5
Biocompatibility of Nanodiamonds
“A diamond is forever,” De Beers’ diamond campaign launched on the New York Times, September 1948, is the same slogan still used today [1]. Funny as it may sound, if asked, scientists working on nanodiamonds (NDs) would say the same. Scientifically, the slogan vividly depicts the exceptionally high physical and chemical stability of the gemstone under ambient conditions. However, some may be wondering: Stable, yes, but is it safe to use? How to tell if it is safe for humans? Can we measure safety in a quantitative way and, if so, how? These are some questions that we try to answer in this chapter.
Diamond, categorized as an inorganic material, is considered both chemically inert and biologically compatible because it is composed of pure sp3‐hybridized carbon atoms, except those on the surface. According to International Union of Pure and Applied Chemistry (IUPAC), the term “biological compatibility” or “biocompatibility” in short is defined in a general context as “the ability to be in contact with a living system without producing an adverse effect” or, in the context of medical therapy, “the ability of a material to perform with an appropriate host response in a specific application” [2]. The exceptionally low chemical reactivity of diamond is well in line with the first definition. This characteristic, together with the facts that diamond can be synthesized by chemical vapor deposition methods for coating of biomedical devices and the surface of diamond can be readily derivatized with various functional groups for bioconjugation, has earned diamond the name “Biomaterial of the 21st century” [3].
Focusing on nanoscale diamonds, we discuss in this chapter the biocompatibility studies of NDs and their comparisons with other members in the nanocarbon family (Section 1.2). How NDs can be used to perform specific medical applications will be the subject of discussion for Chapter 13. Here, we begin with a brief review of some representative protocols used in research laboratories to assess the safety of nanoparticles in cells and organisms.
5.1 Biocompatibility Testing
The purpose of testing for biocompatibility is to determine whether or not a substance or material when introduced to a living host would harm or even kill the host. The events can occur at the cellular (cyto) or animal levels. To examine the damages to cells, for example, we may want to test “cytotoxicity” by measuring the proportion of cells suffered from the introduction of a certain substance under study to the sample. A quantitative analysis of this kind will provide a fair comparison for the biocompatibility of different materials in a study. Several well‐established assays are discussed here, some being more specific than the others, and all together serve as a general tool to evaluate how safe a material is to use in a biological system.
5.1.1 Cytotoxicity
Cultured cells grown under controlled conditions are simple living systems routinely used for assessing the biocompatibility of a material under study in vitro [4]. The cells can be put through various testing procedures with the chemicals of interest in laboratories, allowing for detailed evaluation of the cytotoxicity or irritancy potential of the material being tested. They also provide an excellent platform for any chemicals and materials prior to in vivo studies. Immortal cell lines are the most commonly used cells for testing. In contrast to normal cells, which have a limited lifespan in culture, these cell lines are derived from multicellular organisms that have undergone mutations to become “immortal” [5]. They can continue to divide and grow for a prolonged period of time in culture. The constant supply of almost identical cells makes immortal cell lines an important tool for research in biochemistry and cell biology.
A major pathway by which a cell dies is necrosis [6], which is a form of cell damage so severe that it eventually leads to a loss of plasma membrane integrity. Some organic dyes such as trypan blue, propidium iodide, and ethidium homodimer are impermeant to healthy cells but can enter membrane‐compromised cells [7]. They are capable of distinguishing live cells from dead cells. Trypan blue (Figure 5.1a), for example, is negatively charged at neutral pH and thus does not interact with the membrane of live cells. When stained by this molecule, dead cells show a distinctive blue color and can be quantified colorimetrically at 607 nm. Propidium iodide and ethidium homodimer (Figure 5.1b and c), on the other hand, are positively charged fluorescent probes at neutral pH. Both dye molecules can bind to nucleic acids by intercalating between base pairs, yielding nuclear‐localized red fluorescence (emission maximum at ~620 nm) with an enhanced intensity by more than one order of magnitude. The technique is suitable for the identification and quantification of dead or damaged cells by fluorescence microscopy, flow cytometry, and fluorometry.
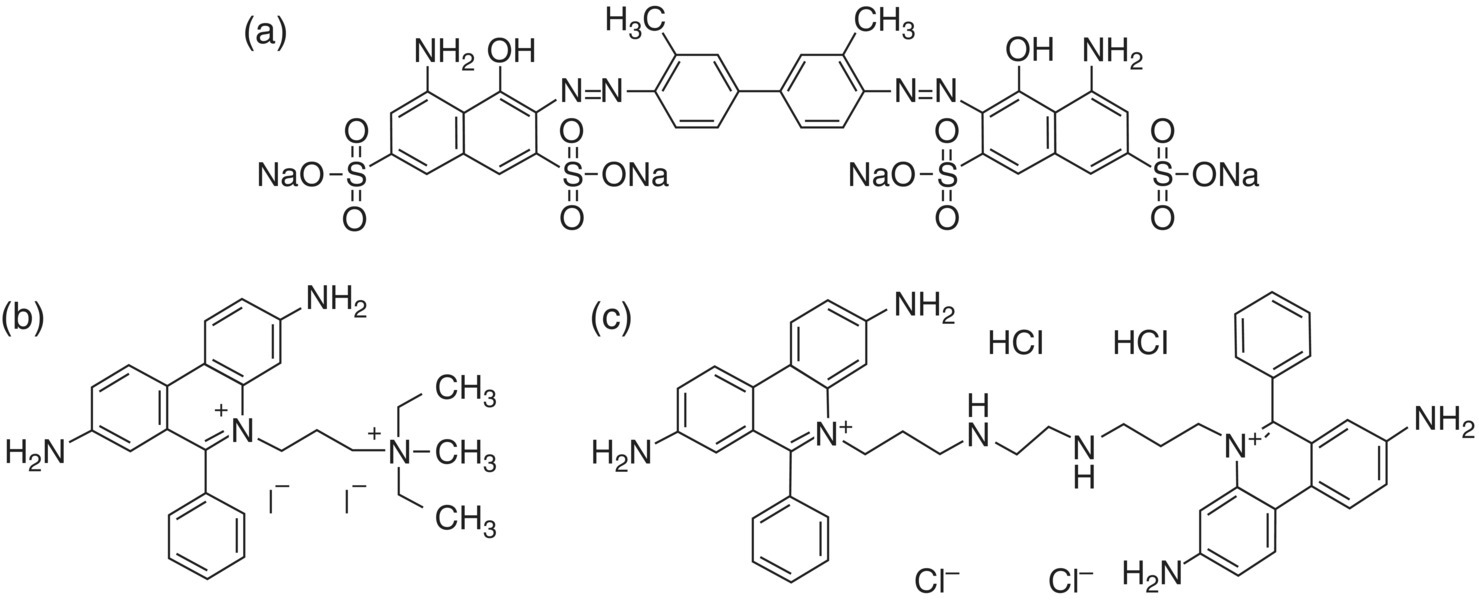
Figure 5.1 Molecular structures of (a) trypan blue, (b) propidium iodide, and (c) ethidium homodimer.
Aside from using nucleic acid stains, the cytotoxicity testing of a nanomaterial can also be conducted by measuring reactive oxygen species (ROS) [6]. The oxygen‐containing species, such as peroxide, superoxide, hydroxyl radical, or singlet oxygen, are routine byproducts of metabolism in biological systems. The ROS level can significantly increase in the presence of environmental stresses (e.g. ultraviolet or heat exposure) that may cause cellular damage to lipids, proteins, and nucleic acids, leading to a myriad of pathological disorders and diseases [8]. A commonly used probe to detect intracellular ROS production is 2′,7′‐dichlorodihydrofluorescein diacetate, a membrane‐permeant molecule. Upon cleavage of the acetate groups by esterases and subsequent oxidation in cells, the nonfluorescent probe is converted to the highly fluorescent 2′,7′‐dichlorofluorescein that can be detected at 514 nm [9].
Apoptosis is another pathway of cell death. It is genetically regulated and occurs in multicellular organisms [6]. Cells undergoing the final stages of apoptosis often display death‐signaling molecules such as phosphatidylserine on their cell surface for phagocytic recognition [10]. The apoptotic cells can be recognized with a Ca2+‐dependent phospholipid‐binding protein called annexin V, which has a high affinity for phosphatidylserine [11]. The assay using fluorescence‐tagged annexin V as the probe can be combined with propidium iodide to distinguish viable cells from apoptotic cells and necrotic cells in a population.
The second method useful for the identification and quantification of apoptotic/necrotic cells is the terminal deoxynucleotidyl transferase mediated dUTP nick end labeling (TUNEL) assay [12]. The method detects DNA fragmentation by labeling the terminal end of nucleic acids with modified dUTPs using terminal deoxynucleotidyl transferase. The modified dUTPs can be later fluorescence‐labeled and probed, allowing for ensuing analysis by both fluorescence microscopy and flow cytometry.
A good indicator of cell health is the cell viability or the cell proliferation rate. The cell viability, defined as the number of healthy cells in a sample, can be measured by several different ways. A commonly used method is the MTT assay, which measures the activity of mitochondrial reductase that reduces the tetrazolium salt (e.g., 3‐[4,5‐dimethylthiazol‐2‐yl]‐2,5‐diphenyltetrazolium bromide, MTT) to formazan (Figure 5.2). After dissolving the reduction end product in acidified isopropyl alcohol [13], the resulting purple solution is measured spectrophotomerically for the absorbance at 570 nm. A more recent advance in the field to measure the cell proliferation is to monitor the impedance of cells cultured on growth‐compatible microplates with prepatterned gold electrodes at the bottom surface [14]. Measurement of the impedance changes over time provides high‐temporal resolution readouts of the cell growth as well as the attachment characteristics.
![Reaction schematic of MTT assay involving the reduction of 3‐[4,5‐dimethylthiazol‐2‐yl]‐2,5‐ diphenyltetrazolium bromide to 1‐(4,5‐dimethylthiazol‐2‐yl)‐3,5‐diphenylformazan by mitochondrial reductase.](http://images-20200215.ebookreading.net/5/2/2/9781119477082/9781119477082__fluorescent-nanodiamonds__9781119477082__images__c05f002.gif)
Figure 5.2 The MTT assay involving the reduction of 3‐[4,5‐dimethylthiazol‐2‐yl]‐2,5‐diphenyltetrazolium bromide (left) to 1‐(4,5‐dimethylthiazol‐2‐yl)‐3,5‐diphenylformazan (right) by mitochondrial reductase.
5.1.2 Genotoxicity
Genotoxicity is a destructive effect caused by chemical agents that damage the genetic makeup within a cell. The damage can occur in either somatic or germline cells, leading to mutations and possibly cancers. One of the most common tests for genotoxicity is single‐cell gel electrophoresis, known as the comet assay [15]. The technique involves lysing cells using detergent and high‐concentration salt to form nucleoids containing supercoiled loops of DNA linked to the nuclear matrix. The lysed cells are then analyzed by gel electrophoresis, yielding comet‐like structures for the individual cells. The comet heads are contributed mainly by undamaged DNA strands, whereas the tails represent DNA fragments that are drawn faster than the intact DNA towards the positively charged electrode. The number of DNA breaks is finally estimated by measuring the fluorescence intensity of the comet tail relative to that of the head after staining and visualization under a microscope.
The micronucleus assay is another useful tool for screening potentially genotoxic compounds [16]. Micronuclei are small membrane‐bound DNA fragments formed during the metaphase‐to‐anaphase transition of cell division. They could originate from acentric chromosome fragments or whole chromosomes that are unable to migrate with the rest of the chromosomes during the anaphase. A positive result from the assay, i.e. more micronuclei found in the treatment groups than in the control groups, is an indication that the tested substance induces chromosomal damage.
5.1.3 Hemocompatibility
Materials used in medical therapy are often in direct contact with blood (hemo) and, therefore, they must be assessed for hemocompatibility to establish their safety. A complete list of the assays to evaluate the blood compatibility of nanoparticles can be found in the literature [17]. Here, we focus only on three major issues pertaining to the hemocompatibility, including (i) disruption of blood cells (hemolysis), (ii) activation of the coagulation pathways (thrombogenicity), and (iii) increase of the cytokine levels in blood (inflammation).
Human erythrocytes, or red blood cells (RBCs), are typical samples used to assess the hemolytic activity of a material [18]. When RBCs are in direct or indirect contact with the materials being tested, hemolysis may be caused by either physical interactions or chemical reactions of the cells with toxins, metal ions, or other compounds. As a result, the erythrocytes are destroyed and the hemoglobin contained within now released. After centrifuging the blood sample, one can easily separate the freed hemoglobin (in supernatant) from the lysed erythrocytes (in precipitate). The heme group in hemoglobin is known to have a strong absorption band, peaking at 410 nm, which is responsible for the deep red‐color in blood. Spectrophotometric measurement of the heme absorption allows quantification for the concentrations of hemoglobin in the supernatant. With water‐treated erythrocytes as the positive control and saline‐treated RBCs as the negative control, one can calculate the hemolysis percentage from the absorbance (A) of hemoglobin at the wavelength (λ) of the measurement as
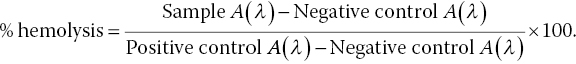
A high percentage of hemolysis signals a high‐risk factor of using the nanomaterial under testing.
Activated partial thromboplastin time (APTT) is a medical test commonly used to evaluate the function of a blood clotting system [19]. The test measures the overall speed at which human blood clots by means of the intrinsic coagulation pathway. It is typically conducted with an APTT kit consisting of phospholipid, a surface activator (e.g. kaolin), and CaCl2. The standard protocol calls for an extraction of plasma from a blood sample. An excess of calcium chloride (in a phospholipid suspension) is then mixed with the plasma and the surface activator is added to activate the intrinsic pathway of coagulation. Finally, the time that the sample takes to clot is optically measured. The APPT test along with the prothrombin time (PT) measurement [19], which evaluates the extrinsic pathway of blood coagulation, allows for detailed characterization of the thrombogenic activity of a nanomaterial.
Cytokines are small glycoproteins (~20 kDa) responsible for cell signaling and cell‐to‐cell communications [6]. They are produced by a broad range of cells including immune cells, endothelial cells, fibroblasts, and various stromal cells. Cytokines are primarily involved in host responses to disease such as infection and inflammation. Interleukins are a class of cytokines acting between leukocytes and other cell types [20]. There are 17 common families of interleukins and some of them are inflammatory mediators including IL‐1, IL‐4, and IL‐6. Evaluation of the cytokine levels in the blood of an animal model (such as a mouse) provides important information for its immune response to the blood‐contacting material. To measure the overall cytokine response elicited, an enzyme‐linked immunosorbent assay (ELISA) [21] is typically used to quantify the number of signal protein molecules in serum with antibodies for colorimetric detection. The antibodies can be either covalently linked to an enzyme (such as horseradish peroxidase) or detected by a secondary antibody linked to the same enzyme through covalent conjugation. An elevating amount of cytokine in the blood sample measured by ELISA is an indication for a higher level of immune response to the material under testing, suggesting a high risk of using it in humans.
5.2 In Vitro Studies
High‐pressure high‐temperature nanodiamonds (HPHT‐NDs) and detonation nanodiamonds (DNDs) are two major types of diamond nanoparticles applied to biological research. DNDs hold great potentials for use as drug delivery devices because of their small size and, therefore, large specific surface area (Chapter 4). HPHT‐NDs, on the other hand, may serve as excellent medical contrast agents due to their imaging capability with built‐in color centers (Chapter 3). We start our discussion with HPHT‐NDs as a step stone toward fluorescent nanodiamonds (FNDs).
5.2.1 HPHT‐ND
Yu et al. [22] was the first group to examine the cytotoxicity of surface‐oxidized HPHT‐NDs in immortal cell lines. The HPHT‐NDs used in the study contained high‐density ensembles of NV centers as fluorophores and thus were called FNDs. Upon feeding human embryonic kidney cells with FNDs (~100 nm in diameter), the researchers found that the particles were avidly taken up by the cells under serum‐free conditions (Figure 5.3). The presence of FNDs in cells was confirmed by detecting the red fluorescence emission from the NV centers. MTT assays showed no noticeable toxicity of the particles at the concentration as high as 100 μg ml−1 (inset in Figure 5.3). Subsequent studies of the cell viability for HeLa cells (a cervical cancer cell line) with MTT assays [23] as well as the cell proliferation (Figure 5.4a) and genotoxicity (Figure 5.4b) of human fibroblasts with impedance sensing and single‐cell gel electrophoresis assays all indicated that the 100‐nm FNDs neither impaired cell growth nor caused DNA damage [24].

Figure 5.3 Confocal fluorescence images of a single 293T human kidney cell after FND uptake. The cross‐sectional image in each three‐dimensional scan (as indicated by the yellow dashed square) has a vertical thickness of 0.25 μm and an area of 42 × 42 μm2. The bright red spots correspond to FNDs. Inset: Cytotoxicity tests with the 293T cells and the MTT reduction assay.
Source: Reprinted with permission from Ref. [22]. Reproduced with permission of American Chemical Society.
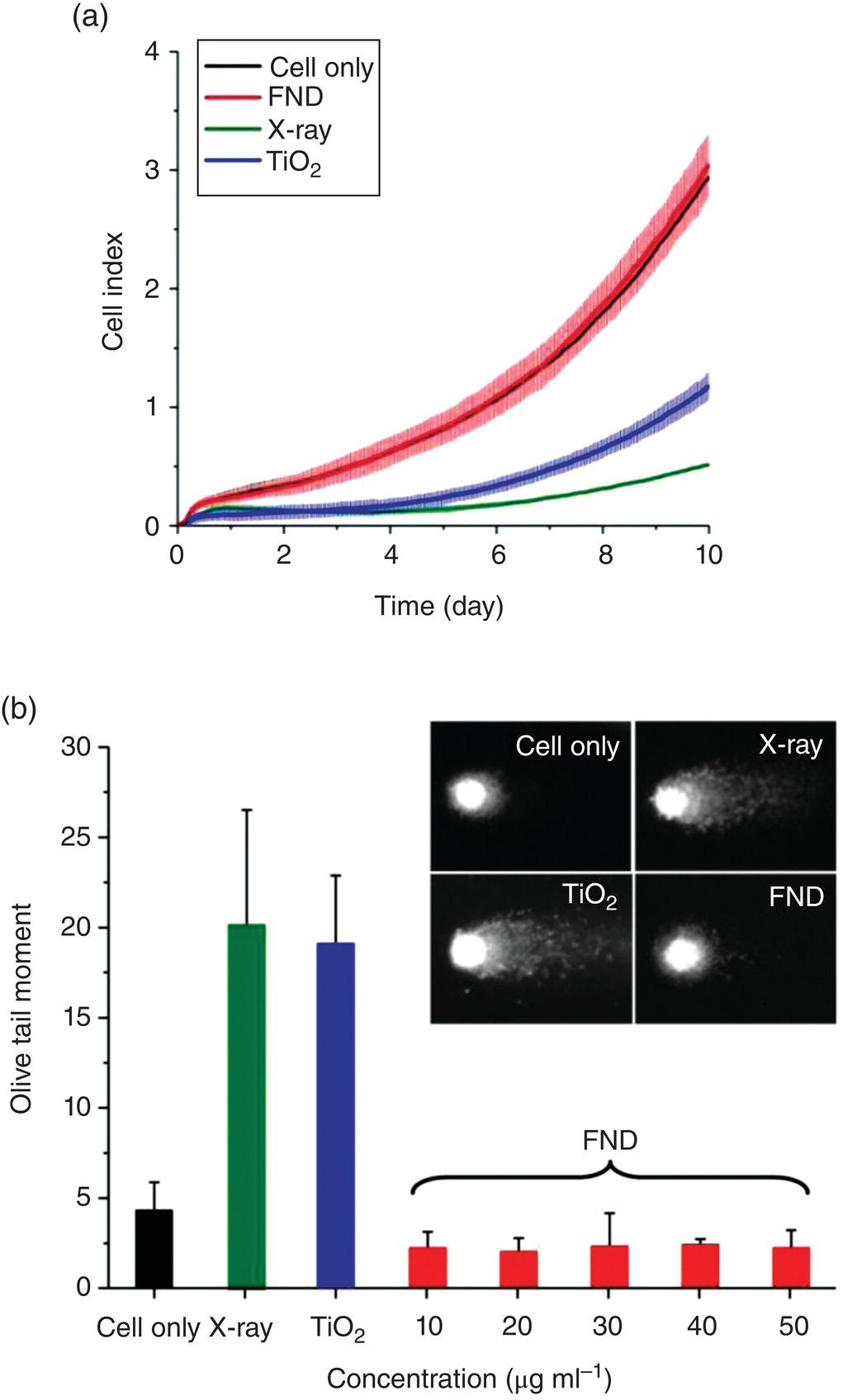
Figure 5.4 (a) Cell proliferation and (b) comet assays of human fibroblasts after treatments with X‐ray, TiO2, and FNDs. The X‐ray and TiO2 treatments served as positive controls.
Source: Adapted with permission from Ref. [24]. Reproduced with permission of John Wiley & Sons.
Paget et al. [25] have recently made a thorough and systematic investigation for the in vitro biocompatibility of HPHT‐NDs. Their work was aimed at a rigorous risk assessment for NDs in human health. The research team examined the cytotoxicity and genotoxicity of two sets of carboxylated HPHT‐NDs with nominal diameters of 20 and 100 nm. Six human cell lines were chosen as representatives of potential target organs: HepG2 and Hep3B (liver), Caki‐1 and HEK293 (kidney), HT29 (intestine), and A549 (lung). The cytotoxicity was assessed by impedance sensing for cell proliferation and flow cytometric analysis for dead cells. The genotoxicity was measured according to the distribution of the number of γ‐H2Ax foci per nucleus, another highly sensitive technique for the study of DNA double‐strand breaks [26]. Their results indicated that the HPHT‐NDs could effectively enter the cells but did not cause any significant cytotoxic or genotoxic effects on the six cell lines even when the dosage went up as high as 250 μg ml−1. Further studies using Chinese hamster ovary cells confirmed that the surface modification of these particles with biomolecules such as peptides [27] did not alter their biocompatibility either.
While HPHT‐NDs have been found to be nontoxic to a wide range of cell lines grown in culture, how they may influence the functions of primary cells remains a concern. Compared to immortal cell lines, primary cells are the more biologically relevant in vitro model. They are isolated directly from tissues and have a finite lifespan and limited expansion capacity. Additionally, they have normal cell morphology and possess many important characteristics originally present in vivo. Two types of primary cells have been employed to address this issue: Mouse lung stem/progenitor cells (LSCs) and mouse embryonal primary neurons. Wu et al. [28] reported that the labeling of LSCs with 100‐nm FNDs did not eliminate the cells’ abilities of self‐renewal and differentiation into type I and type II pneumocytes. Huang et al. [29] reported that the FND labeling did not cause any noticeable toxicity in primary neurons derived from either central or peripheral nervous systems. However, a decrease of the neurite length in both types of the cells was found, which was attributed to the spatial hindrance by the FND particles in advancing axonal growth cones.
In a separate study, FNDs have also been tested for their effects on the differentiation of embryonal carcinoma stem (ECS) cells, e.g. mouse P19 and human NT2/D1 ECS cells, into neuronal cells [30]. It was found that the 100‐nm FNDs could be effectively internalized by the ECS cells, but their physical presence in the cytoplasm did not significantly alter the cells’ morphology and growth ability. Moreover, the FNDs caused no noticeable changes in the protein expression of the stem cell marker, stage‐specific embryonic antigen‐1 [31], and induced no cytotoxicity (including apoptosis) during the neuronal differentiation. In the differentiated neuronal cells, the FNDs did not reduce the cell viability or affect the expression of the neuron‐specific marker, β‐III‐tubulin [32], presumably due to the exceptional chemical inertness of the nanoparticles. Altogether, the results have highlighted the great potential of HPHT‐NDs for nanomedicine and their practical use as negative controls in nanotoxicology studies for both immortal and primary cell lines.
5.2.2 DND
DNDs are synthesized by detonation of an oxygen‐deficient explosive mixture in a closed chamber under extremely high temperature and pressure, achieved at the front of the detonation wave in several microseconds (Section 2.3.3). The primary particles of DNDs are small, approximately 5 nm in diameter, but contain a significant amount of impurities including N, O, and H from the reactants. The typical content of C atoms in the particles is in the range of 90–99% by weight, depending on the manufacturing processes [33]. Unlike HPHT‐NDs, DNDs are predominantly polycrystalline in structure and their surface is always covered with a shell of graphitic carbon atoms. Elemental analysis showed that more than 1% metal impurities (including Fe, Cu, Cr, Ti, and several others) are incorporated into the samples [34]. These impurities appear to come from the interactions of the explosion wave with the reaction chamber walls and the instrumentation corrosion during purification. The high impurity content raises considerable concerns about the biocompatibility of this nanocarbon material.
A large number of experiments have been carried out to study the in vitro toxicity of DNDs [35–44]. Schrand et al. [36, 37] conducted the first experiments to test the differential biocompatibility of different carbon nanoparticles. Using two different cell lines (neuroblastoma cells and rat alveolar macrophage) and the MTT assays, they found that DND had a greater biocompatibility than carbon black (CB), multi‐walled carbon nanotubes (MWCNTs), and single‐walled carbon nanotubes (SWCNTs) (Figure 5.5a and b). The biocompatibility trend followed the order of DND > CB > MWCNT > SWCNT for both cell lines. A later study using HeLa cells treated with MWCNTs, graphene oxides (GOs), and NDs reached a similar conclusion, with a decreasing biocompatibility of DND > MWCNT ≈ GO [38]. The low toxicity of DND makes it a more desirable candidate than other carbon nanoparticles as a drug delivery vehicle for biomedical applications, including personalized medicine [45] (cf., Chapter 13 for details).
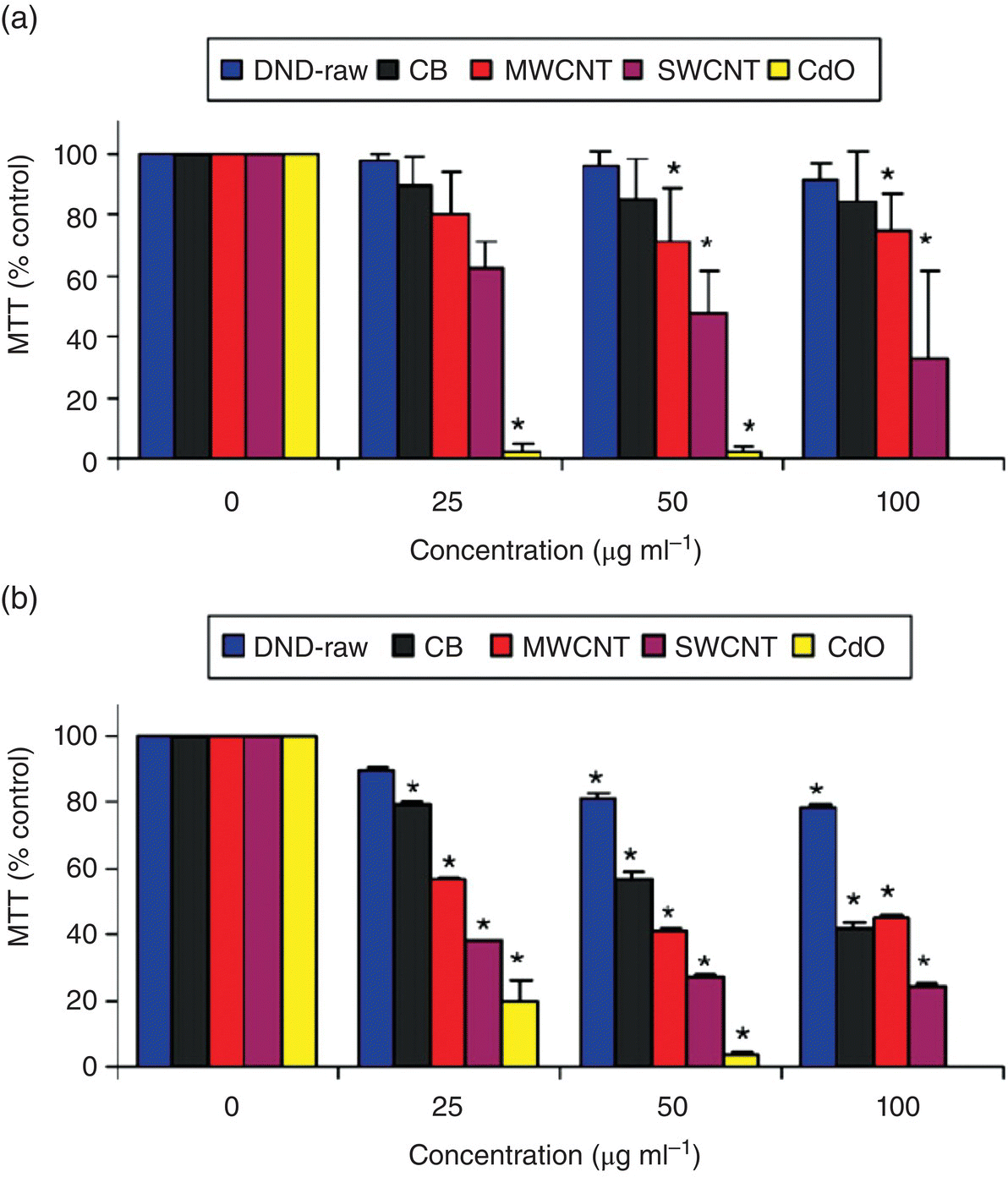
Figure 5.5 Cytotoxicity measurements after 24‐h incubation of various nanocarbons in (a) neuroblastoma cells and (b) macrophages. GdO nanoparticles served as the positive control.
Source: Adapted with permission from Ref. [36]. Reproduced with permission of Elsevier.
The high biocompatibility and low cytotoxicity of DNDs were well founded as per the studies of Schrand et al. [35–37] on the subject. The research team did not observe any disruption of mitochondrial membrane permeability, morphological alterations, or viability changes when exposing the cells to 5–100 μg ml−1 DNDs. Moreover, the DNDs were discovered to neither induce ROS generation nor cause oxidative stress, which could have led to membrane dysfunction, protein degradation, or DNA damage. They also confirmed the lack of change in the expression level of genes that served as the indicators of inflammation and protection against apoptosis in macrophages and neuroblastoma cells when incubated with DNDs. Their results are in accord with other cytotoxicity and genotoxicity tests, showing that the carbon‐based nanoparticles are well tolerated by multiple cell types at both functional and gene expression levels [38–41].
While several experiments have provided evidences for the innate biocompatibility of DNDs, some studies refute this finding, arguing that DNDs can induce both genotoxicity and cytotoxic responses under certain conditions [42–44]. Researchers making such an argument found that the toxicity of DNDs varied, depending on the dosage and surface chemistry of the particles, the type of cell lines used for the assessments, as well as the composition of the treatment medium. Concerns about the toxicity typically arose from the small size of DNDs and their ability to enter cells and localize in critical organelles [42]. Also, the oxidative stress induced by elevation of ROS after the DND treatment could result in DNA damage [43]. Furthermore, the graphitic surface content is a possible determinant of the bioactivity of these carbonaceous nanoparticles [44]. A comprehensive account of the cellular response to FNDs, DNDs, and other functionalized NDs can be found in the work by Moore et al. [46].
5.3 Ex Vivo Studies
Different from in vitro studies with cultured cells under controlled environment, ex vivo biocompatibility evaluations employ tissues isolated from organisms to explore the potential impacts of the tested materials on human health. When tested in animal models such as mice and rats, the majority of nanoparticles are administered through the bloodstream. Therefore, understanding the particles’ blood compatibility is vitally important [16]. Figure 5.6a displays a typical result of the testing for the hemolytic activity of surface‐oxidized HPHT‐NDs of various sizes (35–500 nm) using human RBCs as the samples [47]. The red color of the solution in panel (a) is due to the release of hemoglobin from damaged RBCs and the red pellets at the bottom of the tubes are intact RBCs precipitated by centrifugation. In this particular experiment, phosphate‐buffered saline and distilled deionized water served as the negative (−) and positive (+) controls, respectively. The results showed that irrespective of the particle size, all HPHT‐NDs caused no significant RBC destruction at the concentration as high as 400 mg ml−1. In contrast, the membrane of the RBCs incubated with GOs was substantially damaged even at a dosage lower than 25 mg ml−1 (Figure 5.6b). Therefore, the influence of HPHT‐NDs on the oxygenation states and microrheological properties of RBCs was negligible [48].
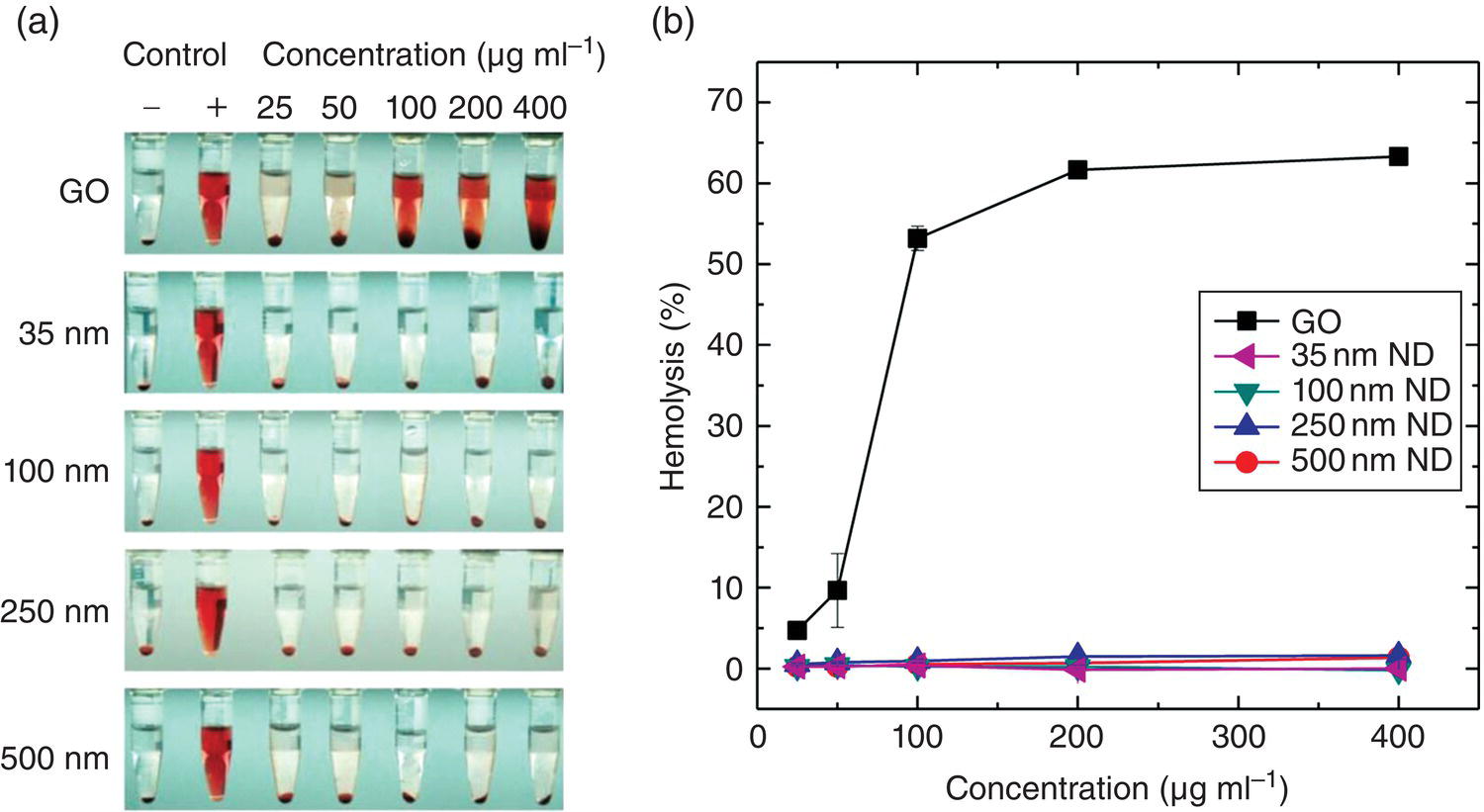
Figure 5.6 Hemolysis studies of GOs and oxidized NDs of different size with human RBCs. (a) Photographs of human RBCs treated with GOs and NDs of four different sizes at the concentration range of 25−400 μg ml−1. (b) Hemolysis percentages measured at the concentration range of 25−400 μg ml−1 for GOs and four different ND samples (35, 100, 250 and 500 nm in diameter) incubated with RBCs at 25 °C for two hours. GOs served as the positive control.
Source: Adapted with permission from Ref. [47]. Reproduced with permission of Nature Publishing Group.
Following the hemolysis study, Li et al. [47] examined the thrombogenicity of surface‐oxidized HPHT‐NDs with human RBCs. In contrast to GOs which exhibited a significant anticoagulant activity, the oxidized NDs (35–500 nm) were completely inert, showing neither thrombogenic potential nor anticoagulant activity at the concentration of up to 400 mg ml−1. Such a remarkable hemocompatibility was attributed to the exceptionally high affinity of the nanoparticles for proteins (Section 4.2.1). A protein corona immediately formed on the surface when the HPHT‐NDs were in contact with serum. It was the so‐called protein corona effect [49] that prevented the RBC membrane from being damaged and the blood clotting from occurring. As to DNDs, controversies exist. Mona et al. [50] reported that there was no delay in time when the coagulation was initiated through the intrinsic pathway in the APPT tests, whereas Kumari et al. [51] claimed that these particles without careful purification could activate blood platelets and induce thromboembolism.
5.4 In Vivo Studies
A number of model organisms have been employed to assess the in vivo biocompatibility of NDs, including Caenorhabditis elegans (C. elegans) [52], zebrafish embryos [53, 54], Xenopus embryos [55], mice [56–59], rats [59, 60], rabbits [61], and monkeys [60]. Covering a wide range of species from the nematode to the primate, these studies used both feeding and microinjection methods to introduce NDs into the living organisms.
Caenorhabditis elegans is a free living soil nematode with simple and well‐defined anatomy. The 1‐mm long adult hermaphrodite consists of an invariable number of 959 cells, which are organized to form complex tissues including intestine, muscle, hypodermis, gonad, and nerve systems [62]. The genome of C. elegans has been completely sequenced [63], making it feasible to study biological processes at the molecular level. It is an ideal model organism to assess the biocompatibility of nanoparticles owing to its short life cycles (about three days), easy handling, and high sensitivity to various types of stresses. Mohan et al. [52] was the first group to introduce surface‐oxidized and protein‐conjugated FNDs into the nematodes. Through feeding and optical imaging, they found that the particles could be readily taken up by the worms and accumulated in lumen. Toxicity assessments, performed by using longevity, reproductive potential, and the ROS level as physiological indicators, showed that the particles were nontoxic and did not cause any detectable stress to the worms.
Extended from their C. elegans work, Chang et al. [53] microinjected FNDs after coating with bovine serum albumin (BSA) into the yolk cells of zebrafish embryos at the one‐cell stage. By fluorescence imaging, they found that the FND particles could be incorporated into the dividing cells in the blastomeres through cytoplasmic streaming [54]. The FND‐labeled larvae were able to develop into whole fishes without any apparent morphological anomalies during their embryogenesis, indicating that the HPHT‐ND particles did not cause any deleterious effects on the development of the vertebrate organism. Parallel to the FND studies above, Marcon et al. [55] used DND to assess the in vivo toxicity of the nanomaterials with different surface modifications (including −OH, −NH2, or −COOH) in Xenopus embryos. They reported that microinjection of DND‐COOH into early‐stage embryos could cause significant embryotoxicity and teratogenicity, despite the fact that DND‐NH2 and DND‐OH were only slightly toxic.
In the studies using murine models to test the biocompatibility of HPHT‐NDs, Li et al. [47] detected no significant elevation of the inflammatory cytokine levels of interleukin‐1β (IL‐1β) and interleukin‐6 (IL‐6) after intravenous injection of the particles into mice through tail veins [47]. Similarly, no visible toxicity and side effects (e.g. stress response) were reported by Vaijayanthimala, et al. [59] for rats subjected to intraperitoneal injection of FNDs over a three‐month period with a total quantity of up to 75 mg kg−1 body weight. The differences in fodder consumption, body weight, and organ index between the control and FND‐treated animals were insignificant. Histopathological examination of tissues revealed that the injected FND particles were engulfed by macrophages, but there was no observable inflammation, necrosis, or tissue reaction surrounding these carbon‐laden macrophages (Figure 5.7).
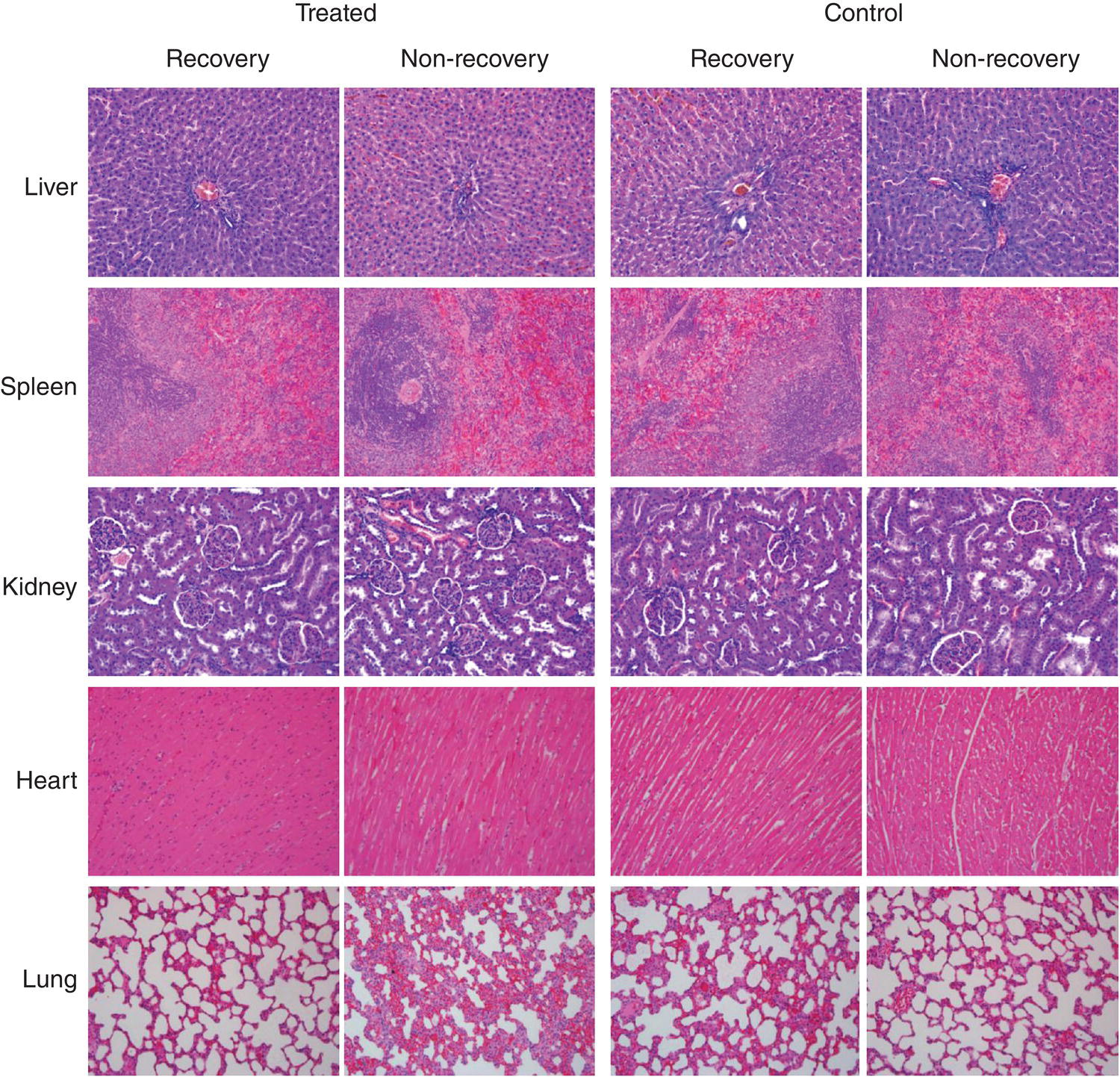
Figure 5.7 Histopathological examination of the tissue sections of FND‐injected (treated) and saline‐injected (control) rats with and without recovery. The results show no specific pathological changes in both FND‐treated and control groups (magnification 200×).
Source: Reprinted with permission from Ref. [59]. Reproduced with permission of Elsevier.
A question often asked is: Where would these nanoparticles end up? Information concerning the biodistribution and fate of the injected nanoparticles is exceedingly important in assessing the in vivo biocompatibility. Yuan et al. [56] carried out experiments pertaining to this study by using HPHT‐NDs labeled with 125I radioisotopes. They found that the particles with a size of 50 nm predominantly accumulated in livers of the mice after intravenous injection, followed by lungs as the target organs. About 37% of the initially injected particles were entrapped in livers and 6% in lungs after 0.5 hour post‐dose (Figure 5.8). High‐resolution transmission electron microscopy and Raman spectroscopy of digested organ solutions confirmed the long‐term entrapment of the BSA‐coated HPHT‐NDs in livers and lungs. However, no mice showed any symptoms of abnormality, such as weight loss, lethargy, anorexia, vomiting, and diarrhea during the course of the treatments.

Figure 5.8 Biodistribution of 125I‐labeled HPHT‐NDs in mice after intravenous injection with a dose of 20 mg kg−1 body weight for 30 minutes.
Source: Adapted with permission from Ref. [56]. Reproduced with permission of Elsevier.
Studies have also been made for the in vivo biocompatibility of DNDs. Zhang et al. [57] found that intratracheal instillation of DNDs did not lead to any differences in body weight or abnormal pathologies of treated mice. However, it did have a negative effect on the lungs, liver, kidneys, and hematological systems. Of these organs, the lungs suffered the worst toxicological effects, becoming inflamed and tissue damaged, as a result of the high uptake and long retention time of the nanoparticles in the lung tissue. In contrast to their findings, the results of Yuan et al. [58] showed no pulmonary toxicity in mice after intratracheal instillation of DNDs. The discrepancies between these two results could be associated with the dosage and sources of the DNDs as well as how their surface might have been modified, as discussed in Section 5.2.2.
Rabbits are one of the earliest animal models used for in vivo nanotoxicity studies. Puzyr et al. [61] gave a high dose (125 mg) of DNDs to the rabbits through intravenous administration and yet did not cause any deaths. Both the RBC count and the hemoglobin level of the rabbits remained stable for 15 min after administration. Later, in a longer time period (48 hours), the levels of biochemical molecules such as total bilirubin, triglyceride, low‐density lipoprotein, etc. changed to an extent that was statistically significant. Three months later, the rabbits showed no signs of inflammation, suggesting a long‐term biocompatibility of the nanoparticles.
Despite the discrepancies mentioned above, a broad spectrum of biocompatibility studies has indicated that DNDs cause no adverse effects on cells and organisms. Aiming for clinical translation of DNDs, Ho and coworkers [60] have recently conducted a comprehensive assessment for the safety of the nanomaterial with both small and large animal preclinical models: rats and monkeys. They performed the studies in two cohorts that lasted for two weeks (rats) or six months (monkeys) based on histological, serum, urine, and body weight analysis. Their results showed that DNDs were well tolerated at the clinically relevant doses of 6.75–13.5 mg kg−1. However, in order for in‐human validation, more detailed biodistribution and pharmacokinetics analysis of the injected nanomaterial is needed. FNDs are expected to make significant contributions to such research studies and developments as discussed in Chapter 9.
References
- 1 Martin, G. (1998). The meaning and origin of the expression: a diamond is forever. http://www.phrases.org.uk/meanings/a‐diamond‐is‐forever.html (accessed 16 April 2018).
- 2 Vert, M., Doi, Y., Hellwich, K.H. et al. (2012). Terminology for biorelated polymers and applications (IUPAC recommendations 2012). Pure Appl Chem 84: 377–410.
- 3 Dion, I., Baquey, C., and Monties, J.R. (1993). Diamond – the biomaterial of the 21st century. Int J Artif Organs 16: 623–627.
- 4 Hodgson, E., Leblanc, G.A., Meyer, S.A., and Smart, R.C. (2010). Introduction to biochemical and molecular methods in toxicology. In: A Textbook of Modern Toxicology, 4e (ed. E. Hodgson), 15–28. New York: Wiley.
- 5 Masters, J.R.W. (2000). Human cancer cell lines: fact and fantasy. Nat Rev Mol Cell Biol 1: 233–236.
- 6 Alberts, B., Johnson, A., Lewis, J. et al. (2014). Molecular Biology of the Cell, 6e. Garland Science.
- 7 Johnson, I. and Spence, M. (2010). Molecular Probes Handbook, a Guide to Fluorescent Probes and Labeling Technologies, 11e. Springer, Sec. 8.1.
- 8 Schieber, M. and Chandel, N.S. (2014). ROS function in redox signaling and oxidative stress. Curr Biol 24: R453–R462.
- 9 Johnson, I. and Spence, M. (2010). Molecular Probes Handbook, a Guide to Fluorescent Probes and Labeling Technologies, 11e. Springer, Sec. 18.2.
- 10 Li, M.O., Sarkisian, M.R., Mehal, W.Z. et al. (2003). Phosphatidylserine receptor is required for clearance of apoptotic cells. Science 302: 1560–1563.
- 11 Johnson, I. and Spence, M. (2010). Molecular Probes Handbook, a Guide to Fluorescent Probes and Labeling Technologies, 11e. Springer, Sec. 15.5.
- 12 Loo, D.T. (2002). TUNEL assay. An overview of techniques. Methods Mol Biol 203: 21–30.
- 13 van Meerloo, J., Kaspers, G.J., and Cloos, J. (2011). Cell sensitivity assays: the MTT assay. Methods Mol Biol 731: 237–245.
- 14 Ke, N., Wang, X., Xu, X., and Abassi, Y.A. (2011). The xCELLigence system for real‐time and label‐free monitoring of cell viability. Methods Mol Biol 740: 33–43.
- 15 Speit, G. and Hartmann, A. (2006). The comet assay: a sensitive genotoxicity test for the detection of DNA damage and repair. Methods Mol Biol 314: 275–286.
- 16 Fenech, M. (2008). The micronucleus assay determination of chromosomal level DNA damage. Methods Mol Biol 410: 185–216.
- 17 Evani, S.J. and Ramasubramanian, A.K. (2011). Hemocompatibility of nanoparticles. In: Nanobiomaterials Handbook (ed. B. Sitharaman). CRC Press Chapter 31.
- 18 Neun, B.W. and Dobrovolskaia, M.A. (2011). Method for analysis of nanoparticle hemolytic properties in vitro. Methods Mol Biol 697: 215–224.
- 19 Neun, B.W. and Dobrovolskaia, M.A. (2011). Method for in vitro analysis of nanoparticle thrombogenic properties. Methods Mol Biol 697: 225–235.
- 20 Meager, A. (2004). Cytokines: interleukins. In: Encyclopedia of Molecular Cell Biology and Molecular Medicine, 2e (ed. R.A. Meyers), 115–151. New York: Wiley.
- 21 Crowther, J.R. (1995). ELISA. Theory and practice. Methods Mol Biol 42: 1–218.
- 22 Yu, S.J., Kang, M.W., Chang, H.C. et al. (2005). Bright fluorescent nanodiamonds: no photobleaching and low cytotoxicity. J Am Chem Soc 127: 17604–17605.
- 23 Vaijayanthimala, V., Tzeng, Y.K., Chang, H.C., and Li, C.L. (2009). The biocompatibility of fluorescent nanodiamonds and their mechanism of cellular uptake. Nanotechnology 20: 425103.
- 24 Lin, H.H., Lee, H.W., Lin, R.J. et al. (2015). Tracking and finding slow‐proliferating/quiescent cancer stem cells with fluorescent nanodiamonds. Small 11: 4394–4402.
- 25 Paget, V., Sergent, J.A., Grall, R. et al. (2014). Carboxylated nanodiamonds are neither cytotoxic nor genotoxic on liver, kidney, intestine and lung human cell lines. Nanotoxicology 8: 46–56.
- 26 Smart, D.J., Ahmedi, K.P., Harvey, J.S., and Lynch, A.M. (2011). Genotoxicity screening via the gammaH2AX by flow assay. Mutat Res 715: 25–31.
- 27 Vial, S., Mansuy, C., Sagan, S. et al. (2008). Peptide‐grafted nanodiamonds: preparation, cytotoxicity and uptake in cells. ChemBioChem 9: 2113–2119.
- 28 Wu, T.J., Tzeng, Y.K., Chang, W.W. et al. (2013). Tracking the engraftment and regenerative capabilities of transplanted lung stem cells using fluorescent nanodiamonds. Nat Nanotechnol 8: 682–689.
- 29 Huang, Y.A., Kao, C.W., Liu, K.K. et al. (2014). The effect of fluorescent nanodiamonds on neuronal survival and morphogenesis. Sci Rep 4: 6919.
- 30 Hsu, T.C., Liu, K.K., Chang, H.C. et al. (2014). Labeling of neuronal differentiation and neuron cells with biocompatible fluorescent nanodiamonds. Sci Rep 4: 5004.
- 31 Solter, D. and Knowles, B.B. (1978). Monoclonal antibody defining a stage‐specific mouse embryonic antigen (SSEA‐1). Proc Natl Acad Sci USA 75: 5565–5569.
- 32 Memberg, S.P. and Hall, A.K. (1995). Dividing neuron precursors express neuron‐specific tubulin. J Neurobiol 27: 26–43.
- 33 Schrand, A.M., Hens, S.A.C., and Shenderova, O.A. (2009). Nanodiamond particles: properties and perspectives for bioapplications. Crit Rev Solid State Mater Sci 34: 18–74.
- 34 Volkov, D., Proskurnin, M., and Korobov, M. (2014). Elemental analysis of nanodiamonds by inductively‐coupled plasma atomic emission spectroscopy. Carbon 74: 1–13.
- 35 Schrand, A.M., Huang, H., Carlson, C. et al. (2006). Are diamond nanoparticles cytotoxic? J Phys Chem B 111: 2–7.
- 36 Schrand, A.M., Dai, L., Schlager, J.J. et al. (2007). Differential biocompatibility of carbon nanotubes and nanodiamonds. Diam Relat Mater 16: 2118–2123.
- 37 Schrand, A.M., Johnson, J., Dai, L. et al. (2009). Cytotoxicity and genotoxicity of carbon nanomaterials. In: Safety of Nanoparticles (ed. T.J. Webster), 159–187. Springer.
- 38 Liu, K.K., Cheng, C.L., Chang, C.C., and Chao, J.I. (2007). Biocompatible and detectable carboxylated nanodiamond on human cell. Nanotechnology 18: 325102.
- 39 Huang, H., Pierstorff, E., Osawa, E., and Ho, D. (2007). Active nanodiamond hydrogels for chemotherapeutic delivery. Nano Lett 7: 3305–3314.
- 40 Xing, Y., Xiong, W., Zhu, L. et al. (2011). DNA damage in embryonic stem cells caused by nanodiamonds. ACS Nano 5: 2376–2384.
- 41 Zhang, X.Y., Hu, W.B., Li, J. et al. (2012). A comparative study of cellular uptake and cytotoxicity of multi‐walled carbon nanotubes, graphene oxide, and nanodiamond. Toxicol Res 1: 62–68.
- 42 Solarska, K., Gajewska, A., Bartosz, G., and Mitura, K. (2012). Induction of apoptosis in human endothelial cells by nanodiamond particles. J Nanosci Nanotechnol 12: 5117–5121.
- 43 Dworaka, N., Wnuk, M., Zebrowski, J. et al. (2013). Genotoxic and mutagenic activity of diamond nanoparticles in human peripheral lymphocytes in vitro. Carbon 68: 763–776.
- 44 Silbajoris, R., Linak, W., Shenderova, O. et al. (2015). Detonation nanodiamond toxicity in human airway epithelial cells is modulated by air oxidation. Diam Relat Mater 58: 16–23.
- 45 Ho, D., Wang, C.H., and Chow, E.K. (2015). Nanodiamonds: the intersection of nanotechnology, drug development, and personalized medicine. Sci Adv 1: e1500439.
- 46 Moore, L., Grobarova, V., Shen, H. et al. (2014). Comprehensive interrogation of the cellular response to fluorescent, detonation and functionalized nanodiamonds. Nanoscale 6: 11712–11721.
- 47 Li, H.C., Hsieh, F.J., Chen, C.P. et al. (2013). The hemocompatibility of oxidized diamond nanocrystals for biomedical applications. Sci Rep 3: 3044.
- 48 Lin, Y.C., Tsai, L.W., Perevedentseva, E. et al. (2012). The influence of nanodiamond on the oxygenation states and micro rheological properties of human red blood cells in vitro. J Biomed Opt 17: 101512.
- 49 Hamad‐Schifferli, K. (2015). Exploiting the novel properties of protein coronas: emerging applications in nanomedicine. Nanomedicine 10: 1663–1674.
- 50 Mona, J., Kuo, C.J., Perevedentseva, E. et al. (2013). Adsorption of human blood plasma on nanodiamond and its influence on activated partial thromboplastin time. Diam Relat Mater 39: 73–77.
- 51 Kumari, S., Singh, M.K., Singh, S.K. et al. (2014). Nanodiamonds activate blood platelets and induce thromboembolism. Nanomedicine (Lond) 9: 427–440.
- 52 Mohan, N., Chen, C.S., Hsieh, H.H. et al. (2010). In vivo imaging and toxicity assessments of fluorescent nanodiamonds in Caenorhabditis elegans. Nano Lett 10: 3692–3699.
- 53 Mohan, N., Zhang, B., Chang, C.C. et al. (2011). Fluorescent nanodiamond − a novel nanomaterial for in vivo applications. MRS Proc 1362: 25–35.
- 54 Chang, C.C., Zhang, B., Li, C.Y. et al. (2012). Exploring cytoplasmic dynamics in zebrafish yolk cells by single particle tracking of fluorescent nanodiamonds. Proc SPIE 8272: 827205.
- 55 Marcon, L., Riquet, F., Vicogne, D. et al. (2010). Cellular and in vivo toxicity of functionalized nanodiamond in Xenopus embryos. J Mater Chem 20: 8064–8069.
- 56 Yuan, Y., Chen, Y., Liu, J.H. et al. (2009). Biodistribution and fate of nanodiamonds in vivo. Diam Relat Mater 18: 95–100.
- 57 Zhang, X., Yin, J., Kang, C. et al. (2010). Biodistribution and toxicity of nanodiamonds in mice after intratracheal instillation. Toxicol Lett 198: 237–243.
- 58 Yuan, Y., Wang, X., Jia, G. et al. (2010). Pulmonary toxicity and translocation of nanodiamonds in mice. Diam Relat Mater 19: 291–299.
- 59 Vaijayanthimala, V., Cheng, P.Y., Yeh, S.H. et al. (2012). The long‐term stability and biocompatibility of fluorescent nanodiamond as an in vivo contrast agent. Biomaterials 33: 7794–7802.
- 60 Moore, L., Yang, J., Lan, T.T. et al. (2016). Biocompatibility assessment of detonation nanodiamond in non‐human primates and rats using histological, hematologic, and urine analysis. ACS Nano 10: 7385–7400.
- 61 Puzyr, A.P., Baron, A.V., Purtov, K.V. et al. (2007). Nanodiamonds with novel properties: a biological study. Diam Relat Mater 16: 2124–2128.
- 62 Sulston, J.E., Schierenberg, E., White, J.G., and Thomson, J.N. (1983). The embryonic cell lineage of the nematode Caenorhabditis elegans. Dev Biol 100: 64–119.
- 63 C. elegans Sequencing Consortium (1998). Genome sequence of the nematode C. elegans: a platform for investigating biology. Science 282: 2012–2018.
