Chapter 3
Environmental Law and Regulations: From End-of-Pipe to Pollution Prevention
3.1 Introduction
Chemical engineers practice a profession and must obey rules governing their professional conduct. One important set of rules that all chemical engineers should be aware of is environmental statutes, which are laws enacted by Congress. Regulations are promulgated by administrative agencies based on authority conferred by the statute. The environmental statutes are designed to protect human health and the environment by placing limits on the quantity and chemical make-up of waste streams that are released from manufacturing processes. For example, one statute places restrictions on how hazardous waste from industry is stored, transported, and treated. Another statute places strict liability on the generators of hazardous waste, requiring responsible parties to clean up disposal sites that fail to protect the environment. For manufacturers of new chemicals, there are regulatory requirements that require filing of a premanufacture notice (PMN) before introducing a new chemical into the marketplace. While many companies have Health, Safety, and Environment (HS&E) staff that can help the engineer interpret and implement environmental requirements, it is nevertheless important that chemical engineers be aware of prominent federal environmental laws, and adhere to the requirements of these statutes.
The purpose of this chapter is to provide an overview of environmental regulation. Much of the material on regulations in this chapter has been adapted from the excellent review of environmental law by Lynch (1995). More comprehensive sources on this topic include the United States Code (U.S.C.) and the Code of Federal Regulations (C.F.R.), which are sets of environmental statutes and regulations, respectively; they are available online at the site maintained by the federal government printing office. The Environmental Law Handbook (Sullivan and Adams, 1997) and West’s Environmental Law Statutes (West Publishing Co.) are compendia of existing statutes. Most of these sources can be found online.
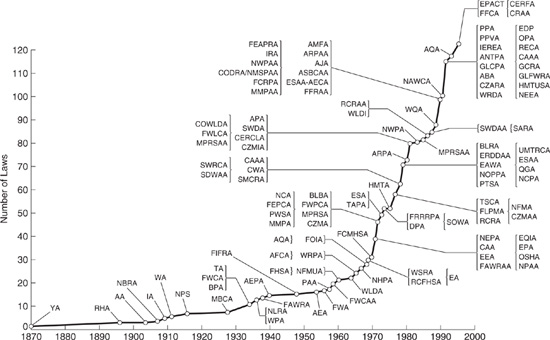
There are approximately 20 major federal statutes, hundreds of state and local ordinances, thousands of federal and state regulations, and even more federal and state court cases and administrative adjudications, etc., that deal with environmental issues. Taken together, they make up the field of environmental law, which has seen explosive growth in the last 30 years, as shown in Figure 3.1-1. Chemical engineers should be familiar with environmental laws and regulations because they affect the operation of chemical processes and the professional responsibilities of chemical engineers. Environmental regulations and the common law system of environmental law require actions by affected entities. For example, the Clean Water Act (an environmental statute) requires facilities which discharge pollutants from a point source into navigable waters of the United States to apply for a National Pollutant Discharge Elimination System (NPDES) permit. In many firms, chemical engineers are responsible for applying for and obtaining these permits. The common law created by judicial decision also encourages chemical engineers to act responsibly when performing their professional duties because environmental laws and regulations do not cover every conceivable environmental wrong. Chemical engineers need to be aware of potential legal liability resulting from violation of environmental laws and regulations to protect their company and themselves from legal and administrative actions.
The sources of environmental law and regulations are legislatures, administrative agencies, and the courts. When drafting environmental laws, federal and state legislatures often use broad language to describe the objectives, regulatory programs, and enforcement provisions of the statute. Often, legislators do not have the time or resources needed to implement the statute and therefore leave the detailed development of regulations to administrative agencies. Administrative agencies, such as the Environmental Protection Agency, give meaning to statutory provisions through a procedure known as rule making. Federal rule making consists of giving notice of proposed new regulations by publication in the Federal Register, providing an opportunity for public comment, altering the proposed rule, where appropriate, to incorporate the comments received, and publishing final regulations in the Federal Register. Final rules have the force of law. As such, administrative agencies fulfill a legislative function delegated to them by Congress.
Administrative agencies can be created by the executive or legislative branches of government. In 1970, President Nixon established the United States Environmental Protection Agency by executive order to consolidate federal programs for regulating air and water pollution, radiation, pesticides, and solid waste disposal. However, administrative agencies are most often established by statute (for example, the Occupational Safety and Health Act established the Occupational Safety and Health Administration), and in these cases, the agency powers are derived from their enabling legislation. Administrative agencies also have the authority to resolve disputes that arise from the exercise of their administrative powers. Regulated entities have the right to appeal decisions made by administrative agencies to an administrative law judge, who is appointed by the agency. Thus administrative agencies have a judicial function in addition to a legislative function.
Figure 3.1-1 Cumulative growth in federal environmental laws and amendments.
Courts are a third government actor that defines the field of environmental law. The role of the courts in environmental law is:
1. To determine the coverage of environmental statutes (which entities are covered by regulations);
2. To review administrative rules and decisions (ensuring that regulations are promulgated following proper procedures and within the limits of statutorily delegated authority); and,
3. To develop the common law (a record of individual court cases and decisions that set a precedent for future judicial decisions).
Section 3.2 provides a brief description of the most important features of nine federal environmental statutes that most significantly affect chemical engineers and the chemical industry. This brief survey is meant to be representative, not comprehensive, and the focus will be on federal laws because they have national scope and often serve as models for state environmental statutes. We begin with three statutes that regulate the creation, use, and manufacture of chemical substances. Next, we cover the key provisions of three statutes that seek to control the discharge of pollutants to specific environmental media—air, water, and soil. Next, a statute that initiated a clean-up program for the many sites of soil and groundwater contamination is discussed. The final two statutes involve the reporting of toxic substance releases and a voluntary program for preventing pollution generation and release at industrial facilities. Section 3.3 describes the evolution in environmental regulation from end-of-pipe pollution control to more proactive pollution prevention approaches. Section 3.4 presents the key features of pollution prevention, including its position in the hierarchy of environmental management alternatives, a short review of terminology, and examples of pollution prevention strategies and applications.
3.2 Nine Prominent Federal Environmental Statutes
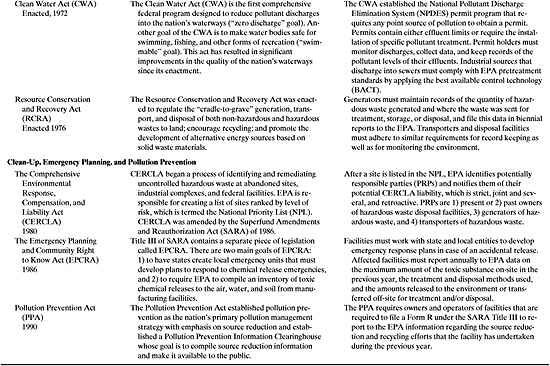
This section provides the key provisions of nine federal environmental statutes that every chemical engineer should know. Taken together, these laws regulate chemicals throughout their life cycle, from creation and production to use and disposal. The nine laws are:
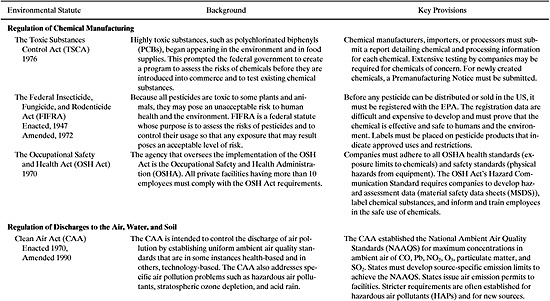
a) The Toxic Substances Control Act (TSCA), 1976 (regulating testing and necessary use restrictions on chemical substances).
b) The Federal Insecticide, Fungicide, and Rodenticide Act (FIFRA), 1972 (the manufacture and use of pesticides).
c) The Occupational Safety and Health Act (OSHA), 1970 (to protect health and safety in the workplace).
d) The Clean Air Act (CAA), 1970 (to protect and enhance the quality of the Nation’s air resources).
e) The Clean Water Act (CWA), 1972 (to restore and maintain the chemical, physical, and biological integrity of the Nation’s water resources).
f) The Resource Conservation and Recovery Act (RCRA), 1976 (the regulation of hazardous and non-hazardous waste treatment, storage, and disposal).
g) The Comprehensive Environmental Response, Compensation, and Liability Act (CERCLA), 1980 (the cleanup of abandoned and inactive hazardous waste sites).
h) The Emergency Planning and Community Right-to-Know Act (EPCRA), 1986 (responding to chemical emergencies and reporting of toxic chemical usage).
i) The Pollution Prevention Act (PPA), 1990 (a proactive approach to reducing environmental impact).
A summary of these prominent federal environmental statutes is provided in Table 3.2–1. The most important regulatory provisions for each statute are stated along with a listing of some key requirements for chemical processing facilities. A more complete description of these federal statutes is included in Appendix A.
3.3 Evolution of Regulatory and Voluntary Programs: From End-of-Pipe to Pollution Prevention
Many of the environmental laws listed in the previous section were enacted to ensure the protection of a single environmental medium. For example, the Clean Air Act instituted a strategy for pollution control on atmospheric emissions. Similarly, the Clean Water Act and the Resource Conservation and Recovery Act provided systems for the protection of the water and the soil environments, respectively. Although these legislative actions have been extremely effective in restoring and maintaining environmental media, they have not ensured that the total amount of hazardous materials entering the environment will eventually decrease. In fact, despite more than twenty years of regulation the volumes and hazards of toxic chemical releases into the environment continued to grow through the 1970s and 1980s (Johnson, 1992).
Beginning in the mid to late 1980s, however, the absolute amounts of toxic releases to the environment in many categories began to decrease. If one uses the Toxics Release Inventory (TRI) as a gauge, the amount of “toxics” released decreased from 3.4 billion pounds in 1986 to less than 2.0 billion pounds in 1998 (USEPA, 2000). The amount released decreased every year from 1988 through 1996 (releases in 1997 were slightly up from 1996 as a result of a booming economy). In addition, concentrations of many categories of pollutants in the environment are going down over time. This is true for ozone, lead, volatile organic compounds (VOCs), and carbon monoxide (CO). Other environmental indicators are also showing improvement. For example, the amount of energy used per dollar of Gross National Product has decreased from about 15,000 to 11,000 Btu/1990 dollar (EIA, 1998) over the last 10 years.
Table 3.2-1 Summary Table for U.S. Environmental Laws
As additional reductions in emissions to individual environmental media are sought, it is important to guard against moving pollutants from one environmental medium into another. For example, traditional air pollution control devices such as scrubbers transfer pollutants from a gaseous stream to a liquid stream. The liquid stream would require further treatment to either remove or destroy the original contaminant. Conversely, some wastewater streams containing volatile organic compounds are contacted with an air stream, transferring the pollutants from the water to air. A more subtle form of media shifting can occur when pollutants are destroyed or trans-formed into less harmful forms by reaction during waste treatment. These processes can be very energy intensive, and energy use can result in the formation of pollutants.
It is clear from the trends just discussed that a complementary strategy is needed to reduce the amounts and the hazardous characteristics of industrial wastes released into all media of the environment. This strategy should also decrease the amounts of contaminants entering traditional waste treatment processes. In the next section of this chapter, we will review the environmental management hierarchy as outlined in the Pollution Prevention Act of 1990 and define important terms, such as pollution prevention, source reduction, and others. These definitions will provide a proper context and categorization for much of the pollution prevention design activities discussed in the remainder of the text.
3.4 Pollution Prevention Concepts and Terminology
A logical starting point for understanding pollution prevention concepts is the waste management hierarchy established in the Pollution Prevention Act of 1990. The waste management hierarchy is defined as follows (U.S.C. §§13101–13109):
The Congress hereby declares it to be the national policy of the United States that pollution should be prevented or reduced at the source whenever feasible; pollution that cannot be prevented should be recycled in an environmentally safe manner, whenever feasible; pollution that cannot be prevented or recycled should be treated in an environmentally safe manner whenever feasible; and disposal or other release into the environment should be employed only as a last resort and should be conducted in an environmentally safe manner.
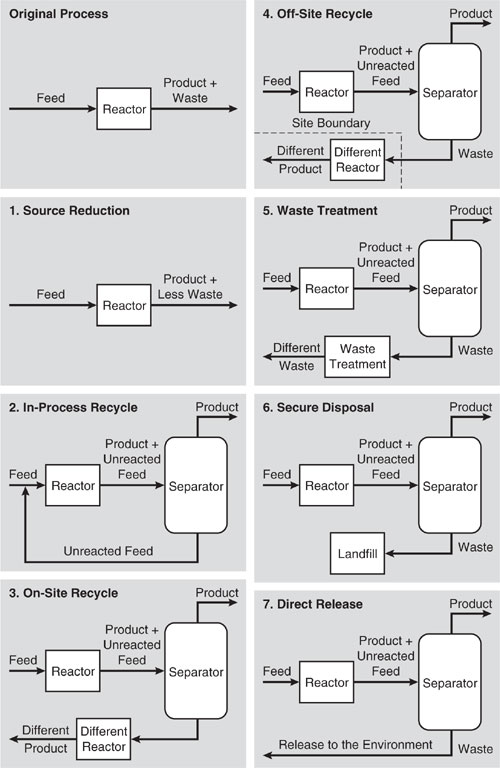
Based on this definition and distinctions between recycle options, we can place the waste management hierarchy in the following descending order, from the most to the least preferable:
2. In-process recycle
3. On-site recycle
4. Off-site recycle
5. Waste treatment
6. Secure disposal
7. Direct release to the environment
The distinction between these seven elements of the waste management hierarchy are shown in Figure 3.4-1, using a simple reactor/separator sequence of units in chemical processes (adapted from Allen and Rosselot, 1997).
1. Source reduction—the reactor is modified so that less waste is generated or so that the waste is less hazardous.
2. In-process recycle—unreacted feed is separated and recycled back to the reactor.
3. On-site recycle—waste from the reactor is converted to a commercial product by a second reactor within the facility.
4. Off-site recycle—waste from the reactor is separated and then transferred off-site where it is converted to a commercial product within another facility.
5. Waste treatment—waste from the reactor is separated and then treated to render it less hazardous.
6. Secure disposal—waste from the reactor is separated and sent to a secure disposal facility (landfill).
7. Direct release to the environment—waste is separated from product and released to the environment.
The waste management hierarchy introduces a number of terms that require definition if the scope of pollution prevention activities is to be understood. In the federal Pollution Prevention Act of 1990, source reduction is defined as:
A. The term “source reduction” means any practice that
1. Reduces the amount of any hazardous substance, pollutant, or contaminant entering any waste stream or otherwise released into the environment (including fugitive emissions) prior to recycling, treatment, or disposal.
2. Reduces the hazards to public health and the environment associated with the release of such substances, pollutants, or contaminants.
The term includes equipment or technology modifications, process or procedure modifications, reformation or redesign of products, substitution of raw materials, and improvements in housekeeping, maintenance, training, or inventory control.
Following are a few examples of source reduction (Hunt, 1995). Inventory control aims to reduce waste generation resulting from “out-of-date” or “off-spec” raw materials or final products. Effective techniques for inventory control might include ordering only the amount of raw material needed for one production run or reviewing purchasing procedures to eliminate hazardous chemicals and substitute environmentally-friendly alternatives. Other techniques for inventory control might be more challenging, such as adopting just-in-time manufacturing techniques. Modifying production procedures can lead to waste reduction and increased profits. A joint DuPont/EPA pollution prevention study showed that a cleaning solvent waste from a specialty chemical multiple batch process could be completely eliminated (US EPA, 1993). A source reduction project installed drains at low points in the process to recover chemicals from the prior campaign, yielding a Net Present Value of $2,212,000. More examples of source reduction methods for all industries are available in several references (US EPA, 1992 and 1993; Hunt, 1995). We will present several examples of unit operation-specific pollution prevention methods in Chapter 9.
The federal legislation continues to define what source reduction is not.
B. The term “source reduction” does not include any practice which alters the physical, chemical, or biological characteristics or the volume of a hazardous substance, pollutant, or contaminant through a process or activity which itself is not integral to and necessary for the production of a product or the providing of a service.
The federal definition of source reduction is controversial because it seems to exclude activities that may reduce the amounts of hazardous substances entering waste streams by processes that may not be “integral to and necessary for the production of a product or the providing of a service.” These potentially beneficial, although excluded, processes would typically fall into the categories of on-site and off-site recycle according to the federal definition. In addition to the federal definition of source reduction, there are many state legislatures and other pertinent bodies having similar definitions that are either more or less exclusive in terms of allowable activities (Foecke, 1992).
In order to help clarify which activities constitute pollution prevention and which do not, the Pollution Prevention Act of 1990 provides a definition (Habitch, 1992).
Pollution prevention means “source reduction,” as defined under the Pollution Prevention Act, and other practices that reduce or eliminate the creation of pollutants through
• Increased efficiency in the use of raw materials, energy, water, or other resources, or
• Protection of natural resources by conservation.
The act (Habitch, 1992) goes further to state what recycling activities are included within pollution prevention activities.
Drawing an absolute line between prevention and recycling can be difficult. “Prevention” includes what is commonly called “in-process recycling,” but not “out-of-process recycling.” Recycling conducted in an environmentally sound manner shares many of the advantages of prevention, such as energy and resource conservation, and reducing the need for end-of-pipe treatment or waste containment. … Some practices commonly described as “in-process recycling” may qualify as pollution prevention.
Thus, the EPA considers the first two elements of the waste management hierarchy as pollution prevention: source reduction and in-process recycling. However, many on-site and off-site recycling activities are consistent with the intent of pollution prevention because of the resulting increased efficiency in the use of raw materials, energy, and other resources. At the state level, there is some consensus in the definition of pollution prevention. For example, 17 states exclude off-site recycling and 14 exclude treatment or incineration (Foecke, 1992). Thus, many state legislatures consider pollution prevention to include the first two and perhaps three elements of the waste management hierarchy. The Pollution Prevention Task force of the American Petroleum Institute, an industry group, provides a more expansive definition of pollution prevention, to include environmentally sound recycling and multimedia reductions in discharges to air, water, and soil (API, 1993). Other definitions exist, some more restrictive and others more expansive (California EPA, 1991).
One of the key concepts that is useful in determining whether a process change is considered pollution prevention or not is defining what is and what is not a process. Is a process only a simple sequence of a reactor and a separator as shown in Figure 3.4-1 or can we consider a process to be comprised of a set of integrated subprocesses? For example, consider the process change shown in Figure 3.4-2 where two reactors are shown (adapted from Allen and Rosselot, 1997). The first reactor converts feeds A and B to product C and byproduct D and a second reactor converts feeds D and E to product F. This modification would be considered in-process recy-cling and thus pollution prevention by the federal definition if the two reactions are considered to comprise a single integrated process. If each reactor is considered to be a separate process, then this modification would be considered on-site (out-ofprocess) recycle and not pollution prevention according to the federal definition.
Another important consideration is what consitiutes a waste that would need to be treated to render the stream less hazardous and what consititues an intermediate stream composed of byproducts that can be transformed into commercial products. Resolving this issue can help categorize process modifications as waste treatment or intermediate recycling. Consider the process flow diagram on Figure 3.4-3 (adapted from Allen and Rosselot, 1997). The top process diagram features in-process recycling of components A and B to the reactor for further reaction and separation of product C from waste D, which is disposed into the environment. After the process modification, the stream containing D is reacted and separated further on-site to render a recycle stream containing A only and one containing E, which is transferred to an off-site recycle operation. The key issue is whether we consider stream D as a waste stream or another intermediate stream which is processed further into salable products. Thus it is difficult to know whether this process modification is waste treatment or recycling. Furthermore, the Pollution Prevention Act of 1990 provides no guidance for this and many other situations that must be considered on a case-by-case basis.
Figure 3.4-1 Waste management modifications for a simple reactor/separator process classified according to the waste management hierarchy. Source: Allen and Rosselot, Pollution Prevention for Chemical Processes © 1997. This material is used by permission of John Wiley & Sons, Inc.
Because of the ambiguities in the definition of pollution prevention and distinguishing between key elements of the waste management hierarchy, we will adopt a more expansive definition of pollution prevention in this text. Process design modification for pollution prevention will constitute the first four elements of the waste management hierarchy: source reduction, in-process recycle, on-site (out of process) recycle, and off-site recycle. The justification for this expanded definition is the many cases where recycle modifications accomplish the primary goals of pollution prevention: improving the efficiency of raw materials conversion and reducing the consumption of energy, water, and other resources.
Figure 3.4-2 A process modification involving two reactors that are part of the same industrial facility. It is in-process recycling or on-site recycling? Source: Allen and Rosselot, Pollution Prevention for Chemical Processes © 1997. This material is used by permission of John Wiley & Sons, Inc.
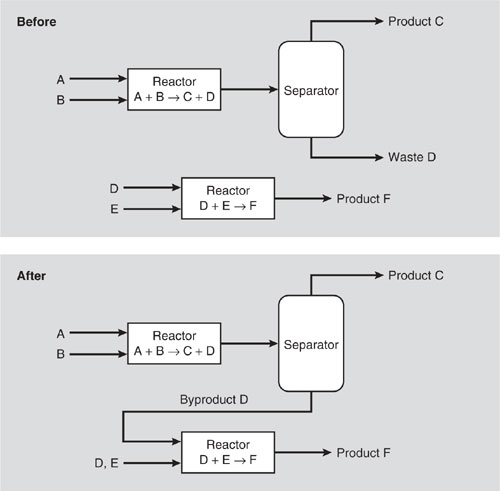
Figure 3.4-3 A process modification. Is it waste treatment or recycling? Source: Allen and Rosselot, Pollution Prevention for Chemical Processes © 1997. This material is used by permission of John Wiley & Sons, Inc.

References
Allen, D.T. and Rosselot, K.S., Pollution Prevention for Chemical Processes, John Wiley & Sons, New York, 1997.
API, American Petroleum Institute, Environmental Design Considerations for Petroleum Refining Crude Processing Units, Publication 311, Feb. 1993.
California EPA (California Environmental Protection Agency), “Report of the 90 Day External Program Review of California’s Toxic Substances Control Program,” 1991.
EIA, Energy Information Administration, US Department of Energy, “International Energy Outlook—1998”, DOE/EIA-0484(98), 1998.
Foecke, T. “Defining pollution prevention and related terms,” Pollution Prevention Review, 2(1), 103–112, Winter 1991/1992.
Habitch, F.H., Memo to all EPA personnel, May 28, 1992.
Hunt, G.E., “Overview of waste reduction techniques leading to pollution prevention,” in Industrial Pollution Prevention Handbook, (ed.) Freeman, H.M., McGraw-Hill, pg 9–26, 1995.
Johnson, S. “From reaction to proaction: The 1990 Pollution Prevention Act,” 17 Columbia Journal of Env. Law, 153, 156 (1992).
Lynch, H., “A Chemical Engineer’s Guide to Environmental Law and Regulation,” National Pollution Prevention Center for Higher Education, University of Michigan, Ann Arbor, MI, 1995. http://css.snre.umich.edu.
Sullivan, T.F.P. and Adams, T.L., Environmental Law Handbook, Government Institutes, Rockville, MD, 1997.
US EPA, “Pollution Prevention Case Studies Compendium,” United States Environmental
Protection Agency, Office of Research and Development, EPA/600/R-92/046, April 1992. US EPA, “DuPont Chambers Works Waste Minimization Project,” United States Environ-mental Protection Agency, Office of Research and Development, EPA/600/R-93/203, pg. 86–91, November 1993.
US EPA, “33/50 Program: The Final Record,” United States Environmental Protection Agency, Office of Pollution Prevention and Toxics, IPA-745-R-99-004, March 1999.
US EPA, United States Environmental Protection Agency, 1997 National Air Quality and Emissions Trends Report, Office of Air Quality Planning and Standards, Research Triangle Park, NC 27711, EPA 454/R-98-016, December 1998, http://www.epa.gov/oar/aqtrnd97/.
US EPA, “1998 Toxics Release Inventory Public Data Release,” United States Environmental Protection Agency, Office of Information Analysis and Access, EPA 745-R-00-007, September 2000.
Problems
1. Provide definitions for the following terms
(a) pollution prevention
(b) source reduction
(c) in-process versus on-site versus off-site recycling
(d) waste treatment
(e) disposal
(f) direct release
2. Categorize the following solvent recovery operation in terms of the waste management hierarchy. Discuss the pollution prevention features of this process. Assess whether this process is pollution prevention, using both the federal definition and also the expanded definition adopted in this text.
Process Description: The automotive industry uses robots to paint automobile bodies before attaching them to the chassis, and installing other components such as the drive train, lights, trim, and upholstery. In order to accommodate different colors, the paint lines must be flushed with a solvent and then re-charged with the new color paint. In the past, this solvent and paint residue was disposed of as hazardous waste or incinerated. The current process of spray painting automobiles uses a closed-loop sol-vent recovery process as outlined in the diagram below (Gage Products, Ferndale, MI).

3. Choose one of the nine federal environmental statutes listed in Table 3.2-1 and then analyze the regulatory provisions for the potential to impact a chemical production facility’s capital and operating costs. What are the key provisions requiring action? What is the nature of those actions? What are the cost implications of those actions? The information contained in Appendix A will be helpful in answering these questions.
4. Categorize the following chemical process source reduction case studies using one or more of the following source reduction categories:
• equipment or technology modifications,
• process or procedure modifications,
• reformation or redesign of products,
• substitution of raw materials,
• improvements in housekeeping,
• maintenance,
• training, or
• inventory control.
(a) A specialty aromatic compound (SAC) process includes a reaction and a distillation train. This process relies on the quality of feed from a separate raw materials process. Due to poor “acid” control from the raw materials process, acid tars are generated within the SAC process, together with thermal tars, at the rate of 0.07 pounds of incinerable tars per pound of SAC product. A relatively large fraction of the tar mass is entrained in the SAC product. Installation of on-line instrumentation for pH control on the raw materials process allowed operators to maintain low acidity levels in the product leaving this processing step. Due to this effort and a lower reactor temperature in the SAC process, SAC waste was reduced by 60% (to 0.03 lb waste/lb SAC product) and had a Net Present Value (12%) of almost $1,000,000. (This case study demonstrates that waste generation can and often does result from complex interactions between separate processing steps in a chemical production facility.)
(b) The crude product from a specialty alcohol process required two washing steps to remove corrosive chlorinated compounds and residual acidity. The wash steps were conducted using two vessels; a wash kettle and an accumulation drum, with the wash solution being composed of water and isopropyl alcohol. The wash solution was sent to an on-site wastewater treatment plant. The washing operations were a severe bottleneck step for the entire process. Over time, the reaction steps leading to the crude specialty alcohol were improved, resulting in a nearly impurity-free crude product with only residual acidity. Because of this realization, the wash steps were completely unnecessary and were replaced with a neutralization step, resulting in elimination of the wastewater stream. The capital cost for this was $40,000 and the project had a NPV (12%) of $272,000.