Appendix B
Molecular Connectivity
Correlations for environmentally relevant physical and chemical properties, described in Chapter 5, are primarily based on bulk properties such as boiling point and octanol-water partition coefficient. While these bulk properties are adequate correlating parameters for many properties, they are not adequate for properties that depend on molecular topology, such as soil sorption. In situations where a description of molecular topology is required, a simple alternative is to utilize the molecular connectivity (χ).
The concept of molecular connectivity initially appeared in the pharmaceutical literature and a variety of molecular connectivity indices have been used in predicting drug behavior (Kier and Hall, 1986). This text uses only the most basic of molecular connectivity indices—the simple first order molecular connectivity (1χ). The goal of this index is to characterize, in a single scalar parameter, the degree of connectedness or the topology of the molecule. A complete description of the rationale behind the molecular connectivity is beyond the scope of this text. The interested reader is referred to Kier and Hall (1986). Instead, the focus here will be on the steps required to calculate 1χ.
The first step in calculating 1χ is to draw the bond structure of the molecule. For example, isopentane would be drawn as:
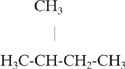
The next step is to count the number of carbon atoms to which each carbon is attached (count any heteroatom as a carbon, but ignore bonds to hydrogen). The assignments of this parameter (δi, the connectedness of carbon atom i) for each carbon in isopentane are given below.
For each bond, identify the connectedness of the carbons connected by the bond (δi, δj). For isopentane, these pairs are:
(1,3),(1,3),(3,2),(2,1)
The value of 1χ is calculated using the equation:
1χ=Σ(δl*δj)-0.5
For isopentane,
![]()
Clearly, this calculation yields a simplistic characterization of complex structural features. Note that isopentene would yield exactly the same value as isopentane, as would 1-chloro, 2 methyl propane. Nevertheless, this simple characterization of molecular topology is often used, as described in Chapter 5, in developing property correlations.
Example B-1
Estimate1χ for 4-chloro-aniline.
Solution: The molecular structure and the connectedness of each carbon or heteroatom are shown below:

The bond pairs, beginning with the amine and continuing clockwise around the molecule, are (1,3), (3,2), (2,2), (2,3), (3,1), (3,2), (2,2), (2,3)
![]()

