12
Hybrid Fluorescent Nanodiamonds
Many natural materials such as wood and bones are made of two or more materials, known as composites. If the materials are mixed at the nanometer or molecular level, these composites are called hybrids [1]. Depending on how they are made, the mixing may lead to the formation of new materials with novel properties and/or multiple functions. For example, the hydroxyapatite (a calcium phosphate) in the bone provides mechanical strength and the collagen (a protein) in the bone promotes the bonding between the inorganic building blocks and the soft tissue.
In the previous chapters, we have discussed the unique chemical and physical properties of fluorescent nanodiamonds (FNDs) alone. Here, we extend the discussion to hydrid FNDs, e.g. FNDs either covalently or noncovalently conjugated with other inorganic nanomaterials such as silica, gold, silver, or iron oxide nanoparticles, all of which have found practical applications for drug delivery and theranostics [2]. In preparing these nanohybrids or nanodevices, the surface chemistry of nanodiamonds, such as that illustrated in Chapter 4, clearly plays a central role. Research and development along this line are expected to add brand new dimensions to the use of FNDs in the life sciences and other research areas. Despite the fact that the field is still at its infant stage, various strategies have been developed and implemented to overcome the obstacles and challenges encountered in sample preparation. In this chapter, we discuss the synthesis and characterization of these nanohybrids, together with their applications in different facets of science and technology.
12.1 Silica/Diamond Nanohybrids
So, where do we begin to find a good hybrid partner for diamond? It all, intuitively at the least, seems that silica ought to be the first choice because both silicon and carbon are in the same family on the Periodic Table, sharing similar bonding configuration and chemistry. It is true that silica and diamond are compatible chemically, able to form stable hybrids in more ways than just one. We will examine two examples of silica/diamond nanohybrids here as a general introduction.
In Section 4.3.2, we have introduced how FNDs can be encapsulated in a silica shell, where two distinct types of nanomaterials are mixed at the molecular level to form a hybrid. Unlike other nanohybrids discussed in the following sections, the encapsulation transforms the irregular shape of the FND into a spheroid (Figure 4.10), a unique aspect of the technique [3]. Another benefit of the encapsulation method is that it renders the FND’s surface readily functionalizable through routine crosslinking chemistry. As an example, Bumb et al. [4] attached biotin to silica‐coated FNDs and quantified their specific binding to a surface passivated with streptavidin‐labeled poly‐ethylene glycol (PEG) using total internal reflection fluorescence microscopy (TIRFM) (Section 8.2.2 and Figure 12.1a). It was found that the binding between the silica‐coated FNDs and the streptavidin‐passivated surface was approximately 15‐fold greater with biotin than without one (Figure 12.1b and c), showing the potential use of these nanohybrids for biosensing applications. Additionally, the researchers applied the method to track the three‐dimensional motion of a biotinylated silica‐FND tethered by a single DNA molecule on a glass slide with both high spatial and temporal resolution. They were able to measure the persistence length of a single DNA molecule after photobleaching the observation area to reduce background fluorescence signals prior to tracking the single DNA‐tethered FND particle. Finally, an application of the nanohybrids was made by the research team to map out the lymph modes in a mouse by background‐free fluorescence imaging of FNDs through magnetic modulation of the emission (Section 9.3.4), demonstrating the versatility of this method [5].
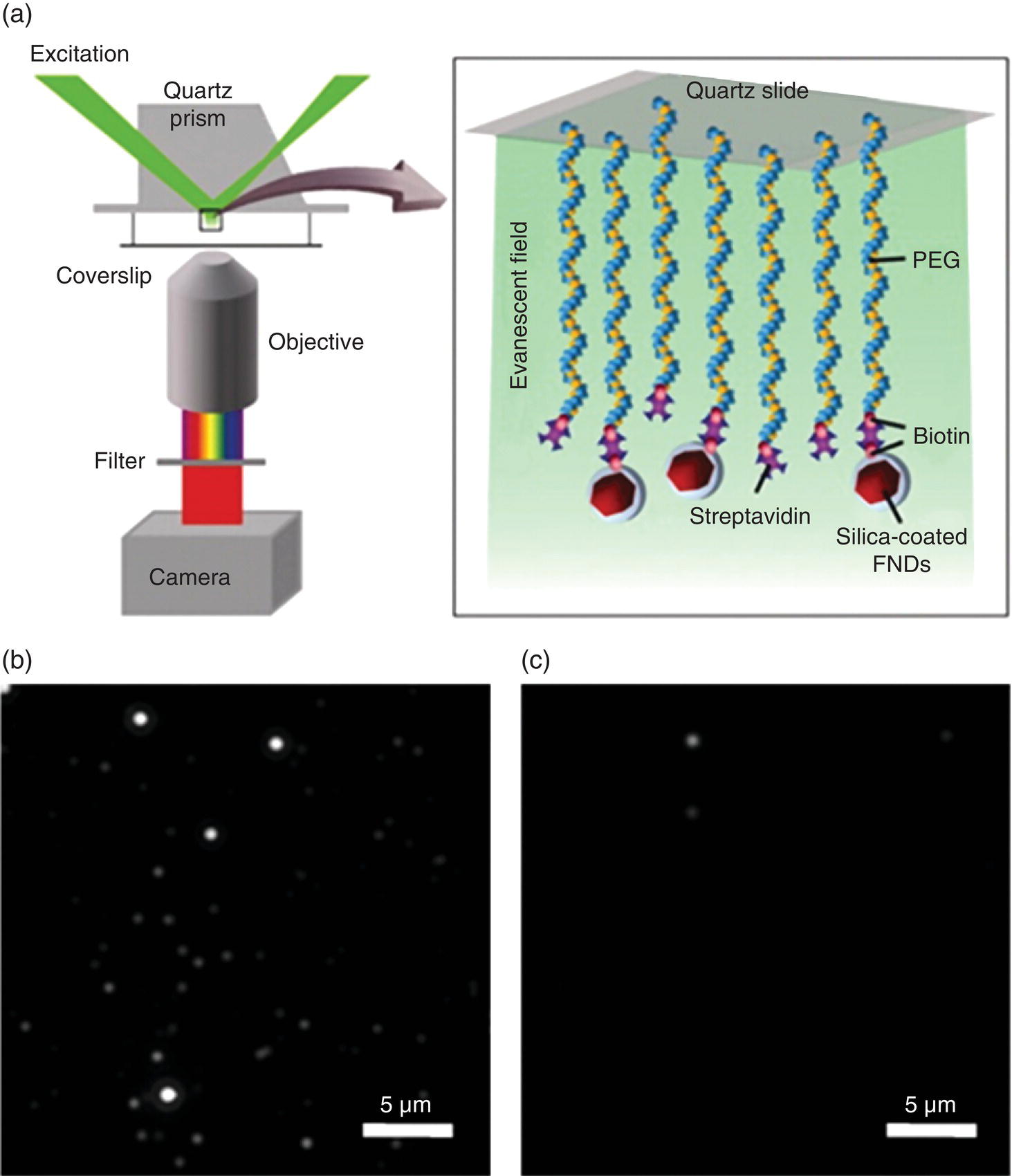
Figure 12.1 (a) Schematic diagram of the biosensing experiment with silica‐coated FNDs. A quartz slide passivated with biotinylated PEG was first saturated with streptavidin. The biotinylated FNDs were then flowed into a microfluidic cell and specific attachment to streptavidin–biotin‐PEG was probed by comparing the number of bound particles before and after washing to remove nonspecifically bound particles. FNDs were excited by the evanescent field (green) in a prism‐type TIRFM. (b, c) Images showing that (b) 60% of the biotinylated silica‐coated FNDs and (c) 4% of the nonbiotinylated silica‐coated FNDs remained attached to the surface after acid–base washes.
Source: Reprinted with permission from Ref. [4]. Reproduced with permission of American Chemical Society.
In another case, Rosenholm and coworkers [6, 7] synthesized silica‐FND nanohybrids for use in both bioimaging and drug delivery. Instead of making a thin shell, they coated the FND core with mesoporous silica nanoparticles (MSNs), which have high porosity and tunable pore sizes, achieved by using a supramolecular templating method. Initially, the FND had an average size of about 50 nm (Figure 12.2a), which increased to more than 300 nm after silica coating (Figure 12.2b–d). Dynamic light scattering (DLS) and transmission electron microscopy (TEM) showed homogeneous coating of the individual FND cores, which in effect removed the undesirable shape irregularity from the primary diamond nanoparticles. The nanohybrid is novel in the sense that it integrates the superb optical properties of FNDs with the high drug delivery capability of MSNs. By conducting cell viability, cargo delivery assays, and live cell imaging, the studies have provided a valuable demonstration of how this novel nanohybrid can be exploited for future theranostic applications in nanoscale biology and medicine.

Figure 12.2 TEM images of FNDs and MSN‐FNDs. (a) Pure FND cores as imaged by TEM and (b) individual MSN‐FNDs imaged by high‐resolution TEM. (c, d) Corresponding (c) bright field and (d) dark field images revealing the presence of a crystalline diamond core.
Source: Reprinted with permission from Ref. [7]. Reproduced with permission of Royal Society of Chemistry.
12.2 Gold/Diamond Nanohybrids
12.2.1 Photoluminescence Enhancement
While silica may have been a logic pick for hybridization, it was gold/diamond that emerged among the first nanohybrids reported in the literature. The development of such nanohybrids was aimed at investigating the plasmonic effect of gold nanoparticles (GNPs) on the optical properties of the nitrogen‐vacancy (NV) centers in FNDs (Section 10.2.4). An early plasmonic study was made by Liu and Sun [8] to couple different‐sized GNPs with FNDs using two complementary DNA strands for physical adsorption. A significant enhancement in the photoluminescence signals was observed when the particles were excited at 488 nm. The enhancement was envisioned useful for biosensing applications.
At a technically more sophisticated level, Schietinger et al. [9] investigated the plasmon‐enhanced effect employing an atomic force microscope (AFM) to achieve controlled coupling of GNPs with a FND that contained only a single NV− center. Either one or two GNPs were assembled with the FND (~30 nm in diameter) in a step‐by‐step manner (Figure 12.3a). The NV− center was observed to have an increase of nearly 10‐fold in both excitation rate and radiative decay rate, a result of plasmonic enhancement as supported by numerical simulations (Figure 12.3b). Following this study, Shen et al. [10] showed that unidirectional emission from a single FND particle could be generated if it was coupled with a single gold nanorod (GNR). The emission patterns were controlled by adjusting the GNR orientation and position with respect to the FND. The emission remained highly unidirectional even when the emitter was positioned away from the GNR antenna by a distance of up to 50 nm. The result was attributed to the interference between the electromagnetic fields produced by the dipole‐like source and the out‐of‐phase dipole induced in the GNR. This hybrid GNR‐FND system, together with GNP‐FND, may serve as an important building block for novel nanophotonic light sources in the advanced plasmonic devices that are stable even at room temperature.
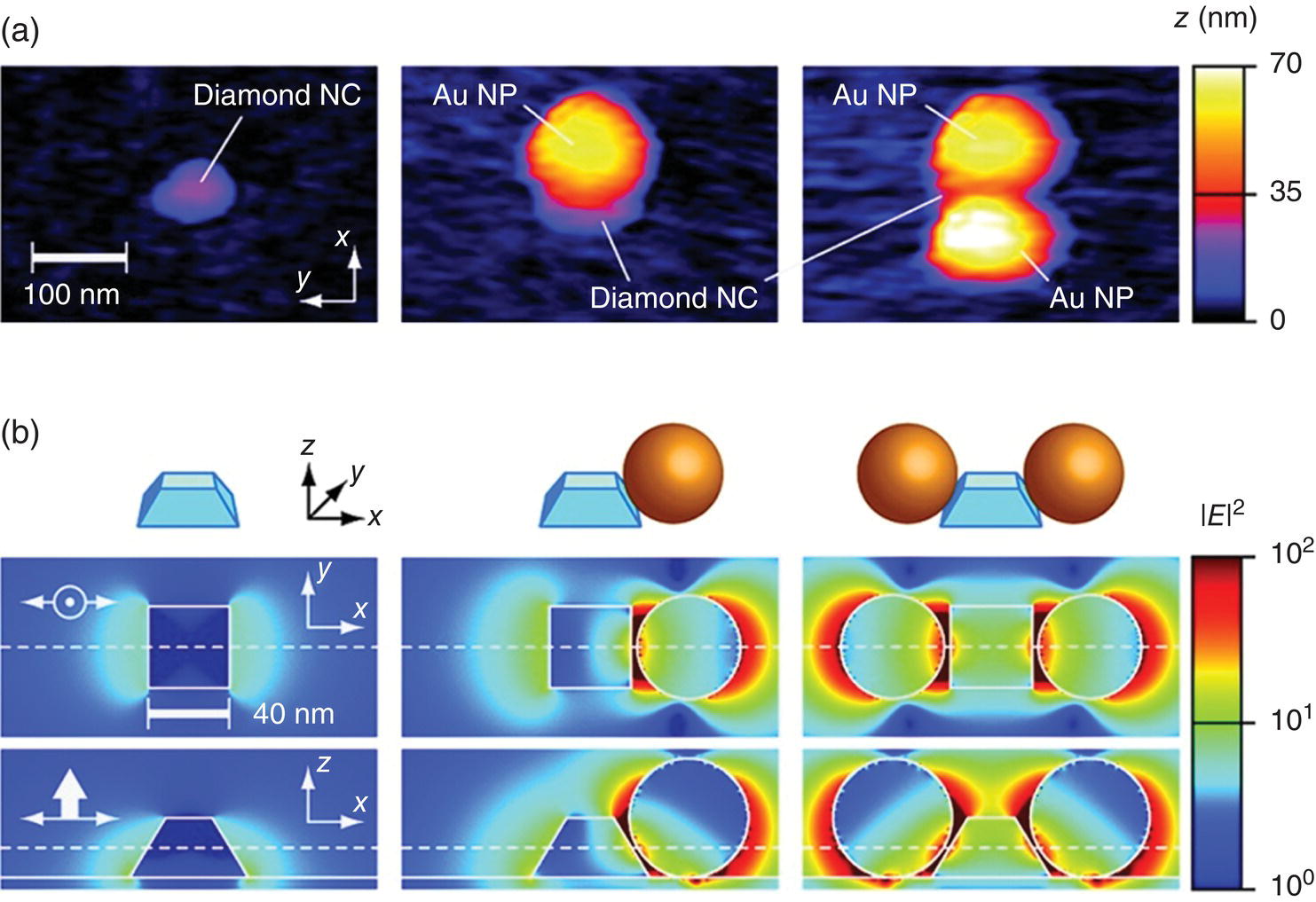
Figure 12.3 Experimental realization and numerical simulation of a GNP‐FND hybrid. (a) AFM images of a single diamond nanocrystal (left), to which one (middle) or two (right) GNPs (or Au NPs) are coupled. (b) Corresponding numerical simulations of the intensity enhancement of the excitation light linearly polarized along the x axis. Upper row: schematic representation of the particle configuration. Middle row: x–y cross section. Lower row: x–z cross section. The field intensity is normalized to the value at the center of the bare FND.
Source: Reprinted with permission from Ref. [9]. Reproduced with permission of American Chemical Society.
12.2.2 Dual‐Modality Imaging
Zhang et al. [11] first used gold/diamond nanohybrids as a dual‐modality contrast agent for combined fluorescence and photoacoustic imaging. Photoacoustic effect is the formation of sound waves upon absorption of light in a sample [12]. Typically, a pulsed nanosecond laser is used for the excitation and a transducer is used to detect the sound wave signals. Compared with all optical methods, the technique allows detection of light‐absorbing chromophores with a greater penetration depth in tissue (Figure 12.4) [13]. Employing a nanosecond optical parametric oscillator operating at 532 nm as the light source, the researchers found that the photoacoustic signals of FNDs (~100 nm in diameter) alone were weak but could be greatly enhanced by a factor of 30 after conjugation with GNPs (~20 nm in diameter). The GNPs were selected here because their surface plasmon resonance (SPR) matches closely with the absorption maximum of FNDs at 560 nm. Moreover, the GNPs were amine‐terminated and thus could be covalently conjugated with carboxylated FNDs through carbodiimide chemistry (Section 4.2.2) to form stable nanohybrids. Furthermore, the red emission of the NV− centers could also be detected when the nanoparticles were excited by green‐yellow light, indicating that the GNP‐attached FNDs were still useful as a fluorescent contrast agent. The method is applicable to conjugate FNDs with GNPs of various sizes and shapes to improve the photoacoustic detection sensitivity.
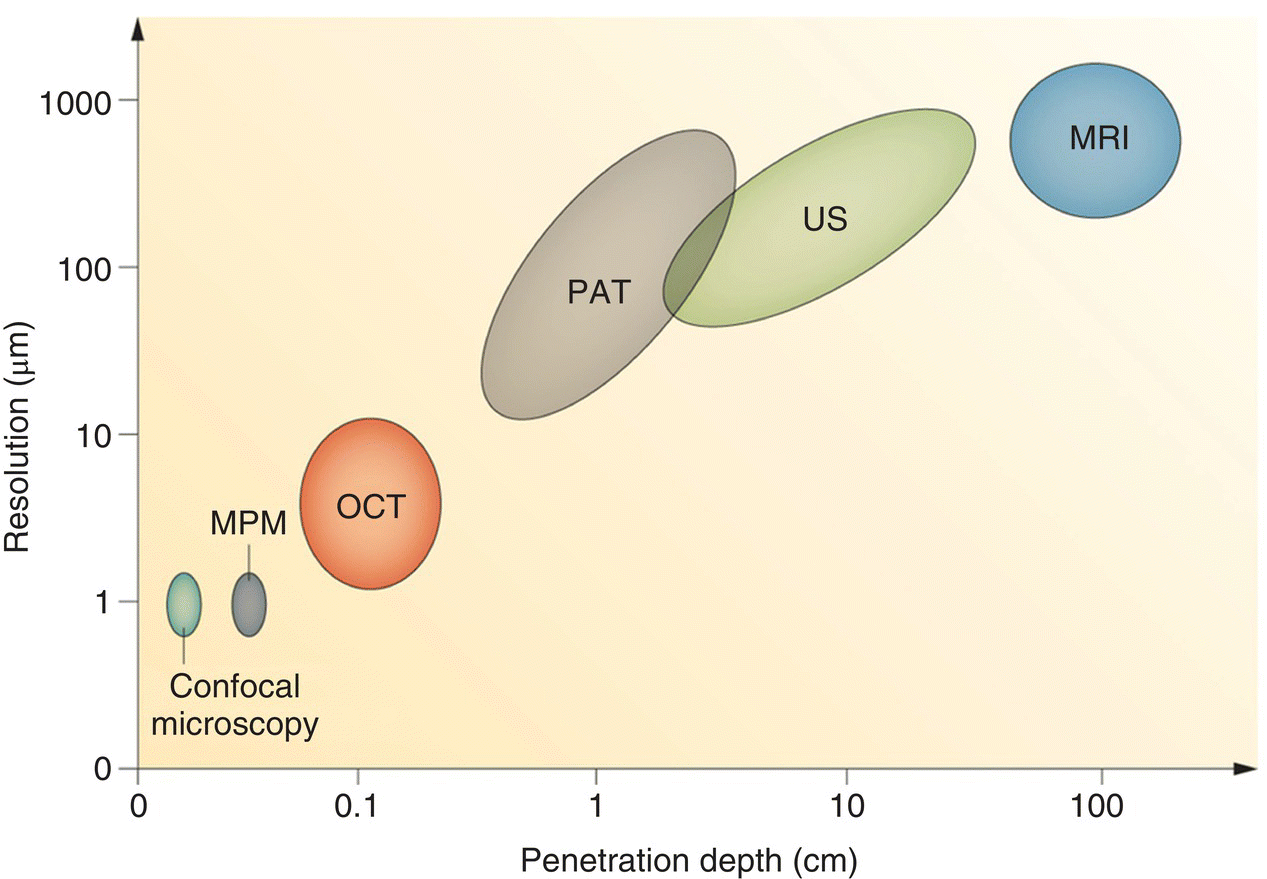
Figure 12.4 Comparison of resolution and penetration depth between different imaging modalities, including confocal microscopy, multiphoton microscopy (MPM), optical coherence tomography (OCT), photoacoustic tomography (PAT), ultrasonography (US), and magnetic resonance imaging (MRI).
Source: Reprinted with permission from Ref. [13]. Reproduced with permission of Nature Publishing Group.
In a recent study, Liu et al. [14] prepared GNP‐FND nanohybrids for combined fluorescence and electron microscopy imaging. The nanohybrids were synthesized via the adsorption of GNPs (~10 nm in diameter) and human serum albumin (HSA) to FNDs (~33 nm in diameter) after careful adjustments of the relative ratios of FND, GNP, and HSA in aqueous solution. To ensure good stability of the GNP‐FND nanohybrids, the carboxyl groups on the surfaces of both FNDs and GNPs were crosslinked with the amine groups of HSA via amide bond formation. TEM revealed that about 1–2 GNPs could be anchored on each FND (Figure 12.5a). The separation between these two types of nanoparticles was approximately 1 nm (Figure 12.5b). An analytical ultracentrifugation method was applied to isolate the GNP‐FNDs in the desired size range. The research team used this nanohybrid as a new class of “all‐in‐one” nanodevice for multimodal applications, including optical microscopy, electron microscopy, and potentially quantum sensing.
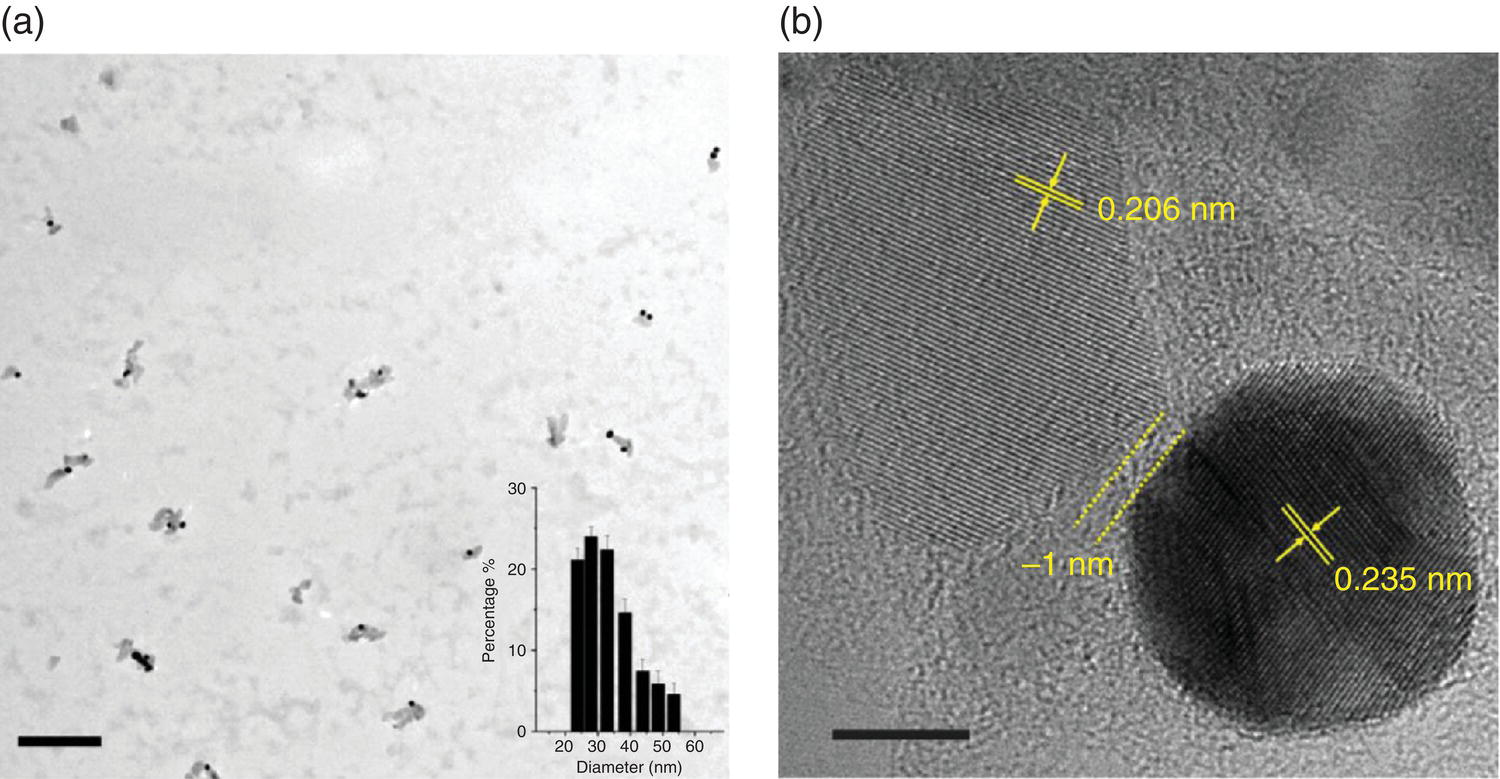
Figure 12.5 Characterization of GNP‐FND nanohybrids. (a) TEM image of GNP‐FNDs. The inset depicts the size distribution of the particles with a mean diameter of 33 nm. (b) High‐resolution TEM image of the GNP‐FND on graphene as a supporting film. The lattice fringes of FND (0.206 nm) and GNP (0.235 nm) are clearly resolved. The separation width between these two nanoparticles is approximately 1 nm. Scale bars: 200 nm in (a) and 5 nm in (b).
Source: Reprinted with permission from Ref. [14]. Reproduced with permission of American Chemical Society.
12.2.3 Hyperlocalized Hyperthermia
Gold particles are one of the most frequently used heating agents in plasmon‐based photothermal therapy [15, 16]. A member of particular interest in this family is the GNR, because its longitudinal surface plasmon resonance (LSPR) band can be conveniently tuned by increasing the aspect ratio of the particle to the near‐infrared range (cf., captions in Figure 12.6) [17], where light has a relatively long penetration depth through tissue (Section 9.3). It is therefore more suitable than spherical GNPs for in vivo photoacoustic imaging and hyperthermia applications. Another outstanding feature of GNR is that its LSPR band has an exceptionally large molar extinction coefficient on the order of 109 M−1 cm−1 at 800 nm [18]. Numerical simulations have shown that the light absorbed by the nanoparticles will be effectively converted into heat and thermal equilibration at the particle/water interface can be rapidly reached within 1 ns due to their small size [19].
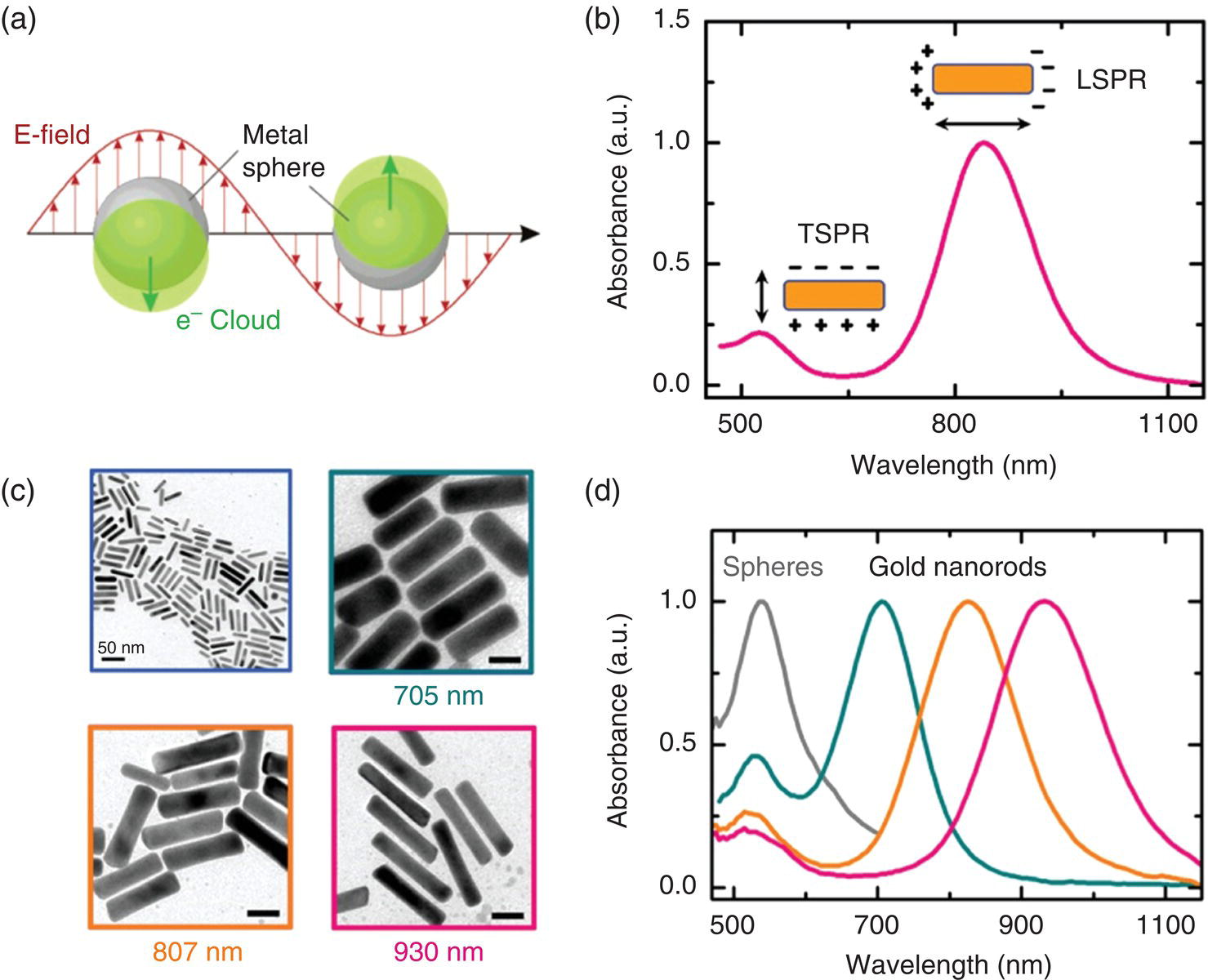
Figure 12.6 (a) Illustration of plasmon oscillation for a metal nanosphere. (b) Measured absorbance spectrum of a GNR solution. The insets show the schematic of the transverse and longitudinal SPR modes responsible for the two absorption bands observed in the spectrum. (c) TEM images of as‐synthesized GNRs with different longitudinal SPR wavelengths as noted. Unlabeled scale bars: 20 nm. (d) Measured absorbance spectra of gold nanospheres and GNRs, whose TEM images are shown in (c).
Source: Reprinted with permission from Ref. [17].
A number of studies have applied GNPs to nanoplasmonic heating of synthetic phospholipid membrane [20] and human cell membrane [21, 22]. The technique, also known as hyperlocalized hyperthermia [23, 24], surpasses conventional thermal therapy by reducing the heating zone down to the nanometer size. When applying the hyperlocalized hyperthermia technique to plasma membrane, for example, only a small heating zone is created on the cell surface. The spatial restriction of the heating zone greatly reduces the energy input required to reach the critical temperature that leads to cell death. However, in order to visualize the particles, which are only weakly fluorescent, either dark‐field microscopy [20] or two‐photon excitation microscopy [21] is necessary, which hinders popular uses of the methods. A way to circumvent this problem is to conjugate GNRs with FNDs, which are both photophysically and thermally stable. To demonstrate the concept, Chang and coworkers [25] prepared GNR‐FND nanohybrids that could be individually imaged and heated at the same time with a single laser. In this experiment, carboxylated FNDs were first covalently linked with polyethylenimine (PEI) through carbodiimide chemistry, followed by decorating the cationic FND surface with citrate‐capped GNRs through electrostatic interactions to form dual‐functional nanodevices. The advantage of this noncovalent conjugation was that it allowed multiple GNRs to be attached on a FND, as demonstrated for GNPs on silica spheres [26, 27]. For GNRs with dimensions of 10 nm × 16 nm (diameter × length) as an example, nanohydrids consisting of more than 50 GNRs on a single 100‐nm FND could be readily synthesized (inset in Figure 12.7a). This large loading made the nanohybrid an efficient light absorber and thus an effective nanoheater, suitable for a wide range of hyperthermia applications.
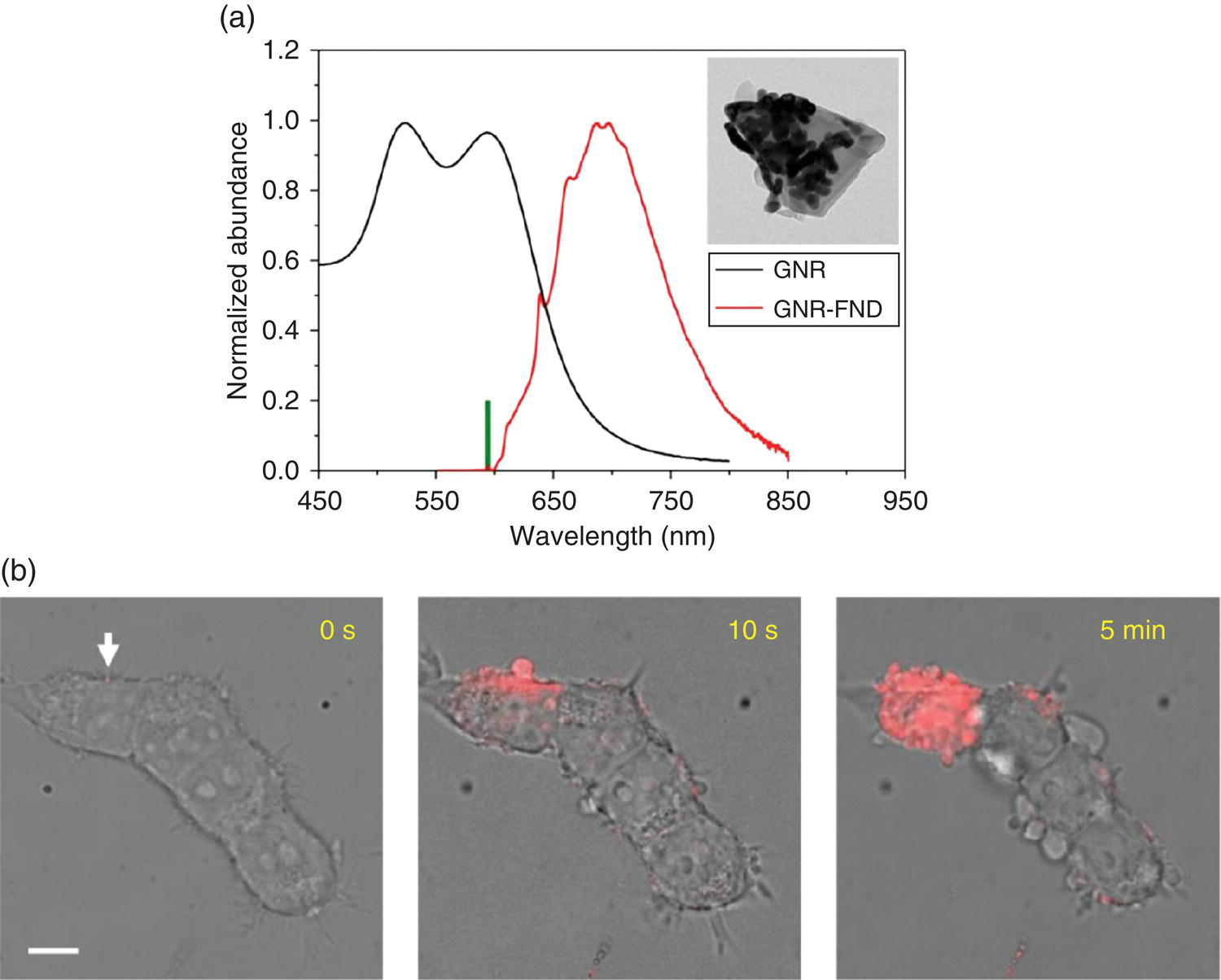
Figure 12.7 (a) Comparison between the absorption spectrum of 10‐nm × 16‐nm GNRs and the emission spectrum of 100‐nm GNR‐FNDs. The thick green bar denotes the excitation wavelength at 594 nm. Inset: TEM image of a GNR‐decorated FND having more than 30 GNRs on the observable hemisphere or a surface coverage of greater than 50%. (b) Merged bright‐field/fluorescence images of an HEK293T cell cluster with GNR‐FNDs attached to the plasma membrane after exposure to the 594 nm laser in the presence of PI at different time points. Intracellular PI was observed almost immediately after laser irradiation of the particle indicated by the white arrow. Many blebs appeared on the cells not being irradiated at five minute after the hyperlocalized hyperthermia treatment. Scale bar: 10 μm.
Source: Adapted with permission from Ref. [25]. Reproduced with permission of John Wiley & Sons.
Figure 12.7a shows an absorption spectrum of 10‐nm × 16‐nm GNRs, which exhibit a LSPR band peaking at approximately 590 nm. The band matches closely with the laser excitation wavelength of 594 nm but does not significantly quench the NV− fluorescence. The nanohybrids can thus be applied as a two‐in‐one nanodevice using a single laser equipped in a fluorescence microscope for imaging and heating at the same time. An example of the application is given in Figure 12.7b, where the GNR‐FNDs were sparsely anchored on the plasma membrane of a cell cluster derived from the human embryonic kidney cell line (HEK293T). These nanohybrids could be readily identified individually by confocal fluorescence microscopy at a laser power as low as 30 μW without damaging the cell’s membrane. To access a viable thermal range, Tsai et al. [25] increased the laser power by a factor of 10 and observed severe membrane blebbing within merely an irradiating time of six seconds. The blebbing was a sign of cell death [28], caused by laser heating of the membrane‐attached GNR‐FNDs. To examine the events more closely, the research team imaged the cells in the presence of propidium iodide (PI) before and after laser irradiation of the nanohybrids. PI is a membrane‐impermeable dye, which increases its fluorescence intensity by more than 10‐fold once bound to nucleic acids in cells. The labeling enabled the observation of the cell membrane damage by detecting the red emission of PI inside the cytoplasm in three dimensions and real time (e.g. zero to five minutes in this case).
While the noncovalently bound GNR‐FND complexes have been demonstrated to be a powerful nanodevice for both hyperlocalized hyperthermia and nanoscale thermometry applications, their chemical stability is a concern. Covalent conjugation of GNPs or GNRs with FNDs to ensure long‐term stability is desired. More preferably, a uniform thin shell of gold should form on the FND surface, similar to that of silica‐encapsulated FNDs. Experiments to realize such nanostructures have been undertaken with promising results so far [29, 30]. Further development of the technology, along with the incorporation of the specific cell targeting functionality [31–33], is expected to make the FND‐assisted photothermal therapy a powerful new tool for cancer treatment.
12.2.4 NV‐Based Nanothermometry
Hyperlocalized hyperthermia differs from conventional local hyperthermia in that it creates a much larger temperature gradient in cells. It is well known in materials science and engineering that a high spatial temperature gradient can cause structural stress detrimental to fragile solids. Hence, the true culprit of the cell killing effect as observed in Figure 12.7b might have been the large change in temperature difference within a short distance, rather than the absolute temperature. With the GNR‐FND nanohybrids becoming available commercially, it is possible to explore the mechanism of the hyperlocalized hyperthermia by conducting nanothermometric measurements within a single cell. The combination of the FND’s temperature sensing ability and the GNR’s heating capability allows in situ temperature tuning while at the same time reading out of the local temperatures of the cell.
Using FNDs, we now have two ways of measuring nanoscale temperatures: One is through optically detected magnetic resonance (ODMR) and the other is by all‐optical detection, as illustrated in Section 11.2. Both methods have been applied to GNR‐FNDs as two‐in‐one nanodevices [23, 25]. Specifically, Tsai et al. [23] conjugated FNDs with GNRs (aspect ratio = 4 : 1) for heating by a near‐infrared laser (808 nm) and measured the resulting temperature rise with the ODMR technique. With less than five GNRs attached to each FND, a temperature rise of 10 K could be readily achieved at a laser power of 0.4 mW in cells. To elucidate the nanoscale heating process, the research team conducted numerical simulations with finite element analysis. Their results showed that the temperature would be distributed almost uniformly over the entire nanohybrid as the number of GNRs on a FND reached 10 or greater. A large thermal gradient would develop right outside the gold shell in water, where the thermal conductivity is 2–3 orders of magnitude lower than that of gold and diamond. By treating the GNR‐FND nanohybrid as a spherical core‐shell structure, the temperature rise relative to ambient temperature around the nanohybrid (i.e. r ≥ r0) could be approximated by
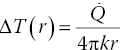
where ![]() is the heat generation rate, k is the thermal conductivity of water, r is the distance from the FND center, and r0 is the outer radius of the gold shell [25].
is the heat generation rate, k is the thermal conductivity of water, r is the distance from the FND center, and r0 is the outer radius of the gold shell [25].
In a later study, Tsai et al. [25] employed the all‐optical method for nanothermometric measurements using GNR‐FND nanohybrids composed of 10‐nm × 16‐nm GNRs as the bifunctional devices (Figure 12.7a). In addition to the hyperthermia experiment as illustrated in Figure 12.7b, the local thermostability (or heat tolerance) of subcellular components was probed at the nanoscale. The researchers chose to study the thermally induced rupture of the membrane tunneling nanotubes (TNTs) of HEK293T cells (Section 7.2.2 and Figure 7.8), because the events of membrane disruption and retraction could be directly visualized with optical microscopy. By using a 594 nm laser for both heating and probing of single GNR‐FNDs confined in the TNTs, they measured the rupture temperatures of the nanotubes one by one (cf., captions in Figure 12.8 for details). A temperature difference as large as 10 °C was found between the results of the local heating by lasers and the global heating with an on‐stage incubator in separate experiments. The local temperature rise leading to cell death could also be measured with this technique. The experiment demonstrated a new paradigm for hyperthermia research and application. It is anticipated that further advancement of the technologies will lead to the production of multifunctional nanoparticles for all‐in‐one hyperthermia therapy with tracking, heating, and sensing capabilities.
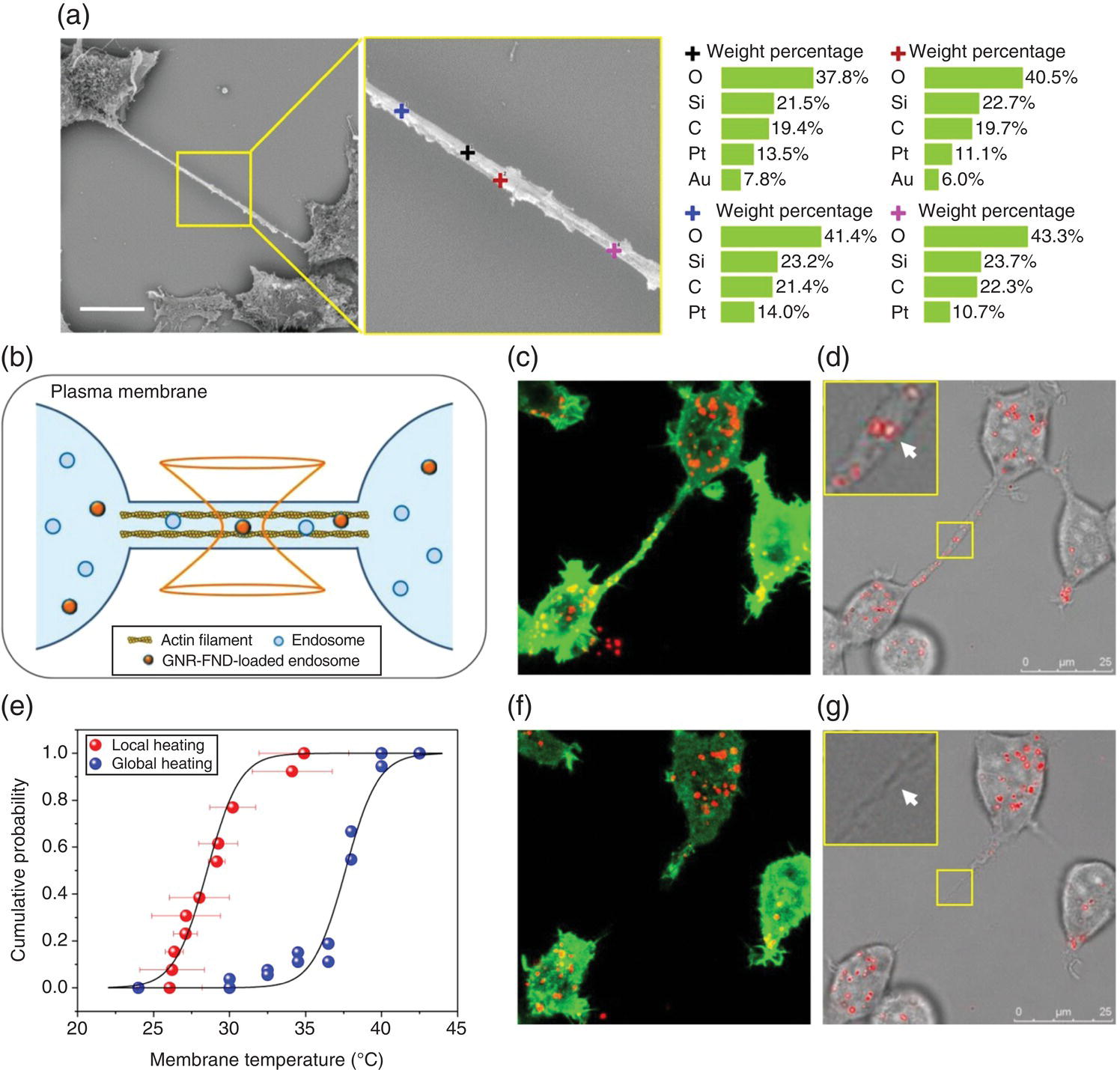
Figure 12.8 Nanohyperthermia with GNR‐FNDs in membrane nanotubes. (a) Scanning electron microscopy (SEM) image of a membrane nanotube formed between two HEK293T cells. Elemental analysis by energy‐dispersive X‐ray spectroscopy (EDS) shown to the right reveals the presence of GNR‐FNDs (denoted by black and red crosses) in the TNT. Scale bar: 10 μm. (b) Pictorial presentation of the experiment using a tightly focused 594 nm laser beam for both heating and temperature sensing of the GNR‐FNDs trapped in the endosomes of a membrane nanotube. (c, d) Fluorescence (c) and merged bright‐field/fluorescence (d) images of HEK293T cells transduced with actin‐GFP fusion proteins (green) and labeled with GNR‐FNDs (red). (e) Empirical cumulative distribution plot of the membrane temperatures at which TNTs are ruptured by local heating and global heating of GFP‐transduced HEK293T cells. Data of two independent global heating experiments (blue dots) are shown in the plot. (f, g) Fluorescence (f) and merged bright‐field/fluorescence (g) images of GNR‐FND‐labeled, GFP‐transduced HEK293T cells after exposure to the 594 nm laser with a power of 330 μW for six seconds. TNT breaking and retraction could be clearly observed after laser irradiation. Insets of (d) and (g): Enlarged views of the regions with the particle irradiated by the 594 nm laser in the yellow boxes. White arrows indicate the particles being irradiated. GFP, green fluorescent protein.
Source: Reprinted with permission from Ref. [25]. Reproduced with permission of John Wiley & Sons.
12.3 Silver/Diamond Nanohybrids
Silver nanostructures are widely recognized to have a profound effect on Raman scattering, known as surface‐enhanced Raman scattering (SERS) [34]. The nanostructures also help increase the photoluminescence intensities of fluorophores in the vicinity [35]. A study in 2009 first reported that the fluorescence intensity of FNDs (35 or 140 nm in size) could be significantly enhanced after deposition on nanocrystalline Ag films without buffer layers [36]. The intrinsic photostability of the FND nanoparticles was preserved even in direct contact with the Ag film. However, the fluorescence enhancement effect diminished when the individual FND particles were wrapped around by DNA molecules, which increased the distance between the NV− centers inside the FNDs and nearby Ag nanoparticles. An enhancement factor of up to 10 was measured for 35‐nm FNDs, confirmed by time‐gated fluorescence imaging (Section 8.2.4) and histogram analysis.
The Ag film mentioned above consisted of Ag nanoparticles in various sizes and shapes, making it difficult to understand the underlying physics of the plasmon‐enhanced effect. To overcome this difficulty, Andersen et al. [37] studied both experimentally and theoretically the spontaneous emission of the NV− centers in a FND placed near a silver nanocube with an AFM. The controlled assembly of the Ag‐FND system allowed the researchers to compare directly its fluorescence properties with that of a single isolated FND (~40 nm in diameter). It was found that the cube‐coupled NV− centers emitted strongly polarized fluorescence. At the optimal pump laser polarization, the rate of photons emitted by the FNDs in the nanohybrids was increased by a factor of 4 due to the local field enhancement. A similar result was obtained by Lourenco‐Martins et al. [38] in a cathodoluminescence study to probe the plasmonic coupling of the NV0 centers in FND aggregates with nearby Ag nanocubes on the nanometer scale using both photons and fast electrons equipped in a scanning transmission electron microscope (Section 10.3).
The Ag‐FND nanohybrids discussed so far are all formed by physical contacts among the individual particles and therefore may not be stable enough for practical applications. To strengthen the bonding, Gong et al. [39] developed a general bottom‐up approach to fabricate freestanding FND‐based hybrid nanostructures. Figure 12.9a displays a schematic diagram of the Ag‐FND synthesis, which started with size‐selected FNDs after extensive acid treatment to passivate their surface with carboxyl groups for good dispersion of the FND particles in water. The tradeoff was that the surface became relatively inert, impeding direct growth of Ag nanoparticles. To enable the nucleation and good control over the coverage of the externally coupled units, the FND surface was first functionalized with poly(vinylpyridine) (PVP) molecules, of which the pyridyl groups could interact with the nonmetallic polar surface containing carboxyl groups through hydrogen bonding. The unbound nitrogen atoms at the end of the pyridyl groups were then utilized to attract and transport Ag+ ions that eventually led to the nucleation and growth of Ag nanoparticles on the FND surface. TEM images confirmed the uniformity of the as‐grown Ag‐FND nanohybrids with the size of the constituent Ag nanoparticles in the range of 10 nm (Figure 12.9b). The research team also investigated in detail the coupling between the NV− centers in FNDs and the SPRs of the Ag subunits by conducting size‐ and coverage‐dependent ODMR, in addition to fluorescence lifetime measurements for the hybrid nanostructures. The strategy, in principle, can be applied to studying the interactions of NV− and other nanostructures (such as quantum dots) as well as the synthesis of a wide range of metal‐FND nanohybrids [40] with improved quantum sensing capabilities (Chapter 11).
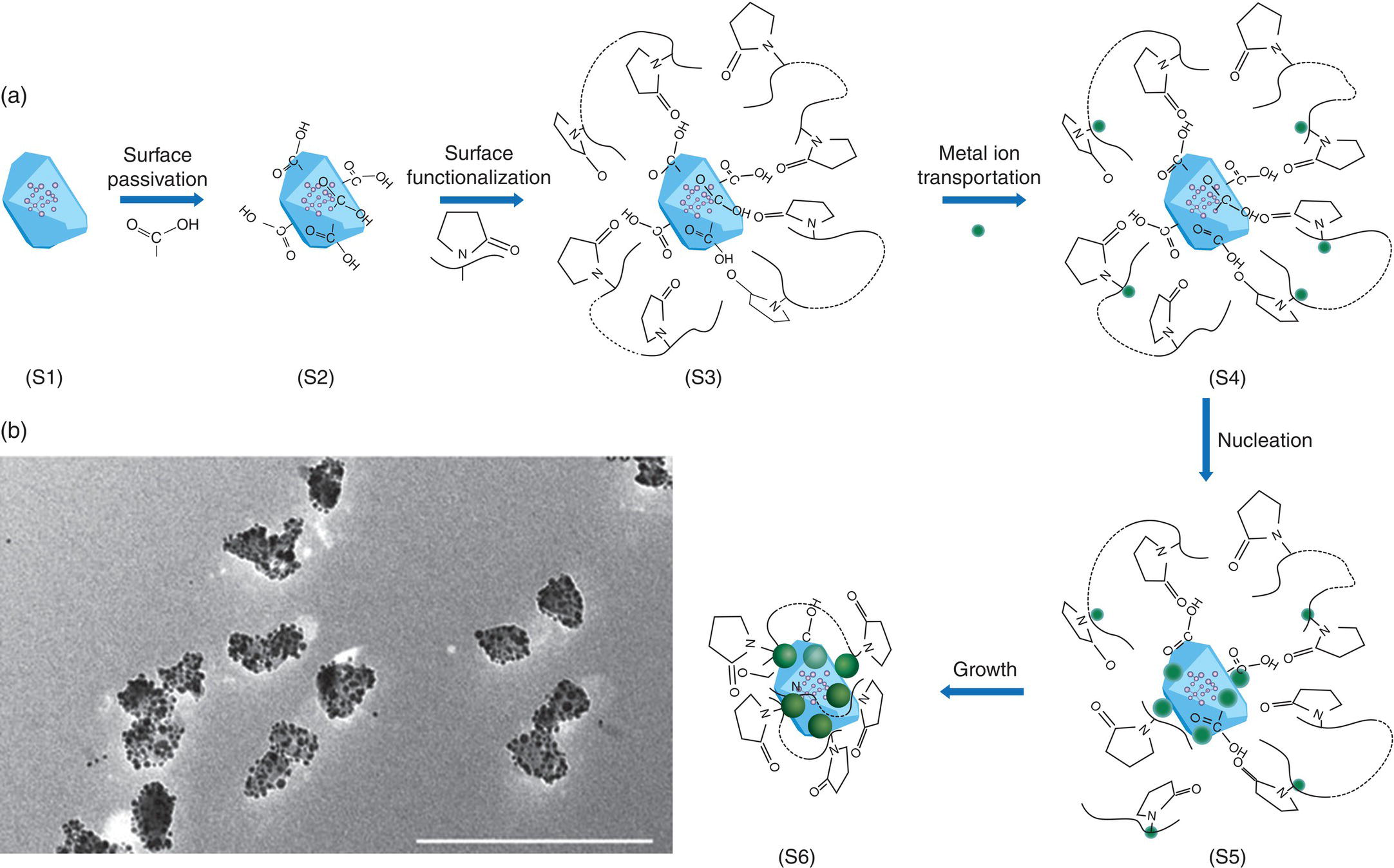
Figure 12.9 (a) Schematic synthetic paradigm illustrating different growth stages (S1‐S6). (b) A typical large‐scale TEM image showing excellent dispersion and uniformity of hybrid Ag‐FND nanostructures made by following synthetic scheme in (a). Scale bar: 200 nm.
Source: Reprinted with permission from Ref. [39].
With regard to biological applications of Ag‐FNDs, a potential use of the nanohybrids is to improve the potency of silver nanoparticles in clinical medicine. Silver is a natural antibiotic material because it kills bacteria. People have used silver products for thousands of years and the material continues to play an important role in hospital as medical and health care appliance nowadays [41]. However, concerns about the possible harm of the heavy metal to human health exist. “Blue blood” is a description for people who are exposed to an excessive amount of silver and suffered from such conditions. The most dramatic symptom is that the skin of the patients turns purple or blue, known as “Argyria” [42]. A solution for Argyria is to use silver nanoparticles, which are more active than bulk silver due to their increased surface‐to‐volume ratios and thus a lower dose is required to achieve the same antimicrobial effects. Studies have shown that some bacteria (such as antibiotic‐resistant bacteria) are unable to develop a resistance to silver nanoparticles even after repeated exposure [43, 44]. The conjugation of FND (or diamond in general) with silver is expected to reduce the toxicity of the metal particles while maintaining their antibacterial activities both in vitro and in vivo.
12.4 Iron Oxide/Diamond Nanohybrids
12.4.1 Single‐Domain Magnetization
Superparamagnetic iron oxides (SPIOs) are ferrimagnetic nanoparticles composed of single magnetic domains [45]. This type of magnetism occurs in small particles (typical diameter < 50 nm), where thermal fluctuations randomly flip their magnetization directions and the average magnetization is zero. However, in the presence of an external magnetic field, the nanoparticles can be magnetized in the superparamagnetic states, developing strong internal magnetization. Unlike ferromagnetic substances, superparamagnetic materials do not retain any net magnetization once the external field is removed and thus have no magnetic memory. Composed of Fe3O4, which is easily oxidized to Fe2O3 in air, SPIOs have found wide applications as contrast agents for magnetic resonance imaging (MRI) [46] and heating agents for hyperthermia therapy [47]. Compared with other nanoparticles made of silica, gold, and silver, SPIOs have a higher level of biocompatibility, posing as the most promising candidate for clinical use. Therefore, when SPIOs are integrated with FNDs, it is anticipated to open up an exciting new horizon for the application of the nanohydrids in biology and medicine.
Plakhotnik et al. [48] presented a method to synthesize this new class of nanohybrids by first modifying the surface of FNDs (~40 nm in diameter) with amino groups, followed by coating of the cationic FNDs with SPIOs (~12 nm in diameter) through electrostatic self‐assembly. TEM imaging showed a high‐density layer of SPIOs on the FND surface. To characterize the magnetic properties of the surface‐bound SPIOs, the researchers utilized the NV− centers embedded in the FNDs for nanomagnetometric measurements. By using the ODMR technique as described in Section 3.4, saturation of the average magnetization was observed for the individual nanomagnets as the external magnetic field strength increased from 10 to 40 mT. The result was in agreement with a simple single‐domain magnetization model, demonstrating the feasibility of forming this novel nanohybrid with the noncovalent conjugation method.
12.4.2 Magnetic Resonance Imaging
MRI is a powerful tool that has revolutionized the practice of medical research since its inception in the early 1970s [49]. The technique is noninvasive and yet able to produce three‐dimensional anatomical and functional images of the human body without the use of harmful ionizing radiation [50]. Compared with other imaging modalities as listed in Figure 12.4, MRI provides a considerably higher penetration depth in tissue but suffers from poor sensitivity and low spatial resolution. To improve the sensitivity, SPIOs and ultrasmall superparamagnetic iron oxides (USPIOs) have been harnessed as MRI contrast agents for their excellent biocompatibility, high biodegradability, and large magnetic susceptibility. By properly conjugating SPIOs or USPIOs with FNDs, high‐sensitivity imaging in vitro and in vivo is possible with these fluorescent magnetic nanohybrids.
In a contemporary work aiming at developing the dual‐modality imaging agents, we covalently linked carboxylated FNDs and aminated USPIOs together by carbodiimide chemistry to form nanohybrids. DLS showed a number‐averaged hydrodynamic diameter of 10.6, 84.9, and 96.4 nm for USPIOs, FNDs, and USPIO‐FNDs, respectively (Figure 12.10a). TEM imaging of USPIO‐FNDs revealed that the surface of FND could be covered by a large number (>100) of USPIO particles with a surface coverage of more than 50% (Figure 12.10b). The content of Fe atoms in the USPIO‐FND nanohybrids was approximately 7% (w/w), as determined by inductively coupled plasma (ICP) mass spectrometry. We then measured the saturation magnetization (Ms) of USPIOs and USPIO‐FNDs separately by using a superconducting quantum interference device (SQUID), and obtained a value of Ms = 63 emu g−1 for USPIOs in the nanohybrids (Figure 12.10c), in agreement with Ms = 60 emu g–1 of free USPIOs. Additionally, the hysteresis curves of USPIO‐FNDs and USPIOs matched closely with each other, indicating that there was no significant difference in the inherent super paramagnetic properties of USPIOs before and after covalent conjugation with FNDs. Further investigations of the magnetic properties of USPIO‐FNDs were carried out by measuring the transverse (spin–spin) relaxation times of water protons with (T2) and without (T2,w) the nanoparticles (Section 11.1). The equation used to quantify the relaxation times was
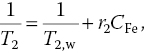
where r2 is the relaxivity and CFe is the Fe concentration calculated from the weight of USPIOs. Relaxation time measurements against CFe yielded r2 = 149 mM−1 s−1 for USPIOs and r2 = 240 mM−1 s−1 for USPIO‐FNDs (Figure 12.10d). The increase of r2 by approximately 60% upon conjugation with FNDs suggested that USPIO‐FNDs may serve as a better contrast agent than USPIOs alone for MRI imaging.
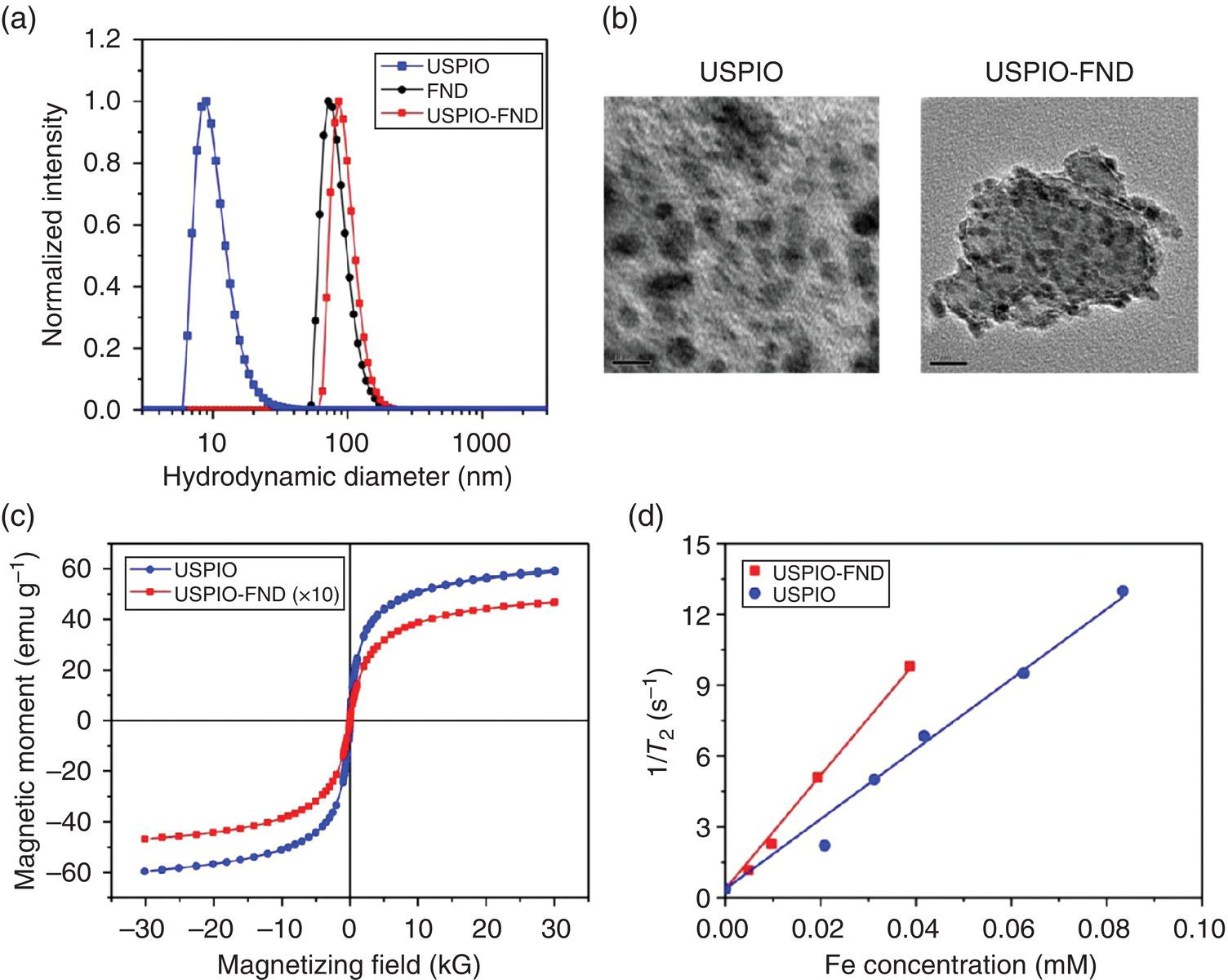
Figure 12.10 Characterization of USPIOs, FNDs, and USPIO‐FNDs. (a) Size distributions, (b) TEM images, (c) magnetizations, and (d) transverse relaxivities (r2) of USPIOs before and after conjugation with FND. Scale bars in (b): 10 nm (USPIO) and 20 nm (USPIO‐FND). The values of the transverse relaxivity (r2) are given by the slopes of the linear fitting to the experimental data in (d).
An application of SPIO‐FNDs with a substantial impact is the tracking of tumor cells in vivo for cancer detection. To demonstrate the feasibility, we labeled human brain tumor cells (U‐87 MG) with USPIO‐FNDs in a culture medium. The USPIO‐FND nanohybrids could be avidly taken up by the cells under serum‐free conditions. To quantify the USPIO‐FND uptake and the Fe loading capacity per cell, we utilized the magnetically modulated fluorescence technique for FNDs as described in Section 9.3.4. For cells labeled at the USPIO‐FND concentration of 200 μg ml−1, the amounts determined were 2.4 × 104 FND particles per cell and 3.15 pg of Fe per cell. The high Fe loading capacity allowed us to conduct in vivo MRI of these labeled cells transplanted through subcutaneous injection into mice. An example of such results is given in Figure 12.11a, where coronal slices (three each) of the T2‐weighted images were acquired at different time points. The sizes of both the unlabeled (right thigh) and USPIO‐FND‐labeled (left thigh) brain tumors were monitored in real time without having to sacrifice the animals. No adverse effects were found in the tumor growth due to the USPIO‐FND labeling. The results proved that the nanohybrid‐enabled MRI was capable of three‐dimensional tracking noninvasive to the growth of glioblastoma tumors (Figure 12.11b). Furthermore, detailed histological analysis of the USPIO‐FND‐labeled glioblastoma tissue sections could be performed by hematoxylin and eosin staining, immunofluorescence, and time‐gated fluorescence imaging of FNDs (Section 9.3.3). Altogether, it is feasible now to extend the spatial resolution of the imaging from in vivo MRI on the centimeter scale to in vitro fluorescence microscopy on the micrometer or even nanometer scale, approaching true nanomedicine.
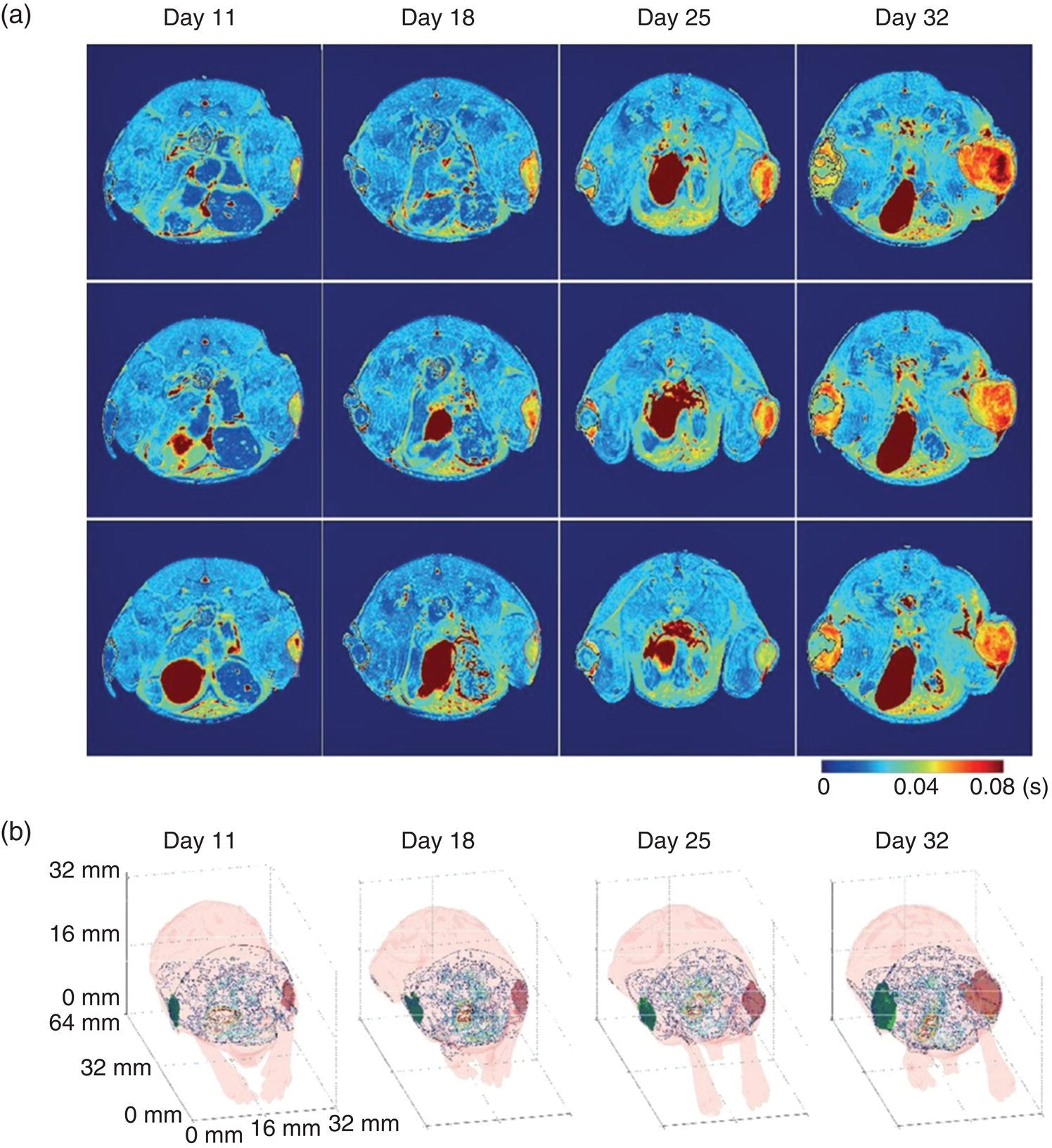
Figure 12.11 MRI for the growth of glioblastoma tumors in a mouse model. (a) T2 mappings of three coronal slices on day 11, 18, 25, and 32. The unlabeled glioblastoma tumors (right thigh) were circled by thin red lines and the USPIO‐FND‐labeled glioblastoma tumors were circled by thin black lines (left thigh). Given shorter T2 times, the contrast‐enhanced regions within the labeled glioblastoma tumors (left thigh) are in dark blue due to the presence of USPIO‐FNDs. (b) Three‐dimensional images of the unlabeled glioblastoma tumor (right thigh, red area) and the USPIO‐FND‐labeled glioblastoma tumor (left thigh, green area) on day 11, 18, 25, and 32.
References
- 1 Kickelbick, G. (2007). Introduction to hybrid materials. In: Hybrid Materials: Synthesis, Characterization, and Applications (ed. G. Kickelbick), 1–48. Wiley.
- 2 Menon, J.U., Jadeja, P., Tambe, P. et al. (2013). Nanomaterials for photo‐based diagnostic and therapeutic applications. Theranostics 3: 152–166.
- 3 Rehor, I., Slegerova, J., Kucka, J. et al. (2014). Fluorescent nanodiamonds embedded in biocompatible translucent shells. Small 10: 1106–1115.
- 4 Bumb, A., Sarkar, S.K., Billington, N. et al. (2013). Silica encapsulation of fluorescent nanodiamonds for colloidal stability and facile surface functionalization. J Am Chem Soc 135: 7815–7818.
- 5 Sarkar, S.K., Bumb, A., Wu, X. et al. (2014). Wide‐field in vivo background free imaging by selective magnetic modulation of nanodiamond fluorescence. Biomed Opt Express 5: 1190–1202.
- 6 von Haartman, E., Jiang, H., Khomich, A.A. et al. (2013). Core‐shell designs of photoluminescent nanodiamonds with porous silica coatings for bioimaging and drug delivery I: fabrication. J Mater Chem B 1: 2358–2366.
- 7 Prabhakar, N., Nareoja, T., von Haartman, E. et al. (2013). Core‐shell designs of photoluminescent nanodiamonds with porous silica coatings for bioimaging and drug delivery II: application. Nanoscale 5: 3713–3722.
- 8 Liu, Y.L. and Sun, K.W. (2011). Plasmon‐enhanced photoluminescence from bioconjugated gold nanoparticle and nanodiamond assembly. Appl Phys Lett 98: 153702.
- 9 Schietinger, S., Barth, M., Aichele, T., and Benson, O. (2009). Plasmon‐enhanced single photon emission from a nanoassembled metal‐diamond hybrid structure at room temperature. Nano Lett 9: 1694–1698.
- 10 Shen, H., Chou, R.Y., Hui, Y.Y. et al. (2016). Directional fluorescence emission from a compact plasmonic‐diamond hybrid nanostructure. Laser Photon Rev 10: 647–655.
- 11 Zhang, B., Fang, C.Y., Chang, C.C. et al. (2012). Photoacoustic emission from fluorescent nanodiamonds enhanced with gold nanoparticles. Biomed Opt Express 3: 1662–1669.
- 12 Tam, A. (1986). Applications of photoacoustic sensing techniques. Rev Mod Phys 58: 381–431.
- 13 Fried, N.M. and Burnett, A.L. (2015). Novel methods for mapping the cavernous nerves during radical prostatectomy. Nat Rev Urol 12: 451–460.
- 14 Liu, W., Naydenov, B., Chakrabortty, S. et al. (2016). Fluorescent nanodiamond‐gold hybrid particles for multimodal optical and electron microscopy cellular imaging. Nano Lett 16: 6236–6244.
- 15 Huang, X., Jain, P.K., El‐Sayed, I.H., and El‐Sayed, M.A. (2008). Plasmonic photothermal therapy (PPTT) using gold nanoparticles. Lasers Med Sci 23: 217–228.
- 16 Jaque, D., Martínez Maestro, L., del Rosal, B. et al. (2014). Nanoparticles for photothermal therapies. Nanoscale 6: 9494–9530.
- 17 Li, J., Guo, H., and Li, Z.Y. (2013). Microscopic and macroscopic manipulation of gold nanorod and its hybrid nanostructures. Photon Res 1: 28–41.
- 18 Park, K., Biswas, S., Kanel, S. et al. (2014). Engineering the optical properties of gold nanorods: independent tuning of surface plasmon energy, extinction coefficient and scattering cross section. J Phys Chem C 118: 5918–5926.
- 19 Ekici, O., Harrison, R.K., Durr, N.J. et al. (2008). Thermal analysis of gold nanorods heated with femtosecond laser pulses. J Phys D Appl Phys 41: 185501.
- 20 Urban, A.S., Fedoruk, M., Horton, M.R. et al. (2009). Controlled nanometric phase transitions of phospholipid membranes by plasmonic heating of single gold nanoparticles. Nano Lett 9: 2903–2908.
- 21 Tong, L., Zhao, Y., Huff, T.B. et al. (2007). Gold nanorods mediate tumor cell death by compromising membrane integrity. Adv Mater 19: 3136–3141.
- 22 Delcea, M., Sternberg, N., Yashchenok, A.M. et al. (2012). Nanoplasmonics for dual‐molecule release through nanopores in the membrane of red blood cells. ACS Nano 6: 4169–4180.
- 23 Tsai, P.C., Chen, O.Y., Tzeng, Y.K. et al. (2015). Gold/diamond nanohybrids for quantum sensing applications. EPJ Quantum Technol 2: 19.
- 24 Epperla, C.P., Chen, O.Y., and Chang, H.C. (2016). Gold/diamond nanohybrids may reveal how hyperlocalized hyperthermia kills cancer cells. Nanomedicine (Lond) 11: 443–445.
- 25 Tsai, P.C., Epperla, C.P., Huang, J.S. et al. (2017). Measuring nanoscale thermostability of cell membranes with single gold‐diamond nanohybrids. Angew Chem Int Ed 56: 3025–3030.
- 26 Sadtler, B. and Wei, A. (2002). Spherical ensembles of gold nanoparticles on silica: electrostatic and size effects. Chem Commun 15: 1604–1605.
- 27 Pastoriza‐Santos, I., Gomez, D., Pérez‐Juste, J. et al. (2004). Optical properties of metal nanoparticle coated silica spheres: a simple effective medium approach. Phys Chem Chem Phys 6: 5056–5060.
- 28 Charras, G.T. (2008). A short history of blebbing. J Microsc 231: 466–478.
- 29 Minati, L., Cheng, L.C., Lin, C.Y. et al. (2015). Synthesis of novel nanodiamonds‐gold core shell nanoparticles. Diam Relat Mater 53: 23–28.
- 30 Matassa, R., Orlanducci, S., Reina, G. et al. (2016). Structural and morphological peculiarities of hybrid Au/nanodiamond engineered nanostructures. Sci Rep 6: 31163.
- 31 Chang, B.M., Lin, H.H., Su, L.J. et al. (2013). Highly fluorescent nanodiamonds protein‐functionalized for cell labeling and targeting. Adv Funct Mater 23: 5737–5745.
- 32 Cheng, L.C., Chen, H.M., Lai, T.C. et al. (2013). Targeting polymeric fluorescent nanodiamond‐gold/silver multi‐functional nanoparticles as a light‐transforming hyperthermia reagent for cancer cells. Nanoscale 5: 3931–3940.
- 33 Rehor, I., Lee, K.L., Chen, K. et al. (2015). Plasmonic nanodiamonds: targeted core‐shell type nanoparticles for cancer cell thermoablation. Adv Healthc Mater 4: 460–468.
- 34 Schlücker, S. (2014). Surface‐enhanced Raman spectroscopy: concepts and chemical applications. Angew Chem Int Ed 53: 4756–4795.
- 35 Aslan, K., Gryczynski, I., Malicka, J. et al. (2005). Metal‐enhanced fluorescence: an emerging tool in biotechnology. Curr Opin Biotechnol 16: 55–62.
- 36 Lim, T.S., Fu, C.C., Lee, H.Y. et al. (2009). Fluorescence enhancement and lifetime modification of single nanodiamonds near a nanocrystalline silver surface. Phys Chem Chem Phys 11: 1508–1514.
- 37 Andersen, S.K.H., Kumar, S., and Bozhevolnyi, S.I. (2016). Coupling of nitrogen‐vacancy centers in a nanodiamond to a silver nanocube. Opt Mater Express 6: 3394–3406.
- 38 Lourenco‐Martins, H., Kociak, M., Meuret, S. et al. (2018). Probing plasmon‐NV0 coupling at the nanometer scale with fast electrons and photons. ACS Photonics 5: 324–328.
- 39 Gong, J., Steinsultz, N., and Ouyang, M. (2016). Nanodiamond‐based nanostructures for coupling nitrogen‐vacancy centres to metal nanoparticles and semiconductor quantum dots. Nat Commun 7: 11820.
- 40 Wang, N., Liu, G.Q., Leong, W.H. et al. (2018). Magnetic criticality‐enhanced hybrid nanodiamond thermometer under ambient conditions. Phys Rev X 8: 011042.
- 41 Alexander, J.W. (2009). History of the medical use of silver. Surg Infect 10: 289–292.
- 42 Wikipedia (2018). Argyria. https://en.wikipedia.org/wiki/Argyria (accessed 16 April 2018).
- 43 Rai, M., Yadav, A., and Gade, A. (2009). Silver nanoparticles as a new generation of antimicrobials. Biotechnol Adv 27: 76–83.
- 44 Lara, H.H., Garza‐Treviño, E.N., Ixtepan‐Turrent, L., and Singh, D.K. (2011). Silver nanoparticles are broad‐spectrum bactericidal and virucidal compounds. J Nanobiotechnol 9: 30.
- 45 Lu, A.H., Salabas, E.L., and Schüth, F. (2007). Magnetic nanoparticles: synthesis, protection, functionalization, and application. Angew Chem Int Ed 46: 1222–1244.
- 46 Wang, Y.X.J. (2011). Superparamagnetic iron oxide based MRI contrast agents: current status of clinical application. Quant Imaging Med Surg 1: 35–40.
- 47 Dutz, S. and Hergt, R. (2014). Magnetic particle hyperthermia – a promising tumor therapy? Nanotechnology 25: 452001.
- 48 Plakhotnik, T., Aman, H., Zhang, S., and Li, Z. (2015). Super‐paramagnetic particles chemically bound to luminescent diamond: single nanocrystals probed with optically detected magnetic resonance. J Phys Chem C 119: 20119–20124.
- 49 The Nobel Prize in Physiology or Medicine for 2003 – Press Release. Nobelprize.org. Nobel Media AB 2014. http://www.nobelprize.org/nobel_prizes/medicine/laureates/2003/press.html (accessed 16 April 2018).
- 50 Storey, P. (2006). Introduction to magnetic resonance imaging and spectroscopy. In: Magnetic Resonance Imaging: Methods and Biologic Applications (ed. P.V. Prasad), 3–57. Humana Press.
