3.4. Aerosol Formation
The physical state of liquid or solid particles suspended in air is known as aerosol. Atmospheric aerosol has long been studied and several classic books exist in this area. Even in industry, hazard exhibited by two-phase mixtures, aerosol, is often overlooked (Bowen and Cameron, 1999). It is widely accepted (Lian et al., 2011) that safety in process industry is also shaped by the presence of aerosol. Since aerosol cannot be treated as gas or liquid, assessing hazard associated with it needs a different approach. Pressurized release of high flash point industrial fluids, such as kerosene, has the potential to create a flammable aerosol. Pressure differential as low as few bars (Bowen and Shirvill, 1994) will suffice to initiate such phenomenon. On the contrary, water aerosol has served as diluent in process industries by providing inerting action (Shebeko et al., 1995) to a process stream. In either case, understanding formation mechanism of aerosol has the potential to provide useful insight and add value to modeling approaches related to flammable aerosols. Current understanding related to complexity of aerosol formation and its corresponding combustion process is not complete (Lian et al., 2011; Bowen and Shirvill, 1994) and to accurately determine associated fire and explosion hazard, there is a need to perform more studies in this area.
As addressed above, aerosol modeling approaches or methodologies in the context of process safety can be classified into three areas of interest:
• Formation
• Dispersion of aerosol
• Combustion of aerosol or aerosol particle reactivity
While most used as air quality models, several dispersion models do exist to address the modeling of aerosols. Models such as Air Force Dispersion Assessment Model (ADAM), modified box and Gaussian dispersion model, HGSYSTEM, and the regional modeling system for aerosol and deposition (REMSAD) need information on aerosol present in the atmosphere as prerequisite. Detailed dispersion modeling, which provides further insight into aerosol dispersion is done through the implementation of computational fluid dynamics approaches.
In addition to dispersion modeling, understanding the formation of aerosol droplets, characterization of these aerosol droplets such as their evaporation characteristics, size and movement velocity is important to perform accurate consequence study. Lian et al. (2011) has studied the effects of aerosol properties such as their size and evaporation rate on characteristics of the flame such as their length and propagating speed using heat transfer fluids. These methods, as mentioned above, deal with post aerosol formation and combustion. Approaches or methodologies to obtain insight on the formation of aerosol and its combustion is primarily done through experiments and modeling at the molecular level. Hence the modeling approaches to assess risk associated with flammable aerosol, can be categorized as:
• Macroscale modeling approaches
• Molecular modeling approaches
As briefed in the above section, various modeling approaches exist to address dispersion of aerosol. Sodium fire and combustion is an example where aerosol is formed.
In Yamaguchi and Tajima (2003) the authors have proposed expressions for aerosol dynamics, radiation heat transfer, and evaporation, arising from liquid sodium combustion phenomena. The multiscale nature of liquid sodium combustion phenomena is best depicted by Figures 3.10 and 3.11.

Figure 3.10 Evaporation from liquid pool (Yamaguchi and Tajima, 2003).

Figure 3.11 Multiscale nature of liquid sodium combustion (Yamaguchi and Tajima, 2003).
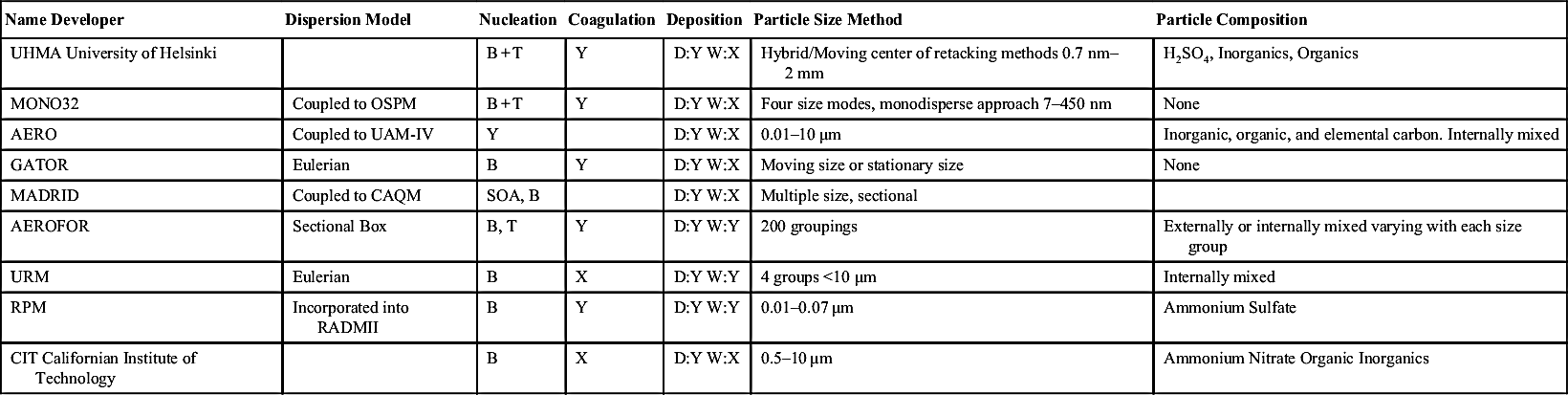
Although the specific work looked into the modeling related to liquid metal fast reactor, the developed model also proposed approaches to capture aerosol generation in the flame zone, coagulation, convection, and deposition. The absorption coefficient, required to calculate the thermal radiation, was adjusted based on aerosol concentration. The authors estimated the evolution of the aerosol particle size distribution by implementing the equation on aerosol density as developed earlier (Wen, 1996). The governing equation, dynamic in nature, helps one to update both the aerosol number density and statistical distribution of particle diameter with time. Incorporation of Brownian movements, relative velocities between aerosol particles including Cunningham correction factor to account noncontinuum effects, and turbulence together builds the model to capture aerosol coagulation (Gelbard and Seinfeld, 1980). Details of aerosol behavior model are not discussed here, and the reader can refer to several references that are available on the subject. Comparative review of various methodologies to model aerosol dynamics, coagulation, growth, nucleation, and gas or particle mass transfer has been done (Zhang et al., 1999). The aerosol modules of CIT (Meng et al., 1998), GATOR (Jacobson, 1997a,b), Models-3 (Binkowski and Shankar, 1995), SAQM-AERO, UAM-AERO (Lurmann et al., 1997), and UAM-AIM (Sun and Wexler, 1998) were reviewed. The reviewers observed that the algorithms available to model coagulation and condensational particle growth can be grouped as sectional or modal, based on particle size distribution. Accurate depiction of coagulation was obtained with a sectional approach, as opposed to modal approach. Within sectional approach, implementation of moving center algorithm as well as hybrid algorithms provided an accurate estimation of condensational growth. A brief comparison of aerosol dynamics is presented in Table 3.6 (Holmes and Morawska, 2006). In all the models referred in the table, modeling of condensation and evaporation processes was included.
The review article (Holmes and Morawska, 2006) looked into various types of models including box models, Gaussian models, Lagrangian/Eulerian models, CFD models, and other miscellaneous models that included aerosol dynamics.
3.4.1. Molecular Modeling Approaches
In determining the hazards associated with aerosols, understanding aerosol reactivity is very important. For example, direct observation (Savee et al., 2015) of highly reactive hydroperoxyalkyl radical species involved in combustion and aerosol chemistry is expected to improve models of atmospheric and combustion chemistry (Kemsley, 2015). In addition to understanding and predicting the formation of aerosol, another major part is to understand how aerosols interact with volatile compounds, say, for example, formaldehyde. In addition to formation mechanism, aerosol chemistry at the air–water interface is particularly still very poorly understood (Martins-Costa and Anglada, 2012). DFT has been used to investigate the mechanism behind the formation of atmospheric aerosols from sulfuric acid and its hydrates in gas phase (Nadykto et al., 2004). The study focused on the nucleation mechanism behind such phenomena, and used DFT to look into various equilibrium structures of sulfuric acid and its hydrate, up to three water molecules per sulfuric acid molecule. The authors calculated dipole moments, vibrational transitions and values of the total bonding energy for several possible structures. The total bonding energy (ETB), difference in energy between the molecule as a whole and its individual constituent atoms, can be equated to:
![]()
Table 3.6
Comparison of Aerosol Dispersion Models (Holmes and Morawska, 2006)
| Name Developer | Dispersion Model | Nucleation | Coagulation | Deposition | Particle Size Method | Particle Composition |
| UHMA University of Helsinki | B + T | Y | D:Y W:X | Hybrid/Moving center of retacking methods 0.7 nm–2 mm | H2SO4, Inorganics, Organics | |
| MONO32 | Coupled to OSPM | B + T | Y | D:Y W:X | Four size modes, monodisperse approach 7–450 nm | None |
| AERO | Coupled to UAM-IV | Y | D:Y W:X | 0.01–10 μm | Inorganic, organic, and elemental carbon. Internally mixed | |
| GATOR | Eulerian | B | Y | D:Y W:X | Moving size or stationary size | None |
| MADRID | Coupled to CAQM | SOA, B | D:Y W:X | Multiple size, sectional | ||
| AEROFOR | Sectional Box | B, T | Y | D:Y W:Y | 200 groupings | Externally or internally mixed varying with each size group |
| URM | Eulerian | B | X | D:Y W:Y | 4 groups <10 μm | Internally mixed |
| RPM | Incorporated into RADMII | B | Y | D:Y W:Y | 0.01–0.07 μm | Ammonium Sulfate |
| CIT Californian Institute of Technology | B | X | D:Y W:X | 0.5–10 μm | Ammonium Nitrate Organic Inorganics |
The coulombic energy term here also incorporates the steric and orbital interaction energies. The study estimated properties of optimized molecular structures of gas-phase sulfuric acid and a small cluster through incorporating one, two, or three water molecule associated with one sulfuric acid molecule. Entropies, enthalpies, and Gibbs free energies were estimated using ADF, a DFT-based software package for these molecules. In addition to looking into optimum structures of various sulfuric acid hydrates, the study looked into possible reaction paths leading to the formation of such molecules. Put differently, the study defined end aerosol structures and looked into the relative ease of one forming over others, including the effect of change in temperature and pressure. This method thus implemented not only provide insight on possible aerosol particle size distribution but also favorable pathways of aerosol formation as a function of temperature and pressure. The ΔG value, change in Gibbs free energy, was calculated to be increasing with increasing pressure and decreasing with increasing temperature. That is, this supports the concern of aerosol formation from pressurized sources of even high flash point liquid. The dipole moment estimation for each structure, which was high in this study for all structures, that is, mono-, di-, and trihydrates of sulfuric acid, provided insight on possibility of nucleation of ultrafine particles in the atmosphere.
The authors used PW91 method to account for exchange correction and correlation parts in conjunction with VWM formal version 5 for local density approximation and the TZP basis set. However, to validate results, the sensitivity of the calculated structural data was investigated against the quality of the basis sets such as DZ, DZP, TZP, TZ2P, ET-pVQZ, and ET-QZ3P-1DIFFUSION.
3.4.1.1. Atomistic simulation–based approaches
Atomistic simulation based methods have been implemented along with quantum mechanical calculations and the Clausius–Clapeyron equation to provide a functional form to temperature-dependent vapor pressure equation (Tong et al., 2004) for oxygenates in atmospheric aerosols. The motivation behind this study was to develop a systematic approach to estimate thermodynamic properties of ambient particulate matter containing multifunctional oxygenates. The system of interest was carboxylic acid with different number of carbon atoms. Low molecular weight carboxylic acids are considered moderate fire hazard (Miller et al., 2010), and hence aerosol formed from carboxylic acids pose as flammable hazard. The approach discussed here looked into estimating liquid vapor pressure of organic compounds through implementation of molecular dynamic simulations.
The vapor pressure of an organic compound can be estimated, as an alternative way to existing empirical equations such as Antoine equation, by intergrating total enthalpy of vaporization over temperature using the Clausius–Clapeyron equation (Baum, 1998), approximating heat of vaporization to be linearly related to temperature and can be expressed as
![]()
where  is heat of vaporization at T,
is heat of vaporization at T, 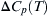 is change in specific heat capacity on phase change, and Tb is normal boiling point.
is change in specific heat capacity on phase change, and Tb is normal boiling point.
Substituting the above equation in vapor pressure equation of Clausius–Claperyon equation generates vapor pressure equation as a function of heat of vaporization.
Problem:
Derive the equation for vapor pressure using the equation for heat of vaporization and Clausius–Clapeyron equation.
The next steps involve accurate estimation of heat of vaporization. It is done through molecular dynamics simulations implemented on the optimized structure including charge distribution, obtained through quantum mechanical calculations, of the molecule of interest. Heat of vaporization can be correlated to cohesive energy density by the following function:
![]()
where Vm is the molar volume, and CED(T) is cohesive energy density.
Cohesive energy density of a pure compound can be calculated using multiple sampling molecular dynamics with periodic boundary conditions. The starting geometry of the molecule along with atomic charges is taken from energy minimized structure as obtained from B3LYP flavor of DFT calculations. The force field used in this particular study was Drieding force field with hydrogen bonding parameters modified based on quantum mechanical calculation results for the dimer using acetic acid dimer. The structural evolution of the system from a reduced density to a target density matching experimentally obtained results was performed in steps, including the use of NVT ensemble in the process of attaining target density and NPT ensemble while equilibrating the system to a final temperature and pressure value after achieving 20% higher than target density. At a specific temperature T, the CED is calculated by subtracting the potential energy of the bulk system from the sum of the potential energies of the individual molecules. Potential energies of individual molecules are estimated through nullifying the effect of nonbonded interaction between inter molecules through separating them by large distances. For a more reliable estimation of CED, a set of 10 samples of the same molecular system went through the set of steps to reduce sampling error. Note that the target temperature for obtaining was set such that the molecule is in liquid state, which, in this particular study was 500 K. Using the correlations (Baum, 1998):
![]()
![]()
along with previously stated results, the authors able to reliably predict vapor pressure of carboxylic acids, which are very important while considering atmospheric aerosols.
3.4.2. Practical Considerations
Multiphase chemical reactions in aerosols and heterogeneous processes and formation mechanism of aerosol are still very poorly understood. Despite the plethora of modeling methods available, owing to challenges in accurate estimation, and appropriate approximation of complexities such as particle size distribution, coagulation, formation, and the like, multiscale modeling methods need to be implemented to access both the detail and the larger time and length scales.
In each scale of modeling and simulation, the user must be aware of applicability and limitations of the method. In Nadykto et al. (2004), the authors investigated sensitivity of using six different DFT methods and six different basis sets. To reduce the sampling error as per the  law, Tong et al. (2004), worked with 10 samples. In the same article, the parameters for hydrogen bonding in Dreiding force field was modified based on DFT results. It was also pointed out that, assumption of linear dependence of heat of vaporization on temperature, can lead to discrepancies in results, as well. However, carefully devised and thought out first principle and atomistic simulation-based fundamental modeling of the system of interest has the potential to provide further insight in understanding flammability hazards arising from aerosol.
law, Tong et al. (2004), worked with 10 samples. In the same article, the parameters for hydrogen bonding in Dreiding force field was modified based on DFT results. It was also pointed out that, assumption of linear dependence of heat of vaporization on temperature, can lead to discrepancies in results, as well. However, carefully devised and thought out first principle and atomistic simulation-based fundamental modeling of the system of interest has the potential to provide further insight in understanding flammability hazards arising from aerosol.
..................Content has been hidden....................
You can't read the all page of ebook, please click here login for view all page.
