6a). It was found that the loading maximized at the isoelectric points of the individual proteins, as a result of the competition between protein–protein and protein–ND interactions. The protein loading capacity varied from 60 to 150 mg g−1, depending on the sizes and molecular weights (12–66 kDa) of the adsorbed proteins. For a 100‐nm ND that weighs approximately 2 fg particle−1, this loading capacity suggests that more than 1000 protein molecules can be attached to the surface per particle. Another significant finding of the same study is that the acid‐treated HPHT‐NDs have an exceptionally high affinity for all proteins investigated. The feature is clearly shown in the isotherms where the 100‐nm HPHT‐ND surface is readily saturated with protein molecules at the protein concentration less than 5 μM (Figure 4.6b). A combination of electrostatic forces, hydrogen bonding, and hydrophobic interactions between adsorbents (NDs) and adsorbates (proteins) may be the origin of this high affinity [33]. The affinity, however, significantly diminishes as the particle size decreases. For DNDs of approximately 5 nm in diameter, both myoglobin and serum albumin exhibit a Langmuir‐type adsorption behavior with the surface saturation occurring only at the protein concentration greater than 100 μm [34]. The particles form 1 : 1 complexes with these two types of proteins at saturation.
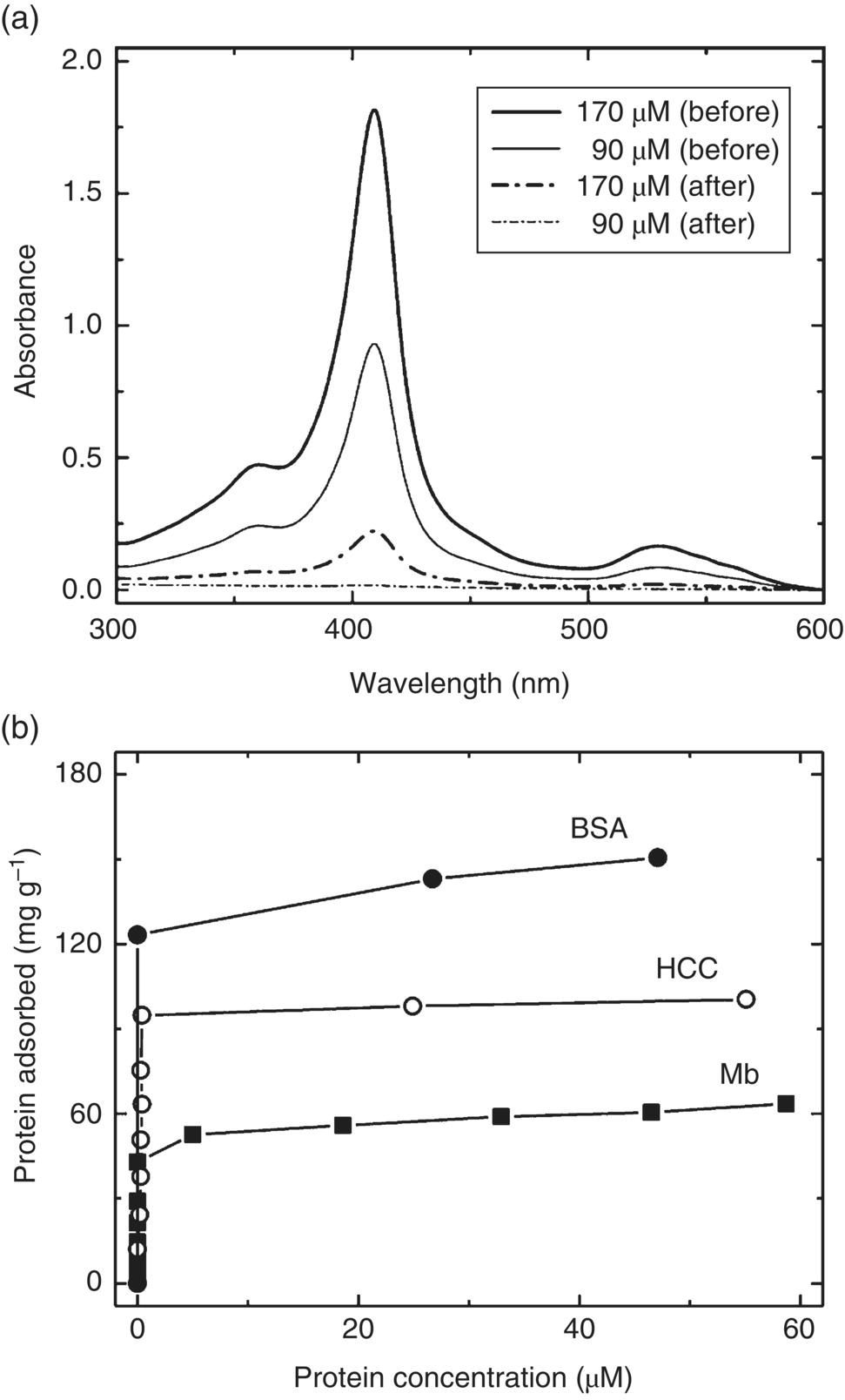
Figure 4.6 (a) UV‐Vis absorption spectra of horse cytochrome c solutions before (solid curves) and after (dash dot curves) exposure to HPHT‐NDs at two different protein concentrations. (b) Adsorption isotherms of horse heart cytochrome c (HCC), horse heart myoglobin (Mb), and bovine serum albumin (BSA) on 100‐nm HPHT‐NDs at pH 10.5, 6.9, and 4.7, respectively.
Source: Reprinted with permission from Refs. [2] and [33]. Reproduced with permission of American Chemical Society.
Given excellent chemical stability, small size, ease of purification, and high affinity for proteins, the acid‐treated HPHT‐NDs have been proposed as a solid‐phase extraction device for bioanalytical applications [35]. Chang and coworkers [36] demonstrated the utility of 100‐nm NDs as a tool to facilitate proteomic analysis by showing that the particles were able to capture proteins from complex medium or highly diluted solution in minutes. The affinity was so high that these protein–ND complexes could sustain repeated washing with deionized water without much loss. Moreover, after separation by centrifugation or filtering, the captured protein molecules could be analyzed immediately by gel electrophoresis or mass spectrometry. A platform called SPEED (solid‐phase extraction and elution on diamond) was developed for proteomics research by the group [36, 37]. A distinct advantage of the SPEED platform is that it facilitates purification and concentration of intact proteins and their enzymatic digests for ensuing sodium dodecyl sulfate‐polyacrylamide gel electrophoresis (SDS‐PAGE) or matrix‐assisted laser desorption/ionization mass spectrometry (MALDI‐MS) analysis without prior removal of the ND adsorbent (Figure 4.7). Moreover, one‐pot workflow involving the reduction of disulfide bonds, protection of free cysteine residues, and proteolytic digestion of the adsorbed proteins can be directly carried out on the particles. The platform, in combination with two‐phase separation techniques, is further applicable to extract membrane proteins in detergent micelles for shotgun proteomic analysis [38, 39]. It is potentially useful as a nucleating agent to facilitate the crystallization of low abundant proteins under unfavorable growth conditions too [40].
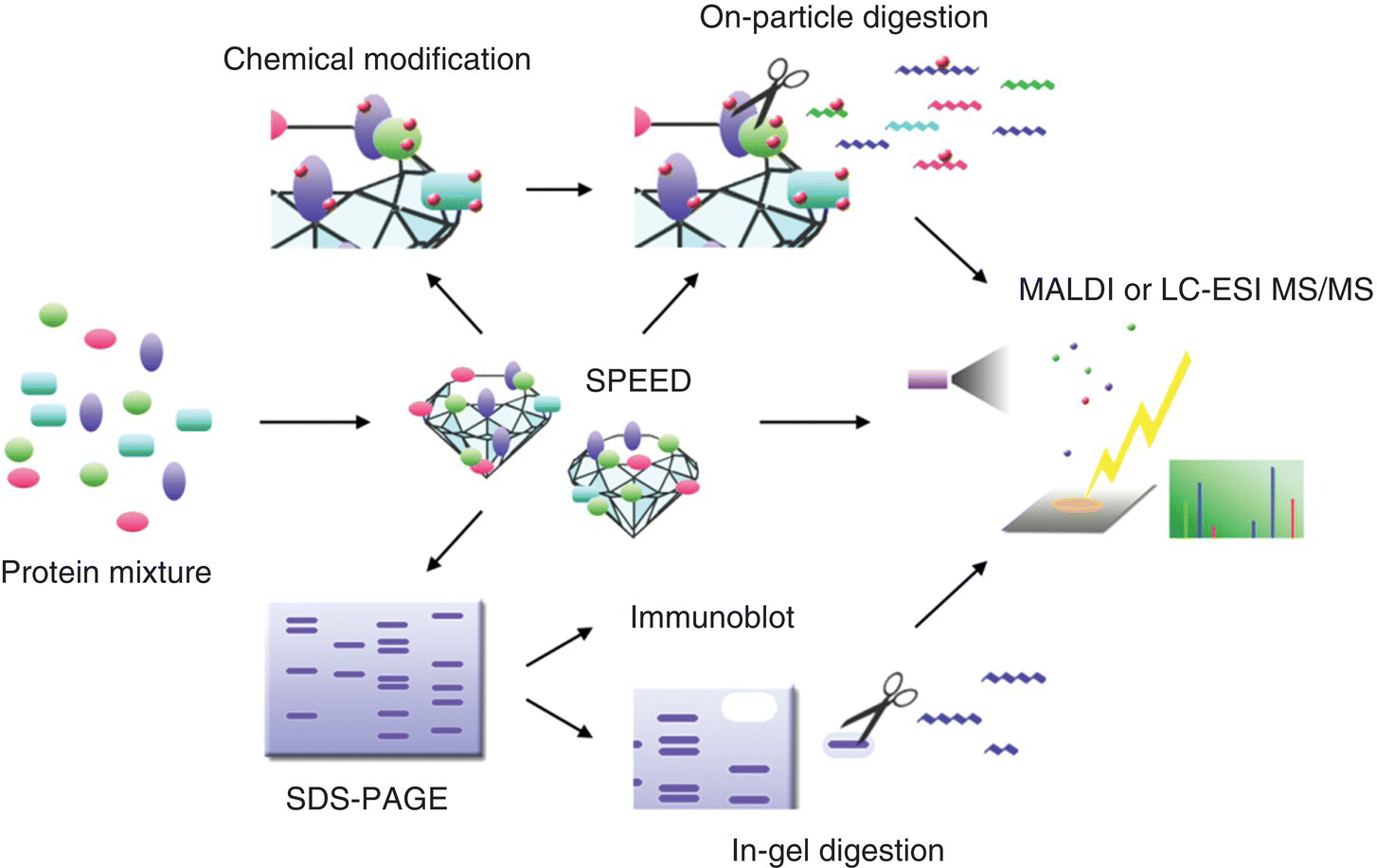
Figure 4.7 Scope of typical applications of the SPEED platform to proteome analysis. ESI, electrospray ionization; LC, liquid chromatography; MALDI, matrix‐assisted laser desorption/ionization; MS, mass spectrometry; PAGE, polyacrylamide gel electrophoresis; and SDS, sodium dodecyl sulfate.
Source: Reprinted with permission from Ref. [35]. Reproduced with permission of John Wiley & Sons.
In applying NDs for biological research, a problem often encountered is the low colloidal stability due to facile aggregation of the nanoparticles in physiological medium. The colloidal stability of surface‐unmodified NDs is not static and, in fact, it drops substantially at high ionic strengths in solution, a behavior commonly found in many nanoparticles [41, 42]. This poses a serious problem since biological buffers such as phosphate‐buffered saline (PBS) and cultivation media all contain high concentrations of salts. Fortunately, difficulties of this kind can be easily overcome by noncovalent conjugation of NDs with protein molecules like albumin [28, 43]. Figure 4.8a shows an example of the DLS measurements for the hydrodynamic sizes of 30‐nm HPHT‐NDs noncovalently conjugated with α‐lactalbumin (α‐LA) and bovine serum albumin (BSA) at different weight ratios in distilled deionized water (DDW) and PBS [43]. The nanoparticle bioconjugates maintain their good dispersibility over weeks without noticeable agglomeration and precipitation in the buffers (Figure 4.8b). Colloidally stable α‐LA‐coated NDs as small as 18 nm can also be prepared with this simple method.
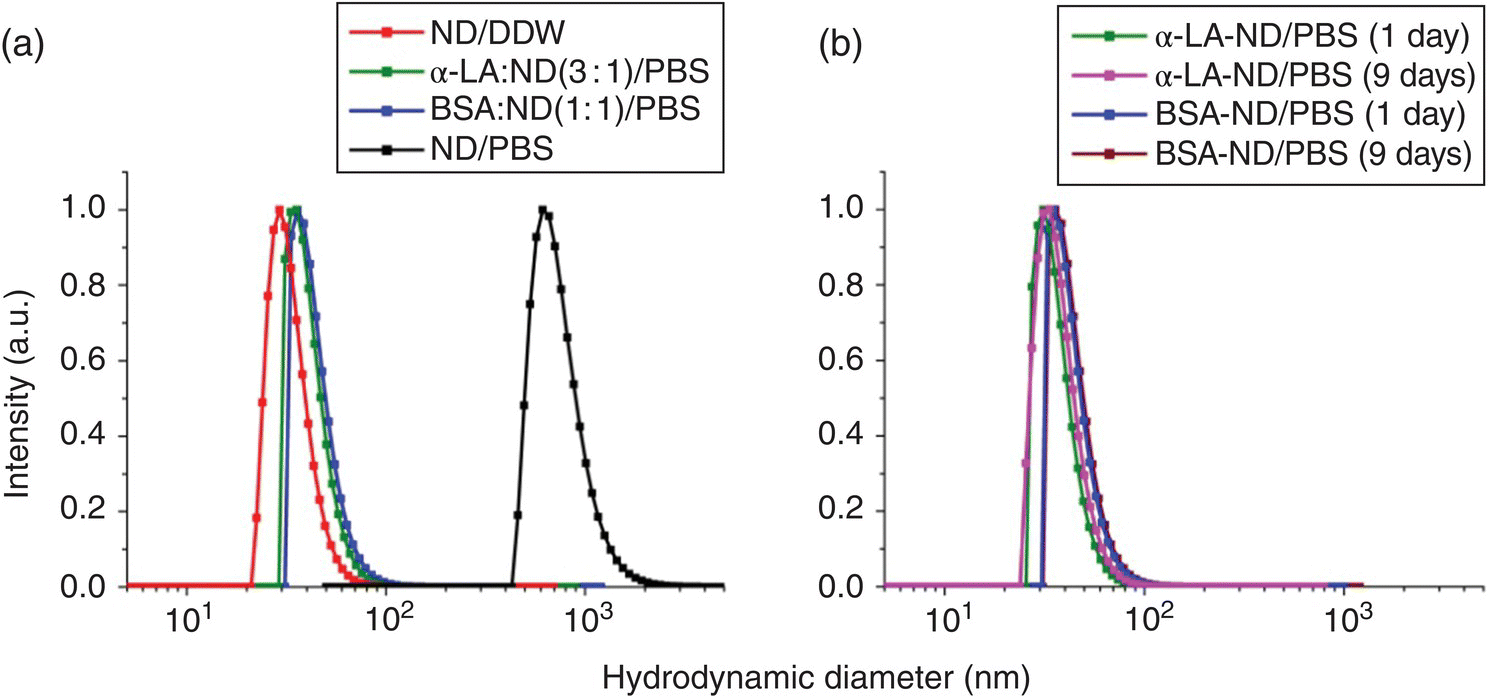
Figure 4.8 (a) Size distributions of HPHT‐NDs before and after noncovalent conjugation with BSA and α‐LA in DDW and PBS, measured by DLS. The mean diameters of the particles with size distributions from left to right are 30.0, 35.1, 41.9, and 752.7 nm, respectively. (b) Stability tests of the colloidal suspensions of BSA‐ and α‐LA‐conjugated HPHT‐NDs in PBS at room temperature for nine days. The mean diameters of the particles with size distributions from left to right are 35.0, 37.0, 41.9, and 43.2 nm, respectively.
Source: Adapted with permission from Ref. [43]. Reproduced with permission of John Wiley & Sons.
4.2.2 Covalent Conjugation
Despite the simple and straightforward approach that noncovalent conjugation may provide, there is a concern for the long‐term stability of the nanoparticle bioconjugates in physiological medium. Covalent conjugation is a more desirable approach. However, direct and chemical bonding of NDs with nanometer‐sized biomolecules such as proteins is nontrivial due to the high steric hindrance of the reactions as discussed in the previous section. To reduce the steric constraints and retain the adsorbate’s activity, a number of methods have been developed to insert spacers between NDs and the biomolecules to be conjugated. The step is particularly crucial for the immobilization of enzymes whose active sites may be sterically hindered after attachment to the ND surface. Additional functions of the spacers are that they help suppress nonspecific interactions and prevent protein conformational changes caused by strong biomolecule–ND interactions.
One of the most commonly used spacers is polyethylene glycol (PEG), which has a high biocompatibility but a low degree of nonspecific interactions with biomolecules [44]. PEG molecules derivatized with amino, carboxyl, and other functional groups are all available commercially. They can be covalently conjugated with carboxylated NDs via the amino groups on their termini by carbodiimide chemistry as follows:

Moreover, these PEG spacers are available in various discrete lengths and also provide additional functional groups for carbodiimide or other crosslinking with drugs such as doxorubicin [24], bioactive ligands such as folic acid [45], or proteins such as streptavidin [28]. Furthermore, they can be noncovalently conjugated with a cationic–hydrophobic block to form (PEG‐b‐poly(2‐(dimethylamino)ethyl methacrylate‐co‐butyl methacrylate)) copolymers on ND surface to avoid aggregation of the nanoparticles in biological buffers [46].
Poly‐lysine is the first polymer covalently grafted on NDs [47]. The grafting terminates the ND surface with amino groups, which are also useful ligands for conjugation with peptides, proteins, and other biomolecules. The method is facile and more effective than high‐temperature gas treatments involving NH3. It allows the formation of multiple amide bonds between surface carboxyl groups and free lysine residues in the polymers, generating a stable overlayer with a high density of amino groups on the surface. The same concept is applicable to other polyelectrolytes such as polyethylenimine and polyallylamine or organosilanes for surface functionalization with amino groups [14]. Further research in the field has also found that atom‐transfer radical‐polymerization is an effective method of attaching polymer brushes onto the ND surface terminated with an initiator [48]. Additionally, coating of NDs with hyperbranched polyglycerol by ring‐opening polymerization can significantly improve their colloidal stability in DDW and PBS [49]. Subsequent reaction of polyglycerol‐conjugated NDs with succinic anhydride and covalent conjugation with antibiotics (such as ampicillin) allows selective targeting of cytokine receptors on cell membrane [50].
Apart from homopolymers, copolymers such as that consisting of 95% N‐(2‐hydroxypropyl)methacrylamide and 5% propargylacrylamide or 3‐(azidopropyl)methacrylamide) have been also developed as biocompatible protein‐resistant coatings on NDs after silica coating as previously described [51]. These polymeric molecules are hydrophilic and highly flexible, allowing bioorthogonal attachment of various molecules by click chemistry,

In this reaction, an azide forms a five‐membered heterogeneous ring with an alkyne group, known as the catalyzed Cu(I)–azide–alkyne cycloaddition [52]. Because of its gentle nature and high specificity, the chemistry is gaining popularity for the bioconjugation of NDs terminated first with azides by carbodiimide chemistry or other methods [53, 54].
Many potent drugs, such as those can potentially treat cancers, are bundled with delivery challenges. For instance, some of the drugs are insoluble in polar protic solvents (such as water) but are soluble in nonpolar solvents (such as tetrahydrofuran) that are harmful to the body. Through surface modification in conjunction with drug loading on NDs, researchers have created new delivery methods to solve this problem. One of such examples is the covalent linkage of DNDs with paclitaxel, a chemotherapy drug, for cancer therapy [23]. The benefits of this approach are many as NDs are inherently biocompatible (Chapter 5) and have the ability to carry a significant amount of drugs. While some studies have already used ND surfaces to conjugate with drugs via chemical bonding [22–24], the majority of the research studies are focusing on physical adsorption procedures [55, 56]. Detailed discussion of the therapeutic applications of NDs can be found in Chapter 13.
4.3 Encapsulation
In nanotechnology, encapsulation is a process that one nanomaterial is enclosed in another material, either a pure element or a compound. The encapsulation can be carried out covalently and/or noncovalently, and the thickness of the overlayers is typically in the range of 1–10 nm. The overlayers may be organic (such as lipids), inorganic (such as silica and metals), or a combination of both. Crosslinking often occurs between the atoms or molecules in the overlayers to form a network on the nanomaterial’s surface and thus stabilize the encapsulation. In this section, we introduce only two types of encapsulation that have been successfully applied to NDs: Lipid layers and silica shells. Further discussions of the silica‐encapsulated NDs and other hybrid ND materials are given in Chapter 12.
4.3.1 Lipid Layers
Liposomization of pharmaceuticals has been a promising technique in drug delivery since its invention in the 1970s [57]. A well‐developed method for liposome preparation in the field is the thin‐film hydration, which involves dissolving powdered lipids and lipid‐soluble drugs in organic solvent, followed by deposition of a thin film of lipids on the surface of a round bottom flask by evaporating the organic phase, and finally hydration of the lipid film in aqueous medium. Ho and coworkers [58] applied the technique to synthesize self‐assembled ND–lipid hybrid nanoparticles that allowed for potent interactions between NDs and small drug molecules. To achieve cell‐specific targeting, the researchers synthesized ND‐based nanohybrids containing cholesterol and biotinylated lipids, which could selectively bind to streptavidin and then with biotinylated antibodies of various specificities (Figure 4.9). Hui et al. [12] reported a similar procedure to encapsulate HPHT‐NDs within cationic cholesterol‐based lipids after surface reduction and silanization of the particles, as illustrated in Figure 4.4a.
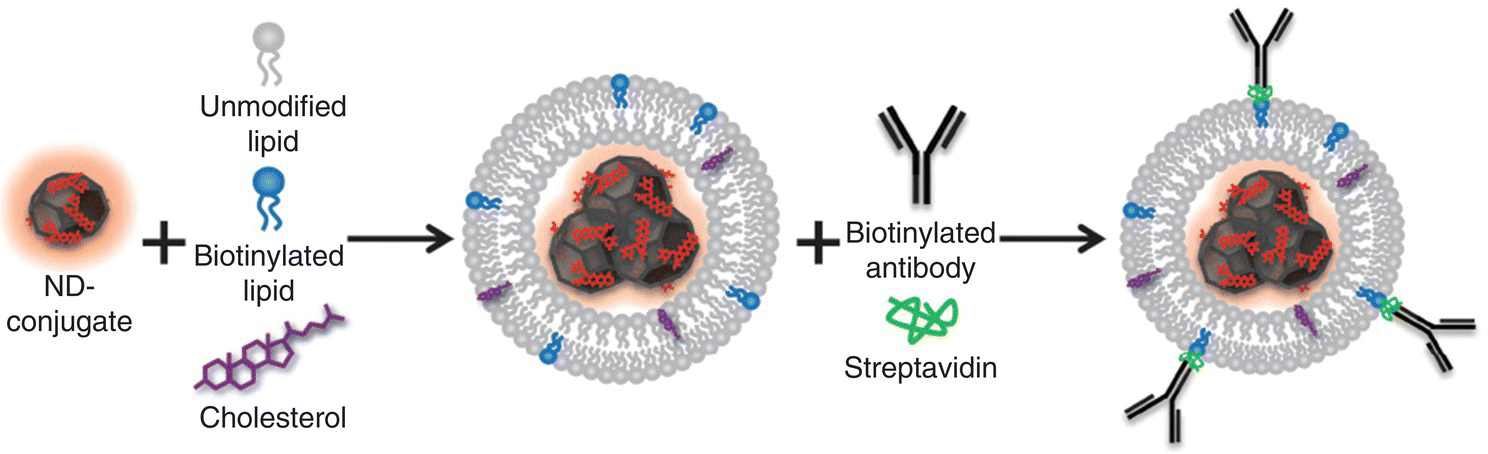
Figure 4.9 Encapsulation of NDs in liposome by rehydration of lipid thin films containing cholesterol and biotinylated lipids in concentrated ND solution. The molecule in red is epirubicin, a chemotherapy drug.
Source: Reprinted with permission from Ref. [58]. Reproduced with permission of John Wiley & Sons.
Recently, a simple and effective method to encapsulate NDs in biofunctionalized lipid layers has been developed by Hsieh et al. [59]. The method takes advantage of the Ouzo effect [60], which involves the addition of a mixture of hydrophobic solute and water‐miscible solvent into water to form stable microdroplets. The hydrophobic solute used in this work is a lipid layer consisting of egg phosphatidylcholine, cholesterol, and PEGylated 1,2‐distearoyl‐sn‐glycero‐3‐phosphoethanolamine, which are dissolved in tetrahydrofuran (i.e. the water‐miscible solvent) and then added to water containing surface‐oxidized NDs to form emulsions. Subsequent evaporation of tetrahydrofuran in a vacuum allows the lipid layer to coat on NDs. The method enables not only robust coating but also the synthesis of NDs with desired functional groups such as biotin. The particles exhibit exceptionally high dispersibility in PBS and cell medium, well suited for biolabeling applications. Further stabilization of the surface coating can be established with photo‐crosslinked lipids [61]. The effective encapsulation of NDs (size of 30–100 nm) in liposomes opens a promising new avenue to conjugate the particles with bioactive ligands or proteins on the lipid layer for specific cell labeling, targeting, and imaging with both light microscopy and electron microscopy (cf., Section 10.4).
4.3.2 Silica Shells
Along with liposomization, researchers have also encapsulated NDs in silica shells [62–68]. The encapsulation provides a novel platform for subsequent chemical treatment based on the silica chemistry. For example, the silica‐encapsulated ND surface may contain a variety of free silanol groups that allow conjugation of the biomolecules of interest with the encapsulated nanoparticles. The approach enhances not only the colloidal stability but also the functionality of NDs by using core‐shell rational designs with the benefit of synthetic versatility [69]. Figure 4.10a and b show, respectively, the transmission electron microscopy (TEM) images of HPHT‐NDs before and after silica coating [65]. The shell thickness can be as thin as 10 nm if the coating is properly prepared [67]. An added benefit of the shell coating is that it normalizes the irregular shape of the prickly ND particles [65, 68], yielding egg‐like spheroids. Moreover, the shell serves as a multifunctional interface for further conjugation with bioactive molecules like biotin for subsequent conjugation with streptavidin, antibodies, and other protein molecules.
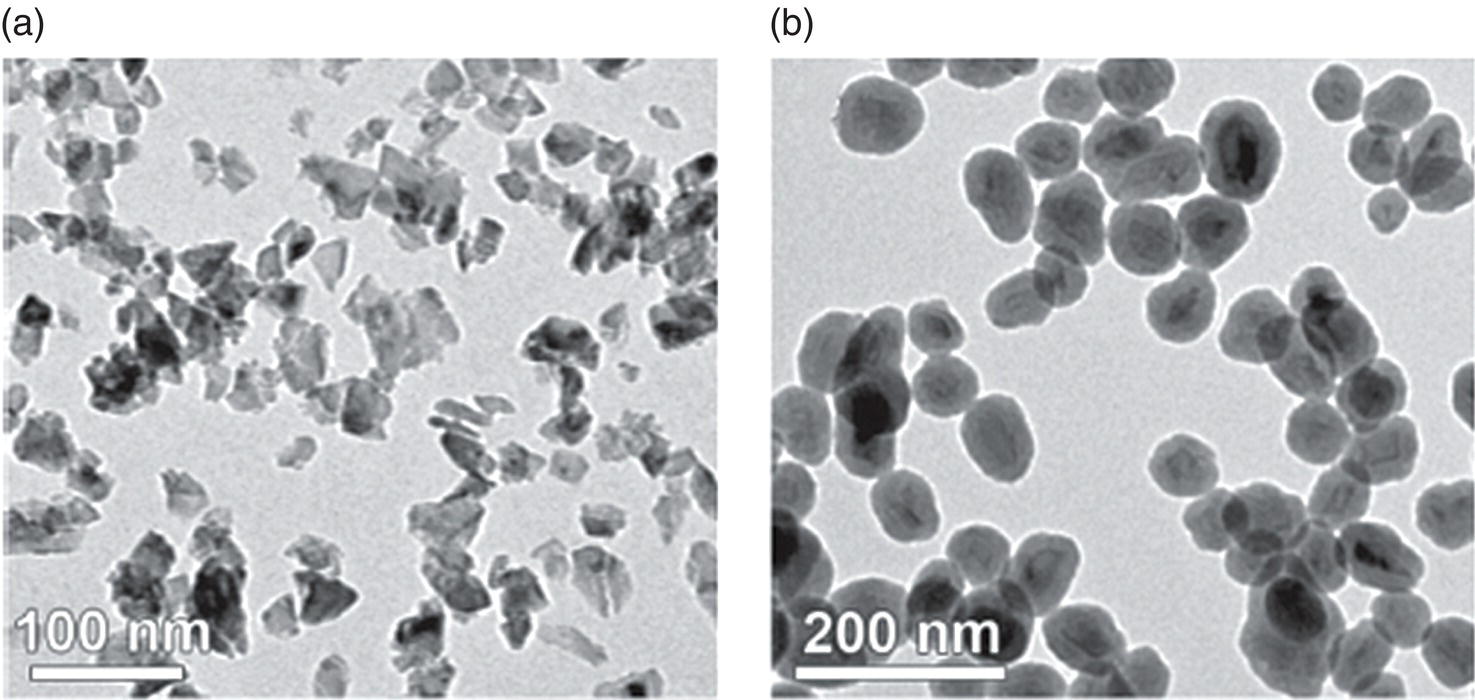
Figure 4.10 TEM images of (a) as‐received and (b) silica‐coated HPHT‐ND particles.
Source: Reprinted with permission from Ref. [65]. Reproduced with permission of John Wiley & Sons.
An example of the synthesis of core‐shell ND‐silica particles through liposome‐based encapsulation is given in Figure 4.11. In this protocol developed by Bumb et al. [64], NDs in a solution of tetraethyl orthosilicate (TEOS) were first trapped in multilamellar vesicles (MLVs) that ranged in size from 500 to 10 000 nm. Ultrasonication broke the MLVs into small unilamellar vesicles (SUVs) with a nominal diameter of approximately 100 nm. TEOS was then converted into silica, catalyzed by triethylamine. After removal of free TEOS and triethylamine by dialysis, a sodium dodecyl sulfate wash ruptured the liposomes to free the coated NDs. The final products consisted of stabilized and monodisperse silica‐encapsulated NDs. Although the surface of these silica‐encapsulated NDs presented mainly free silanol groups, attachment of biomolecules to the nanoparticles could be readily achieved by replacing these silanol groups with amino groups. The same strategy has been applied to grafting copolymers on the silica shell for further conjugation with fluorescent probes and targeting peptides via click chemistry [67]. With this combined approach, it is possible to selectively attach bioactive ligands to NDs of various sizes and concurrently improve the colloidal stability of these nanoparticles in biological buffers.
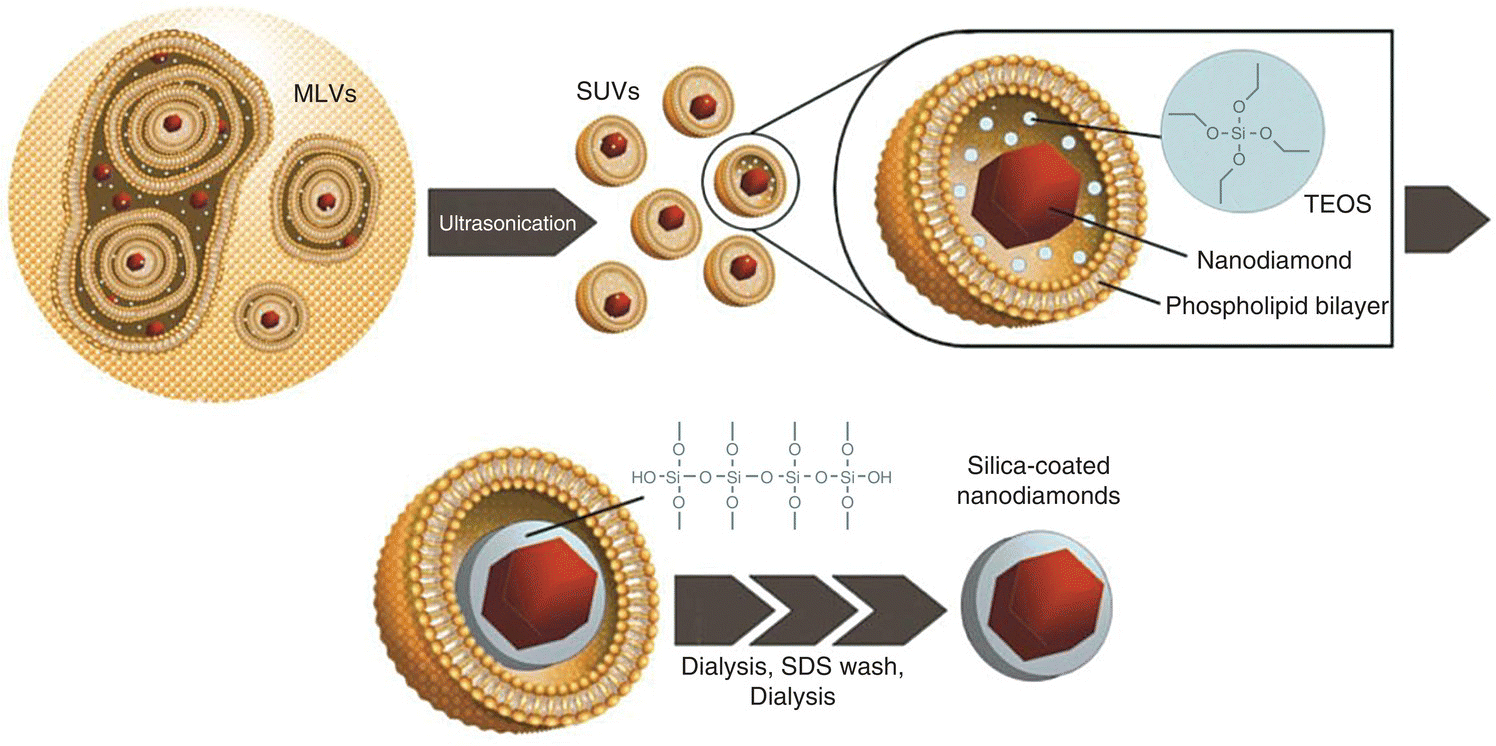
Figure 4.11 Synthesis of silica‐coated NDs by liposome‐based encapsulation. MLVs, multilamellar vesicles; SUVs, unilamellar vesicles; TEOS, tetraethyl orthosilicate; and SDS, sodium dodecyl sulfate.
Source: Adapted with permission from Ref. [64]. Reproduced with permission of American Chemical Society.
References
- 1 Krueger, A. (2014). The chemistry of nanodiamond. In: Nanodiamonds (ed. O.A. Williams), 49–88. Royal Society of Chemistry.
- 2 Huang, L.C.L. and Chang, H.C. (2004). Adsorption and immobilization of cytochrome c on nanodiamonds. Langmuir 20: 5879–5884.
- 3 Shenderova, O., Koscheev, A., Zaripov, N. et al. (2011). Surface chemistry and properties of ozone‐purified detonation nanodiamonds. J Phys Chem C 115: 9827–9837.
- 4 Sotoma, S., Akagi, K., Hosokawa, S. et al. (2015). Comprehensive and quantitative analysis for controlling the physical/chemical states and particle properties of nanodiamonds for biological applications. RSC Adv 5: 13818–13827.
- 5 Krueger, A., Liang, Y.J., Jarre, G., and Stegk, J. (2006). Surface functionalisation of detonation diamond suitable for biological applications. J Mater Chem 16: 2322–2328.
- 6 Osswald, S., Yushin, G., Mochalin, V. et al. (2006). Control of sp2/sp3 carbon ratio and surface chemistry of nanodiamond powders by selective oxidation in air. J Am Chem Soc 128: 11635–11642.
- 7 Krueger, A. and Lang, D. (2012). Functionality is key: recent progress in the surface modification of nanodiamond. Adv Funct Mater 22: 890–906.
- 8 Mochalin, V.N., Shenderova, O., Ho, D., and Gogotsi, Y. (2012). The properties and applications of nanodiamonds. Nat Nanotechnol 7: 11–23.
- 9 Arnault, J.C. and Girard, H.A. (2017). Hydrogenated nanodiamonds: synthesis and surface properties. Curr Opin Solid State Mater Sci 21: 10–16.
- 10 Tsubota, T., Tanii, S., Ida, S. et al. (2004). Chemical modification of diamond surface with various carboxylic acids by radical reaction in liquid phase. Diam Relat Mater 13: 1093–1097.
- 11 Chang, I.P., Hwang, K.C., Ho, J.A. et al. (2010). Facile surface functionalization of nanodiamonds. Langmuir 26: 3685–3689.
- 12 Hui, Y.Y., Zhang, B.L., Chang, Y.C. et al. (2010). Two‐photon fluorescence correlation spectroscopy of lipid‐encapsulated fluorescent nanodiamonds in living cells. Opt Express 18: 5896–5905.
- 13 Moore, W.J. (1963). Physical Chemistry. New York: Longmans Green.
- 14 Vial, S., Mansuy, C., Sagan, S. et al. (2008). Peptide‐grafted nanodiamonds: preparation, cytotoxicity and uptake in cells. ChemBioChem 9: 2113–2119.
- 15 Williams, O.A., Hees, J., Dieker, C. et al. (2010). Size‐dependent reactivity of diamond nanoparticles. ACS Nano 4: 4824–4830.
- 16 Kaur, R. and Badea, I. (2013). Nanodiamonds as novel nanomaterials for biomedical applications: drug delivery and imaging systems. Int J Nanomed 8: 203–220.
- 17 Nguyen, T.T.B., Chang, H.C., and Wu, V.W.K. (2007). Adsorption and hydrolytic activity of lysozyme on diamond nanocrystallites. Diam Relat Mater 16: 872–876.
- 18 Vogel, A.I. (1989). Vogel’s Textbook of Quantitative Chemical Analysis. New York: Wiley.
- 19 Berne, B.J. and Pecora, R. (2000). Dynamic Light Scattering: With Applications to Chemistry, Biology, and Physics. Dover Publications.
- 20 Bohren, C.F. and Huffman, D.R. (1983). Absorption and Scattering of Light by Small Particles. Wiley.
- 21 Huang, H., Pierstorff, E., Osawa, E., and Ho, D. (2007). Active nanodiamond hydrogels for chemotherapeutic delivery. Nano Lett 7: 3305–3314.
- 22 Li, J., Zhu, Y., Li, W.X. et al. (2010). Nanodiamonds as intracellular transporters of chemotherapeutic drug. Biomaterials 31: 8410–8418.
- 23 Liu, K.K., Zheng, W.W., Wang, C.C. et al. (2010). Covalent linkage of nanodiamond‐paclitaxel for drug delivery and cancer therapy. Nanotechnology 21: 315106.
- 24 Wang, D.X., Tong, Y.L., Li, Y.Q. et al. (2013). PEGylated nanodiamond for chemotherapeutic drug delivery. Diam Relat Mater 36: 26–34.
- 25 Hartmann, M., Betz, P., Sun, Y.C. et al. (2012). Saccharide‐modified nanodiamond conjugates for the efficient detection and removal of pathogenic bacteria. Chem‐Eur J 18: 6485–6492.
- 26 Barras, A., Martin, F.A., Bande, O. et al. (2013). Glycan‐functionalized diamond nanoparticles as potent E. coli anti‐adhesives. Nanoscale 5: 12678–12678.
- 27 Shimkunas, R.A., Robinson, E., Lam, R. et al. (2009). Nanodiamond‐insulin complexes as pH‐dependent protein delivery vehicles. Biomaterials 30: 5720–5728.
- 28 Chang, B.M., Lin, H.H., Su, L.J. et al. (2013). Highly fluorescent nanodiamonds protein‐functionalized for cell labeling and targeting. Adv Funct Mater 23: 5737–5745.
- 29 Chen, M., Zhang, X.Q., Man, H.B. et al. (2010). Nanodiamond vectors functionalized with polyethylenimine for siRNA delivery. J Phys Chem Lett 1: 3167–3171.
- 30 Alhaddad, A., Adam, M.P., Botsoa, J. et al. (2011). Nanodiamond as a vector for siRNA delivery to Ewing sarcoma cells. Small 7: 3087–3095.
- 31 Zhang, X.Q., Chen, M., Lam, R. et al. (2009). Polymer‐functionalized nanodiamond platforms as vehicles for gene delivery. ACS Nano 3: 2609–2616.
- 32 Petrakova, V., Benson, V., Buncek, M. et al. (2016). Imaging of transfection and intracellular release of intact, non‐labeled DNA using fluorescent nanodiamonds. Nanoscale 8: 12002–12012.
- 33 Kong, X.L., Huang, L.C.L., Hsu, C.M. et al. (2005). High‐affinity capture of proteins by diamond nanoparticles for mass spectrometric analysis. Anal Chem 77: 259–265.
- 34 Lin, C.L., Lin, C.H., Chang, H.C., and Su, M.C. (2015). Protein attachment on nanodiamonds. J Phys Chem A 119: 7704–7711.
- 35 Wu, C.C., Han, C.C., and Chang, H.C. (2010). Applications of surface‐functionalized diamond nanoparticles for mass‐spectrometry‐based proteomics. J Chin Chem Soc‐Taip 57: 583–594.
- 36 Chen, W.H., Lee, S.C., Sabu, S. et al. (2006). Solid‐phase extraction and elution on diamond (SPEED): a fast and general platform for proteome analysis with mass spectrometry. Anal Chem 78: 4228–4234.
- 37 Sabu, S., Yang, F.C., Wang, Y.S. et al. (2007). Peptide analysis: solid phase extraction‐elution on diamond (SPEED) combined with atmospheric pressure MALDI‐FTICR mass spectrometry. Anal Biochem 367: 190–200.
- 38 Pham, M.D., Yu, S.S.F., Han, C.C., and Chan, S.I. (2013). Improved mass spectrometric analysis of membrane proteins based on rapid and versatile sample preparation on nanodiamond particles. Anal Chem 85: 6748–6755.
- 39 Pham, M.D., Wen, T.C., Li, H.C. et al. (2016). Streamlined membrane proteome preparation for shotgun proteomics analysis with Triton X‐100 cloud point extraction and nanodiamond solid phase extraction. Materials 9: 385.
- 40 Chen, Y.W., Lee, C.H., Wang, Y.L. et al. (2017). Nanodiamonds as nucleating agents for protein crystallization. Langmuir 33: 6521–6527.
- 41 Lim, J.K., Majetich, S.A., and Tilton, R.D. (2009). Stabilization of superparamagnetic iron oxide core‐gold shell nanoparticles in high ionic strength media. Langmuir 25: 13384–13393.
- 42 Zhang, W. (2014). Nanoparticle aggregation: principles and modeling. Adv Exp Med Biol 811: 19–43.
- 43 Tzeng, Y.K., Faklaris, O., Chang, B.M. et al. (2011). Superresolution imaging of albumin‐conjugated fluorescent nanodiamonds in cells by stimulated emission depletion. Angew Chem Int Ed 50: 2262–2265.
- 44 Zhang, X.Y., Fu, C.K., Feng, L. et al. (2012). PEGylation and polyPEGylation of nanodiamond. Polymer 53: 3178–3184.
- 45 Zhang, B.L., Li, Y.Q., Fang, C.Y. et al. (2009). Receptor‐mediated cellular uptake of folate‐conjugated fluorescent nanodiamonds: a combined ensemble and single‐particle study. Small 5: 2716–2721.
- 46 Lee, J.W., Lee, S., Jang, S. et al. (2013). Preparation of non‐aggregated fluorescent nanodiamonds (FNDs) by non‐covalent coating with a block copolymer and proteins for enhancement of intracellular uptake. Mol Biosyst 9: 1004–1011.
- 47 Fu, C.C., Lee, H.Y., Chen, K. et al. (2007). Characterization and application of single fluorescent nanodiamonds as cellular biomarkers. Proc Natl Acad Sci USA 104: 727–732.
- 48 Dahoumane, S.A., Nguyen, M.N., Thorel, A. et al. (2009). Protein‐functionalized hairy diamond nanoparticles. Langmuir 25: 9633–9638.
- 49 Zhao, L., Takimoto, T., Ito, M. et al. (2011). Chromatographic separation of highly soluble diamond nanoparticles prepared by polyglycerol grafting. Angew Chem Int Ed 50: 1388–1392.
- 50 Sotoma, S., Iimura, J., Igarashi, R. et al. (2016). Selective labeling of proteins on living cell membranes using fluorescent nanodiamond probes. Nanomaterials 6: 56.
- 51 Rehor, I., Mackova, H., Filippov, S.K. et al. (2014). Fluorescent nanodiamonds with bioorthogonally reactive protein‐resistant polymeric coatings. ChemPlusChem 79: 21–24.
- 52 Kolb, H.C., Finn, M.G., and Sharpless, K.B. (2001). Click chemistry: diverse chemical function from a few good reactions. Angew Chem Int Ed 40: 2004–2021.
- 53 Barras, A., Szunerits, S., Marcon, L. et al. (2010). Functionalization of diamond nanoparticles using “click” chemistry. Langmuir 26: 13168–13172.
- 54 Meinhardt, T., Lang, D., Dill, H., and Krueger, A. (2011). Pushing the functionality of diamond nanoparticles to new horizons – orthogonally functionalized nanodiamond using click chemistry. Adv Funct Mater 21: 494–500.
- 55 Chen, M., Pierstorff, E.D., Lam, R. et al. (2009). Nanodiamond‐mediated delivery of water‐insoluble therapeutics. ACS Nano 3: 2016–2022.
- 56 Chow, E.K., Zhang, X.Q., Chen, M. et al. (2011). Nanodiamond therapeutic delivery agents mediate enhanced chemoresistant tumor treatment. Sci Transl Med 3: 73ra21.
- 57 Gregoriadis, G. and Ryman, B.E. (1971). Liposomes as carriers of enzymes or drugs: a new approach to the treatment of storage diseases. Biochem J 124: 58.
- 58 Moore, L., Chow, E.K., Osawa, E. et al. (2013). Diamond‐lipid hybrids enhance chemotherapeutic tolerance and mediate tumor regression. Adv Mater 25: 3532–3541.
- 59 Hsieh, F.J., Chen, Y.W., Hui, Y.Y. et al. (2018). Correlative light‐electron microscopy of lipid‐encapsulated fluorescent nanodiamonds for nanometric localization of cell surface antigens. Anal Chem 90: 1566–1571.
- 60 Vitale, S.A. and Katz, J.L. (2003). Liquid droplet dispersions formed by homogeneous liquid‐liquid nucleation: “the ouzo effect”. Langmuir 19: 4105–4110.
- 61 Sotoma, S., Hsieh, F.J., Chen, Y.W. et al. (2018). Highly stable lipid‐encapsulation of fluorescent nanodiamonds for bioimaging applications. Chem Commun 54: 1000–1003.
- 62 von Haartman, E., Jiang, H., Khomich, A.A. et al. (2013). Core‐shell designs of photoluminescent nanodiamonds with porous silica coatings for bioimaging and drug delivery I: fabrication. J Mater Chem B 1: 2358–2366.
- 63 Prabhakar, N., Nareoja, T., von Haartman, E. et al. (2013). Core‐shell designs of photoluminescent nanodiamonds with porous silica coatings for bioimaging and drug delivery II: application. Nanoscale 5: 3713–3722.
- 64 Bumb, A., Sarkar, S.K., Billington, N. et al. (2013). Silica encapsulation of fluorescent nanodiamonds for colloidal stability and facile surface functionalization. J Am Chem Soc 135: 7815–7818.
- 65 Rehor, I., Slegerova, J., Kucka, J. et al. (2014). Fluorescent nanodiamonds embedded in biocompatible translucent shells. Small 10: 1106–1115.
- 66 Rehor, I., Lee, K.L., Chen, K. et al. (2015). Plasmonic nanodiamonds: targeted core‐shell type nanoparticles for cancer cell thermoablation. Adv Healthc Mater 4: 460–468.
- 67 Slegerova, J., Hajek, M., Rehor, I. et al. (2015). Designing the nanobiointerface of fluorescent nanodiamonds: highly selective targeting of glioma cancer cells. Nanoscale 7: 415–420.
- 68 Chu, Z., Zhang, S., Zhang, B. et al. (2014). Unambiguous observation of shape effects on cellular fate of nanoparticles. Sci Rep 4: 4495.
- 69 Guerrero‐Martínez, A., Pérez‐Juste, J., and Liz‐Marzán, L.M. (2010). Silica‐coated nanomaterials: recent progress on silica coating of nanoparticles and related nanomaterials. Adv Mater 22: 1182–1195.
