13
Nanodiamond‐Enabled Medicine
Equipped with specific targeting ability (Chapter 4), inherent biocompatibility (Chapter 5), and versatile imaging capability (Chapters 7–12), surface‐functionalized fluorescent nanodiamonds (FNDs) have a promising role to play in the magical land of medicine. We mean the medicine of this generation and the future. It seems fitting to have an overview here, specifically, in the areas of precision medicine and nanomedicine where nanodiamonds (NDs) have found their place.
Precision medicine, best described as “prevention and treatment strategies that take individual variability into account” [1], may not be a new concept but is boosted mainly by big data becoming available (and accessible), emerging in recent years as a current trend in modern medicine. In his annual State of The Union address in 2015, the U.S. President Obama launched a new Precision Medicine Initiative and stated that the goal of such an initiative was “to give all of us access to the personalized information we need to keep ourselves and our families healthier” [2]. From that point onward, although not exactly the equivalent, precision medicine is synonymous with personalized medicine. The goal, to put it bluntly, is to provide the right drug at the right dose to the right person. With the participation of nanomedicine, the statement may be expanded to include “in the right place and at the right time”.
Nanomedicine is both young and technological, cohesively integrated from multiple disciplines. A nicely drafted definition is provided by the European Science Foundation: “Nanomedicine uses nano‐sized tools for the diagnosis, prevention, and treatment of disease and to gain increased understanding of the complex underlying patho‐physiology of disease. The ultimate goal is to improve quality‐of‐life” [3]. A comprehensive study of the nanotechnologies in nanomedicine over a 10‐year period has concluded three major areas of applications, including (i) diagnostics, sensors, and surgical tools used outside the patients, (ii) imaging agents and monitoring technologies applied at the cell level and up to the whole body, and (iii) nanomaterials and devices for drug delivery and therapeutics [4]. As unveiled in the previous chapters, FNDs and their nanohydrids pose a remarkable prospect as a competent candidate for tasks (i) and (ii) stated above. For the sake of drug delivery and therapeutics (task (iii)), NDs (both fluorescent and nonfluorescent) are useful as a biocompatible and nontoxic platform for nanomedicine, which is aimed at delivering only the amount of active ingredient needed for treating the exact spots that are infected at the molecular or cellular level over a designated period of time necessary for curing disease without overdoses or side effects. We start the discussion in this chapter with NDs as therapeutic carriers.
13.1 NDs as Therapeutic Carriers
Therapeutics is a branch of medicine that deals with the treatment of disease and the action of remedial agents [5]. Nanotechnology is becoming an integral part of this medical development because nanoparticle‐based therapeutics hold the promises to overcome biological barriers, deliver hydrophobic drugs and biologics, and selectively target cancer cells [6]. However, in general, nanoparticles themselves may be highly reactive due to their small sizes and large specific surface areas (Chapter 4). Interactions of them with biomolecules in cells, tissues, and even extracellular environments can sometimes trigger a sequence of unexpected, undesired, or lethal effects (Chapter 5). These dynamic characteristics determine the biocompatibility of the nanoparticles as well as the efficacy of the intended treatments. Therefore, despite their potential benefits, the approved cases of nanoparticle‐based medicines for clinical use are few and far between [7, 8]. The inherently biocompatible NDs are indeed in the right position to offer a possible solution to overcome these hurdles and challenges.
Detonation nanodiamonds (DNDs) and high‐pressure high‐temperature nanodiamonds (HPHT‐NDs) are two most popular types of diamond nanoparticles used in the life sciences research [9]. They both are capable of binding with biomolecules and bioactive molecules (such as drugs) as a result of the interactions between the functional groups on their surface and the molecules of interest (Chapter 4). For acid‐treated NDs as an example, most functional groups derivatized on the particles can carry charges in aqueous solution and the overall charge density depends on the pKa values of these groups and the pH of the solution. The forces involved in the carrier–cargo interactions encompass electrostatic, hydrogen bonding, hydrophobic, and van der Waals forces. These acid‐treated NDs stand out as an appealing drug carrier for their high loading capacities and the abilities to protect and retain the inherent therapeutic effects of the noncovalently bound molecules. Equally important, if not more, is the versatile surface chemistry that has enabled the development of novel delivery methods for optimal loading, specific targeting, and controlled release of cargo molecules on the ND surface for therapeutic treatments. NDs have been harnessed for the delivery of many classes of molecules, including small molecules, peptides and proteins, and nucleic acids, with a major focus on the use of chemotherapeutic agents for biomedical applications [9]. While some studies have covalently conjugated drug molecules with surface‐functionalized NDs, which may impose some challenges on drug release, the majority of the present research has employed physical adsorption procedures.
Aside from the surface properties, the structure of the nanoparticles is another factor in favor of NDs for therapeutic applications. As discussed in Section 2.3.3, DNDs are synthesized by shock wave compression using explosive compounds such as 2,4,6‐trinitrotoluene (TNT) and 1,3,5‐trinitro‐1,3,5‐triazinane (RDX). With proper explosive mixture ratios, the shock wave can produce NDs with an average size of 4–5 nm in diameter for the primary particles. However, DNDs always agglomerate to form covalently bound clusters during the synthesis, with many nanometer‐sized cavities present in the interiors of the clusters (Figure 2.7), as revealed by transmission electron microscopy (TEM) [10, 11]. These cavities are disordered in structure and size, allowing the particles to be filled with assorted molecules. Similar to mesoporous silica nanoparticles [12], they are useful as drug delivery devices.
The most unique feature that makes NDs stand out among other nanoparticle‐based drug carriers is, perhaps, their fluorescence property. FNDs made of HPHT‐NDs are appealing in this aspect because their fluorescence is bright and their spectroscopic properties are largely unaffected by the attachment of various types of therapeutic agents to the surface. Once properly conjugated with the therapeutic agents, FNDs can be used to measure, monitor, and even alter the biochemical processes within cells. They have the potential to serve as a treatment tool (e.g. with the carried drugs), and more significantly, a preventative monitoring device (e.g. for detecting pre‐cancerous changes). Possessing several key properties necessary for clinical applications, i.e. stability and compatibility in biological environments as well as scalability in production, surface‐functionalized FNDs are emerging as a powerful and versatile drug delivery platform [13, 14].
13.2 Drug Delivery
Drug delivery at the nanoscale is an interdisciplinary field that spans across chemistry, biology, and medicine. A variety of organic and inorganic nanoparticles has been tested as the drug carriers, including lipid‐based vehicles (such as liposomes and micelles), polymeric nanoparticles (such as hydrogels and dendrimers), metal and metal oxide nanoparticles (such as gold or iron oxides), silica nanoparticles (such as amorphous or mesoporous silica), and carbon nanostructures (such as fullerenes, nanotubes, graphenes, and NDs) [15, 16]. To be a suitable drug delivery device, the carrier should possess the following characteristics: (i) sufficient loading capacity in proportion to the weight of the carrier, (ii) versatile binding with bioactive molecules, (iii) functional mechanism for targeted release, and (iv) high‐sensitivity tracking by noninvasive methods. With good biocompatibility, large specific surface areas, high bioconjugation ability, and unique magneto‐optical properties as discussed in the previous chapters, FNDs comply with all these requirements.
13.2.1 Small Molecules
Doxorubicin (DOX), an apoptosis‐inducing drug widely used for cancer chemotherapy, is the first drug tested with NDs as the carriers [17]. Containing an amino group (Figure 13.1a), the molecule is fluorescent and therefore can be used as a theranostic (therapeutic and diagnostic) agent [18]. Ho and coworkers [17, 19–24] made considerable efforts to develop ND‐based therapeutics with the DOX molecule. Instead of using DND agglomerates as shown in Figure 2.7, the experiments adopted monodisperse DNDs (2–8 nm in size) produced by wet ball milling [11]. Cytotoxicity tests through quantitative real‐time polymerase chain reaction (RT‐PCR) along with the DNA fragmentation assays all confirmed the innate biocompatibility of the monodisperse DND particles. To load DNDs with DOX, the researchers took advantage of the fact that DNDs could easily agglomerate in high‐concentration salt solution (e.g. NaCl) to form noncovalently bound clusters. These DND clusters were then bound with DOX through ionic interactions between the negatively charged carboxyl groups on DNDs and the positively charged amino group of DOX (Figure 13.2a and b). It was found that the DND hydrogels loaded with DOX could efficiently deliver the drugs into living cells such as murine macrophages and human colorectal carcinoma cells by endocytosis. The same group further investigated the rate of DOX–DND movement into cells by using DNDs physically bound with fluorescently labeled poly‐L‐lysine observable by confocal fluorescence microscopy. Cytotoxicity and genotoxicity assays proved that the DOX–DND complexes prompted the cell death, showing clearly that DND could serve as an effective carrier therapeutically significant for both systemic and localized drug delivery [25].
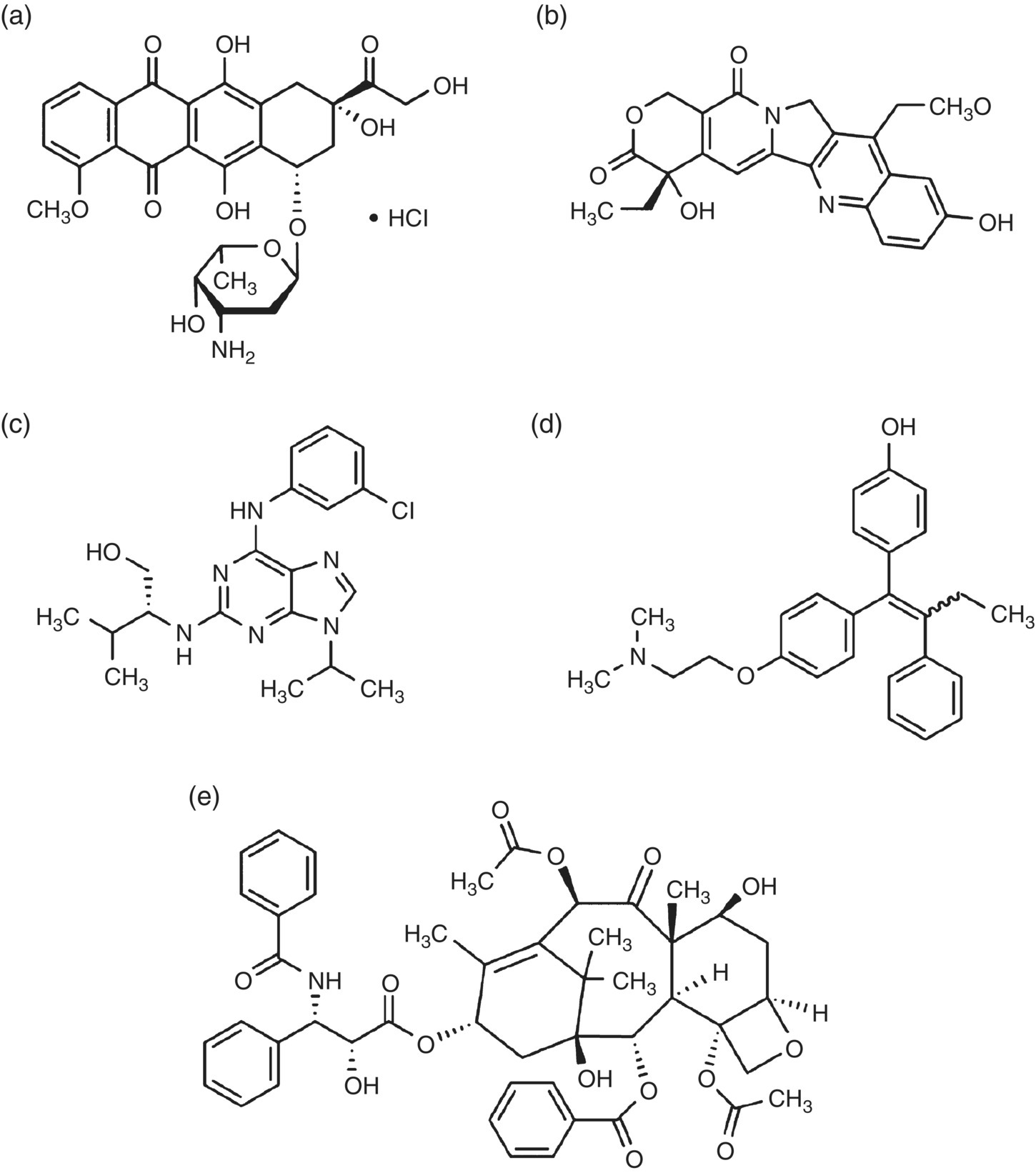
Figure 13.1 Chemical structures of some commonly used drugs loaded on NDs. (a) Doxorubicin, (b) 10‐hydroxycamptothecin, (c) purvalanol A, (d) 4‐hydroxytamoxifen, and (e) Paclitaxel.
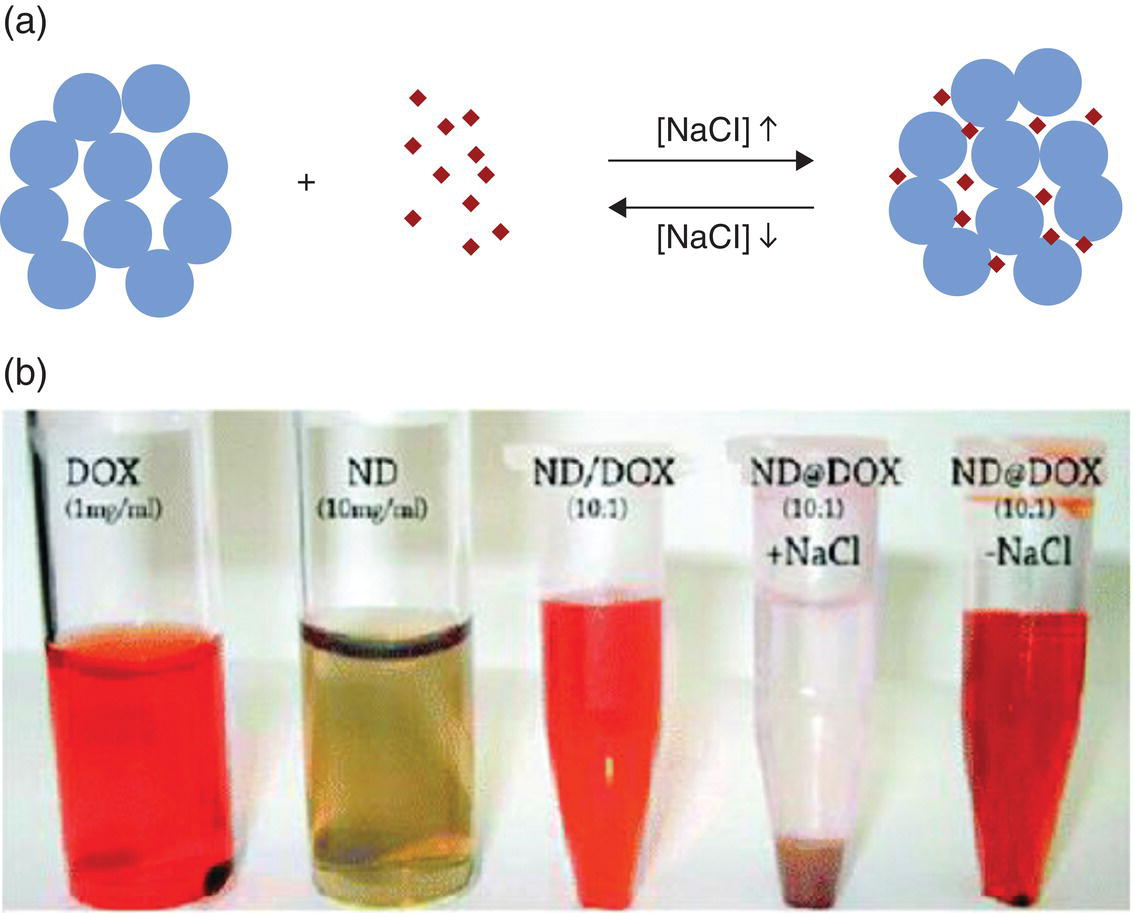
Figure 13.2 (a) Schematic drawing and (b) photograph of NaCl‐mediated loading and release of doxorubicin. Blue spheres denote DNDs and red dots denote DOX.
Source: Adapted with permission from Ref. [17]. Reproduced with permission of American Chemical Society.
The findings that the salt addition facilitates the loading of DOX into the DND clusters and conversely the salt removal triggers its release suggest a simple switching mechanism that has great potential for use in medical practice. In a supplementary study, both theoretical and experimental, Adnan et al. [21] looked into the influence of pH on the degree of the DOX loading and found that DOX was only able to bind with DNDs at a high pH (pH > 10). The amount of the bound DOX increased with the ionic strength and basicity of the environment, a trend in line with the strengthening of the electrostatic interactions [26]. Similar studies were carried out by other research groups using DNDs or HPHT‐NDs surface‐coated with polyethylene glycol to achieve drug delivery and slow release of DOX [27, 28]. Indeed, the delivery strategies thus developed all showed the transport of DOX into cancer cells, as confirmed by confocal fluorescence microscopy and flow cytometric analysis utilizing the intrinsic fluorescence of DOX. However, as the fluorescence properties of DOX depended sensitively on its local environment [29], FNDs were additionally used as references to support their findings [28]. Following the same approach, Wang et al. [30] performed targeting therapy of cancer cells using transferrin‐conjugated FNDs as the DOX carrier and proved successful delivery and release of DOX at the targeted sites.
Inspired by the studies of DOX, Guan et al. [31] loaded DNDs with cis‐dichlorodiamineplatinum (II) (Pt(NH3)22+, CDDP), another widely used anticancer drug. The loading was made by surface functionalization of DNDs with carboxyl groups, followed by ionic interactions of the surface‐bound –COO− with the Pt(II) of CDDP. It was found that CDDP could be released from the composite in phosphate‐buffered saline (PBS) of pH 6.0 at a rate twice faster than that of pH 7.4. This pH‐responsive release property implied a reduction of toxic side effects. For example, the CDDP–DND composites may release a small amount of CDDP during their circulation period in the blood (pH 7.4) but deliver a larger amount to acidic compartments such as lysosomes (pH < 6) in cells.
In another study, Li et al. [32] investigated the dose‐dependent behaviors of DNDs as an anticancer drug delivery vehicle. The researchers demonstrated that 10‐hydroxycamptothecin (HCPT) (Figure 13.1b) was capable of attaching to DNDs through the NaOH‐aided solubility enhancement of HCPT at an increased diffusion rate when the drug entered the interior of the covalently agglomerated DNDs. The sustained release of HCPT from the DND agglomerates occurred at pH < 6. The chemotherapeutic effect of the HCPT–DND complexes was found to be higher than that of the stand‐alone HCPT by a significant margin (Figure 13.3), suggesting that DNDs were a promising drug delivery platform for cancer therapy.
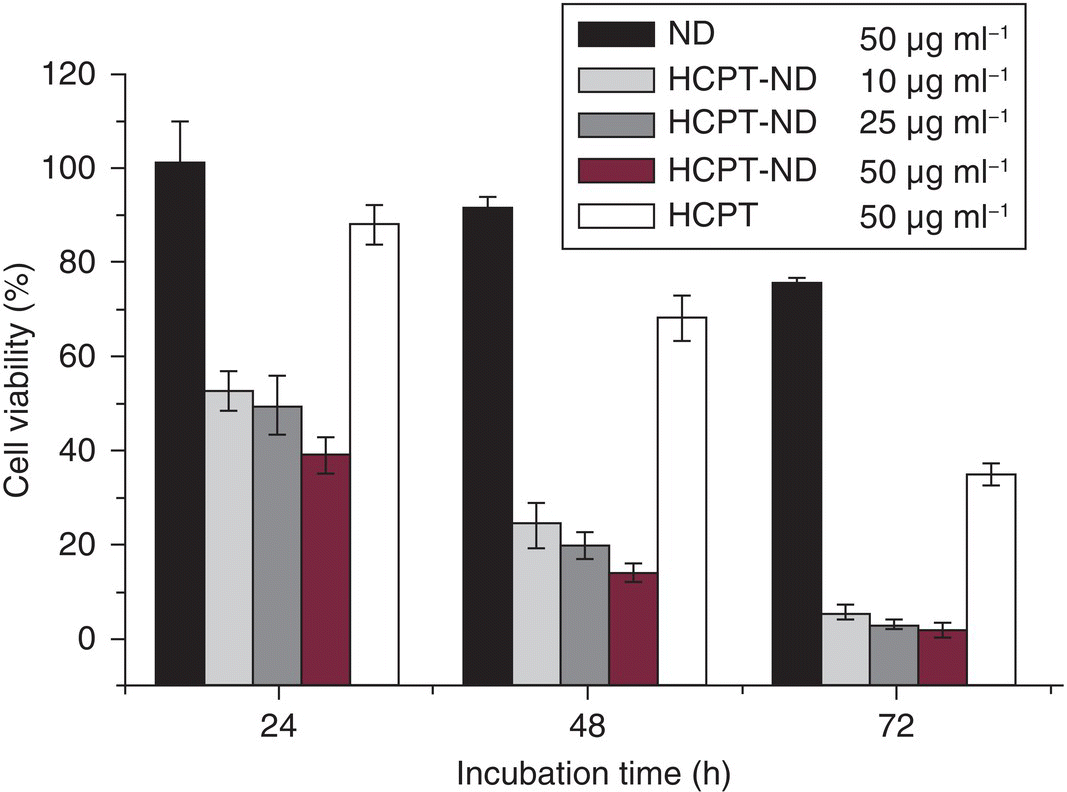
Figure 13.3 Viability of HeLa cells treated with DNDs, HCPT‐DNDs, and HCPT alone at different concentrations and time points.
Source: Reprinted with permission from Ref. [32]. Reproduced with permission of Elsevier.
A main limitation in the systemic administration is the low water solubility of certain drugs. Take two commonly used drugs for liver and breast cancer treatments, Purvalanol A (Figure 13.1c) and 4‐hydroxytamoxifen (Figure 13.1d) for example; they both are soluble in dimethyl sulfoxide (DMSO) but have a poor solubility in water. Chen et al. [33] chose these two as the model compounds to study the improvement of the drug solubility by complexation with acid‐treated, monodisperse DNDs. A visible enhancement in the dispersibility was found for both drugs in the DMSO/water solution (Figure 13.4a–f). The study provided a useful strategy for applying water‐insoluble drug molecules to treatment‐relevant scenarios through the complexation with DNDs (Figure 13.4g and h).
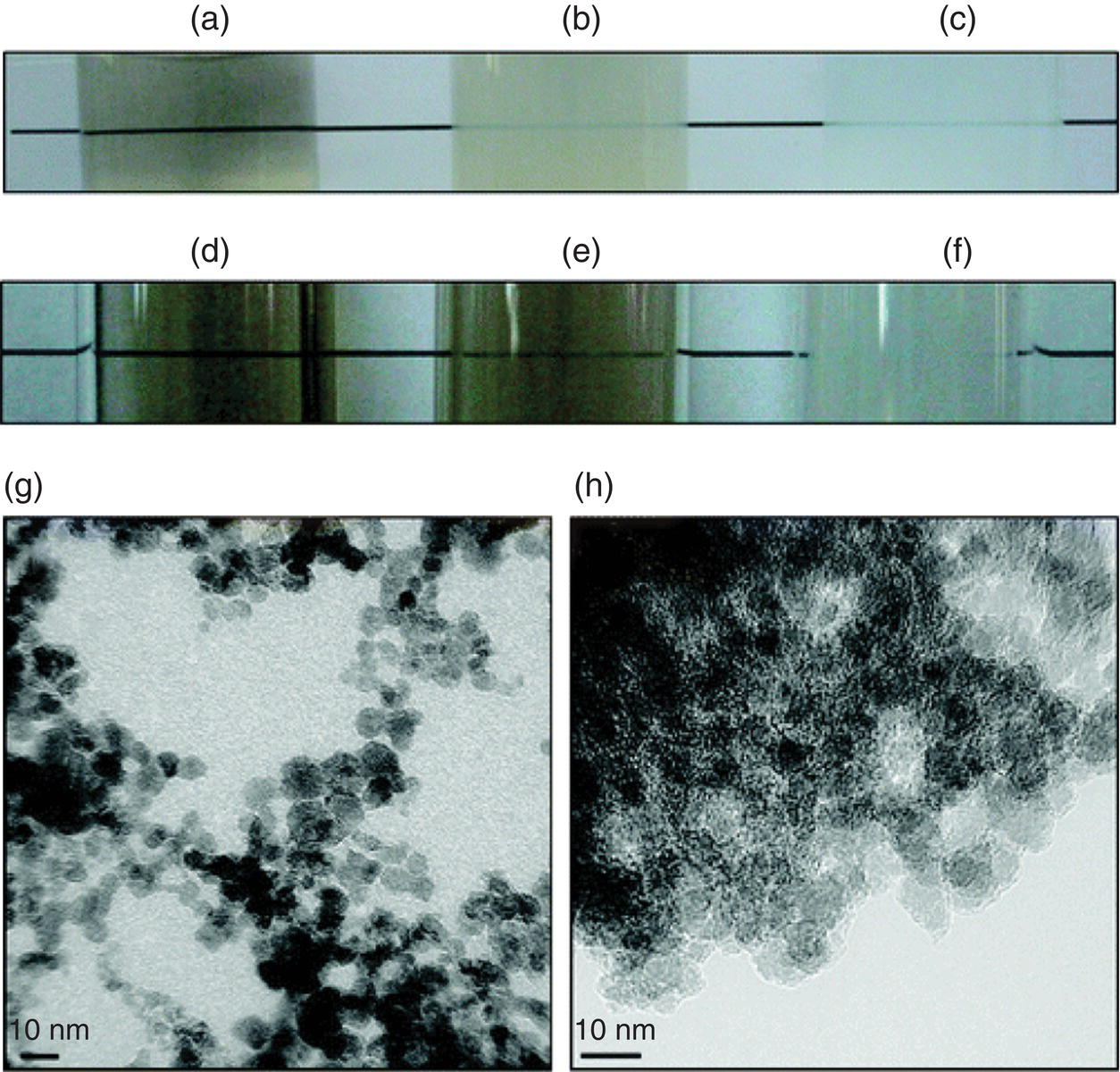
Figure 13.4 Dispersion of Purvalanol A and 4‐hydroxytamoxifen (4‐OHT) in water before and after complexation with monodisperse DNDs. Vials were prepared against background, and the reduction in turbidity mediated by the DNDs was confirmed under the following conditions: (a) 1 mg ml−1 DND in 5% DMSO; (b) 1 mg ml−1 DND, 0.1 mg ml−1 Purvalanol A in 5% DMSO; (c) 0.1 mg ml−1 Purvalanol A in 5% DMSO; (d) 1 mg ml−1 DND in 25% DMSO; (e) 1 mg ml−1 DND, 0.1 mg ml−1 4‐OHT in 25% DMSO; (f) 0.1 mg ml−1 4‐OHT in 25% DMSO. (g, h) TEM images of pristine DNDs (g) and 4‐OHT–DND complexes (h).
Source: Reprinted with permission from Ref. [33]. Reproduced with permission of American Chemical Society.
Someone may ask: Could drug molecules be delivered through covalent conjugation with the carriers? What are the pros and cons of a covalent delivery? Indeed, researchers in the field have also covalently grafted DOX onto the surface of NDs to avoid premature release and enhance its delivery. This was made by covalent conjugation of the particles with DOX and the cell‐penetrating peptide TAT, a HIV trans‐activator of the transcription protein, in sequence by carbodiimide chemistry (Section 4.2.2) [34]. The same approach was further carried out by Zhang et al. [35] who presented a multimodal DND drug delivery system for the targeting, imaging, and enhanced treatment of Paclitaxel (PTX) (Figure 13.1e), a drug commonly used to treat ovarian, breast, and lung cancers. The novelty of this work is that it performed heterofunctionalization of DNDs by grafting fluorescently labeled PTX–DNA conjugates as well as monoclonal antibodies for epidermal growth factor receptor (EGFR) on the same DND surface for specific targeting and imaging purposes. The integration of therapeutics and diagnostics provides a transition from conventional medicine to contemporary personalized and precision medicine, as discussed at the beginning of this chapter.
13.2.2 Proteins
Beyond small molecules, NDs can also play a significant role in protein therapeutics [36]. In a typical application, protein molecules are first loaded on the surface of the carrier, transported, and then released at a specific target site. As pointed out in Section 4.2, care must be taken to conserve their functionalities when loading the biological macromolecules onto NDs. A spectroscopic study of bovine serum albumin (BSA) on DNDs showed that most structural features of the protein were preserved, although the adsorbed BSA might have undergone some minor conformational changes due to the protein‐surface interactions [37]. In another study for lysozyme physically anchored on HPHT‐NDs (~100 nm in diameter), the hydrolytic activity of the adsorbed protein was found to be retained but reduced to 15–70% of the activity of free lysozyme in solution [38]. Similarly, for α‐bungarotoxin, a neurotoxin from Bungarus multicinctus, the protein could maintain its bioactivity even after physical adsorption onto carboxylated HPHT‐NDs [39]. The activity was confirmed by blocking the membrane protein, α‐7‐nicotinic acetylcholine receptor, expressed in oocytes from Xenopus laevis. The same conclusion was reached for rabbit anti‐mouse antibodies covalently immobilized on DNDs [40].
Insulin was the first recombinant human protein therapeutic developed in the 1980s [41]. The therapeutics employs recombinant insulin synthesized by protein engineering to replace its natural counterpart deficient in diabetes mellitus. The human insulin has a molecular mass of 5808 Da, consisting 51 amino acids as shown in Figure 13.5a. Shimkunas et al. [42] explored the feasibility of using monodisperse DNDs for insulin therapy, where the protein molecules served as a potential promoting agent for wound healing and vascularization for severe burns and other possible conditions. The study demonstrated an efficient method for noncovalent conjugation of insulin to the surface of DNDs and, when exposed to an alkaline environment, the attached insulin could be released from the complexes (Figure 13.5b). The research team confirmed the effective binding and release of insulin from the DND carriers through imaging and adsorption/desorption assays. Both cytotoxicity and RT‐PCR analysis revealed that the protein’s functionality remained active after desorption but was inactive when adsorbed onto the DND surface, a behavior attributed to the rapid formation of stable insulin aggregates at interfaces [43, 44]. The result demonstrated that DND could play an effective role in insulin delivery. In addition to insulin, the same research group also investigated the feasibility of using DNDs as a protein delivery vehicle for the transforming growth factor beta antibody (anti‐TGF‐β), which is a potential anti‐scarring agent [45]. While the anti‐TGF‐β‐DND complexes were stable in water, the antibodies could be triggered to release upon their incubation in serum‐containing media. Enzyme‐linked immunosorbent assays (ELISA) verified the preservation of the protein activity after release.
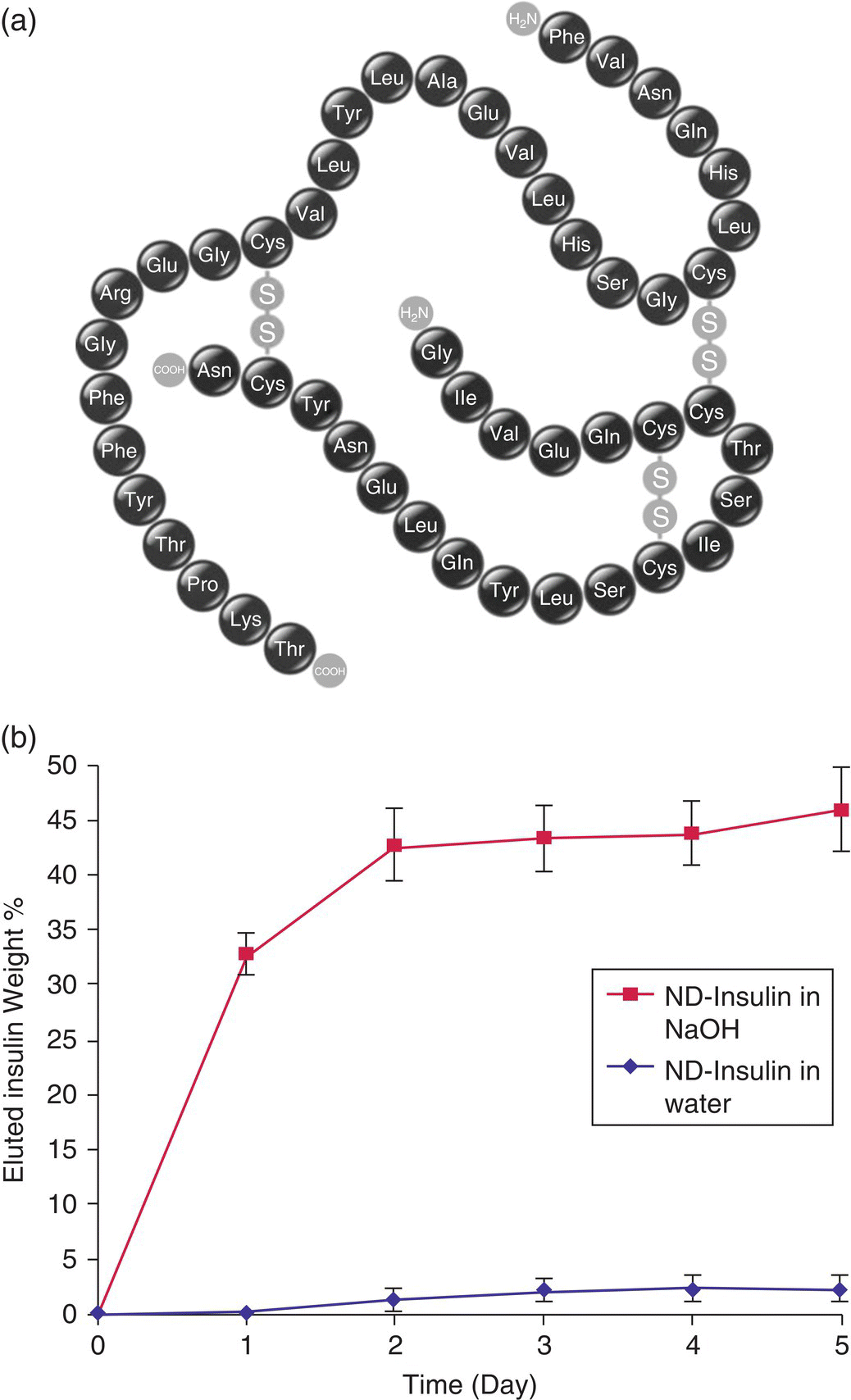
Figure 13.5 (a) Amino acid sequence of human insulin. (b) Five‐day insulin desorption test of ND–insulin complexes treated with NaOH (pH 10.5) and water, showing insulin release in an alkaline pH environment.
Source: Adapted with permission from Ref. [42]. Reproduced with permission of Elsevier.
Pushing the research further along, Moore et al. [46] developed novel nanoparticle suspensions that could be used in oral surgery as injectable alternatives for the delivery of bone morphogenetic proteins (BMP‐2) and low doses of basic fibroblast growth factors (b‐FGF). The study evaluated the efficacy of DNDs for simultaneous and targeted delivery of both proteins as well as their ability to hasten localized bone growth. The high adsorption ability of DNDs allowed BMP‐2 and b‐FGF to be promptly loaded into the DND clusters by physical adsorption, as confirmed by Fourier transform infrared spectroscopy and ELISA. The results successfully demonstrated that DNDs were indeed qualified for the simultaneous delivery of these two functional proteins (BMP‐2 and b‐FGF), both required for bone healing in vivo. All the above studies together support the notion that ND is a useful protein delivery vehicle.
A promising advance in the field is the development of ND‐based vaccines. Nanovaccine, defined as any vaccines containing nanoparticles, is a new kind of immunotherapy. It has attracted considerable interest recently because using nanoparticles for vaccine delivery allows the improvement of antigen stability and the enhancement of antigen immunogenicity [47]. Moreover, it enables targeted delivery and slow release of antigens as discussed in the previous sections. Pham et al. [48] demonstrated the use of NDs to facilitate the development of vaccine against H7N9 viruses with the antigen, hemagglutinin subtype 7 (H7). Hemagglutinin is a glycoprotein on the surface of influenza viruses, responsible for both viral attachment and viral/host membrane fusion. It is included in all currently approved human influenza vaccines. The research team first mixed trimeric H7 with HPHT‐NDs (100 nm in diameter) at a weight ratio of 1 : 12 to form noncovalently bound H7–ND complexes and then determined their activities by the hemagglutination assay [49]. A 64‐fold increase in the activity was found for H7–ND, compared with that of free trimeric H7. Additionally, the H7–ND complex elicited a significantly higher level of H7‐specific antibodies than the free trimeric H7 did after the second and third immunization in mice, as revealed by ELISA and Western blotting. The results suggested an important role of NDs in developing better and more cost‐effective nanovaccines than traditional vaccine formulations.
13.3 Gene Therapy
13.3.1 RNA
Gene therapy is a technique that potentially treats a disease at its genetic roots [50]. The primary goal of gene therapy is to introduce exogenous genetic materials into cells to swap out abnormal genes or to enable additional functions. The conventional approach of gene therapy is to transfect cells with a polymer‐encapsulated DNA plasmid that can replace a defective gene in the target‐cell genome. RNA interference has recently emerged as a new therapeutic pathway that can silence harmful genes by delivering complementary short interfering RNA (siRNA) to the targeted cells [51]. However, the siRNA delivery suffers from many of the same difficulties found in the DNA delivery as discussed in the next section. One of the difficulties is that the actual application of gene therapy to humans must rely on the use of benign and effective carriers for the genetic materials. Researchers have explored an array of materials to address the challenges associated with the delivery. Materials that have been applied include polymers, lipids, peptides, antibodies, aptamers, and small molecules [52]. Most of these compounds ease the delivery of genetic information by encapsulating or condensing nucleic acids into nanosized particles to increase their stability in the bloodstream or facilitate their uptake by cells.
Polyethylenimine (PEI) is one of the most commonly used polymers for nucleic acid delivery. An important feature of PEI is that it contains a high concentration of protonated amines, which makes it suitable for condensing large, negatively charged molecules such as RNA to form polyplexes [53]. The polyplexes first enter cells via endocytosis. Within endosomes, the unprotonated amine groups of PEI absorb protons from the cytosol, leading to an osmotic swelling of the vesicles and an increased influx of Cl− ions and water. The swelling may eventually cause disruption of the endosomal membrane, which subsequently releases the contents into the cytoplasm. This is known as the proton sponge effect [54]. However, the PEI treatment has some toxic side effects, which are directly related to the polymer size and thus limit its practical therapeutic application.
In an effort to establish NDs as a siRNA delivery vehicle, Chen et al. [55] mixed monodisperse DNDs with excess PEI800 to form noncovalently bound DND–PEI complexes, followed by incubation of the complexes with siRNA for the delivery purpose. They chose PEI with a molecular mass of 800 Da (thus, PEI800) for the conjugation to avoid the toxicity effect often accompanied by high‐molecular‐weight PEI to cells. The strong electrostatic interactions between the oppositely charged DND–PEI and siRNA allowed the particle to act as an effective nucleic acid carrier. Examined with both confocal fluorescence microscopy (Figure 13.6a and b) and flow cytometry (Figure 13.6c), knockdown experiments using human breast cancer cells (M4A4) transfected with green fluorescent protein (GFP) showed an efficiency exceeding 20%.
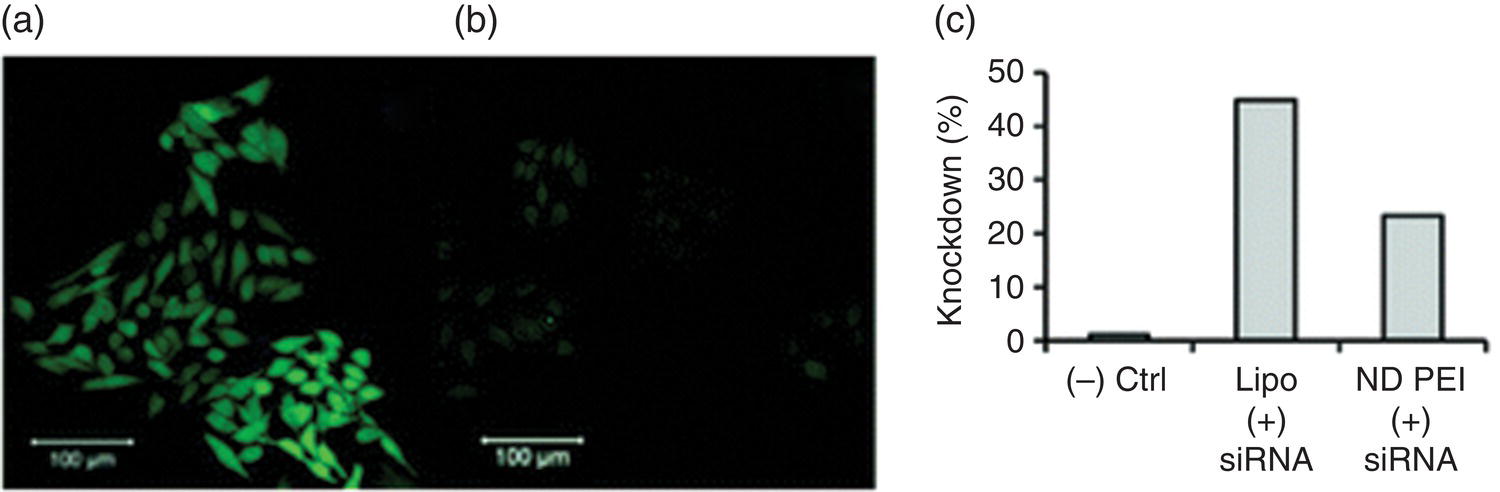
Figure 13.6 (a, b) Confocal fluorescence microscopy and (c) flow cytometric analysis of GFP knockdown in M4A4 cells transfected with GFP‐expressing plasmids. Images shown in (a, b) are that of the negative control (a) and the treatment group with ND‐PEI + siRNA (b). Lipo stands for lipofectamine, a commonly used transfection reagent.
Source: Reprinted with permission from Ref. [55]. Reproduced with permission of American Chemical Society.
Research studies along this line continued the coating of NDs with basic amino acids such as lysine [56] and other cationic polymers such as poly(allylamine hydrochloride) (PAH) [57] through covalent immobilization to carry anionic siRNA. Alhaddad et al. [57] demonstrated the feasibility of using PEI‐ or PAH‐coated FNDs to deliver siRNA into Ewing sarcoma cells. In this experiment, the siRNA sequences able to target the oncogene junction EWS‐Fli1 in the chimeric mRNA were attached to the PEI‐ or PAH‐coated FNDs with an average size of approximately 50 nm. Cellular uptake of the particles was evidenced by the intrinsic fluorescence of FNDs from the hosted color centers. Confocal fluorescence imaging confirmed the colocalization between the FND vectors and siRNA labeled with fluorescein isothiocyanate (FITC) (Figure 13.7a and b). With the use of FNDs, the researchers were able to evaluate the desorption kinetics of siRNA from the carriers in living cells. For cells cultured at five different time points, the signals of FITC were stronger than that of FNDs at the initial stage, a signature that siRNA was bound to both PEI‐ and PAH‐coated carriers (Figure 13.7c). The FITC intensity, however, drastically decreased in the case of PEI coating at 24 hours, while it was only slightly reduced in the PAH coating, indicating a higher affinity of siRNA for PAH‐FNDs. A specific inhibition of the EWS/Fli‐1 gene expression was found at the mRNA and protein levels, proving the efficacy of this method.
13.3.2 DNA
Gene delivery is a process to introduce foreign DNA into host cells. It falls into two categories: viral and nonviral [58]. The former has an unrivaled level of gene transfection efficiency. However, it fails to become an all‐utility vector due to the problems associated with formulation, storage, gene‐carrying capacity, and residual viral elements that can potentially cause insertional mutagenesis, cytotoxicity, immunogenicity, and tumorigenicity. The field of nonviral vectors began as a response to these problems by including biocompatible materials designed and fabricated through innovative synthesis schemes. The preparation of nonviral vectors is relatively easy, less immunogenic and oncogenic, and allows all gene sizes. Nanoparticles have been increasingly popular as a nanoconstruct for the gene delivery [59]. However, because of the complexities involved in the precess, bringing nanoparticle‐based gene therapy from the benchtop to the bedside still requires a comprehensive understanding of the advanced drug and gene delivery systems as well as how to apply nanoparticles in the therapy.
Various surface‐modified NDs have been proposed as potential gene carriers in the literature [60–63]. Specifically, Zhang et al. [60] demonstrated that DNDs surface‐functionalized (either covalently or noncovalently) with PEI800 served well as an effectual plasmid DNA delivery vehicle. The composite material DND‐PEI800 showed low cytotoxicity with a transfection efficiency similar to that of DND‐PEI25k, which had higher toxicity. A favorable property of the cross‐linked ND‐PEI800 was that it bound with plasmid DNA through electrostatic interactions and protected the plasmid from degradation in solution. Compared with PEI800, DND‐PEI800 mediated a 70‐fold upsurge in transfection efficiency while at the same time maintaining good biocompatibility with the cells. Also, the enhancement factor increased to 400 and 800 over that of amine‐ and carboxyl‐terminated NDs, respectively. The transfection efficiency followed the trend of DND‐PEI800 > PEI800 > DND‐NH2 > DND‐COOH > naked DNA. The increased transfection efficiency of ND‐PEI800 over ND‐NH2 was attributed to the proton sponge effect as discussed previously.
A bottleneck of the nanoparticle‐based gene delivery is that the DNA molecules on the carriers must escape from endosomes in order to be available for cells to replicate or express. The proton sponge effect is one of the strategies. Interestingly, with HPHT‐NDs as the carriers, another possible mechanism may exist. It was reported by Chu et al. [62] that prickly FNDs made of HPHT‐NDs could easily enter cells via endocytosis, followed by a quick endosomal escape to the cytoplasm. Confocal fluorescence microscopy and TEM identified endosomal membrane rupturing to be the major route of the particles escaping from the confinement due to the unusual shape effect [64]. Little cytotoxicity was observed for such cytosolic release. The researchers demonstrated a viable application of the method by transfecting HepG2 cells with the GFP gene.
A nice piece of work to demonstrate the feasibility of tracking gene delivery in real time with FNDs was conducted by Petrakova et al. [63], who monitored the DNA transfection and payload release in cells over 12 hours using PEI‐coated FNDs as the carriers (Figure 13.8a). They observed the events based on the changes in the fluorescence spectra of nitrogen‐vacancy centers (including NV− and NV0) hosted in the FNDs of 35 nm in diameter. Specifically, the reduction of the fluorescence intensity at 638 nm relative to that at 576 nm served as an indicator for the release of DNA from the PEI‐coated FND surface (Figure 13.8b). The changes of the NV−/NV0 fluorescence intensity ratios with or without DNA attachment were attributed to the modifications of the surface electric fields coupled with the electronic states of NV centers in the matrix [65]. Their results highlight the potential use of FNDs not only as nontoxic biolabels, but also as non‐photobleachable fluorescent nanosensors to reveal complex intracellular events.
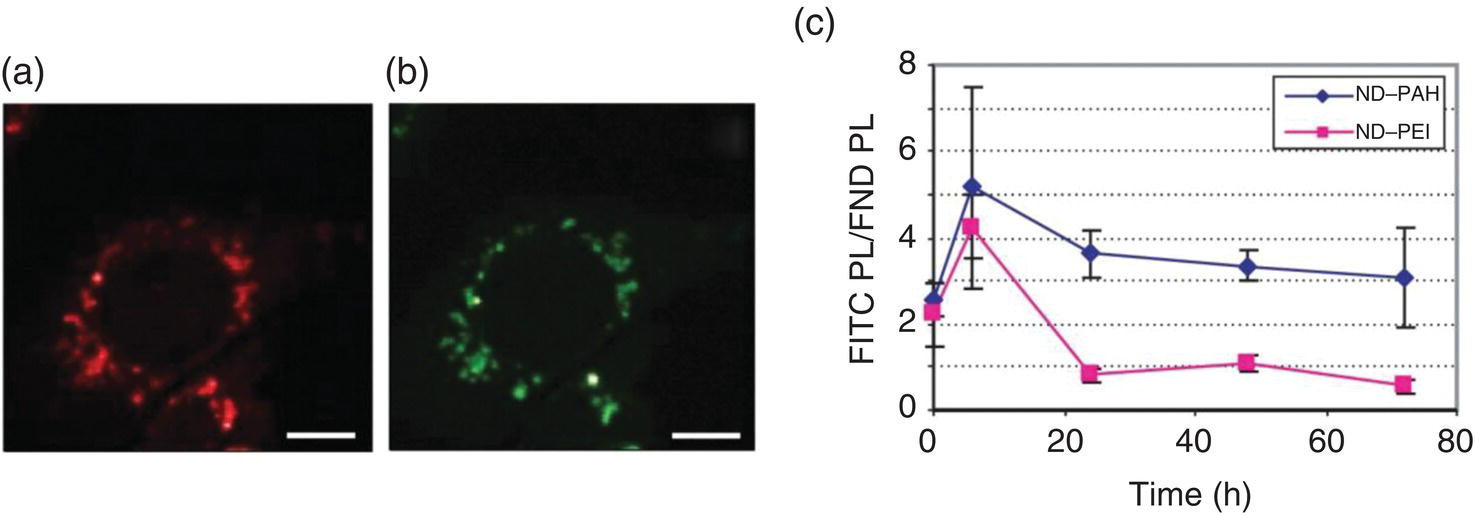
Figure 13.7 Colocalization studies between (a) PEI‐FND vectors and (b) siRNA labeled by FITC in NIH/3T3 EF cells. (c) Quantitative estimate of the siRNA release time, using the photoluminescence intensities (PL) of FITC over the whole cell, normalized to that of FND.
Source: Adapted with permission from Ref. [57]. Reproduced with permission of John Wiley & Sons.
It is now well supported by multiple evidences that the advantages of using FND as a gene delivery device are many‐fold. First, the nanomaterial is highly fluorescent and thus can be used to monitor the transfection process by optical imaging of live cells. Second, being an electron‐dense material, it can be detected by TEM for high‐resolution localization (Section 10.4). Third, the spiky and rough shape of the nanomaterial facilitates endosomal membrane rupturing and escape of the carried nucleic acids in cells.
13.4 Animal Experiments
Mice represent the most commonly used model animals for the efficacy testing of drugs. Compared with other model organisms, mice have several advantages: (i) similar (99%) genome to the humans’, (ii) good genetic/molecular toolboxes available, and (iii) small size that allows large scale and high throughput studies [66]. Liu et al. [67] explored the feasibility of using mouse models to validate the ND‐based therapy. The researchers first demonstrated that HPHT‐NDs covalently linked with PTX could significantly reduce the cell viability of A549 human lung carcinoma cells. The ND–PTX complexes induced both mitotic arrest and apoptosis in A549 cells, in addition to inhibiting tumorigenesis and the formation of lung cancer cells in the xenografts of severe combined immunodeficiency mice. The conjugated chemotherapeutic drug still preserved its anticancer activities, which could induce mitotic blockage, apoptosis, and anti‐tumorigenesis in human lung carcinoma cells.
In the treatment of cancer, chemotherapy resistance is one main obstruction. The resistance often leads to the recurrence of tumors and cross‐resistance against other chemotherapeutic drugs. Along with the rapid development of nanotechnology, scalable and biocompatible nanotherapies have emerged in an effort to overcome the drug resistances while, at the same time, improving the antitumor drug efficacy. Chow et al. [20] reported that ND‐DOX could overcome drug efflux and increase apoptosis in both murine liver and mammary carcinoma cell models. Additionally, the new ND‐DOX system exhibited a lower level of toxicity than the standard DOX treatment in vivo. Furthermore, compared with DOX alone, ND‐DOX significantly prolonged the drug retention in tumor cells with improved safety and efficacy.
Other opportunities for NDs abound, such as in the identification of key extracellular receptors and signaling pathways in cancer research. Researchers in this field are developing targeted drug delivery strategies capable of enhancing efficacy and reducing toxicity in chemotherapy of patients with malignant tumors. For instance, receptor‐targeted therapies are now routinely integrated into the breast cancer chemotherapy programs. To address the receptor‐targeting efficiency, Moore et al. [68] developed ND‐lipid hybrid particles consisting of NDs noncovalently bound with epirubicin (a chemotherapy drug) and then encapsulated in a lipid double layer containing a small amount of biotinylated lipid molecules, as shown in Figure 4.9. Through biotin–streptavidin interactions, the lipid‐encapsulated particles were then conjugated with biotinylated antibodies that targeted EGFRs on breast cancer cells and tumors implanted in mice. Their result showed a marked improvement in the treatment efficacy and chemotherapeutic tolerance. The scalability, chemical stability, and biocompatibility were all strongly favorable, suggesting a promising future of the approach for chemotherapeutic delivery applications.
Glioblastoma is the most common and lethal malignant brain tumor today [69]. Because of the difficulty of penetrating the blood–brain barrier (BBB) with standard drugs and their poor retention in conventional treatments, researchers have been investigating alternative options. To overcome the obstacles in treating glioblastoma, Xi et al. [24] applied a convection‐enhanced delivery method to administer DOX adsorbed on monodisperse DNDs. In this experiment, drugs were delivered through catheter(s) placed stereotactically directly within the tumor mass, around the tumor, or in the resection cavity of glioma‐bearing rats to bypass the BBB. The conjugation with DNDs was proven to enhance the DOX uptake and retention in the glioma cell line and in normal rodent parenchyma (cf., captions of Figure 13.9 for details). Furthermore, through the convection‐enhanced delivery, the conjugation with NDs successfully localized the toxicity and significantly prolonged the killing efficacy of DOX to the brain tumors.

Figure 13.8 Imaging DNA release from the FND–PEI–DNA complexes in IC‐21 macrophages. (a) Confocal fluorescence images of the cells incubated with FND–PEI–DNA for 30 minutes, where DNA was labeled with fluorescein amidite (FAM) before forming complexes with FND–PEI. (b) Fluorescence spectra of NV centers measured by 532 nm laser excitation, measured for FND–PEI–DNA in water, medium, and cells after incubation for 30, 60, and 120 minutes. TDI, transmission/bright field image.
Source: Adapted with permission from Ref. [63].
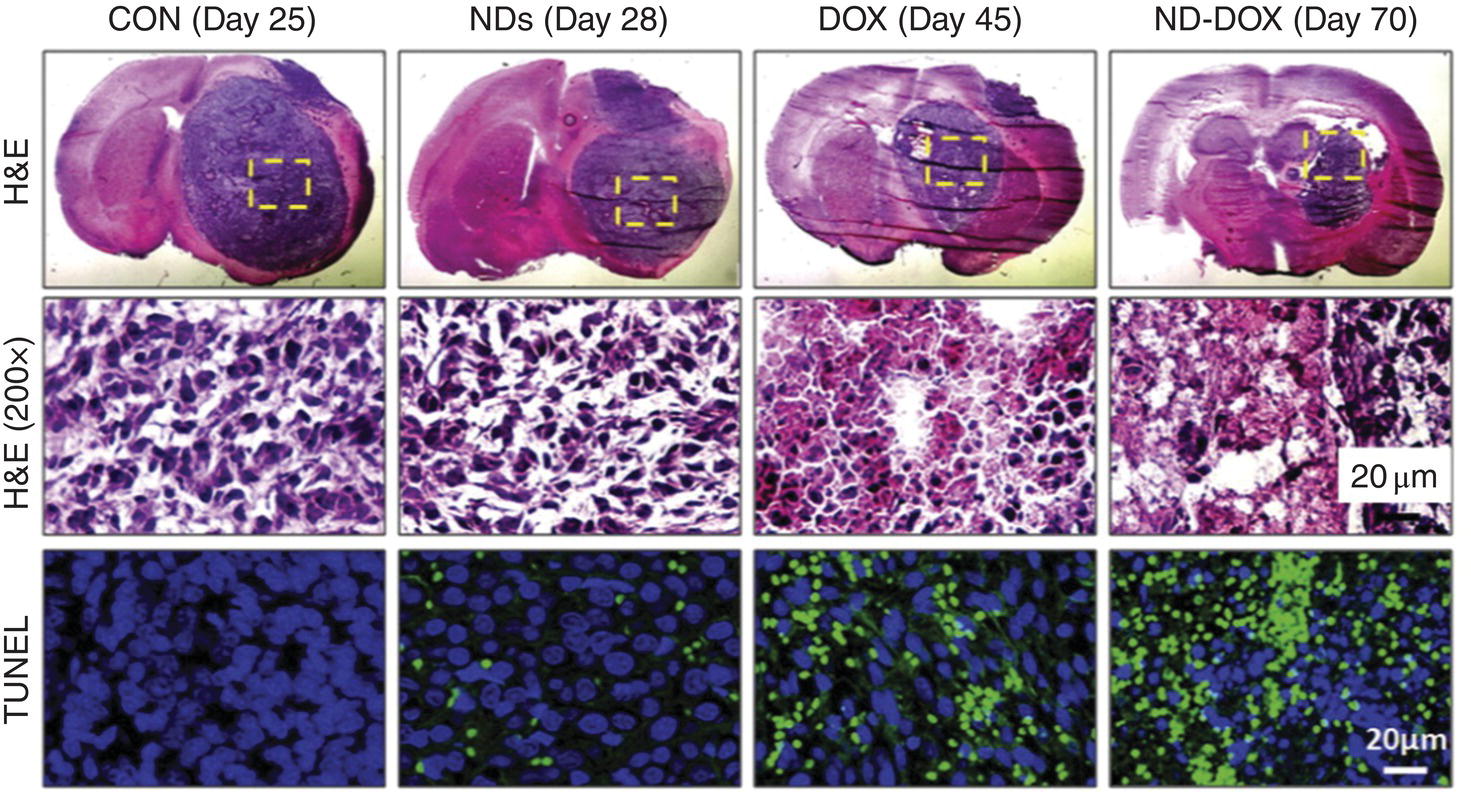
Figure 13.9 Improving glioblastoma therapy efficiency via convection‐enhanced delivery of DND‐DOX. (Top) Representative images of H&E staining from each treatment group, showing tumor size regression at the indicated day after tumor inoculation in glioma‐bearing rats. CON: 0.9% saline; NDs: 5 mg ml−1 DNDs, DOX: 1 mg ml−1 DOX; DND‐Dox: 5 mg ml−1 DND and 1 mg ml−1 DOX. (Middle) 200 × magnification of boxed areas. (Bottom) Apoptotic cell death, measured by TUNEL staining of the boxed areas. Nuclei were counterstained with DAPI (blue). H&E, hematoxylin and eosin; TUNEL, terminal deoxynucleotidyl transferase mediated dUTP nick end labeling; DAPI, 4’,6‐diamidino‐2‐phenylindole.
Source: Reprinted with permission from Ref. [24]. Reproduced with permission of Elsevier.
Finally, as a footnote, for readers who are interested in delving further into the applications of NDs for personalized and precision medicine, please refer to the review article of Ho et al. [70].
References
- 1 Collins, F.S. and Varmus, H. (2015). A new initiative on precision medicine. N Engl J Med 372: 793–795.
- 2 The White House, President Barack Obama (2016). The precision medicine initiative. https://obamawhitehouse.archives.gov/node/333101 (accessed 16 April 2018).
- 3 European Science Foundation’s (2005). Forward look nanomedicine: an EMRC consensus opinion. www.esf.org (accessed 16 April 2018).
- 4 Duncan, R. and Gaspar, R. (2011). Nanomedicine(s) under microscope. Mol Pharm 8: 2101–2141.
- 5 The Oxford Dictionaries (2016). The Oxford Dictionaries. Oxford: Oxford University Press.
- 6 Desai, N. (2012). Challenges in development of nanoparticle‐based therapeutics. AAPS J 14: 282–295.
- 7 Bae, Y.H. and Park, K. (2011). Targeted drug delivery to tumors: myths, reality and possibility. J Control Release 153: 198–205.
- 8 De Jong, W.H. and Borm, P.J.A. (2008). Drug delivery and nanoparticles: applications and hazards. Int J Nanomedincine 3: 133–149.
- 9 Mochalin, V.N., Shenderova, O., Ho, D., and Gogotsi, Y. (2012). The properties and applications of nanodiamonds. Nat Nanotechnol 7: 11–23.
- 10 Krüger, A., Kataoka, F., Ozawa, M. et al. (2005). Unusually tight aggregation in detonation nanodiamonds: identification and disintegration. Carbon 43: 1722–1730.
- 11 Osawa, E. (2008). Monodisperse single nanodiamond particles. Pure Appl Chem 80: 1365–1379.
- 12 Slowing, I.I., Vivero‐Escoto, J.L., Wu, C.W., and Lin, V.S. (2008). Mesoporous silica nanoparticles as controlled release drug delivery and gene transfection carriers. Adv Drug Deliv Rev 60: 1278–1288.
- 13 Vaijayanthimala, V. and Chang, H.C. (2009). Functionalized fluorescent nanodiamonds for biomedical applications. Nanomedicine 4: 47–55.
- 14 Vaijayanthimala, V., Lee, D.K., Kim, S.V. et al. (2015). Nanodiamond‐mediated drug delivery and imaging: challenges and opportunities. Expert Opin Drug Deliv 12: 735–749.
- 15 Koo, O.M., Rubinstein, I., and Onyuksel, H. (2005). Role of nanotechnology in targeted drug delivery and imaging: a concise review. Nanomedicine 1: 193–212.
- 16 Chow, E.K. and Ho, D. (2013). Cancer nanomedicine: from drug delivery to imaging. Sci Transl Med 5: 216rv4.
- 17 Huang, H., Pierstorff, E., Osawa, E., and Ho, D. (2007). Active nanodiamond hydrogels for chemotherapeutic delivery. Nano Lett 7: 3305–3314.
- 18 Kelkar, S.S. and Reineke, T.M. (2011). Theranostics: combining imaging and therapy. Bioconjugate Chem 22: 1879–1903.
- 19 Pierstorff, E. and Ho, D. (2008). Nanomembrane‐driven co‐elution and integration of active chemotherapeutic and anti‐inflammatory agents. Int J Nanomedicine 3: 425–433.
- 20 Lam, R., Chen, M., Pierstorff, E. et al. (2008). Nanodiamond‐embedded microfilm devices for localized chemotherapeutic elution. ACS Nano 2: 2095–2102.
- 21 Adnan, A., Lam, R., Chen, H. et al. (2011). Atomistic simulation and measurement of pH dependent cancer therapeutic interactions with nanodiamond carrier. Mol Pharm 8: 368–374.
- 22 Chow, E.K., Zhang, X.Q., Chen, M. et al. (2011). Nanodiamond therapeutic delivery agents mediate enhanced chemoresistant tumor treatment. Sci Transl Med 3: 73ra21.
- 23 Man, H.B., Kim, H., Kim, H.J. et al. (2014). Synthesis of nanodiamond‐daunorubicin conjugates to overcome multidrug chemoresistance in leukemia. Nanomedicine 10: 359–369.
- 24 Xi, G., Robinson, E., Mania‐Farnell, B. et al. (2014). Convection‐enhanced delivery of nanodiamond drug delivery platforms for intracranial tumor treatment. Nanomedicine 10: 381–391.
- 25 Lam, R. and Ho, D. (2009). Nanodiamonds as vehicles for systemic and localized drug delivery. Expert Opin Drug Deliv 6: 883–895.
- 26 Yan, J.J., Guo, Y., Altawashi, A. et al. (2012). Experimental and theoretical evaluation of nanodiamonds as pH triggered drug carriers. New J Chem 36: 1479–1484.
- 27 Zhang, X.Y., Wang, S.Q., Fu, C.K. et al. (2012). PolyPEGylated nanodiamond for intracellular delivery of a chemotherapeutic drug. Polym Chem 3: 2716–2719.
- 28 Wang, D.X., Tong, Y.L., Li, Y.Q. et al. (2013). PEGylated nanodiamond for chemotherapeutic drug delivery. Diam Relat Mater 36: 26–34.
- 29 Karukstis, K., Thompson, E., Whiles, J., and Rosenfeld, R. (1998). Deciphering the fluorescence signature of daunomycin and doxorubicin. Biophys Chem 73: 249–263.
- 30 Wang, D., Li, Y., Tian, Z. et al. (2014). Transferrin‐conjugated nanodiamond as an intracellular transporter of chemotherapeutic drug and targeting therapy for cancer cells. Ther Deliv 5: 511–524.
- 31 Guan, B., Zou, F., and Zhi, J.F. (2010). Nanodiamond as the pH‐responsive vehicle for an anticancer drug. Small 6: 1514–1519.
- 32 Li, J., Zhu, Y., Li, W. et al. (2010). Nanodiamonds as intracellular transporters of chemotherapeutic drug. Biomaterials 31: 8410–8418.
- 33 Chen, M., Pierstorff, E.D., Lam, R. et al. (2009). Nanodiamond‐mediated delivery of water‐insoluble therapeutics. ACS Nano 3: 2016–2022.
- 34 Li, X.X., Shao, J.Q., Qin, Y. et al. (2011). TAT‐conjugated nanodiamond for the enhanced delivery of doxorubicin. J Mater Chem 21: 7966–7973.
- 35 Zhang, X.Q., Lam, R., Xu, X. et al. (2011). Multimodal nanodiamond drug delivery carriers for selective targeting, imaging, and enhanced chemotherapeutic efficacy. Adv Mater 23: 4770–4775.
- 36 Leader, B., Baca, Q.J., and Golan, D.E. (2008). Protein therapeutics: a summary and pharmacological classification. Nat Rev Drug Discov 7: 21–39.
- 37 Wang, H.D., Niu, C.H., Yang, Q., and Badea, I. (2011). Study on protein conformation and adsorption behaviors in nanodiamond particle‐protein complexes. Nanotechnology 22: 145703.
- 38 Nguyen, T.T.B., Chang, H.C., and Wu, V.W.K. (2007). Adsorption and hydrolytic activity of lysozyme on diamond nanocrystallites. Diam Relat Mater 16: 872–876.
- 39 Liu, K.K., Chen, F., Chen, P.Y. et al. (2008). Alpha‐bungarotoxin binding to target cell in a developing visual system by carboxylated nanodiamond. Nanotechnology 19: 205102.
- 40 Purtov, K.V., Petunin, A.I., Burov, A.E. et al. (2010). Nanodiamonds as carriers for address delivery of biologically active substances. Nanoscale Res Lett 5: 631–636.
- 41 Carter, P.J. (2011). Introduction to current and future protein therapeutics: a protein engineering perspective. Exp Cell Res 317: 1261–1269.
- 42 Shimkunas, R.A., Robinson, E., Lam, R. et al. (2009). Nanodiamond‐insulin complexes as pH‐dependent protein delivery vehicles. Biomaterials 30: 5720–5728.
- 43 Li, S. and Leblanc, R.M. (2014). Aggregation of insulin at the interface. J Phys Chem B 118: 1181–1188.
- 44 Lin, C.L., Lin, C.H., Chang, H.C., and Su, M.C. (2015). Protein attachment on nanodiamonds. J Phys Chem A 119: 7704–7711.
- 45 Smith, A.H., Robinson, E.M., Zhang, X.Q. et al. (2011). Triggered release of therapeutic antibodies from nanodiamond complexes. Nanoscale 3: 2844–2848.
- 46 Moore, L., Gatica, M., Kim, H. et al. (2013). Multi‐protein delivery by nanodiamonds promotes bone formation. J Dent Res 92: 976–981.
- 47 Zhao, L., Seth, A., Wibowo, N. et al. (2014). Nanoparticle vaccines. Vaccine 32: 327–337.
- 48 Pham, N.B., Ho, T.T., Nguyen, G.T. et al. (2017). Nanodiamond enhances immune responses in mice against recombinant HA/H7N9 protein. J Nanobiotechnol 15: 69.
- 49 Killian, M.L. (2008). Hemagglutination assay for the avian influenza virus. Methods Mol Biol 436: 47–52.
- 50 Naldini, L. (2015). Gene therapy returns to centre stage. Nature 526: 351–360.
- 51 Pai, S.I., Lin, Y.Y., Macaes, B. et al. (2006). Prospects of RNA interference therapy for cancer. Gene Ther 13: 464–477.
- 52 Kanasty, R., Dorkin, J.R., Vegas, A., and Anderson, D. (2013). Delivery materials for siRNA therapeutics. Nat Mater 12: 967–977.
- 53 Dunlap, D.D., Maggi, A., Soria, M.R., and Monaco, L. (1997). Nanoscopic structure of DNA condensed for gene delivery. Nucleic Acids Res 25: 3095–3101.
- 54 Behr, J. (1997). The proton sponge: a trick to enter cells the viruses did not exploit. Chimia 51: 34–36.
- 55 Chen, M., Zhang, X.Q., Man, H.B. et al. (2010). Nanodiamond vectors functionalized with polyethylenimine for siRNA delivery. J Phys Chem Lett 1: 3167–3171.
- 56 Alwani, S., Kaur, R., Michel, D. et al. (2016). Lysine‐functionalized nanodiamonds as gene carriers: development of stable colloidal dispersion for in vitro cellular uptake studies and siRNA delivery application. Int J Nanomedicine 11: 687–702.
- 57 Alhaddad, A., Adam, M.P., Botsoa, J. et al. (2011). Nanodiamond as a vector for siRNA delivery to Ewing sarcoma cells. Small 7: 3087–3095.
- 58 Mintzer, M.A. and Simanek, E.E. (2009). Nonviral vectors for gene delivery. Chem Rev 109: 259–302.
- 59 Jin, S., Leach, J.C., and Ye, K. (2009). Nanoparticle‐mediated gene delivery. Methods Mol Biol 544: 547–557.
- 60 Zhang, X.Q., Chen, M., Lam, R. et al. (2009). Polymer‐functionalized nanodiamond platforms as vehicles for gene delivery. ACS Nano 3: 2609–2616.
- 61 Martin, R., Alvaro, M., Herance, J.R., and Garcia, H. (2010). Fenton‐treated functionalized diamond nanoparticles as gene delivery system. ACS Nano 4: 65–74.
- 62 Chu, Z., Miu, K., Lung, P. et al. (2015). Rapid endosomal escape of prickly nanodiamonds: implications for gene delivery. Sci Rep 5: 11661.
- 63 Petrakova, V., Benson, V., Buncek, M. et al. (2016). Imaging of transfection and intracellular release of intact, non‐labeled DNA using fluorescent nanodiamonds. Nanoscale 8: 12002–12012.
- 64 Chu, Z., Zhang, S., Zhang, B. et al. (2014). Unambiguous observation of shape effects on cellular fate of nanoparticles. Sci Rep 4: 4495.
- 65 Petrakova, V., Taylor, A., Kratochvílova, I. et al. (2012). Luminescence of nanodiamond driven by atomic functionalization: towards novel detection principles. Adv Funct Mater 22: 812–819.
- 66 Vandamme, T.F. (2014). Use of rodents as models of human diseases. J Pharm Bioallied Sci 6: 2–9.
- 67 Liu, K.K., Zheng, W.W., Wang, C.C. et al. (2010). Covalent linkage of nanodiamond‐paclitaxel for drug delivery and cancer therapy. Nanotechnology 21: 315106.
- 68 Moore, L., Chow, E.K., Osawa, E. et al. (2013). Diamond‐lipid hybrids enhance chemotherapeutic tolerance and mediate tumor regression. Adv Mater 25: 3532–3541.
- 69 Davis, M.E. (2016). Glioblastoma: overview of disease and treatment. Clin J Oncol Nurs 20: S2–S8.
- 70 Ho, D., Wang, C.H., and Chow, E.K. (2015). Nanodiamonds: the intersection of nanotechnology, drug development, and personalized medicine. Sci Adv 1: e1500439.
