Towards Molecular Medicine
Philips Research, High Tech Campus 34, Eindhoven, The Netherlands
1. Introduction
Historically, the human species has lived with the expectation of dying quite young from violent external factors or from infectious diseases. Considerable progress in world health arose eventually from improvements in living conditions – hygiene, access to safe drinking water, improved quality and variety of nutrition. However, many major infectious diseases, such as tuberculosis or syphilis, were not understood, and could not really be treated. In most cases, the medical profession could only offer palliative care. As Lewis Thomas recalls the years of his training to become a medical doctor in the early 1930’s:” … it gradually dawned upon us that we didn’t know much that was really useful, that we could do nothing to change the course of the great majority of the diseases we were so busy analyzing, that medicine, for all its façade as a learned profession, was in real life a profoundly ignorant occupation.”1 Medicine only started to be a science with the realization that diseases have their origin in microbiological processes. Since the 1930’s, much progress has been made, with the discovery of antibiotics and the development of very effective vaccines.
As a result, life expectancy has increased dramatically, first in Europe and North America, but now also in countries such as China. Unfortunately, the increased life expectancy is going hand in hand with an increase in the number of people affected by chronic or degenerative diseases, which also are at the origin of the current explosion of costs in the healthcare system. Again, treatment for these diseases is typically palliative in nature – mostly there is no cure and at best the progression of the disease can be delayed. In addition, the development and regulatory approval of new drugs is hampered by the fact that the effectiveness and side effects are not the same for all patient groups, leading to very costly trials and late-stage drug withdrawals. Furthermore, bacterial and viral infections still pose very serious threats, ranging from hospital infections to viral pandemics.
Further progress can only be expected from a much more sophisticated understanding and exploitation of molecular biology. It is now well accepted that most diseases have their origin in disturbances of the delicate balance in molecular processes taking place at the cellular and sub-cellular level. This is currently leading to a paradigm shift in medicine: from a focus on dysfunctional organs to an understanding of disease pathways at the cellular and molecular level. The vision is that genetic predisposition testing, early diagnosis using molecular tests, and personalized treatment will transform clinical practice, and lead to improved patient outcomes. Aspects of this vision are referred to as evidence-based medicine, personalized medicine, molecular medicine, or nanomedicine.2,3
The promise of molecular medicine is illustrated in Fig. 1: early and faster diagnosis, better prognosis, and tailored therapy with higher efficacy and reduced side effects as compared to the present state-of-the-art, which is based on treatment of the patient on a trial and error basis after serious symptoms have developed.
It is our firm belief that the vision of nanomedicine can only be realized by matching the progress in molecular biology with advances in medical microdevices, nanotechnology and physical instrumentation, and by inventing new ways of dealing with complex data in support of decision-taking, a field referred to as bioinformatics.
2. System biology and biomarkers
The central dogma of molecular biology is that the genetic make-up of the individual, primarily given by hereditary factors, is laid down in the DNA, and subsequently transcribed by RNA into proteins, the molecules that are instrumental in all major biological processes taking place in human cells, tissues and organs.
Figure 1. Molecular diagnostics and molecular imaging, coupled to therapy, will change the current practice of health care.
The basis for nanomedicine is the explosive growth in knowledge of the structure of the human genome, and its translation into functional elements, the proteins. Advances in technology have led to elucidation of the genetic make-up of many species, including humans as a result of the ambitious human genome project. Similarly, efforts are ongoing to establish RNA patterns (the “transcriptome”) and – more challenging still – get insight into the range of proteins present in a human being (the “proteome”).
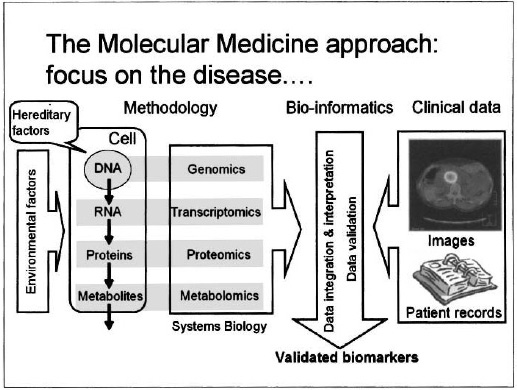
Knowledge of the genome, transcriptome and proteome by itself is not sufficient, however. The next step requires relating the knowledge of the molecular translation cycle to the onset, development and ultimately treatment of disease. This step is still extremely complex and poorly understood. Gaining insight into the functioning of protein signalling, and its impact on cell multiplication, interaction and transformation forms the main challenge of an emerging branch of science called systems biology. In systems biology, the aim is to integrate all relevant genomic, transcriptomic, and proteomic information with the metabolic processes in our cells and organs (“metabolomics”) into a consistent model of the intricate biological processes that describe the functioning of the human body. Disturbances of these intrinsic processes are at the origin of disease. External factors, such as nutrition and lifestyle, play an important role as well, as they can have profound effects on the structure of the genome (e.g. modification of DNA by methylation, “epigenetics”), and will translate into the proteome. A proper understanding of these environmental factors is required to really get a handle on the origin of most diseases, and to be able to devise effective cures.
In an effort to reach this goal one tries to relate the wealth of information, which is available from patient samples (information from healthy and diseased tissue, generally comprising DNA, RNA, proteins and metabolites), to clinical information. The key challenge is to discover biomarkers, which are observable signatures of relevant disease pathways.
Biomarkers are so important because they are characteristic of a particular disease, and can be used for early diagnosis. Such biomarkers can be discovered in bodily fluids, e.g. in blood or serum, so that they may be determined by in-vitro diagnostic approaches, but also in tissue or organs, providing handles for targeted contrast agents, which can be visualized in-vivo by making use of advanced imaging instrumentation.
Specific biomarkers can be employed for early diagnosis and for monitoring of diseases, but they can also be used to accelerate the process of drug discovery and development: by using biomarkers as “surrogate endpoints” in clinical trials, drug effectiveness (and toxicology, or other side effects) can be detected much earlier than by such conventional metrics as the five-year survival rate. Here the challenge is to link the rich “molecular” information to the relatively scarce patient data, which is furthermore complicated by the inherent biological variability and the environmental factors mentioned before. Bioinformatics plays a central role in coming to grips with this complexity, as illustrated in Fig. 2.
Figure 2. Validated biomarkers are key in the successful introduction of molecular medicine. Their identification requires the interpretation of large and complicated data sets, with the help of bio-informatics tools.
3. Molecular medicine
The major life-threatening and chronic diseases, such as cardiovascular disease, cancer, diabetes, and infectious diseases (tuberculosis, malaria, AIDS) are at least partially genetically determined. The same is true for the major debilitating diseases, such as neuro-degenerative diseases (Alzheimer’s, Parkinson’s) and autoimmune diseases (rheumatoid arthritis). Early detection of these diseases greatly improves the therapeutic success rate, leading to a prolongation of the healthy and productive lifespan of the individual, and treatment with fewer side effects. In addition, it has a potential cost-containment effect as well: particularly, delaying the onset of debilitating diseases results in a significant reduction of the very high personnel costs associated with nursing the patients.
A more personalized approach of tailored care for every individual will become the standard. Introduction of targeted drugs to block receptors in the membranes of tumor cells, for instance, may result in slowing down tumor cell proliferation or even their elimination. Apart from more effective treatment, some cancer types may well be contained – effectively turning cancer in a manageable “chronic” disease. The first successful targeted drugs have already been introduced. An example is the drug imatinib (Gleevec, by Novartis), developed after the discovery of a chromosome translocation creating a new gene structure, the abl-bcr gene, in chronic myeloid leukemia patients. Gleevec binds specifically to the abl-bcr protein, and can alleviate leukemia in patients for whom other treatments have failed.4 Other examples of targeted drugs are Herceptin (produced by Genentech/Roche to treat metastatic breast cancer) and the non-Hodgkin’s lymphoma drugs Bexxar (GlaxoSmithKline) and Zevalin (Biogenldec). All these drugs are based on monoclonal antibodies, which bind selectively to tumor cells, and may be equipped with toxic substances to enhance their efficiency (e.g. in Bexxar radioactive 131I is present to invoke radio immunotherapy). Such targeted or “smart” drugs can be quite expensive. For instance, a course of treatment with Zevalin and Bexxar amounts to $25–30K per patient for the medication. It is therefore very important for financial reasons to identify the patients who are likely to respond well to the medication prior to the treatment, and this can be done with molecular diagnostic tests.
Traditional medical practice, based on trial-and-error, results both in under-treatment and over-treatment, multiple office visits, the need for drug monitoring, frequent regimen changes, and a staggering number of deaths attributed to adverse drug reactions (more than 100,000 annually in the U.S.A. alone).2 We expect that molecular medicine will dramatically change the healthcare system, by alleviating many of these problems.
A secondary opportunity may be the application of molecular medicine techniques to accelerate and simplify the drug discovery and development process, driven by collaboration of pharmaceutical and biotech companies on the one hand, and medical technology companies on the other.
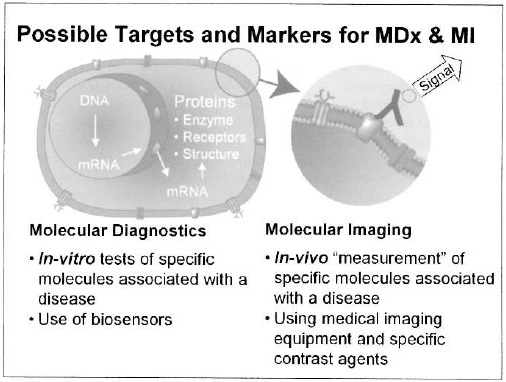
Molecular medicine is enabled by two key medical technologies, see Figure 3. In-vitro molecular diagnostics is a technique for screening and monitoring to enable early and precise detection of disease. In-vivo molecular imaging is a technique to localize, image, and interpret the disease as it takes place in the body. This requires the right combination of advanced imaging equipment with targeted and/or functional contrast agents. Molecular imaging offers unique opportunities for combination with (targeted) therapy, which can be much better planned and monitored with the help of advanced hardware and especially software tools utilizing pharmacodynamic modelling. Typically, molecular diagnostics and molecular imaging will be applied in tandem, with the goal of providing tailored solutions for a wide range of diseases.
4. Molecular diagnostics
In-vitro diagnostic approaches will become indispensable for early diagnosis, for the selection of personalized therapy, and for effective follow-up, after completion of the treatment or to support management of a chronic condition. A distinction should be made between techniques applied for the identification of genomic fingerprints and methods suitable for identification of particular biomarkers.
Figure 3. Molecular diagnostics (MDx) and molecular imaging (MI) are the key technologies enabling molecular medicine. In both technologies characteristic and validated biomarkers are needed. The diagram illustrates an antibody with a signalling label, bound selectively to a disease specific molecule expressed at the cell membrane.
Genomic fingerprints thus far have been mostly applied to identify pathogens. In particular, tests are commercially available for the human papilloma virus, for various forms of the human immunodeficiency virus, and for hepatitis B and C. Diagnostic products for infectious diseases therefore dominate the market at present. Detection is predominantly based on amplification of characteristic nucleotide sequences using the polymerase chain reaction (PCR), followed by a hybridization assay.
However, genomic fingerprints are increasingly being utilized to assay the molecular make-up of the host, rather than the pathogen. They are applied to phenotype individuals to identify their predisposition to particular diseases or to tailor individual therapeutic interventions (e.g. selection of the appropriate dose of medication on the basis of metabolic characteristics). The resulting “pharmacogenomic” fingerprints rely on the application of high-density arrays (e.g. the GeneChips provided by Affymetrix, or the DNA Microarrays sold by Agilent). Typically, these high-density arrays contain many thousands of different oligonucleotide strings found at different known locations. The presence of complementary oligonucleotides in the sample can be measured optically, through sensitive detection of fluorescent labels; even single mismatches, so-called single nucleotide polymorphisms, can be identified. By careful execution of the measurement protocol, genetic expression profiles highlighting up-regulation or down-regulation of certain parts of DNA or RNA can also be made visible. The observed features can be applied for diagnostic classification, treatment selection and prognostic assessment.5 In Fig. 1 an image of (part of) a DNA “chip” is shown. Other technologies currently gaining ground, particularly for cancer diagnostics, are in-situ hybridization and fluorescent in-situ hybridization.
Alternatively, the measurement can be focused on the identification of proteomic biomarkers. Proteomic biomarkers are proteins, such as membrane proteins, triggered by or synthesized in response to disease. Examples are the proteins that signal apoptosis, or programmed cell death, and the enzymes that are released following a stroke or a myocardial infarction. Generally, well-established immunological techniques, such as the widely applied enzyme-linked immunosorbent assay (“ELISA”), are used for protein diagnostics, all based on the application of highly specific antibodies. For many diseases it is necessary to determine a multitude of proteins and, sometimes, additional biomarkers, which requires development of new methodologies. High-throughput analysis of proteins can be applied for the detection of novel drug targets, diagnostic markers, and for the investigation of biological event.5 Proteomics has the potential of becoming a very powerful tool in modern medicine, but it is still very much under development.
Another kind of biomarker is the presence of a particular kind of pathogen, which can be identified following the approach described above, so that immediately the cause of the infection and the optimal cure can be established.
Essential for the massive introduction of molecular diagnostics is the availability of cheaper and more accessible technologies. Rapid, simple to use, self-contained systems are needed. Miniaturized, integrated, “lab-on-a-chip” tools, based on microfluidic solutions and enabled by advances in micro- and nanotechnology, may serve this need. An example is the work done at Philips Research laboratories on magnetic biosensors, which utilize the highly sensitive magnetic field sensor based on the giant magnetoresistance (GMR) effect in conjunction with paramagnetic nanoparticles as labels.7
5. Molecular imaging
Medical imaging modalities have become highly advanced systems, combining high performance image acquisition with sophisticated data and image processing to provide ever increasing quality of information to the medical professional. Molecular imaging (MI) is enabled by such systems, in combination with functional and targeted contrast agents.8, 9
Two nuclear imaging technologies – single photon emission computed tomography (SPECT) and positron emission tomography (PET) – appear very promising. In SPECT, a tomographic picture is reconstructed from data obtained using an orthogonal set of gamma cameras rotated around the body, to which a radioactive agent has been administered that decays by emitting gamma quanta. In PET, a positron-emitting agent is used instead. On recombination of the positron with an electron, two high-energy photons are emitted in opposite directions, and detected in coincidence. Both imaging modalities have a relatively low resolution, but a very high sensitivity, since no background signals are present. Consequently, SPECT and PET can localize and quantify extremely low concentrations of targets, down to the nanomolar or even picomolar concentration regime. As it intrinsically relies on contrast agents, nuclear imaging offers poor anatomical information, but is strong in functional imaging. An important example is the imaging of increased metabolic rates, related to tumor growth. This is achieved in PET using the modified glucose-based agent SDG, which is taken up by cells as part of the metabolic process.
The combination of PET and SPECT as functional or molecular imaging tools with a complementary imaging modality such as computed tomography (CT), which does provide high-resolution morphological images, leads to very powerful MI tools. Once suitable biomarkers are discovered, it is expected to be relatively easy to develop a targeted contrast agent for PET or for SPECT.
Magnetic resonance imaging (MRI) also offers excellent morphological images, with very good soft tissue imaging capability. In addition, MRI can be used as a functional imaging tool, e.g. to measure brain activity. Although MRI is far less sensitive than PET or SPECT, impressive improvements in sensitivity have been achieved in recent years. Thus, it is conceivable that MRI will also develop into a molecular imaging tool. For this purpose, one needs targeted agents present at sub-micromolar concentration. A promising route towards this goal is to use nanoparticles as a scaffold.10
An emerging imaging modality is optical imaging. Due to the limited penetration rate of photons in human tissue, the application range is expected to be restricted. A promising application that is being explored by Philips Research in an alliance with Schering is optical mammography.
6. Molecular medicine in the “care cycle”
The full-fledged introduction of molecular medicine is the basis for an integrated approach of tomorrow’s healthcare, with the following characteristics:
- earlier detection of disease, by careful screening of people with elevated genetically inherited and/or lifestyle-related risks using highly specific biomarkers;
- better diagnosis for better treatment, based on the individual patient’s own biochemistry; and
- targeted and minimally invasive treatment with improved efficacy and fewer side effects.
These advances will be enabled by new technology, and by molecular biology and biomedical science, but they will require important progress in information technology as well.
We are convinced that an optimal way to benefit from nanomedicine requires an approach to healthcare in an integrated fashion, addressing all aspects of the “care cycle”, as schematically depicted in Fig. 4. The care cycle approach starts with determination of the individual’s predisposition to identified genetically inherited or lifestyle-related risks using molecular diagnostics (MDx). It then moves to focused screening of people at risk, initially employing MDx technologies, but in combination with MI for confirmation, localization and quantification, aiming at early detection of the onset of disease. Subsequently, when needed, individualized therapy is started, guided by treatment planning and monitoring of the therapeutic results with the aid of (molecular) imaging. In addition, imaging techniques can be invoked for minimally invasive treatment, providing more directed surgery and treatment. Finally, post-treatment MDx and MI can be utilized to monitor for recurrence or for active containment of the disease. The proposed approaches, which of course need to be combined with established clinical procedures, lead to an explosive increase of data, both qualitative and quantitative (enabling more objective, “evidence-based” medicine), which makes taking the right decisions more and more complex. Hence, attention needs to be paid to derive transparent information from the rich data sets, in order to support the physician in coming to the correct diagnosis and therapy selection. To this purpose, a clinical decision support system will need to be developed.
The first elements of molecular medicine have already been introduced into clinical practice. As an example, consider the case of breast cancer. Focused screening of women at risk may result in detection of breast cancer at an early stage, when it is still localized, with close to 100% treatment success. Screening for predisposition for breast cancer is offered, for example, by Myriad Genetics. Once breast cancer has been correctly diagnosed, the optimum treatment needs to be selected. The Dutch start-up Agendia, based on the pioneering work of van ’t Veer and co-workers, has developed a tool for stratification of breast cancer patients, based on 70 marker genes, allowing for the administering of the best treatment to the individual patient.11 Genentech has developed an antibody-based targeted drug, which can be applied to cure women with metastasised breast cancer, provided they show over-expression of the Her2/Neu membrane receptor. Vysis has developed a molecular diagnostic test, which can in fact screen for this receptor, to identify the patients who would benefit from the treatment.
Even though individual tests are available, it will take time to introduce molecular medicine throughout the care cycle. For many diseases, no comprehensive insight is available into their origin, and no unambiguous biomarkers have yet been identified. To counter this challenge, a tremendous effort is required, involving advanced academic research, together with contributions from pharmaceutical and biotech companies, and from medical technology companies, which should join forces to realize breakthroughs. At the same time, it is crucial to link the increasing insights in the fundamental biochemistry of disease to clinical observations. In particular, MI can play a crucial role in this translational challenge. Finally, the medical profession is (rightly) conservative; therefore, convincing evidence for the efficacy of the molecular medicine approaches needs to be provided, before they will be accepted. The challenges have been recognized by NIH director Zerhouni, who identifies in his description of the NIH Roadmap the most compelling opportunities in three arenas: new pathways to discoveries, (highly multidisciplinary) research teams of the future, and re-engineering the clinical research enterprise.12
We are optimistic, nevertheless, that these challenges can be overcome, and that within the next decades an increasing number of MDx and MI approaches will be introduced, providing molecular medicine-based care cycles for many important diseases.
Figure 4. The future care cycle of molecular medicine, based on the joint application of molecular diagnostics (MDx) and molecular imaging (MI) to enable timely and targeted treatment.
References
- Lewis Thomas, The Youngest Science: Notes of a Medicine Watcher, New York: The Viking Press, 1983.
- G. S. Ginsburg and J. J. McCarthy, “Personalized medicine: Revolutionizing drug discovery and patient care,” Trends Biotechnol. 19, 491 (2001).
- R. I. Pettigrew, C. A. Fee, and K. C. Li, “Changes in the world of biomedical research are moving the field of ‘personalized medicine’ from concept to reality,” J. Nucl. Med. 45, 1427 (2004).
- B. J. Druker, “Imatinib alone and in combination for chronic myeloid leukemia,” Semin. Hematol. 40, 50 (2003).
- R. Simon, “Using DNA microarrays for diagnostic and prognostic prediction,” Expert Rev. Mol. Diagn. 3, 587 (2003).
- M. Fountoulakis, “Proteomics in drug discovery: Potential and limitations,” Biomed. Health Res. 55, 279 (2002).
- M. Megens and M. W. J. Prins, “Magnetic biochips: A new option for sensitive diagnostics,” J. Magn. Magn. Mat. 293, 702 (2005).
- R. Weissleder and U. Mahmood, “Molecular imaging,” Radiology 219, 316 (2001).
- F. A. Jaffer and R. Weissleder, “Molecular imaging in the clinical arena,” J. Amer. Med. Assoc. 293, 855 (2005).
- S. A. Wickline and G. Lanza, “Nanotechnology for molecular imaging and targeted therapy,” Circulation 107, 1092 (2003).
- L. van ’t Veer, H. Dai, M. van de Vijver, et al., “Gene expression and profiling predicts clinical outcome of breast cancer,” Nature 415, 530 (2002).
- E. Zerhouni, “Medicine. The NIH Roadmap,” Science 302, 63 (2003).